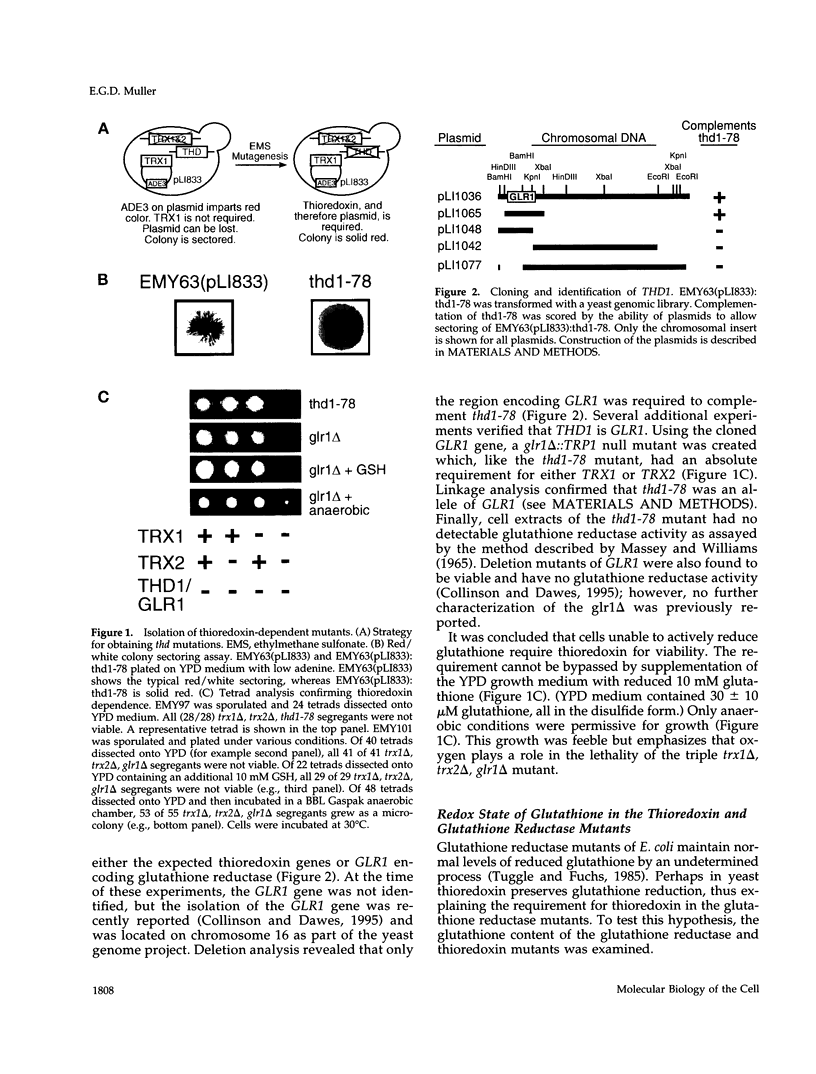
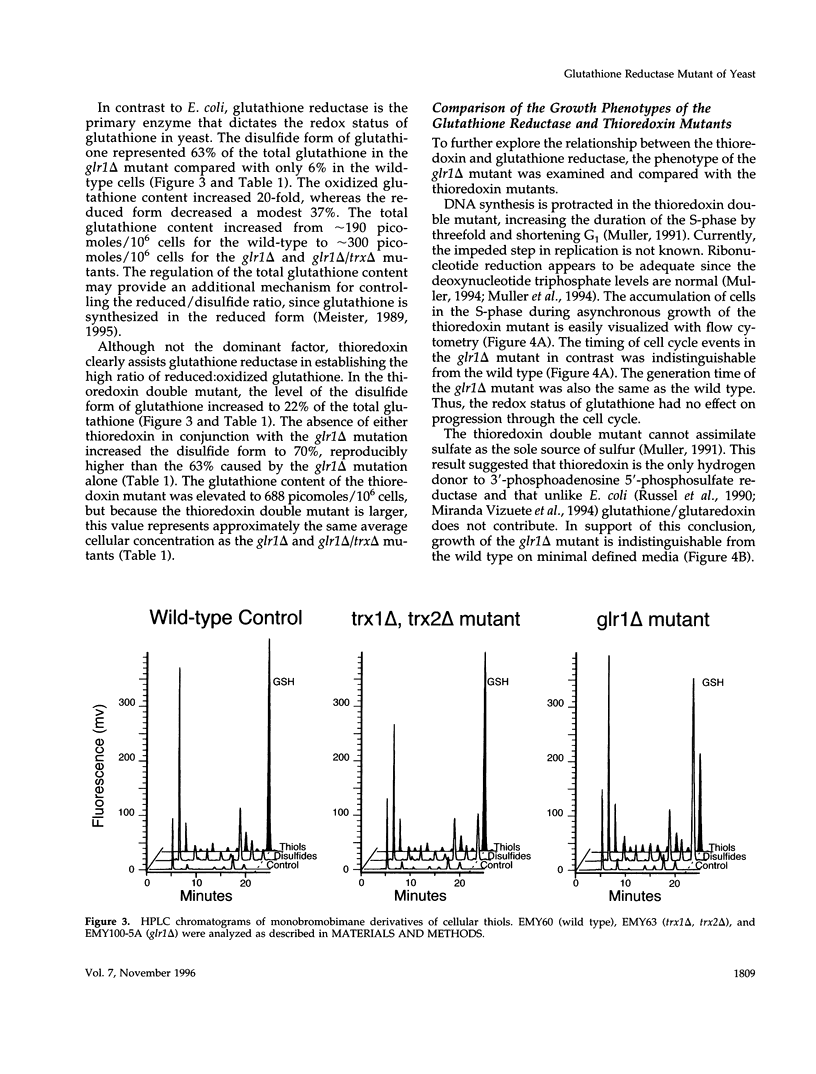
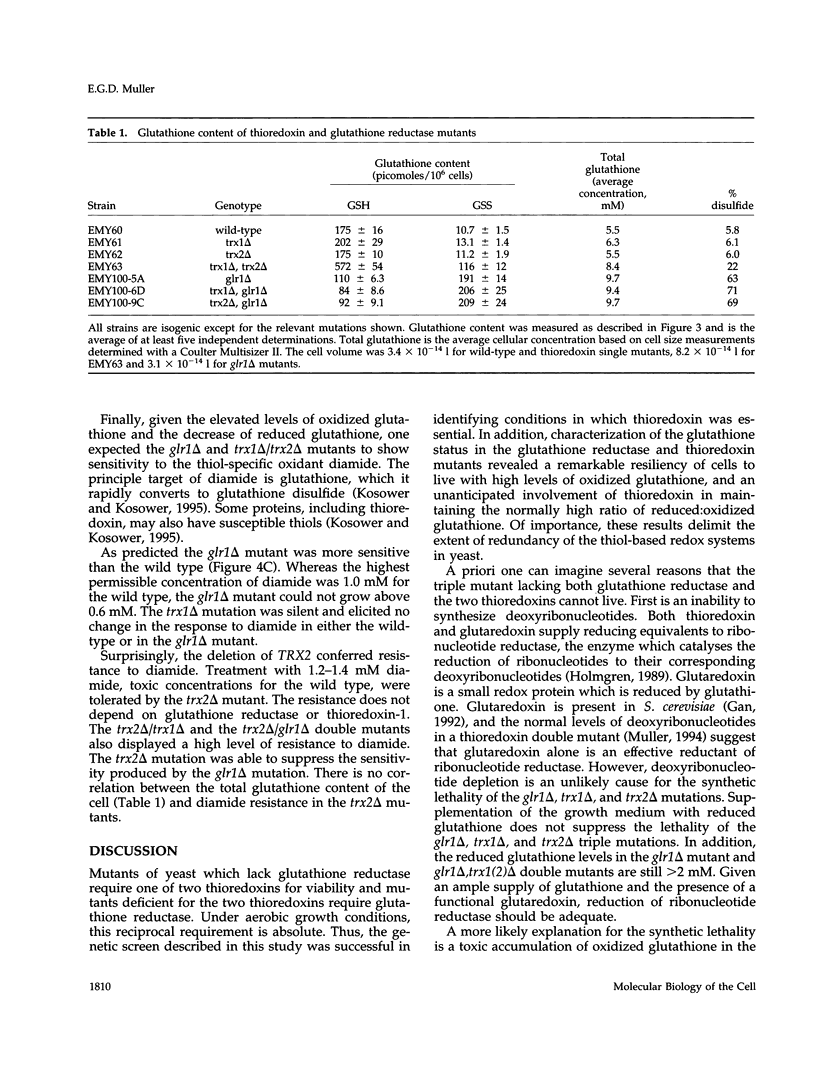
Abstract
A glutathione reductase null mutant of Saccharomyces cerevisiae was isolated in a synthetic lethal genetic screen for mutations which confer a requirement for thioredoxin. Yeast mutants that lack glutathione reductase (glr1 delta) accumulate high levels of oxidized glutathione and have a twofold increase in total glutathione. The disulfide form of glutathione increases 200-fold and represents 63% of the total glutathione in a glr1 delta mutant compared with only 6% in wild type. High levels of oxidized glutathione are also observed in a trx1 delta, trx2 delta double mutant (22% of total), in a glr1 delta, trx1 delta double mutant (71% of total), and in a glr1 delta, trx2 delta double mutant (69% of total). Despite the exceptionally high ratio of oxidized/reduced glutathione, the glr1 delta mutant grows with a normal cell cycle. However, either one of the two thioredoxins is essential for growth. Cells lacking both thioredoxins and glutathione reductase are not viable under aerobic conditions and grow poorly anaerobically. In addition, the glr1 delta mutant shows increased sensitivity to the thiol oxidant diamide. The sensitivity to diamide was suppressed by deletion of the TRX2 gene. The genetic analysis of thioredoxin and glutathione reductase in yeast runs counter to previous studies in Escherichia coli and for the first time links thioredoxin with the redox state of glutathione in vivo.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. D., Jr, Wang B., Klaidman L. K., LeBel C. P., Odunze I. N., Shah D. New aspects of brain oxidative stress induced by tert-butylhydroperoxide. Free Radic Biol Med. 1993 Aug;15(2):195–202. doi: 10.1016/0891-5849(93)90059-4. [DOI] [PubMed] [Google Scholar]
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Aslund F., Ehn B., Miranda-Vizuete A., Pueyo C., Holmgren A. Two additional glutaredoxins exist in Escherichia coli: glutaredoxin 3 is a hydrogen donor for ribonucleotide reductase in a thioredoxin/glutaredoxin 1 double mutant. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):9813–9817. doi: 10.1073/pnas.91.21.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukrust P., Svardal A. M., Müller F., Lunden B., Berge R. K., Ueland P. M., Frøland S. S. Increased levels of oxidized glutathione in CD4+ lymphocytes associated with disturbed intracellular redox balance in human immunodeficiency virus type 1 infection. Blood. 1995 Jul 1;86(1):258–267. [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Jacquot J. P. Thioredoxin and metabolic regulation. Semin Cell Biol. 1994 Oct;5(5):285–293. doi: 10.1006/scel.1994.1035. [DOI] [PubMed] [Google Scholar]
- Chae H. Z., Chung S. J., Rhee S. G. Thioredoxin-dependent peroxide reductase from yeast. J Biol Chem. 1994 Nov 4;269(44):27670–27678. [PubMed] [Google Scholar]
- Collinson L. P., Dawes I. W. Isolation, characterization and overexpression of the yeast gene, GLR1, encoding glutathione reductase. Gene. 1995 Apr 14;156(1):123–127. doi: 10.1016/0378-1119(95)00026-3. [DOI] [PubMed] [Google Scholar]
- Davis T. N. Mutational analysis of calmodulin in Saccharomyces cerevisiae. Cell Calcium. 1992 Jun-Jul;13(6-7):435–444. doi: 10.1016/0143-4160(92)90056-x. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Regulation of cellular glutathione. Am J Physiol. 1989 Oct;257(4 Pt 1):L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Derman A. I., Prinz W. A., Belin D., Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993 Dec 10;262(5140):1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- Fahey R. C., Newton G. L. Determination of low-molecular-weight thiols using monobromobimane fluorescent labeling and high-performance liquid chromatography. Methods Enzymol. 1987;143:85–96. doi: 10.1016/0076-6879(87)43016-4. [DOI] [PubMed] [Google Scholar]
- Fuchs J. A., Warner H. R. Isolation of an Escherichia coli mutant deficient in glutathione synthesis. J Bacteriol. 1975 Oct;124(1):140–148. doi: 10.1128/jb.124.1.140-148.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiazzo F., Schiesser A., Rotilio G. Glutathione peroxidase in yeast. Presence of the enzyme and induction by oxidative conditions. Biochem Biophys Res Commun. 1987 Sep 30;147(3):1200–1205. doi: 10.1016/s0006-291x(87)80197-3. [DOI] [PubMed] [Google Scholar]
- Gan Z. R. Cloning and sequencing of a gene encoding yeast thioltransferase. Biochem Biophys Res Commun. 1992 Sep 16;187(2):949–955. doi: 10.1016/0006-291x(92)91289-3. [DOI] [PubMed] [Google Scholar]
- Geiser J. R., Sundberg H. A., Chang B. H., Muller E. G., Davis T. N. The essential mitotic target of calmodulin is the 110-kilodalton component of the spindle pole body in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Dec;13(12):7913–7924. doi: 10.1128/mcb.13.12.7913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert H. F. Thiol/disulfide exchange equilibria and disulfide bond stability. Methods Enzymol. 1995;251:8–28. doi: 10.1016/0076-6879(95)51107-5. [DOI] [PubMed] [Google Scholar]
- Gounalaki N., Thireos G. Yap1p, a yeast transcriptional activator that mediates multidrug resistance, regulates the metabolic stress response. EMBO J. 1994 Sep 1;13(17):4036–4041. doi: 10.1002/j.1460-2075.1994.tb06720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B. Free radicals and antioxidants: a personal view. Nutr Rev. 1994 Aug;52(8 Pt 1):253–265. doi: 10.1111/j.1753-4887.1994.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Holmgren A. Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem. 1979 Sep 25;254(18):9113–9119. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin and glutaredoxin systems. J Biol Chem. 1989 Aug 25;264(24):13963–13966. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin. Annu Rev Biochem. 1985;54:237–271. doi: 10.1146/annurev.bi.54.070185.001321. [DOI] [PubMed] [Google Scholar]
- Hussain M., Lenard J. Characterization of PDR4, a Saccharomyces cerevisiae gene that confers pleiotropic drug resistance in high-copy number: identity with YAP1, encoding a transcriptional activator [corrected]. Gene. 1991 May 15;101(1):149–152. doi: 10.1016/0378-1119(91)90238-7. [DOI] [PubMed] [Google Scholar]
- Hwang C., Sinskey A. J., Lodish H. F. Oxidized redox state of glutathione in the endoplasmic reticulum. Science. 1992 Sep 11;257(5076):1496–1502. doi: 10.1126/science.1523409. [DOI] [PubMed] [Google Scholar]
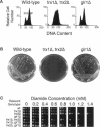
- Kosower N. S., Kosower E. M. Diamide: an oxidant probe for thiols. Methods Enzymol. 1995;251:123–133. doi: 10.1016/0076-6879(95)51116-4. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Pfeifer U., Machnik G., Klinger W. Glutathione homeostasis and turnover in the totally hepatectomized rat: evidence for a high glutathione export capacity of extrahepatic tissues. Exp Toxicol Pathol. 1992 Sep;44(5):273–281. doi: 10.1016/S0940-2993(11)80244-7. [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J. 1994 Feb 1;13(3):655–664. doi: 10.1002/j.1460-2075.1994.tb06304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundström-Ljung J., Holmgren A. Glutaredoxin accelerates glutathione-dependent folding of reduced ribonuclease A together with protein disulfide-isomerase. J Biol Chem. 1995 Apr 7;270(14):7822–7828. doi: 10.1074/jbc.270.14.7822. [DOI] [PubMed] [Google Scholar]
- Lundström J., Krause G., Holmgren A. A Pro to His mutation in active site of thioredoxin increases its disulfide-isomerase activity 10-fold. New refolding systems for reduced or randomly oxidized ribonuclease. J Biol Chem. 1992 May 5;267(13):9047–9052. [PubMed] [Google Scholar]
- Lyles M. M., Gilbert H. F. Catalysis of the oxidative folding of ribonuclease A by protein disulfide isomerase: dependence of the rate on the composition of the redox buffer. Biochemistry. 1991 Jan 22;30(3):613–619. doi: 10.1021/bi00217a004. [DOI] [PubMed] [Google Scholar]
- Massey V., Williams C. H., Jr On the reaction mechanism of yeast glutathione reductase. J Biol Chem. 1965 Nov;240(11):4470–4480. [PubMed] [Google Scholar]
- Meister A. Glutathione metabolism. Methods Enzymol. 1995;251:3–7. doi: 10.1016/0076-6879(95)51106-7. [DOI] [PubMed] [Google Scholar]
- Michiels C., Raes M., Toussaint O., Remacle J. Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med. 1994 Sep;17(3):235–248. doi: 10.1016/0891-5849(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Miranda-Vizuete A., Martinez-Galisteo E., Aslund F., Lopez-Barea J., Pueyo C., Holmgren A. Null thioredoxin and glutaredoxin Escherichia coli K-12 mutants have no enhanced sensitivity to mutagens due to a new GSH-dependent hydrogen donor and high increases in ribonucleotide reductase activity. J Biol Chem. 1994 Jun 17;269(24):16631–16637. [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D., Parker C. S. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev. 1989 Mar;3(3):283–292. doi: 10.1101/gad.3.3.283. [DOI] [PubMed] [Google Scholar]
- Muller E. G. A redox-dependent function of thioredoxin is necessary to sustain a rapid rate of DNA synthesis in yeast. Arch Biochem Biophys. 1995 Apr 20;318(2):356–361. doi: 10.1006/abbi.1995.1240. [DOI] [PubMed] [Google Scholar]
- Muller E. G. Deoxyribonucleotides are maintained at normal levels in a yeast thioredoxin mutant defective in DNA synthesis. J Biol Chem. 1994 Sep 30;269(39):24466–24471. [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin deficiency in yeast prolongs S phase and shortens the G1 interval of the cell cycle. J Biol Chem. 1991 May 15;266(14):9194–9202. [PubMed] [Google Scholar]
- Muller E. G. Thioredoxin genes in Saccharomyces cerevisiae: map positions of TRX1 and TRX2. Yeast. 1992 Feb;8(2):117–120. doi: 10.1002/yea.320080206. [DOI] [PubMed] [Google Scholar]
- Müller S., Senn H., Gsell B., Vetter W., Baron C., Böck A. The formation of diselenide bridges in proteins by incorporation of selenocysteine residues: biosynthesis and characterization of (Se)2-thioredoxin. Biochemistry. 1994 Mar 22;33(11):3404–3412. doi: 10.1021/bi00177a034. [DOI] [PubMed] [Google Scholar]
- Newton G. L., Aguilera J. A., Fahey R. C., Ward J. F., Radkowsky A. E., Kosower E. M. para-sulfobenzoyloxybromobimane: a new membrane-impermeable reagent useful for the analysis of thiols and their export from cells. Anal Biochem. 1992 Feb 14;201(1):30–42. doi: 10.1016/0003-2697(92)90170-c. [DOI] [PubMed] [Google Scholar]
- Nicotera P., Moore M., Bellomo G., Mirabelli F., Orrenius S. Demonstration and partial characterization of glutathione disulfide-stimulated ATPase activity in the plasma membrane fraction from rat hepatocytes. J Biol Chem. 1985 Feb 25;260(4):1999–2002. [PubMed] [Google Scholar]
- Perham R. N. Glutathione reductase from Escherichia coli: mutation, cloning and sequence analysis of the gene. Biochem Soc Trans. 1987 Aug;15(4):730–733. doi: 10.1042/bst0150730. [DOI] [PubMed] [Google Scholar]
- Russel M., Holmgren A. Construction and characterization of glutaredoxin-negative mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1988 Feb;85(4):990–994. doi: 10.1073/pnas.85.4.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P. Direct cloning of the trxB gene that encodes thioredoxin reductase. J Bacteriol. 1985 Jul;163(1):238–242. doi: 10.1128/jb.163.1.238-242.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russel M., Model P., Holmgren A. Thioredoxin or glutaredoxin in Escherichia coli is essential for sulfate reduction but not for deoxyribonucleotide synthesis. J Bacteriol. 1990 Apr;172(4):1923–1929. doi: 10.1128/jb.172.4.1923-1929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk H., Klein M., Erdbrügger W., Dröge W., Schulze-Osthoff K. Distinct effects of thioredoxin and antioxidants on the activation of transcription factors NF-kappa B and AP-1. Proc Natl Acad Sci U S A. 1994 Mar 1;91(5):1672–1676. doi: 10.1073/pnas.91.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell N., Krems B., Entian K. D. The PAR1 (YAP1/SNQ3) gene of Saccharomyces cerevisiae, a c-jun homologue, is involved in oxygen metabolism. Curr Genet. 1992 Apr;21(4-5):269–273. doi: 10.1007/BF00351681. [DOI] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuggle C. K., Fuchs J. A. Glutathione reductase is not required for maintenance of reduced glutathione in Escherichia coli K-12. J Bacteriol. 1985 Apr;162(1):448–450. doi: 10.1128/jb.162.1.448-450.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis J. W., Chrebet G., Brodsky G., Rolfe M., Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989 Jul 28;58(2):409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- Wells W. W., Yang Y., Deits T. L., Gan Z. R. Thioltransferases. Adv Enzymol Relat Areas Mol Biol. 1993;66:149–201. doi: 10.1002/9780470123126.ch4. [DOI] [PubMed] [Google Scholar]
- Wu A. L., Moye-Rowley W. S. GSH1, which encodes gamma-glutamylcysteine synthetase, is a target gene for yAP-1 transcriptional regulation. Mol Cell Biol. 1994 Sep;14(9):5832–5839. doi: 10.1128/mcb.14.9.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]