Summary
Growth hormone plays an important role in regulating numerous functions in vertebrates. Several pathways that negatively regulate the magnitude and duration of its signaling (including expression of tyrosine phosphatases, SOCS and PIAS proteins) are shared between signaling induced by growth hormone itself and by other cytokines. Here we overview downregulation of the growth hormone receptor as the most specific and potent mechanism of restricting cellular responses to growth hormone and analyze the role of several proteolytic systems and, specifically, ubiquitin-dependent pathways in this regulation.
Growth Hormone Actions and Regulation of Receptor Abundance
Growth hormone (GH) is a 22,000 Da peptide hormone derived mainly from the anterior pituitary gland that is critical in promotion of growth and regulation of metabolism and energy balance and may have a role in longevity in humans and other vertebrates [1, 2]. Clinically, disruptions in GH action are most evident when either GH levels are either too high or too low. GH excess found in the setting of a GH-secreting pituitary adenoma yields the syndrome of acromegaly with its characteristic connective tissue and bony overgrowth, visceromegaly, and insulin resistance [3]; if present prior to closure of the epiphyseal growth plates, gigantism results. Conversely, GH deficiency in childhood yields shortness of stature, altered fat distribution, and a tendency to hypoglycemia [4]. In addition to these scenarios, an emerging experimental literature suggests potential roles for both pituitary-derived GH and autocrine/paracrine GH produced in extra-pituitary sites in potentiating or aberrantly promoting certain cancers [5–9].
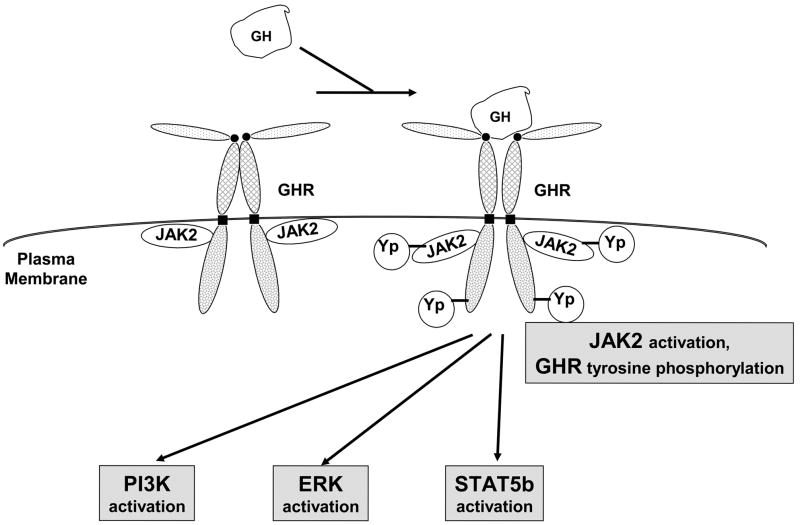
Observations in patients with GH resistance (Laron syndrome) [10] and analysis of mice with targeted deletion of the GH receptor (GHR) [11] indicate that the in vivo biological actions of GH are transduced by the GHR, a member of the cytokine receptor superfamily [12, 13]. GHR is a widely distributed ~620 residue type 1 single membrane-spanning glycoprotein, expressed in many species, that likely exists as a dimer at the cell surface where it binds GH in its extracellular domain and signals by regulated interaction of its ~350 residue intracellular domain with signaling molecules [13–16]. The GHR couples physically and functionally the Janus kinase, JAK2, which is a cytoplasmic tyrosine kinase that is also utilized by other cytokine receptor family members [17, 18]. JAK2 associates non-covalently with the dimerized GHR during its biogenesis and at the cell surface via the receptor’s proline-rich perimembranous intracellular domain Box 1 element and the N-terminal FERM domain of JAK2 [19–25]. GH-triggered activation of JAK2 causes GHR and JAK2 tyrosine phosphorylation and induces signaling systems including STATs (most notably STAT5b), ERKs, and PI3-kinase [14–16] (Figure 1). GH-induced STAT5b activation requires receptor tyrosine phosphorylation and promotes gene transcription (eg., IGF-1, acid-labile subunit (ALS) of the IGF binding protein complex, SOCS proteins, hepatic P450 enzymes, and serine protease inhibitor 2.1 (Spi2.1) [26–36]). Unlike STAT5b, GH-induced ERK and PI3K activation do not require the entire GHR cytoplasmic domain, but only JAK2 coupling [21–23, 37–39]. ERK activity is critical for GH-induced c-fos transcription [40], enhances GH-stimulated proliferation [41], and mediates crosstalk with EGF signaling [42–44]. GH-induced PI3K activity is implicated in anti-apoptosis and/or proliferation and likely contributes to GH-induced ERK, p70 S6 kinase, and phosphodiesterase activity [40, 41, 45–49].
Figure 1.
GH signaling pathways. GH binds to the cell surface dimerized GHR, causing JAK2 activation and activation of downstream pathways, including STAT5, PI3-kinase, and ERK pathways.
GH sensitivity is substantially affected by the abundance of GHR available for ligand engagement at particular target cells and tissues. Surface GHR availability is regulated at several levels, including transcriptional, post-transcriptional, and post-translational. Transcriptional and post-transcriptional GHR regulation have been reviewed extensively [50–52] and will not be further dealt with in this review. Post-translational regulation of GHR abundance is exerted at the levels of receptor biosynthesis and trafficking to the cell surface, stability and constitutive (non-GH-dependent) downregulation of the surface receptor, metalloprotease-mediated GHR processing, and GH-induced GHR downregulation. Elements of each of these processes are impacted upon by the ubiquitin-proteasome system and each will be further discussed below in this context.
The ubiquitin-proteasome system is critical in maintenance of cellular physiology and the health of humans and other organisms. With regard to GH action and the GH axis, there are as yet no examples of specific disruptions of the ubiqutin-proteasome system that themselves account for disease states, but two interesting examples of mutational alteration of GH signaling elements that affect protein turnover in disease states have recently been defined that may relate to this important system.
One of these pertains to the syndrome of acromegaly. As alluded to above, the basis for this syndrome is in almost all instances a somatotroph adenoma that secretes excessive GH in a dysregulated fashion. Nearly twenty years ago, investigators uncovered a pituitary-specific activating mutation of the G-protein that couples to the GH releasing hormone receptor, thereby underlying the pathogenesis of many so-called “densely granulated” GH-secreting anterior pituitary adenomas [53–56]. A recent analysis of pituitary tumor specimens also demonstrated that nearly one-half of densely granulated tumors harbored such an activating mutation, but none were found in any of the fourteen “sparsely granulated” GH-secreting tumors examined [57]. Interestingly, six (43%) of these sparsely granulated tumors harbored a heterozygous mutation of the GHR that predicts a change of His to Leu in codon 49 of the extracellular domain; none of the densely granulated tumors exhibited any mutations of GHR. Functional analysis of a site-directed H49L mutant GHR in a reconstitution system (more below) revealed that this mutant receptor exhibited altered posttranslational processing with accumulation of the precursor form of the receptor at the expense of the mature form. Notably, however, the mutant precursor was markedly longer-lived than the wild-type precursor and the mature form of the mutant that found its way to the cell surface displayed decreased GH binding capacity and diminished GH signaling [57]. This work is important in that it suggests an autofeedback loop of GH on the anterior pituitary somatotrophs via the somatotroph GHR, the disruption of which leads to hypersecretion of GH; this is a novel concept in pituitary-GH physiology. Furthermore, it will be important to determine if the buildup of the precursor (which, as seen below, is normally either rapidly processed to the mature form or undergoes endoplasmic reticulum-associated degradation) is due to or causative of disruption of the ubiquitin-proteasome system in these cells.
Another recent example of alteration of a GH signaling component relating to the function of the ubiquitin-proteasome system derives from investigation of a naturally-occurring mutation of STAT 5b [58, 59]. Targeted deletion of Stat 5b in mice leads to disruption of key GH-dependent growth and metabolic functions [34]. Kofoed, et al [59] described a young woman with a homozygous mutation of the Stat 5b gene that predicts a change of alanine to proline at residue 630 in the phosphotyrosine-binding SH2 domain. This patient had shortness of stature and evidenced clinical and biochemical GH insensitivity. When the mutation was studied in the context of cellular reconstitution experiments [58], this A630P Stat 5b mutant was found to have a dramatically shortened half-life and failed to undergo activation in response to GH. Furthermore, the mutant displayed markedly enhanced aggregation and formed cytoplasmic inclusions. Perhaps most notably, expression of the mutant Stat 5b conferred defective proteasome function on the cells in which it was expressed, such that other proteins normally degraded by the proteasome were less susceptible to this activity. Thus, mutation of this critical GH signal transducing element not only caused GH resistance, but also disrupted proteasomal function because of its aberrant folding.
Trafficking of the Newly Synthesized GHR: Roles of JAK2 and the Proteasome
The GHR is synthesized as a non-glycosylated nascent precursor that is transported from the endoplasmic reticulum (ER) to the Golgi apparatus. It is now clear that GHR dimerizes in the ER early in the process of biogenesis, thus accounting for the receptor dimers detected at the cell surface even in the absence of GH engagement [19, 60, 61]. Recent studies undertook to determine the role, if any, that association of the GHR with JAK2 has on the receptor’s trafficking to the cell surface [62, 63]. These studies yielded not only an appreciation of JAK2’s role as a potential chaperone early in the biosynthetic pathway, but also revealed an interesting role for the proteasome with regard to GHR trafficking.
As for many other surface glycoprotein receptors, the progress of GHR biogenesis and trafficking can be tracked by monitoring biochemically the degree and type of glycosylation it undergoes. In the process of transport through the Golgi, the GHR acquires carbohydrate in a characteristic fashion, in which high-mannose sugars added in the ER are ultimately removed during the transition from the early to late Golgi to yield the mature glycosylated GHR that populates the cell surface. This process can be assessed by determining the receptor’s sensitivity to in vitro deglycosylation by endoglycosidase H (endoH). Mature GHR is endoglycosidase H (endoH)-resistant while the high-mannose GHR precursor is sensitive to deglycosylation by endoH [25, 30, 62, 64, 65]. Using this feature, as well as a robust cellular stable reconstitution system in which GHR was expressed either in the presence or absence of JAK2 and immunoblotting and pulse-chase metabolic labeling techniques, it was first observed that although JAK2 is not required for detectable surface GHR expression, cells expressing JAK2 exhibited enhanced surface GHR abundance and an increased mature:precursor GHR ratio compared with JAK2-deficient cells, suggesting that JAK2 fosters GHR maturation [25, 62].
Furthermore, in cells that lack JAK2, a GHR fragment was detected with antibodies to the receptor intracellular domain, but not the extracellular domain [63, 66]. The abundance of this fragment and the steady state level of GHR precursor relative to the mature form were dramatically lessened by expression of wild-type JAK2 or JAK2 mutants that were capable of interacting with GHR, independent of whether such mutants possessed tyrosine kinase activity. Similarly, cells that harbored a GHR mutant that lacks the ability to interact with JAK2 (by virtue of mutation of the receptor Box 1 region) also manifested the same intracellular domain-containing GHR fragment and a decreased mature:precursor GHR ratio, independent of whether JAK2 was expressed. These data point to the ability of GHR to associate with JAK2 as enabling efficient receptor maturation and indicate that the presence of the intracellular domain-containing fragment of the receptor reflects inefficiency in this process.
Further studies focused on factors that affected the level of the intracellular domain-containing fragment in JAK2-deficient cells [63]. Notably, treatment of these cells with a proteasome inhibitor (lactacystin), but not two separate lysosome inhibitors, dramatically decreased the level of this receptor fragment and this was accompanied by decreased precursor GHR and increased mature GHR abundance. When brefeldin A (BFA) was used to disrupt ER-to-Golgi transport, the abundance of the intracellular domain fragment was also reduced; washout of BFA allowed regeneration of the fragment along with the GHR precursor. Interestingly, washout of BFA in the presence of cycloheximide (to prevent new protein synthesis) blocked reappearance of both the intracellular domain fragment and the precursor GHR, but washout of BFA in the presence of lactacystin prevented reappearance only of the intracellular domain fragment. Thus, the intracellular domain fragment appeared to derive from the precursor GHR in a proteasome-dependent fashion.
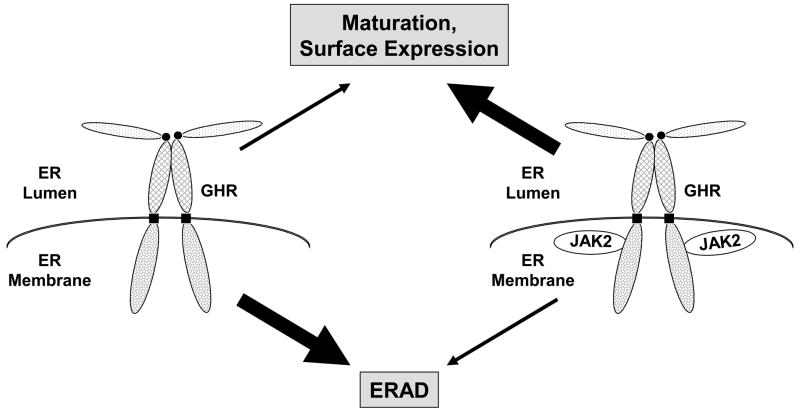
The data in these studies suggest that in cells that lack JAK2 the nascent precursor GHR is a target for endoplasmic reticulum-associated degradation (ERAD) and represent the first example of ERAD-associated cleavage of a cytokine receptor family member that stems from a lack of its cognate JAK (Figure 2). ERAD is a process whereby proteins that fail to fold properly or otherwise fail quality control mechanisms in the ER undergo retrotranslocation and proteasomal degradation in the cytosol [67–69]. JAK2, by virtue of its association with the GHR, rather than via its kinase activity, apparently “chaperones” the dimerized precursor so as to avoid quality control and proceed with efficient processing to mature GHR in the secretory pathway. How does JAK2 exert this chaperone effect? Multiple possibilities exist, including the notion that a receptor region that might otherwise be seen as defective or unfolded to the quality control apparatus is hidden by JAK2 binding. In a similar fashion, JAK2 binding might allosterically alter a GHR site outside of the region that interacts with JAK2 to make that site appear less defective. Unraveling of such possibilities awaits further investigation.
Figure 2.
JAK2 association affects endoplasmic reticulum to cell surface GHR trafficking. In cells harboring GHR and JAK2 molecules that can associate, GHR moves from the ER to the Golgi, matures, and reaches the cell surface efficiently. In cells lacking JAK2 or with GHR molecules that cannot associate with JAK2, GHR undergoes endoplasmic reticulum-associated degradation (ERAD) and inefficiently matures and trafficks to the cell surface.
Stabilizing effects on the cell surface levels of respective cytokine receptors have been shown for all members of JAK family including TYK2 (for IFNAR1 [70, 71] and thrombopoietin receptor (TpoR) [72]), JAK1 (for oncostatin M receptor [73, 74] and IL-9Rα, and IL-2Rβ [72] and Fuchs, et al, unpublished data), JAK2 (for TPOR [72], erythropoietin receptor [75], and GHR [62]) and JAK3 (for the common gamma chain of IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21 receptor complexes [76]). Little is known about the mechanisms by which different JAK family members increase the cell surface levels of different receptor chain; models proposed so far include a chaperone-like assistance in receptor folding, maturation and delivery to the cell surface, inhibition of basal endocytosis and/or post-internalization sorting into the late endosomes/lysosomes, as well as protection from proteasome-mediated degradation.
A novel aspect of the work on JAK2 and GHR biosynthesis that extends broadly into the arena of ERAD and proteasome function is the fact that the apparent product of this GHR degradation is a discrete intracellular domain-containing receptor fragment that is stable enough to be detected by immunoblotting. Assuming that the proteasome (rather than a separate proteasome-dependent protease) directly catalyzes GHR ERAD under JAK2-deficient circumstances, such an apparently discrete cleavage is somewhat unusual. Proteasomal degradation is usually thought to involve the threading of target proteins through the proteasome complex’s gated substrate channel. Relatively few instances of incomplete or discrete cleavage by the proteasome have been described. Examples include the incomplete cleavage of NF-κB p105 yielding the p50 subunit due to the presence of a “processing stop signal” [77, 78] and the endoproteolytic cleavage of certain fusion proteins [79]. Whether either of these mechanisms is in play to explain the ERAD-generated, proteasome-dependent production of the intracellular domain-containing GHR is as yet unknown.
Proteolysis of the GHR: Roles of TACE, γ-secretase, and the Proteasome
Over the last decade, it has become appreciated that GHR, like some other surface receptors, is a target for regulated sequential proteolysis, the first step of which (“α-secretase cleavage”) occurs in the proximal extracellular domain stem region 8–9 residues (depending on species) outside the plasma membrane [65, 80, 81]. This α-secretase cleavage results in loss of full-length GHR, appearance of a cell-associated transmembrane domain (TMD)/intracellular domain (ICD)-containing receptor fragment (the “remnant”), and a soluble GHR extracellular domain (ECD) (called GH binding protein (GHBP), in correspondence with the high affinity GH binding protein that circulates in many species, including humans [82, 83]). GHR α-secretase cleavage is constitutive, but can be further induced in various cell types by a protein kinase C activator (the phorbol ester, PMA), platelet-derived growth factor (PDGF), or serum [65, 80, 81, 84–87]. This cleavage is catalyzed mainly by the extracellular domain of the transmembrane metalloprotease, TACE (tumor necrosis factor-α converting enzyme; ADAM-17) [66, 88]. Importantly, inducible α-secretase cleavage likely regulates GH sensitivity; that is, GH-induced signaling is dampened after cells are exposed to stimuli that promote GHR α-secretase cleavage, but not in the presence of metalloprotease inhibitors or if noncleavable receptor mutants are expressed, suggesting that metalloproteolysis modulates GH responsiveness in part by regulating surface GHR levels [65, 83, 84]. Further, recent in vivo experiments indicate that administration of bacterial endotoxin leads to downregulation of hepatic GHR abundance and hepatic insensitivity to GH at least in part by inducing receptor proteolysis, suggesting that this may constitute a physiologically-relevant mechanism of regulation of GH action [89]. Notably, GH itself does not promote GHR α-secretase cleavage; indeed, GH inhibits subsequent GHR proteolysis, apparently by altering GHR conformation, rather than by causing signaling [66].
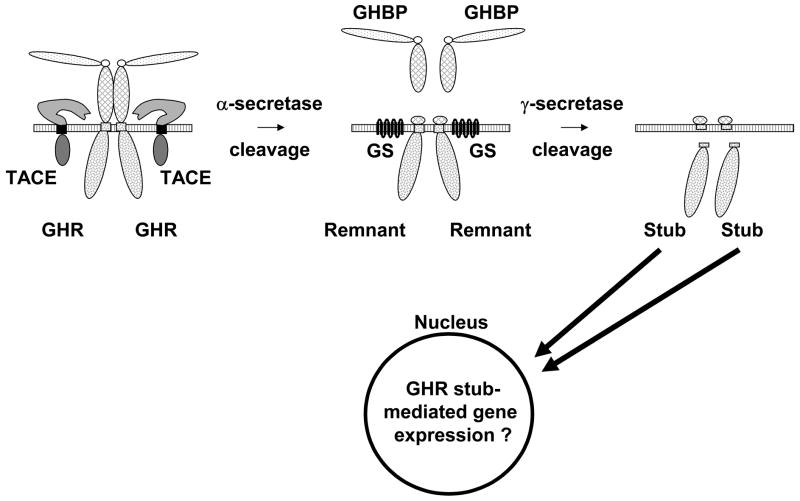
Recent studies have shown that the α-secretase-generated GHR TMD/ICD remnant is further cleaved by an enzyme activity termed “γ-secretase” within the TMD, which liberates the ICD, a protein termed the “GHR stub” [87]. γ-secretase consists of four molecules, including presenilin, which forms the aspartyl protease core and facilitates a process known as regulated intramembrane proteolysis (RIP) [90]. The GHR stub was detected by immunoblotting most readily when cells were pretreated with either of the proteasome inhibitors, lactacystin or epoxomicin [87], but such treatment had no effect on either the formation or degradation of the remnant from which the stub derives. Thus, the stub is selectively degraded in a proteasome-dependent fashion. Furthermore, the stub can be detected in both the cytosol and the nucleus [87]. The data are consistent with the notion that inducible α-secretase cleavage generates remnant, which is converted to stub by γ-secretase. The stub is labile and can accumulate in the nucleus; proteasome inhibition prevents stub degradation (Figure 3).
Figure 3.
GHR undergoes sequential TACE and gamma-secretase cleavage. Surface GHR undergoes constitutive and inducible cleavage in the extracellular domain stem region by TACE in a process called “alpha-secretase” cleavage. This yields the shed GHBP and the GHR remnant. Remnant is then cleaved by gamma-secretase within the membrane to yield the GHR stub (soluble intracellular domain), which localizes to the nucleus, where it may affect gene expression.
The two-step α-/γ-secretase GHR processing is analogous to that seen for amyloid precursor protein, Notch, and others; in those systems, nuclear-localized ICD fragments affect gene expression [90]. It is not yet known whether the GHR stub also regulates gene expression. However, as α-/γ-secretase GHR processing is inhibited, rather than stimulated, by GH, such GHR-mediated gene expression could constitute a unique mechanism by which GH may affect gene expression; that is, by negatively regulating stub formation, rather than by promoting via GHR signaling the activation of STAT5 and STAT5-mediated gene transcription, for example. If such an alternative pathway of GH-modulated gene expression does exist, it will likely be modulated by factors that affect proteasome activity. Future studies will likely address whether the stub indeed associates with chromatin/DNA and, if so, which genes might be regulated by the stub.
Surface GHR Stability: Roles of JAK2 and Proteasome Inhibitors
Once at the cell surface, the GHR could, in principle, achieve several fates. If engaged by GH, signaling is triggered and the receptor undergoes ligand-dependent downregulation (more below). However, in the natural milieu, GH is released from the pituitary gland in a pulsatile fashion such that GH levels are quite low in periods between pulses. Thus, it is critical to understand factors that govern GHR abundance independent of GH. It is believed that mature GHRs are cleared from the cell surface by constitutive or inducible proteolytic shedding and by constitutive downregulation. GHR proteolysis was discussed above. Recent work has revealed important features relating to constitutive downregulation and has, in particular, focused on effects of JAK2 on this process.
Elegant work over the past decade, emanating mainly from the Strous laboratory and performed largely in one cell system (a stably GHR-transfected temperature-sensitive Chinese hamster lung fibroblast that has a thermolabile ubiquitin activating enzyme E1), has suggested that constitutive (and GH-induced) GHR endocytosis requires an intact ubiquitin-proteasome system; an important conclusion drawn was that, although GHR is ubiquitinated, this ubiquitination is not necessary for its endocytosis [91–93] (more below). The so-called ubiquitin-dependent endocytosis (UbE) motif, a conserved ten residue region in the proximal third of the receptor’s cytoplasmic domain, is believed to be necessary for efficient GHR endocytosis [94–97]. Furthermore, these studies suggest that even though an intact ubiquitin-proteasome system is required, constitutive GHR endocytosis results in lysosomal, rather than proteasomal, degradation.
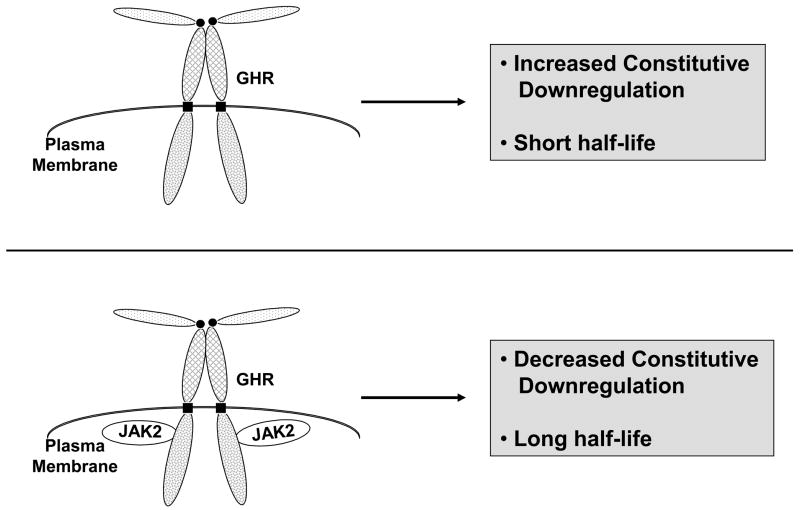
As above, JAK2 affects the fate of the cell surface GHR and in cells lacking JAK2, the ratio of mature (cell surface):precursor GHR was substantially reduced in comparison to JAK2-replete cells [25, 62]. This finding is partly explained by the chaperone effect of JAK2 during GHR biogenesis [63]. However, notable JAK2-dependent differences in the constitutive fate of mature GHRs are found as well [62]. In the context of a stable reconstitution system, the half-life (t1/2) of the receptor was estimated by anti-GHR immunoblotting after 0–4 hours of treatment with cycloheximide (CHX) to inhibit new protein synthesis. The results of such a “CHX chase” assay indicated that the precursor GHR abundance dropped precipitously and to a similar degree with increasing duration of CHX treatment both in cells that did or did not express JAK2. For the mature receptor, however, there was a dramatic effect of JAK2. As measured by this assay, the GHR t1/2 increased from roughly one hour in cells that lack JAK2 to roughly four hours in cells expressing JAK2 [62]. Thus, in the absence of GH, it appears that, in addition to its role in shepherding the GHR through the secretory pathway and lessening the degree to which it is targeted for ERAD, JAK2 also extends the receptor’s presence at or near the cell surface, presumably by interfering with constitutively active cellular machinery that functions to internalize and downregulate the receptor (Figure 4). Notably, this latter effect has also been detected for another JAK family member, TYK2, with regard to its associated type I interferon-α receptor (IFNAR1) [71], but not for some other cytokine receptor/JAKs, including the erythropoietin receptor/JAK2 [75] and the oncostatin-M receptor/JAK1 [73].
Figure 4.
JAK2 association affects the constitutive (GH-independent) fate of surface GHR. In cells harboring GHR and JAK2 molecules that can associate, surface GHR is downregulated at a low constitutive rate and its half-life is long. In cells that lack JAK2 or have GHR and JAK2 molecules that cannot associate, GHR undergoes enhanced constitutive downregulation and exhibits a short half-life.
The degradative pathways operative in conferring the rapid downregulation of mature (presumably cell surface) GHR in cells lacking JAK2 were also studied [62]. In particular, both a proteasome inhibitor (clasto-lactacystin β-lactone, an active analog of lactacystin) and lysosome inhibitors (ammonium chloride and chloroquine) were tested and inhibition of each pathway similarly prevented loss of the mature GHR upon CHX treatment in the cells that lack JAK2, but no effect was seen in cells that express JAK2 (as the mature GHR t1/2 was already quite prolonged in those cells). The results of these studies are consistent with the idea that JAK2 affects surface GHR availability being at the level of constitutive endocytosis and lysosomal degradation and that proteasome inhibition in cells that lack JAK2 blocks the ability of the receptor to enter this pathway. Whether proteasome inhibitor exerts this effect by a more “global” mechanism (eg., by depleting available cellular stores of ubiquitin by blocking turnover of ubiquitinated proteins) vs. specifically impacting the turnover of the GHR or other proteins involved in its constitutive downregulation is as yet unclear. Likewise, further research will be required to definitively discern whether JAK2 functions in this process by inhibiting entry into the endocytic pathway vs. promoting recycling of already endocytosed receptors.
GH-induced GHR Downregulation: Roles of JAK2, GHR Tyrosine Phosphorylation, and Ubiquitination
Like many surface receptors, GHR undergoes important trafficking events in response to binding of its ligand. The net effect is substantial GH-induced receptor downregulation, which serves to limit or alter the receptor’s signaling capacity and perhaps thereby further emphasize the physiologic effects of pulsatile GH release from the pituitary gland. Work as early as the 1970s–1980s and since that time suggested that GH-induced GHR downregulation proceeds via clathrin coated pit-mediated endocytosis and lysosomal degradation [91, 98–100]. As mentioned above, mutagenesis studies have pointed to a cytoplasmic domain region of the receptor that includes the UbE motif as required for efficient GH-induced GHR endocytosis and downregulation [92, 94–97]. While many studies have indicated that GH markedly augments the constitutive rate of GHR downregulation described above, some have suggested only a modest GH-induced increase [93, 101]. Similarly, the roles of GH signaling and, in particular, JAK2 activity, in promoting receptor downregulation have been debated. Studies in which chemical kinase inhibitors have been employed suggested that JAK2 kinase activation is required for GH-induced receptor downregulation [102, 103]. Like all such inhibitor studies, potential nonspecific effects of the compounds used could hamper interpretation. A different conclusion was drawn by Strous and colleagues in the GHR-transfected temperature-sensitive Chinese hamster lung fibroblast system mentioned above; in this case, a receptor with a mutated Box 1 element (which disrupts GHR-JAK2 association) was downregulated in response to GH similarly to the wild-type GHR, suggesting to the authors that GHR degradation is independent of signal transduction via JAK2 [104].
This question of the role of JAK2 and its activation in GH-induced GHR downregulation compared to constitutive downregulation, was approached recently, again using the reconstitution system described above [105]. In cells that harbor JAK2, GH markedly enhanced GHR degradation; however, in cells that lack JAK2, GH had no effect on receptor degradation (which proceeded at a high level constitutively in the absence of JAK2). Thus, in this system, GH caused receptor downregulation in a JAK2-dependent fashion. Expression of a GHR mutant that lacks the ability to interact with JAK2 resulted in enhanced constitutive receptor downregulation and a loss of GH-induced downregulation. Similarly, the ability of JAK2 to both allow GH-induced receptor loss and to protect GHR from constitutive degradation depended on the presence of an intact (GHR-associating) FERM domain within JAK2. In distinction, JAK2 mutants lacking the kinase-like and kinase domains did not mediate GH-induced GHR downregulation, despite their ability to protect the receptor from constitutive downregulation. A kinase-deficient JAK2 mutant was also unable to mediate GH-induced GHR downregulation, confirming that kinase activity is required. Notably, a GHR mutant in which all the cytoplasmic tyrosine residues were changed to phenylalanines was also resistant to GH-induced GHR downregulation; this powerful observation indicates that tyrosine phosphorylation of the receptor (rather than JAK2 kinase activation only) is required for GH-induced receptor loss. Interestingly, GH-induced GHR ubiquitination was detected in cells expressing wild-type GHR and JAK2, but not in cells that expressed wild-type GHR and a kinase-deficient JAK2 or in those with wild-type JAK2 and the GHR mutant with all tyrosines changed to phenylalanine, indicating that GH-induced receptor ubiquitination depends on both JAK2 activity and the ability of the receptor to be tyrosine phosphorylated.
Pharmacologic studies in this system showed that a lysosome inhibitor (chloroquine) blocked GH-induced GHR downregulation in cells harboring wild-type GHR and JAK2, consistent with previous findings in other systems that GH ultimately causes its receptor to be degraded in lysosomes. However, as we found for constitutive downregulation of GHR in JAK2-deficient cells (above), a proteasome inhibitor (lactacystin) also blocked GH-induced GHR downregulation. This is consistent with the work of Strous and colleagues (described above) in that intact proteasome activity is needed for effective GH-induced GHR downregulation. But, importantly, in contrast to previous reports, the findings in this system are also consistent with the possibility that GHR ubiquitination itself might play a role in its downregulation.
Tyrosine phosphorylation is essential for downregulation of various cell surface receptors. One of the best studied is the epidermal growth factor receptor (EGFR), which autophosphorylates in response to EGF, allowing association with ubiquitinating machinery, ubiquitination of the receptor itself, and its post-endocytic lysosomal degradation [106]. For GHR, several binding partners have been shown to associate via their SH2 domains with the tyrosine phosphorylated intracellular domain [30, 107–111]. One of them, the protein tyrosine phosphatase, SHP-2, may contribute modestly to GH-induced GHR downregulation [108]. More recently, it has been appreciated that the SOCS family protein CIS (cytokine inducible SH2 domain-containing protein), which interacts with tyrosine phosphorylated GHR [109, 110] and is likely linked to Cullin5-based E3 ubiquitin ligase complex that can recruit proteins to the proteasome for degradation [112], promotes GH-induced GHR internalization and thus can desensitize GH signaling [113]. Whether CIS regulates GHR downregulation at a level following internalization is not known. Another E3 ubiquitin ligase, β-TrCP (β-transducin repeats-containing protein), may function in the ligand-induced ubiquitination and proteolysis of cytokine receptor family members including interferon-α receptor 1 (IFNAR1), prolactin receptor (PRLR) and erythropoietin receptor [114–118]. Phosphorylation of specific serine residues within the cytoplasmic domain of these receptors allows ligand-induced association with β-TrCP, receptor ubiquitination, and downregulation [115, 117]. In the case of IFNAR1 and the PRLR, receptor phosphorylation within the β-TrCP-binding motif required the catalytic activity of the associated JAK (TYK2 for IFNAR1 and JAK2 for PRLR) [119, 120] and, like our findings for GHR, ubiquitination of EpoR requires JAK2 activity [121].
β-TrCP has also recently been implicated in GHR downregulation in that knockdown of this molecule retards receptor internalization and increases its steady-state abundance [122]. Furthermore, the GHR cytoplasmic tail and β-TrCP were shown to associate in in vitro binding assays, intriguingly via the receptor’s UbE motif, rather than via the canonical DSG motif seen for other β-TrCP-binding proteins and also present in the GHR [122]. Whether this novel association and/or β-TrCP itself are the critical determinants of GH-induced receptor downregulation remains unknown in the intact cellular setting. Based on our data above with the GHR that cannot undergo tyrosine phosphorylation, our view is that future studies that identify proteins that differentially associate with a wild-type vs. tyrosine mutated GHR in response to GH stimulation might shed light on the reasons underlying the defective ligand-induced downregulation of the latter receptor and whether it is due to its inability to be tyrosine phosphorylated, ubiquitinated, or both. This is an interesting area that may yield substantial insight into the mechanisms of downregulation of GHR and, by extension, other cytokine receptor family members.
Mechanistic links between ubiquitination and endocytosis: lessons from GHR and other cytokine/hormone receptors
For the past ten years, an important role of receptor ubiquitination in regulating the rate of their endocytosis has emerged. During this time span, it has been demonstrated that monoubiquitinated cargo receptors are recruited to the proteins containing ubiquitin-binding domains. Accordingly, a prevalent line of thought (supported by experiments utilizing a linear fusion of ubiquitin to the intracellular tail of the receptor) was that such proteins link receptor cargo to the components of endocytic and sorting machinery thereby enabling receptor internalization and post-internalization sorting. Under this scenario, neither polyubiquitination nor the topology of ubiquitin chains nor the site of ubiquitin conjugation should be important for the efficiency of endocytosis [123–128].
However, recent studies on mechanisms that govern internalization of the interferon alpha receptor revealed that site-specific polyubiquitination promotes internalization of this receptor via exposing a linear endocytic motif within the receptor to the interaction with the AP2 adaptin complex which is essential for efficient internalization. The lines of this new paradigm, therefore, suggest that, in the absence of ligand-stimulated ubiquitination, a specific linear motif within the receptor might be masked by receptor-interacting proteins that shield such motif from the AP2 adaptin complex. Upon ligand addition, stimulation of a site- and topology-specific ubiquitination of the intracellular domain of a receptor may result in exposure of the linear motif to adaptin complex either via rearranging the masking protein complex or by changing the conformation of the intracellular tail of a given receptor in a way that enables such interaction. Whereas additional mechanisms leading to an exposure of a linear endocytic motif cannot be ruled out, ubiquitination-stimulated interaction of adaptin with such motif on one hand and clathrin lattices on another hand should promote efficient internalization of a given cytokine/hormone receptor.
TYK2 has recently been identified as a component of this masking complex for interferon alpha receptor (S.Y.F., unpublished data). In cells lacking TYK2 or under the conditions where either TYK2 recruitment is impaired, the receptor undergoes very efficient basal endocytosis that is totally independent of receptor ubiquitination. Other masking determinants that protect various endocytic motifs and, hence, limit basal internalization of other signaling receptors to preserve a physiological density of surface receptors should be eventually identified. In case of GHR, although the identity of linear motifs and masking proteins remain to be determined, the evaluation of this scenario might be useful to understand the counterintuitive results obtained by the Strous group on GHR.
While, in some cases, interacting proteins will been involved (similar to the function of TYK2 for interferon alpha receptor), masking could also be achieved by intramolecular folding of the receptor itself. Thus, the functional role of a given endocytic determinant should always be investigated in the context of the full length receptor. There might be a need to revise the interpretation of data obtained from studies that relied on deletions of the intracellular domains as a strategy for delineating either ubiquitination-related or linear endocytic motifs of signaling receptors. Such deletions might potentially remove not only important positive regulators of endocytosis (such as recognition signal for a ubiquitin ligase or a ubiquitin-acceptor site) but also docking sequences or surfaces involved in recruitment of masking proteins. In support of this hypothesis, the ubiquitin-independent role of di-leucine linear endocytic motifs that emerge in a truncated GHR has been reported [95].
As per the role of either ubiquitination or linear motifs or both in clathrin-mediated internalization of cell surface receptors, GHR will likely fall into the group for which cooperation between ubiquitination and linear endocytic motifs might serve as a major mechanism for promoting the internalization of these receptors. While a candidate linear motif that might be important for such interaction within the intracellular domain of GHR has been revealed [129], and GHR has been the first mammalian receptor for which overall role of ubiquitination for its endocytosis and degradation has been demonstrated [95] and further delineated by an elegant work of the Strous group [19, 91–94, 96, 104, 129–136], the role of GHR ubiquitination per se (as opposed to ubiquitination of the components of endocytic machinery) in internalization and degradation of GHR remains controversial. The bulk of available data strongly suggest a logical connection between GHR ubiquitination followed by its endocytosis followed by the proteolytic turnover in the lysosomes. Furthermore, similarly to IFNAR1 [114, 137] and PRLR [116, 120], β-TrCP-based E3 ubiquitin ligase interaction (via a non-canonical UbE domain instead of the canonical phosphodegron) and Cullin1-dependent E3 ligase activity governs the endocytosis and degradation of GHR [95]. However, the latter report also suggests that ubiquitination of GHR itself does not largely affect the rate of GHR endocytosis. This conclusion is based on the observation that mutation of all of lysine residues within the cytoplasmic tail of a truncated rabbit GHR into arginines did not affect its endocytosis [96]. However, while ubiquitination of truncated receptor (whose linear motifs might be already exposed to interacting with AP2) for its internalization might be dispensable, the same is not necessarily true for the full length receptor. Work of the Pellegrini group has demonstrated that IFNAR1 mutants lacking Tyk2-binding sites (but retaining phospho-degron and, therefore, ubiquitination) undergo very efficient endocytosis [71]. It remains to be seen whether abrogation of the full length GHR ubiquitination by means that do not involve its extensive mutagenesis, which might activate alternative or downstream internalization pathways, will not be essential for its efficient endocytosis. Another possibility is that, in the absence of lysines, ubiquitination might occur on the cysteine residues. Furthermore, such ubiquitination can stimulate endocytosis as has been demonstrated for the major histocompatibility complex class I molecules decorated by viral E3 ubiquitin ligases [138]. In all, given these relatively recent developments, the issue of the role of GHR ubiquitination in its internalization, post-internalization sorting and lysosomal degradation might worth being revisited.
Acknowledgments
The authors appreciate helpful conversations with Drs. K. Loesch, K. He, J. Jiang, L. Deng, J. Cowan, N. Yang, and X. Wang. This work was supported by NIH grant DK58259 and in part by NIH grant DK46395, and a VA Merit Review Award (to S.J.F.) and by NIH grant CA115281 (to S.Y.F.).
Footnotes
Disclosure Summary: S.J.F. and S.Y.F. have nothing to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Isaksson OG, Eden S, Jansson JO. Mode of action of pituitary growth hormone on target cells. Annu Rev Physiol. 1985;47:483–499. doi: 10.1146/annurev.ph.47.030185.002411. [DOI] [PubMed] [Google Scholar]
- 2.Bartke A. Minireview: role of the growth hormone/insulin-like growth factor system in mammalian aging. Endocrinology. 2005;146:3718–3723. doi: 10.1210/en.2005-0411. [DOI] [PubMed] [Google Scholar]
- 3.Melmed S. Medical progress: Acromegaly. N Engl J Med. 2006;355:2558–2573. doi: 10.1056/NEJMra062453. [DOI] [PubMed] [Google Scholar]
- 4.Vance ML, Mauras N. Growth hormone therapy in adults and children. N Engl J Med. 1999;341:1206–1216. doi: 10.1056/NEJM199910143411607. [DOI] [PubMed] [Google Scholar]
- 5.Perry JK, Mohankumar KM, Emerald BS, Mertani HC, Lobie PE. The Contribution of Growth Hormone to Mammary Neoplasia. J Mammary Gland Biol Neoplasia. 2008 doi: 10.1007/s10911-008-9070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway-Campbell BL, Wooh JW, Brooks AJ, Gordon D, Brown RJ, Lichanska AM, Chin HS, Barton CL, Boyle GM, Parsons PG, Jans DA, Waters MJ. Nuclear targeting of the growth hormone receptor results in dysregulation of cell proliferation and tumorigenesis. Proc Natl Acad Sci U S A. 2007;104:13331–13336. doi: 10.1073/pnas.0600181104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swanson SM, Unterman TG. The growth hormone-deficient Spontaneous Dwarf rat is resistant to chemically induced mammary carcinogenesis. Carcinogenesis. 2002;23:977–982. doi: 10.1093/carcin/23.6.977. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Mehta RG, Lantvit DD, Coschigano KT, Kopchick JJ, Green JE, Hedayat S, Christov KT, Ray VH, Unterman TG, Swanson SM. Inhibition of estrogen-independent mammary carcinogenesis by disruption of growth hormone signaling. Carcinogenesis. 2007;28:143–150. doi: 10.1093/carcin/bgl138. [DOI] [PubMed] [Google Scholar]
- 9.Wang Z, Luque RM, Kineman RD, Ray VH, Christov KT, Lantvit DD, Shirai T, Hedayat S, Unterman TG, Bosland MC, Prins GS, Swanson SM. Disruption of Growth Hormone Signaling Retards Prostate Carcinogenesis in the Probasin/TAg Rat. Endocrinology. 2008;149:1366–1376. doi: 10.1210/en.2007-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Laron Z. Laron syndrome (primary growth hormone resistance or insensitivity): the personal experience 1958–2003. J Clin Endocrinol Metab. 2004;89:1031–1044. doi: 10.1210/jc.2003-031033. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse) Proc Natl Acad Sci U S A. 1997;94:13215–13220. doi: 10.1073/pnas.94.24.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazan JF. Structural design and molecular evolution of a cytokine receptor superfamily. Proc Natl Acad Sci U S A. 1990;87:6934–6938. doi: 10.1073/pnas.87.18.6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987;330:537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- 14.Frank SJ, Messina JL. Growth Hormone Receptor. In: Oppenheim JJ, Feldman M, editors. Cytokine Reference On-Line, vol. Academic Press; Harcourt, London, UK: 2002. pp. 1–21. Website: www.academicpress.com/cytokinereference, 24-hour free access. [Google Scholar]
- 15.Carter Su C, Schwartz J, Smit LS. Molecular mechanism of growth hormone action. Annu Rev Physiol. 1996;58:187–207. doi: 10.1146/annurev.ph.58.030196.001155. [DOI] [PubMed] [Google Scholar]
- 16.Frank SJ, O’Shea JJ, editors. Recent advances in cytokine signal transduction: lessons from growth hormone and other cytokines. Greenwich, CT: 1999. [Google Scholar]
- 17.Silvennoinen O, Witthuhn BA, Quelle FW, Cleveland JL, Yi T, Ihle JN. Structure of the murine Jak2 protein-tyrosine kinase and its role in interleukin 3 signal transduction. Proc Natl Acad Sci USA. 1993;90:8429–8433. doi: 10.1073/pnas.90.18.8429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Argetsinger LS, Campbell GS, Yang X, Witthuhn BA, Silvennoinen O, Ihle JN, Carter-Su C. Identification of JAK2 as a growth hormone receptor-associated tyrosine kinase. Cell. 1993;74:237–244. doi: 10.1016/0092-8674(93)90415-m. [DOI] [PubMed] [Google Scholar]
- 19.Gent J, van Kerkhof P, Roza M, Bu G, Strous GJ. Ligand-independent growth hormone receptor dimerization occurs in the endoplasmic reticulum and is required for ubiquitin system-dependent endocytosis. Proc Natl Acad Sci U S A. 2002;99:9858–9863. doi: 10.1073/pnas.152294299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank SJ, Gilliland G, Kraft AS, Arnold CS. Interaction of the growth hormone receptor cytoplasmic domain with the JAK2 tyrosine kinase. Endocrinology. 1994;135:2228–2239. doi: 10.1210/endo.135.5.7956946. [DOI] [PubMed] [Google Scholar]
- 21.Sotiropoulos A, Perrot-Applanat M, Dinerstein H, Pallier A, Postel-Vinay MC, Finidori J, Kelly PA. Distinct cytoplasmic regions of the growth hormone receptor are required for activation of JAK2, mitogen-activated protein kinase, and transcription. Endocrinology. 1994;135:1292–1298. doi: 10.1210/endo.135.4.7925092. [DOI] [PubMed] [Google Scholar]
- 22.Vanderkuur JA, Wang X, Zhang L, Campbell GS, Allevato G, Billestrup N, Norstedt G, Carter-Su C. Domains of the growth hormone receptor required for association and activation of JAK2 tyrosine kinase. J Biol Chem. 1994;269:21709–21717. [PubMed] [Google Scholar]
- 23.Frank SJ, Yi W, Zhao Y, Goldsmith JF, Gilliland G, Jiang J, Sakai I, Kraft AS. Regions of the JAK2 tyrosine kinase required for coupling to the growth hormone receptor. J Biol Chem. 1995;270:14776–14785. doi: 10.1074/jbc.270.24.14776. [DOI] [PubMed] [Google Scholar]
- 24.Tanner JW, Chen W, Young RL, Longmore GD, Shaw AS. The conserved box 1 motif of cytokine receptors is required for association with JAK kinases. J Biol Chem. 1995;270:6523–6530. doi: 10.1074/jbc.270.12.6523. [DOI] [PubMed] [Google Scholar]
- 25.He K, Wang X, Jiang J, Guan R, Bernstein KE, Sayeski PP, Frank SJ. Janus kinase 2 determinants for growth hormone receptor association, surface assembly, and signaling. Mol Endocrinol. 2003;17:2211–2227. doi: 10.1210/me.2003-0256. [DOI] [PubMed] [Google Scholar]
- 26.Hansen LH, Wang X, Kopchick JJ, Bouchelouche P, Nielsen JH, Galsgaard ED, Billestrup N. Identification of tyrosine residues in the intracellular domain of the growth hormone receptor required for transcriptional signaling and Stat5 activation. J Biol Chem. 1996;271:12669–12673. doi: 10.1074/jbc.271.21.12669. [DOI] [PubMed] [Google Scholar]
- 27.Smit LS, Meyer DJ, Billestrup N, Norstedt G, Schwartz J, Carter-Su C. The role of the growth hormone (GH) receptor and JAK1 and JAK2 kinases in the activation of Stats 1, 3, and 5 by GH. Mol Endocrinol. 1996;10:519–533. doi: 10.1210/mend.10.5.8732683. [DOI] [PubMed] [Google Scholar]
- 28.Sotiropoulos A, Moutoussamy S, Renaudie F, Clauss M, Kayser C, Gouilleux F, Kelly PA, Finidori J. Differential activation of Stat3 and Stat5 by distinct regions of the growth hormone receptor. Molec Endocrinol. 1996;10:998–1009. doi: 10.1210/mend.10.8.8843416. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Darus CJ, Xu BC, Kopchick JJ. Identification of growth hormone receptor (GHR) tyrosine residues required for GHR phosphorylation and JAK2 and STAT5 activation. Mol Endocrinol. 1996;10:1249–1260. doi: 10.1210/mend.10.10.9121492. [DOI] [PubMed] [Google Scholar]
- 30.Yi W, Kim SO, Jiang J, Park SH, Kraft AS, Waxman DJ, Frank SJ. Growth hormone receptor cytoplasmic domain differentially promotes tyrosine phosphorylation of signal transducers and activators of transcription 5b and 3 by activated JAK2 kinase. Mol Endocrinol. 1996;10:1425–1443. doi: 10.1210/mend.10.11.8923468. [DOI] [PubMed] [Google Scholar]
- 31.Bergad PL, Shih HM, Towle HC, Schwarzenberg SJ, Berry SA. Growth hormone induction of hepatic serine protease inhibitor 2.1 transcription is mediated by a Stat5-related factor binding synergistically to two gamma-activated sites. J Biol Chem. 1995;270:24903–24910. doi: 10.1074/jbc.270.42.24903. [DOI] [PubMed] [Google Scholar]
- 32.Davey HW, McLachlan MJ, Wilkins RJ, Hilton DJ, Adams TE. STAT5b mediates the GH-induced expression of SOCS-2 and SOCS-3 mRNA in the liver. Mol Cell Endocrinol. 1999;158:111–116. doi: 10.1016/s0303-7207(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 33.Davey HW, Xie T, McLachlan MJ, Wilkins RJ, Waxman DJ, Grattan DR. STAT5b is required for GH-induced liver IGF-I gene expression. Endocrinology. 2001;142:3836–3841. doi: 10.1210/endo.142.9.8400. [DOI] [PubMed] [Google Scholar]
- 34.Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci U S A. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ooi GT, Hurst KR, Poy MN, Rechler MM, Boisclair YR. Binding of STAT5a and STAT5b to a single element resembling a gamma-interferon-activated sequence mediates the growth hormone induction of the mouse acid-labile subunit promoter in liver cells. Molecular Endocrinology. 1998;12:675–687. doi: 10.1210/mend.12.5.0115. [DOI] [PubMed] [Google Scholar]
- 36.Woelfle J, Billiard J, Rotwein P. Acute control of insulin-like growth factor-I gene transcription by growth hormone through Stat5b. J Biol Chem. 2003;278:22696–22702. doi: 10.1074/jbc.M301362200. [DOI] [PubMed] [Google Scholar]
- 37.Moller C, Hansson A, Enberg B, Lobie PE, Norstedt G. Growth hormone (GH) induction of tyrosine phosphorylation and activation of mitogen-activated protein kinases in cells transfected with rat GH receptor cDNA. J Biol Chem. 1992;267:23403–23408. [PubMed] [Google Scholar]
- 38.Argetsinger LS, Hsu GW, Myers MGJ, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-gamma, and leukemia inhibitory factor promoted tyrosyl phosphorylation of insulin receptor substrate-1. J Biol Chem. 1995;270:14685–14692. doi: 10.1074/jbc.270.24.14685. [DOI] [PubMed] [Google Scholar]
- 39.Argetsinger LS, Norstedt G, Billestrup N, White MF, Carter-Su C. Growth hormone, interferon-gamma, and leukemia inhibitory factor utilize insulin receptor substrate-2 in intracellular signaling. J Biol Chem. 1996;271:29415–29421. doi: 10.1074/jbc.271.46.29415. [DOI] [PubMed] [Google Scholar]
- 40.Hodge C, Liao J, Stofega M, Guan K, Carter-Su C, Schwartz J. Growth hormone stimulates phosphorylation and activation of elk-1 and expression of c-fos, egr-1, and junB through activation of extracellular signal-regulated kinases 1 and 2. J Biol Chem. 1998;273:31327–31336. doi: 10.1074/jbc.273.47.31327. [DOI] [PubMed] [Google Scholar]
- 41.Liang L, Zhou T, Jiang J, Pierce JH, Gustafson TA, Frank SJ. Insulin receptor substrate-1 enhances growth hormone-induced proliferation. Endocrinology. 1999;140:1972–1983. doi: 10.1210/endo.140.5.6724. [DOI] [PubMed] [Google Scholar]
- 42.Kim SO, Houtman JC, Jiang J, Ruppert JM, Bertics PJ, Frank SJ. Growth hormone-induced alteration in ErbB-2 phosphorylation status in 3T3-F442A fibroblasts. J Biol Chem. 1999;274:36015–36024. doi: 10.1074/jbc.274.50.36015. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Chang Y, Wang X, Jiang J, Frank SJ. Growth hormone alters epidermal growth factor receptor binding affinity via activation of ERKs in 3T3-F442A cells. Endocrinology. 2004;145:3297–3306. doi: 10.1210/en.2003-1658. [DOI] [PubMed] [Google Scholar]
- 44.Huang Y, Kim SO, Jiang J, Frank SJ. Growth hormone-induced phosphorylation of epidermal growth factor (EGF) receptor in 3T3-F442A cells. Modulation of EGF-induced trafficking and signaling. J Biol Chem. 2003;278:18902–18913. doi: 10.1074/jbc.M300939200. [DOI] [PubMed] [Google Scholar]
- 45.Costoya JA, Finidori J, Moutoussamy S, Searis R, Devesa J, Arce VM. Activation of growth hormone receptor delivers an antiapoptotic signal: evidence for a role of Akt in this pathway. Endocrinology. 1999;140:5937–5943. doi: 10.1210/endo.140.12.7209. [DOI] [PubMed] [Google Scholar]
- 46.Jeay S, Sonenshein GE, Kelly PA, Postel-Vinay MC, Baixeras E. Growth hormone exerts antiapoptotic and proliferative effects through two different pathways involving nuclear factor-kappaB and phosphatidylinositol 3-kinase. Endocrinology. 2001;142:147–156. doi: 10.1210/endo.142.1.7892. [DOI] [PubMed] [Google Scholar]
- 47.Kilgour E, Gout I, Anderson NG. Requirement for phosphoinositide 3-OH kinase in growth hormone signalling to the mitogen-activated protein kinase and p70s6k pathways. Biochem J. 1996;315:517–522. doi: 10.1042/bj3150517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.MacKenzie SJ, Yarwood SJ, Peden AH, Bolger GB, Vernon RG, Houslay MD. Stimulation of p70S6 kinase via a growth hormone-controlled phosphatidylinositol 3-kinase pathway leads to the activation of a PDE4A cyclic AMP-specific phosphodiesterase in 3T3-F442A preadipocytes. Proc Natl Acad Sci U S A. 1998;95:3549–3554. doi: 10.1073/pnas.95.7.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liang L, Jiang J, Frank SJ. Insulin receptor substrate-1-mediated enhancement of growth hormone-induced mitogen-activated protein kinase activation. Endocrinology. 2000;141:3328–3336. doi: 10.1210/endo.141.9.7673. [DOI] [PubMed] [Google Scholar]
- 50.Schwartzbauer G, Menon RK. Regulation of growth hormone receptor gene expression. Mol Genet Metab. 1998;63:243–253. doi: 10.1006/mgme.1998.2685. [DOI] [PubMed] [Google Scholar]
- 51.Talamantes F, Ortiz R. Structure and regulation of expression of the mouse GH receptor. J Endocrinol. 2002;175:55–59. doi: 10.1677/joe.0.1750055. [DOI] [PubMed] [Google Scholar]
- 52.Edens A, Talamantes F. Alternative processing of growth hormone receptor transcripts. Endocr Rev. 1998;19:559–582. doi: 10.1210/edrv.19.5.0347. [DOI] [PubMed] [Google Scholar]
- 53.Landis CA, Harsh G, Lyons J, Davis RL, McCormick F, Bourne HR. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990;71:1416–1420. doi: 10.1210/jcem-71-6-1416. [DOI] [PubMed] [Google Scholar]
- 54.Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340:692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- 55.Lyons J, Landis CA, Harsh G, Vallar L, Grunewald K, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne HR, et al. Two G protein oncogenes in human endocrine tumors. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- 56.Spada A, Arosio M, Bochicchio D, Bazzoni N, Vallar L, Bassetti M, Faglia G. Clinical, biochemical, and morphological correlates in patients bearing growth hormone-secreting pituitary tumors with or without constitutively active adenylyl cyclase. J Clin Endocrinol Metab. 1990;71:1421–1426. doi: 10.1210/jcem-71-6-1421. [DOI] [PubMed] [Google Scholar]
- 57.Asa SL, Digiovanni R, Jiang J, Ward ML, Loesch K, Yamada S, Sano T, Yoshimoto K, Frank SJ, Ezzat S. A growth hormone receptor mutation impairs growth hormone autofeedback signaling in pituitary tumors. Cancer Res. 2007;67:7505–7511. doi: 10.1158/0008-5472.CAN-07-0219. [DOI] [PubMed] [Google Scholar]
- 58.Chia DJ, Subbian E, Buck TM, Hwa V, Rosenfeld RG, Skach WR, Shinde U, Rotwein P. Aberrant folding of a mutant Stat5b causes growth hormone insensitivity and proteasomal dysfunction. J Biol Chem. 2006;281:6552–6558. doi: 10.1074/jbc.M510903200. [DOI] [PubMed] [Google Scholar]
- 59.Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med. 2003;349:1139–1147. doi: 10.1056/NEJMoa022926. [DOI] [PubMed] [Google Scholar]
- 60.Brown RJ, Adams JJ, Pelekanos RA, Wan Y, McKinstry WJ, Palethorpe K, Seeber RM, Monks TA, Eidne KA, Parker MW, Waters MJ. Model for growth hormone receptor activation based on subunit rotation within a receptor dimer. Nat Struct Mol Biol. 2005;12:814–821. doi: 10.1038/nsmb977. [DOI] [PubMed] [Google Scholar]
- 61.van den Eijnden MJ, Lahaye LL, Strous GJ. Disulfide bonds determine growth hormone receptor folding, dimerisation and ligand binding. J Cell Sci. 2006;119:3078–3086. doi: 10.1242/jcs.03036. [DOI] [PubMed] [Google Scholar]
- 62.He K, Loesch K, Cowan JW, Li X, Deng L, Wang X, Jiang J, Frank SJ. JAK2 enhances the stability of the mature GH receptor. Endocrinology. 2005;145:4755–4765. doi: 10.1210/en.2005-0514. [DOI] [PubMed] [Google Scholar]
- 63.Loesch K, Deng L, Wang X, He K, Jiang J, Frank SJ. Endoplasmic reticulum-associated degradation of growth hormone receptor in Janus kinase 2-deficient cells. Endocrinology. 2007;148:5955–5965. doi: 10.1210/en.2007-0455. [DOI] [PubMed] [Google Scholar]
- 64.Silva CM, Day RN, Weber MJ, Thorner MO. Human growth hormone (GH) receptor is characterized as the 134-kilodalton tyrosine-phosphorylated protein activated by GH treatment in IM-9 cells. Endocrinology. 1993;133:2307–2312. doi: 10.1210/endo.133.5.7691587. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, He K, Gerhart M, Huang Y, Jiang J, Paxton RJ, Yang S, Lu C, Menon RK, Black RA, Baumann G, Frank SJ. Metalloprotease-mediated GH receptor proteolysis and GHBP shedding. Determination of extracellular domain stem region cleavage site. J Biol Chem. 2002;277:50510–50519. doi: 10.1074/jbc.M208738200. [DOI] [PubMed] [Google Scholar]
- 66.Loesch K, Deng L, Cowan JW, Wang X, He K, Jiang J, Black RA, Frank SJ. JAK2 influences growth hormone receptor metalloproteolysis. Endocrinology. 2006;147:2839–2849. doi: 10.1210/en.2005-1484. [DOI] [PubMed] [Google Scholar]
- 67.Romisch K. Endoplasmic reticulum-associated degradation. Annu Rev Cell Dev Biol. 2005;21:435–456. doi: 10.1146/annurev.cellbio.21.012704.133250. [DOI] [PubMed] [Google Scholar]
- 68.Ahner A, Brodsky JL. Checkpoints in ER-associated degradation: excuse me, which way to the proteasome? Trends Cell Biol. 2004;14:474–478. doi: 10.1016/j.tcb.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 69.Schmitz A, Herzog V. Endoplasmic reticulum-associated degradation: exceptions to the rule. Eur J Cell Biol. 2004;83:501–509. doi: 10.1078/0171-9335-00412. [DOI] [PubMed] [Google Scholar]
- 70.Gauzzi MC, Barbieri G, Richter MF, Uze G, Ling L, Fellous M, Pellegrini S. The amino-terminal region of Tyk2 sustains the level of interferon alpha receptor 1, a component of the interferon alpha/beta receptor. Proc Natl Acad Sci U S A. 1997;94:11839–11844. doi: 10.1073/pnas.94.22.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ragimbeau J, Dondi E, Alcover A, Eid P, Uze G, Pellegrini S. The tyrosine kinase Tyk2 controls IFNAR1 cell surface expression. Embo J. 2003;22:537–547. doi: 10.1093/emboj/cdg038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Royer Y, Staerk J, Costuleanu M, Courtoy PJ, Constantinescu SN. Janus kinases affect thrombopoietin receptor cell surface localization and stability. J Biol Chem. 2005;280:27251–27261. doi: 10.1074/jbc.M501376200. [DOI] [PubMed] [Google Scholar]
- 73.Radtke S, Hermanns HM, Haan C, Schmitz-Van De Leur H, Gascan H, Heinrich PC, Behrmann I. Novel role of Janus kinase 1 in the regulation of oncostatin M receptor surface expression. J Biol Chem. 2002;277:11297–11305. doi: 10.1074/jbc.M100822200. [DOI] [PubMed] [Google Scholar]
- 74.Radtke S, Jorissen A, de Leur HS, Heinrich PC, Behrmann I. Three dileucine-like motifs within the interbox1/2 region of the human oncostatin M receptor prevent efficient surface expression in the absence of an associated Janus kinase. J Biol Chem. 2006;281:4024–4034. doi: 10.1074/jbc.M511779200. [DOI] [PubMed] [Google Scholar]
- 75.Huang LJ, Constantinescu SN, Lodish HF. The N-terminal domain of Janus kinase 2 is required for Golgi processing and cell surface expression of erythropoietin receptor. Mol Cell. 2001;8:1327–1338. doi: 10.1016/s1097-2765(01)00401-4. [DOI] [PubMed] [Google Scholar]
- 76.Hofmann SR, Lam AQ, Frank S, Zhou YJ, Ramos HL, Kanno Y, Agnello D, Youle RJ, O’Shea JJ. Jak3-independent trafficking of the common gamma chain receptor subunit: chaperone function of Jaks revisited. Mol Cell Biol. 2004;24:5039–5049. doi: 10.1128/MCB.24.11.5039-5049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Moorthy AK, Savinova OV, Ho JQ, Wang VY, Vu D, Ghosh G. The 20S proteasome processes NF-kappaB1 p105 into p50 in a translation-independent manner. Embo J. 2006;25:1945–1956. doi: 10.1038/sj.emboj.7601081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cohen S, Lahav-Baratz S, Ciechanover A. Two distinct ubiquitin-dependent mechanisms are involved in NF-kappaB p105 proteolysis. Biochem Biophys Res Commun. 2006;345:7–13. doi: 10.1016/j.bbrc.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 79.Liu CW, Corboy MJ, DeMartino GN, Thomas PJ. Endoproteolytic activity of the proteasome. Science. 2003;299:408–411. doi: 10.1126/science.1079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alele J, Jiang J, Goldsmith JF, Yang X, Maheshwari HG, Black RA, Baumann G, Frank SJ. Blockade of growth hormone receptor shedding by a metalloprotease inhibitor. Endocrinology. 1998;139:1927–1935. doi: 10.1210/endo.139.4.5906. [DOI] [PubMed] [Google Scholar]
- 81.Wang X, He K, Gerhart M, Jiang J, Paxton RJ, Menon RK, Black RA, Baumann G, Frank SJ. Reduced proteolysis of rabbit growth hormone (GH) receptor substituted with mouse GH receptor cleavage site. Mol Endocrinol. 2003;17:1931–1943. doi: 10.1210/me.2003-0120. [DOI] [PubMed] [Google Scholar]
- 82.Baumann G. Growth hormone binding protein 2001. J Pediatr Endocrinol Metab. 2001;14:355–375. doi: 10.1515/jpem.2001.14.4.355. [DOI] [PubMed] [Google Scholar]
- 83.Baumann G, Frank SJ. Metalloproteinases and the modulation of GH signaling. J Endocrinol. 2002;174:361–368. doi: 10.1677/joe.0.1740361. [DOI] [PubMed] [Google Scholar]
- 84.Guan R, Zhang Y, Jiang J, Baumann CA, Black RA, Baumann G, Frank SJ. Phorbol ester- and growth factor-induced growth hormone (GH) receptor proteolysis and GH-binding protein shedding: relationship to GH receptor down-regulation. Endocrinology. 2001;142:1137–1147. doi: 10.1210/endo.142.3.8030. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Y, Guan R, Jiang J, Kopchick JJ, Black RA, Baumann G, Frank SJ. Growth hormone (GH)-induced dimerization inhibits phorbol ester-stimulated GH receptor proteolysis. J Biol Chem. 2001;276:24565–24573. doi: 10.1074/jbc.M101281200. [DOI] [PubMed] [Google Scholar]
- 86.Jiang J, Wang X, He K, Li X, Chen C, Sayeski PP, Waters MJ, Frank SJ. A Conformationally-sensitive GHR (Growth Hormone (GH) Receptor) Antibody: Impact on GH Signaling and GHR Proteolysis. Mol Endocrinol. 2004;18:2981–2996. doi: 10.1210/me.2004-0102. [DOI] [PubMed] [Google Scholar]
- 87.Cowan JW, Wang X, Guan R, He K, Jiang J, Baumann G, Black RA, Wolfe MS, Frank SJ. Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage. J Biol Chem. 2005;280:19331–19342. doi: 10.1074/jbc.M500621200. [DOI] [PubMed] [Google Scholar]
- 88.Zhang Y, Jiang J, Black RA, Baumann G, Frank SJ. TACE is a growth hormone binding protein sheddase: the metalloprotease TACE/ADAM-17 is critical for (PMA-induced) growth hormone receptor proteolysis and GHBP generation. Endocrinology. 2000;141:4324–4348. doi: 10.1210/endo.141.12.7858. [DOI] [PubMed] [Google Scholar]
- 89.Wang X, Jiang J, Warram J, Baumann G, Gan Y, Menon RK, Denson LA, Zinn KR, Frank SJ. Endotoxin-Induced Proteolytic Reduction in Hepatic Growth Hormone Receptor: A Novel Mechanism for GH Insensitivity. Mol Endocrinol. 2008 doi: 10.1210/me.2007-0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wolfe MS, Kopan R. Intramembrane proteolysis: theme and variations. Science. 2004;305:1119–1123. doi: 10.1126/science.1096187. [DOI] [PubMed] [Google Scholar]
- 91.Strous GJ, van Kerkhof P. The ubiquitin-proteasome pathway and the regulation of growth hormone receptor availability. Mol Cell Endocrinol. 2002;197:143–151. doi: 10.1016/s0303-7207(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 92.van Kerkhof P, Govers R, Alves dos Santos CM, Strous GJ. Endocytosis and degradation of the growth hormone receptor are proteasome-dependent. J Biol Chem. 2000;275:1575–1580. doi: 10.1074/jbc.275.3.1575. [DOI] [PubMed] [Google Scholar]
- 93.van Kerkhof P, Smeets M, Strous GJ. The ubiquitin-proteasome pathway regulates the availability of the GH receptor. Endocrinology. 2002;143:1243–1252. doi: 10.1210/endo.143.4.8755. [DOI] [PubMed] [Google Scholar]
- 94.van Kerkhof P, Sachse M, Klumperman J, Strous GJ. Growth hormone receptor ubiquitination coincides with recruitment to clathrin-coated membrane domains. J Biol Chem. 2001;276:3778–3784. doi: 10.1074/jbc.M007326200. [DOI] [PubMed] [Google Scholar]
- 95.Strous GJ, van Kerkhof P, Govers R, Ciechanover A, Schwartz AL. The ubiquitin conjugation system is required for ligand-induced endocytosis and degradation of the growth hormone receptor. Embo J. 1996;15:3806–3812. [PMC free article] [PubMed] [Google Scholar]
- 96.Govers R, ten Broeke T, van Kerkhof P, Schwartz AL, Strous GJ. Identification of a novel ubiquitin conjugation motif, required for ligand-induced internalization of the growth hormone receptor. Embo J. 1999;18:28–36. doi: 10.1093/emboj/18.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Allevato G, Billestrup N, Goujon L, Galsgaard ED, Norstedt G, Postel-Vinay MC, Kelly PA, Nielsen JH. Identification of phenylalanine 346 in the rat growth hormone receptor as being critical for ligand-mediated internalization and down- regulation. J Biol Chem. 1995;270:17210–17214. doi: 10.1074/jbc.270.29.17210. [DOI] [PubMed] [Google Scholar]
- 98.Roupas P, Herington AC. Intracellular processing of growth hormone receptors by adipocytes in primary culture. Mol Cell Endocrinol. 1988;57:93–99. doi: 10.1016/0303-7207(88)90037-8. [DOI] [PubMed] [Google Scholar]
- 99.Lesniak MA, Roth J. Regulation of receptor concentration by homologous hormone. Effect of human growth hormone on its receptor in IM-9 lymphocytes. J Biol Chem. 1976;251:3720–3729. [PubMed] [Google Scholar]
- 100.Hizuka N, Gorden P, Lesniak MA, Van Obberghen E, Carpentier JL, Orci L. Polypeptide hormone degradation and receptor regulation are coupled to ligand internalization. A direct biochemical and morphologic demonstration. J Biol Chem. 1981;256:4591–4597. [PubMed] [Google Scholar]
- 101.Takagi K, Saito Y, Sawada JI. Proteasomes are involved in the constitutive degradation of growth hormone receptors. Biol Pharm Bull. 2001;24:744–748. doi: 10.1248/bpb.24.744. [DOI] [PubMed] [Google Scholar]
- 102.Moulin S, Bouzinba-Segard H, Kelly PA, Finidori J. Jak2 and proteasome activities control the availability of cell surface growth hormone receptors during ligand exposure. Cell Signal. 2003;15:47–55. doi: 10.1016/s0898-6568(02)00054-2. [DOI] [PubMed] [Google Scholar]
- 103.Saito Y, Teshima R, Yamazaki T, Ikebuchi H, Sawada J. Ligand-induced internalization and phosphorylation-dependent degradation of growth hormone receptor in human IM-9 cells. Mol Cell Endocrinol. 1994;106:67–74. doi: 10.1016/0303-7207(94)90187-2. [DOI] [PubMed] [Google Scholar]
- 104.Alves dos Santos CM, ten Broeke T, Strous GJ. Growth hormone receptor ubiquitination, endocytosis, and degradation are independent of signal transduction via Janus kinase 2. J Biol Chem. 2001;276:32635–32641. doi: 10.1074/jbc.M103583200. [DOI] [PubMed] [Google Scholar]
- 105.Deng L, He K, Wang X, Yang N, Thangavel C, Jiang J, Fuchs SY, Frank SJ. Determinants of growth hormone receptor down-regulation. Mol Endocrinol. 2007;21:1537–1551. doi: 10.1210/me.2007-0138. [DOI] [PubMed] [Google Scholar]
- 106.Burke P, Schooler K, Wiley HS. Regulation of epidermal growth factor receptor signaling by endocytosis and intracellular trafficking. Mol Biol Cell. 2001;12:1897–1910. doi: 10.1091/mbc.12.6.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moutoussamy S, Renaudie F, Lago F, Kelly PA, Finidori J. Grb10 identified as a potential regulator of growth hormone (GH) signaling by cloning of GH receptor target proteins. J Biol Chem. 1998;273:15906–15912. doi: 10.1074/jbc.273.26.15906. [DOI] [PubMed] [Google Scholar]
- 108.Stofega MR, Herrington J, Billestrup N, Carter-Su C. Mutation of the SHP-2 binding site in growth hormone (GH) receptor prolongs GH-promoted tyrosyl phosphorylation of GH receptor, JAK2, and STAT5B. Mol Endocrinol. 2000;14:1338–1350. doi: 10.1210/mend.14.9.0513. [DOI] [PubMed] [Google Scholar]
- 109.Ram PA, Waxman DJ. SOCS/CIS protein inhibition of growth hormone-stimulated STAT5 signaling by multiple mechanisms. J Biol Chem. 1999;274:35553–35561. doi: 10.1074/jbc.274.50.35553. [DOI] [PubMed] [Google Scholar]
- 110.Du L, Frick GP, Tai LR, Yoshimura A, Goodman HM. Interaction of the growth hormone receptor with cytokine-induced Src homology domain 2 protein in rat adipocytes. Endocrinology. 2003;144:868–876. doi: 10.1210/en.2002-220830. [DOI] [PubMed] [Google Scholar]
- 111.Kim SO, Jiang J, Yi W, Feng GS, Frank SJ. Involvement of the Src homology 2-containing tyrosine phosphatase SHP-2 in growth hormone signaling. J Biol Chem. 1998;273:2344–2354. doi: 10.1074/jbc.273.4.2344. [DOI] [PubMed] [Google Scholar]
- 112.Zhang JG, Farley A, Nicholson SE, Willson TA, Zugaro LM, Simpson RJ, Moritz RL, Cary D, Richardson R, Hausmann G, Kile BJ, Kent SB, Alexander WS, Metcalf D, Hilton DJ, Nicola NA, Baca M. The conserved SOCS box motif in suppressors of cytokine signaling binds to elongins B and C and may couple bound proteins to proteasomal degradation. Proc Natl Acad Sci U S A. 1999;96:2071–2076. doi: 10.1073/pnas.96.5.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Landsman T, Waxman DJ. Role of the cytokine-induced SH2 domain-containing protein CIS in growth hormone receptor internalization. J Biol Chem. 2005;280:37471–37480. doi: 10.1074/jbc.M504125200. [DOI] [PubMed] [Google Scholar]
- 114.Kumar KG, Tang W, Ravindranath AK, Clark WA, Croze E, Fuchs SY. SCF(HOS) ubiquitin ligase mediates the ligand-induced down-regulation of the interferon-alpha receptor. Embo J. 2003;22:5480–5490. doi: 10.1093/emboj/cdg524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kumar KG, Krolewski JJ, Fuchs SY. Phosphorylation and specific ubiquitin acceptor sites are required for ubiquitination and degradation of the IFNAR1 subunit of type I interferon receptor. J Biol Chem. 2004;279:46614–46620. doi: 10.1074/jbc.M407082200. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Kumar KG, Tang W, Spiegelman VS, Fuchs SY. Negative regulation of prolactin receptor stability and signaling mediated by SCF(beta-TrCP) E3 ubiquitin ligase. Mol Cell Biol. 2004;24:4038–4048. doi: 10.1128/MCB.24.9.4038-4048.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Y, Clevenger CV, Minkovsky N, Kumar KG, Raghunath PN, Tomaszewski JE, Spiegelman VS, Fuchs SY. Stabilization of prolactin receptor in breast cancer cells. Oncogene. 2005 doi: 10.1038/sj.onc.1209214. [DOI] [PubMed] [Google Scholar]
- 118.Meyer L, Deau B, Forejtnikova H, Dumenil D, Margottin-Goguet F, Lacombe C, Mayeux P, Verdier F. Beta-Trcp mediates ubiquitination and degradation of the erythropoietin receptor and controls cell proliferation. Blood. 2007 doi: 10.1182/blood-2006-10-055350. [DOI] [PubMed] [Google Scholar]
- 119.Marijanovic Z, Ragimbeau J, Kumar KG, Fuchs SY, Pellegrini S. TYK2 activity promotes ligand-induced IFNAR1 proteolysis. Biochem J. 2006;397:31–38. doi: 10.1042/BJ20060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Swaminathan G, Varghese B, Thangavel C, Carbone CJ, Plotnikov A, Kumar KG, Jablonski EM, Clevenger CV, Goffin V, Deng L, Frank SJ, Fuchs SY. Prolactin stimulates ubiquitination, initial internalization, and degradation of its receptor via catalytic activation of Janus kinase 2. J Endocrinol. 2008;196:R1–7. doi: 10.1677/JOE-07-0554. [DOI] [PubMed] [Google Scholar]
- 121.Walrafen P, Verdier F, Kadri Z, Chretien S, Lacombe C, Mayeux P. Both proteasomes and lysosomes degrade the activated erythropoietin receptor. Blood. 2005;105:600–608. doi: 10.1182/blood-2004-03-1216. [DOI] [PubMed] [Google Scholar]
- 122.van Kerkhof P, Putters J, Strous GJ. The ubiquitin ligase SCF(betaTrCP) regulates the degradation of the growth hormone receptor. J Biol Chem. 2007;282:20475–20483. doi: 10.1074/jbc.M702610200. [DOI] [PubMed] [Google Scholar]
- 123.d’Azzo A, Bongiovanni A, Nastasi T. E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic. 2005;6:429–441. doi: 10.1111/j.1600-0854.2005.00294.x. [DOI] [PubMed] [Google Scholar]
- 124.Sigismund S, Polo S, Di Fiore PP. Signaling through monoubiquitination. Curr Top Microbiol Immunol. 2004;286:149–185. doi: 10.1007/978-3-540-69494-6_6. [DOI] [PubMed] [Google Scholar]
- 125.Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- 126.Raiborg C, Rusten TE, Stenmark H. Protein sorting into multivesicular endosomes. Curr Opin Cell Biol. 2003;15:446–455. doi: 10.1016/s0955-0674(03)00080-2. [DOI] [PubMed] [Google Scholar]
- 127.Polo S, Confalonieri S, Salcini AE, Di Fiore PP. EH and UIM: endocytosis and more. Sci STKE. 2003;2003:re17. doi: 10.1126/stke.2132003re17. [DOI] [PubMed] [Google Scholar]
- 128.Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol. 2003;19:141–172. doi: 10.1146/annurev.cellbio.19.110701.154617. [DOI] [PubMed] [Google Scholar]
- 129.Govers R, van Kerkhof P, Schwartz AL, Strous GJ. Di-leucine-mediated internalization of ligand by a truncated growth hormone receptor is independent of the ubiquitin conjugation system. J Biol Chem. 1998;273:16426–16433. doi: 10.1074/jbc.273.26.16426. [DOI] [PubMed] [Google Scholar]
- 130.Strous GJ, van Kerkhof P, Govers R, Rotwein P, Schwartz AL. Growth hormone-induced signal tranduction depends on an intact ubiquitin system. J Biol Chem. 1997;272:40–43. doi: 10.1074/jbc.272.1.40. [DOI] [PubMed] [Google Scholar]
- 131.Govers R, van Kerkhof P, Schwartz AL, Strous GJ. Linkage of the ubiquitin-conjugating system and the endocytic pathway in ligand-induced internalization of the growth hormone receptor. Embo J. 1997;16:4851–4858. doi: 10.1093/emboj/16.16.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alves dos Santos CM, van Kerkhof P, Strous GJ. The signal transduction of the growth hormone receptor is regulated by the ubiquitin/proteasome system and continues after endocytosis. J Biol Chem. 2001;276:10839–10846. doi: 10.1074/jbc.M003635200. [DOI] [PubMed] [Google Scholar]
- 133.van Kerkhof P, Strous GJ. The ubiquitin-proteasome pathway regulates lysosomal degradation of the growth hormone receptor and its ligand. Biochem Soc Trans. 2001;29:488–493. doi: 10.1042/bst0290488. [DOI] [PubMed] [Google Scholar]
- 134.Sachse M, van Kerkhof P, Strous GJ, Klumperman J. The ubiquitin-dependent endocytosis motif is required for efficient incorporation of growth hormone receptor in clathrin-coated pits, but not clathrin-coated lattices. J Cell Sci. 2001;114:3943–3952. doi: 10.1242/jcs.114.21.3943. [DOI] [PubMed] [Google Scholar]
- 135.Gent J, Van Den Eijnden M, Van Kerkhof P, Strous GJ. Dimerization and signal transduction of the growth hormone receptor. Mol Endocrinol. 2003;17:967–975. doi: 10.1210/me.2002-0261. [DOI] [PubMed] [Google Scholar]
- 136.Strous GJ, Gent J. Dimerization, ubiquitylation and endocytosis go together in growth hormone receptor function. FEBS Lett. 2002;529:102–109. doi: 10.1016/s0014-5793(02)03187-3. [DOI] [PubMed] [Google Scholar]
- 137.Kumar KG, Barriere H, Carbone CJ, Liu J, Swaminathan G, Xu P, Li Y, Baker DP, Peng J, Lukacs GL, Fuchs SY. Site-specific ubiquitination exposes a linear motif to promote interferon-alpha receptor endocytosis. J Cell Biol. 2007;179:935–950. doi: 10.1083/jcb.200706034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cadwell K, Coscoy L. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science. 2005;309:127–130. doi: 10.1126/science.1110340. [DOI] [PubMed] [Google Scholar]