Abstract
The control and regulation of the lower urinary tract (LUT) is partly mediated by purinergic signaling. This study investigated the distribution and function of P2Y receptors in the rat urinary bladder. Application of P2Y agonists to rat urothelial cells evoked increases in intracellular calcium; the rank order of agonist potency (pEC50 ± S.E.M.) was ATP (5.10 ± 0.07)>UTP (4.91 ± 0.14)>UTPγS (4.61 ± 0.16) = ATPγS (4.70 ± 0.05) > 2MeSADP = NECA = ADP (<3.5). The rank order potency for these agonists indicates that urothelial cells functionally express P2Y2/P2Y4 receptors, with a relative lack of contribution from other P2Y or adenosine receptors. Real-time PCR, western blotting and immunocytochemistry, confirmed the expression of P2Y2, and to a lesser extent P2Y4 in the urothelium. Immunocytochemical studies revealed expression of P2Y2 staining in all layers of the urothelium, with relative absence of P2Y4. P2Y2 staining was also present in sub-urothelial nerve bundles and underlying detrusor smooth muscle. Addition of UTP and UTPγS was found to evoke ATP release from cultured rat urothelial cells. These findings indicate that cultured rat urothelial cells functionally express P2Y2/P2Y4 receptors. Activation of these receptors could have a role in autocrine and paracrine signaling throughout the urothelium. This could lead to the release of bioactive mediators such as additional ATP, nitric oxide and acetylcholine, which can modulate the micturition reflex by acting on sub-urothelial myofibroblasts and/or pelvic afferent fibers.
Keywords: Purinergic receptors, urinary bladder, epithelium, lower urinary tract
Introduction
The control and regulation of lower urinary tract (LUT) functions are regulated by the complex integration of sympathetic, parasympathetic and afferent pathways (18). These highly regulated processes are mediated by neural controls involving many neurotransmitters including acetylcholine, amino acids, nitric oxide, neuropeptides and monoamines, as well as ATP acting on purinergic receptors (18). Kasakov and Burnstock (1982) initially demonstrated that parasympathetic neural contractions of the bladder were in part mediated by non-adrenergic-non-cholinergic (NANC) atropine resistant purinergic transmission. Purinergic transmission is also involved in transducing bladder mechanosensation and other forms of afferent information to the CNS (17, 18, 22). For example, intravesical administration of ATP or α,β-methylene ATP into the bladder evokes bladder hyperactivity, an effect that is blocked with selective purinergic receptor antagonists (34, 40, 49).
P2 purinergic and pyrimidinergic receptors can be divided into two major families, ionotropic ligand-gated P2X and metabotropic G-protein coupled P2Y receptors. To date, seven P2X receptors have been identified (P2X1-7) and eight P2Y receptors have been recognized as molecularly distinct proteins which can produce functional responses (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14). Urinary bladders of a number of species, such as human (35), rat (30) and cat (9) are known to express purinergic receptors including P2X1 on detrusor smooth muscle (30, 48) and P2X3 on sub-urothelial nerve plexi and urothelium (9, 16, 30).
As with many hollow organs and sacs, distention or mechanical stretch evokes the release of ATP from the urothelium lining the urinary bladder (21, 44, 49). Urothelial ATP release in response to distention/mechanical stimulation occurs from both mucosal and serosal compartments (31). Urothelial-released ATP is thought to activate P2X3 receptors expressed on sub-urothelial nerves in a paracrine manner, which convey afferent information to the CNS, leading to altered micturition reflexes. Indeed, P2X3 deficient mice exhibit normal distention-evoked urothelial ATP release, but marked urinary bladder hyporeflexia, characterized by decreased voiding frequency and increased bladder capacity (16, 49). The ability of the urothelium to sense mechanical distention and convey information to afferent nerves supports the notion that the urothelium plays an important sensory role in the urinary bladder (6, 10, 11, 18, 29, 50).
The pyrimidine nucleotide, UTP and dinucleotides, ADP and UDP bind to the P2Y family of metabotropic heptahelical G-protein coupled receptors (GPCRs). Birder et al (2004) reported the constitutive expression of P2Y1, P2Y2, and P2Y4 in feline urothelium, and reduction of P2Y2 in a naturally occurring model of feline interstitial cystitis (FIC), suggesting that P2Y receptors may play a role in urothelial function. P2Y6 receptors have also been reported to be expressed on the guinea-pig urothelium (43). Relatively little however, is known about the distribution and function of P2Y receptors in the rat bladder. This study investigated the expression of P2Y receptors on the rat urothelium.
Methods
All procedures were approved by the University of Pittsburgh Animal Care and Use Committee.
Rat urothelial cell culture
Preparation of rat urothelial cultures have been previously described (5, 6, 47). In brief, urinary bladders were rapidly excised from euthanized Sprague-Dawley rats, gently stretched (urothelial side up) and incubated overnight in DMEM containing penicillin/streptomycin/fungizone and dispase (2.5 mg ml-1, Invitrogen, Carlsbad, CA). The urothelium was then gently scraped, treated with trypsin-EDTA (0.25 %, Invitrogen, Carlsbad, CA) and following gentle pipetting, resuspended in serum-free keratinocyte medium (Invitrogen, Carlsbad, CA). The cell suspension was plated onto either collagen-coated black walled 96-well FLIPR plates (30,000 cells/well) or onto collagen-coated glass coverslips (50,000 cells/coverslip) and incubated in a humidified atmosphere of 5 % CO2 at 37 °C. The majority of cultured urothelial cells were cytokeratin 17 positive (Dako, Carpentaria, CA) and regarded as from epithelial origin, as previously reported (7).
Intracellular calcium imaging methods
Fluorometric Imaging Plate Reader (FLIPR)
Urothelial cells plated onto collagen-coated black walled 96-well FLIPR plates were grown to 90 % confluence and washed in FLIPR buffer, composed of Ca2+/Mg2+ free HBSS supplemented with HEPES (10 mM), CaCl2 (2 mM) and probenecid (2.5 mM). Fluo-3 (2 μM; Molecular Probes, Eugene, OR) in FLIPR buffer (final volume = 200 μl) was added to each well, incubated for 1 h at 37 °C and cells washed 4 times with FLIPR buffer. Purinergic receptor agonists were diluted and added to additional assay plates (agonist plates) at concentrations twice those needed to construct E/[A] curves, ranging from 100 nM to 1 mM final. In studies in which antagonist profiles were studied, urothelial cells were pre-treated for at least 10 min prior to agonist application. The agonist, antagonist and FLIPR cell plates were placed in the FLIPR incubation chamber. A baseline fluorescence measurement (excitation wavelength 488 nm; emission wavelength 530 nm) was obtained, and reactions started with the addition to FLIPR cell plates of 100 μl/well from the agonist plates. Fluorescence was measured for 3-5 min at 1-5 s intervals, with readings taken until a plateau phase was reached. Ionomycin (5 μM) was added at the end of each experiment to determine cell viability and maximum fluorescence of dye-bound cytosolic calcium.
Fura-2
Cultured rat urothelial cells (18-72 h following plating) were incubated with the fluorescent Ca2+ indicator, fura-2-acetoxymethyl (AM) (5 μM, Molecular Probes, Eugene, OR) in HBSS containing bovine serum albumin (5 mg ml-1) for 30 min at 37 °C in an atmosphere containing 5 % CO2. Cells were washed in HBSS (containing in mM; NaCl 138, KCl 5, KH2PO4 0.3, NaHCO3 4, CaCl2 2, MgCl2 1, HEPES 10, Glucose 5.6, pH=7.35, titrated with NaOH, 310 mosm l-1), transferred to a perfusion chamber and mounted onto an epifluorescence microscope (Olympus IX70). In Ca2+-free HBSS, the Ca2+ was substituted with additional NaCl (2 mM) and EGTA (0.5 mM). Measurement of [Ca2+]i was performed by ratiometric imaging of fura-2 at 340 and 380 nm (100 Hz) and the emitted light monitored at 510 nm. The fluorescence ratio (F340/F380) was calculated and acquired by C-Imaging systems (Compix Inc., Cranberry, PA) and background fluorescence subtracted. All test agents were bath applied (flow rate=1.5ml min-1). Thapsigargin (SigmaAldrich, St. Louis, MO), and U73122 (Jena Bioscience, Jena, Germany) was pre-incubated with urothelial cells for at least 10 min prior to agonist application. Data were obtained from at least 3 independent urothelial cultures and from at least 4 sets of experiments from each culture. Data were analyzed using Student's unpaired t-test and expressed as a mean percentage of the maximum response ± S.E.M to ionomycin (5 μM).
RNA extraction and quantitative real-time-PCR
Sprague-Dawley rats were euthanized by inhalation of medical grade CO2 followed by thoracotomy and cardiac puncture, and urinary bladders excised. Bladders were cut open longitudinally and pinned urothelial side up in sylgard coated dishes and covered with oxygenated Krebs solution. The urothelium was then gently teased away from the underlying tissue using fine forceps and scissors under a dissecting microscope. The urothelium and remaining smooth muscle tissue were placed separately into Trizol (Invitrogen, Carlsbad, CA). RNA was extracted according to manufacturer's guidelines and contaminating genomic DNA was removed using Turbo DNA-free (Ambion, Austin, TX). First strand synthesis was performed using Omniscript RT kit (Qiagen, Valencia, CA), using 1μg of RNA and random hexamer primers. Quantitative PCR was performed using iQ SYBR Green Supermix Kit (Bio-Rad, Hercules, CA) using an iCycler thermal cycler with the MyiQ optical attachment. The primers used were as follows (100 nM, each): P2Y2: (left) AGCTCTGTCATGCTGGGTCT, (right) GTAATAGAGGGTGCGGGTGA; P2Y4: (left) GCAAGTTTGTCCGCTTTCTC, (right) AGGCAGCCAGCTACTACCAA; β-actin: (left) ATGGTGGGTATGGGTCAGAA, (right) GCTGTGGTGGTGAAGCTGTA. The experimental protocol was 95°C for 3 min followed by 40 cycles of 95°C for 15 s and 60°C for 60 s. For each sample, serial dilutions of 1μg cDNA (1/10) were used to generate a standard curve, and run in triplicate. Results are expressed as a ratio of the threshold cycle (CT) of each receptor to the CT of β-actin.
Western blotting
Sprague-Dawley rats were euthanized by inhalation of medical grade CO2 followed by thoracotomy and cardiac puncture, and urinary bladders excised. Bladders were cut open longitudinally and pinned urothelial side up in sylgard coated dishes and covered with oxygenated Krebs solution containing protease inhibitor cocktail (Roche, Indianapolis, USA). The urothelium was then gently teased away from the underlying tissue using fine forceps and scissors under a dissecting microscope. Thereafter, urothelial, underlying smooth muscle and whole bladder tissues were cut into smaller pieces using dissecting scissors. Tissue samples were then placed into a lysis buffer containing tris-HCl (125mM pH 7.4), glycerol (20% v/v), sodium dodecyl sulphate (2% w/v), sodium fluoride (50 mM), sodium orthovanadate (2mM), tetra-sodium pyrophosphate (30mM), dithiothreitol (0.2% v/v) and protease inhibitor cocktail (Roche, Indianapolis, USA). Protein lysates were homogenized and sonicated prior to centrifugation at 4,500 rpm for 30 min at 4°C. Protein concentrations were determined by the Coomassie Plus Protein assay (Pierce, supplied by Fisher Scientific, Pittsburgh, USA). Whole rat brain cell lysate (5μg/lane; Abcam, Cambridge, MA) was used as a positive control for antibody binding. Cell extracts were resolved electrophoretically on NuPage® 4-12% bis-tris acrylamide gels using 3-(n-morpholino)-propanesulphonic acid (MOPS) buffer (Invitrogen, Carlsbad, CA) and transferred electrophoretically onto 0.45μm polyvinylidene fluoride (PVDF) membrane (GE Healthcare, Piscataway, NJ) in 25mM Tris base containing 192mM glycine at 4°C, 25V for 90 min. Membranes were probed with primary antibodies overnight at 4°C, and bound antibody detected with either goat anti-rabbit or rabbit anti-mouse immunoglobulins conjugated to horseradish peroxidase (GE Heathcare, Piscataway, NJ). Immunolabelled proteins were analysed using chemiluminescence (ECL-plus detection kit, GE Healthcare, Piscataway, NJ).
Immunocytochemistry
Adult Sprague-Dawley rats (250-350 g) were euthanized by inhalation of medical grade CO2 followed by thoracotomy and cardiac puncture. Urinary bladders were excised, embedded in OCT Tissue-Tek (Sakura Finetek, Torrance, CA), rapidly frozen over liquid nitrogen and stored at -80 °C prior to use. Frozen urinary bladder sections (10 μm) were sectioned using a cryostat (Hacker-Bright Instruments, Fairfield, NJ), mounted onto microscope slides and air-dried. Tissue sections were then fixed using 4 % paraformaldehyde and washed in phosphate buffered saline (PBS). Tissue sections were placed in a tissue permeabilizing solution (0.5 % Triton X-100 and 10 % goat serum) and washed in PBS prior to incubation in primary antisera.
Tissue sections were incubated in rabbit-polyclonal anti-rat P2Y2 or P2Y4 receptor antibodies (5 μg. ml-1, 4 °C overnight, Alomone Labs, Jerusalem, Israel). Colocalization studies were also conducted with P2Y receptor antibodies and urothelial cell markers, cytokeratin 17 (basal cells) and cytokeratin 20 (apical cells) and the neuronal marker PGP 9.5. Mouse anti-human cytokeratin 17 (1:2000) and cytokeratin 20 (1:1000) were obtained from Dako Cytomation (Carpinteria, CA). Mouse monoclonal PGP 9.5 antibody (1:50) was obtained from Abcam (Cambridge, MA).
Primary antibodies were removed and tissue sections washed in PBS prior to incubation in fluorescein isothiocyanate (FITC)-conjugated anti-rabbit IgG and/or texas-red (TR)-conjugated anti-mouse IgG (1:500, Jackson ImmunoResearch, West Grove, PA) for 2 h at room temperature. For studies examining P2Y2 colocalization with PGP 9.5, Cy3-conjugated anti-rabbit IgG (1:500, Jackson ImmunoResearch) and FITC-conjugated anti-mouse IgG (1:500, Jackson ImmunoResearch) were used. Tissue sections were then washed in PBS and mounted with glass coverslips using a glycerol based aqueous antifade mountant, Citifluor (Ted Pella Inc., Redding, CA). Background immunofluorescence was assessed in the absence of primary antibodies and secondary only.
Measurement of ATP release
Cultured rat urothelial cells (18-72 h following plating onto glass coverslips) were transferred into a perfusion chamber and superfused with an oxygenated Krebs solution (containing in mM; KCl 4.8, NaCl 120, MgCl2 1, CaCl2 2, glucose 11 and HEPES 10, pH 7.4) at room-temperature (flow rate = 0.5ml min-1), until a stable baseline level of ATP release was measured; all test agents were bath applied. Perfusate was collected (100 μl) at 30 s intervals following agonist stimulation and ATP levels quantified using a luciferin-luciferase reagent and ATP concentrations extrapolated from a standard-curve (ATP assay, SigmaAldrich, St. Louis, MO). Only selected purinergic receptor agonists could be tested in this system to evaluate release of ATP from cultured urothelial cells. ATPγS, ADP, UDP, 2-meSADP, suramin and PPADS were all found to interfere with the luciferin-luceriferase based ATP assay mix and were not tested further. Data was obtained from at least 3 independent cultures and at least n = 3 from each culture. Data are expressed as mean ± S.E.M., analyzed using Student unpaired t-test and statistical significance accepted when P < 0.05.
Materials
All standard chemicals were obtained from Sigma-Aldrich or Fisher and were either analytical or laboratory grade.
Results
Intracellular calcium imaging
FLIPR
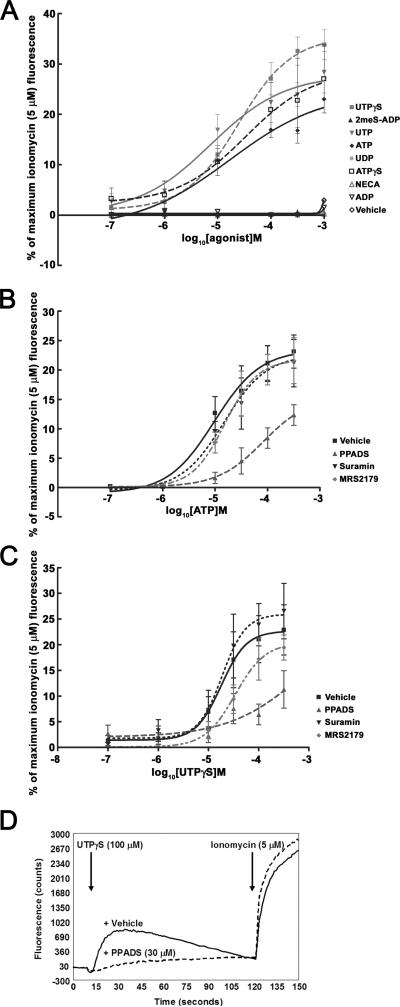
FLIPR analysis of cultured rat urothelial cells following stimulation with purinergic receptor agonists revealed that these agents evoke increases in intracellular calcium. These responses typically reached peak within 30 s and fully recovered to baseline levels between 2-3 min following application (Figure 1D). The rank order of agonist potency (pEC50 ± S.E.M.) was ATP (5.10 ± 0.07) ≥ UTP (4.91 ± 0.14) > UTPγS (4.61 ± 0.16) = ATPγS (4.70 ± 0.05) >> 2MeSADP = NECA = ADP = UDP (<3.5; see Figure 1A). Curve shift analysis with a number of P2 receptor antagonists, revealed that suramin (30 μM) and the selective P2Y1 receptor antagonist, MRS2179 (30 μM) had little or no effect on either UTP- or UTPγS-evoked increases in intracellular calcium (Figure 1B, C). In contrast, PPADS (30 μM) produced a rightward shift in UTP- and UTPγS-evoked changes in intracellular calcium (Figure 1B-D). Furthermore, PPADS (30 μM) produced an inhibition of cytosolic calcium increases evoked by UTP (30 μM) and/or UTPγS (30 μM), with a pIC50 value of 4.8 (data not shown). These findings demonstrate that UTP-and UTPγS-evoked responses in cultured rat urothelial cells are sensitive to PPADS.
Figure 1. FLIPR analysis of changes in intracellular calcium in cultured rat urothelial cells by purinergic receptor agonists and antagonists.
(A) Illustrates the concentration-response (100 nM to 1 mM) of a range of purinergic receptor agonists in cultured rat urothelial cells. (B, C) Effect of P2 receptor antagonists, PPADS (30 μM), suramin (30 μM) and MRS2179 (30 μM) on ATP and UTPγS evoked changes in intracellular calcium in cultured rat urothelial cells. (D) Representative continuous traces of changes in fluo-3 fluorescence in response to UTPγS (100 μM) alone and in the presence of PPADS (30 μM).
Fura-2
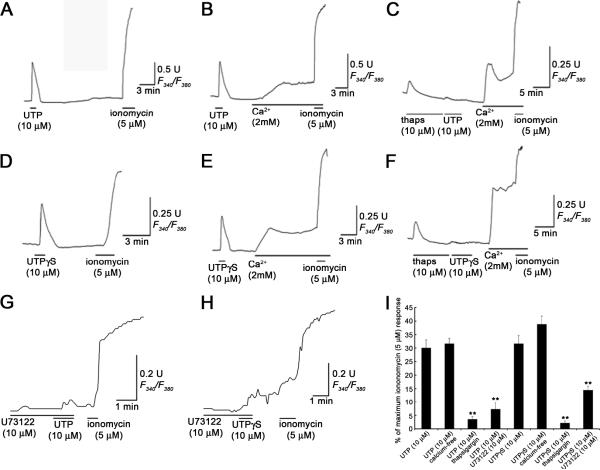
Bath application of either UTP (10 μM) or UTPγS (10 μM) evoked a rapid increase in [Ca2+]i in cultured rat urothelial cells. These responses typically reached peak within 1 min and fully recovered to baseline levels between 2-3 min following application. The mean UTP and UTPγS (10 μM) responses were 30 ± 3% (n=30) and 31.6 ± 3.0% (n=32) of the peak ionomycin (5 μM) response, respectively (Figure 2A, D). In the absence of extracellular calcium, the amplitudes of UTP (10 μM; 31.6 ± 1.9%) and UTPγS (10 μM; 38.8 ± 3.0%) evoked responses were not significantly different than those evoked in medium containing normal (2 mM) extracellular calcium (Figure 2B, E). Thapsigargin (10 μM) was used to deplete intracellular calcium stores by inhibiting intracellular calcium reuptake by the SERCA pump. Both UTP (10 μM) and UTPγS (10 μM) evoked responses were significantly attenuated under these conditions (Figure 2C, F). UTP and UTPγS responses following pre-treatment with thapsigargin (10 μM) were 3.5 ± 1.1% (n=30; P<0.01) and 2.1 ± 0.8% (n=30; P<0.01) of the peak ionomycin (5 μM) response, respectively. These findings indicate UTP- and UTPγS-evoked changes in intracellular calcium in cultured rat urothelial cells result from the release of calcium from intracellular stores. Inhibition of phospholipase C (PLC) with U73122 (10 μM) significantly attenuated UTP (10 μM) and UTPγS (10 μM); the evoked responses were 7.3 ± 2.7% (n=14; P<0.01)and 14.4 ± 1.5% (n=17; P<0.01) of the peak ionomycin (5 μM) responses, respectively (Figure 2G, H, I), suggesting UTP and UTPγS evoked responses are mediated by PLC linked processes.
Figure 2. UTP and UTPγS evoked changes in intracellular calcium in cultured rat urothelial cells are significantly attenuated by inhibition of the SERCA pump and phospholipase C.
(A) and (D) illustrate UTP (10 μM) and UTPγS (10 μM) evoked changes in [Ca2+]i normal physiological calcium (2 mM). (B) and (E) in the absence of extracellular calcium, the amplitude of UTP (10 μM) and UTPγS (10 μM) evoked responses were not significantly different that those in normal extracellular calcium. (C) and (F) thapsigargin (10 μM) was used to deplete intracellular calcium stores by inhibiting the SERCA pump. Both UTP (10 μM) and UTPγS (10 μM) responses were abolished under these conditions. (G) and (H) Pre-treatment of rat urothelial cells with the PLC inhibitor, U73122 (10 μM) significantly attenuated both UTP (10 μM) and UTPγS (10 μM) evoked changes in [Ca2+]i. (I) Histograms illustrating the mean changes in [Ca2+]i evoked by UTP (10 μM) and UTPγS (10 μM) as a percentage of the maximum ionomycin (5 μM) response in rat urothelial cells alone and following pre-treatment with thapsigargin (10 μM) and U73122 (10 μM); ** P<0.01.
P2Y receptor expression in the rat urinary bladder
(i) Western blotting
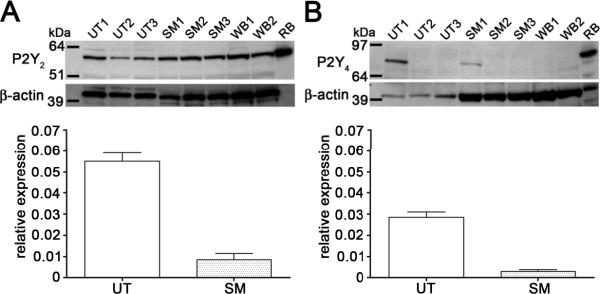
Expression of P2Y receptors in urothelial, detrusor smooth muscle and whole bladder protein lysates was assessed using western blotting; whole rat brain protein lysates were used as positive controls for antibody binding. Strong P2Y2 immunoblotting (60 kDa) was also present in all tissue samples assessed. P2Y4 immunoblotting (80-85 kDa) was observable to a lesser extent relative to the other P2Y subtypes. In 1/3 rats assessed P2Y4 immunoblotting was observable in rat urothelium and detrusor smooth muscle, and absent in 2 other rats assessed (Figure 3A & B, upper panels).
Figure 3. Gene and protein expression of P2Y2,4 receptors in the rat urinary bladder.
Expression of P2Y2 (A, upper panel) and P2Y4 (B, upper panel) receptors in urothelial (UT1-3), detrusor smooth muscle (SM1-3) and whole bladder (WB1-2) protein lysates was assessed using western blotting; whole rat brain (RB) protein lysate was used as positive control for antibody binding. Immunoblotted proteins corresponding to P2Y2 (60 kDa) and P2Y4 (85 kDa) were detected. P2Y4 immunoblotting was only detected in 1/3 rat urinary bladders tested. Relative expressions of P2Y2 (A, lower panel) and P2Y4 (B, lower panel) mRNA in the urothelium and detrusor smooth muscle compared to β-actin; n=3 rats was assessed using quantitative PCR. UT = urothelium; SM = smooth muscle.
(ii) Expression of P2Y mRNA in the urothelium and smooth muscle
The relative expression of P2Y receptor mRNA compared to β-actin in the urothelium and detrusor smooth muscle was assessed using quantitative real-time PCR. The rank order for expression of urothelial P2Y mRNA was P2Y2>P2Y4 (Figures 3A & B, lower panels). However, no statistical significance was found between the levels of the 2 receptors. Levels of P2Y receptor mRNA was between 2-7 times lower in the detrusor smooth muscle compared to urothelium (Figures 3A and B, lower panel). The rank order for expression of P2Y mRNA in the detrusor was P2Y2>P2Y4.
(iii) Immunocytochemistry
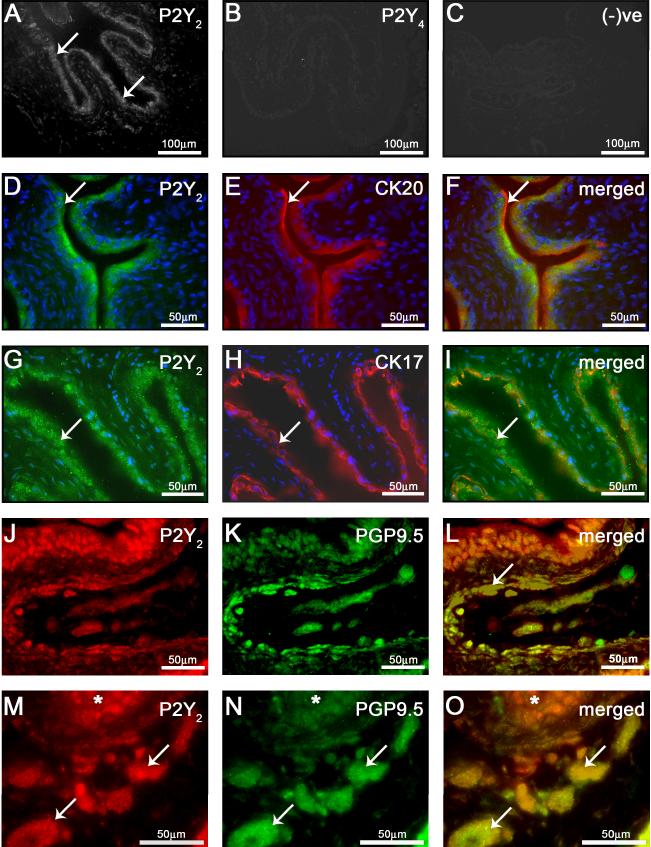
Immunocytochemical studies provided evidence for the expression of P2Y2 with very little/no detectable P2Y4 receptor staining in the normal rat urinary bladder (Figure 4). P2Y2 immunoreactivity was present in the urothelium (Figure 4A), putative nerve fibres/plexi, as indicated by PGP9.5 staining (Figure 4J-O) and detrusor smooth muscle (data not shown). Co-localization experiments with putative markers of the urothelium, cytokeratin 17 (basal cells) and cytokeratin 20 (apical cells), revealed P2Y2 immunoreactivity to be present in all layers of the urothelium and restricted primarily to the plasma membrane and cytoplasm (Figure 4F, I). P2Y4 receptor staining was not present in the normal rat urinary bladder (Figure 4B). P2Y1 receptor expression was not assessed in the present study due to concerns about antibody binding specificity. Omission of primary antibodies from the incubation buffer completely attenuated secondary antibody labeling (Figure 4C).
Figure 4. Expression of P2Y2,4 in the rat urinary bladder.
(A) P2Y2 immunoreactivity was present in the bladder urothelium (arrows). (B) Little/no P2Y4 immunoreactivity was detected in the rat bladder. (C) Background immunofluorescence was assessed in the absence of primary antibodies and secondary only. Colocalization of P2Y2 receptor with cytokeratin 20 (D-F) and cytokeratin 17 (G-I) revealed P2Y2 expression in both apical and basal cells of the urothelium. Further colocalization studies with P2Y2 with PGP9.5 revealed P2Y2 receptor expression within submucosal nerve fibres (J-L) and nerve bundles (M-O; * denotes localization of the urothelium).
P2Y receptor evoked ATP release from cultured rat urothelial cells
Serosal release of ATP from the urothelium has been reported to activate underlying pelvic afferent fibers in a paracrine manner (16, 21, 49). We assessed whether activation of P2Y receptors can evoke the release of ATP from cultured rat urothelial cells.
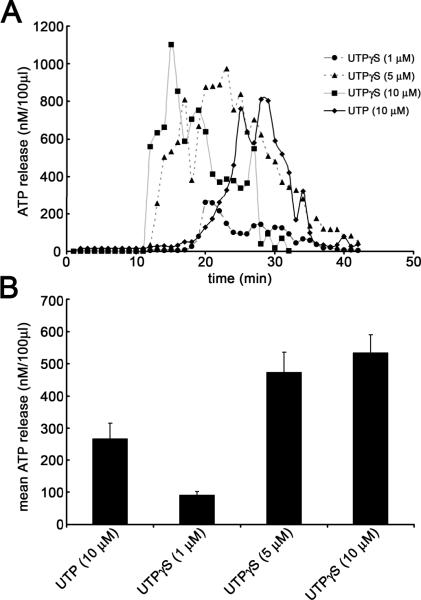
Due to interference with the luciferin-luceriferase based ATP assay mix, numerous purine nucleotides were not assessed, including ATP, ATPγS, ADP, UDP and 2meSADP; in addition, PPADS and suramin were also not tested. Agonists were bath-applied for 60 s and typical responses reached a peak between 10-30 min after application and returned to baseline levels 15 min post-application (Figure 5A). In many cases the ATP release evoked from rat urothelial cells following addition of agonists, exhibited an oscillatory release profile over the time period assessed (Figure 5A). UTP (10 μM) consistently and reproducibly evoked the release of ATP from cultured rat urothelial cells; average ATP release evoked was 268 ± 47 nM.100μl-1; n=3 independent cultures (Figure 5A, B). The selective P2Y2/4 receptor agonist, UTPγS, also evoked ATP release from cultured rat urothelial cells in a dose-related manner; the average levels of ATP released were 534 ± 56 nM.100μl-1, 473 ± 62 nM.100μl-1 and 91 ± 12nM.100μl-1 following application of UTPγS 10, 5 and 1μM, respectively (Figure 5A, B).
Figure 5. UTP and UTPγS evoke ATP release from cultured rat urothelial cells.
(A) Representative time course recordings illustrating ATP release evoked from cultured rat urothelial cells following stimulation with varying concentrations of UTPγS (1, 5 & 10 μM) and UTP (10 μM). Agonists were applied at t = 0. (B) Histograms illustrating mean release of ATP from cultured rat urothelial cells as described above. Data were obtained from at least 3 independent cultures and at least n = 3 from each culture.
Discussion
The data presented in this study demonstrates the functional presence and distribution of P2Y receptors in the rat urothelium. Based on data obtained with intracellular calcium imaging techniques and assessment of ATP release using a luciferin-luciferase assay, we have demonstrated that cultured rat urothelial cells are responsive to exogenously applied P2Y receptor agonists. The agonist profile of these responses provides functional evidence for the presence of P2Y2/4 receptors in cultured rat urothelial cells. Furthermore, PCR, western blotting and immunocytochemical studies of the rat bladder indicates the constitutive expression of P2Y2 receptors with relatively little P2Y4 receptor expression in the normal rat bladder.
Purinergic receptors have previously been demonstrated to be expressed on the urinary bladders of a number of species (9, 30, 35). In the present study, we demonstrate the functional presence of metabotropic P2Y receptors on cultured rat urothelial cells. FLIPR analysis revealed the rank order of P2 agonist potency to be ATP ≥ UTP > UTPγS = ATPγS >> 2MeSADP = NECA = ADP = UDP. Based on current pharmacological profiling, this rank order is not consistent with that reported for P2Y1 (ADP > UTP), P2Y6 (UDP > UTP), P2Y11 (ATP >> UTP), P2Y12 (ADP > UTP), P2Y13 (2-MeSADP = ADP > UTP, ATP) or P2Y14 (UDP-glucose >> UTP, ATP, ADP) (1, 14, 41). Only the rat P2Y2 and P2Y4 receptors have been reported to be activated preferentially and equipotently by UTP and ATP (14, 26). Closer analysis revealed that UTP- and UTPγS-evoked increases in cytosolic calcium in rat urothelial cells were mediated by the release of calcium from intracellular stores and via PLC-linked mechanisms, consistent with the mode of action of putative P2Y2/4 receptors (3, 12). Therefore, the rank order of these agonists indicates that rat urothelial cells functionally express P2Y2 and/or P2Y4 receptors with a relative lack of functional contribution from other P2Y or adenosine receptors. The expression of adenosine receptors have recently been reported in the rat urinary bladder urothelium (53), however, results obtained from the present study did not reveal the presence of functional adenosine receptors, as putative A1 and A2 receptor agonists, adenosine and NECA, at the concentrations tested did not induce changes in levels of intracellular calcium in cultured rat urothelial cells. Nevertheless, since the function of adenosine receptors is perhaps better assessed through a more direct measure of receptor activation (e.g. through quantification of cAMP accumulation via these Gs-and Gi/o-protein coupled receptors), the presence of adenosine receptors can not be ruled out.
Antagonist studies revealed that PPADS (30 μM), which blocks most P2X and some P2Y receptors significantly attenuated ATP- and UTP-evoked responses in cultured rat urothelial cells. Recombinant rat P2Y2 or P2Y4 receptors expressed in oocytes have been reported to be relatively insensitive to antagonism by PPADS (IC50 > 1 mM and 10 mM, respectively, (51)). However, other studies have demonstrated that PPADS can antagonize UTP-evoked Ca2+ responses in human astrocytoma cells expressing recombinant P2Y2 receptors (IC50 = 24 μM, (24)). Suramin (30 μM), which is a general blocker of purinergic receptors and is effective at most P2Y receptors, but reported to have selectivity for P2Y2 receptors (IC50 = 8.9 μM, (51)) had relatively little/no effect on ATP-, UTP- or UTPγS-evoked [Ca2+]i transients, as assessed by FLIPR. The findings from these antagonist studies provide somewhat stronger evidence for the functional presence of P2Y4 than P2Y2 receptors. This variance in the data however, may be due to the relative lack of specificity of these P2 receptor antagonists. In the present study, PPADS produced a significant shift of UTP- and ATP-evoked calcium transients at a test concentration of 30 μM, consistent with these previous findings. Given the lack of specificity of PPADS and suramin, but convergence from immunocytochemical, PCR, western blotting and P2 agonist profiles, it is likely that P2Y2 is the predominant P2 receptor subtype functionally expressed in cultured rat urothelial cells.
Immunofluorescence studies of rat urinary bladder revealed the presence of P2Y2 receptors in the rat urothelium, with little/no expression of P2Y4. P2Y2 immunoreactivity was present throughout the rat urinary bladder, including detrusor smooth muscle, underlying nerve fibres/plexi and urothelium. Previous studies conducted in cat urinary bladder have revealed constitutive expression of P2Y1, P2Y2 and P2Y4 in the urothelium (9). P2Y6 receptor expression has also been reported in the guinea pig urothelium (43). Additionally, the presence of P2Y2 was strongly indicated in urothelium, and to a lesser extent in detrusor smooth muscle, by measurement of both mRNA and protein levels. In contrast, P2Y4 had lower mRNA expression in both urothelium and detrusor smooth muscle relative to P2Y2, and when assessed by western blot, only one of three rat bladders tested provided evidence for the presence of P2Y4. Functional P2Y1 receptors, as well as mRNA transcripts have previously been reported in the rat urinary bladder detrusor smooth muscle(28, 37). In the current study, we observed P2Y1 receptor mRNA in the bladder urothelium and detrusor smooth muscle (data not shown).
Activation of P2Y receptors with UTP and UTPγS in cultured rat urothelial cells evoked the release of ATP. Distention of the urinary bladder evokes ATP release from both mucosal and serosal sides of the urothelium (31). These findings have important implications for the action of urothelial ATP release in the urinary bladder. Mucosal release of ATP has the potential to act in a paracrine/juxtacrine manner on urothelial cells. This could lead to activation of purinergic receptors expressed on urothelial cells which may further evoke the release of other bioactive mediators, such as nitric oxide (5), prostacyclin (20, 32), bradykinin (15), acetylcholine (4), neurokinins (25), and additional ATP from urothelial cells. Furthermore, urothelially expressed P2Y receptors may be involved in regulating changes in urothelial membrane capacitance following bladder distention. Wang et al (2005) demonstrated that application of UTP onto the mucosal side of the rabbit urothelium increased urothelial membrane capacitance, by stimulating exocytosis and fusion of discoidal/fusiform vesicles but not on the serosal side. In addition, application of 2MeSADP and 2MeSATP to the serosal urothelium also caused an increase in membrane capacitance, suggesting involvement of P2Y1 receptors.
Serosal release of ATP from the urothelium following bladder distention, may act on underlying pelvic afferent fibres. Indeed, in P2X3 deficient mice, distention of the urinary bladder resulted in decreased afferent nerve activity, suggesting a role for urothelially derived ATP release acting as a sensor and conveying information to afferent fibres (16, 49). Strong immunolabelling of P2Y2 was evident on sub-urothelial PGP9.5 positive nerve bundles. P2Y2/4 receptors have been reported to activate and regulate capsaicin-sensitive cutaneous afferent nerve activity with relatively little effect on thinly myelinated A-mechanoreceptors (42). These findings suggest that endogenous ATP release from serosal urothelium may contribute to regulating pelvic afferent activity by acting at P2Y2/4 receptors.
Moreover, a recently discovered cell type in the human and guinea pig bladder, myofibroblasts, have emerged as a potential modulator of sensory signaling between the urothelium and pelvic afferents (43, 52). These cells are small, spindle shaped cells, which are responsive to ATP, express connexin 43 and may serve to transfer information between urothelium and bladder sensory nerves. Recent studies in guinea pig bladder, revealed strong expression of P2Y6 and weaker labeling of P2X3, P2Y2 and P2Y4 on the surface of myofibroblasts (43). Myofibroblasts are also found in the gastrointestinal tract and have been demonstrated to evoke ATP release in response to mechanical stimulation (23, 39). The released ATP activates P2Y receptors on the surrounding cells and propagated calcium waves with a concomitant transient contraction (23). These findings suggest that distention of the urinary bladder and subsequent release of ATP from the serosal side of the urothelium may activate myofibroblasts in addition to pelvic nerve afferents. Potential cross talk between these cell types via the release of bioactive mediators, such as further ATP, or release of other neurogenic compounds, may provide a local circuit which could regulate bladder tone.
The expression of purinergic receptors are known to be altered in a range of debilitating urological conditions such as interstitial cystitis (38, 46), idiopathic detrusor instability (2, 13, 36) and urge incontinence (33). Histologically, there is increased P2X2 and P2X3 receptor expression levels in human bladder urothelium obtained from patients diagnosed with interstitial cystitis (IC) compared to control patients (46) and urothelial cells isolated from patients with IC and cats diagnosed with a comparable disorder (FIC) release significantly greater amounts of ATP following stretch/mechanical stimuli (8, 44, 45). In the model of FIC, there is also a concomitant reduction of P2X1 and loss of P2Y2 expression in the urothelium (9). The combination of these physiological and molecular changes may contribute to the underlying symptoms associated with interstitial cystitis.
The emerging profile of purinergic receptors expressed on the urothelium in a range of species, suggests that these receptors play an important role in bladder function. The data presented in this study demonstrates the expression of P2Y receptors in the rat urothelium. These findings further confirm that the urothelium, which was commonly perceived to act as a passive barrier in the urinary bladder, is a dynamic tissue which has sensory properties conferred by the expression of a wide range of receptors and its ability to release bioactive mediators in response to changes in its local environment.
Acknowledgements
This work was supported in part by grants from Roche (Palo Alto) and National Institutes of Health Grant RO1 DK54824.
References
- 1.Abbracchio MP, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Miras-Portugal MT, King BF, Gachet C, Jacobson KA, Weisman GA, Burnstock G. Characterization of the UDP-glucose receptor (re-named here the P2Y14 receptor) adds diversity to the P2Y receptor family. Trends Pharmacol Sci. 2003;24:52–55. doi: 10.1016/S0165-6147(02)00038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apostolidis A, Popat R, Yiangou Y, Cockayne D, Ford AP, Davis JB, Dasgupta P, Fowler CJ, Anand P. Decreased sensory receptors P2X3 and TRPV1 in suburothelial nerve fibers following intradetrusor injections of botulinum toxin for human detrusor overactivity. J Urol. 2005;174:977–982. doi: 10.1097/01.ju.0000169481.42259.54. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Burnstock G, Webb TE. G protein-coupled receptors for ATP and other nucleotides: a new receptor family. Trends Pharmacol Sci. 1994;15:67–70. doi: 10.1016/0165-6147(94)90280-1. [DOI] [PubMed] [Google Scholar]
- 4.Beckel JM, Meyers S, Giesselman BR, de Groat WC, Birder LA. Acetylcholine release from rat bladder epithelial cells and cholinergic modulation of bladder reflexes. Exp Biol Abstracts. 2005;863:2. [Google Scholar]
- 5.Birder LA, Apodaca G, de Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol. 1998;275:F226–229. doi: 10.1152/ajprenal.1998.275.2.F226. [DOI] [PubMed] [Google Scholar]
- 6.Birder LA. Involvement of the urinary bladder urothelium in signaling in the lower urinary tract. Proc West Pharmacol Soc. 2001;44:85–86. [PubMed] [Google Scholar]
- 7.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci. 2002;22:8063–8070. doi: 10.1523/JNEUROSCI.22-18-08063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birder LA, Barrick SR, Roppolo JR, Kanai AJ, de Groat WC, Kiss S, Buffington CA. Feline interstitial cystitis results in mechanical hypersensitivity and altered ATP release from bladder urothelium. Am J Physiol Renal Physiol. 2003;285:F423–F429. doi: 10.1152/ajprenal.00056.2003. [DOI] [PubMed] [Google Scholar]
- 9.Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, Roppolo JR, Ford AP, de Groat WC, Burnstock G. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- 10.Birder LA. More than just a barrier: urothelium as a drug target for urinary bladder pain. Am J Physiol Renal Physiol. 2005;289:F489–F495. doi: 10.1152/ajprenal.00467.2004. [DOI] [PubMed] [Google Scholar]
- 11.Birder LA. Role of the urothelium in urinary bladder dysfunction following spinal cord injury. Prog Brain Res. 2006;152:135–146. doi: 10.1016/S0079-6123(05)52009-0. [DOI] [PubMed] [Google Scholar]
- 12.Boarder MR, Weisman GA, Turner JT, Wilkinson GF. G protein coupled P2 purinoceptors: from molecular biology to functional responses. Trends Pharmacol Sci. 1995;16:133–139. doi: 10.1016/s0165-6147(00)89001-x. [DOI] [PubMed] [Google Scholar]
- 13.Brady CM, Apostolidis A, Yiangou Y, Baecker PA, Ford AP, Freeman A, Jacques TS, Fowler CJ, Anand P. P2X3-immunoreactive nerve fibres in neurogenic detrusor overactivity and the effect of intravesical resiniferatoxin. Eur Urol. 2004;46:247–253. doi: 10.1016/j.eururo.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Brunschweiger A, Muller CE. P2 receptors activated by uracil nucleotides--an update. Curr Med Chem. 2006;13:289–312. doi: 10.2174/092986706775476052. [DOI] [PubMed] [Google Scholar]
- 15.Chopra B, Barrick SR, Meyers S, Beckel JM, Zeidel ML, Ford AP, de Groat WC, Birder LA. Expression and function of bradykinin B1 and B2 receptors in normal and inflamed rat urinary bladder urothelium. J Physiol. 2005;562:859–871. doi: 10.1113/jphysiol.2004.071159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 17.de Groat WC. The urothelium in overactive bladder: passive bystander or active participant? Urology. 2004;64:7–11. doi: 10.1016/j.urology.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 18.de Groat WC. Integrative control of the lower urinary tract: preclinical perspective. British Journal of Pharmacology. 2006;147:S25–S40. doi: 10.1038/sj.bjp.0706604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Groat WC, Yoshimura N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog Brain Res. 2006;152:59–84. doi: 10.1016/S0079-6123(05)52005-3. [DOI] [PubMed] [Google Scholar]
- 20.Downie JW, Karmazyn M. Mechanical trauma to bladder epithelium liberates prostanoids which modulate neurotransmission in rabbit detrusor muscle. J Pharmacol Exp Ther. 1984;230:445–449. [PubMed] [Google Scholar]
- 21.Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes--a possible sensory mechanism? J Physiol. 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ford APDW, Gever JR, Nunn PA, Zhong Y, Cefalu JS, Dillon MP, Cockayne DA. Purinoceptors as therapeutic targets for lower urinary tract dysfunction. British Journal of Pharmacology. 2006;147:S132–S143. doi: 10.1038/sj.bjp.0706637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furuya K, Sokabe M, Furuya S. Characteristics of subepithelial fibroblasts as a mechano-sensor in the intestine: cell-shape-dependent ATP release and P2Y1 signaling. J Cell Sci. 2005;118:3289–3304. doi: 10.1242/jcs.02453. [DOI] [PubMed] [Google Scholar]
- 24.Gallagher CJ, Salter MW. Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci. 2003;23:6728–6739. doi: 10.1523/JNEUROSCI.23-17-06728.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizuka O, Mattiasson A, Andersson KE. Tachykinin effects on bladder activity in conscious normal rats. J Urol. 1995;154:257–261. [PubMed] [Google Scholar]
- 26.Jacobson KA, Costanzi S, Ivanov AA, Tchilibon S, Besada P, Gao ZG, Maddileti S, Harden TK. Structure activity and molecular modeling analyses of ribose- and base-modified uridine 5'-triphosphate analogues at the human P2Y2 and P2Y4 receptors. Biochem Pharmacol. 2006;71:540–549. doi: 10.1016/j.bcp.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasakov L, Burnstock G. The use of the slowly degradable analog, alpha, beta-methylene ATP, to produce desensitisation of the P2-purinoceptor: effect on nonadrenergic, non-cholinergic responses of the guinea-pig urinary bladder. Eur. J. Pharmacology. 1982;86:291–294. doi: 10.1016/0014-2999(82)90330-2. [DOI] [PubMed] [Google Scholar]
- 28.King BF, Knowles ID, Burnstock G, Ramage AG. Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetized rats. Br J Pharmacol. 2004;142:519–530. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lazzeri M. The physiological function of the urothelium--more than a simple barrier. Urol Int. 2006;76:289–295. doi: 10.1159/000092049. [DOI] [PubMed] [Google Scholar]
- 30.Lee HY, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and the ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- 31.Lewis SA, Lewis JR. Kinetics of urothelial ATP release. Am J Physiol Renal Physiol. 2006;291:F332–F340. doi: 10.1152/ajprenal.00340.2005. [DOI] [PubMed] [Google Scholar]
- 32.Maggi CA. Prostanoids as local modulators of reflex micturition. Pharmacol Res. 1992;25:13–20. doi: 10.1016/s1043-6618(05)80059-3. [DOI] [PubMed] [Google Scholar]
- 33.Moore KH, Ray FR, Barden JA. Loss of purinergic P2X(3) and P2X(5) receptor innervation in human detrusor from adults with urge incontinence. J Neurosci. 2001;21:RC166. doi: 10.1523/JNEUROSCI.21-18-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namasivayam S, Eardley I, Morrison JF. Purinergic sensory neurotransmission in the urinary bladder: an in vitro study in the rat. BJU Int. 1999;84:854–860. doi: 10.1046/j.1464-410x.1999.00310.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly BA, Kosaka AH, Chang TK, Ford AP, Popert R, Rymer JM, McMahon SB. A quantitative analysis of purinoceptor expression in human fetal and adult bladders. J Urol. 2001;165:1730–1734. [PubMed] [Google Scholar]
- 36.O'Reilly BA, Kosaka AH, Knight GF, Chang TK, Ford AP, Rymer JM, Popert R, Burnstock G, McMahon SB. P2X receptors and their role in female idiopathic detrusor instability. J Urol. 2002;167:157–164. [PubMed] [Google Scholar]
- 37.Obara K, Lepor H, Walden PD. Localization of P2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J Urol. 1998;160:587–591. [PubMed] [Google Scholar]
- 38.Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- 39.Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am J Physiol. 1999;277:C183–201. doi: 10.1152/ajpcell.1999.277.2.C183. [DOI] [PubMed] [Google Scholar]
- 40.Rong W, Spyer KM, Burnstock G. Activation and sensitisation of low and high threshold afferent fibres mediated by P2X receptors in the mouse urinary bladder. J Physiol. 2002;541:591–600. doi: 10.1113/jphysiol.2001.013469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sak K, Illes P. Neuronal and glial cell lines as model systems for studying P2Y receptor pharmacology. Neurochem Int. 2005;47:401–412. doi: 10.1016/j.neuint.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Stucky CL, Medler KA, Molliver DC. The P2Y agonist UTP activates cutaneous afferent fibers. Pain. 2004;109:36–44. doi: 10.1016/j.pain.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Sui GP, Wu C, Fry CH. Characterization of the purinergic receptor subtype on guinea-pig sub-urothelial myofibroblasts. BJU Int. 2006;97:1327–1331. doi: 10.1111/j.1464-410X.2006.06200.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun Y, Keay S, DeDeyne P, Chai T. Stretch-activated release of adenosine triphosphate by bladder uroepithelia is augmented in interstitial cystitis. Urology. 2001;57:131. doi: 10.1016/s0090-4295(01)01106-2. [DOI] [PubMed] [Google Scholar]
- 45.Sun Y, Chai TC. Augmented extracellular ATP signaling in bladder urothelial cells from patients with interstitial cystitis. Am J Physiol Cell Physiol. 2006;290:C27–C34. doi: 10.1152/ajpcell.00552.2004. [DOI] [PubMed] [Google Scholar]
- 46.Tempest HV, Dixon AK, Turner WH, Elneil S, Sellers LA, Ferguson DR. P2X and P2X receptor expression in human bladder urothelium and changes in interstitial cystitis. BJU Int. 2004;93:1344–1248. doi: 10.1111/j.1464-410X.2004.04858.x. [DOI] [PubMed] [Google Scholar]
- 47.Truschel ST, Ruiz WG, Shulman T, Pilewski J, Sun TT, Zeidel ML, Apodaca G. Primary uroepithelial cultures. A model system to analyze umbrella cell barrier function. J Biol Chem. 1999;274:15020–15029. doi: 10.1074/jbc.274.21.15020. [DOI] [PubMed] [Google Scholar]
- 48.Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X(1) receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, Ford AP, Burnstock G. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wein AJ. The urothelium in overactive bladder: passive bystander or active participant? J Urol. 2005;173:2199–2200. [PubMed] [Google Scholar]
- 51.Wildman SS, Unwin RJ, King BF. Extended pharmacological profiles of rat P2Y2 and rat P2Y4 receptors and their sensitivity to extracellular H+ and Zn2+ ions. Br J Pharmacol. 2003;140:1177–1186. doi: 10.1038/sj.bjp.0705544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiseman OJ, Fowler CJ, Landon DN. The role of the human bladder lamina propria myofibroblast. BJU Int. 2003;91:89–93. doi: 10.1046/j.1464-410x.2003.03802.x. [DOI] [PubMed] [Google Scholar]
- 53.Yu W, Zacharia LC, Jackson EK, Apodaca G. Adenosine receptor expression and function in bladder uroepithelium. Am J Physiol Cell Physiol. 2006;291:C254–265. doi: 10.1152/ajpcell.00025.2006. [DOI] [PubMed] [Google Scholar]