Abstract
OBJECTIVES
Our objectives were to identify factors associated with the duration of the first antibiotic course initiated in the first 3 postnatal days and to assess associations between the duration of the initial antibiotic course and subsequent necrotizing enterocolitis or death in extremely low birth weight infants with sterile initial postnatal culture results.
METHODS
We conducted a retrospective cohort analysis of extremely low birth weight infants admitted to tertiary centers in 1998–2001. We defined initial empirical antibiotic treatment duration as continuous days of antibiotic therapy started in the first 3 postnatal days with sterile culture results. We used descriptive statistics to characterize center practice, bivariate analyses to identify factors associated with prolonged empirical antibiotic therapy (≥5 days), and multivariate analyses to evaluate associations between therapy duration, prolonged empirical therapy, and subsequent necrotizing enterocolitis or death.
RESULTS
Of 5693 extremely low birth weight infants admitted to 19 centers, 4039 (71%) survived >5 days, received initial empirical antibiotic treatment, and had sterile initial culture results through the first 3 postnatal days. The median therapy duration was 5 days (range: 1–36 days); 2147 infants (53%) received prolonged empirical therapy (center range: 27%–85%). Infants who received prolonged therapy were less mature, had lower Apgar scores, and were more likely to be black. In multivariate analyses adjusted for these factors and center, prolonged therapy was associated with increased odds of necrotizing enterocolitis or death and of death. Each empirical treatment day was associated with increased odds of death, necrotizing enterocolitis, and the composite measure of necrotizing enterocolitis or death.
CONCLUSION
Prolonged initial empirical antibiotic therapy may be associated with increased risk of necrotizing entero-colitis or death and should be used with caution.
Keywords: antibiotic use, bloodstream infection, extremely low birth weight infants, necrotizing enterocolitis, death
Antibiotics are the most commonly prescribed medications in intensive care nurseries.1,2 Virtually all extremely low birth weight (ELBW) infants (birth weight of <1000 g) admitted to intensive care nurseries receive empirical antibiotic treatment in the first postnatal days, although cultures from normally sterile sites usually do not yield any bacterial agents and the incidence of culture-proven bacterial sepsis is low in this population.2,3 A previous study suggested that selection of cefotaxime instead of gentamycin for the first 3 postnatal days was associated with higher mortality risk, even for the most preterm infants.2 In addition to selecting which antibiotics to use for initial therapy in the first postnatal days, clinicians caring for ELBW infants must choose the duration of the initial empirical antibiotic course. One concern regarding prolonged empirical therapy and treatment with broadly acting therapeutic agents such as cefotaxime is that prolonged therapy perturbs the colonization of the neonatal intestinal flora.4 It is not known whether variations in the duration of the initial antibiotic course in ELBW infants, in the absence of positive blood or cerebrospinal fluid culture results, are associated with the risk of subsequent death or necrotizing enterocolitis (NEC). Our hypotheses were that multiple factors, including gestational age and center, would be associated with variations in the duration of the initial empirical antibiotic therapy provided for ELBW infants with sterile initial postnatal culture results and that prolonged duration of the initial empirical antibiotic course would be associated with the risk of death or NEC among ELBW infants with sterile initial postnatal culture results.
METHODS
Cohort
Data were collected prospectively for ELBW neonates (weighing 401–1000 g at birth) who were born between September 1, 1998, and December 31, 2001, and were cared for at one of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Neonatal Research Network sites. The network maintains a registry of infants with birth weights of 401 to 1500 g who are admitted to NICUs at participating centers within 14 days after birth. Trained research personnel collect maternal sociodemographic, pregnancy, and delivery data soon after birth and infant data from birth until 120 postnatal days, discharge, or death, using common definitions described previously.5 The institutional review board at each center approved participation in the registry.
The registry includes data on early-onset sepsis (EOS) (defined as in previous network reports of EOS,3 on the basis of positive blood culture results obtained within the first 3 postnatal days and treated for ≥5 days), late-onset sepsis (LOS) (culture-positive infections after 3 postnatal days), and infecting organisms. During the study period, infection surveillance was expanded to include detailed data on maternal intrapartum antibiotic use, results of all blood and cerebrospinal fluid cultures, and all antibiotic use for newborns regardless of culture status. Data on intrapartum maternal fever were not collected. Blood cultures were processed by the clinical microbiology laboratory at each center, with either Bactec (Becton Dickinson, Sparks, MD) or BacT/Alert (Organon Teknika, Durham, NC) systems. Decisions regarding the number of blood culture samples collected and whether to obtain samples through catheters or peripherally were at the discretion of the bedside clinician. The usual practice among participating centers was to inoculate blood culture bottles with ≥0.5 mL of blood. The decision to collect other culture samples, including cerebrospinal fluid, was at the clinician’s discretion. Neonates included in the current analysis weighed 401 to 1000 g at birth, survived beyond postnatal day 5, were inborn or admitted within the first 24 hours after birth, had no major birth defects or congenital anomalies, received initial empirical treatment with ≥1 antibacterial agent in the first 3 postnatal days, and did not have EOS.
Definitions
We defined initial empirical antibiotic treatment as the first antibiotic treatment initiated within the first 3 postnatal days. If administration of 2 antibiotics was started on postnatal day 1 and administration of 1 antibiotic was started on postnatal day 3, then we included only antibiotics for which administration was started on postnatal day 1. We defined prolonged initial empirical antibiotic treatment as ≥5 days of initial empirical antibiotic treatment with sterile culture results. The duration of initial empirical antibiotic treatment was defined as the number of days until administration of all initially administered antibiotics was discontinued. Dates but not times of therapy initiation and discontinuation were available; therefore, days of initial antibiotic use might have been overestimated by as much as 1 day in some cases. For example, if administration of antibiotics for a patient born Monday at 8:00 am was started at 9:00 am Monday and stopped at 9:00 am Wednesday, this was counted as 3 days of antibiotic administration. For infants with positive blood or cerebrospinal fluid culture results after the first 3 postnatal days, the duration of initial empirical antibiotic treatment was calculated by using the date of the first positive culture result as the end date (because this likely represented nursery-associated infection and not intrapartum infection); therefore, the duration of initial empirical antibiotic therapy stopped with the first positive culture result even if antibiotic administration was continued after that time. NEC was classified according to the modified Bell criteria, and stage ≥2 NEC was included in the outcome definitions.6
Analysis
To identify factors associated with the duration of initial empirical antibiotic treatment, the median duration and frequency of prolonged initial empirical antibiotic treatment were examined for all infants and for infants at individual centers. Maternal and neonatal baseline characteristics collected within the first 3 postnatal days were compared for infants who did and did not receive prolonged initial empirical antibiotic treatment. Statistical significance for comparisons was determined with χ2 tests for categorical variables and with Wilcoxon tests for continuous variables. Nonparametric tests were used to determine significance for comparisons involving continuous variables because some of the continuous variables were not normally distributed.
Logistic regression models were used to evaluate associations between the duration of initial empirical antibiotic treatment and the use of prolonged initial empirical antibiotic treatment and the 3 outcomes of primary interest, that is, NEC or death, NEC alone, and death alone. In addition to including either duration of initial empirical antibiotic treatment or use of prolonged antibiotic therapy, models included demographic, maternal, perinatal, and neonatal variables shown previously to be associated with NEC and death.7–9 These variables included network center, gestational age, small-for-gestational age status, race, gender, rupture of membranes for >24 hours, prenatal steroid treatment, prenatal antibiotic treatment, maternal hypertension, maternal hemorrhage, multiple births, Apgar score at 5 minutes of <5, and outborn versus inborn. All variables were entered as categorical variables except for gestational age and duration of initial empirical antibiotic treatment, which were entered as continuous variables. A quadratic term for duration of initial empirical antibiotic treatment was initially included in each model with this variable, to assess whether the relationship between duration and outcome was linear. The term was statistically significant for the outcome of death alone; therefore, it was retained in this model but dropped from the models for the other 2 outcomes.
Therapy use may indicate sicker infants who are most at risk for the outcomes studied. In an attempt to minimize this concern, associations between duration of initial empirical antibiotic treatment and outcomes were assessed among the sickest infants, in a subgroup analysis. For this analysis, the sickest infants were defined as infants for whom mechanical ventilation was started on postnatal day 1 and continued through at least the first 7 days or until death (if before day 7). Each outcome model was reanalyzed with this indicator of severity of illness and the interaction between this indicator and the initial antibiotic variable added. For all infants and for those in the 7-day ventilation subgroup, the number needed to harm was calculated for each outcome by using the adjusted odds ratio (OR) associated with prolonged initial empirical antibiotic treatment in the logistic regression model fit to the outcome of interest. To assess the impact of defining prolonged antibiotic treatment beyond the somewhat-arbitrary cutoff point of 5 days, the risk-adjusted overall and ventilation subgroup analyses were repeated by using cutoff points of ≥4 days (when virtually all negative blood culture results would be expected to represent true-negative results and most clinicians would have completed the minimal course to rule out sepsis),10 ≥7 days, and ≥10 days to define prolonged initial empirical antibiotic treatment.
Secondary analyses were conducted to assess associations between prolonged initial empirical antibiotic treatment and LOS. A logistic regression model that included the same covariates as for the primary analysis and prolonged initial therapy defined as ≥5 days was fit to the combined outcome of LOS or death, and analysis was repeated by using a cutoff point of 4 days to define prolonged therapy. Both models were repeated with exclusion of LOS caused by coagulase-negative Staphylococcus from the outcome. Other supplementary analyses examined the impact of early enteral feedings on associations between the duration of initial empirical antibiotic treatment and prolonged initial antibiotic treatment and each primary outcome, as well as the secondary outcome of LOS or death. The logistic regression models for each outcome were reanalyzed to include an indicator for early enteral feedings (started in the first 4 days of life versus started on day 5 or later), as well as an interaction between this indicator and the initial empirical antibiotic treatment variable in the model. All data were analyzed by using SAS 9.1 (SAS Institute, Cary, NC), at RTI International (Research Triangle Park, NC).
RESULTS
Study Group
A total of 5693 infants with birth weights of 401 to 1000 g were born between September 1, 1998, and December 31, 2001, cared for at 19 neonatal network centers, and included in the database. Of those, 1654 infants were excluded from analysis, including 1161 infants who died <5 days after birth, 174 outborn infants who were admitted >24 hours after birth, 125 infants who had major birth defects, 80 infants who had EOS, and 114 infants who did not receive any initial empirical antibiotic treatment in the first 3 postnatal days. Therefore, 4039 infants who had no major birth defects, survived ≥5 days, did not have EOS, and received empirical antibiotic treatment in the first 3 postnatal days were included.
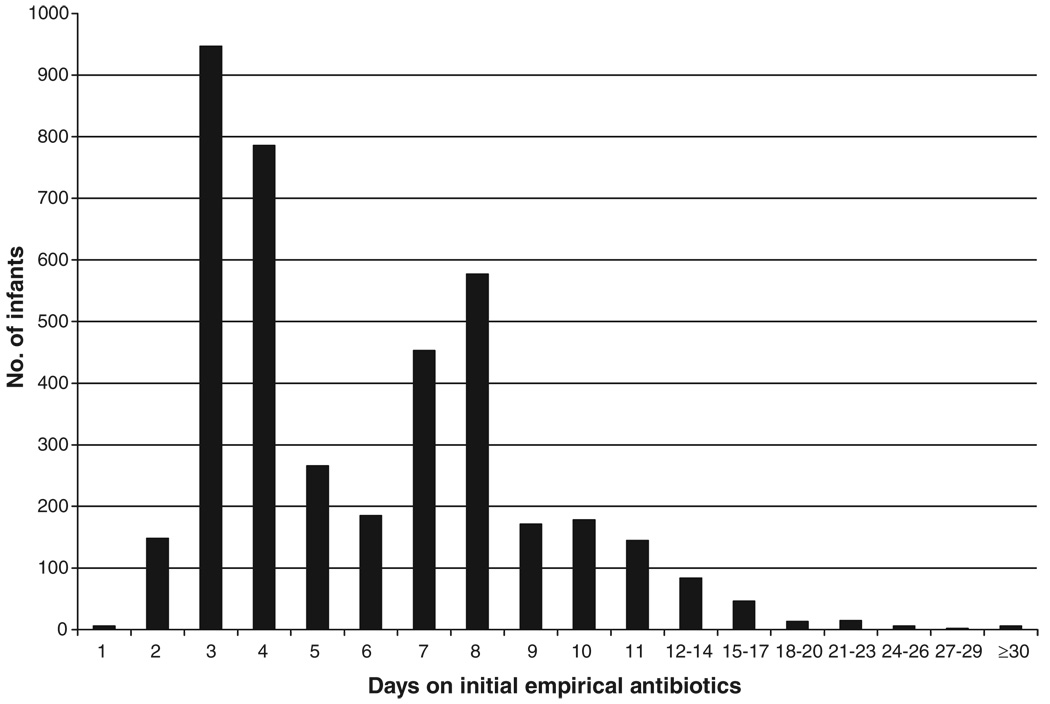
Of the 4039 infants, 3881 (96%) were treated with a combination of 2 antibiotics. Ampicillin and gentamicin were the most commonly prescribed combination (used for 83% of those 4039 infants). Three centers accounted for virtually all cephalosporin use (Table 1). Overall, the median duration of initial empirical antibiotic treatment for individual infants was 5 days (Table 2). The median duration of initial empirical antibiotic treatment varied significantly among centers, from 3 to 9.5 days (P < .001). More than one half of the infants (2147 infants; 53% of 4039 infants) in the study cohort received initial empirical antibiotic treatment for ≥5 days (Fig 1). The range among centers for the proportion of infants who received ≥5 days of empirical antibiotic treatment in the absence of positive culture results was 27% to 85%.
TABLE 1.
Description of Initial Empirical Antibiotic Therapy According to Neonatal Research Network Center (N=4039)
| Center | Proportion, % (n)a | |||||
|---|---|---|---|---|---|---|
| Ampicillin + Gentamycin | Ampicillin + Cefotaxime | Ampicillin + Other Aminoglycosidesb | Ampicillin Only | Gentamycin + Other Antibiotic | Other Antibioticsc | |
| 1 | 85 | 0 | 0 | 2 | 0 | 13 |
| 2 | 99 | 0 | 0 | 0 | 0 | 1 |
| 3 | 1 | 95 | 0 | 1 | 0 | 2 |
| 4 | 32 | 0 | 29 | 2 | 32 | 5 |
| 5 | 78 | 9 | 4 | 1 | <1 | 7 |
| 6 | 97 | 0 | <1 | 0 | <1 | 3 |
| 7 | 90 | 1 | 3 | 2 | 1 | 3 |
| 8 | 94 | 1 | 0 | 2 | 1 | 2 |
| 9 | 83 | 0 | 11 | 4 | 0 | 2 |
| 10 | 91 | 0 | 4 | 3 | 0 | 2 |
| 11 | 97 | 0 | 0 | 1 | 0 | 2 |
| 12 | 92 | 0 | 2 | 4 | 0 | 2 |
| 13 | 96 | 0 | <1 | 3 | 0 | 1 |
| 14 | 97 | 0 | 0 | 1 | 0 | 1 |
| 15 | 94 | 1 | 2 | 1 | 0 | 2 |
| 16 | 100 | 0 | 0 | 0 | 0 | 0 |
| 17 | 92 | 0 | 0 | 2 | 2 | 3 |
| 18 | 97 | 0 | 0 | 0 | 2 | 2 |
| 19 | 30 | 65 | 0 | 0 | 0 | 6 |
| Total | 83 (3334) | 7 (297) | 3 (137) | 2 (69) | 2 (73) | 3 (129) |
Row percentages may not total 100 because of rounding.
Other aminoglycosides indicate amikacin or tobramycin.
Other antibiotics include carbenicillin, oxacillin, penicillin G, piperacillin, nafcillin, cefazolin, ceftazadime, ceftriaxone, cefotetan, vancomycin, clindamycin, and erythromycin. All antibiotics listed were administered to ≥1 infant in the study cohort.
TABLE 2.
Duration of Initial Empirical Antibiotic Treatment and Frequency of Prolonged Initial Empirical Antibiotic Treatment According to Network Center (N = 4039)
| Center | Duration of Initial Empirical Antibiotic Treatment, Median (Range), d |
Prolonged Initial Empirical Antibiotic Course (≥5 d), %a |
|---|---|---|
| 1 | 6 (2–21) | 55 |
| 2 | 4 (2–11) | 49 |
| 3 | 8 (1–33) | 73 |
| 4 | 5 (1–33) | 56 |
| 5 | 7 (1–36) | 73 |
| 6 | 7 (3–29) | 68 |
| 7 | 4 (2–17) | 38 |
| 8 | 6 (1–26) | 55 |
| 9 | 4 (2–26) | 47 |
| 10 | 6 (1–15) | 55 |
| 11 | 3 (2–26) | 29 |
| 12 | 4 (2–19) | 49 |
| 13 | 4 (2–22) | 43 |
| 14 | 4.5 (2–23) | 50 |
| 15 | 5 (2–32) | 54 |
| 16 | 9.5 (2–17) | 85 |
| 17 | 4 (2–33) | 27 |
| 18 | 4.5 (2–15) | 50 |
| 19 | 7 (3–21) | 72 |
| Total | 5 (1–36) | 53 (N = 2147) |
Proportion of infants included in the study cohort (P <.001 for center differences).
FIGURE 1.
Numbers of study infants according to duration of initial empirical antibiotic treatment.
Identification of Risk Factors for Prolonged Initial Empirical Antibiotic Treatment
Infants who received initial empirical antibiotic treatment for ≥5 days in the absence of positive culture results were more likely to be of younger gestational age, to be of lower birth weight, to be black, to have lower Apgar scores, and to have been born >24 hours after rupture of membranes. Their mothers were more likely to have received intrapartum antibiotic treatment. Other variables that were tested are listed in Table 3.
TABLE 3.
Characteristics Associated With Prolonged Initial Empirical Antibiotic Treatment
| Prolonged Initial Empirical Antibiotic Treatment |
p | ||
|---|---|---|---|
| No (N = 1892) |
Yes (N = 2147) |
||
| Maternal demographic features | |||
| Married, n (%) | 873 (47) | 914 (43) | .02 |
| Gravida, mean ± SD | 2.62 ± 1.88 | 2.87 ± 2.05 | <.001 |
| Multiple gestation, n (%) | 459 (24) | 435 (20) | .002 |
| Hypertension, n (%) | 539 (29) | 422 (20) | <.001 |
| Prenatal steroid treatment, n (%) | 1538 (82) | 1685 (79) | .02 |
| >1 course of prenatal steroid treatment,n(%) |
144 (8) | 131 (6) | .07 |
| Intrapartum antibiotic treatment, n (%) |
1213 (64) | 1515 (71) | <.001 |
| Rupture of membranes for >24 h, n (%) | 336 (18) | 569 (27) | <.001 |
| Cesarean section, n (%) | 1207 (64) | 1184 (55) | <.001 |
| Neonatal demographic features | |||
| Birth weight, mean ± SD, g | 790 ± 139 | 752 ± 144 | <.001 |
| Gestational age, mean ± SD, wk | 26.3 ± 2.01 | 25.6 ± 1.89 | <.001 |
| Female, n (%) | 1036 (55) | 1049 (49) | <.001 |
| Race, n (%) | |||
| Black | 783 (42) | 1031 (48) | <.001 |
| White | 771 (41) | 762 (35) | |
| Hispanic | 270 (14) | 299 (14) | |
| Other | 62 (3) | 53 (2) | |
| Small for gestational age, n (%) | 366 (19) | 308 (14) | <.001 |
| 5-min Apgar score of<5, n (%) | 205 (11) | 391 (18) | <.001 |
Variables tested but not associated with prolonged initial empirical antibiotic treatment with P <.1 included maternal age of<25 years, prenatal care (defined as≥1 visit before delivery), maternal diabetes mellitus, maternal hemorrhage, completed ≥1 course of prenatal steroid treatment, and maternal use of tocolytics.
Analysis of Duration of Initial Empirical Antibiotic Treatment and Prolonged Initial Empirical Antibiotic Treatment for Association With NEC or Death
A total of 440 (11%) of the 4039 study infants were diagnosed as having NEC, 203 (46% of infants with NEC) with Bell stage 2a, 2b, or 3a NEC (medical NEC) and 237 (54% of infants with NEC) with Bell stage 3b NEC (surgical NEC). In addition, 658 study infants (16% of 4039 infants) died after postnatal day 5; 919 (23%) of the 4039 infants developed the composite outcome of NEC or death. A larger proportion of infants with this composite outcome had received prolonged initial antibiotic treatment, compared with infants without this outcome (61% vs 51%; P < .001) (Table 4). Results were similar for infants with NEC alone or death alone.
TABLE 4.
Duration of Initial Empirical Antibiotic Treatment and Proportion of Infants Who Received Prolonged Initial Empirical Antibiotic Treatment According to the Outcome of NEC and/or Death
| Outcome | P | ||
|---|---|---|---|
| No | Yes | ||
| NEC or death, N | 3099 | 918 | |
| Duration of initial treatment, median (range), d |
5 (1–36) | 6 (1–33) | <.001 |
| Prolonged initial treatment, n (%) | 1577 (51) | 563 (61) | <.001 |
| NEC,N | 3594 | 440 | |
| Duration of initial treatment, median (range), d |
5 (1–36) | 6 (2–33) | <.001 |
| Prolonged initial treatment, n (%) | 1892 (53) | 255 (58) | .04 |
| Death, N | 3359 | 657 | |
| Duration of initial treatment, median (range), d |
5 (1–36) | 6 (1–22) | <.001 |
| Prolonged initial treatment, n (%) | 1716 (51) | 412 (64) | <.001 |
Data on duration of antibiotic use were missing for 5 infants, data on the death outcome were missing for 18 infants, and data on the composite NEC or death outcome were missing for 17 infants.
In risk-adjusted multivariate analyses, longer durations of initial empirical antibiotic treatment were more likely to be associated with NEC or death and NEC alone, compared with shorter durations of initial empirical antibiotic treatment (Table 5). We observed a ~4% increase in the odds of an infant in this study cohort having NEC or dying with each additional day of initial empirical antibiotic treatment. The increase with each additional day was almost doubled for NEC alone, with a ~7% increase in the odds for each additional day of initial empirical antibiotic treatment. Duration of initial empirical antibiotic use was strongly associated with increased mortality rates, with a ~16% increase in the adjusted odds for each additional day of initial empirical antibiotic treatment; however, the statistically significant quadratic term suggests that this effect was attenuated as duration increased beyond 22 days. Odds of NEC or death and odds of death were increased with prolonged initial empirical antibiotic treatment of ≥5 days, and a trend toward increased odds was demonstrated for NEC. The number needed to harm, that is, the number of infants who would need to be treated with prolonged initial empirical antibiotic treatment before 1 infant developed NEC or died who would not have otherwise, was 22 infants. For death alone, the number needed to harm was 21 infants; for NEC, the value was 54 infants. Center also was associated with the odds of NEC or death, as well as the odds of NEC alone and death alone, as noted in previous analyses.7,8 When prolonged initial empirical antibiotic treatment was defined as ≥4 days of initial empirical antibiotic treatment, the results were similar to, if not more striking than, the ≥5-day treatment results for NEC or death (OR: 1.50 [95% confidence interval (CI): 1.22–1.83]), NEC alone (OR: 1.34 [95% CI: 1.04–1.73]), and death alone (OR: 1.86 [95% CI: 1.45–2.39]). Results were similar but the association was reduced when cutoff points of ≥7 or ≥10 days were used.
TABLE 5.
Multivariate Logistic Regression Analysis of Antibiotic Duration and NEC or Death
| Outcome | Duration of Initial Empirical Antibiotic Treatment (Odds per Day) |
Prolonged Initial Empirical Antibiotic Treatment |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| NEC or death (total, N = 3883; with outcome, n = 884) |
1.04 (1.02–1.06) | <.01 | 1.30 (1.10–1.54) | <.01 |
| NEC (total, N = 3899; with outcome, n = 427) |
1.07 (1.04–1.10) | <.001 | 1.21 (0.98–1.51) | .08 |
| Death (total, N = 3882; with outcome, n = 631) |
1.16 (1.08–1.24) | <.001 | 1.46 (1.19–1.78) | <.001 |
ORs were adjusted for study center, gestational age, small-for-gestational age status, gender, black race, 5-minute Apgar score of <5, rupture of membranes for >24 hours, outborn, prenatal steroid treatment, intrapartum antibiotic treatment, maternal hypertension, maternal hemorrhage, and multiple birth. The total numbers of infants shown represent the numbers of infants with nonmissing outcome and covariate data who were included in each model.
Additional multivariate analyses were performed for the infants who were intubated for 7 of the first 7 postnatal days, in an attempt to account for severity of illness. Among infants who were intubated and receiving mechanical ventilation for the first 7 postnatal days, the associations with prolonged initial empirical antibiotic treatment remained strong, with multivariate logistic regression analyses demonstrating higher odds of NEC, death, and the composite outcome of NEC or death for infants who received prolonged initial empirical antibiotic treatment (Table 6). The number needed to harm (the number of infants from this higher-risk cohort who would need to be treated with prolonged initial empirical antibiotic treatment before 1 infant developed NEC or died who would not have otherwise) was 14 infants. For death alone, the number needed to harm was 16 infants; for NEC, the value was 25 infants. The associations remained strong (NEC or death: OR: 1.73 [95% CI: 1.32–2.27]; NEC alone: OR: 1.70 [95% CI: 1.15–2.51]; death alone: OR: 1.85 [95% CI: 1.36–2.50]) when prolonged initial empirical antibiotic treatment was defined as ≥4 days.
TABLE 6.
Multivariate Logistic Regression Analysis of Antibiotic Duration and NEC or Death Among Infants Intubated for the First 7 Postnatal Days
| Outcome | Duration of Initial Empirical Antibiotic Treatment (Odds per Day) |
Prolonged Initial Empirical Antibiotic Treatment |
||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| NEC or death (total, N = 2128; with outcome, n = 635) |
1.04 (1.01–1.07) | .01 | 1.41 (1.14–1.75) | <.01 |
| NEC (total, N = 2141; with outcome, n = 238) |
1.09 (1.05–1.13) | <.001 | 1.50 (1.11–2.02) | <.01 |
| Death (total, N = 2127; with outcome, n = 509) |
1.13 (1.05–1.22) | <.01 | 1.42 (1.13–1.80) | <.01 |
Infants who were intubated continuously until death but who died before day 7 (n = 52) were included in this subgroup. ORs were adjusted for study center, gestational age, small-for-gestational age status, gender, black race, 5-minute Apgar score of <5, rupture of membranes for > 24 hours, outborn, prenatal steroid treatment, intrapartum antibiotic treatment, maternal hypertension, maternal hemorrhage, and multiple birth. The total numbers of infants shown represent the numbers of infants in this subset with nonmissing outcome and covariate data.
Supplemental Analyses of LOS and Impact of Early Enteral Feedings
In addition to the primary analyses assessing associations between initial empirical antibiotic therapy and NEC or death, we assessed associations with LOS or death. With the same multivariate model, a significant association was found between initial antibiotic course of ≥4 days and the combined outcome of LOS or death (OR: 1.21 [95% CI: 1.03–1.42]), although statistical significance was not obtained for initial therapy lasting ≥5 days (OR: 1.09 [95% CI: 0.95–1.26]). Both ≥4 and ≥5 days of initial empirical antibiotic treatment were associated with increased risk of the combined outcome of LOS caused by organisms other than coagulase-negative Staphylococcus or death (≥4 days: OR: 1.32 [95% CI: 1.11–1.58]; ≥5 days: OR: 1.24 [95% CI: 1.06–1.44]).
We found no evidence that the associations of duration of initial empirical antibiotic treatment or prolonged initial empirical antibiotic treatment with the primary outcome of NEC or death were different among infants who began enteral feedings in the first 4 postnatal days versus after 4 days (nonsignificant interactions; P = .17 and P = .22, respectively); however, the associations of NEC alone with duration of initial empirical antibiotic treatment and with prolonged initial empirical antibiotic treatment (defined as ≥5 days) were stronger for the infants who began enteral feedings on postnatal day 5 or later than for those who started enteral feedings in the first 4 postnatal days (prolonged initial empirical antibiotic treatment versus not: feedings started on postnatal day 5 or later: OR: 1.50 [95% CI: 1.14–1.97]; feedings started before postnatal day 5: OR: 0.99 [95% CI: 0.93–1.06]).
DISCUSSION
The use of empirical antibiotic therapy for premature infants in the first postnatal days is based on the immaturity of these infants’ immune systems, the high mortality rate among infants who have invasive bacterial infections, and the higher prevalence of EOS among preterm infants, compared with term infants.3,11 Our data suggest that prolonged initial empirical antibiotic therapy for infants with sterile culture results may be associated with subsequent death or NEC.
Cordero and Ayers12 investigated initial empirical antibiotic practices for 790 ELBW infants, including 695 with sterile initial culture results, from a consortium of 30 academic hospitals in 24 states. Those investigators did not statistically assess center variation in initial empirical antimicrobial practices but did report that, in one half of the 30 hospitals, ≥50% of the infants with suspected EOS were given antibiotics for >3 days after documented sterile blood culture results. Our results are consistent with theirs, because no advantage of longer empirical antibiotic courses was apparent. Their study also included a measure of severity of illness, the Clinical Risk Index for Babies.13 Clinical Risk Index for Babies scores were not different for infants with sterile culture results who received <3 days of antibiotic treatment and infants who received >7 days of antibiotic treatment. This result suggests that the decision to use longer versus shorter courses of empirical antibiotic treatment among infants with sterile culture results is more an institutional decision than one based on severity of illness.12
The choice of antibiotic duration is likely to affect colonization of the neonatal intestine.4,14 This colonization, at least in animal models, seems to contribute to physiologic development of the intestine, immunologic development, and absorbance of nutrients.15,16 Alterations and variations in any or all of these areas could increase the risk of NEC.17,18 In addition, exclusion of commensal flora could allow for colonization with fungal species, and intestinal colonization by fungi has been associated with greater risk of neonatal candidiasis.19,20 The association in our data between prolonged initial antibiotic course and higher risk of NEC among infants who started enteral feedings on day ≥5 of life warrants further investigation, with more-complete information on the type and volume of feedings than was available in this retrospective cohort analysis.
Our data suggest that use of ≥5 days of empirical antibiotic therapy initiated in the first 3 postnatal days despite sterile culture results may not be benign, although we are not proposing that all ELBW infants with sterile culture results have antibiotic coverage limited to <5 postnatal days, because blood culture volumes of 1 to 2 mL needed for optimal sensitivity may not be consistently available.21 In the near future, the decision to treat ELBW infants with sterile culture results and without signs of sepsis beyond the first 2 to 3 postnatal days may be more informed with use of automated blood culture systems that provide positive results in the first 48 hours after initiation of culture,22 and with development of adjunctive diagnostic tests such as those reviewed by Mishra et al.23 In that review, the authors described the relative value of multiple tests and combinations of tests, including leukocyte indices and levels of C-reactive protein, procalcitonin, cell surface markers CD11b and CD64, granulocyte colony-stimulating factor, numerous cytokines, including interleukin 6, interleukin 8, and members of the interleukin 1 family, and various leukocyte adhesion factors. Sensitivity, specificity, and positive and negative predictive values are promising for many of these tests alone and most compellingly in combinations, but most are not readily available in clinical settings. Mishra et al23 also described the potential use of microbial molecular genetics methods and the use of probes for the common bacterial genome marker 16S rRNA. Although culture methods might have improved in sensitivity (not all viral, fungal, or bacterial organisms grow in clinical laboratory conditions), additional microbial molecular methods may be useful.24 Such current methods were described by Palacios et al25 in a recent report on adapting high-throughput DNA pyrosequencing to pathogen discovery for 3 patients who developed lethal febrile illnesses after receiving visceral organ transplants from the same donor. In that report, the authors discussed the innovations in specimen preparation and bioinformatics that permitted them to identify the novel arenavirus infection in those 3 patients. With evidence supporting risk associated with duration of initial empirical antibiotic treatment, enhancing clinicians’ ability to distinguish ELBW infants who would benefit from prolonged antibiotic treatment in the absence of positive culture results justifies the ongoing investigation and the development of adjunctive diagnostic tests. When these new techniques of analyzing the complex combinations of host and pathogen molecular markers become available to clinicians, we are likely to see a significant change in antimicrobial practice.
Several study design decisions were made during this analysis, including the use of 5 days of antibiotic exposure as a categorical definition of prolonged duration of antibiotic treatment. This arbitrary decision was somewhat validated by the finding that it was the median duration for the study cohort. Given the possibility that duration of initial therapy was overestimated by 1 day, because we had no information on the time of day of antibiotic dosing, we wanted to ensure that our definition of prolonged antimicrobial therapy went beyond the usual shorter rule-out duration of 2 to 3 days of antibiotic treatment. Testing associations of NEC or death with prolonged antibiotic treatment defined as 7 or 10 days of exposure yielded similar results. Earlier investigators examined the use of cephalosporins in the first empirical antibiotic course and associations with mortality rates.2 We had hoped to assess the independent effect of exposure to cephalosporins in the initial antibiotic regimen on the primary outcome but were limited because the use of cephalosporins was virtually exclusive to 3 centers (Table 1), with 1 in particular using it for 95% of infants. The strong correlation between cephalosporin use and center in our cohort prohibited examination of the effects of cephalosporin use independent of center effects.
This investigation focused on associations between antibiotic practice differences and NEC or death. We did not analyze all early postnatal practices that could influence the risk of NEC or death. Although data on the timing of first enteral feedings were available, we lacked detailed information such as type (human milk versus formula) and amount of enteral nutrition received on any particular day.26,27 Risk association with longer antibiotic courses in our retrospective analyses may indicate that a sicker group of infants received longer antibiotic courses. Multivariate logistic regression analyses were used in attempts to correct for some of those differences by assessing the significance of factors, such as gestational age, that contribute to the overall risk of a specific outcome, but they were unable to correct fully for all possible study group differences. Additional subgroup analyses of a proxy for the sickest infants, that is, those who required continuous mechanical ventilation throughout the first 7 postnatal days, showed results similar to those for analysis of the whole cohort; however, even this approach is imperfect. Not all ventilated infants have the same severity of illness, and some infants who are weaned off ventilator support early may be more likely to have minor hypoxic events that predispose them to gut ischemia and thus NEC. This might have created significant “risk misclassification.”
CONCLUSIONS
The duration of initial empirical antibiotic treatment in the first postnatal days for ELBW infants whose cultures from sterile sites failed to yield evidence of infection varied widely among the centers studied. The data from this study demonstrated increased individual risk of NEC or death with prolonged initial empirical antibiotic treatment in this study population. Further prospective study is needed to determine causation and to determine whether limiting the duration of initial antibiotic treatment with sterile culture results might reduce the risk of NEC and death for ELBW infants.
What’s Known on This Subject
Antibiotics are among the most commonly prescribed medications in intensive care nurseries, especially in the first postnatal days, when most culture results are negative. Antimicrobial agents perturb colonization of the intestinal flora and may influence health outcomes.
What This Study Adds
This study provides evidence of an association between longer duration of initial empirical antibiotic courses started in the first postnatal days and death and NEC for ELBW infants whose initial blood and cerebrospinal fluid culture results are sterile.
ACKNOWLEDGMENTS
This work was supported by grants from the NICHD (grants U10 HD21364, U10 HD21373, U10 HD21385, U10 HD21397, U10 HD21415, U10 HD27851, U10 HD27853, U10 HD27856, U10 HD27871, U10 HD27880, U10 HD27881, U10 HD27904, U10 HD34216, U10 HD36790, U10 HD40461, U10 HD40492, U10 HD40498, U10 HD40521, U10 HD40689, U10 HD53089, U10 HD53109, U10 HD53119, and U10 HD53124) and from the National Institutes of Health (grants CCTS UL1 RR24128, CCTS UL1 RR24148, CCTS KL2 RR24149, GCRC M01 RR30, GCRC M01 RR32, GCRC M01 RR39, GCRC M01 RR44, GCRC M01 RR54, GCRC M01 RR59, GCRC M01 RR64, GCRC M01 RR70, GCRC M01 RR80, GCRC M01 RR633, GCRC M01 RR750, GCRC M01 RR997, GCRC M01 RR2588, GCRC M01 RR6022, GCRC M01 RR7122, and GCRC M01 RR8084). Dr Benjamin received support from grant HD-044799-01, Drs Cotten and Goldberg received support from National Institutes of Health grant 1U10 H040492-01, Dr Stoll received support from National Institutes of Health grant U10 HD27851, Dr Sánchez received support from National Institutes of Health grant U10 HD40689, and Dr Ambalavanan received support from National Institutes of Health grant U10 HD34216.
The following investigators participated in the NICHD Neonatal Research Network (1998–2001): Chair: Alan H. Jobe, MD, PhD, University of Cincinnati; Brown University, Women and Infants Hospital of Rhode Island: William Oh, MD, and Angelita Hensman, BSN, RNC; Case Western Reserve University, Rainbow Babies and Children’s Hospital: Avroy A. Fanaroff, MD, and Nancy S. Newman, BA, RN; Duke University, University Hospital, Alamance Regional Medical Center, Duke Raleigh Hospital, and Durham Regional Hospital: Kathy J. Auten, BS; Emory University, Grady Memorial Hospital, Emory Crawford Long Hospital, and Children’s Healthcare of Atlanta: Ellen C. Hale, RN; Indiana University, Indiana University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services: James A. Lemons, MD, Diana D. Appel, RN, BSN, Dianne Herron, RN, Lucy Miller, RN, BSN, CCRC, and Leslie Dawn Wilson, RN, BSN; Eunice Kennedy Shriver National Institute of Child Health and Human Development: Linda L. Wright, MD, and Elizabeth M. McClure, MEd; RTI: W. Kenneth Poole, PhD, and Betty K. Hastings; Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital: David K. Stevenson, MD, and M. Bethany Ball, BS CCRC; University of Alabama at Birmingham, Health System and Children’s Hospital of Alabama: Waldemar A. Carlo, MD, Monica V. Collins, RN, BSN, MaEd, and Shirley S. Cosby, RN, BSN; University of California, San Diego, Medical Center and Sharp Mary Birch Hospital for Women: Neil N. Finer, MD, Kathy Arnell, RN, Clarence Demetrio, RN, and Chris Henderson, RCP, CRTT; University of Cincinnati, University Hospital, Cincinnati Children’s Hospital Medical Center, and Good Samaritan Hospital: Edward F. Donovan, MD, Barb Alexander, RN, Cathy Grisby, BSN, CCRC, Marcia Mersmann, RN, Holly Mincey, RN, BSN, and Jody Shively, RN; University of Miami, Holtz Children’s Hospital: Shahnaz Duara, MD, Charles R. Bauer, MD, and Ruth Everett-Thomas, RN, MSN; University of New Mexico, Health Sciences Center: Lu-Ann Papile, MD, and Conra Backstrom Lacy, RN; University of Rochester, Golisano Children’s Hospital at Strong: Dale L. Phelps, MD, Linda J. Reubens, RN, and Erica Burnell, RN; University of Tennessee: Sheldon B. Korones, MD, Henrietta S. Bada, MD, and Tina Hudson, RN, BSN; University of Texas Southwestern Medical Center at Dallas, Parkland Health and Hospital System and Children’s Medical Center Dallas: Abbot R. Laptook, MD, and Susie Madison, RN; University of Texas at Houston, Health Science Center and Children’s Memorial Hermann Hospital: Jon E. Tyson, MD, MPH, Esther G. Akpa, RN, BSN, Patty A. Cluff, RN, Claudia Y. Franco, RN, BSN, MSN, NNP, Anna E. Lis, RN, BSN, and Georgia E. McDavid, RN; Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children’s Hospital: T. Michael O’Shea, MD, MPH, and Nancy J. Peters, RN; Wayne State University, Hutzel Women’s Hospital and Children’s Hospital of Michigan: Seetha Shankaran, MD, Rebecca Bara, RN, BSN, and Geraldine Muran, RN, BSN; Yale University, Yale-New Haven Children’s Hospital: Richard A. Ehrenkranz, MD, Patricia Gettner, RN, and Monica Konstantino, RN, BSN.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Abbreviations
- CI
confidence interval
- ELBW
extremely low birth weight
- NICHD
Eunice Kennedy Shriver National Institute of Child Health and Human Development
- EOS
early-onset sepsis
- LOS
late-onset sepsis
- NEC
necrotizing enterocolitis
- OR
odds ratio
Footnotes
The authors have indicated they have no financial relationships relevant to this article to disclose.
Reprints Information about ordering reprints can be found online: http://www.pediatrics.org/misc/reprints.shtml
REFERENCES
- 1.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics. 2006;117(6):1979–1987. doi: 10.1542/peds.2005-1707. [DOI] [PubMed] [Google Scholar]
- 2.Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Empiric use of ampicillin and cefotaxime, compared with ampicillin and gentamicin, for neonates at risk for sepsis is associated with an increased risk of neonatal death. Pediatrics. 2006;117(1):67–74. doi: 10.1542/peds.2005-0179. [DOI] [PubMed] [Google Scholar]
- 3.Stoll BJ, Hansen NI, Higgins RD, et al. Very low birth weight preterm infants with early onset neonatal sepsis: the predominance of Gram-negative infections continues in the National Institute of Child Health and Human Development Neonatal Research Network, 2002–2003. Pediatr Infect Dis J. 2005;24(7):635–639. doi: 10.1097/01.inf.0000168749.82105.64. [DOI] [PubMed] [Google Scholar]
- 4.Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. 1999;80(3):F167–F173. doi: 10.1136/fn.80.3.f167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lemons JA, Bauer CR, Oh W, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development Neonatal Research Network, January 1995 through December 1996. Pediatrics. 2001;107(1) doi: 10.1542/peds.107.1.e1. Available at www.pediatrics.org/cgi/content/full/107/1/e1. [DOI] [PubMed] [Google Scholar]
- 6.Walsh M, Kleigman R. Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am. 1986;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uauy RD, Fanaroff AA, Korones SB, Phillips EA, Phillips JB, Wright LL. Necrotizing enterocolitis in very low birth weight infants: biodemographic and clinical correlates. J Pediatr. 1991;119(4):630–638. doi: 10.1016/s0022-3476(05)82418-7. [DOI] [PubMed] [Google Scholar]
- 8.Ambalavanan N, Carlo WA, Bobashev G, et al. Prediction of death for extremely low birth weight neonates. Pediatrics. 2005;116(6):1367–1373. doi: 10.1542/peds.2004-2099. [DOI] [PubMed] [Google Scholar]
- 9.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolits. Pediatrics. 2005;115(3):696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 10.Kurlat I, Stoll BJ, McGowen JE., Jr Time to positivity for detection of bacteremia in neonates. J Clin Microbiol. 1989;27(5):1068–1071. doi: 10.1128/jcm.27.5.1068-1071.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17(3):638–680. doi: 10.1128/CMR.17.3.638-680.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cordero L, Ayers LW. Duration of empiric antibiotics for suspected early-onset sepsis in extremely low birth weight infants. Infect Control Hosp Epidemiol. 2003;24(9):662–666. doi: 10.1086/502270. [DOI] [PubMed] [Google Scholar]
- 13.de Courcy-Wheeler RHB, Wolfe CDA, Fitzgerald A, Spencer M, Goodman JDS, Gamsu HR. Use of the CRIB (Clinical Risk Index for Babies) score in prediction of neonatal mortality and morbidity. Arch Dis Child Fetal Neonatal Ed. 1995;73(1):F32–F36. doi: 10.1136/fn.73.1.f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitajima H, Sumida Y, Tanaka R, Yuki N, Takayama H, Fujimura M. Early administration of Bifidobacterium breve to preterm infants: randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 1997;76(2):F101–F107. doi: 10.1136/fn.76.2.f101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118(2):229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 16.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Caicedo RA, Schanler RJ, Li N, Neu J. The developing intestinal ecosystem: implications for the neonate. Pediatr Res. 2005;58(4):625–628. doi: 10.1203/01.PDR.0000180533.09295.84. [DOI] [PubMed] [Google Scholar]
- 18.Jilling T, Simon D, Lu J, et al. The roles of bacteria and TLR4 in rat and murine models of necrotizing enterocolitis. J Immunol. 2006;177(5):3273–3282. doi: 10.4049/jimmunol.177.5.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pappu-Katikaneni LD, Rao KP, Banister E. Gastrointestinal colonization with yeast species and Candida septicemia in very low birth weight infants. Mycoses. 1990;33(1):20–23. doi: 10.1111/myc.1990.33.1.20. [DOI] [PubMed] [Google Scholar]
- 20.Rangel-Frausto MS, Wiblin T, Blumberg HM, et al. National Epidemiology of Mycoses Survey (NEMIS): variations in rates of bloodstream infections due to Candida species in seven surgical intensive care units and six neonatal intensive care units. Clin Infect Dis. 1999;29(2):253–258. doi: 10.1086/520194. [DOI] [PubMed] [Google Scholar]
- 21.Schelonka RL, Chai MK, Yoder BA, Hensley D, Brockett RM, Ascher DP. Volume of blood required to detect common neonatal pathogens. J Pediatr. 1996;129(2):275–278. doi: 10.1016/s0022-3476(96)70254-8. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Prats JA, Cooper TR, Schneider VF, Stager CE, Hansen TN. Rapid detection of microorganisms in blood cultures of newborn infants utilizing an automated blood culture system. Pediatrics. 2000;105(3):523–527. doi: 10.1542/peds.105.3.523. [DOI] [PubMed] [Google Scholar]
- 23.Mishra UK, Jacobs SE, Doyle LW, Garland SM. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F208–F212. doi: 10.1136/adc.2004.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitley R. The new age of molecular diagnostics for microbial agents. N Engl J Med. 2008;358(10):988–989. doi: 10.1056/NEJMp0708085. [DOI] [PubMed] [Google Scholar]
- 25.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358(10):991–998. doi: 10.1056/NEJMoa073785. [DOI] [PubMed] [Google Scholar]
- 26.Berseth CL, Bisquera JA, Paje VU. Prolonging small feeding volumes early in life decreases the incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics. 2003;111(3):529–534. doi: 10.1542/peds.111.3.529. [DOI] [PubMed] [Google Scholar]
- 27.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. 2005;116(2):400–406. doi: 10.1542/peds.2004-1974. [DOI] [PubMed] [Google Scholar]