Abstract
Purpose
This review outlines the anatomical and functional bases of somatosensory influences on auditory processing in the normal brainstem and midbrain. Thereafter, it explores how interactions between the auditory and somatosensory system are modified through deafness and their impact on tinnitus is discussed.
Methods
literature-review, tract-tracing, immunohistochemistry, in vivo electrophysiological recordings
Results
Somatosensory input originates in the dorsal root ganglia (DRG) and trigeminal ganglia (TG) and is transmitted directly and indirectly through second order nuclei to the ventral and dorsal cochlear nucleus (VCN, DCN) and inferior colliculus (IC). The glutamatergic somatosensory afferents can be segregated from auditory nerve inputs by the type of vesicular glutamate transporters present in their terminals. Electrical stimulation of the somatosensory input results in a complex combination of excitation and inhibition and alters the rate and timing of responses to acoustic stimulation.
Deafness increases the spontaneous rates of those neurons that receive excitatory somatosensory input, and results in a greater sensitivity of DCN neurons to trigeminal stimulation.
Conclusions
Auditory-somatosensory bimodal integration is already present in first order auditory nuclei. The balance of excitation and inhibition elicited by somatosensory input is altered following deafness. The increase in somatosensory influence on auditory neurons when their auditory input is diminished could be due to cross modal re-innervation or increased synaptic strength, and may contribute to mechanisms underlying somatic tinnitus.
Keywords: Auditory system, Cochlear nucleus, Inferior colliculus, Trigeminal, Reticular formation, Somatosensory, Non-auditory projections, Tinnitus, Deafness, Bimodal plasticity
Introduction
In a simple experiment Jousmäki and Hari (1998) described how auditory input can modulate or even determine touch sensation. Subjects were asked to rub their hands and the thereby evoked sounds were played back to them. When the high frequency content of the played back signals was increased, the subjects felt the skin on their palms becoming dry as parchment paper. This so called parchment-skin illusion is an impressive example of auditory-somatosensory integration. The converse was shown by Levine et al. (2003): forceful manipulations or contractions of the muscles of the jaw, head or neck elicited the perception of sounds physically not present, i.e. tinnitus, in 58% of the subjects. The neurobiological basis of how somatosensory inputs influence neuronal activity of auditory neurons as part of their normal functioning will be reviewed in this chapter. We will give a short overview of the concepts of bimodal interaction and the presumed functions of the auditory-somatosensory interactions. We will introduce the somatic tinnitus syndrome. We will present the anatomical basis for the auditory-somatosensory interactions and show how auditory neurons respond to somatosensory stimulation, and how somatosensory stimulation influences auditory coding in animal experiments. Imbalance in this interaction in deafened animals will be described and its relation to somatic tinnitus discussed.
Cross-modal convergence or interaction of multisensory neurons refers to the responsiveness of a single neuron to stimulation of different sensory modalities and/or the modulation of activity evoked by one modality on that evoked by another (Kayser & Logothetis, 2007). Typically, this influence has been described in terms of changes in the response rate of the neuron, being either suppressive or enhancing. The strength of the effect depends on the measure used. The definition of bimodal enhancement is based on either the magnitude of the bimodal response to the sum of the unimodal responses (King & Palmer, 1985; Populin & Yin, 2002) or on the magnitude of the bimodal response to the larger of the unimodal responses (Meredith & Stein, 1986). A criterion for bimodal suppression was derived from the latter definition as a bimodal response that is smaller than the larger of the unimodal responses (Populin & Yin, 2002). As pointed out by Populin and Yin (2002) there is no single measure that can be applied for bimodal suppression and enhancement, to assess both equally. Clearly, as addressed by Stanford and co-workers (2005) the investigation of bimodal processing should not only describe its effects but should take into account possible underlying mechanisms of neuronal processing, which can be done most effectively by varying the stimulus strength and temporal relationships of the bimodal stimuli.
Cross-modal integration has been described extensively in cortical areas and in the superior colliculus (Reviews: Stein, 1998; Kayser & Logothetis, 2007; Shore, 2008). Principles of cross-modal integration have been formulated by Stein and co-workers based on results from recordings in the superior colliculus (reviewed in Kayser & Logothetis, 2007). These principles of spatial and temporal coincidence demand that the inputs from the different modalities originate from the same location in space and occur simultaneously. This would be the case when the same object stimulates the different modalities. The third principle is that of inverse effectiveness in which unimodal stimuli, which themselves elicit no or only weak responses, can evoke strong responses when presented simultaneously, i.e. elicit strong bimodal interactions. That these principles are not restricted to higher structures, such as the superior colliculus and cortex, but are also observed in the auditory brainstem will be addressed below. The cooperation of multimodal neurons in the superior colliculus with descending inputs from the cortex is one mechanism underlying improvements in response, such as decreased reaction time, increased stimulus detectibility and enhanced perceptual reliability, when information from two modalities is used in fulfilling a task, (Reviews: Stein & Meredith, 1993; Ernst & Bülthoff, 2004; Kayser & Logothetis, 2007).
The relative contribution of auditory-somatosensory interactions in cross-modal integration is less well documented than auditory-visual interactions at the level of cortex and superior colliculus (Review: Stein, 1998). However, the fundamental prerequisite of bimodal integration, the anatomical convergence of auditory and somatosensory inputs onto single neurons is abundant and found at different levels of the auditory pathway including the lowest levels, as exemplified by the pathway from the TG to the cochlea and cochlear nucleus (CN) and the dorsal column nuclei to the CN. The function of this convergence, i.e. its significance for the behavior of the animal is yet to be elucidated, but several hypotheses exist concerning its function. These are inspired by the cerebellar-like nature of the DCN, similar to that of the octavolateral system of the weakly electric fish (Lorente de No, 1981; Mugnaini & Morgan, 1987; Bell et al., 1997; Devor, 2000; reviewed in Oertel and Young, 2004). These structures have in common a system of granule cells that receives inputs from multiple sensory modalities, including somatosensory input from the face, head, neck, trunk and limbs. In the electrosensory lateral-line lobe of electric fish, proprioceptive inputs are used to cancel out electrical signals originating from movements of the fish. Similarly, it was hypothesized that propriocptive information about the pinna is conveyed to the DCN in order to cancel changes in the spectrum of auditory signals due to pinna movements, thus supporting the assumed role of the DCN in in coding sound source elevation using the spectrum of the sound (Nelken & Young, 1996; Kanold & Young, 2001). Alternatively, it has been suggested that proprioceptive inputs from intra oral structures could be used to cancel responses to auditory stimuli evoked by internally generated sounds as self vocalization and respiration (Bell et al., 1997; Shore, 2005). In both these hypotheses the functioning of the DCN in detection and processing external auditory signals, which are relevant to e.g. prey detection or enemy avoidance and communication, would be improved by reducing the response to irrelevant signals generated by the animal itself.
Cross-modal interactions between auditory and non-auditory systems such as the somatosensory system appear to take place within in the non-classical or extralemniscal pathway of the auditory system (DCN, external nucleus of IC, dorsal/medial thalamus, AII; reviews: Møller, 2001; Møller, 2006; Bartels et al., 2007). Common characteristics of auditory neurons in this pathway are their broader frequency tuning, resulting in a preference for spectral complex stimuli, and their extensive inputs from non-auditory modalities (reviewed in: Møller & Rollins, 2002). The extralemniscal pathway is also connected to structures of the limbic system (reviewed in: Møller, 2001; Kaltenbach, 2006; Bartels et al., 2007). The multimodal processing within this pathway has been one factor in its presumed role in certain forms of tinnitus (somatic tinnitus), and its projection to the limbic system has been discussed in connection with the emotional disorders often associated with tinnitus (Reviews: Møller, 2001; Eggermont, 2005; Kaltenbach, 2006; Bartels et al., 2007; Saunders, 2007).
Plastic changes in the mature neural system have now been accepted as a common underlying mechanism used to adapt to altered demands while learning or after trauma. However, plastic changes can also lead to disorders, such as tinnitus, and in particular, somatic tinnitus. The somatic tinnitus syndrome was defined by Levine et al. (2003, p.643) as “unilateral tinnitus temporally associated with, and ipsilateral to, a somatic disorder involving the head or upper neck”, which can also occur without a hearing loss. This form of tinnitus is hypothesized to occur when the balance of bimodal integration of the somatosensory and auditory modalities is impaired by alterations in somatosensory inputs, thus altering the integration of excitatory and inhibitory inputs from both modalities (Levine, 1999; review: Eggermont, 2005; Saunders, 2007). After noise induced hearing loss, somatic tinnitus is the second largest type of tinnitus (review: Eggermont, 2005). Somatic disorders of the head and upper neck that are often associated with somatic tinnitus include e.g. whiplash, temporomandibular joint syndrome, and other diseases involving the jaw or teeth. The sites of these disorders are innervated by the TG and DRG, as will be summarized in the next part of this chapter. Besides these manifestations of somatosensory influence on the auditory system in pathological conditions, its influence can also be shown in the normal system. Levine et al. (2003) showed that more than half of their subjects without tinnitus developed tinnitus upon active, forceful contractions involving muscles of the jaw, head, and neck. The same manipulations can also modulate the loudness, pitch and location of a pre-existing tinnitus in other subjects (Sanchez, et al., 2002; Levine et al., 2003). In normal subjects, Møller and Rollins (2002) showed that somatosensory stimulation (in this case, of the median nerve) can change the percept of external sounds.
Overview of non-auditory projections to the CN and IC
The following sections will give an overview of the projections from non-auditory systems to multisensory neurons within the auditory system (Fig. 1).
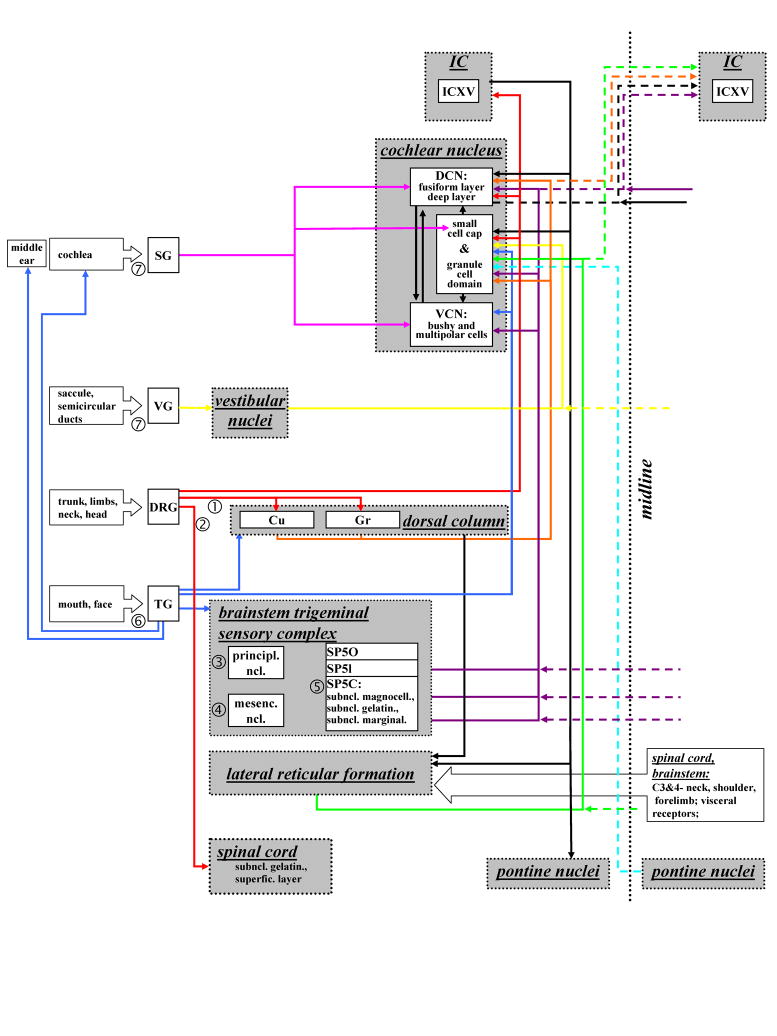
Figure 1.
Schematic overview of the pathways connecting the somatosensory and auditory system. Arrows connect the origins and target of inputs. Ascending inputs arise in the spiral ganglion (pink, SG), vestibular ganglion (yellow, VG), dorsal root ganglia (red, DRG), trigeminal ganglion (dark blue, TG), lateral reticlar formation (green, RVL, LPGi) and pontine nuclei (light blue). Projections ascending from the brainstem trigeminal complex are marked purple and from the dorsal column are marked orange. Dashed lines mark the contralateral projectons, crossing midline (dotted line). ➀: Dorsal column-medial lemniscal system; ➁: Spinothalamic pathway of the anterolateral system. The brainstem trigeminal sensory omplex is comprised of three nuclei: ➂: principle nucleus; ➃: mesecephalic nucleus, ➄: spinal trigeminal nucleus (SP5). The SP5 consists of three parts: pars oralis (SP5O), pars interpolaris (SP5I), pars caudalis (SP5C), SP5C has three subnuclei: subnucleus magnocellularis, subnucleus gelatinosus, subnucleus marginalis. ➅: three braches of the trigeminal nerve:1-ophthalmic branch: innervates forehead, upper eyelid, extraocular muscles; 2-maxillar branch: innervates upper lip, lower eyelid, upper jaw, roof of the mouth; 3-mandibular branch: innervates lower lip, mucous membranes of the lower jaw, floor of the mouth, anterior two thirds of the tongue; ➆: The two branches of the vestibulocochlear nerve: vestibular nerve and cochlear nerve. (Abbreviations: DCN – dorsal cochlear nucleus; DRG – dorsal root ganglia; IC – inferior colliculus; ICVX - ventrolateral border region of IC; mesenc. ncl. –mesencephalic nucleus; Cu – cuneate nucleus; Gr – gracile nucleus; principl. ncl. – principle nucleus; SG – spiral ganglion; Sp5 – spinal trigeminal nucleus; Sp5C - pars caudalis of Sp5; Sp5I - pars interpolaris of Sp5; Sp5O - pars oralis of Sp5; Subncl. gelatin. - subnucleus gelatinosus; subncl. magnocell. – subnucleus magnocellularis; subncl. marginal. – subnucleus marginalis; superfic. layer – superficial layer; TG trigeminal ganglion; VG – vestibular ganglion; VCN - ventral cochlear nucleus).
The somatosensory system
The somatosensory system is an important source of non-auditory inputs to different auditory nuclei. It conveys touch, temperature, pain, proprioception, and joint position from the body surface and inner organs. The primary neurons that link to the auditory system reside in the DRG (Fig. 1) of the spinal nerve at the C (cervical nerve) 2 level, which innervates the head and neck, and C7/8 which innervate the trunk and shoulders and in the TG, which innervates the face and mouth e.g. vocal tract/intra oral structures as tongue muscles, jaw and the temporomandibular joint (Fig. 1).
Two pathways ascend from the DRG (➀ ➁, Fig. 1): I. the spinothalamic pathway of the anterolateral system and II. the dorsal column-medial lemniscal system. Each pathway carries different sensory modalities: the anterolateral system mediates itch, crude touch, temperature and pain, its nociceptive secondary neurons are predominantly found in the superficial layer (lamina I) and the substantia gelatinosa (lamina II) of the spinal cord. The dorsal column-medial lemniscal system carries proproceptive and fine touch information and projects to the two dorsal column nuclei, nucleus cuneatus and nucleus gracilis. Nucleus cuneatus receives its input from the head, upper trunk and limbs whereas the nucleus gracilis receives inputs from the lower trunk and limbs. The dorsal column nuclei are involved in relaying proprioceptive information from the pinna to the DCN, to provide information relevant to orienting towards sound sources (Kanold & Young, 2001; Rice et al., 1992; Young et al., 1996).
Neurons of the TG project to the brainstem trigeminal sensory complex. This complex consists of three nuclei that convey different sensory modalities (➂ ➃ ➄, Fig. 1): I. the principle (main) nucleus, which receives information about light touch and position sensation; II. the mesencephalic nucleus, which receives proprioceptive information from the jaw, and III. the spinal trigeminal nucleus (Sp5), which receives information about pain, temperature, gentle pressure, vibrissa deflection (Hayashi et al., 1984; Jacquin et al., 1989) and proprioception from the vocal tract and intra oral structures e.g. the temporo-mandibular joint and the tongue muscles (Romfh et al., 1979; Jacquin et al., 1989; Nazruddin et al., 1989; Takemura et al., 1991; Suemune et al., 1992). The Sp5 is comprised of three divisions: pars oralis (Sp5O), pars interpolaris (Sp5I), and pars caudalis (Sp5C). Sp5C consists of three layers: the subnucleus magnocellularis, the outermost subnucleus marginalis, and the subnucleus gelatinosus. All three subdivisions of the Sp5 (Sp5O, S5I, S5C) receive either nociceptive or non-nociceptive afferents from head/face and proprioceptive inputs from vocal tract/intra oral structures. Nociceptive neurons concentrate in the subnucleus gelatinosus of Sp5C, which is analogous to the lamina II (substantia gelatinosa) in the spinal cord, which also receives primarily nociceptive inputs (Darian-Smith et al., 1963; Usunoff et al., 1997).
It is now well established that somatosensory information is fed into the auditory system via the cochlear nucleus (CN; Haenggeli et al., 2005; Itoh et al., 1987; Weinberg & Rustioni, 1987; Wright & Ryugo, 1996; Li & Mizuno, 1997; Shore et al., 2000; Shore et al., 2003; Zhou & Shore, 2004; Zhou et al., 2007; Shore, 2005) and IC (Li & Mizuno, 1997; Zhou & Shore, 2006). The next section will focus on the anatomy of the pathways from the somatosensory system to the CN and IC.
Direct somatosensory innervations: projections from the DRG and TG to the CN and IC
The primary neurons of the somatosensory system in the DRG and TG project to the CN (Pfaller & Arvidsson, 1988; Shore et al., 2000; Zhan et al., 2006). The projection neurons in the DRG are small (15~20 μm in diameter; Zhan et al., 2006). Those in the TG are small-medium (10~45 μm in diameter; Shore et al., 2000) and may belong to the category of type B neurons, which usually give rise to unmyelinated or lightly myelinated fibers.
The terminal fields of the DRG projection neurons are concentrated within the medial edge of the VCN and dorsal ridge of the anteroventral cochlear nucleus (AVCN), i.e. subpeduncular corner (between the AVCN and the inferior cerebellar peduncle) and lamina of the granule cell domain (GCD), which includes the shell region and the fusiform cell layer of the DCN and is comprised of numerous small cells (Pfaller & Arvidsson, 1988; Weedman et al., 1996; Zhou & Shore, 2004; Zhan et al., 2006). Postsynaptic targets of the DRG projection include the primary dendrites of unipolar brush cells and the distal dendrites of granule cells. The terminal endings of DRG projection neurons have distinct sizes and shapes, and cannot be simply characterized as small boutons or mossy fibers (Zhan et al., 2006).
Neurons of the TG that project to the CN are located in the medial part of the ganglion and at the origin of the ophthalmic nerve, as well as in the mandibular division of the ganglion (➅, Fig. 1). A few projection neurons are located in the maxillary division (Shore et al., 2000). The locations of these projection neurons overlap with the regions that innervate both the cochlea and the middle ear: The ophthalmic division innervates the cochlea, and the mandibular region innervates the middle ear (Vass et al., 1997, 1998). The TG neurons that project to the CN are generally smaller, with a smaller nucleus (compared to those labeled by skin injections) and have uneven surfaces (Shore et al., 2000). The TG projection fibers are thin (~ 1 μm) and form en passant boutons mainly in the medial and lateral edges of VCN (PVCN and AVCN) i.e. shell regions and the GCD of the CN, but there are also terminals in the magnocellular regions of the VCN. Postsynaptic targets of TG terminals in the VCN include dendrites of granule cells and somata of large cells, and lumina of blood vessels (Shore et al., 2000), implying that the TG-to-CN pathway plays a role in the regulation of blood flow or metabolism in the CN.
IC projection neurons in the TG have not been reported, however, the TG also sends a minor projection to the superior olivary complex (Shore et al., 2000; Zhou & Shore, 2006).
Innervation of CN and IC from secondary somatosensory neurons: Sp5 and dorsal column nuclei
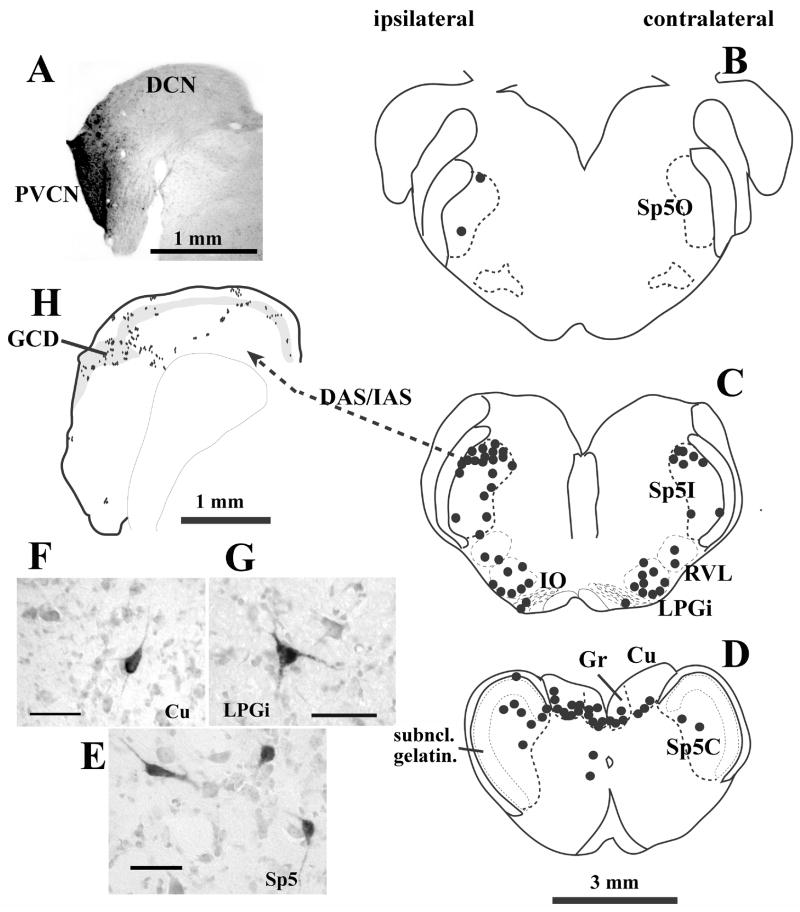
Secondary neurons of the trigeminal system are located in the Sp5. The majority of CN-projection neurons are located in the dorsomedial and marginal areas of Sp5I and in the subnucleus marginalis or the subnucleus magnocellularis of Sp5C (Fig. 2; Wolff & Kunzle, 1997; Zhou & Shore, 2004; Haenggeli et al., 2005). Few projection neurons are located in the subnucleus gelatinosus of Sp5 (Fig. 2), suggesting that the Sp5 projection to the CN carries mainly non-nociceptive information (Zhou & Shore, 2004; Shore & Zhou, 2006). There are two distinct morphological types of Sp5 neurons projecting to the CN (Fig. 2): I. polygonal neurons (somata ranging from 10 × 12 μm to 25 × 28 μm in diameter), and II. elongated neurons (somata 10 × 30 μm to 7 × 40 μm; Wolff & Kunzle, 1997; Zhou & Shore, 2004; Haenggeli et al., 2005). Their projection fibers and terminal endings are small-medium sized en passant and large, irregular swellings that resemble mossy fibers, which have been identified using EM in Haenggeli et al. (2005). Sp5 small terminal endings are scattered across the entire CN, making synaptic contacts with granule cells or large principal cells. The Sp5 mossy fibers concentrate in the GCD, making synaptic contacts with granule cells (Zhou & Shore, 2004; Haenggeli et al., 2005).
Figure 2.
Spinal trigeminal nucleus, dorsal column and lateral reticular formation project to the CN. (A) –(G): Retrograde labeling in the brainstem after an injection of biotinylated dextran amine into the CN. (A) Photomicrograph of the injection site. The injection site is virtually restricted to granule cell domain of the PVCN. (B) – (D): Drawings of 1 mm transverse sections across the medulla. Each dot represents one labeled neuron. The labeled neurons are located primarily in the ipsilateral Sp5I and Sp5C. Very few labeled cells, if any, are located in the subnucleus gelatinosus (D). Labeled neurons are also found in the medullar reticular formation (RVL and LPGi, C), inferior olive (IO, C), and dorsal column nuclei (Gr and Cu, D). Projection neurons in Sp5 have either polygonal or elongated somata (E). Projection neurons in dorsal column nuclei and reticular formation are multipolar (F and G). (H): Terminal labeling in the CN after placement of an anterograde tracer into Sp5I. Most Sp5 fibers enter the CN via the DAS/IAS and terminate primarily in the granule cell domain (grey shaded), but also in deep DCN. Each dot represents one to three labeled terminal endings.
Scale bars = 25 μm (E – G). (Abbreviations: CN – cochlear nucleus; Cu – cuneate nucleus; DAS - dorsal acoustic striae; DCN - dorsal cochlear nucleus; GCD - granule cell domain; Gr – gracile nucleus; IAS - intermediate acoustic striae; IO - inferior olive; LPGi - lateral paragigantocellular reticular nucleus; PVCN - posteroventral cochlear nucleus; RVL - rostral ventrolateral reticular formation; subncl. gelatin. - subnucleus gelatinosus; Sp5 – spinal trigeminal nucleus; Sp5C -pars caudalis of Sp5; Sp5I - pars interpolaris of Sp5; Sp5O - pars oralis of Sp5). (Adapted with permission from Shore & Zhou, 2006)
Dorsal column nuclei as key non-auditory structures also project to the CN. CN projection neurons are located in both the cuneate and gracile nuclei and aggregate in the ventral edge of the dorsal column nuclei. The projection neurons have polygonal somata. They project mainly to the GCD of the CN and terminate as mossy fibers and boutons (Itoh et al., 1987, Weinberg & Rustioni, 1987, Wright & Ryugo, 1996; Wolff & Kunzle, 1997; Zhou & Shore, 2004). The postsynaptic targets of mossy fibers include dendrites of granule cells (Wright & Ryugo, 1996).
The dorsal column nuclei and Sp5 also project to the IC (Zhou & Shore, 2006). Some neurons in the Sp5 and the dorsal column nuclei project to both CN and external cortex of IC by way of axon collaterals (Li & Mizuno, 1997). Projection fibers from these somatosensory neurons form a laminar pattern of en passant terminal endings from ventromedial to dorsolateral within the ventrolateral IC, the ventral border of IC, and the ventromedial edge of IC. These regions are collectively termed “the ventrolateral border region of IC” (ICXV; Zhou & Shore, 2006). The ICXV is the primary region that receives convergent projections from the CN and the somatosensory system, and thus is likely to be involved in multimodal integration in the IC (Jain & Shore, 2006).
Other non-auditory inputs to the CN
Neurons in the reticular formation also project to the CN. The lateral reticular formation (LRF) is located in the rostral ventrolateral medulla, which receives projections from diverse brain stem pathways and the spinal cord (Van Bockstaele et al., 1989; Caicedo & Herbert, 1993; Hermann et al., 2003). The LRF conveys somatosensory information from the neck, head, forelimb, vocal tract, and respiratory system and is part of the circuit controlling the startle reflex (Bowker et al., 1981; Kamiya et al., 1988) and signals movement of the head (reviewed in Zhan & Ryugo, 2007). Moreover, it is assumed that the LRF is involved in visceral pain and analgesia mechanisms and cardiovascular regulation (Van Bockstaele et al., 1989; Shintani et al., 2003; Babic & Ciriello, 2004). The multipolar projection neurons are found in the ipsilateral and contralateral lateral paragigantocellular and rostroventrolateral reticular formation (LPGi, RVL; Fig. 2). The terminals of the LRF projection distribute across the GCD of the CN (shell region of VCN and fusiform cell layer of DCN; Zhan & Ryugo 2007). The endings are either small, en passant boutons (< 2.5μm) or large, irregular swellings, typical for mossy fibers (≥2.5μm). The mossy-fiber boutons from the LRF in the CN outnumber the small boutons (Cui & Shore, 2008). This is in contrast to the Sp5 input to the GCD, for which small bouton type endings predominate (Cui & Shore, 2008). These distinct patterns suggest functional differences in signal transfer from each nucleus to the CN (Cui & Shore 2008).
Projections from the primary somatosensory cortex (Wolff & Kunzle, 1997) and primary auditory cortex to the CN (Weedman & Ryugo, 1996) may play a role in modulating orienting responses. In addition to somatosensory connections, neurons in the pontine nuclei (Ohlrogge et al., 2001) and vestibular system project to the CN (Burian et al., 1989; Kevetter & Perachio, 1989; Gstoettner et al., 1991).
Somatosensory pathways to the CN are glutamatergic
Numerous studies using light and electron microscopy have established that projections from somatosensory neurons to the CN are excitatory (Wright & Ryugo, 1996; Shore et al., 2000; Zhou & Shore, 2004; Zhan et al., 2006). Mossy fibers in the CN that originate in the cuneate nucleus and Sp5 contain round synaptic vesicles and make asymmetric contacts with postsynaptic targets, features commonly associated with excitatory synapses (Wright & Ryugo, 1996; Haenggeli et al., 2005). Terminals from TG and DRG in the CN have similar excitatory ultrastructural characteristics (Shore et al., 2000; Zhan et al., 2006). Mossy fibers from the cuneate nucleus are non-immunoreactive to choline acetyltransferase or GABA, providing indirect support for this being a glutamatergic pathway (Wright & Ryugo, 1996).
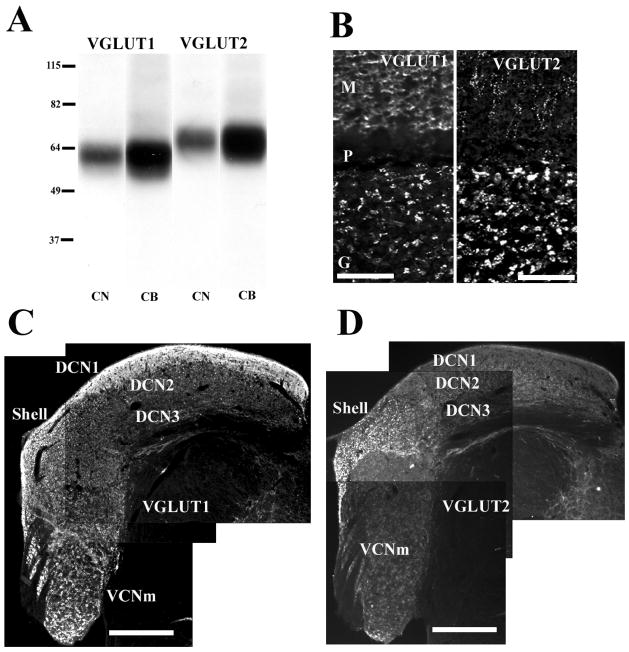
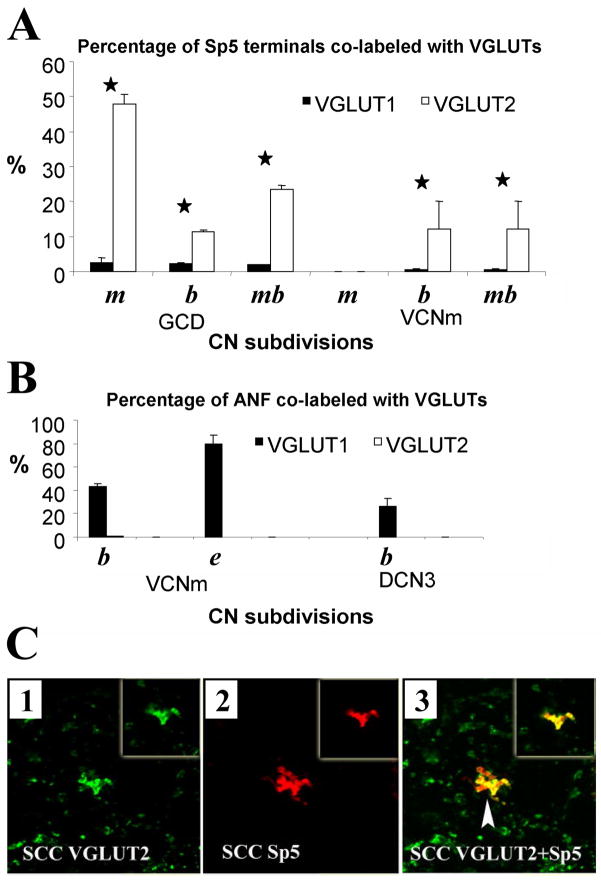
Recently, the use of antibodies against vesicular glutamate transporters (VGLUTs) has confirmed that somatosensory pathways to the CN are glutamatergic (Zhou et al., 2007). Three types of VGLUTS, VGLUT1, VGLUT2 and VGLUT3 are expressed in the adult mammalian brain. In the CN, VGLUT1 and VGLUT2 show distinct distributions in the CN (Fig. 3; Zhou et al., 2007). VGLUT1 is expressed primarily in the magnocellular regions of the VCN and the molecular layer of the DCN, whereas the most intense VGLUT2 labeling is found in the GCD. BDA-labeled presumed mossy fiber endings and small bouton endings from the Sp5 colabel predominantly with VGLUT2 in the GCD, whereas fluorogold labeled auditory nerve endings, including calyceal terminals, colabel only with VGLUT1 in the magnocellular regions of the VCN and deep layer of the DCN (Fig. 4). Additionally, BDA-labeled LRF terminals colabel with VGLUT2 in the GCD (Cui & Shore, 2008). The distinct co-localization of VGLUT1 and VGLUT2 with auditory nerve, and Sp5/LRF terminals, respectively, suggests different characteristics of the excitatory synaptic transmission, depending on the input source (Fremeau et al, 2001; Varoqui et al., 2002; Gras et al., 2002). Auditory nerve terminals transmit acoustic signals with high temporal precision. Thus, VGLUT1 is likely to be involved in fast transport of glutamate of the auditory nerve terminals in the CN in order to convey this precise temporal information. Like cerebellar mossy fibers that adjust synaptic strength by modulating their release of neurotransmitter (Sola et al., 2004), CN mossy fibers in the GCD, originating in non-auditory regions, could play a similar role in adjusting synaptic strength. The adjustable mossy fiber input to the GCD could modulate CN output activity, and therefore is well suited to play a key role in the plastic processes underlying the initiation of tinnitus. Interestingly, VGLUT2 is implicated in the mediation of neuropathic or phantom pain (Moechars et al., 2006), which has some characteristics in common with tinnitus.
Figure 3.
VGLUT1 and VGLUT2 show distinct expression in the CN. (A): Western blot analysis of proteins from CN and cerebellum (CB) with anti-VGLUT1 and anti-VGLUT2 antibodies. Anti-VGLUT1 antibody is recognized a single band at ~60 kDa, and anti-VGLUT2 antibody is recognized a single band at ~65 kDa, corresponding to the molecular weights predicted for VGLUT1 and VGLUT2, respectively. Molecular weight standards are indicated at left (kDa). (B): VGLUT1-ir and VGLUT2-ir in the cerebellar cortex, as a positive control (M, molecular layer; P, Purkinje cell layer; G, granular layer). (C): VGLUT1-ir in the CN at low magnification (x10). VGLUT1 is intensely expressed in DCN1 and VCNm; weak to moderate VGLUT1-ir is found in the shell and DCN2; weak VGLUT1-ir is seen in DCN3. (D): VGLUT2-ir in the CN at low magnification (x10). VGLUT2 is expressed predominantly in the shell; moderate VGLUT2-ir is found in DCN2; very weak to weak staining is found in DCN1, VCNm, and DCN3. Scale bars = 50 μm in (B); 0.5 mm in (C), (D). (Adapted with permission from Zhou et al., 2007)
Figure 4.
Co-labeling of VGLUT1-ir and VGLUT2-ir with terminal endings of SP5 and auditory nerve in the CN (n=2). (A, B) Percentages of SP5 terminals (A) and auditory nerve terminals (ANF, (B)) co-labeling with VGLUT1-ir (black columns) and VGLUT2-ir (white columns). (A) About 50% of Sp5 mossy fibers (m) and 12% of small Sp5 boutons (b) colabeled with VGLUT2. Significantly fewer mossy fibers and boutons colabeled with VGLUT1 (paired t-test, P < 0.05); 23.6% ± 1% of total Sp5 terminal endings (mb, both mossy fibers and boutons) colabeled with VGLUT2, which is significantly greater than the endings colabeled with VGLUT1 (2% ± 0.1%; paired t-test, P < 0.05). (B) In contrast, many labeled ANF terminals colocalized with VGLUT1 in the VCNm (79.5% ± 7% endbulb-like terminals (e), 43.0% ± 3% bouton terminals (b)) as well as in the DCN3 (26.0% ± 5%). Neither ANF endbulb-like terminals nor small boutons were colocalized with VGLUT2. Error bars represent SEM. Asterisks indicate significant differences (see text). (C) High-magnification confocal images (×63) showing colocalization of anterogradely labeled Sp5 terminal endings with VGLUT2-ir in the small cell cap (SCC) of the CN. Green, VGLUT-ir; red, Sp5 labeling; yellow, double-labeled terminals. Images were obtained from Z projections of stacks of serial 1-μm confocal images. Insets show a single 1-μm confocal image. Mossy fibers are labeled with BDA from Sp5 and VGLUT2 in the shell. Colocalization of Sp5 MFs with VGLUT2-ir is indicated by arrowhead in C3. (Abbreviations: m – mossy fibers; b -boutons; mb - total (mossy fibers and boutons); e - endbulb-like terminals). (Adapted with permission from Zhou et al., 2007)
Auditory-somatosensory interactions in the normal auditory system
The extensive somatosensory innervation of auditory nuclei has substantial functional consequences. Stimulation of the various somatosensory input pathways, in vivo, can evoke complex patterns of inhibition and excitation even in second order neurons of the auditory system. Moreover, somatosensory stimulation influences the coding of acoustic stimuli by auditory neurons. These results will be summarized in the following section and will be discussed in terms of their functional significance and their contribution to the understanding of the mechanisms initiating tinnitus.
Auditory neurons respond to stimulation of the dorsal column – medial lemniscal pathway
Electrical stimulation of the dorsal column nuclei and Sp5 together (collectively termed medullary somatosensory nuclei, MSN), primarily inhibits spontaneous activity in DCN type IV units (pyramidal and giant cells; Young et al., 1995). Using repetitive stimulation, this inhibition can be accompanied by a transient excitatory and an early inhibitory response (Young et al., 1995; Davis et al., 1996). Interestingly, the early inhibitory component is not evoked by parallel fiber stimulation, thus other sources than cartwheel cells must be responsible for the fast acting inhibition including glycinergic multipolar neurons of the VCN (discussed in Davis et al., 1996). Kanold and Young (2001) also obtained responses in DCN to stimulation of C2, C7 and the mandibular branch of the trigeminal nerve. In the cat, responses to dorsal column stimulation were stronger than responses to trigeminal nerve stimulation, in contrast to the guinea pig (see next section). The DCN responses to MSN stimulation and C2 stimulation are similar, suggesting that the MSN stimulation reflects the C2 inputs to MSN. In these experiments, Kanold and Young (2001) identified passive pressing or stretching of the muscles of the cats’ pinna as the most effective somatic stimulus. These results were interpreted in terms of one proposed functional role of the DCN, to encode the orientation of the pinna, thereby enabling the system to subtract changes in the spectrum due to pinna movements, from changes due to sound source movement. This hypotheses demands experiments showing how the coding of acoustic stimuli is modified by the C2/MSN input and how both inputs are integrated during active movements or for different orientations of the pinna and/or head. Experiments addressing the bimodal interaction of somatosensory and auditory inputs to the CN were first performed by Saadé and coworkers. They used combined auditory and dorsal column stimulation while recording from DCN chopper units (Saadé et al., 1989). They demonstrated that the unit’s responses to acoustic stimulation were either facilitated or inhibited and the magnitude of the effect depended on the delay between the two stimuli. However, the data presented by Saadé et al. were limited and further experiments are needed to explore the influence of the dorsal column on auditory coding in the DCN. Interactions between auditory and dorsal column input are also evident within the external and central nucleus of the IC (Aitkin et al., 1978; Tawil et al., 1983). In those studies the prevailing effect was suppression of sound evoked activity by electrical stimulation of the dorsal column. Further experiments are needed to describe the details and function of bimodal interaction in the IC. Furthermore, it seems worthwhile to reconsider the function of the C2 dorsal column afferents as these also exist in animals that do not direct their pinnas towards a sound source. Indeed, in contrast to the cat, stimulation of C2 in guinea pig does not result in observable responses in DCN (Shore et al., unpublished observations).
Auditory neurons respond to stimulation of the trigeminal pathway
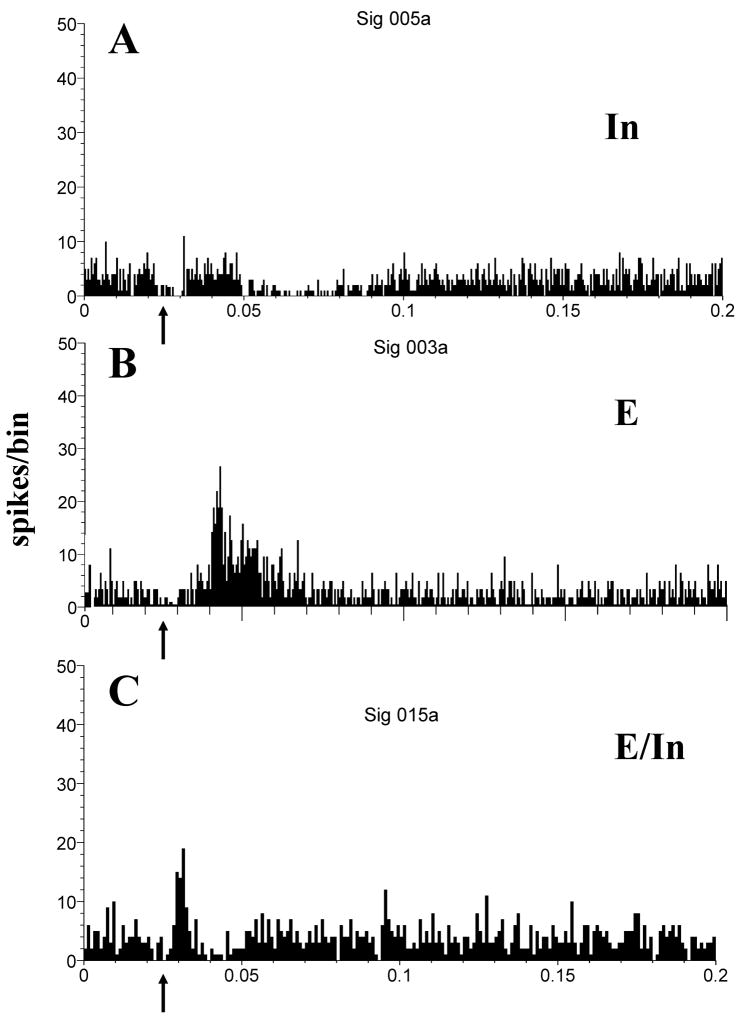
In addition to the dorsal column nuclei/C2/C7/C8 pathway, which carries mainly proprioceptive input from the trunk, shoulders, head (pinna) and neck, another major input pathway of somatosensory stimuli to auditory nuclei is the trigeminal system (➅, Fig. 1), which conveys information from face and oral structures, e.g. vocal tract/intra oral structures such as tongue muscles, temporomandibular joint and jaw (Romfh et al., 1979; Capra, 1987; Jacquin et al., 1989; Nazruddin et al., 1989; Takemura et al., 1991; Suemune et al., 1992). In a series of studies, Shore and co-workers verified the functionality of the trigeminal input to the auditory system of the guinea pig, demonstrating responses of DCN, VCN and IC neurons to TG and Sp5 stimulation. Single bipolar shocks applied to the TG evoked responses in 29% of DCN units (Fig. 5), most of which showed excitation (11% excitation alone or 9% excitation followed by inhibition); a smaller percentage showed inhibition of spontaneous activity (8%; Shore, 2005). The latencies of the inhibitory responses were on average 3 ms longer than the excitatory responses and the inhibitory responses were typically long lasting (up to 70ms; Shore, 2005). The longer latencies of the inhibitory responses presumably reflect the additional synapse needed to switch the direct excitatory input from the parallel fibers into inhibition via inhibitory interneurons such as the cartwheel cells (Young et al., 1995; Davis & Young, 1997). Response thresholds depended on the site of stimulation, being lowest for sites within the ophthalmic and mandibular divisions of the TG, which project to the CN (Shore et al. 2000; Kanold & Young, 2001) and that carry proprioceptive information from lower lip, lower teeth and gums, floor of the mouth, anterior of the tongue, chin and jaw. Comparing these results to the experiments of Young and co-workers reveals that the C2 pathway predominantly inhibits neurons in the DCN while the TG predominantly excites DCN neurons (which, as will be explained below, results in a suppressive effect during bimodal integration). Some of these differences could be explained by differences in the experimental setup, for example stimulating sensory receptors may elicit less synchronous activation than stimulating brainstem sensory nuclei.
Figure 5.
Electrical stimulation of the trigeminal ganglion elicits inhibitory (In), excitatory (E) and mixed excitatory–inhibitory (E/In) responses of dorsal cochlear nucleus neurons. (A) Post-stimulus time histogram (PSTH) for In type response. Arrow indicates stimulus onset. Inhibition occurs with a latency around 20 ms and lasts for approximately 70 ms. (B) PSTH for E type response, which occurs with a latency of approximately 15 ms and lasts for around 25 ms, returning to the pre-stimulation spike rate. (C) PSTH for E/In type response. Excitation with a shorter latency than B is followed by inhibition that recovers after approximately 20 ms. Current level of trigeminal ganglion stimulation: 80 μA; bin width: 1 ms; 200 presentations. Responses are from sorted single units in the fusiform cell layer. (Adapted with permission from Shore, 2005)
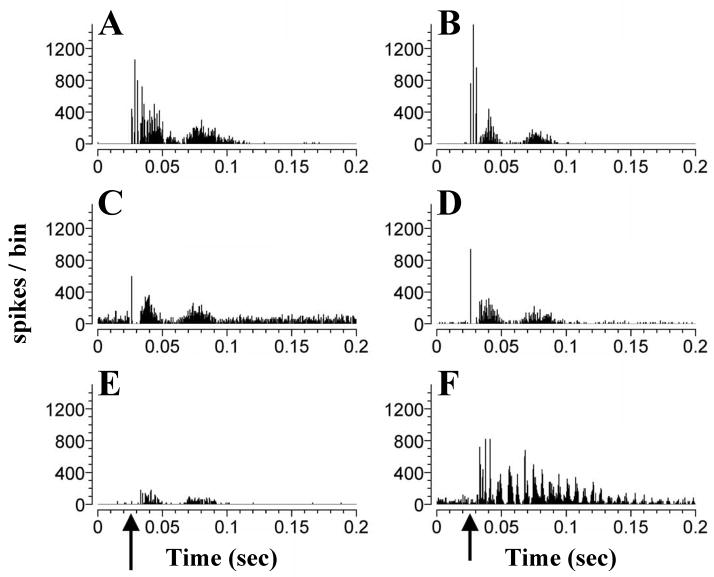
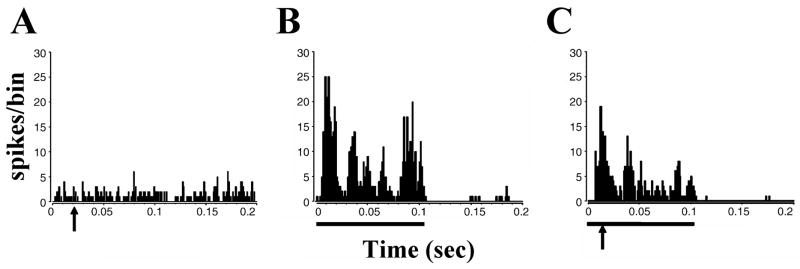
Electrical stimulation of the TG also evokes responses in the VCN, predominantly excitatory responses in primary-like, onset-L and chopper neurons (Shore et al., 2003). The responses consisted of one or several peaks of excitation (Fig. 6), which could be followed by a long lasting inhibition of up to 200 ms (Fig. 7). The first spike latencies of the responses varied between 5 and 17 ms. Such a large spread of latencies and the varying number of peaks in the responses indicate that responses were transmitted through diverse circuits differing in their speed and neurons of origin. The shorter latencies would be compatible with direct innervation of the magnocellular or shell region of the VCN (Shore et al., 2000). Longer latencies could be derived from relaying the inputs through Sp5 or other auditory nuclei to the DCN or IC, which project back to the VCN. The functional significance of the somatosensory inputs to the VCN has not yet been addressed. Interestingly, Levine et al. (2003) described a case of somatic tinnitus, in which a binaural percept of tinnitus “throughout the head” could shift to a unilateral percept, when the subject manipulated muscles innervated by the somatosensory system (here the dorsal column at C2/C3) on the same side of the unilateral percept. Such and other changes in the location of the tinnitus percept with time have been reported by approximately 13% of tinnitus patients (Kaltenbach et al., 2005), but their underlying mechanisms remain to be explored. It is well documented that VCN primary-like, onset-L and chopper neurons are the origins of pathways encoding interaural differences in intensity and timing of binaural stimuli (review: Cant & Benson, 2003). Therefore, the direct input of the TG/SP5 to the VCN could, in principle, be capable of influencing the coding of sound location in azimuth in the auditory brainstem. Studying the temporal fine structure of responses of units in the VCN to iterated rippled noise Winter et al. (2001) concluded that responses of different primarylike and chopper units encode the pitch of different frequency ranges (Winter et al., 2001). Whether changes in the pitch percept of tinnitus after stimulating the somatosensory system (Levine et al., 2003) could be caused by changing the response properties of VCN neurons by somatosensory input, remains to be investigated. Trigeminal input to the VCN could also influence the activity of DCN neurons via projections from VCN multipolar neurons to the DCN (Adams, 1983; Ostapoff et al., 1999; Doucet et al., 1999).
Figure 6.
Neurons in the VCN respond to trigeminal stimulation with single- or multipeaked excitatory discharges. (A to F): PSTHs of single unit responses to trigeminal ganglion stimulation (50μA), arrows indicate onset of trigeminal ganglion stimulation at 0.025ms. One, two or more peaks of excitation are seen, lasting for up to 80 ms. Some neurons show chopped discharges at the beginning (A, B, F) or for the entire duration of their response (F). (Adapted with permission from Shore at al., 2003)
Figure 7.
In some VCN units an excitatory response is followed by a depression of SR, that can last for 100–200 ms. Shown are PSTHs of single unit responses to trigeminal ganglion stimulation, arrows indicate onset of trigeminal ganglion stimulation (0.025ms). (Adapted with permission from Shore at al., 2003)
Auditory inputs from the CN and somatosensory inputs from the Sp5 converge in the ventrolateral region of the ICX (Aitkin et al., 1981; Zhou & Shore, 2006). Single electrical pulses delivered to Sp5 evoked little response from neurons recorded in the ICX. This apparent lack of response does not predict the strong influence of the Sp5 input to the IC on sound driven responses, and as will be shown below is a strong example of bimodal acoustic-somatosensory integration in which the response to Sp5 alone is subthreshold (Jain & Shore, 2006).
We have thus far described the temporal complexity of responses of neurons within the auditory pathway to stimulation of their trigeminal inputs. Although the predominant response is excitatory, it can also be followed by inhibitory responses or cause inhibition without excitation. The response properties to TG stimulation in VCN are more complex and variable than those seen in the DCN while in the IC, mostly sub threshold responses were seen to Sp5 stimulation (see below). This indicates the involvement of a multitude of pathways that differ in their target neurons within the auditory system. The question of how these somatosensory inputs modify the responses of auditory neurons to acoustic stimuli, i.e. how both modalities are integrated, will be addressed in the next section.
Multisensory integration of auditory and trigeminal inputs
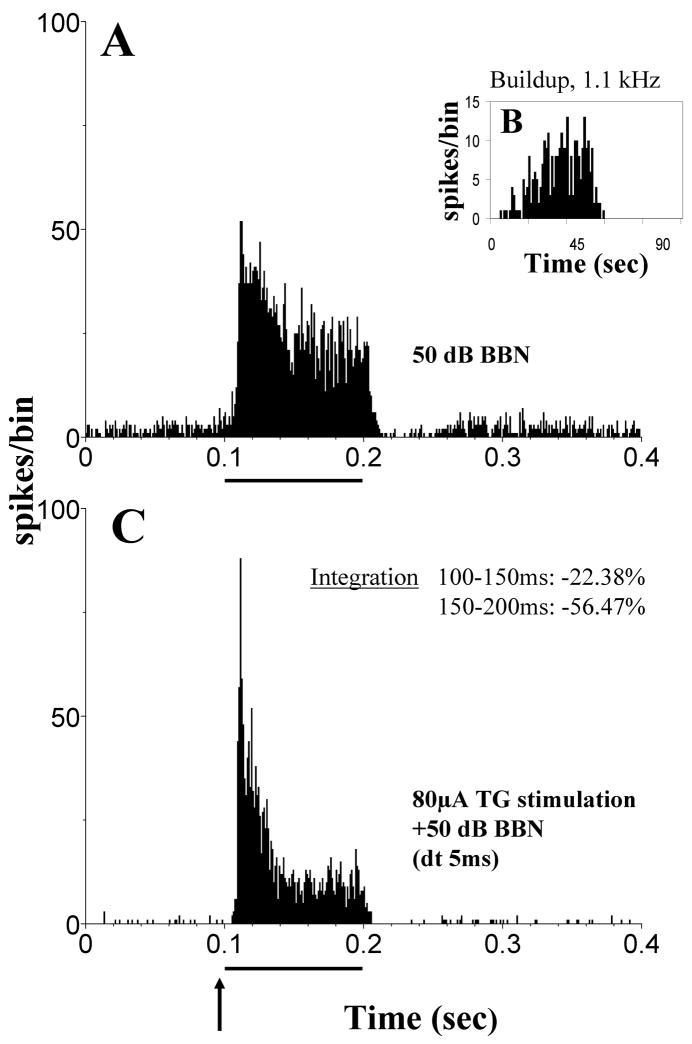
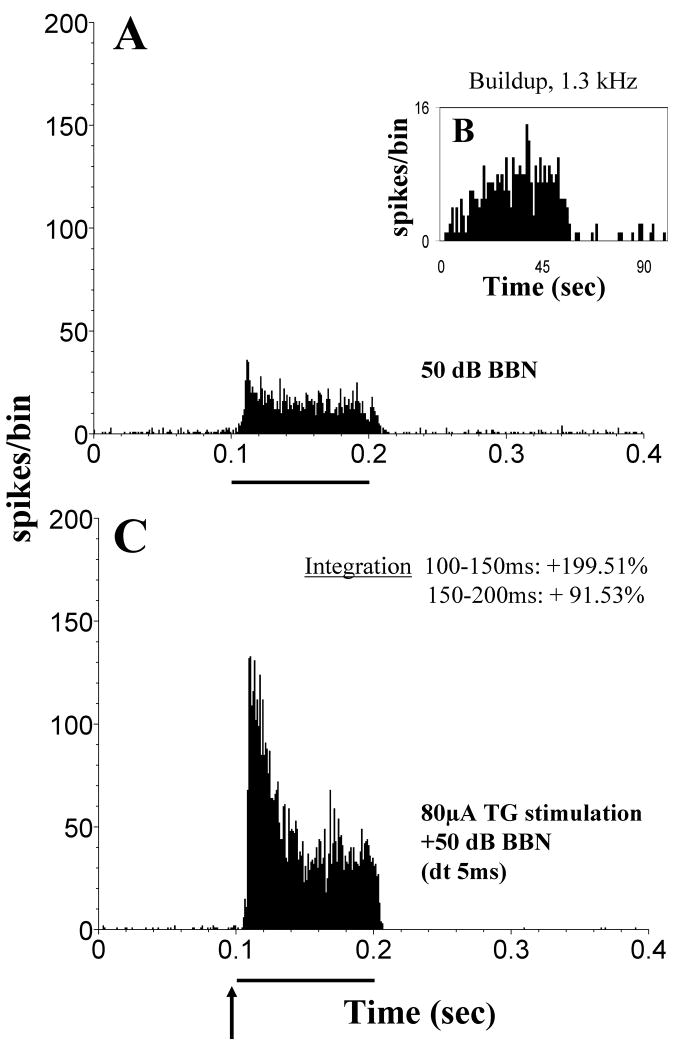
Multisensory integration of inputs from the auditory and somatosensory systems has been studied by Shore and coworkers in the auditory brainstem and midbrain nuclei using electrical stimulation of the trigeminal system (TG and Sp5) combined with acoustic stimulation (broad band noise). Responses to this bimodal stimulation were compared to the responses to acoustic and trigeminal stimulation alone. The difference in responses between bimodal and unimodal stimulation allows to draw conclusions about the extent and nature of multisensory integration, whereas the constancy of the recordings, i.e. if the same single neurons were recorded during both stimulations, was assured with analysis of the recorded waveforms, principle component analysis and cluster analysis (e.g. Shore et al., 2008a). The majority of units in the DCN showed multisensory integration in response to acoustic and TG stimulation (78% of 115 single units; Shore, 2005). This is a larger percentage compared to the units showing a response to TG stimulation alone (41% of 126 single units in the same animals; Shore, 2005). The large difference indicates that subthreshold responses from the trigeminal system contribute to bimodal integration. In most units, bimodal suppression was observed (Fig. 8) with a much smaller fraction of units showing bimodal enhancement (Fig. 9; 60% vs. 18%, Shore, 2005). The percentages of units showing bimodal interaction, as well as its strength and quality, varied depending on the time window in which the bimodal response was examined and depended on the time interval between both stimuli (Fig. 10). These findings illuminate the particular importance of the temporal relationship of both stimuli in achieving auditory-somatosensory integration in the naturally behaving animal. This is an example of the principle of temporal coincidence formulated for bimodal integration (see Introduction). The overwhelming suppressive effect of trigeminal inputs could underly the DCNs’ proposed role in filtering out components of the auditory input that are related to self-generated body sounds. Changes in the perceived loudness of tinnitus during somatosensory manipulations of the face/head/neck (Sanchez et al., 2002; Levine et al., 2003) could be mediated by these rate changes. Even though the effect of TG stimulation alone was predominantly excitatory, the effect of the TG input on acoustically evoked responses was mainly suppressive. The excitatory effect of TG stimulation reflects the glutamatergic input from the parallel fibers to pyramidal and cartwheel cells, the latter probably responsible for suppressing the responses of these neurons to combined auditory-somatosensory stimulation. A possible contributing factor to this bimodal integration is LTP/LTD, which has been shown in the cerebellum and DCN (Fujino & Oertel, 2003; Tzounopoulos et al., 2004). In vitro small depolarizing currents before a depolarizing puls change the regularity of pyramidal cells (Kanold and Manis, 1999). This could resemble a somatosensory stimulus followed by an auditory stimulus changing the temporal finestructure of the response in vivo.
Figure 8.
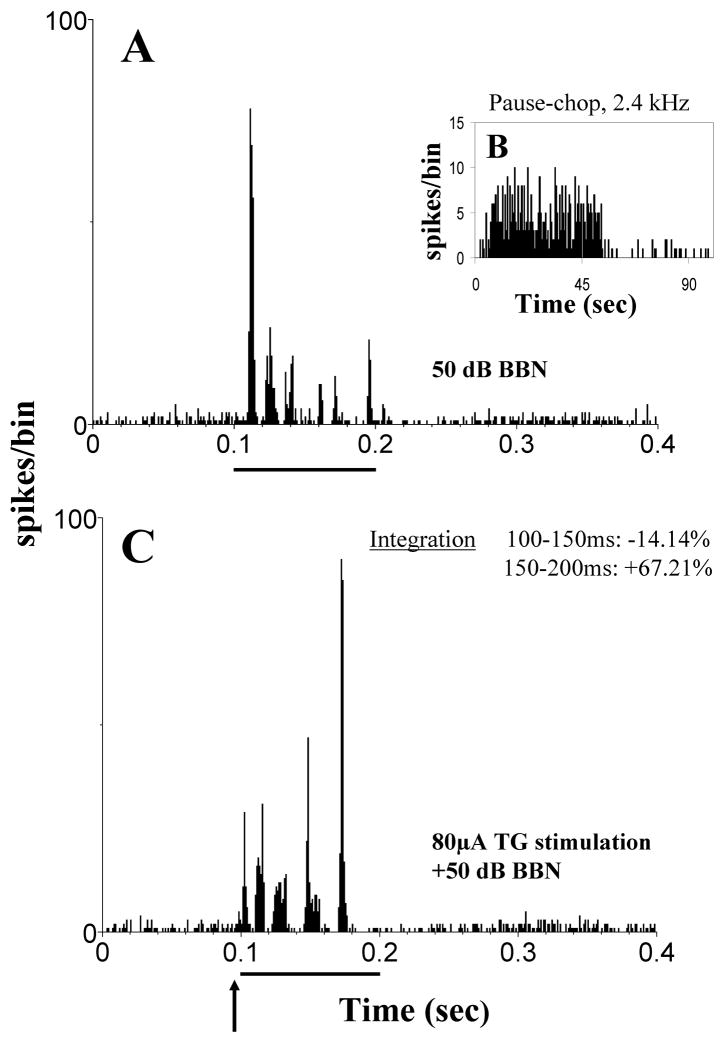
Trigeminal ganglion stimulation suppresses acoustically evoked responses in many DCN neurons. (A) Post-stimulus time histograms (PSTHs) of a single unit responding to a broadband noise (BBN, 50dB SPL, 100 ms). (B) This unit shows a buildup response to a best frequency tone burst (50-ms, onset at 0 ms, bin width: 1ms; 100 repetitions). (C) PSTH of the bimodal response of the same unit as in (A): electrical stimulation of the trigeminal ganglion (80 μA; 100 μs/phase) precedes the BBN noise stimulus by 5ms (dt). Arrow indicates onset of electrical stimulation at 95 ms; solid bar indicates 100 ms duration of BBN. PSTHs were calculated for 200 presentations, with bin width, 0.5 ms. Multisensory integration to the bimodal stimulus is calculated for times 100–150 and 150–200 ms (inset in (B)). Multisensory integration occurs as bimodal enhancement (positive percentages; BE=[(Bi−T−A)/T+A)] × 100, Bi: response rate to the bimodal stimulation, T and A: response rates to the unimodal trigeminal or acoustic stimulation respectively) or bimodal supression (negative percentages; BS=[(Bi−Unimax)/Unimax] × 100, Bi: response rate to the bimodal stimulation, Unimax: response rate to the maximal effective unimodal stimulation; suppression occurs when BS<0). Suppression of more than 50% occurs during the period 150–200 ms but not during the period 100–150 ms in this unit, indicating a delayed maximal suppression. (Adapted with permission from Shore, 2005)
Figure 9.
In some units DCN units trigeminal ganglion stimulation enhances responses to sound. Design of figure as Figure 8. (A) Unimodal stimulation: PSTH of a single unit responding to a broadband noise (BBN) stimulus (50 dB SPL, 100 ms). (B) Buildup PSTH of the same unit’s response to a 50-ms, best frequency tone burst. 1ms bin width; 100 repetitions; tone onset at 0 ms. (C) Bimodal stimulation: BBN noise stimulus is preceded by 5ms with an electrical stimulation of the trigeminal ganglion (80 μA; 100 μs/phase). 200 presentations. Bin width, 0.5 ms. Enhancement of almost 200% occurs during the period 150–200 ms and enhancement of almost 100% occurs during the period 100–150 ms in this unit, indicating a more rapid effect than demonstrated for the suppression in Fig. 8. The enhancement lasts for the duration of the stimulus. (Adapted with permission from Shore, 2005)
Figure 10.
The temporal fine structure of acoustically evoked responses in the DCN is altered by trigeminal ganglion stimulation. Design of figure as for Figure 8. (A) Unimodal stimulation: PSTH of a single unit responding to a broadband noise (BBN) stimulus (50 dB SPL, 100 ms). (B) pauser-chopper PSTH of the same unit’s response to a 50-ms, best frequency toneburst. 1ms bin width; 100 repetitions; tone onset at 0 ms. (C) Bimodal stimulation: BBN noise stimulus is preceded by 5ms with an electrical stimulation of the trigeminal ganglion (80 μA; 100 μs/phase). 200 presentations. Bin width, 0.5 ms. Slight suppression occurs during the period 150–200 ms and enhancement of almost 70% occurs during the period 100–150 ms in this unit, with the ultimate effect producing a reversal of the temporal pattern evoked by the BBN after trigeminal stimulation. (Adapted with permission from Shore, 2005)
Although IC neurons showed little change in their response rates to Sp5 stimulation alone, a large number of units showed bimodal integration when Sp5 stimulation was combined with acoustic stimulation (66% of 126 single units, mostly On and On sustained units; Jain & Shore, 2006). This again indicates that subthreshold responses from the trigeminal system play a role in bimodal integration, fulfilling the principle of inverse effectiveness for bimodal interaction (see introduction). As in the DCN, the majority of affected IC units showed bimodal suppression, with the bimodal response smaller than the acoustically evoked response (48%, 60/126 units in 5 animals; (Fig. 11, 12). Given the similarity of the bimodal processing in the DCN and IC it remains to be determined which part reflects processing already completed in the DCN and transferred to the IC via the projection of the DCN to the ICXV, and which part of this processing reflects the influence of the trigeminal system via the direct input from the Sp5 to the IC (Zhou & Shore, 2006).
Figure 11.
The predominant effect of bimodal integration in the IC is suppression. Responses to broad band noise (BBN) are suppressed due to trigeminal ganglion stimulation. (A) Post-stimulus time histogram (PSTH) of a single unit to electrical stimulation alone at 50 μA: this neuron does show no response to trigeminal stimulation. (B) PSTH of the same neuron to BBN at 30 dB SPL. (C) Responses to BBN are suppressed when paired with trigeminal stimulation with 50 μA. The solid bar indicates the duration of the BBN burst. The arrow indicates the onset of the electrical stimulus. Bin width 1 ms, 100 presentations. (Adapted with permission from Jain & Shore, 2006)
Figure 12.
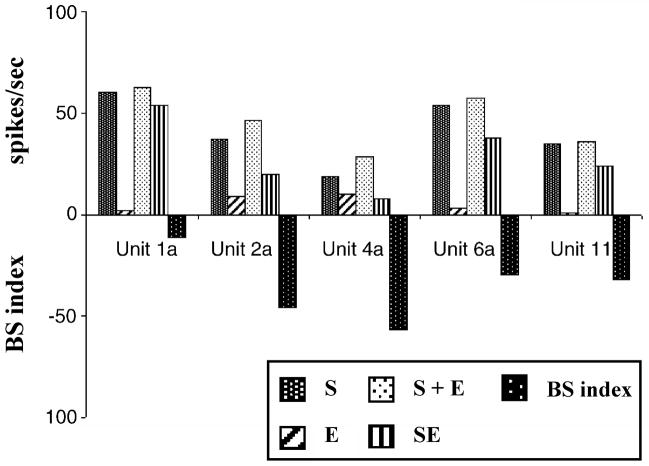
Bimodal supression in neurons of the ICx. Bar diagrams demonstrating multisensory integration in five ICx units. S, unimodal acoustic stimulation (BBN, 40 dB SPL); E, unimodal somatosensory stimulation (electrical stimulation of trigeminal nucleus with 50 μA); S + E, algebraic sum of responses to acoustic and electrical stimulation; SE, actually observed response to combined trigeminal-BBN stimulation. Bimodal suppression is quantified with the BS index (see legend figure 8): responses to bimodal stimulaton (SE) are smaller than those redicted by summing individual unimodal responses (S + E), resulting in negative BS indices, which indicates bimodal suppression. (Adapted with permission from Jain & Shore, 2006)
Clues about the function of the somatosensory input to the ICXV are given by experiments of Tammer et al. (2004), who studied audio-vocal interactions in the IC in freely behaving squirrel monkeys. They found neurons in the external nucleus of IC (but not the central nucleus) that responded to vocalizations of group mates but not to self-vocalizations. In the same species, Lüthe et al. (2000) recorded the neuronal activity in various nuclei of the medulla oblongata during self-vocalization. In the Sp5, 80% of the neurons responded to self-vocalization. Among these were units that responded before vocalization onset, and continued to respond for the duration of the vocalization with the same time structure as the vocalization. The early onset of the activity in the Sp5 is most likely due to activation of larygeal muscles before vocalization onset, or by a feedforward input from vocal motor centers to Sp5 (discussed in Lüthe et al., 2000). In both cases, the early onset of Sp5 activity and its precise temporal relationship to self-vocalization would enable the simultaneous arrival of acoustic and somatosensory activity to the IC, which would precisely encode the self-vocalization. The interaction of both inputs in the ICXV would then serve to filter out the auditory response to self-vocalization while transmitting other auditory signals at the same time. The same process could take place in the CN, where cancellation of self-vocalizationis likely to begin.
Auditory-somatosensory interaction in the deafened auditory system
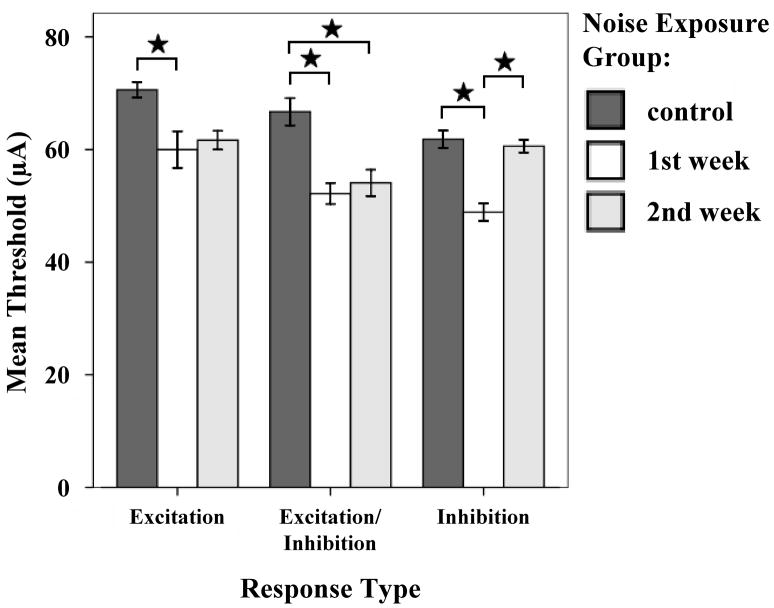
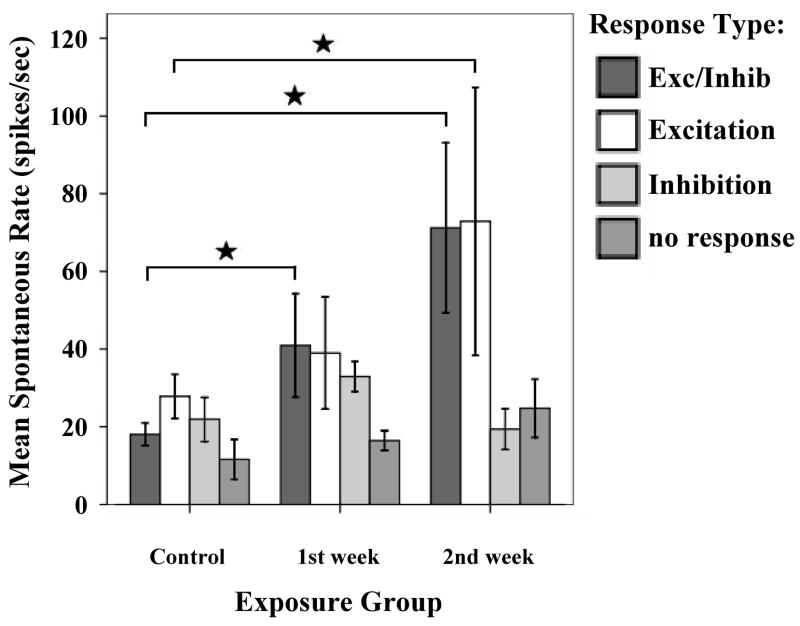
Studies of neuronal plasticity in the auditory system have shown that sensory deprivation or over-stimulation results in morphological reorganization of inputs to the deprived area (Michler & Illing, 2002; Kim et al., 2004a, b; Hsieh & Cramer, 2006; reviews: Cacace, 2003; Kaltenbach et al., 2005). Such plasticity can occur across different modalties, not only in the cortex, but also in sub-cortical areas such as the thalamus (Batzri-Izraeli et al., 1990; Pichert et al., 1997). The extensive cross-modal interactions between auditory and somatosensory inputs in the normal auditory system suggested that auditory-somatosensory cross-modal plasticity may occur after auditory deprivation: Shore and coworkers investigated the possibility of cross modal compensation from the trigeminal system in noise damaged guinea pigs (Shore et al., 2008a). Recordings from DCN single units within the first two weeks after noise exposure demonstrated decreased thresholds and latencies to TG stimulation (Fig. 13), and a greater amount of bimodal integration in the noise damaged animals, providing evidence for increased responsiveness to the somatosensory system co-incident with decreased responsiveness to auditory stimulation. The noise damaged animals also showed an increase in spontaneous rates (Fig. 14; Shore et al., 2008a). When analyzed with respect to the response types to TG stimulation, the increases in spontaneous rate were restricted to those units that received an excitatory input from the trigeminal system (Shore et al., 2008a). The increased SRs for those units was larger than in the whole population and was comparable to the increase of spontaneous rate shown with superficial recordings of the DCN 14 days after noise exposure (Kaltenbach et al., 2000). The increased spontaneous rates of neurons receiving excitatory input from the trigeminal system could reflect strengthened excitatory input from the trigeminal system via the granule cells to pyramidal cells. Indeed, recent studies demonstrate an upregulation in VGLUT2 in regions of the CN that receive somatosensory inputs, suggesting greater glutamatergic transmission in this system to compensate for loss of glutamatergic inputs from the VIIIth nerve (Shore et al., 2008b). Concurrent with this increase in VGLUT2 was an increase in the synaptic vesicle protein, SV2, suggesting an increase in synaptic inputs from somatosensory regions to the CN following cochlear damage (Zeng et al., 2008).
Figure 13.
Thresholds to trigeminal stimulation decrease following noise damage. Mean thresholds for all response types to trigeminal ganglion stimulation are decreased one week and two weeks after noise exposure. Stars indicate significant differences (one week: E, p=0.021; E/In, p<0.001; In, p<0.001, two weeks: E/in, p=0.003). Error bars indicate +/− 1 standard error. (Adapted with permission from Shore et al., 2007)
Figure 14.
Mean spontaneous rates (SR) for DCN single units one and two weeks after noise exposure at 120 dB SPL. The distribution of SRs by responses to trigeminal stimulation indicated that only units with E and E/I responses to trigeminal stimulation showed increased SRs after noise exposure. Units with In or no responses did not show increased SR after noise damage. (Adapted with permission from Shore et al., 2007)
The increased influence of the somatosensory system on cochlear nucleus function might not only be relevant to the generation and/or maintenance of tinnitus, but could be of functional significance for the animal in coping with impaired auditory input. The increased sensitivity of DCN neurons to somatosensory simulation as well as their increased bimodal suppression could allow for greater filtering of unwanted information, even more important to an individual with a hearing loss, which has to struggle to seggregate the sparse auditory information from non-information such as self generated sounds.
Summary and conclusions
The anatomical and physiological results summarized in this chapter describe the widespread influence of the somatosensory system on auditory brainstem and midbrain nuclei, namely the DCN, VCN and IC. Tracer studies show that the predominant influence of somatosensory neurons is transmitted via the GCD, which can be considered to be part of the extralemniscal system together with the external nucleus of the IC. Connections of the extralemniscal system with the limbic system have been linked to affects that may accompany tinnitus.
The experiments using bimodal stimulation show that bimodal interaction occurs already in second-order auditory neurons, and that the results of this interaction are well suited to raise the detectibility of relevant acoustic signals. The different neuronal responses to stimulation of somatosensory inputs in vivo are complex and depend not only on the stimulation site (dorsal column, TG, Sp5) and recording site (DCN, VCN, ICXV), but also on the temporal relationship between the inputs from both modalities as well as the strength of stimulation. The specific function of the diverse somatosensory input pathways to the auditory system is not yet well understood and requires further investigation.
Changes in auditory-somatosensory interactions in the DCN of deafened animals include an increase in spontaneous discharge rate, which may be caused by an increased excitatory influence of the somatosensory system after input from the auditory system is reduced. These findings stress the importance of considering the somatosensory influence in the extralemniscal auditory system in defining mechanisms underlying tinnitus. The impact of the compensated auditory-somatosensory interactions has not been studied in other nuclei receiving bimodal inputs, nor has the question of how this imbalance is transferred from the CN to the IC. Furthermore, while bimodal auditory-somatosensory interactions have been demonstrated in the VCN, (Shore et al., 2003), further studies are needed to explore how plastic changes after noise induced hearing loss in the VCN (Michler & Illing, 2002; Kim et al. 2004a,b; Shore et al., 2007; Zeng et al., 2008) might contribute to the generation of tinnitus.
Given the widespread somatosensory influence on the auditory system, changes in auditory-somatosensory coding after auditory or somatosensory deprivation or deafferentation deafness will occur not only in the DCN, but in all nuclei receiving bimodal input including the inferior colliculus, medial geniculate nucleus and auditory cortex. Therefore, it seems unlikely that treatments concentrating on a single nucleus or pathway could alleviate tinnitus. Furthermore, the increased somatosensory influence on the DCN after auditory deprivation highlights the importance of considering cross-modal plasticity as an important factor underlying tinnitus. These results support the contention that evaluation of the tinnitus patient should include a careful examination of the somatosensory system (Levine et al., 2007). Subsequent treatments would then involve modification of somatosensory inputs to the auditory system, or treatment of an underlying disorder of the somatosensory system using acupuncture, transcutaneous stimulation of the scalp and auricle or temporomandibular joint, cervical manupulations and craniosacral or trigger point therapy. Use of these therapies in modifying tinnitus needs to be explored systematically taking into accout the factors presented in this chapter.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 DC004825; P30 DC05188 and the Tinnitus Research Consortium.
Abbreviations
- AVCN
anteroventral cochlear nucleus
- C2-8
cervical nerves 2–8
- CN
cochlear nucleus
- Cu
cuneate nucleus
- DAS
dorsal acoustic striae
- DCN
dorsal cochlear nucleus
- DRG
dorsal root ganglion
- GCD
granule cell domain
- Gr
gracile nucleus
- IAS
intermediate acoustic striae
- IC
inferior colliculus
- ICXV
ventrolateral border region of IC
- IO
inferior olive
- LPGi
lateral paragigantocellular reticular nucleus
- LRF
lateral reticular formation
- mesenc. ncl
mesencephalic nucleus of the brainstem trigeminal sensory complex
- MSN
medullary somatosensory nuclei
- principl. ncl
principle nucleus of the brainstem trigeminal sensory complex
- PVCN
posteroventral cochlear nucleus
- RVL
rostral ventrolateral reticular formation
- SG
spiral ganglion
- Sp5
spinal trigeminal nucleus
- Sp5C
pars caudalis of Sp5
- Sp5I
pars interpolaris of Sp5
- Sp5O
pars oralis of Sp5
- Subncl. gelatin
subnucleus gelatinosus of Sp5 and spinal cord
- subncl. magnocell
subnucleus magnocellularis of Sp5
- subncl. marginal
subnucleus marginalis of Sp5
- superfic. layer
superficial layer of the spinal cord
- TG
trigeminal ganglion
- VCN
ventral cochlear nucleus
- VG
vestibular ganglion
- VGLUT
vesicular glutamate transporte
References
- Adams JC. Multipolar cells in the ventral cochlear nucleus project to the dorsal cochlear nucleus and the inferior colliculus. Neuroscience Letters. 1983;37(3):205–208. doi: 10.1016/0304-3940(83)90431-7. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Dickhaus H, Schult W, Zimmermann M. External nucleus of inferior colliculus: Auditory and spinal somatosensory afferents and their interactions. J Neurophysiol. 1978;41(4):837–847. doi: 10.1152/jn.1978.41.4.837. [DOI] [PubMed] [Google Scholar]
- Aitkin LM, Kenyon CE, Philpott P. The representation of the auditory and somatosensory systems in the external nucleus of the cat inferior colliculus. J Comp Neurol. 1981;196(1):25–40. doi: 10.1002/cne.901960104. [DOI] [PubMed] [Google Scholar]
- Babic T, Ciriello J. Medullary and spinal cord projections from cardiovascular responsive sites in the rostral ventromedial medulla. J Comp Neurol. 2004;469(3):391–412. doi: 10.1002/cne.11024. [DOI] [PubMed] [Google Scholar]
- Bartels H, Staal MJ, Albers FW. Tinnitus and neural plasticity of the brain. Otol Neurotol. 2007;28(2):178–184. doi: 10.1097/MAO.0b013e31802b3248. [DOI] [PubMed] [Google Scholar]
- Batzri-Izraeli R, Kelly JB, Glendenning KK, Masterton RB, Wollberg Z. Auditory cortex of the long-eared hedgehog (hemiechinus auritus). I. Boundaries and frequency representation. Brain Behav Evol. 1990;36(4):237–248. doi: 10.1159/000115310. [DOI] [PubMed] [Google Scholar]
- Bell C, Bodznick D, Montgomery J, Bastian J. The generation and subtraction of sensory expectations within cerebellum-like structures. Brain Behav Evol. 1997;50(Suppl 1):17–31. doi: 10.1159/000113352. [DOI] [PubMed] [Google Scholar]
- Bowker RM, Westlund KN, Coulter JD. Origins of serotonergic projections to the spinal cord in rat: An immunocytochemical-retrograde transport study. Brain Res. 1981;226(1–2):187–199. doi: 10.1016/0006-8993(81)91092-1. [DOI] [PubMed] [Google Scholar]
- Burian M, Gstoettner W, Zundritsch R. Saccular afferent fibers to the cochlear nucleus in the guinea pig. Arch Otorhinolaryngol. 1989;246(5):238–241. doi: 10.1007/BF00463563. [DOI] [PubMed] [Google Scholar]
- Cacace AT. Expanding the biological basis of tinnitus: Crossmodal origins and the role of neuroplasticity. Hear Res. 2003;175(1–2):112–132. doi: 10.1016/s0378-5955(02)00717-7. [DOI] [PubMed] [Google Scholar]
- Caicedo A, Herbert H. Topography of descending projections from the inferior colliculus to auditory brainstem nuclei in the rat. J Comp Neurol. 1993;328(3):377–392. doi: 10.1002/cne.903280305. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Parallel auditory pathways: Projection patterns of the different neuronal populations in the dorsal and ventral cochlear nuclei. Brain Res Bull. 2003;60(5–6):457–474. doi: 10.1016/s0361-9230(03)00050-9. [DOI] [PubMed] [Google Scholar]
- Capra NF. Localization and central projections of primary afferent neurons that innervate the temporomandibular joint in cats. Somatosens Res. 1987;4(3):201–213. doi: 10.3109/07367228709144607. [DOI] [PubMed] [Google Scholar]
- Cui Y, Shore SE. Abstracts for the association for research in otolaryngology. Phoenix, AZ: 2008. Topography of projections from the lateral reticular formation and spinal trigeminal nucleus to the cochlear nucleus in guinea pigs. [Google Scholar]
- Darian-Smith I, Phillips G, Ryan RD. Functional organization in the trigeminal main sensory and rostral spinal nuclei of the cat. J Physiol. 1963;168:129–146. doi: 10.1113/jphysiol.1963.sp007182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KA, Miller RL, Young ED. Effects of somatosensory and parallel-fiber stimulation on neurons in dorsal cochlear nucleus. J Neurophysiol. 1996;76(5):3012–3024. doi: 10.1152/jn.1996.76.5.3012. [DOI] [PubMed] [Google Scholar]
- Davis KA, Young ED. Granule cell activation of complex-spiking neurons in dorsal cochlear nucleus. J Neurosci. 1997;17(17):6798–6806. doi: 10.1523/JNEUROSCI.17-17-06798.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devor A. Is the cerebellum like cerebellar-like structures? Brain Res Brain Res Rev. 2000;34(3):149–56. doi: 10.1016/s0165-0173(00)00045-x. [DOI] [PubMed] [Google Scholar]
- Doucet JR, Ross AT, Gillespie MB, Ryugo DK. Glycine immunoreactivity of multipolar neurons in the ventral cochlear nucleus which project to the dorsal cochlear nucleus. Journal of Comparative Neurology. 1999;408(4):515–531. [PubMed] [Google Scholar]
- Eggermont JJ. Tinnitus: Neurobiological substrates. Drug Discov Today. 2005;10(19):1283–1290. doi: 10.1016/S1359-6446(05)03542-7. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Bulthoff HH. Merging the senses into a robust percept. Trends Cogn Sci. 2004;8(4):162–169. doi: 10.1016/j.tics.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Fremeau RT, Jr, Troyer MD, Pahner I, Nygaard GO, Tran CH, Reimer RJ, et al. The expression of vesicular glutamate transporters defines two classes of excitatory synapse. Neuron. 2001;31(2):247–260. doi: 10.1016/s0896-6273(01)00344-0. [DOI] [PubMed] [Google Scholar]
- Gras C, Herzog E, Bellenchi GC, Bernard V, Ravassard P, Pohl M, et al. A third vesicular glutamate transporter expressed by cholinergic and serotoninergic neurons. J Neurosci. 2002;22(13):5442–5451. doi: 10.1523/JNEUROSCI.22-13-05442.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gstoettner W, Burian M, Zundritsch R, Mayr R. The origin of the vestibulo-cochlear projection in the guinea pig. Neurosci Lett. 1991;122(2):163–166. doi: 10.1016/0304-3940(91)90848-n. [DOI] [PubMed] [Google Scholar]
- Haenggeli CA, Pongstaporn T, Doucet JR, Ryugo DK. Projections from the spinal trigeminal nucleus to the cochlear nucleus in the rat. J Comp Neurol. 2005;484(2):191–205. doi: 10.1002/cne.20466. [DOI] [PubMed] [Google Scholar]
- Hayashi H, Sumino R, Sessle BJ. Functional organization of trigeminal subnucleus interpolaris: Nociceptive and innocuous afferent inputs, projections to thalamus, cerebellum, and spinal cord, and descending modulation from periaqueductal gray. J Neurophysiol. 1984;51(5):890–905. doi: 10.1152/jn.1984.51.5.890. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Holmes GM, Rogers RC, Beattie MS, Bresnahan JC. Descending spinal projections from the rostral gigantocellular reticular nuclei complex. J Comp Neurol. 2003;455(2):210–221. doi: 10.1002/cne.10455. [DOI] [PubMed] [Google Scholar]
- Hsieh CY, Cramer KS. Deafferentation induces novel axonal projections in the auditory brainstem after hearing onset. J Comp Neurol. 2006;497(4):589–599. doi: 10.1002/cne.21002. [DOI] [PubMed] [Google Scholar]
- Itoh K, Kamiya H, Mitani A, Yasui Y, Takada M, Mizuno N. Direct projections from the dorsal column nuclei and the spinal trigeminal nuclei to the cochlear nuclei in the cat. Brain Res. 1987;400(1):145–150. doi: 10.1016/0006-8993(87)90662-7. [DOI] [PubMed] [Google Scholar]
- Jacquin MF, Barcia M, Rhoades RW. Structure-function relationships in rat brainstem subnucleus interpolaris: Iv. Projection neurons. J Comp Neurol. 1989;282(1):45–62. doi: 10.1002/cne.902820105. [DOI] [PubMed] [Google Scholar]
- Jain R, Shore S. External inferior colliculus integrates trigeminal and acoustic information: Unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neurosci Lett. 2006;395(1):71–75. doi: 10.1016/j.neulet.2005.10.077. [DOI] [PubMed] [Google Scholar]
- Jousmäki V, Hari R. Parchment-skin illusion: Sound-biased touch. Curr Biol. 1998;8(6):R190. doi: 10.1016/s0960-9822(98)70120-4. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA. The dorsal cochlear nucleus as a participant in the auditory, attentional and emotional components of tinnitus. Hear Res. 2006:216–217. 224–234. doi: 10.1016/j.heares.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Afman CE. Plasticity of spontaneous neural activity in the dorsal cochlear nucleus after intense sound exposure. Hear Res. 2000;147(1–2):282–292. doi: 10.1016/s0378-5955(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Kaltenbach JA, Zhang J, Finlayson P. Tinnitus as a plastic phenomenon and its possible neural underpinnings in the dorsal cochlear nucleus. Hear Res. 2005;206(1–2):200–226. doi: 10.1016/j.heares.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Itoh K, Yasui Y, Ino T, Mizuno N. Somatosensory and auditory relay nucleus in the rostral part of the ventrolateral medulla: A morphological study in the cat. J Comp Neurol. 1988;273(3):421–435. doi: 10.1002/cne.902730311. [DOI] [PubMed] [Google Scholar]
- Kanold PO, Young ED. Proprioceptive information from the pinna provides somatosensory input to cat dorsal cochlear nucleus. J Neurosci. 2001;21(19):7848–7858. doi: 10.1523/JNEUROSCI.21-19-07848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser C, Logothetis NK. Do early sensory cortices integrate cross-modal information? Brain Struct Funct. 2007;212(2):121–132. doi: 10.1007/s00429-007-0154-0. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Perachio AA. Projections from the sacculus to the cochlear nuclei in the mongolian gerbil. Brain Behav Evol. 1989;34(4):193–200. doi: 10.1159/000116505. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Morest DK, Potashner SJ. Quantitative study of degeneration and new growth of axons and synaptic endings in the chinchilla cochlear nucleus after acoustic overstimulation. J Neurosci Res. 2004a;77(6):829–842. doi: 10.1002/jnr.20211. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Gross J, Potashner SJ, Morest DK. Fine structure of long-term changes in the cochlear nucleus after acoustic overstimulation: Chronic degeneration and new growth of synaptic endings. J Neurosci Res. 2004b;77(6):817–828. doi: 10.1002/jnr.20212. [DOI] [PubMed] [Google Scholar]
- King AJ, Palmer AR. Integration of visual and auditory information in bimodal neurones in the guinea-pig superior colliculus. Exp Brain Res. 1985;60(3):492–500. doi: 10.1007/BF00236934. [DOI] [PubMed] [Google Scholar]
- Levine RA. Somatic (craniocervical) tinnitus and the dorsal cochlear nucleus hypothesis. Am J Otolaryngol. 1999;20(6):351–362. doi: 10.1016/s0196-0709(99)90074-1. [DOI] [PubMed] [Google Scholar]
- Levine RA, Abel M, Cheng H. CNS somatosensory-auditory interactions elicit or modulate tinnitus. Exp Brain Res. 2003;153(4):643–648. doi: 10.1007/s00221-003-1747-3. [DOI] [PubMed] [Google Scholar]
- Levine RA, Nam EC, Oron Y, Melcher JR. Evidence for a tinnitus subgroup responsive to somatosensory based treatment modalities. Prog Brain Res. 2007;166:195–207. doi: 10.1016/S0079-6123(07)66017-8. [DOI] [PubMed] [Google Scholar]
- Li H, Mizuno N. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: A fluorescent retrograde double-labeling study in the rat. Neurosci Res. 1997;29(2):135–142. doi: 10.1016/s0168-0102(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Lorente de No R. The primary acoustic nuclei. New York: Raven Press; 1981. [Google Scholar]
- Lüthe L, Häusler U, Jürgens U. Neuronal activity in the medulla oblongata during vocalization. A single-unit recording study in the squirrel monkey. Behav Brain Res. 2000;116(2):197–210. doi: 10.1016/s0166-4328(00)00272-2. [DOI] [PubMed] [Google Scholar]
- Meredith MA, Stein BE. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J Neurophysiol. 1986;56(3):640–662. doi: 10.1152/jn.1986.56.3.640. [DOI] [PubMed] [Google Scholar]
- Michler SA, Illing RB. Acoustic trauma induces reemergence of the growth- and plasticity-associated protein gap-43 in the rat auditory brainstem. J Comp Neurol. 2002;451(3):250–266. doi: 10.1002/cne.10348. [DOI] [PubMed] [Google Scholar]
- Moechars D, Weston MC, Leo S, Callaerts-Vegh Z, Goris I, Daneels G, et al. Vesicular glutamate transporter VGLUT2 expression levels control quantal size and neuropathic pain. J Neurosci. 2006;26(46):12055–12066. doi: 10.1523/JNEUROSCI.2556-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AR. Symptoms and signs caused by neural plasticity. Neurol Res. 2001;23(6):565–572. doi: 10.1179/016164101101199009. [DOI] [PubMed] [Google Scholar]
- Møller AR. Neural plasticity in tinnitus. Prog Brain Res. 2006;157:365–372. doi: 10.1016/S0079-6123(06)57022-0. [DOI] [PubMed] [Google Scholar]
- Møller AR, Rollins PR. The non-classical auditory pathways are involved in hearing in children but not in adults. Neurosci Lett. 2002;319(1):41–44. doi: 10.1016/s0304-3940(01)02516-2. [DOI] [PubMed] [Google Scholar]
- Mugnaini E, Morgan JI. The neuropeptide cerebellin is a marker for two similar neuronal circuits in rat brain. Proc Natl Acad Sci U S A. 1987;84(23):8692–6. doi: 10.1073/pnas.84.23.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazruddin, Suemune S, Shirana Y, Yamauchi K, Shigenaga Y. The cells of origin of the hypoglossal afferent nerves and central projections in the cat. Brain Res. 1989;490(2):219–235. doi: 10.1016/0006-8993(89)90240-0. [DOI] [PubMed] [Google Scholar]
- Nelken I, Young ED. Why do cats need a dorsal cochlear nucleus? J Basic Clin Physiol Pharmacol. 1996;7(3):199–220. doi: 10.1515/jbcpp.1996.7.3.199. [DOI] [PubMed] [Google Scholar]
- Oertel D, Young ED. What’s a cerebellar circuit doing in the auditory system? Trends Neurosci. 2004;27(2):104–110. doi: 10.1016/j.tins.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Ohlrogge M, Doucet JR, Ryugo DK. Projections of the pontine nuclei to the cochlear nucleus in rats. J Comp Neurol. 2001;436(3):290–303. [PubMed] [Google Scholar]
- Ostapoff EM, Morest DK, Parham K. Spatial organization of the reciprocal connections between the cat dorsal and anteroventral cochlear nuclei [see comments] Hearing Research. 1999;130(1–2):75–93. doi: 10.1016/s0378-5955(98)00224-x. [DOI] [PubMed] [Google Scholar]
- Pfaller K, Arvidsson J. Central distribution of trigeminal and upper cervical primary afferents in the rat studied by anterograde transport of horseradish peroxidase conjugated to wheat germ agglutinin. J Comp Neurol. 1988;268(1):91–108. doi: 10.1002/cne.902680110. [DOI] [PubMed] [Google Scholar]
- Pichert JW, Hickson GB, Bledsoe S, Trotter T, Quinn D. Understanding the etiology of serious medical events involving children: Implications for pediatricians and their risk managers. Pediatr Ann. 1997;26(3):160–164. 167–168, 170–162. doi: 10.3928/0090-4481-19970301-06. [DOI] [PubMed] [Google Scholar]
- Populin LC, Yin TC. Bimodal interactions in the superior colliculus of the behaving cat. J Neurosci. 2002;22(7):2826–2834. doi: 10.1523/JNEUROSCI.22-07-02826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice JJ, May BJ, Spirou GA, Young ED. Pinna-based spectral cues for sound localization in cat. Hear Res. 1992;58(2):132–152. doi: 10.1016/0378-5955(92)90123-5. [DOI] [PubMed] [Google Scholar]
- Romfh JH, Capra NF, Gatipon GB. Trigeminal nerve and temporomandibular joint of the cat: A horseradish peroxidase study. Exp Neurol. 1979;65(1):99–106. doi: 10.1016/0014-4886(79)90251-6. [DOI] [PubMed] [Google Scholar]
- Saade NE, Frangieh AS, Atweh SF, Jabbur SJ. Dorsal column input to cochlear neurons in decerebrate-decerebellate cats. Brain Res. 1989;486(2):399–402. doi: 10.1016/0006-8993(89)90532-5. [DOI] [PubMed] [Google Scholar]
- Sanchez TG, Guerra GC, Lorenzi MC, Brandão AL, Bento RF. The influence of voluntary muscle contractions upon the onset and modulation of tinnitus. Audiol Neurootol. 2002;7(6):370–375. doi: 10.1159/000066155. [DOI] [PubMed] [Google Scholar]
- Saunders JC. The role of central nervous system plasticity in tinnitus. J Commun Disord. 2007;40(4):313–334. doi: 10.1016/j.jcomdis.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Mori RL, Yates BJ. Locations of neurons with respiratory-related activity in the ferret brainstem. Brain Res. 2003;974(1–2):236–242. doi: 10.1016/s0006-8993(03)02592-7. [DOI] [PubMed] [Google Scholar]
- Shore S. Auditory/somatosensory interactions. In: Adelman G, Smith BH, editors. The new encyclopedia of neuroscience. Elsevier Science; Amsterdam and New York: 2008. [Google Scholar]
- Shore SE. Multisensory integration in the dorsal cochlear nucleus: Unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci. 2005;21(12):3334–3348. doi: 10.1111/j.1460-9568.2005.04142.x. [DOI] [PubMed] [Google Scholar]
- Shore S, Koehler S, Oldakowski M, Hughes L, Syed S. Dorsal cochlear nucleus reponses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008a;27(1):155–68. doi: 10.1111/j.1460-9568.2007.05983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Zeng C, Zhou J, Nannapaneni N. Abstracts of the Society for Neuroscience. San Diego. CA: 2007. Deafferentation alters the distribution of vessicular glutamate transporters in the guinea pig cochlear nucleus. [Google Scholar]
- Shore S, Zhou J, Koehler S. Neural mechanisms underlying somatic tinnitus. Prog Brain Res. 2007;166:107–23. doi: 10.1016/S0079-6123(07)66010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore SE, Zhou J. Somatosensory influence on the cochlear nucleus and beyond. Hear Res. 2006:216–217. 90–99. doi: 10.1016/j.heares.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Shore SE, El Kashlan H, Lu J. Effects of trigeminal ganglion stimulation on unit activity of ventral cochlear nucleus neurons. Neuroscience. 2003;119(4):1085–1101. doi: 10.1016/s0306-4522(03)00207-0. [DOI] [PubMed] [Google Scholar]
- Shore SE, Vass Z, Wys NL, Altschuler RA. Trigeminal ganglion innervates the auditory brainstem. J Comp Neurol. 2000;419(3):271–285. doi: 10.1002/(sici)1096-9861(20000410)419:3<271::aid-cne1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Sola E, Prestori F, Rossi P, Taglietti V, D’Angelo E. Increased neurotransmitter release during long-term potentiation at mossy fibre-granule cell synapses in rat cerebellum. J Physiol. 2004;557(Pt 3):843–861. doi: 10.1113/jphysiol.2003.060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford TR, Quessy S, Stein BE. Evaluating the operations underlying multisensory integration in the cat superior colliculus. J Neurosci. 2005;25(28):6499–6508. doi: 10.1523/JNEUROSCI.5095-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BE. Neural mechanisms for synthesizing sensory information and producing adaptive behaviors. Exp Brain Res. 1998;123(1–2):124–135. doi: 10.1007/s002210050553. [DOI] [PubMed] [Google Scholar]
- Stein BE, Meredith MA. The merging of the senses. Cambridge: MIT Press; 1993. [Google Scholar]
- Suemune S, Nishimori T, Hosoi M, Suzuki Y, Tsuru H, Kawata T, et al. Trigeminal nerve endings of lingual mucosa and musculature of the rat. Brain Res. 1992;586(1):162–165. doi: 10.1016/0006-8993(92)91389-v. [DOI] [PubMed] [Google Scholar]
- Takemura M, Sugimoto T, Shigenaga Y. Difference in central projection of primary afferents innervating facial and intraoral structures in the rat. Exp Neurol. 1991;111(3):324–331. doi: 10.1016/0014-4886(91)90099-x. [DOI] [PubMed] [Google Scholar]
- Tammer R, Ehrenreich L, Jürgens U. Telemetrically recorded neuronal activity in the inferior colliculus and bordering tegmentum during vocal communication in squirrel monkeys (saimiri sciureus) Behav Brain Res. 2004;151(1–2):331–336. doi: 10.1016/j.bbr.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Tawil RN, Saade NE, Bitar M, Jabbur SJ. Polysensory interactions on single neurons of cat inferior colliculus. Brain Res. 1983;269(1):149–152. doi: 10.1016/0006-8993(83)90972-1. [DOI] [PubMed] [Google Scholar]
- Usunoff KG, Marani E, Schoen JH. The trigeminal system in man. Adv Anat Embryol Cell Biol. 1997;136:I–X. 1–126. doi: 10.1007/978-3-642-60779-0. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele EJ, Pieribone VA, Aston-Jones G. Diverse afferents converge on the nucleus paragigantocellularis in the rat ventrolateral medulla: Retrograde and anterograde tracing studies. J Comp Neurol. 1989;290(4):561–584. doi: 10.1002/cne.902900410. [DOI] [PubMed] [Google Scholar]
- Varoqui H, Schäfer MK, Zhu H, Weihe E, Erickson JD. Identification of the differentiation-associated Na+/PI transporter as a novel vesicular glutamate transporter expressed in a distinct set of glutamatergic synapses. J Neurosci. 2002;22(1):142–155. doi: 10.1523/JNEUROSCI.22-01-00142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vass Z, Shore SE, Nuttall AL, Jancso G, Brechtelsbauer PB, Miller JM. Trigeminal ganglion innervation of the cochlea--a retrograde transport study. Neuroscience. 1997;79(2):605–615. doi: 10.1016/s0306-4522(96)00641-0. [DOI] [PubMed] [Google Scholar]
- Vass Z, Shore SE, Nuttall AL, Miller JM. Direct evidence of trigeminal innervation of the cochlear blood vessels. Neuroscience. 1998;84(2):559–567. doi: 10.1016/s0306-4522(97)00503-4. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Pongstaporn T, Ryugo DK. Ultrastructural study of the granule cell domain of the cochlear nucleus in rats: Mossy fiber endings and their targets. J Comp Neurol. 1996;369(3):345–360. doi: 10.1002/(SICI)1096-9861(19960603)369:3<345::AID-CNE2>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Weedman DL, Ryugo DK. Projections from auditory cortex to the cochlear nucleus in rats: Synapses on granule cell dendrites. J Comp Neurol. 1996;371(2):311–324. doi: 10.1002/(SICI)1096-9861(19960722)371:2<311::AID-CNE10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Weinberg RJ, Rustioni A. A cuneocochlear pathway in the rat. Neuroscience. 1987;20(1):209–219. doi: 10.1016/0306-4522(87)90013-3. [DOI] [PubMed] [Google Scholar]
- Winter IM, Wiegrebe L, Patterson RD. The temporal representation of the delay of iterated rippled noise in the ventral cochlear nucleus of the guinea-pig. J Physiol. 2001;537(Pt 2):553–566. doi: 10.1111/j.1469-7793.2001.00553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A, Kunzle H. Cortical and medullary somatosensory projections to the cochlear nuclear complex in the hedgehog tenrec. Neurosci Lett. 1997;221(2–3):125–128. doi: 10.1016/s0304-3940(96)13305-x. [DOI] [PubMed] [Google Scholar]
- Wright DD, Ryugo DK. Mossy fiber projections from the cuneate nucleus to the cochlear nucleus in the rat. J Comp Neurol. 1996;365(1):159–172. doi: 10.1002/(SICI)1096-9861(19960129)365:1<159::AID-CNE12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Young ED, Nelken I, Conley RA. Somatosensory effects on neurons in dorsal cochlear nucleus. J Neurophysiol. 1995;73(2):743–765. doi: 10.1152/jn.1995.73.2.743. [DOI] [PubMed] [Google Scholar]
- Young ED, Rice JJ, Tong SC. Effects of pinna position on head-related transfer functions in the cat. J Acoust Soc Am. 1996;99(5):3064–3076. doi: 10.1121/1.414883. [DOI] [PubMed] [Google Scholar]
- Zeng C, Nannapaneni N, Zhou J, Shore S. Abstracts of Assocation for Research in Otolaryngology. Phoenix, AZ: 2008. Deafferentation changes the distribution of vesicular glutamate transporters associated with auditory nerve or non-auditory inputs to the cochlear nucleus. [Google Scholar]
- Zhan X, Pongstaporn T, Ryugo DK. Projections of the second cervical dorsal root ganglion to the cochlear nucleus in rats. J Comp Neurol. 2006;496(3):335–348. doi: 10.1002/cne.20917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Ryugo DK. Projections of the lateral reticular nucleus to the cochlear nucleus in rats. J Comp Neurol. 2007;504(5):583–598. doi: 10.1002/cne.21463. [DOI] [PubMed] [Google Scholar]
- Zhou J, Nannapaneni N, Shore S. Vessicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol. 2007;500(4):777–787. doi: 10.1002/cne.21208. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Projections from the trigeminal nuclear complex to the cochlear nuclei: A retrograde and anterograde tracing study in the guinea pig. J Neurosci Res. 2004;78(6):901–907. doi: 10.1002/jnr.20343. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S. Convergence of spinal trigeminal and cochlear nucleus projections in the inferior colliculus of the guinea pig. J Comp Neurol. 2006;495(1):100–112. doi: 10.1002/cne.20863. [DOI] [PubMed] [Google Scholar]