Abstract
The biological significance of protective CD8 T-cell-mediated responses against non-traditional alternative reading frame epitopes remains relatively unknown. Cytolytic CD8 T cells (CTL) specific for a non-traditional cryptic MHC class I epitope, SYNTGRFPPL, are critically involved in the protection of mice during infection with the LP-BM5 murine retrovirus. The goal of this study was to determine the functional properties of the protective SYNTGRFPPL-specific CTL during LP-BM5 infection of susceptible BALB/c CD8−/− mice. Direct infection experiments and adoptive transfer of CD8 T cells derived from perforin (pfp)−/−, IFNγ−/−, FasL−/− and, as a positive control, wild-type BALB/c mice, were utilized to assess the effector mechanisms responsible for protection. Our results indicate that SYNTGRFPPL-specific effector CTL preferentially utilize perforin-mediated cytolysis to provide protection against LP-BM5-induced pathogenesis, whereas CTL production of IFNγ is not required. Our results also suggest a minimal contribution of FasL/Fas-mediated lysis during the effector response. Collectively, these results provide insight into effector mechanisms utilized by protective CTL directed against non-traditional cryptic epitopes during disease protection.
Keywords: LP-BM5 murine retrovirus, pathogenesis, protective effector CD8 T-cell response, alternative reading frame, cryptic epitopes, perforin, interferon gamma, FasL, cytotoxicity
Introduction
Clearance and subsequent protection against many viral pathogens are often mediated by the effector functions of activated cytolytic CD8 T cells (CTL) capable of antigen-mediated cytokine secretion and directed cytolysis of virally infected cells. Early studies using perforin deficient mice indicated that upon engagement of the T-cell receptor, activated CTL could trigger apoptosis in target cells through one of two distinct molecular mechanisms (Kagi et al., 1994a; Kagi et al., 1994b; Walsh et al., 1994a; Walsh et al., 1994b): the granule exocytosis pathway involving directed release of cytoplasmic granules containing pore-forming perforin and granzymes into the target cell, or via upregulation of Fas ligand (CD95L), facilitating binding to the Fas receptor (CD95) expressed upon target cells. Initially it was hypothesized that CTL preferentially utilized the perforin/granzyme exocytosis pathway during infection with non-cytolytic viruses, such as during acute LCMV infection in mice. What has become apparent is that CTL have the capability to utilize multiple effector mechanisms to eliminate/control viral infection.
In addition to alternative FasL-mediated cytolysis (Kagi and Hengartner, 1996; Kagi et al., 1995), secretion of inflammatory cytokines such as IFNγ and TNFα by effector CTL have also been shown to contribute to the protective CD8-mediated immune response, through inhibition of viral protein expression, recruitment of antigen presenting cells (APC), and/or upregulation of MHC expression on the surface of infected cells and APC, resulting in the consequent increased activation of effector CD8 T cells (Guidotti and Chisari, 2001; Harty, Tvinnereim, and White, 2000). Indeed, during infection with acutely cytopathic viruses -- such as vaccinia, vesicular stomatitis virus, Semliki Forest virus or influenza-- it has been reported that CTL mediate anti-viral responses through secretion of cytokines, rather than through perforin or FasL/Fas-mediated cytolysis (Kagi and Hengartner, 1996; Kagi et al., 1995).
In the absence of either, but not both, of the perforin- or FasL-mediated cytolytic pathways, effector CTL effectively controlled primary influenza infection (Topham, Tripp, and Doherty, 1997). Studies in other viral systems have also demonstrated similar cytolytic “flexibility”. During the acute phase of Friend virus infection, when virus levels have peaked, perforin and granzymes A and B are utilized by CTL to mediate virus clearance (Zelinskyy et al., 2004), whereas during a low-level Friend infection, FasL/Fas interactions seem to be required for effective anti-viral CTL responses (Zelinskyy et al., 2007). Virus clearance during pulmonary infection with murine gammaherpesvirus is also mediated through CTL utilizing perforin- or FasL-mediated cytolysis (Topham et al., 2001; Usherwood et al., 1997). However, CD8 T-cell clearance of intestinal rotavirus infection in adult mice was shown to be independent of not only perforin or FasL/Fas cytotoxicity, but also secretion of IFNγ (Franco et al., 1997), suggesting that alternative CTL mechanisms are utilized within the mouse intestine during this viral infection. Thus, virus type, anatomical location of infection, and level of viral infection may be factors that influence the effector mechanisms utilized by CD8 T cells during the anti-viral response.
Interaction between the effector CD8 T cell receptor (TCR) and an MHC class-I molecule complexed with an 8–11 amino acid peptide epitope is required to trigger the cytolytic and/or cytokine-mediated effector mechanisms of CD8 T cells during infection. Historically, peptide epitopes were automatically assumed to be derived from the primary open reading frame of the pathogen’s genome, while the rare expression of cryptic epitopes was generally considered to be immunologically insignificant. However, evidence has accumulated demonstrating that CTL are able to recognize and respond to cryptic antigens using a murine model of leukemia (Uenaka et al., 1994) and during infection of mice with influenza (Elliott, Bodmer, and Townsend, 1996). To our knowledge, our lab, utilizing the murine retrovirus LP-BM5, the causative agent of murine AIDS (MAIDS), was the first to define a CD8 CTL-defined major immunodominant cryptic epitope in a retroviral system (Mayrand et al., 2000; Mayrand, Schwarz, and Green, 1998; Schwarz and Green, 1994). This LP-BM5 cryptic epitope, SYNTGRFPPL, was encoded in an alternative reading frame (ARF) of the retroviral gag gene, apparently by a frame-shifting mechanism(s) (Mayrand, Schwarz, and Green, 1998). Despite the increasing evidence that cryptic peptide epitopes contribute significantly to effector CTL responses against tumors and viruses (Bain et al., 2004; Basu et al., 2004; Chen, Bennink, and Yewdell, 2003; Chen et al., 2001; Ho and Green, 2006b; Mayrand et al., 2000; Mayrand, Schwarz, and Green, 1998), there is a relative lack of information available detailing the functional features of CTL specific for these unique antigens.
Although CD8 T-cell effector responses appear to be critical for the control of retroviral infections such as HIV-1, it is well documented that recognition of HIV-1 dominant epitopes by epitope-specific CTL drives the generation of virus escape mutants (Borrow et al., 1997; Geels et al., 2003; Goulder et al., 2001a; Goulder et al., 2001b; Goulder et al., 1997; Price et al., 1997). The ability of retroviruses to escape CD8 T-cell mediated cytotoxicity has made the design of a protective or therapeutic vaccine quite challenging. Cryptic epitopes represent a relatively underappreciated reservoir of immunogenic antigens when considering the design of multi-epitope vaccines that are intended to reinvigorate the protective CD8 CTL response in patients with progressive AIDS (Schirmbeck et al., 2005; Yokomaku et al., 2004). Indeed, the generation of immunologically relevant cryptic epitopes during natural HIV-1 infection in humans has recently been demonstrated. Following in vitro recognition of synthetic peptides derived from epitopes located within ARFs of the gag, pol, and env genes of HIV-1, CD8 T cells isolated from HIV-1 infected individuals were able to produce IFNγ and were highly lytic towards APC pulsed with the cryptic epitopes, indicating natural in vivo priming to these ARF-encoded determinants (Cardinaud et al., 2004). Additionally, there was a high degree of intra- and inter-clade conservation of these ARF epitopes amongst various isolates of the HIV-1 virus, demonstrating that functional preservation of the protein encoded by the primary reading frame may ultimately drive the conservation of ARF epitopes within the genome (Cardinaud et al., 2004).
Similar to CD8 CTL responses in HIV, such CTL-driven immune selection of virus escape mutants has also been observed during infection of primates with SIV (Allen et al., 2000; Barouch et al., 2002; Evans et al., 1999), and following passage of murine retrovirus LP-BM5 in MAIDS-susceptible B6 mice (Gaur and Green, 2003). Detailed sequence comparisons of the LP-BM5 BM5eco cloned retroviral genome to its progenitor murine retrovirus revealed amino-acid substitutions only in the known H-2b restricted epitopes, resulting in diminished CD8 T-cell mediated immunity to LP-BM5 in B6 mice (Gaur and Green, 2003). Further analogous to HIV infection in humans and our system of LP-BM5 infection in mice, significant CD8 effector responses directed against cryptic epitopes in the SIV retroviral infection model have also been reported. Elite (i.e. infrequent) controllers of the SIVmac239 retroviral-isolate were found to have dominant CTL responses against a cryptic epitope located within the +2 ORF of the gag gene (Maness et al., 2007), suggesting that, at least for certain MHC haplotypes, protection against this retrovirus-induced immunodeficiency may require CTL recognition of cryptic ARF antigens.
Although studies with HIV and SIV cryptic antigens have provided evidence that CD8 T cells are able to mount dominant effector responses against cryptic antigens, there has been little direct insight into whether these responses result in protection against virus-induced disease. Previously, we have reported that CD8 CTL directed against the cryptic epitope SYNTGRFPPL are sufficient to induce protection in MAIDS-susceptible BALB/c CD8−/− mice infected with LP-BM5 retrovirus (Ho and Green, 2006b). This study directly demonstrated that CTL recognition of cryptic epitopes in vivo resulted in protection against retrovirus-induced pathogenesis. Due to the similarities between HIV in humans and the disease manifestations of MAIDS (Jolicoeur, 1991; Liang, Wang, and Watson, 1996; Morse et al., 1992), infection of mice with LP-BM5 represents an opportunity for a detailed and controlled study of these unique CD8 effector T-cell responses. Our goal was to further characterize the molecular mechanism(s) involved in the protective CD8 T-cell response against the ARF-epitope SYNTGRFPPL during infection of MAIDS-resistant wild-type (w.t.) BALB/c mice with LP-BM5. This question was approached both by direct infection experiments and by utilizing the previously described adoptive transfer approach that results in essentially full protection against LP-BM5-induced disease when w.t. BALB/c anti-SYNTGRFPPL effector CTL are transferred into MAIDS-susceptible CD8−/− BALB/c mice (Ho and Green, 2006b). In particular, we employed donor BALB/c knockout mice to approach the question of whether the perforin/granzyme and/or FasL cytolytic pathways, and/or the production of IFNγ, was essential for protection.
Materials and methods
Mice
Female BALB/c w.t. mice (6–8 weeks old) were purchased from the National Cancer Institute (NCI, Bethesda, MD). Breeding pairs for knockout mice on the BALB/c background were kindly provided as follows: CD8−/− from P. Stuart (Washington University, St. Louis, MO) and T. Mak (Ontario Cancer Institute, Toronto, Canada); FasL−/− from W. Davidson (American Red Cross, Rockville, MD); IFNγ−/− from J. Gorham (Dartmouth Medical School, Lebanon, NH); and Perforin −/− mice (pfp−/−) from T. Sayers (NCI, Bethesda, MD) and M. Smyth (Peter MacCallum Cancer Institute, Australia). Mice were bred and housed in specific pathogen free conditions. All experimental procedures were approved by and performed under the requirements set forth by the AAALAC accredited Animal Care and Use Program of Dartmouth College.
Cell lines, viruses, and reagents
The P815B mouse mastocytoma cell line (H-2d), provided by J. Bennink (NIH/NIAID, Bethesda, MD), was maintained in RPMI 1640 supplemented with 5% fetal calf serum, 2 mM L-glutamine, 30 µg/ml penicillin, 20 µg/ml streptomycin, and 33 µg/ml gentamicin. The mouse lymphocytic leukemia cell lines L1210Fas− and L1210Fas+ were kindly provided by R. Dutton (Trudeau Institute) and maintained in DMEM supplemented with 5% fetal calf serum, 2 mM L-glutamine, 30 µg/ml penicillin, 20 µg/ml streptomycin, and 200 µg/ml gentamicin. The mouse anti-SYNTGRFPPL CTL line, clone D7, was generated as previously described (Mayrand, Schwarz, and Green, 1998), maintained by restimulation every two weeks with 106/mL irradiated syngeneic splenocytes (3000rads), and 10µg/mL SYNTGRFPPL peptide. The LP-BM5 retroviral isolate, originally provided by J. W. Hartley and H. C. Morse (National Institutes of Health/National Institute of Allergy and Infectious Diseases, Bethesda, MD), was propagated in our laboratory as previously described (Green et al., 2008; Klinken et al., 1988). Vac-DG, the recombinant vaccinia virus with the gag gene of the LP-BM5 retrovirus inserted, was generated as previously described (Schwarz and Green, 1994). The LP-BM5 ORF2 peptide (SYNTGRFPPL) was purchased from Invitrogen Life Technologies at > 95% purity. The control gammaherpesvirus latent peptide M291–99 (GFNKLRSTL) was a generous gift from E. Usherwood, at Dartmouth Medical School. Tetramer, consisting of Kd folded with SYNTGRFPPL peptide and labeled with allophycocyanin, was provided by the NIH Tetramer Core Facility (Atlanta, GA). The control allophycocyanin-labeled tetramer, consisting of Kd folded with GFNKLRSTL, was a generous from E. Usherwood (Dartmouth College).
Purification of splenocyte subpopulations
Splenocyte suspensions derived from FasL−/− or w.t. BALB/c mice, or from BALB/c mice primed with Vac-DG, were labeled with antibody-coupled paramagnetic beads (MACS; Miltenyi Biotec, Auburn, CA) and subjected to column purification according to the manufacturer’s protocol. Purification was verified by flow cytometry: ≥ 95% purity for CD8 enrichment, >99% for CD4 or CD8 depletion, and ≥ 96% purity for CD4 enrichment. For adoptive transfer experiments, recipient CD8−/− mice were infected with LP-BM5 retrovirus 48 hours post-transfer. For in vitro restimulation, 0.1 µg/ml synthetic SYNTGRFPPL peptide was added to splenocytes cultures, as detailed below.
Infections and adoptive transfers
For all LP-BM5 infections, mice were infected intraperitoneally with 5 × 104 plaque forming units (pfu). For polyclonal anti-viral CTL generation, w.t. BALB/c or BALB/c IFNγ−/−, pfp−/−, and FasL−/− mice were immunized i.p. with 3 × 107 pfu of Vac-DG as previously reported (Ho and Green, 2006b). Briefly, antigen-sensitized splenocytes were isolated approximately 3 weeks post-priming: the mice were sacrificed and splenocytes were collected by homogenization through mesh screens followed by lysis of red blood cells. Splenocytes were cultured in media with 0.1 µg/ml of SYNTGRFPPL peptide for 6 days. For short-term maintenance of the cultures, splenocytes were carried for an additional 3 days with 5U/ml of rIL-2 (Cetus Corporation, Everyville, CA). Prior to adoptive transfer into BALB/c CD8−/− recipients, CTL were characterized for antigen specificity by staining with tetramer. SYNTGRFPPL-specific cytotoxicity and IFNγ production were assessed by using the standard 51Cr release assay (Schwarz and Green, 1994), and intracellular cytokine staining, respectively. Three, 6, 9, and 12 days post LP-BM5 infection, 8–15 × 106 polyclonal CTL, or CTL containing approximately 5 × 105 ORF2/SYNTGRFPPL-specific CTL (enumerated by tetramer staining), were transferred intravenously into naïve or LP-BM5 infected CD8−/− recipients. Alternatively, when indicated, approximately 1–3 × 107 purified naïve CD8 T cells were transferred into CD8−/− recipients.
Chromium release cytotoxicity assays
ORF2/SYNTGRFPPL-specific cytolytic activity was determined in a standard 51Cr release assay using P815B or L1210Fas+ or L1210Fas− cells pulsed with 200 µCi of 51Cr, followed by pulsing cells with or without 100 ng/ml SYNTGRFPPL peptide at 37°C for 30 minutes in RPMI supplemented with 10% calf serum and 30µg/ml penicillin, 20 µg/ml streptomycin. Blockade of perforin-mediated cytotoxicity was achieved by incubation of target cells in medium containing 3mM EGTA. In this case, to promote the interaction of effector T cells with L1210Fas+/− target cells mediated by LFA adhesion molecules, 2mM MgCL2 was added to the assay medium during incubation of target cells with effector cells.
Intracellular cytokine stain of IFNγ
ORF2/SYNTGRFPPL-specific CTL were incubated in complete medium plus 1µg/ml SYNTGRFPPL or control GFNKLRSTL peptides, 10 U/ml rIL-2 and 10 µg/ml brefeldin A for 6 hours at 37°C. Cultured cells were subsequently incubated with a monoclonal antibody (mAb) directed against the FcγII/III receptors (2.4G2, BD Pharmingen) for 10 minutes on ice followed by surface staining with PE Cy-5-conjugated-anti-mouse CD44 (IM7, BD Pharmingen) and FITC-conjugated-anti-mouse CD8 (53–6.7, BD Pharmingen) for 20 minutes on ice. Cells were then fixed in a 1 % solution of paraformaldehyde for 20 minutes at room temperature and rendered permeable with buffer containing 0.5 % saponin (Sigma) for 10 minutes at room temperature. After permeabilization, cells were cytoplasmically stained with PE-conjugated-anti-mouse IFNγ (XMG1.2, eBioscience) or isotype control mAb for 30 minutes at room temperature. Stained cells were analyzed on a FACSCalibur flow cytometer using CellQuest software (BD Bioscience).
Tetramer staining
ORF2/SYNTGRFPPL-specific CTL were incubated with anti-FcγII/III on ice for 10 minutes. Cells were then incubated with APC- conjugated Kd/SYNTGRFPPL tetramer or control APC-conjugated Kd/GFNKLRSTL at room temperature for one hour, followed by labeling with FITC-anti-CD8α and PE-anti-CD44 for 20 minutes on ice. Stained cells were then analyzed on a FACSCalibur as previously stated.
LP-BM5 disease measurement
For analysis of LP-BM5-induced lymphoproliferation, spleen weight was determined, and serum, separated from the collected peripheral blood of sacrificed mice, was tested for total levels of IgG2A and IgM in a standard ELISA assay, as previously described in detail (Green et al., 2008; Li and Green, 2006). Immunodeficiency was measured using Con A and LPS mitogen-induced proliferation assays of isolated splenocytes as previously described (Green et al., 2008; Li and Green, 2006). For calculation of viral load, mRNA encoding the BM5 defective and ecotropic gag genes was amplified from purified splenic RNA by real-time RT-PCR as previously described (Cook et al., 2003).
Calculation of disease index
Disease index was determined by calculating the % disease of all experimental mice for each disease parameter (splenomegaly, LPS and ConA mitogen response, and serum levels of IgG2A and IgM). Percent disease was calculated using the formula: ((experimental value – mean value of non-infected controls)/(mean value of LP-BM5-infected controls – mean value of non-infected controls)) × 100. CD8−/− mice were used as infected and non-infected controls. Mice with 0% or calculated “negative” disease for any one parameter were assigned a value of 0, mice with 1–20% disease were assigned a value of 0.5, mice with 21–40% disease were assigned a value of 1, mice with 41–60% disease were assigned a value of 2, mice with 61–80% disease were assigned a value of 3, mice with 81–100% disease were assigned a value of 4, and mice with greater than 100% disease were assigned a value of 5. Values for all parameters were combined for each experimental set of mice and graphed.
Statistics
Prism (GraphPad; San Diego, CA) was used for all statistical tests of significance (P values of ≤ 0.05).
Results
Direct infection of mice deficient in perforin results in enhanced susceptibility to LP-BM5 retrovirus
Previously we have shown that resistance to LP-BM5-induced pathogenesis in non-susceptible strains of mice, such as the BALB/c mouse, is mediated by a protective CD8 T-cell response against a cryptic epitope located within ORF2 of the LP-BM5 gag gene, termed SYNTGRFPPL (Ho and Green, 2006b; Mayrand et al., 2000; Mayrand, Schwarz, and Green, 1998; Schwarz and Green, 1994). Conventional effector mechanisms such as perforin-mediated cytotoxicity and secretion of IFNγ are commonly associated with enhancing the protective response during infection of mice with Friend retrovirus (Stromnes et al., 2002; Zelinskyy et al., 2004) and infection of humans with HIV (Benito, Lopez, and Soriano, 2004). Therefore, we initially used perforin (pfp−/−), or IFNγ (IFNγ−/−), deficient mice to determine which effector mechanisms were involved during the CD8-mediated response against the cryptic epitope SYNTGRFPPL during infection with LP-BM5. Activational and immunodeficiency parameters associated with infection were assessed in pfp−/− and IFNγ−/− mice and compared to disease in susceptible BALB/c CD8−/−mice and resistant BALB/c w.t. mice.
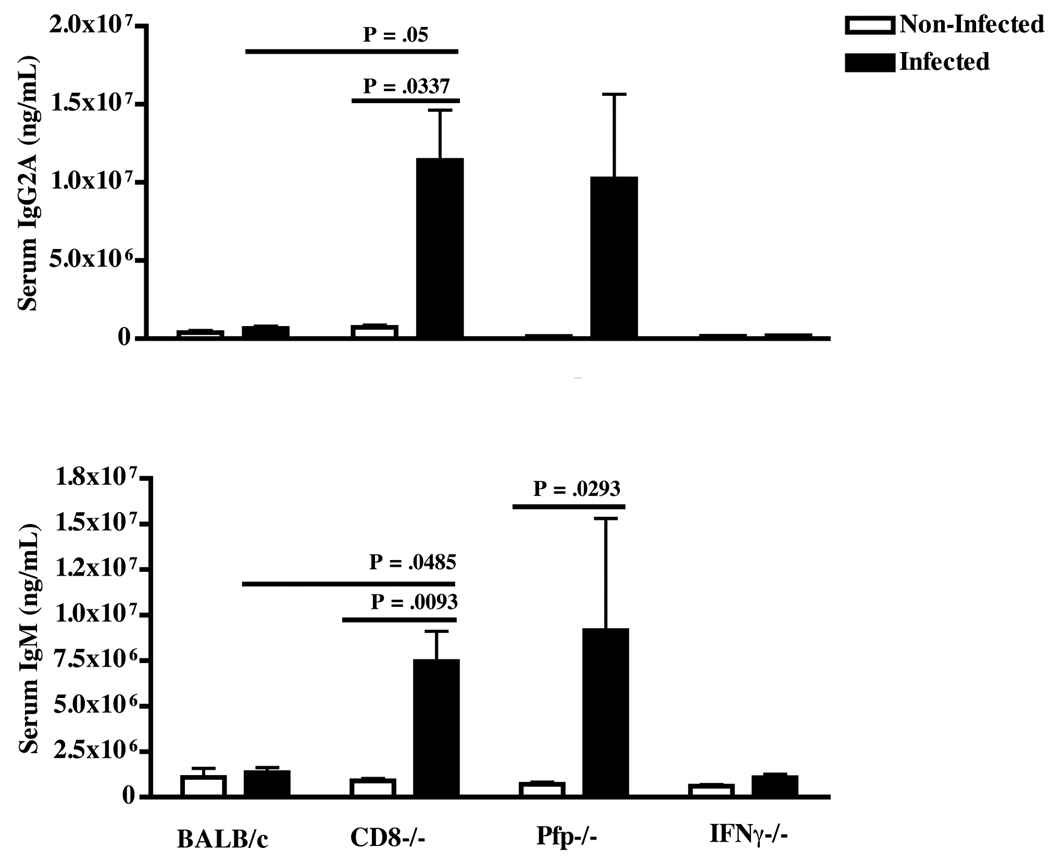
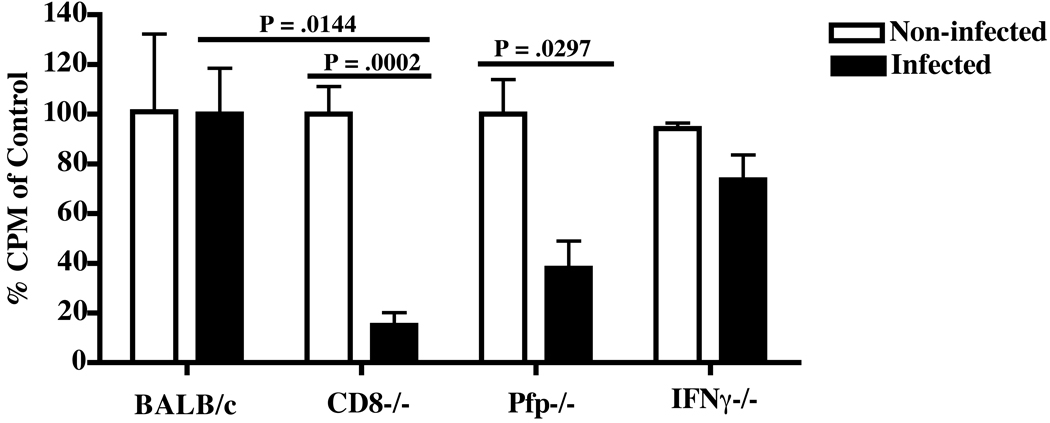
As expected, by measurement of activational parameters such as splenomegaly (P = .0049) and hypergammglobulinemia, with respect to both IgG2A and IgM (P = .0337 and P = .0093 respectively); in addition to measurement of immunodeficiency parameters, such as impaired lymphocyte responsiveness to ConA (P = .0002), CD8−/− mice infected with LP-BM5 had significantly more disease than non-infected controls (Figs. 1A–1C). Compared to resistant w.t. BALB/c mice infected with LP-BM5, infection in CD8−/− mice resulted in significantly more splenomegaly (P = .006), serum levels of IgG2A (P = .05) and IgM (P = .0485), and a significantly reduced T cell response to ConA (P = .0144) and reduced B-cell responses to LPS (data not shown). As expected, and in direct correlation to LP-BM5 disease parameters, LP-BM5-infected CD8−/− mice had significantly greater load of viral RNA as determined by RT-qPCR analysis for both the LP-BM5 defective and ecotropic helper viruses, as detected in the spleen, compared to non-infected controls and infected w.t. BALB/c mice (Fig. 1E).
Figure 1. Direct infection of BALB/c mice deficient in perforin results in increased incidence of disease following infection with LP-BM5.
BALB/c, CD8−/−, pfp−/−, and IFNγ−/− mice were directly infected with LP-BM5 for 9 weeks, after which disease was assessed. A: Spleen weights of individual mice with or without infection by LP-BM5. Bars represent the mean weight for each group of animals. B: Serum levels of IgG2A and IgM immunoglobulin measured by standard ELISA. C: T-cell responsiveness to ConA stimulation. The data are represented as the percentage counts per minute relative to the appropriate non-infected control. Students T tests were used to measure disease significance of infected mice in comparison to non-infected controls, or to infected BALB/c mice. D: Disease index measurement considering all parameters of LP-BM5-induced disease. Statistics were calculated using the non-parametric Mann-Whitney test of significance. E: Viral RNA expression of BM5def and BM5eco from spleen-derived RNA samples isolated from LP-BM5-infected mice, quantified using real-time RT-PCR. Values of BM5def and BM5eco are shown as expression levels relative to values obtained for β-actin controls. The data are representative of at least 2 experiments with N = 4–9 mice/group. * indicates P ≤ .05, ** indicates P ≤ .01.
Deficiencies in IFNγ did not result in a conversion to significant disease, as BALB/c IFNγ−/− mice infected with LP-BM5 had similar spleen weights, serum levels of both IgG2A and IgM, mitogen responsiveness to stimulation with ConA and LPS, and negligible amounts of viral RNA present, as the non-infected control mice (Fig. 1A–1E and data not shown). These data suggested that IFNγ was not an important effector mechanism during the protective anti- ORF2/SYNTGRFPPL response during MAIDS pathogenesis. However, it has been reported by others that induction of LP-BM5 pathogenesis is correlated to high levels of IFNγ: specifically that B6 mice lacking IFNγ may have reduced MAIDS disease or require a prolonged time course to develop disease equivalent to w.t. susceptible B6 mice (Giese et al., 1996; Morawetz et al., 1998). To verify that the lack of disease observed in BALB/c IFNγ−/− mice was not instead due to an IFNγ-dependent pathogenic mechanism, we infected susceptible B6 mice lacking IFNγ with equivalent amounts of LP-BM5 and assessed disease severity, compared to w.t. B6 controls. With this experimental system, we found that IFNγ−/− B6 mice had similar splenomegaly and immunosuppression as compared to infected w.t. B6 controls (Supplementary Figs. 1A and 1B). Thus, the absence of disease susceptibility in BALB/c IFNγ−/− mice infected with LP-BM5 suggested that IFNγ may not be required during the protective response against LP-BM5-induced pathogenesis.
In contrast, LP-BM5 infection of mice lacking perforin resulted in a significant increase in spleen weight compared to either non-infected pfp−/− controls (P = .033) or to the w.t. BALB/c mice infected with LP-BM5 (P = .0452) (Fig. 1A). There was a trend towards an increased, although not significantly greater, amount of serum IgG2A in pfp−/− mice infected with LP-BM5 compared to non-infected pfp−/− mice (Fig. 1B). However, it was clear that the infected pfp−/− mice converted to disease susceptibility based on the hyper Ig criterion, as serum levels of IgM were significantly greater in pfp−/− infected mice compared to the pfp−/− uninfected controls (P = .0293). Confirming a conversion to disease susceptibility in BALB/c pfp−/− mice, T cell responsiveness to ConA was significantly reduced (P = .0297) upon infection (Fig. 1C), and correspondingly, viral load was significantly increased compared to non-infected controls and both infected IFNγ−/− and w.t. BALB/c mice (Fig. 1E). Overall, summation of all disease parameters into a measurement of disease index clearly indicated that a deficiency in perforin resulted in significantly greater MAIDS disease compared to the disease index of both pfp−/− non-infected controls (P = .0007), and, the disease index of infected w.t. BALB/c mice (P = .0235) (Fig. 1D). Collectively, these results provide evidence that the ability to express perforin is an important factor in the resistance of w.t. BALB/c mice to LP-BM5-induced pathogenesis and specifically to the spread of retroviral infection.
FasL/Fas-mediated CTL mechanisms do not significantly contribute to the CD8 T-cell response during LP-BM5 infection
Based upon the phenotype of the FasLgld mutation, it is well known that homozygous mutant mice develop enlarged spleens, lymphadenopathy, and systemic autoimmunity, with significant onset of symptoms by 20 weeks of age (Roths, Murphy, and Eicher, 1984). In agreement with the literature, and, despite evidence of a trend towards elevated spleen weights and levels of serum IgG2A and IgM and increased immunosuppression during infection of FasL−/− mice, non-infected FasL−/− mice also had a significantly greater disease index than uninfected CD8−/− mice (Supplementary Fig. 2). Based upon these data, it was apparent that the direct infection approach could not be used to decipher whether the FasL/Fas cytolytic pathway is involved in reducing LP-BM5-mediated pathogenesis.
Alternatively, in order to approach the possibility that the FasL/Fas pathway may be utilized as a cytolytic effector mechanism by protective CTL directed against the ORF2/SYNTGRFPPL epitope, we compared in vitro lysis of the Fas expressing, L1210Fas+, or Fas negative, L1210Fas−, lymphocytic leukemia lines as target cells by effector CTL, derived from Vac-DG primed w.t. BALB/c, pfp−/− or FasL−/− mice after secondary in vitro restimulation with SYNTGRFPPL. Roughly equivalent lysis of Fas-expressing L1210Fas+, versus Fas-deficient L1210Fas−, target cells pulsed with SYNTGRFPPL peptide was observed using ORF2/SYNTGRFPPL-specific effector CTL generated from w.t. BALB/c mice (Fig. 2A), suggesting that Fas expression on the targets was not required for SYNTGRFPPL-mediated cytolysis in the presence of perforin. Additionally, substantial lysis of SYNTGRFPPL-pulsed L1210Fas+ and L1210Fas− target cells was observed using effector CTL derived from FasL−/− mice (Fig. 2A). In agreement with this data, viral load in FasL−/− mice infected with LP-BM5 was significantly reduced compared to that detected in susceptible CD8−/− mice (Supplementary Fig. 2). In sharp contrast, the absence of perforin expression by the effector cells resulted in ablation of target-cell lysis, as essentially no lysis was observed with either the Fas1210+ or Fas1210−targets pulsed with peptide (Fig. 2A). There was, at best, a slight increase in the lysis of Fas1210+ target cells by pfp−/− effector cells, however this lysis did not appear to be peptide specific, as there was no difference in lysis of Fas1210+ targets pulsed with SYNTGRFPPL peptide, compared to the mock pulsed control targets.
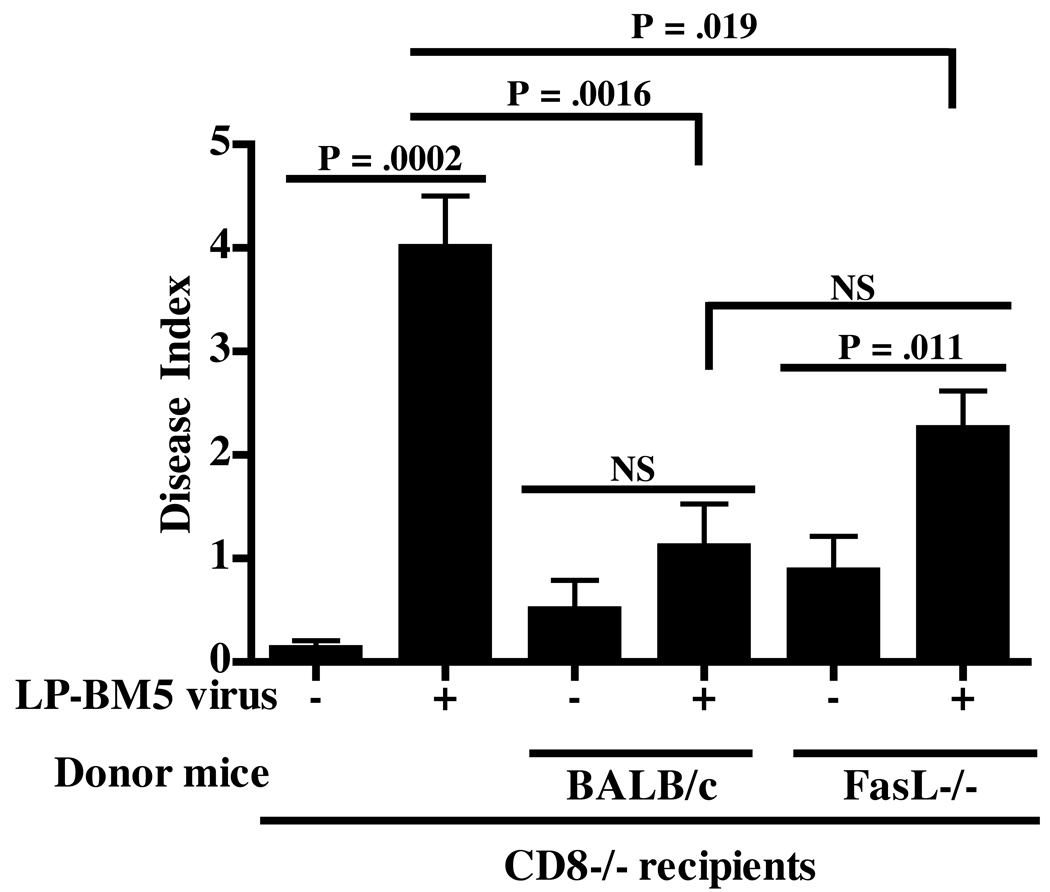
Figure 2. FasL/Fas-mediated cytolysis does not substantially contribute to the effector activity of ORF2/SYNTGRFPPL-specific CD8 T cells.
A: Lysis of L1210Fas+/− target cells, alone or pulsed with SYNTGRFPPL peptide, was measured by titration with ORF2/SYNTGRFPPL-specific splenocytes, obtained from BALB/c, FasL−/−, or pfp−/− mice primed with DG-Vac/SYNTGRFPPL followed by secondary re-stimulation of splenocytes with synthetic peptide. B: ORF2/SYNTGRFPPL-specific cytotoxicity by the highly lytic ORF2/SYNTGRFPPL-specific CTL clone, D7, and by CTL derived from DG-Vac/SYNGRFPPL stimulated w.t. BALB/c mice. CTL activity was measured against P815B targets incubated with or without the addition of the relatively calcium-specific chelating agent EGTA and MgCL2. The data are representative of at least 2 independent experiments. C: Disease index of CD8−/− recipients receiving adoptive transfer of 1–3×107 purified naïve CD8 T cells isolated from FasL−/− or w.t. BALB/c mice and infected with LP-BM5. Disease was assessed at 11 weeks post-infection. NS indicates non-significant differences between experimental groups. Students T tests were used to measure disease significance of infected mice in comparison to non-infected controls, or to infected BALB/c mice.
This apparent perfoin-mediated cytolysis was also examined under conditions in which the perforin/granule exocytosis pathway was inhibited by blockade with calcium-specific chelating agent EGTA. Lysis of SYNTGRFRPPL-pulsed P815B target cells by ORF2/SYNTGRFPPL-specific w.t. polyclonal CTL, and the cell line D7, the highly lytic SYNTGRFPPL-specific CD8 CTL clone derived from polyclonal BALB/c CD8 T cell effectors of the same specificity (Mayrand, Schwarz, and Green, 1998), was nearly abolished when EGTA was added to the assay mixture (Fig. 2B), further supporting the preferential use of perforin by protective CTL directed against the cryptic epitope SYNTGRFPPL.
Given the report that there are limitations in utilizing the 51Cr cytotoxicity assay to examine the FasL/Fas-mediated cytotlytic pathways (Liu et al., 2002) and in order to verify the in vitro data, we chose to examine CD8 effector mechanisms with FasL-deficient CTL generated in vivo. In order to diminish the pre-existing systemic lymphoproliferative and immunosuppressive effects of FasL deficiency observed using the direct in vivo infection model, the genetic effect of the FasL mutation was isolated solely to the CD8 compartment. MAIDS-susceptible CD8−/− mice were reconstituted with purified naïve CD8 T cells derived from FasL−/− or w.t. BALB/c mice. Similar to what was observed following direct infection of BALB/c mice, LP-BM5 infected CD8−/− mice receiving naïve w.t. BALB/c CD8 T cells had a significantly lower disease index than LP-BM5-infected CD8−/− recipients that did not receive transfer of naïve CD8 T cells (P = .0016), indicating that transfer of w.t. BALB/c CD8 T cells resulted in sufficient protection from MAIDS pathogenesis. Infected CD8−/− mice receiving naïve FasL−/− CD8 T cells had a significantly greater disease index, compared to non-infected controls receiving FasL−/− CD8 T cells (P = .011). However, in comparison to the disease index of non-reconstituted CD8−/− mice infected with LP-BM5, FasL−/− CD8 T cells were sufficiently and significantly able to protect infected CD8−/− recipients from LP-BM5-induced pathogenesis (P = .019). Additionally, transfer of FasL−/− CD8 T cells did not result in a significant increase in disease index or viral load (data not shown) compared to CD8−/− mice receiving w.t. BALB/c CD8 T cells (Fig. 2C). These data, taken together, indicate that cytolysis through the FasL/Fas pathway has, at best, a minor contribution to the CTL response during LP-BM5 infection in BALB/c mice, and that an absence of FasL in the CD8 cellular compartment leads to perhaps partial disease in comparison to susceptible CD8−/− mice, but not relative to CD8−/− mice reconstituted with w.t. CD8 T cells.
Isolation of effector deficiencies results in the inability of perforin-, but not IFNγ-, deficient effector CD8 T cells to effectively control LP-BM5-induced pathogenesis
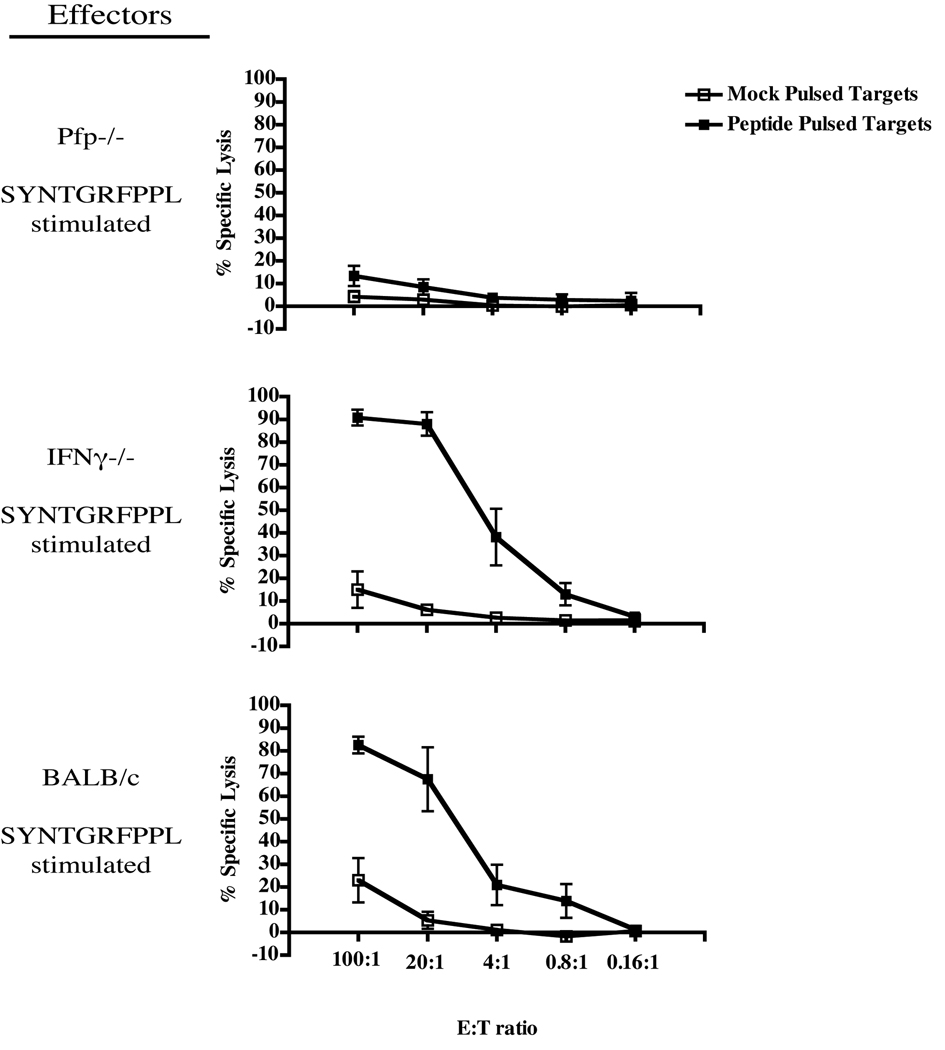
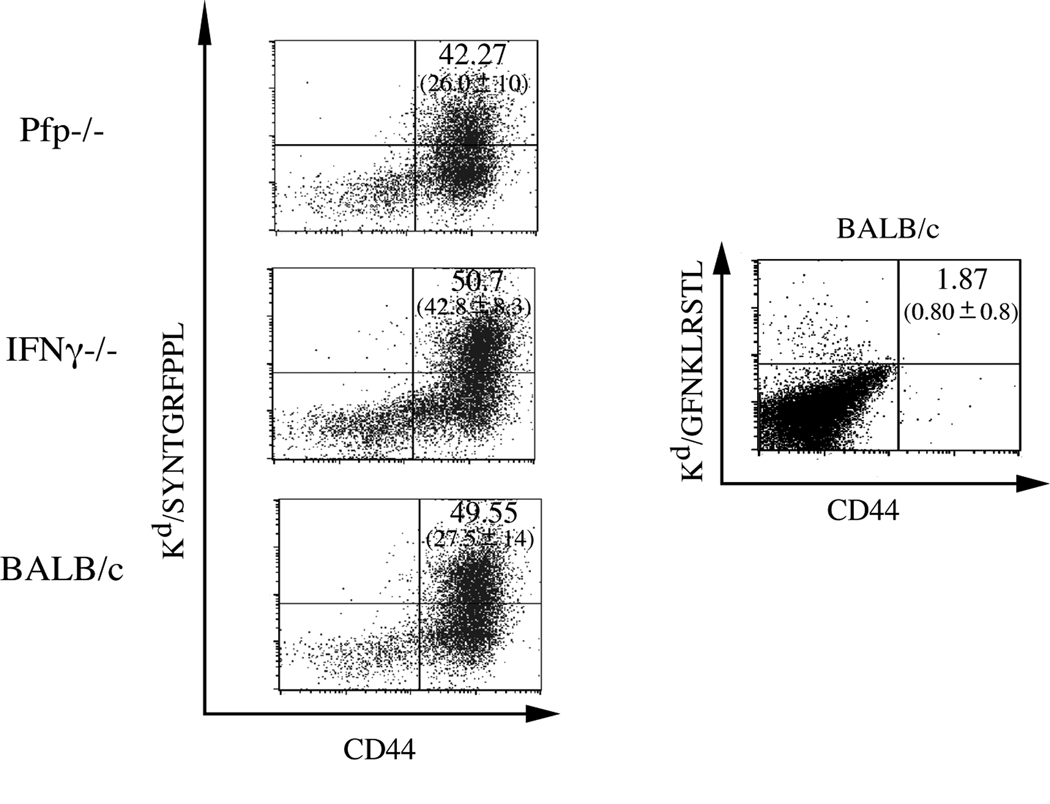
Since direct-infection experiments demonstrated that perforin-deficient mice are more susceptible to LP-BM5-induced pathogenesis than w.t. BALB/c mice, we wanted to determine whether in vivo disease susceptibility was specifically due to a defect in certain effector-cell functional capabilities by the CD8 T cells specific for ORF2/SYNTGRFPPL. To this end, ORF2/SYNTGRFPPL-restimulated polyclonal effector cells derived from IFNγ−/− or pfp−/−mice previously immunized with Vac-DG were adoptively transferred into LP-BM5-infected CD8−/− recipients. Prior to transfer, to confirm the functional effector phenotype of transferred CTL, cytolytic capabilities, IFNγ cytokine production, and antigen specificity were assessed. Cytolysis of SYNTGRFPPL-pulsed P815B target cells was demonstrated at high levels by w.t. BALB/c and IFNγ−/−effectors; however, as expected from the data of Figure 2, was not detected at significant levels with pfp−/− effectors (Fig. 3A). Similarly, peptide-specific IFNγ production, while not observed by effectors derived from IFNγ−/− mice, was robust for pfp−/− and w.t. polyclonal effectors (Fig. 3B). Tetramer staining of the polyclonal effector cells derived from w.t, pfp−/−, and IFNγ−/− BALB/c mice indicated that 40–50% of total CD8 T cells were similarly specific for SYNTGRFPPL, irrespective of deficiencies in perforin or IFNγ (Fig. 3C).
Figure 3. Phenotypic characterization of ORF2/SYNTGRFPPL effector CTL prior to transfer into CD8−/− recipients.
A: Lytic activity of BALB/c, pfp−/−, or IFNγ−/−ORF2/SYNTGRFPPL CTL titrated against P815B targets pulsed with or without SYNTGRFPPL peptide. B: Antigen-specific IFNγ expression of ORF2/SYNTGRFPPL CTL. Numbers indicate the total percentage of lymphocytes that are positive for both CD8 and expression of IFNγ. The numbers in parenthesis represent the mean, plus or minus the standard deviation, of the total percentages of CD8 T cells expressing IFNγ from 2 separate experiments. C: Tetramer staining of ORF2/SYNTGRFPPL-specific CTL. The numbers represent the total percentage Kd/SYNTGRFPPL tetramer-specific CD8 T lymphocytes. Numbers in parenthesis represent the mean, plus or minus the standard deviation, of the total percentage of CD44 high CD8 T cells stained with Kd/SYNTGRFPPL tetramer from 8 individual experiments. To control for non-specific binding of the H-2Kd tetramer, BALB/c CTL were also stained with the non-specific M2 tetramer, Kd/GFNKLRSTL.
As previously described (Ho and Green, 2006b), w.t BALB/c CD8 effector cells of SYNTGRFPPL specificity provided essentially complete protection from LP-BM5-induced MAIDS (Figs. 4A and B). Thus, both activational and immunodeficiency readouts, measured by spleen weight and mitogen-induced proliferation, respectively, indicated minimal disease in CD8−/− mice reconstituted with BALB/c ORF2/SYNTGRFPPL-specific polyclonal CTL. Similar protection from disease was observed following infection of CD8−/− mice subsequently reconstituted in parallel with IFNγ−/− SYNTGRFPPL-specific CTL. However, and strikingly, reconstitution of CD8−/−mice with pfp−/− effector CTL resulted in significantly greater LP-BM5-induced disease with respect to all MAIDS parameters: splenomegaly (P < .01) and immunosuppression (P < .01), measured by lymphocyte responsiveness to both ConA and LPS, compared to non-infected CD8−/− mice (Figs. 4A and B). In support of previously published results (Ho and Green, 2006b) demonstrating that CD8 effector CTL per-se were responsible for mediating disease resistance, transfer of purified CD4 T cells by positive selection just prior to transfer (pCD4+) resulted in a similar level of disease, compared to CD8−/− mice not receiving a transfer. Conversely, purification of CD8 T cells from bulk CTL cultures via either positive selection (pCD8+) or CD4 depletion (CD4p−), also just prior to transfer, resulted in significant disease protection, similar to the protection observed for the transfer of bulk CTL preparations. Interestingly, the splenomegaly of infected CD8−/−mice receiving pfp−/− donor cells was less severe compared to control LP-BM5-infected, but non-reconstituted, CD8−/− mice (P = 0.02). These data suggested the possibility of one or more residual pfp-independent mechanisms of protection, and/or a differential ability to resolve the level of disease by this, compared to the other, disease parameters. Collectively, these findings suggest that perforin function, specifically by ORF2/SYNTGRFPPL-specific CD8 T cells, is critical for full MAIDS resistance.
Figure 4. ORF2/SYNTGRFPPL-specific effector CTL lacking perforin are unable to confer full protection against LP-BM5-induced MAIDS in CD8−/− recipients.
Mice shown were infected with LP-BM5 as previously described. Mice received 4 transfers at 3-day intervals consisting of approximately 0.6–1 × 107 total cells per transfer. Effector cells from Vac-DG primed w.t. BALB/c mice, with or without prior positive selection for CD8 or CD4 T cells (pCD8+ or pCD4+) or negative selection for CD8 T cells (pCD4) following SYNTGRFPPL restimulation, were also transferred as controls for ORF2/SYNTGRFPPL-specific CD8-dependent protection. Disease was measured 9 weeks post-infection. A: Spleen weight. B: T cell responsiveness to ConA and B cell responsiveness to LPS stimulation. Students T tests were used to measure disease significance of infected CD8−/− recipients in comparison to the non-infected CD8−/− or infected CD8−/− controls.
Discussion
The essential role of CD8 T cells in protection against MAIDS, based upon CD8 T-cell recognition of a non-traditional cryptic epitope, has been previously demonstrated, as gag-specific CD8 CTL directed against an epitope located within ORF2 of the gag gene are critical for mediating resistance to LP-BM5-induced pathogenesis (Mayrand et al., 2000; Schwarz and Green, 1994). Thus, we demonstrated that reconstitution of disease susceptible BALB/c CD8−/− mice with CD8 CTL directed against this cryptic epitope restored resistance to virus-induced pathogenesis, including viral immunosuppression (Ho and Green, 2006b). This essentially complete level of protection was remarkable, considering that the very nature of the generation of cryptic ARF-encoded epitopes may suggest a diminished density of cell surface viral peptide-MHC class I complex presented, in this case, on LP-BM5-infected target cells. Consequently, either a decreased frequency of CD8 CTL capable of recognizing these presumably infrequent antigens, and/or the possibility that CTL generated against these epitopes may have qualitatively and/or quantitatively impaired effector functions, may well limit the effectiveness of CD8-driven anti-ARF epitope immune responses. However, it has been extrapolated that target cells with an average of three epitope-MHC-I complexes expressed upon the surface are sufficient to induce a half-maximal cytolytic response by effector CD8 T cells (Sykulev et al., 1996), presumably due to the selective expansion of high avidity TCR- bearing CD8 T cells (Zook et al., 2006). It is tempting to speculate that the dominant CD8 T cell response against the Kd-restricted gag ARF cryptic epitope SYNTGRFPPL arose because of an absence of functional CTL epitopes encoded in the primary retroviral reading frames, due to immune selection against these typical epitopes. Indeed, in the B6 strain, passage of LP-BM5 in vivo has resulted in epitope-crippling mutations in three of four known ORF1 epitopes (Gaur and Green, 2003). Evolution of CD8 T cell responses to recognize cryptic ARF epitopes is not uncommon, as a similar effect was observed upon deletion of the major ORF1 CTL determinant in herpes simplex virus, resulting in an overall increase of previously undetectable CD8 T cells specific for cryptic determinants (Wallace et al., 1999).
In this report, we studied BALB/c mice deficient in effector mechanisms associated with CD8 T-cell function, namely the apoptosis-inducing FasL/Fas and perforin/granzyme cytolytic pathways, and the secretion of the antiviral cytokine IFNγ. The results herein indicate a conventional, albeit selective, engagement of CD8 T-cell protective mechanism(s) in response to a cryptic ARF immunodominant epitope. Direct infection of mice deficient in perforin, FasL, or IFNγ, in addition to experiments involving isolation of effector deficiencies to the CD8 compartment, indicated that perforin-mediated cytolysis is the dominant effector mechanism during the CD8-directed protective response against the ARF epitope SYNTGRFPPL. To a lesser degree, our in vivo data suggested that FasL/Fas signaling may contribute slightly to the effector function of protective CD8 T cells during the anti-LP-BM5 response.
Although direct infection of FasL−/− mice with LP-BM5 suggested a trend towards increased disease pathology, the significant disease susceptibility of pfp−/− mice, both by incidence and severity, indicated that a systemic deficiency in perforin results in an enhanced susceptibility to LP-BM5-induced pathogenesis (Figs. 1A–D). Susceptibility in pfp−/− mice was somewhat variable, as a small proportion of directly infected pfp−/− mice did not appear to display significant MAIDS pathogenesis (Fig. 1A). Additionally, CD8−/−mice receiving adoptive transfer of ORF2/SYNTGRFPPL-specific pfp−/− CD8 CTL had significantly less lymphoproliferation, as measured by spleen weight, compared to the highly susceptible CD8−/− mice infected with LP-BM5 (Fig. 4A). However, in support of our overall conclusion that perforin-mediated cytolysis is the dominant effector mechanism of CTL directed against the ARF epitope SYNTGRFPPL, this reduced level of protection was not observed for the other disease parameters, whether tested by the adoptive transfer approach (Figure 4) or the direct infection approach (Fig. 1). Preferential use of perforin by CTL resulting in incomplete protection from disease, as evidenced by some parameters of pathogenesis, could be due to two possible, non-exclusive mechanisms: 1) disease kinetics of splenomegaly in pfp−/− deficient mice are reduced in comparison to the highly susceptible CD8−/− deficient mice, and/or 2) SYNTGRFPPL-specific effector CTL are polyfunctional, requiring multiple effector mechanisms to impart full protection from disease in infected mice.
Regarding reduced disease kinetics in pfp−/− mice, a previous study examining mechanisms in MAIDS resistant mice suggested that pfp deficiency in B10.D2 (H-2d) mice resulted in susceptibility to LP-BM5-induced pathogenesis: e.g. about 12% developed robust disease by 16 weeks of infection (Tang et al., 1997). Therefore, the partial susceptibility of pfp−/− mice could putatively depend upon the stage or level of LP-BM5 infection. Similar observations have been made during natural HIV infection in humans (Rehr et al., 2008) or during Friend retroviral infection of mice (Zelinskyy et al., 2004; Zelinskyy et al., 2007). Polyfunctionality of SYNTGRFPPL-specific effector CTL is an additional explanation for this varying level of disease susceptibility in pfp−/− mice. HIV-1 patients receiving antiretroviral therapy had an increase in polyfunctional CD8 T cells that were able to degranulate and secrete IFNγ, TNFα, and IL-2 in response to infection, resulting in further reduced viral loads. In contrast, in patients with high viral loads, or prior to the establishment of antiretroviral therapy, CD8 T cells were dysfunctional with respect to secretion of effector cytokines (Rehr et al., 2008). During infection of mice with Friend murine retrovirus, depending upon the level of virus replication, perforin/granzymes A and B, or FasL/Fas-mediated cytolysis of target cells was preferentially utilized by CTL to mediate virus clearance (Zelinskyy et al., 2004; Zelinskyy et al., 2007). Polyfunctional effector CD8 T cells have been documented in several other viral systems. Using a murine model of influenza infection, perforin deficient CD8 T cells were only able to mediate virus clearance in the presence of Fas, demonstrating that both lytic pathways were utilized by CTL during virus clearance (Topham, Tripp, and Doherty, 1997). Similar findings were described with murine gammaherpesvirus (Topham et al., 2001). Alternatively, it is possible that perforin-independent killing of target cells by granzymes A and B might contribute to the variable susceptibility of perforin-deficient mice. Recent studies support this notion, that, in the absence of perforin, granzymes A and B are capable of mediating cytolysis (Gondek et al., 2005; Kurschus et al., 2004; Zelinskyy et al., 2004).
In regards to the role of IFNγ as an important effector mechanism, we have conclusively demonstrated that IFNγ−/− CTL are effectively able to lyse SYNTGRFPPL-pulsed target cells and that BALB/c IFNγ−/− mice are not susceptible to LP-BM5-mediated pathogenesis. Additionally, we demonstrated by adoptive transfer and thereby localizing the IFNγ deficiency to the CD8 effector cells, that BALB/c IFNγ−/− CTL were able to mediate full protection of CD8−/− recipients infected with LP-BM5. However, as a possible confounding effect, other reports have proposed that IFNγ may be an important mediator of disease in susceptible B6 mice, resulting in diminished pathogenesis in IFNγ−/− mice. Treatment of B6 mice with neutralizing mAb to IFNγ before and during the period of infection (Uehara et al., 1994), or utilizing B6 IFNγ−/− mice (Giese et al., 1996), was reported to significantly delay the progression of some, but not all, parameters of MAIDS. This notion could be extrapolated, albeit with difficulty, to the context herein of CD8−/− mice receiving IFNγ−/− effector CTL. However, during similar experimental conditions as our direct infection of BALB/c IFNγ−/− mice, we found that infection of B6 IFNγ−/− mice clearly resulted in significant splenomegaly and immunosuppression, comparable to what was observed in w.t. B6 mice (Supplementary Fig. 1). These data demonstrate, that in our infection model of BALB/c-background knock-out mouse strains, the lack of disease observed in BALB/c IFNγ−/− mice was not due to an effect that IFNγ had upon LP-BM5 pathogenesis per se, but rather was explained by the observation that adoptively transferred BALB/c IFNγ−/− CD8 T cells could impart disease protection similar to that observed using w.t. BALB/c CTL.
This study provides an important insight into the effector mechanisms utilized by protective CTL directed against a cryptic epitope and further underscores the physiological significance of ARF epitopes in disease protection. Collectively, the findings presented herein strongly implicate perforin function, and suggest, to a much lesser degree, FasL function, by SYNTGRFPPL-induced CD8 T cells, are required for full MAIDS resistance. In terms of vaccination strategies utilizing vectors that can translate from cryptic reading frames, knowing the preferential effector mechanisms utilized by CTL to control viral replication is crucial, as inserting additional immunogenic components that result in enhanced and polyfunctional effector activity should result in a multipronged and highly effective attack on virally infected target cells. Due to the genetic constraints associated with encoding multiple proteins critical for viral pathogenesis and replication within overlapping reading frames of a viral genome that is limited in size, retroviruses and many other viruses may have a particularly low tolerance for amino acid sequence variations within embedded cryptic ORF2 (or conventional ORF1) epitopes in these regions of overlap (Ho and Green, 2006a). In support of this notion, conservation of cryptic epitopes within the genome of HIV-1 has been demonstrated amongst multiple isolates of circulating virus (Cardinaud et al., 2004). Thus, cryptic epitopes present an attractive source of additional immunogenic determinants that may be utilized to elicit protective CD8 T-cell responses (Ho and Green, 2006b) during infection with viruses prone to antigenic variation due to immune selection pressures.
Supplementary Material
W.t. or IFNγ−/− B6 mice were infected with LP-BM5 for 11 weeks after which disease was assessed. A: Spleen weight. B: B-cell responsiveness to LPA and T-cell responsiveness to ConA, with the data represented as the percentage counts per minute of non-infected controls. Students T tests were used to measure significance.
FasL−/−, CD8−/−, and w.t. BALB/c mice were infected with LP-BM5 for 11 weeks. A: Spleen weight. B: Serum levels of IgM and IgG2A. C: T-cell responsiveness to ConA stimulation, with the data represented as the percentage counts per minute of non-infected controls. Students T tests were used to measure disease significance. D: Disease index of mice with or without prior infection with LP-BM5. Statistics were calculated using the non-parametric Mann-Whitney test of significance. E: Viral RNA expression of BM5def and BM5eco from spleen-derived RNA samples isolated from LP-BM5-infected mice, quantified using real-time RT-PCR. NS indicates non-significant differences between experimental groups. Students T tests were used to measure disease significance. The data are representative of 3 experiments with 4–6 mice/group.
Acknowledgments
The authors would like to thank Wen Li for helpful discussions and Kathy Green for discussions and preparation of all LP-BM5 virus stocks. This work was supported by NIH grants CA50257 and AI059580. M Rutkowski was supported by training grants T32AI07363 and T32AR007576.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allen TM, O’Connor DH, Jing P, Dzuris JL, Mothe BR, Vogel TU, Dunphy E, Liebl ME, Emerson C, Wilson N, Kunstman KJ, Wang X, Allison DB, Hughes AL, Desrosiers RC, Altman JD, Wolinsky SM, Sette A, Watkins DI. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407(6802):386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- Bain C, Parroche P, Lavergne JP, Duverger B, Vieux C, Dubois V, Komurian-Pradel F, Trepo C, Gebuhrer L, Paranhos-Baccala G, Penin F, Inchauspe G. Memory T-cell-mediated immune responses specific to an alternative core protein in hepatitis C virus infection. J Virol. 2004;78(19):10460–10469. doi: 10.1128/JVI.78.19.10460-10469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch DH, Kunstman J, Kuroda MJ, Schmitz JE, Santra S, Peyerl FW, Krivulka GR, Beaudry K, Lifton MA, Gorgone DA, Montefiori DC, Lewis MG, Wolinsky SM, Letvin NL. Eventual AIDS vaccine failure in a rhesus monkey by viral escape from cytotoxic T lymphocytes. Nature. 2002;415(6869):335–339. doi: 10.1038/415335a. [DOI] [PubMed] [Google Scholar]
- Basu A, Steele R, Ray R, Ray RB. Functional properties of a 16 kDa protein translated from an alternative open reading frame of the core-encoding genomic region of hepatitis C virus. J Gen Virol. 2004;85(Pt 8):2299–2306. doi: 10.1099/vir.0.80028-0. [DOI] [PubMed] [Google Scholar]
- Benito JM, Lopez M, Soriano V. The role of CD8+ T-cell response in HIV infection. AIDS Rev. 2004;6(2):79–88. [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3(2):205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Cardinaud S, Moris A, Fevrier M, Rohrlich PS, Weiss L, Langlade-Demoyen P, Lemonnier FA, Schwartz O, Habel A. Identification of cryptic MHC I-restricted epitopes encoded by HIV-1 alternative reading frames. J Exp Med. 2004;199(8):1053–1063. doi: 10.1084/jem.20031869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Bennink JR, Yewdell JW. Systematic search fails to detect immunogenic MHC class-I-restricted determinants encoded by influenza A virus noncoding sequences. Virology. 2003;305(1):50–54. doi: 10.1006/viro.2002.1744. [DOI] [PubMed] [Google Scholar]
- Chen W, Calvo PA, Malide D, Gibbs J, Schubert U, Bacik I, Basta S, O’Neill R, Schickli J, Palese P, Henklein P, Bennink JR, Yewdell JW. A novel influenza A virus mitochondrial protein that induces cell death. Nat Med. 2001;7(12):1306–1312. doi: 10.1038/nm1201-1306. [DOI] [PubMed] [Google Scholar]
- Cook WJ, Green KA, Obar JJ, Green WR. Quantitative analysis of LP-BM5 murine leukemia retrovirus RNA using real-time RT-PCR. J Virol Methods. 2003;108(1):49–58. doi: 10.1016/s0166-0934(02)00256-2. [DOI] [PubMed] [Google Scholar]
- Elliott T, Bodmer H, Townsend A. Recognition of out-of-frame major histocompatibility complex class I-restricted epitopes in vivo. Eur J Immunol. 1996;26(5):1175–1179. doi: 10.1002/eji.1830260532. [DOI] [PubMed] [Google Scholar]
- Evans DT, O’Connor DH, Jing P, Dzuris JL, Sidney J, da Silva J, Allen TM, Horton H, Venham JE, Rudersdorf RA, Vogel T, Pauza CD, Bontrop RE, DeMars R, Sette A, Hughes AL, Watkins DI. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat Med. 1999;5(11):1270–1276. doi: 10.1038/15224. [DOI] [PubMed] [Google Scholar]
- Franco MA, Tin C, Rott LS, VanCott JL, McGhee JR, Greenberg HB. Evidence for CD8+ T-cell immunity to murine rotavirus in the absence of perforin, fas, and gamma interferon. J Virol. 1997;71(1):479–486. doi: 10.1128/jvi.71.1.479-486.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaur A, Green WR. Analysis of the helper virus in murine retrovirus-induced immunodeficiency syndrome: evidence for immunoselection of the dominant and subdominant CTL epitopes of the BM5 ecotropic virus. Viral Immunol. 2003;16(2):203–212. doi: 10.1089/088282403322017938. [DOI] [PubMed] [Google Scholar]
- Geels MJ, Cornelissen M, Schuitemaker H, Anderson K, Kwa D, Maas J, Dekker JT, Baan E, Zorgdrager F, van den Burg R, van Beelen M, Lukashov VV, Fu TM, Paxton WA, van der Hoek L, Dubey SA, Shiver JW, Goudsmit J. Identification of sequential viral escape mutants associated with altered T-cell responses in a human immunodeficiency virus type 1-infected individual. J Virol. 2003;77(23):12430–12440. doi: 10.1128/JVI.77.23.12430-12440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese NA, Gazzinelli RT, Actor JK, Morawetz RA, Sarzotti M, Morse HC., 3rd Retrovirus-elicited interleukin-12 and tumour necrosis factor-alpha as inducers of interferon-gamma-mediated pathology in mouse AIDS. Immunology. 1996;87(3):467–474. doi: 10.1046/j.1365-2567.1996.492569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gondek DC, Lu LF, Quezada SA, Sakaguchi S, Noelle RJ. Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J Immunol. 2005;174(4):1783–1786. doi: 10.4049/jimmunol.174.4.1783. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Brander C, Tang Y, Tremblay C, Colbert RA, Addo MM, Rosenberg ES, Nguyen T, Allen R, Trocha A, Altfeld M, He S, Bunce M, Funkhouser R, Pelton SI, Burchett SK, McIntosh K, Korber BT, Walker BD. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature. 2001a;412(6844):334–338. doi: 10.1038/35085576. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Pasquier C, Holmes EC, Liang B, Tang Y, Izopet J, Saune K, Rosenberg ES, Burchett SK, McIntosh K, Barnardo M, Bunce M, Walker BD, Brander C, Phillips RE. Mother-to-child transmission of HIV infection and CTL escape through HLA-A2-SLYNTVATL epitope sequence variation. Immunol Lett. 2001b;79(1–2):109–116. doi: 10.1016/s0165-2478(01)00272-3. [DOI] [PubMed] [Google Scholar]
- Goulder PJ, Phillips RE, Colbert RA, McAdam S, Ogg G, Nowak MA, Giangrande P, Luzzi G, Morgan B, Edwards A, McMichael AJ, Rowland-Jones S. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat Med. 1997;3(2):212–217. doi: 10.1038/nm0297-212. [DOI] [PubMed] [Google Scholar]
- Green KA, Okazaki T, Honjo T, Cook WJ, Green WR. The programmed death-1 and interleukin-10 pathways play a down-modulatory role in LP-BM5 retrovirus-induced murine immunodeficiency syndrome. J Virol. 2008;82(5):2456–2469. doi: 10.1128/JVI.01665-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001;19:65–91. doi: 10.1146/annurev.immunol.19.1.65. [DOI] [PubMed] [Google Scholar]
- Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Annu Rev Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- Ho O, Green WR. Alternative translational products and cryptic T cell epitopes: expecting the unexpected. J Immunol. 2006a;177(12):8283–8289. doi: 10.4049/jimmunol.177.12.8283. [DOI] [PubMed] [Google Scholar]
- Ho O, Green WR. Cytolytic CD8+ T cells directed against a cryptic epitope derived from a retroviral alternative reading frame confer disease protection. J Immunol. 2006b;176(4):2470–2475. doi: 10.4049/jimmunol.176.4.2470. [DOI] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. Faseb J. 1991;5(10):2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- Kagi D, Hengartner H. Different roles for cytotoxic T cells in the control of infections with cytopathic versus noncytopathic viruses. Curr Opin Immunol. 1996;8(4):472–477. doi: 10.1016/s0952-7915(96)80033-1. [DOI] [PubMed] [Google Scholar]
- Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen KJ, Podack ER, Zinkernagel RM, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature. 1994a;369(6475):31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- Kagi D, Seiler P, Pavlovic J, Ledermann B, Burki K, Zinkernagel RM, Hengartner H. The roles of perforin- and Fas-dependent cytotoxicity in protection against cytopathic and noncytopathic viruses. Eur J Immunol. 1995;25(12):3256–3262. doi: 10.1002/eji.1830251209. [DOI] [PubMed] [Google Scholar]
- Kagi D, Vignaux F, Ledermann B, Burki K, Depraetere V, Nagata S, Hengartner H, Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994b;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Klinken SP, Fredrickson TN, Hartley JW, Yetter RA, Morse HC., 3rd Evolution of B cell lineage lymphomas in mice with a retrovirus-induced immunodeficiency syndrome, MAIDS. J Immunol. 1988;140(4):1123–1131. [PubMed] [Google Scholar]
- Kurschus FC, Kleinschmidt M, Fellows E, Dornmair K, Rudolph R, Lilie H, Jenne DE. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562(1–3):87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]
- Li W, Green WR. The role of CD4 T cells in the pathogenesis of murine AIDS. J Virol. 2006;80(12):5777–5789. doi: 10.1128/JVI.02711-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Wang JY, Watson RR. Murine AIDS, a key to understanding retrovirus-induced immunodeficiency. Viral Immunol. 1996;9(4):225–239. doi: 10.1089/vim.1996.9.225. [DOI] [PubMed] [Google Scholar]
- Liu L, Chahroudi A, Silvestri G, Wernett ME, Kaiser WJ, Safrit JT, Komoriya A, Altman JD, Packard BZ, Feinberg MB. Visualization and quantification of T cell-mediated cytotoxicity using cell-permeable fluorogenic caspase substrates. Nat Med. 2002;8(2):185–189. doi: 10.1038/nm0202-185. [DOI] [PubMed] [Google Scholar]
- Maness NJ, Valentine LE, May GE, Reed J, Piaskowski SM, Soma T, Furlott J, Rakasz EG, Friedrich TC, Price DA, Gostick E, Hughes AL, Sidney J, Sette A, Wilson NA, Watkins DI. AIDS virus specific CD8+ T lymphocytes against an immunodominant cryptic epitope select for viral escape. J Exp Med. 2007;204(11):2505–2512. doi: 10.1084/jem.20071261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayrand SM, Healy PA, Torbett BE, Green WR. Anti-Gag cytolytic T lymphocytes specific for an alternative translational reading frame-derived epitope and resistance versus susceptibility to retrovirus-induced murine AIDS in F(1) mice. Virology. 2000;272(2):438–449. doi: 10.1006/viro.2000.0339. [DOI] [PubMed] [Google Scholar]
- Mayrand SM, Schwarz DA, Green WR. An alternative translational reading frame encodes an immunodominant retroviral CTL determinant expressed by an immunodeficiency-causing retrovirus. J Immunol. 1998;160(1):39–50. [PubMed] [Google Scholar]
- Morawetz RA, Giese NA, Gabriele L, Rothman P, Horak I, Ozato K, Morse HC., 3rd Relationship of cytokines and cytokine signaling to immunodeficiency disorders in the mouse. Braz J Med Biol Res. 1998;31(1):61–67. doi: 10.1590/s0100-879x1998000100008. [DOI] [PubMed] [Google Scholar]
- Morse HC, 3rd, Chattopadhyay SK, Makino M, Fredrickson TN, Hugin AW, Hartley JW. Retrovirus-induced immunodeficiency in the mouse: MAIDS as a model for AIDS. Aids. 1992;6(7):607–621. doi: 10.1097/00002030-199207000-00001. [DOI] [PubMed] [Google Scholar]
- Price DA, Goulder PJ, Klenerman P, Sewell AK, Easterbrook PJ, Troop M, Bangham CR, Phillips RE. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc Natl Acad Sci U S A. 1997;94(5):1890–1895. doi: 10.1073/pnas.94.5.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehr M, Cahenzli J, Haas A, Price DA, Gostick E, Huber M, Karrer U, Oxenius A. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. J Virol. 2008;82(7):3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roths JB, Murphy ED, Eicher EM. A new mutation, gld, that produces lymphoproliferation and autoimmunity in C3H/HeJ mice. J Exp Med. 1984;159(1):1–20. doi: 10.1084/jem.159.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmbeck R, Riedl P, Fissolo N, Lemonnier FA, Bertoletti A, Reimann J. Translation from cryptic reading frames of DNA vaccines generates an extended repertoire of immunogenic, MHC class I-restricted epitopes. J Immunol. 2005;174(8):4647–4656. doi: 10.4049/jimmunol.174.8.4647. [DOI] [PubMed] [Google Scholar]
- Schwarz DA, Green WR. CTL responses to the gag polyprotein encoded by the murine AIDS defective retrovirus are strain dependent. J Immunol. 1994;153(1):436–441. [PubMed] [Google Scholar]
- Stromnes IM, Dittmer U, Schumacher TN, Schepers K, Messer RJ, Evans LH, Peterson KE, Race B, Hasenkrug KJ. Temporal effects of gamma interferon deficiency on the course of Friend retrovirus infection in mice. J Virol. 2002;76(5):2225–2232. doi: 10.1128/jvi.76.5.2225-2232.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a single peptide-MHC complex on a target cell can elicit a cytolytic T cell response. Immunity. 1996;4(6):565–571. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- Tang Y, Hugin AW, Giese NA, Gabriele L, Chattopadhyay SK, Fredrickson TN, Kagi D, Hartley JW, Morse HC., 3rd Control of immunodeficiency and lymphoproliferation in mouse AIDS: studies of mice deficient in CD8+ T cells or perforin. J Virol. 1997;71(3):1808–1813. doi: 10.1128/jvi.71.3.1808-1813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham DJ, Cardin RC, Christensen JP, Brooks JW, Belz GT, Doherty PC. Perforin and Fas in murine gammaherpesvirus-specific CD8(+) T cell control and morbidity. J Gen Virol. 2001;82(Pt 8):1971–1981. doi: 10.1099/0022-1317-82-8-1971. [DOI] [PubMed] [Google Scholar]
- Topham DJ, Tripp RA, Doherty PC. CD8+ T cells clear influenza virus by perforin or Fas-dependent processes. J Immunol. 1997;159(11):5197–5200. [PubMed] [Google Scholar]
- Uehara S, Hitoshi Y, Numata F, Makino M, Howard M, Mizuochi T, Takatsu K. An IFN-gamma-dependent pathway plays a critical role in the pathogenesis of murine immunodeficiency syndrome induced by LP-BM5 murine leukemia virus. Int Immunol. 1994;6(12):1937–1947. doi: 10.1093/intimm/6.12.1937. [DOI] [PubMed] [Google Scholar]
- Uenaka A, Ono T, Akisawa T, Wada H, Yasuda T, Nakayama E. Identification of a unique antigen peptide pRL1 on BALB/c RL male 1 leukemia recognized by cytotoxic T lymphocytes and its relation to the Akt oncogene. J Exp Med. 1994;180(5):1599–1607. doi: 10.1084/jem.180.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usherwood EJ, Brooks JW, Sarawar SR, Cardin RD, Young WD, Allen DJ, Doherty PC, Nash AA. Immunological control of murine gammaherpesvirus infection is independent of perforin. J Gen Virol. 1997;78(Pt 8):2025–2030. doi: 10.1099/0022-1317-78-8-2025. [DOI] [PubMed] [Google Scholar]
- Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73(9):7619–7626. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CM, Glass AA, Chiu V, Clark WR. The role of the Fas lytic pathway in a perforin-less CTL hybridoma. J Immunol. 1994a;153(6):2506–2514. [PubMed] [Google Scholar]
- Walsh CM, Matloubian M, Liu CC, Ueda R, Kurahara CG, Christensen JL, Huang MT, Young JD, Ahmed R, Clark WR. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci U S A. 1994b;91(23):10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokomaku Y, Miura H, Tomiyama H, Kawana-Tachikawa A, Takiguchi M, Kojima A, Nagai Y, Iwamoto A, Matsuda Z, Ariyoshi K. Impaired processing and presentation of cytotoxic-T-lymphocyte (CTL) epitopes are major escape mechanisms from CTL immune pressure in human immunodeficiency virus type 1 infection. J Virol. 2004;78(3):1324–1332. doi: 10.1128/JVI.78.3.1324-1332.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelinskyy G, Balkow S, Schimmer S, Schepers K, Simon MM, Dittmer U. Independent roles of perforin, granzymes, and Fas in the control of Friend retrovirus infection. Virology. 2004;330(2):365–374. doi: 10.1016/j.virol.2004.08.040. [DOI] [PubMed] [Google Scholar]
- Zelinskyy G, Balkow S, Schimmer S, Werner T, Simon MM, Dittmer U. The level of friend retrovirus replication determines the cytolytic pathway of CD8+ T-cell-mediated pathogen control. J Virol. 2007;81(21):11881–11890. doi: 10.1128/JVI.01554-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook MB, Howard MT, Sinnathamby G, Atkins JF, Eisenlohr LC. Epitopes derived by incidental translational frameshifting give rise to a protective CTL response. J Immunol. 2006;176(11):6928–6934. doi: 10.4049/jimmunol.176.11.6928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
W.t. or IFNγ−/− B6 mice were infected with LP-BM5 for 11 weeks after which disease was assessed. A: Spleen weight. B: B-cell responsiveness to LPA and T-cell responsiveness to ConA, with the data represented as the percentage counts per minute of non-infected controls. Students T tests were used to measure significance.
FasL−/−, CD8−/−, and w.t. BALB/c mice were infected with LP-BM5 for 11 weeks. A: Spleen weight. B: Serum levels of IgM and IgG2A. C: T-cell responsiveness to ConA stimulation, with the data represented as the percentage counts per minute of non-infected controls. Students T tests were used to measure disease significance. D: Disease index of mice with or without prior infection with LP-BM5. Statistics were calculated using the non-parametric Mann-Whitney test of significance. E: Viral RNA expression of BM5def and BM5eco from spleen-derived RNA samples isolated from LP-BM5-infected mice, quantified using real-time RT-PCR. NS indicates non-significant differences between experimental groups. Students T tests were used to measure disease significance. The data are representative of 3 experiments with 4–6 mice/group.