Abstract
Although nicotinic acetylcholine receptors in both the central and peripheral nervous systems play a prominent role in the control of urinary bladder function, little is known regarding expression or function of nicotinic receptors in the bladder epithelium, or urothelium. Nicotinic receptors have been described in epithelial cells lining the upper gastrointestinal tract, respiratory tract, and the skin. Thus the present study examined the expression and functionality of nicotinic receptors in the urothelium, as well as the effects of stimulation of nicotinic receptors on the micturition reflex. mRNA for the α3, α5, α7, β3, and β4 nicotinic subunits was identified in rat urothelial cells using RT-PCR. Western blotting also confirmed urothelial expression of the α3- and α7-subunits. Application of nicotine (50 nM) to cultured rat urothelial cells elicited an increase in intracellular Ca2+ concentration, indicating that at least some of the subunits form functional channels. These effects were blocked by the application of the nicotinic antagonist hexamethonium. During in vivo bladder cystometrograms in urethane-anesthetized rats, intravesical administration of nicotine, choline, or the antagonists methyllycaconitine citrate and hexamethonium elicited changes in voiding parameters. Intravesical nicotine (50 nM, 1 μM) increased the intercontraction interval. Intravesical choline (1–100 μM) also affected bladder reflexes similarly, suggesting that α7 nicotinic receptors mediate this effect. Intravesical administration of hexamethonium (1–100 μM) potentiated the nicotine-induced changes in bladder reflexes. Methyllycaconitine citrate, a specific α7-receptor antagonist, prevented nicotine-, choline-, and hexamethonium-induced bladder inhibition. These results are the first indication that stimulation of nonneuronal nicotinic receptors in the bladder can affect micturition.
Keywords: intracellular Ca2+, ion channels, RT-PCR
The afferent nerves in the urinary bladder respond to a variety of stimuli including distension and contraction of the detrusor muscle as well as noxious chemicals contained in the urine or released during infection (11). Activity in afferent nerves initiates sensations of bladder fullness or pain and can also trigger voiding and urine storage reflexes (1, 12). Previous studies of sensory mechanisms in the bladder have primarily focused on the properties of the afferent nerves (1, 12).
Recently, however, it has been shown that urothelial cells may influence afferent nerve activity by expressing “neuronal-like” properties including 1) release of neurotransmitters (e.g., NO and ATP) (40); 2) expression of receptors normally found in nerves, such as vanilloid receptors (TRPV1) (6) and calcium-activated K+ channels (SK channels) (20); and 3) sensitivity to neurotransmitters, such as acetylcholine (5), norepinephrine (7), and ATP (4). The urothelium may also release acetylcholine in a fashion similar to that already reported for other nonneural tissues including pulmonary endothelial cells (24), placental epithelial cells (39), and bronchial epithelial cells (8), where it acts in an autocrine fashion to influence cellular functions (16, 24). In this context, recent data suggest that urothelial cells may release acetylcholine during the filling phase of micturition (2). Given the potential role for nicotinic acetylcholine receptors (nAChRs) in the neural control of bladder function, the current study was aimed at determining whether nAChRs are also expressed in the urothelium and whether activation of these receptors can alter voiding reflexes.
The nicotinic acetylcholine receptor family is currently known to consist of at least 17 different subunits (α1–10, β1–4, γ, δ, and ε) (29, 30). These subunits form pentameric channels that can be categorized into two different groups; neuronal nicotinic receptors (consisting of α2–10 and α2–4 subunits) and muscle nicotinic receptors (consisting of α1-, β1-, γ-, δ-, and ε-subunits). Neuronal nAChRs can be further classified into three groups: 1) homomeric pentamers (such as α7 or α9), 2) simple heteromeric pentamers consisting of one type of α-subunit and one type of β-subunit (e.g., α3β2 receptors), and 3) complex heteromeric pentamers consisting of three or more different subunits (e.g., α3α5β2 receptors) (22, 23). Each type of receptor has different electrophysiological and pharmacological properties, which have been hypothesized to be responsible for the widely varying effects of acetylcholine throughout the central and peripheral nervous system (8, 9, 13, 15, 32, 35, 37). The present study detected, in the urothelium, mRNA for several nicotinic receptor subunits known to form functional receptors. Activation of these receptors in cultured urothelial cells with nicotine increased intracellular calcium. In addition, intravesical administration of nicotinic agonists also altered reflex bladder function. Preliminary accounts of these data were first reported in an abstract (3).
METHODS AND MATERIALS
Animals
All experiments used female Sprague-Dawley (SD) rats (250–300 g) that were fed a standard diet with free access to water before experimentation. Tissues were harvested from rats that were euthanized by inhalation of 100% CO2. All studies were carried out with the approval of the University of Pittsburgh Institutional Animal Care and Use Committee and maintained according to the standards set forth in the American Physiological Society’s handbook on the care and use of laboratory animals.
Rat urothelial cell culture
Rat urinary bladders were excised and stored in oxygenated Krebs solution. The bladder was cut open, gently stretched with the epithelial side up, and pinned in a Sylgard-coated dish. The bladder was incubated overnight in minimal essential medium (Invitrogen), penicillin/streptomycin/fungizone, and 2.5 mg/ml dispase (Invitrogen). The epithelium was then gently scraped from the underlying tissue and treated with enzyme (0.25% trypsin) to dissociate urothelial cells. The cells were resuspended in keratinocyte medium (Invitrogen), and 0.1 ml of the cell suspension (50,000–250,000 cells/ml) was added to the surface of collagen-coated plates. Cells were used within the first 3 days following plating.
RNA extraction and RT-PCR
mRNA for RT-PCR experiments was obtained from both cultured urothelial cells and whole urothelial tissue. To collect whole urothelial tissue, bladders were obtained from three female SD rats, cut open, and pinned flat on Sylgard dishes. The urothelium was then gently teased away from the underlying tissue using fine forceps and scissors and placed in RNase-free buffer. A RNeasy RNA extraction kit from Qiagen was used to extract RNA following the published protocol for tissue samples or cultured cells. The RNA was resuspended in RNAse-free water and tested for quality, purity, and concentration by UV spectrophotometry and gel electrophoresis (1.2% formaldehyde agarose gel). First-strand cDNA synthesis was performed using a Roche Molecular Biochemicals First-Strand cDNA Synthesis Kit for RT-PCR, using an oligo-dT15 primer. PCR was performed using Qiagen’s Taq PCR Core Kit, using the optional Q-solution, sequence-specific primers designed for each subunit, and annealing temperatures were determined by the sequence of the primers. Primers for PCR were as follows: α2 (forward) 5′-CGAGTCGGGGAGTATGGTAA-3′ (reverse) 5′-AGGAAGTGGCTTCTCAGTCG-3′; α3 (forward) 5′-GTGAATTCAGCCGTGCAGACTCCA-3′ (reverse) 5′-ATA-AGCTTGGCAACGTACTTCCAATCATC-3′; α4 (forward) 5′-GTGAATTCCACAGGTCGTACACGGGTCG-3′ (reverse) 5′-ATAAGCTTGCGAGCCCGGCATCTTGAGT-3′; α5 (forward) 5′-AGTGGGCTGGACCTAAATCTCG-3′ (reverse) 5′-CAA-AAAGCCCTAAAGTCCCAATGA-3′; α7 (forward) 5′-GT-GAATTCAAGAGGCCCGGAGAGGACAA-3′ (reverse) 5′-AT-AAGCTTCGCCACATACGACCCCAGAG-3′; β2 (forward) 5′-GTGAATTCAGGGCGAGGCGGTTTTCTT-3′ (reverse) 5′-ATAAGCTTGCGTACGCCATCCACTGCT-3′; β3 (forward) 5′-GTGAATTCTGGGTGAAGAGGCTGTT-3′ (reverse) 5′-ATAAGCTTATCGCTGGCGGGAGTCTGTT-3′; β4 (forward) 5′-GTGAATTCCATGGCATCCTGGGTCAAG-3′ (reverse) 5′-ATAAGCTTCTGGGGAGGCCTGCTGTGT-3′. PCR was performed using the GeneAmp 9700 thermocycler from Applied Biosystems. PCR products were run on a 1.2% agarose gel using ethidium bromide to visualize bands. Results were analyzed using the Eagle Eye II digital imaging system (Stratagene). Positive results were sequenced using an ABI PRISM 3100 Automated Genetic Analyzer in the University of Pittsburgh DNA Sequencing Core Facility.
Western blots
Cultured urothelial cells grown for 3 days were lysed in RIPA buffer, and protein was collected by centrifugation (15 min at 13,000 rpm). Protein was quantified using a BCA Protein Assay Kit (Pierce). Fifty micrograms of total protein were loaded onto an 8–20% polyacrylamide gel (Gradipore) and run for 1 h at 150 V using the Mini-Protean System (Bio-Rad). Proteins were then transferred to PVDF membranes using the Transblot system (Bio-Rad) at 250 mA for 1 h. The membranes were then blocked using 5% nonfat milk in TBS-T buffer and incubated with the primary antibody (1:1,000 dilution) overnight at 4°C. After being rinsed in TBS-T buffer three times for 15 min each, the membranes were incubated with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature with shaking. Following additional washes (3 × 15 min), the membrane was developed using the ECL plus system (Immersion) and exposed to film to visualize banding. All antibodies and blocking peptides were purchased from Santa Cruz Biotechnology (goat polyclonal α3: sc-1771, goat polyclonal α7: sc-1447, bovine anti-goat-HRP sc-2350).
Calcium imaging (Fura-2)
Urothelial cells were maintained in vitro in Krebs solution and placed in a flow chamber. Cells were loaded with fura 2-AM (1 μM dye, 40-min incubation at 25°C), alternatively illuminated at 340 and 380 nm by using a xenon lamp and imaged with a Dage-MTI cooled CCD camera with 640 × 480-pixel resolution. A Dage-MTI Gen. II system image intensifier and software package from Compix (Cranberry, PA) was used to collect data over a time span of ~10 min for each experiment, with no cell being exposed to light for longer than 30 min to minimize the effect of photobleaching.
Rat in vivo bladder cystometrogram
Female SD rats (250–275 g) were anesthetized with urethane (1.2 g/kg sc). A midline incision was made, and a catheter (intramedic tubing, PE 50) was inserted into the bladder lumen through a small incision in the bladder dome and secured with a suture. The catheter was connected by way of a three-way stopcock to a syringe pump for fluid infusion and a pressure transducer was connected to a computer to record changes in bladder pressure. The ureters were also tied and cut to prevent filling of the bladder from the kidneys. A wick of sterile gauze was inserted into the midline incision and sewn in place to drain urine from the abdominal cavity. Saline was infused intravesically (0.04 ml/min) for 1 h to allow the bladder to recover from surgery. Saline infusion continued for an additional hour while bladder pressure was recorded to obtain control data. The bladder infusate was then switched to a drug solution and recorded for at least another hour before the switch to another drug solution. In four animals, epibatidine was administered (0.05 μg/kg ip injection) at the end of the experiment and bladder pressure recordings were obtained for 2 h.
Reagents
All cell culture reagents as well as nicotine hydrogen tartrate and hexamethonium were obtained from Sigma (St. Louis, MO) and Invitrogen (Grand Island, NY). Epibatidine and methyllycaconitine citrate were obtained from Tocris Chemicals (Ellisville, MO).
Statistical analysis
Statistical significance was assessed in all experiments using unpaired, two-tailed Student’s t-tests. Statistical significance was accepted when P < 0.05, compared with controls.
RESULTS
Nicotinic subunit expression in the urothelium
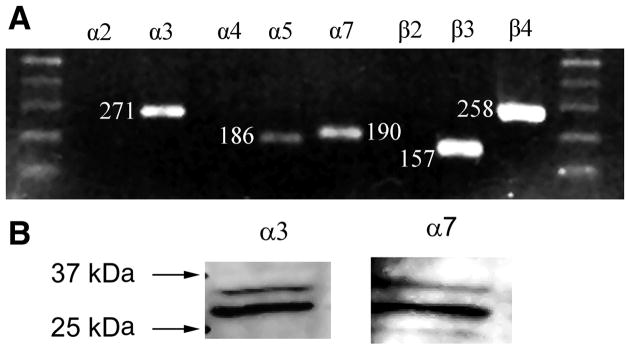
RT-PCR experiments indicated the presence of mRNA for α3, α5, α7, β3, and β4 nicotinic receptor subunits in both cultured cells (Fig. 1) and whole urothelial tissue (results not shown). Each band corresponded to the expected product size. Products for α2, α4, and β2 subunits were not detected. Each product’s identity was confirmed through DNA sequencing. These results are consistent with the types of subunits that normally associate together and form functional receptors (e.g., α3β3β4, α3α5β4, β7). Identical results were obtained using urothelial cells from three separate rat bladders. Positive controls were obtained for α2, α4, and β2 using RNA from rat brain extract (Clontech, results not shown).
Fig. 1.
A: RT-PCR detects presence of nicotinic acetylcholine receptor (nAChR) subunits. Notice positive results in α3, α5, α7, β3, and β4 lanes; 1.2% agarose gel in 1× TBE buffer stained with ethidium bromide. Results are typical of cells cultured from 3 rat bladders or urothelial tissue surgically removed from the bladder. B: Western blot of cultured urothelial cells detects the presence of the α3 and α7 nAChR subunits. Results are typical for blots performed with cultured cells, surgically stripped urothelium, or dorsal root ganglion cells used as a control. Visualization of bands is blocked by the addition of an antigen to each antibody.
To determine whether nAChR subunits are expressed in urothelial cells, cell lysates from cultured urothelial cells were probed in Western blots. Because our PCR experiments indicated that all possible receptors expressed in the urothelium would contain either the α3 or α7 subunits, antibodies for both were used. Western blots of cell lysates from cultured urothelial cells or surgically removed urothelial tissue revealed a major band of 30 kDa and minor band of 35 kDa for both the α3 and α7 antibodies, (Fig. 1B), which were selectively blocked by their respective antigens (data not shown). These bands were identical to those observed using cell lysates from dorsal root ganglion cells as a control (data not shown).
Nicotine increases intracellular calcium in urothelial cells
To assess whether the nicotinic receptor subunits present in the urothelium form functional receptors, we examined the ability of nicotine to increase cytosolic calcium ([Ca+2]i) in cultured urothelial cells. Application of nicotine (50 nM, n = 34 cells from 5 independent cultures) elicited an increase in [Ca+2]i (4-fold increase, representative trace shown in Fig. 2A) in rat urothelial cells. This increase in [Ca+2]i evoked by nicotine was abolished by pretreatment with the nicotinic antagonist, hexamethonium (C6, 10 min, 20 μM, n = 18; Fig. 2B).
Fig. 2.
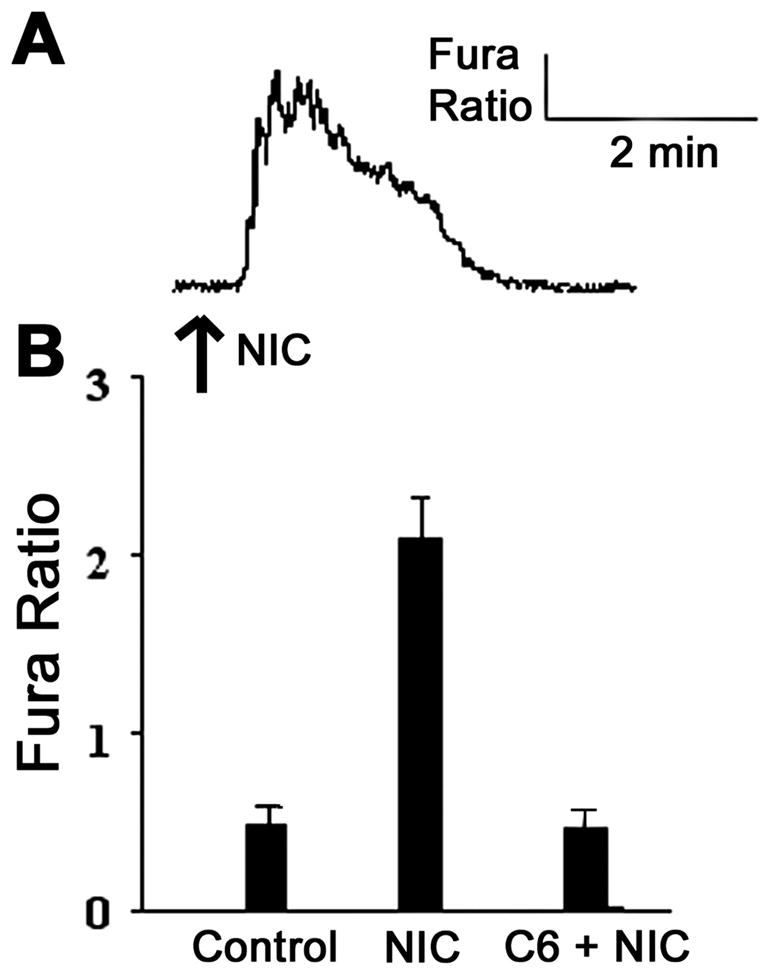
Application of nicotine to urothelial cells causes an increase in the fura 2 signal, indicating an increase in intracellular calcium. A: representative trace of fura 2 ratio. B: application of nicotine (NIC; 50 nM, n = 34 cells from 5 independent cultures) causes a 2.5-fold increase in the fura 2 ratio, which is blocked by the pretreatment of the nicotinic antagonist hexamethonium (20 μM, C6, n = 18, from 3 separate cultures).
Intravesical administration of nicotinic agonists elicits changes in voiding patterns in the anesthetized rat
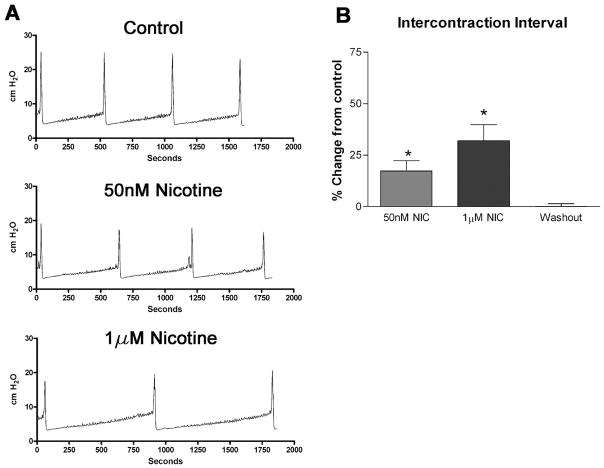
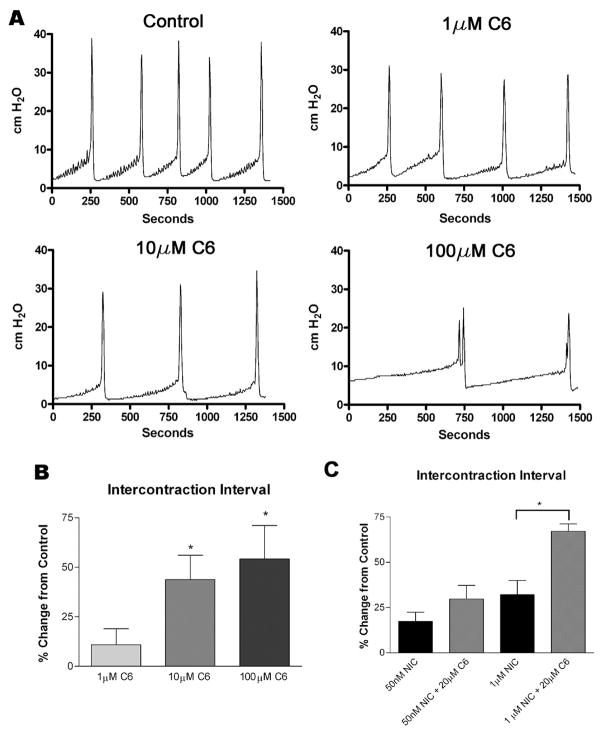
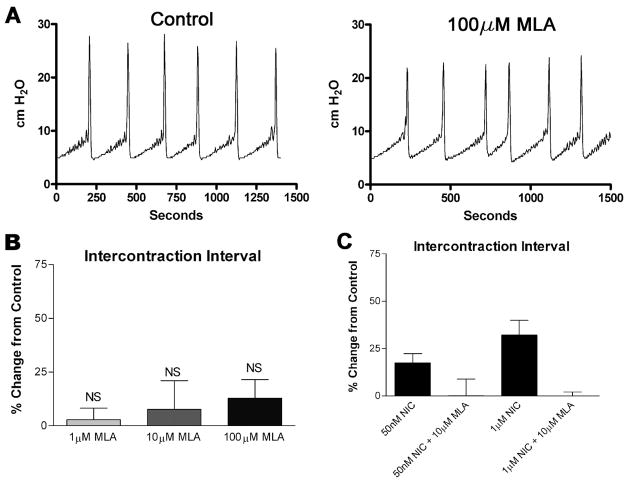
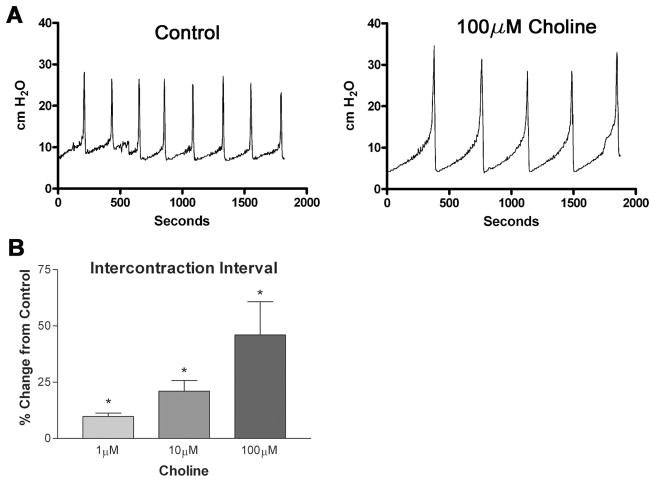
To determine whether activation of urothelial nicotinic receptors causes changes in reflex bladder contractions, we infused a saline solution containing nicotine hydrogen tartrate into the bladders of anesthetized rats and examined changes in voiding parameters. As shown in Fig. 3, A and B, instillation of nicotine (50 nM and 1 μM, 0.04 ml/min, n = 6) increased the intercontraction interval (ICI) in a concentration-dependent manner (17.4 ± 4.9 and 32.0 ± 7.5%, respectively, P < 0.05 each) over saline infusion alone. Switching the infusate back to saline after 1 h resulted in a complete reversal of the increase in ICI (Fig. 3B). Intravesical instillation of the nicotinic antagonist hexamethonium (1, 10, 100 μM, n = 6 each) also increased the ICI (10.9 ± 8.1, 33.7 ± 8.5, and 39.3 ± 10.3%, respectively, P < 0.05; Fig. 4A) in a concentration-dependent manner. Additionally, concurrent instillation of hexamethonium (20 μM) and nicotine (50 nM, 1 μM) had additive effects to inhibit reflex voiding (Fig. 4C). Pretreatment with the specific α7 antagonist methyllycaconitine citrate (MLA; 10 μM) completely blocked the nicotine-induced changes in ICI (Fig. 5C) without significantly changing the voiding parameters when administered alone (1, 10, 100 μM; Fig. 5, A and B). Concurrent intravesical application of MLA (100 μM) and hexamethonium (10, 100 μM, n = 6) significantly reduced the hexamethonium-induced inhibition of reflex voiding (Fig. 6). To further determine whether changes in bladder reflexes were influenced by activation of α7 receptors, choline (1, 10, 100 μM, n = 6), an agonist for α7 receptors, was instilled intravesically. Choline elicited effects similar to those of nicotine, increasing the ICI by 9.8 ± 1.5, 21.0 ± 5.7, and 46.0 ± 14.8%, respectively, P < 0.05 (Fig. 7).
Fig. 3.
Effects of intravesical nicotine on voiding function in the rat. A: 3 representative tracings of CMG recordings. All tracings were recorded from the same animal. Intravesical administration of NIC (50 nM and 1 μM, n = 6 each) increases the interval between voiding bladder contractions (intercontraction interval or ICI). B: effect is reversed by saline washout. *P < 0.05.
Fig. 4.
Effect of hexamethonium (C6) on voiding function in the rat. A: representative traces of CMG recordings during intravesical administration of hexamethonium (C6). B: intercontraction interval changes following instillation of hexamethonium (C6) intravesically, n = 8 for each concentration. C: effects on ICI of nicotine infusion alone (50 nM and 1 μM) compared with simultaneous infusion of C6 (20 μM) and NIC; n = 6 for each. Bars 1 and 3 are taken from different rats than bars 2 and 4. All results are shown as a percent change from saline-infused controls. *P < 0.05 compared with control.
Fig. 5.
Effect of methyllycaconitine citrate (MLA) on voiding function in the rat. A: representative traces of CMG recordings during intravesical administration of MLA. B: intercontraction interval following instillation of MLA intravesically, n = 8 for each MLA concentration. C: effects on ICI of nicotine infusion alone (50 nM and 1 μM, columns 1 and 3) compared with simultaneous infusion of MLA (10 μM) and NIC (columns 2 and 4), n = 6 for each, columns 1 and 3 are taken from different rats than columns 2 and 4. All results are shown as a percent change from saline-infused controls. NS, not statistically significant compared with control.
Fig. 6.
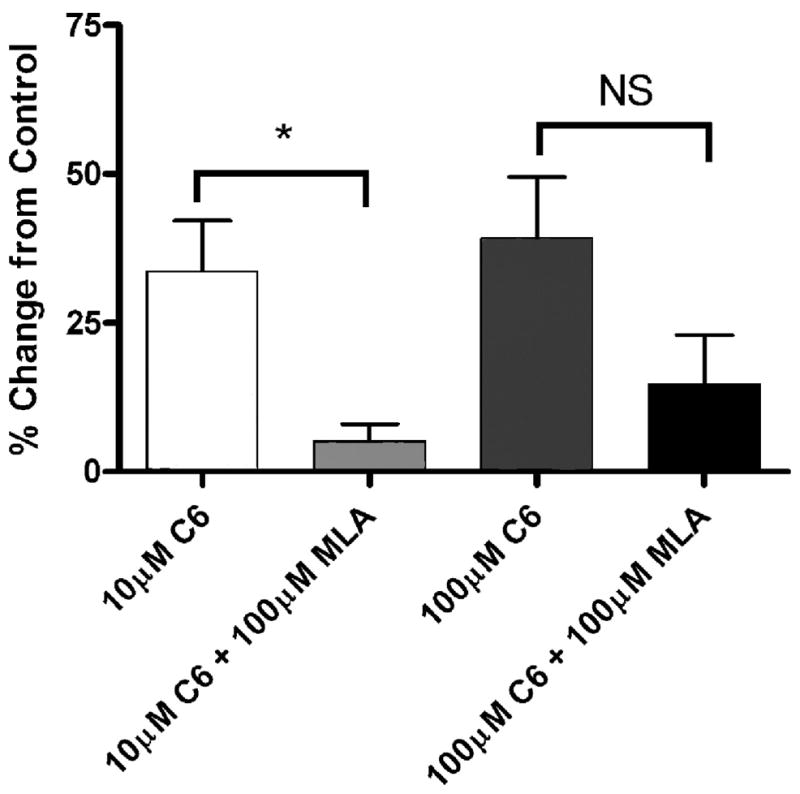
Effect of simultaneous infusion of methyllycaconitine citrate and hexamethonium on micturition. Comparison of the percent change in ICI from saline control following instillation of hexamethonium (C6) alone or concurrent instillation of C6 and MLA, n = 6 for each. *P < 0.05. NS, not significant.
Fig. 7.
Effect of the α7-specific agonist, choline, on ICI in the rat. Choline (1, 10, 100 μM) was infused intravesically for 1 h each and ICI was measured. A: representative tracings of CMG recordings during intravesical administration of choline. B: graph shows change in ICI as a percent change from saline-infused controls. *P < 0.05 compared with control, n = 8 for each concentration.
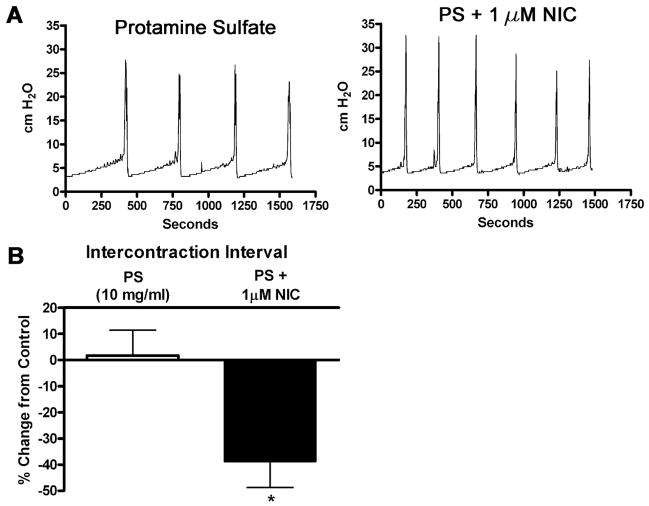
To determine whether nicotinic agents may be acting on afferent nerves that lie immediately beneath the urothelium, we instilled protamine sulfate intravesically to disrupt the urothelial barrier (25). Following 1 h of protamine sulfate (10 mg/ml) infusion, the infusate was switched to nicotine. Protamine sulfate treatment did not significantly alter the ICI (Fig. 8B). However, nicotine (1 μM) infusion following protamine sulfate treatment decreased the ICI 38.8 ± 9.9% (Fig. 8, A and B). This effect could not be reversed by washout.
Fig. 8.
Effect of permeabilization of the urothelial barrier by protamine sulfate (PS) on nicotine-induced changes in bladder reflexes. A: representative tracings of CMG recordings taken during a control period of protamine sulfate (10 mg/ml) infusion and PS infusion with simultaneous NIC (1 μM) infusion. B: graph depicting changes in ICI during PS infusion or simultaneous PS and nicotine infusion as a change from saline-infused controls, n = 6 for each column. *P < 0.05 compared with control.
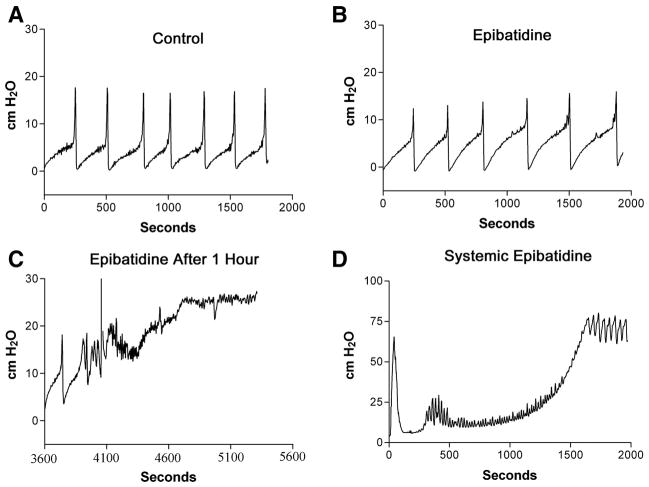
Intravesical administration of epibatidine (250 nM, 0.04 ml/min, n = 4), an ultrapotent, highly lipophillic nicotinic agonist, elicited an immediate increase in voiding pressure (~50%) without significantly changing the ICI (Fig. 9, A and B). Continuous infusion of epibatidine for 1 h resulted in complete urinary retention and overflow incontinence (Fig. 9C). Systemic administration of epibatidine (0.05 μg/kg ip, n = 4) also suppressed reflex bladder contractions and produced urinary retention (Fig. 9D); however, the onset occurred much more rapidly (onset of ~10 min compared with 1 h following intravesical administration).
Fig. 9.
A: effect of the potent nicotinic agonist, epibatidine, on bladder function. B: control recordings after 250 nM epibatidine instillation into the bladder (continuous infusion, 0.04 ml/min). C: after 1 h of continuous instillation of epibatidine. D: cystometrogram of bladder pressure following systemic administration of epibatidine (0.01 μg ip). A–C were recorded from the same animal, D from a separate animal.
DISCUSSION
Acetylcholine is an important transmitter in the neural pathways controlling bladder function, being responsible for neurotransmission in the brain, spinal cord, autonomic ganglia, and detrusor smooth muscle (10, 11, 21, 26, 34). The present study raises the possibility of an additional site for cholinergic modulation of bladder function. RT-PCR revealed that urothelial cells express the appropriate nicotinic receptor subunits that can interact to generate functional receptors, while calcium imaging experiments indicated that the receptors were indeed functional. In vivo experiments also demonstrated that activation of these receptors can affect reflex bladder function.
RT-PCR and Western blot analysis indicate that there may be more than one type of nicotinic receptor present in the urothelium. Possible functional receptors include α7 homomeric receptors and α3β4, α3β3β4, α3α5β4, and α3α5β3β4 heteromeric receptors (commonly referred to collectively as α3*). Research in other laboratories suggests that β3-subunits can only form functional receptors when expressed together with another β-subunit (e.g., α3β3β4) (17). Each subtype of receptor has specific electrophysiological properties that may be responsible for mediating different effects in the bladder. Subtype-specific agonists have become available, which will allow further study of the specific activities of each putative receptor subtype present in the urothelium (31–33).
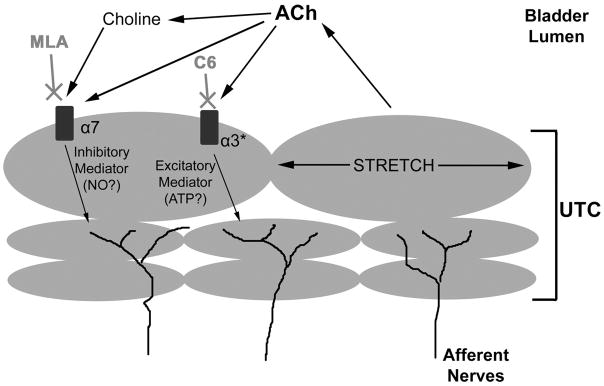
Increases in intracellular calcium in cultured cells following application of nicotine were blocked by pretreatment with the nicotinic antagonist hexamethonium. However, intravesical administration of hexamethonium did not block the nicotine-induced changes in voiding in vivo. To the contrary, hexamethonium amplified the inhibition of bladder reflexes caused by nicotine, an effect reversed, at least in part, by blocking α7 receptors using MLA. It is therefore possible that different types of nicotinic receptors in the bladder have directly opposing effects. Activation of α7 receptors has an inhibitory effect on bladder reflexes, as evidenced by the inhibitory effect of choline, an α7-specific agonist, in vivo. It is possible that this inhibitory effect is mediated by the release of a urothelial-derived inhibitory mediator (Fig. 10) (19, 41). The identity of this mediator is unknown, but one possibility is nitric oxide (NO), as α7 receptors have been shown to activate NO synthase in dorsal root ganglion cells (18). Recent studies have shown that NO is released from cultured urothelial cells following cholinergic or adrenergic stimulation (5, 7) and that NO donors, when instilled into the bladder, can increase the ICI (36). Additionally, oxyhemoglobin, a NO scavenger, induces bladder overactivity in unanesthetized rats when applied intravesically (1). It is thought that NO released from the urothelium can act on underlying afferent nerves, decreasing excitability and promoting storage. It should be noted that the α7-inhibitory pathway demonstrated in our study does not appear to be tonically active, as instillation of MLA into the bladder did not decrease the ICI. However, it should be noted that MLA was only tested in anesthetized animals. It is possible that while inhibition of α7 receptors does not affect reflex voiding, loss of its inhibitory signal in the awake animal may lead to increased bladder sensations, leading to increased voiding.
Fig. 10.
Hypothetical model of nicotinic cholinergic signaling in the urothelium. ACh, acetylcholine; ATP, adenosine triphosphate; C6, hexamethonium; NO, nitric oxide; UTC, urothelial cells (urothelium).
Our experiments also suggest that the urothelium possesses an excitatory nicotinic pathway, mediated through α3* receptors. This is evidenced by our results using hexamethonium, an antagonist which is known to block α3* receptors in the autonomic ganglia. Blockade of α3* receptors in the urothelium by hexamethonium would allow the inhibitory α7 pathway, activated by ACh released from the urothelium by stretch or nicotine in the infusate, to be unmasked (Fig. 10). The α3* pathway may act through the release of an excitatory mediator from the urothelium, such as ATP. ATP can be released from urothelial cells following stretch or various chemical stimuli (5, 7, 40). It has been suggested that ATP can act on bladder afferent nerves through purinergic receptors to increase afferent nerve excitability (1). Indeed, intravesical administration of ATP, ATP analogs, or specific purinergic receptor agonists decreases the ICI, indicating bladder hyperactivity (1).
It is unclear as to why the urothelium would exhibit such a system of opposing nicotinic signaling pathways. One hypothesis is that these pathways represent a novel way of determining the extent that the bladder is filled. ACh released from the urothelium as it stretches (2, 42) could act back on the urothelium in an autocrine or paracrine manner, activating urothelial nicotinic receptors. When the bladder is empty, small amounts of ACh would be released and levels in the urothelial layer would be low. At low concentrations of ACh, α3β4 receptors are almost exclusively desensitized (27, 28). However, low levels of ACh, or its metabolite choline, are sufficient to activate α7 receptors without desensitization (38). Results of this study suggest that activation of α7 receptors could result in an inhibition of bladder reflexes and promotion of urine storage. As the bladder fills with urine and the urothelium stretches, ACh levels would increase and activate urothelial α3* receptors, triggering the release of an excitatory mediator and stimulating afferent nerves to promote voiding.
One potential problem with the present experiments is the possibility that intravesically administered agents act not only on urothelial receptors but also at other sites in the bladder or in the peripheral nervous system by passing through the urothelium and into the bladder wall or blood. The urothelium forms a highly effective barrier to the potential toxins in the urine with a permeability comparable to that of the blood-brain barrier. Therefore, we would expect that polar, hydrophilic agents, such as nicotine (in its hydrogen tartrate form), MLA, and the quaternary amine hexamethonium would not cross the urothelial barrier. This is supported by our experiments showing that nicotine’s effects can be rapidly washed out. To test what might happen if a nicotinic agent did cross the urothelial barrier to work in the periphery, we examined the effect of protamine sulfate, which has been shown to disrupt the urothelial barrier and allow intravesically administered agents to pass into the underlying tissue. Before protamine sulfate treatment, nicotine has an inhibitory effect, increasing the ICI. However, when administered after protamine sulfate treatment, nicotine has the opposite effect, exciting the bladder reflex and decreasing the ICI. This switch in nicotine’s effects following permeability of the urothelial barrier suggests that protamine sulfate allows nicotine to act on suburothelial targets such as afferent nerve terminals while nicotine infusion without protamine sulfate treatment activates receptors in the urothelium.
Additionally, we examined the effects of the nicotinic agonist epibatidine, which readily crosses the blood-brain barrier and has a greater affinity for α3β4 receptors than α7 receptors [EC50: 0.01–0.02 vs. 1.0–2.0 μM (14)]. Initially, intravesically administered epibatidine had an excitatory effect on the bladder. However, after 2 h of continuous infusion, voiding was blocked, leading to a state of urinary retention and overflow incontinence. The onset of overflow incontinence was much more rapid following a systemic dose of epibatidine, indicating that urinary retention is a result of effects of the drug outside the bladder, most likely on nicotinic receptors in the autonomic ganglia. We believe that the other nicotinic agents used in these experiments are acting primarily on urothelial receptors because of 1) the chemicals’ structures; which suggest a reduced or limited ability to diffuse across the urothelial barrier; 2) the ability to wash out the nicotine effect and return to normal, suggesting that the compound did not cross the urothelial barrier; 3) the reversal of nicotine’s effects following disruption of the urothelial barrier, allowing nicotine to penetrate to suburothelial afferent nerves; and 4) a lack of a biphasic response that would indicate an early action on urothelial receptors followed by diffusion through the barrier and subsequent action at other sites.
These data represent the first demonstration that stimulation of nicotinic receptors in the urothelium can alter bladder reflexes. Our research raises the possibility that alterations or dysfunctions in urothelial nicotinic receptors may contribute to the etiology of bladder diseases such as overactive bladder and these receptors may represent another target for the treatment of these disorders.
Acknowledgments
GRANTS
The work contained in this manuscript was funded by National Institutes of Health Grants RO1–54824 (to L. A. Birder), RO1-DK-064280 (to A. Kanai), and RO1-DK-49430 (to W. C. de Groat). The work contained in this manuscript was partially funded by a research grant from Roche Palo Alto to L. A. Birder.
References
- 1.Andersson KE. Bladder activation: afferent mechanisms. Urology. 2002;59:43–50. doi: 10.1016/s0090-4295(01)01637-5. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Yoshida M. Antimuscarinics and the overactive detrusor–which is the main mechanism of action? Eur Urol. 2003;43:1–5. doi: 10.1016/s0302-2838(02)00540-7. [DOI] [PubMed] [Google Scholar]
- 3.Beckel JM, Birder LA, Kiss S, Kanai AJ, Lee SJ, Yoshiyama M, de Groat WC. Expression of nicotinic acetylcholine receptors in the rat urothlieum (Abstract) Soc Neurosci Abstr. 2002;32:538.510. [Google Scholar]
- 4.Birder LA. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc West Pharmacol Soc. 2001;44:85–86. [PubMed] [Google Scholar]
- 5.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA, Kanai AJ, de Groat WC, Kiss S, Nealen ML, Burke NE, Dineley KE, Watkins S, Reynolds IJ, Caterina MJ. Vanilloid receptor expression suggests a sensory role for urinary bladder epithelial cells. Proc Natl Acad Sci USA. 2001;98:13396–13401. doi: 10.1073/pnas.231243698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. β-Adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conti-Fine BM, Navaneetham D, Lei S, Maus AD. Neuronal nicotinic receptors in non-neuronal cells: new mediators of tobacco toxicity? Eur J Pharmacol. 2000;393:279–294. doi: 10.1016/s0014-2999(00)00036-4. [DOI] [PubMed] [Google Scholar]
- 9.Cuevas J, Roth AL, Berg DK. Two distinct classes of functional 7-containing nicotinic receptor on rat superior cervical ganglion neurons. J Physiol. 2000;525:735–746. doi: 10.1111/j.1469-7793.2000.t01-1-00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Biasi M, Nigro F, Xu W. Nicotinic acetylcholine receptors in the autonomic control of bladder function. Eur J Pharmacol. 2000;393:137–140. doi: 10.1016/s0014-2999(00)00008-x. [DOI] [PubMed] [Google Scholar]
- 11.De Groat WC, Yoshimura N. Pharmacology of the lower urinary tract. Annu Rev Pharmacol Toxicol. 2001;41:691–721. doi: 10.1146/annurev.pharmtox.41.1.691. [DOI] [PubMed] [Google Scholar]
- 12.Fowler CJ. Bladder afferents and their role in the overactive bladder. Urology. 2002;59:37–42. doi: 10.1016/s0090-4295(02)01544-3. [DOI] [PubMed] [Google Scholar]
- 13.Genzen JR, Van Cleve W, McGehee DS. Dorsal root ganglion neurons express multiple nicotinic acetylcholine receptor subtypes. J Neurophysiol. 2001;86:1773–1782. doi: 10.1152/jn.2001.86.4.1773. [DOI] [PubMed] [Google Scholar]
- 14.Gerzanich V, Peng X, Wang F, Wells G, Anand R, Fletcher S, Lindstrom J. Comparative pharmacology of epibatidine: a potent agonist for neuronal nicotinic acetylcholine receptors. Mol Pharmacol. 1995;48:774–782. [PubMed] [Google Scholar]
- 15.Gerzanich V, Wang F, Kuryatov A, Lindstrom J. α5 Subunit alters desensitization, pharmacology, Ca2+ permeability and Ca2+ modulation of human neuronal α3 nicotinic receptors. J Pharmacol Exp Ther. 1998;286:311–320. [PubMed] [Google Scholar]
- 16.Grando SA, Horton RM, Pereira EF, Diethelm-Okita BM, George PM, Albuquerque EX, Conti-Fine BM. A nicotinic acetylcholine receptor regulating cell adhesion and motility is expressed in human keratinocytes. J Invest Dermatol. 1995;105:774–781. doi: 10.1111/1523-1747.ep12325606. [DOI] [PubMed] [Google Scholar]
- 17.Groot-Kormelink PJ, Luyten WH, Colquhoun D, Sivilotti LG. A reporter mutation approach shows incorporation of the “orphan” subunit β3 into a functional nicotinic receptor. J Biol Chem. 1998;273:15317–15320. doi: 10.1074/jbc.273.25.15317. [DOI] [PubMed] [Google Scholar]
- 18.Haberberger RV, Henrich M, Lips KS, Kummer W. Nicotinic receptor α7-subunits are coupled to the stimulation of nitric oxide synthase in rat dorsal root ganglion neurons. Histochem Cell Biol. 2003;120:173–181. doi: 10.1007/s00418-003-0550-3. [DOI] [PubMed] [Google Scholar]
- 19.Hawthorn MH, Chapple CR, Cock M, Chess-Williams R. Urothelium-derived inhibitory factor(s) influences on detrusor muscle contractility in vitro. Br J Pharmacol. 2000;129:416–419. doi: 10.1038/sj.bjp.0703068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishiura Y, Yoshiyama M, Yokoyama O, Namiki M, de Groat WC. Central muscarinic mechanisms regulating voiding in rats. J Pharmacol Exp Ther. 2001;297:933–939. [PubMed] [Google Scholar]
- 22.Itier V, Bertrand D. Neuronal nicotinic receptors: from protein structure to function. FEBS Lett. 2001;504:118–125. doi: 10.1016/s0014-5793(01)02702-8. [DOI] [PubMed] [Google Scholar]
- 23.Karlin A. Emerging structure of the nicotinic acetylcholine receptors. Nat Rev Neurosci. 2002;3:102–114. doi: 10.1038/nrn731. [DOI] [PubMed] [Google Scholar]
- 24.Klapproth H, Reinheimer T, Metzen J, Munch M, Bittinger F, Kirkpatrick CJ, Hohle KD, Schemann M, Racke K, Wessler I. Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol. 1997;355:515–523. doi: 10.1007/pl00004977. [DOI] [PubMed] [Google Scholar]
- 25.Lavelle J, Meyers S, Ramage R, Doty D, Bastacky S, Apodaca G, Zeidel M. Protamine sulfate-induced cystitis: a model of selective cytodestruction of the urothelium. Urology. 2001;57:113. doi: 10.1016/s0090-4295(01)01047-0. [DOI] [PubMed] [Google Scholar]
- 26.Lee SJ, Nakamura Y, de Groat WC. Effect of (+/−)-epibatidine, a nicotinic agonist, on the central pathways controlling voiding function in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;285:R84–R90. doi: 10.1152/ajpregu.00109.2003. [DOI] [PubMed] [Google Scholar]
- 27.Lester RA. Activation and desensitization of heteromeric neuronal nicotinic receptors: implications for non-synaptic transmission. Bioorg Med Chem. 2004;14:1897–1900. doi: 10.1016/j.bmcl.2004.02.081. [DOI] [PubMed] [Google Scholar]
- 28.Lester RA, Dani JA. Acetylcholine receptor desensitization induced by nicotine in rat medial habenula neurons. J Neurophysiol. 1995;74:195–206. doi: 10.1152/jn.1995.74.1.195. [DOI] [PubMed] [Google Scholar]
- 29.Lindstrom J, Anand R, Gerzanich V, Peng X, Wang F, Wells G. Structure and function of neuronal nicotinic acetylcholine receptors. Prog Brain Res. 1996;109:125–137. doi: 10.1016/s0079-6123(08)62094-4. [DOI] [PubMed] [Google Scholar]
- 30.Mamalaki A, Tzartos SJ. Nicotinic acetylcholine receptor: structure, function and main immunogenic region. Adv Neurol. 1994;4:339–354. doi: 10.1016/0960-5428(94)00032-j. [DOI] [PubMed] [Google Scholar]
- 31.McIntosh JM, Gardner S, Luo S, Garrett JE, Yoshikami D. Conus peptides: novel probes for nicotinic acetylcholine receptor structure and function. Eur J Pharmacol. 2000;393:205–208. doi: 10.1016/s0014-2999(99)00887-0. [DOI] [PubMed] [Google Scholar]
- 32.Meyer EL, Xiao Y, Kellar KJ. Agonist regulation of rat α3β4 nicotinic acetylcholine receptors stably expressed in human embryonic kidney 293 cells. Mol Pharmacol. 2001;60:568–576. [PubMed] [Google Scholar]
- 33.Meyer MD, Decker MW, Rueter LE, Anderson DJ, Dart MJ, Kim KH, Sullivan JP, Williams M. The identification of novel structural compound classes exhibiting high affinity for neuronal nicotinic acetylcholine receptors and analgesic efficacy in preclinical models of pain. Eur J Pharmacol. 2000;393:171–177. doi: 10.1016/s0014-2999(00)00041-8. [DOI] [PubMed] [Google Scholar]
- 34.Nakamura Y, Ishiura Y, Yokoyama O, Namiki M, de Groat WC. Role of protein kinase C in central muscarinic inhibitory mechanisms regulating voiding in rats. Neuroscience. 2003;116:477–484. doi: 10.1016/s0306-4522(02)00658-9. [DOI] [PubMed] [Google Scholar]
- 35.Nelson ME, Lindstrom J. Single channel properties of human alpha3 AChRs: impact of β2, β4 and α5 subunits. J Physiol. 1999;516:657–678. doi: 10.1111/j.1469-7793.1999.0657u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol. 1999;162:2211–2216. doi: 10.1016/S0022-5347(05)68161-X. [DOI] [PubMed] [Google Scholar]
- 37.Palma E, Maggi L, Barabino B, Eusebi F, Ballivet M. Nicotinic acetylcholine receptors assembled from the α7 and β3 subunits. J Biol Chem. 1999;274:18335–18340. doi: 10.1074/jbc.274.26.18335. [DOI] [PubMed] [Google Scholar]
- 38.Papke RL, Porter Papke JK. Comparative pharmacology of rat and human α7 nAChR conducted with net charge analysis. Br J Pharmacol. 2002;137:49–61. doi: 10.1038/sj.bjp.0704833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sastry BV. Human placental cholinergic system. Biochem Pharmacol. 1997;53:1577–1586. doi: 10.1016/s0006-2952(97)00017-8. [DOI] [PubMed] [Google Scholar]
- 40.Sun Y, Keay S, DeDeyne P, Chai T. Stretch-activated release of adenosine triphosphate by bladder uroepithelia is augmented in interstitial cystitis. Urology. 2001;57:131. doi: 10.1016/s0090-4295(01)01106-2. [DOI] [PubMed] [Google Scholar]
- 41.Templeman L, Chapple CR, Chess-Williams R. Urothelium derived inhibitory factor and cross-talk among receptors in the trigone of the bladder of the pig. J Urol. 2002;167:742–745. doi: 10.1016/S0022-5347(01)69137-7. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida M, Miyamae K, Iwashita H, Otani M, Inadome A. Management of detrusor dysfunction in the elderly: changes in acetylcholine and adenosine triphosphate release during aging. Urology. 2004;63:17–23. doi: 10.1016/j.urology.2003.11.003. [DOI] [PubMed] [Google Scholar]