Abstract
Functional MRI (fMRI) studies of mild cognitive impairment (MCI) and Alzheimer’s disease (AD) have begun to reveal abnormalities in memory circuit function in humans suffering from memory disorders. Since the medial temporal lobe (MTL) memory system is a site of very early pathology in AD, a number of studies, reviewed here, have focused on this region of the brain. By the time individuals are diagnosed clinically with AD dementia, the substantial memory impairments appear to be associated with not only MTL atrophy but also hypoactivation during memory task performance. Prior to dementia, when individuals are beginning to manifest signs and symptoms of memory impairment (MCI), the hippocampal formation and other components of the MTL memory system exhibit substantial functional abnormalities during memory task performance. It appears that, early in the course of MCI when memory deficits and hippocampal atrophy are less prominent, there may be hyperactivation of MTL circuits, possibly representing inefficient compensatory activity. Later in the course of MCI, when considerable memory deficits are present, MTL regions are no longer able to activate during attempted learning, as is the case in AD dementia. Recent fMRI data in MCI and AD are beginning to reveal relationships between abnormalities of functional activity in the MTL memory system and in functionally connected brain regions, such as the precuneus. As this work continues to mature, it will likely contribute to our understanding of fundamental memory processes in the human brain and how these are perturbed in memory disorders. We hope these insights will translate into the incorporation of measures of task-related brain function into diagnostic assessment or therapeutic monitoring, such as for use in clinical trials.
Keywords: Alzheimer’s disease, mild cognitive impairment, functional magnetic resonance imaging, hippocampus, entorhinal cortex, parahippocampal gyrus
Introduction
Alzheimer’s disease (AD) is the most common cause of dementia (Kukull and Bowen, 2002). Typically, the symptoms of the disease begin with mild memory difficulties after the sixth decade of life and progress slowly toward significant impairment in memory, executive function, visuospatial abilities, language, and other domains of cognition. Eventually, impaired cognitive abilities interfere with complex activities of daily life and ultimately result in the loss of independent function. Current treatments are symptomatic, in that clinical trials demonstrate short-term benefits in cognitive function but not a slowing of the rate of decline (Cummings, 2004). Increasing emphasis is being placed on the development of disease-modifying therapies to impede the underlying neurodegenerative process of AD and thereby slow the rate of cognitive decline.
By the time AD is typically diagnosed, substantial neuronal loss and neuropathologic change have damaged many brain regions. Although it may be possible to reverse some aspects of this damage, it would be ideal to initiate treatment with neuroprotective medications at a time when—or even before—AD is mildly symptomatic (DeKosky and Marek, 2003). To approach this goal, our capability needs to be improved to identify individuals with very mild symptoms prior to dementia (Dickerson and Sperling, 2005). Currently, individuals are classified as having mild cognitive impairment (MCI) when symptoms suggestive of AD are present but mild enough that traditional diagnostic criteria (which require functional impairment consistent with dementia) are not fulfilled. This gradual transitional state may last for a number of years, and diagnostic criteria have been developed (Petersen et al., 1999) and operationalized (Grundman et al., 2004). Efforts are currently underway by international groups of experts to revise the diagnostic criteria for AD with the goal of diagnosis prior to dementia—one proposed criteria set already makes explicit use of imaging and cerebrospinal fluid biomarkers (Dubois et al., 2007).
The cortex of the medial temporal lobe (MTL) subserves fundamental mnemonic functions, providing critical input from heteromodal association cortices to the hippocampal formation and receiving reciprocal afferents from the hippocampal formation (Van Hoesen and Pandya, 1975a; Van Hoesen et al., 1975; Van Hoesen and Pandya, 1975b). Even at a pre-dementia clinical stage of MCI, significant AD pathology is present in the MTL memory system. The entorhinal and perirhinal cortices are devastated by neurofibrillary pathology and cell loss very early in the course of AD, “disconnecting” the hippocampal formation from neocortical afferents and efferents, and the disease is thought to spread quickly to involve fields of the hippocampal formation (Hyman et al., 1984; Braak and Braak, 1991; Price et al., 1991; Gomez-Isla et al., 1996; Van Hoesen et al., 2000; Kordower et al., 2001).
Since an amnesic syndrome is typically the earliest symptom of AD, it is critical to further our understanding of abnormalities of the function of the medial temporal lobe memory system early in the course of AD. One promising technique for this purpose is functional magnetic resonance imaging (fMRI), which is thought to provide an in vivo correlate of neural activity. Given the growing body of evidence that alterations in synaptic function are present very early in the disease process, possibly long before the development of clinical symptoms and even significant neuropathology (Selkoe, 2002; Coleman et al., 2004), fMRI may be particularly useful for detecting alterations in brain function that may be present very early in the course of AD. In this article, we will review fMRI data regarding functional abnormalities of the medial temporal lobe memory system in MCI and AD.
Functional MRI as a tool to probe brain activity in MCI and AD
Since functional neuroimaging tools assess inherently dynamic processes that may change over short time intervals in relation to a host of factors, these measures have unique characteristics that may offer both strengths and weaknesses as potential biomarkers of neurologic disease. Functional neuroimaging measures may be affected by transient brain and body states at the time of imaging, such as arousal, attention, sleep deprivation, sensory processing of irrelevant stimuli, or the effects of substances with pharmacologic central nervous system activity. Imaging measures of brain function may also be more sensitive than structural measures to constitutional or chronic differences between individuals, such as genetics, intelligence or educational level, learning, mood, or medication use. While these may be effects of interest in certain experimental settings, they need to be controlled when the focus is on disease-related changes and differences between subject groups or within individuals over time.
Among functional neuroimaging techniques, fMRI has many potential advantages in studying patients with neurodegenerative disorders, as it is a non-invasive imaging technique that does not require the injection of contrast agent. It can be repeated many times over the course of a longitudinal study and thus lends itself well as a measure in clinical drug trials. It has relatively high spatial and temporal resolution, and the use of event-related designs enables the hemodynamic correlates of specific behavioral events, such as successful memory formation (Dickerson et al., 2007a), to be measured.
There are, however, significant challenges to performing fMRI studies in cognitively impaired patients. The technique is particularly sensitive to even small amounts of head motion. Differences in task performance between patient and control groups complicate data interpretation (Price and Friston, 1999). Finally, it is critical to complete further reliability experiments if fMRI is to be used in longitudinal or pharmacologic studies. Although there are now a few studies of fMRI test-retest reliability in young subjects (Machielsen et al., 2000; Manoach et al., 2001; Sperling et al., 2002), reproducibility studies are only beginning to be performed in MCI and AD patients.
FMRI in MCI and AD
Functional MRI has been used to investigate abnormalities in patterns of regional brain activation during a variety of cognitive tasks in patients diagnosed with mild AD compared to control subjects. It is important to keep in mind that the particular abnormalities found in an fMRI study of an AD or other patient group are heavily dependent on the type of behavioral task used in the study—if the task does not engage a particular circuit, functional abnormalities will not likely be observed. Also, the nature of functional abnormalities may depend on whether the activated brain regions are directly affected by the disease, are indirectly affected via connectivity, or are not pathologically affected. Tools are now available to directly investigate the overlap of disease-related alterations in brain structure and task-related functional activity (see Figure 1). Yet it should also be kept in mind that even brain regions not usually thought to be affected by AD (sensorimotor areas) have been shown to exhibit abnormal function in AD patients (Buckner et al., 2000; D’Esposito et al., 2003).
Figure 1.
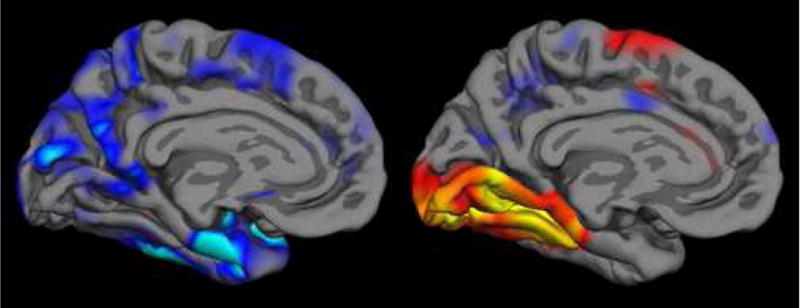
The localization, magnitude, and extent of abnormalities observed in fMRI studies of patients with neurologic diseases depend on both localization and severity of pathology and on functional networks engaged by the particular fMRI task, as well as participant performance on the task. In this illustration, regions of cortical thinning in Alzheimer’s disease from structural MRI (left, (Dickerson et al., 2007b)) are compared with cortical areas activated, as measured with fMRI, in normals during an event-related study of successful learning of new information that was able to later be freely recalled (right, (Dickerson et al., 2005a)). Analytic tools are emerging that enable the direct investigation of relationships between functional and structural abnormalities in MCI/AD and other disorders. Figure used with permission. (WE NEED TO REQUEST PERMISSION FROM NeuroRx, Elsevier—Dickerson BC, July 2007)
Abnormalities in activation of the medial temporal lobe memory system in AD and MCI
With respect to memory, a number of fMRI studies in patients with clinically diagnosed AD, using a variety of visually presented stimuli, have identified decreased activation in hippocampal and parahippocampal regions compared to control subjects during episodic encoding tasks (Small et al., 1999; Rombouts et al., 2000; Kato et al., 2001; Machulda et al., 2003; Sperling et al., 2003b). Neocortical abnormalities in AD have also been demonstrated using fMRI, including decreased activation in temporal and prefrontal regions. In addition to AD-related differences in task-related blood-oxygen level dependent (BOLD) signal amplitude or spatial extent, the temporal dynamics of activation appear to be altered in patients with AD (Rombouts et al., 2005). And as has been observed in other types of tasks, increased activation in prefrontal and other regions has also been found in AD patients performing memory tasks (Sperling et al., 2003b).
Our fMRI studies of MCI and AD have employed primarily two memory tasks, one item-based and one associative task. These paradigms focus on the encoding of stimuli using three conditions grouped in blocks: “Novel” stimuli, seen only once during the scanning procedure; “Repeated” stimuli, shown to subjects prior to the scanning procedure and then shown repeatedly during specific blocks; and “Fixation” on a crosshair as a relatively passive baseline condition. The primary comparison of interest is the Novel vs. Repeated contrast, which holds the visual complexity of the stimuli constant, and provides information about new learning (i.e., processing relevant to the encoding of stimuli for later memory testing as well as processing relevant to novelty). Subjects are instructed explicitly to try to remember the stimuli for later testing. All of the scanning is performed during the encoding phase, and subjects are tested with a post-scan recognition test.
Depending on the task, different components of the MTL memory system have been activated. In our studies of the encoding of complex indoor and outdoor scenes, the caudal parahippocampal cortex and body of the hippocampal formation were activated, while in our studies of the encoding of face-name paired associates, the rostral hippocampal formation and entorhinal cortex were activated. In addition, both paradigms activate ventral temporal and inferior prefrontal cortices. Given that AD pathology is thought to progress along a rostro-caudal gradient in the MTL, there has been surprisingly little study of the relative sensitivity (to disease effects) of fMRI paradigms that activate rostral vs. caudal MTL regions. Little comparison has been made of tasks that activate different regions of the MTL memory system (hippocampal vs. entorhinal vs. perirhinal) in MCI/AD. The paucity of this sort of data is a result, in large part, of the notorious technical difficulties involved in obtaining fMRI data in the ventromedial regions of the brain due to susceptibility artifacts and distortions. Advances in fMRI technology will be critical for the field to achieve the goal of robustly testing hypotheses about the activity of MTL subregions (Small et al., 2001; Zeineh et al., 2003; Dickerson, 2007).
In a study of mild AD using the face-name paradigm, the mild AD subjects showed lesser activation in the hippocampal formation bilaterally compared to cognitively intact control subjects (Sperling et al., 2003b). Several neocortical regions, including frontal cortices, showed increased activation in the mild AD patients compared to controls. These findings are consistent with other recent reports demonstrating a relative lack of MTL activation in patients with clinical AD dementia, and suggest that additional regions, not typically activated in the task in young and older controls, may be recruited during performance of this task in individuals with AD dementia.
With respect to MCI, a handful of fMRI studies have been published to date and the results, thus far, have been inconsistent (see Table for summary). In comparison to older controls, Machulda et al. reported that, during the encoding of novel pictures, MTL activation was decreased to a similar degree in patients with MCI and AD patients (Machulda et al., 2003). In a face-encoding paradigm, Small et al. reported heterogeneity in MTL activation in memory-impaired subjects, with some showing hypoactivation similar to that of AD patients. Other subjects showed entorhinal and hippocampal activation that was similar to controls, but had decreased activation in the subiculum (Small et al., 1999). Using a face-name associative paradigm, Petrella et al. (Petrella et al., 2006) found no differences between MCI and controls in MTL activation during encoding, but observed left hippocampal hypoactivation in MCI vs. controls during the retrieval (forced-choice recognition) condition. Hippocampal hypoactivation in MCI was no longer seen when memory performance accuracy was included as a covariate in the analysis. Using an item-based old/new recognition retrieval paradigm, Johnson et al. found right hippocampal hypoactivation in MCI patients compared to controls (Johnson et al., 2006). In a separate study, Johnson et al. used a paradigm involving the repetitive presentation of faces to demonstrate that MCI patients do not show the same slope of decreasing hippocampal activation with face repetition that is seen in older controls, suggesting disruption of this “adaptive” response in the medial temporal lobe (Johnson et al., 2004).
TABLE.
| Study | Populatio n/ Design |
Level of impairment |
Memory Task |
Level of performa nce |
Analysis | Contrast | Measure | Findings | Morphometry |
|---|---|---|---|---|---|---|---|---|---|
| Small et al. (1999) | Isolated memory impairment (N=12; age=78.3) vs. NC vs. AD; community-based longitudinal cohort | N.R. | Encoding:novel faces (block) | N.R. | Individual subject native space, anatomic ROI constrained (hippocampus, subiculum, entorhinal cortex ROIs) | Novel vs. Fixation | Extent (# voxels above threshold) | Heterogeneity of activation in IMI; one subgroup with hypoactivation in all ROIs; one with hypoactivation only in subiculum | N.R. |
| Machulda et al. (2003) | MCI (N=9; age=76.5) vs. NC vs. AD; clinic-based cohort | MMSE 28.4; memory performance of MCI group similar to that of AD group (AVLT) | Encoding:novel line drawings of people (block) | Impaired vs. controls (post-scan recognition memory similar to that of AD group) | Individual subject native space, anatomic ROI constrained (large ventromedial temporal lobe ROI) | Novel vs. Fixation | Extent (# voxels above threshold); receiver operating curve analysis used to assess range of thresholds | Hypoactivation in MCI | N.R. |
| Dickerson et al. (2004) | Within-group MCI (N=32; age=75); community-based longitudinal cohort | Range of impairment within MCI group | Encoding:novel vs. repeated scenes (block) | Range of performance within MCI group | Individual subject native space, anatomic ROI constrained (hippocampus, parahippocampal gyrus) | Novel vs. Repeated; Novel vs. Fixation | Extent of activation (# voxels activated controlling statistically for volume of ROI); % signal change | Hyperactivation (extent) in relatively more impaired MCI | Range of hippocampal volume within MCI group, relating directly to CDR-SB and memory performance |
| Johnson et al. (2004) | MCI (N=12; age=79.1) vs. NC vs. AD; community-based longitudinal cohort | MMSE 27.3±1.4; memory performance > 2 S.D. below NC | Encoding:multiple repetitions of faces | N.R. | Group template space, voxel-based whole brain SPM analysis; signal modeled using adaptation slopes | Encoding vs. Fixation | Magnitude/extent composite (SPM) | Hypoactivation | No atrophy of ventromedial temporal lobe (VBM) |
| Dickerson et al. (2005) | Very mild MCI (N=9; age=73.9) vs. NC vs. AD; community-based longitudinal cohort | MMSE 29.6±0.5; CDR-SB 0.94±0.5; memory performance similar to NC | Encoding:novel vs. repeated face-name pairs (block) | Similar to NC | Individual subject native space, anatomic ROI constrained (hippocampus, entorhinal cortex ROIs) | Novel vs. Repeated; Novel vs. Fixation | Extent of activation (# voxels activated controlling statistically for volume of ROI); also % signal change | Hyperactivation (extent) in very mild MCI vs. NC | Minimally reduced entorhinal and hippocampal volumes in MCI compared to NC |
| Celone et al. (2006) | Very mild MCI (N=15; age=75.1) vs. MCI (N=12; age=80) vs. NC vs. AD; community-based longitudinal cohort | vMCI: MMSE29.3±0.9; CDR-SB 1.1±0.4; mildly impaired memory performance; MCI: MMSE 28.6±1.2; CDR-SB 2.5±0.6; mildly impaired memory performance | Encoding:novel vs. repeated face-name pairs (block) | Similar to NC | Group template space, voxel-based whole brain SPM analysis | Independent component analysis (ICA) | Extent of activation (# voxels activated) | Hyperactivation in bilateral hippocampus in very mild MCI; hypoactivation in more impaired MCI | N.R. |
| Hamlainen et al. (2006) | MCI (N=14; age=72.4) vs. NC vs. AD; community-based longitudinal cohort MCI (N=14; age=73.7) | MMSE 25.6±3.1; CDR-SB 1.6±0.6, memory performance > 1.5 S.D. below NC | Encoding:novel verbally cued line drawings of objects (block) | Mildly impaired vs. NC but not as impaired as AD | Group template space, voxel-based whole brain SPM analysis | Novel vs. Fixation | Magnitude/extent composite (SPM) | Hyperactivation of left hippocampus and bilateral parahippocamp al gyrus vs. NC | Bilateral hippocampal atrophy in MCI using VBM but no difference in hippocampal volumes between MCI and NC using manual volumetrics |
| Johnson et al. (2006) | MCI (N=14; age=73.7) vs. NC; clinic-based group | MMSE 28.6±1.5; memory performance > 2 S.D. below NC | Retrieval:line drawings; old/new recognition decision (block) | Similar to controls | Group template space, voxel-based whole brain SPM analysis | Novel vs. Previously Learned | Magnitude/extent composite (SPM) | Hypoactivation in right hippocampus vs. NC | N.R. |
| Petrella et a. (2006) | MCI (N=20; age=65) vs. NC; community-based group | MMSE 26.7±1.5; memory performance > 2 S.D. below NC | Encoding:novel vs. repeated face-name pairs; Retrieval:forced choice (block) | Impaired vs. controls | Group template space, voxel-based whole brain SPM analysis | Novel vs. Repeated | Magnitude/extent composite (SPM) | No MTL differences between MCI and NC during encoding; hypoactivation in left hippocampus during retrieval (but no MTL differences between MCI and NC when task performance was regressed out) | N.R. |
| Kircher et al. (2007) | MCI (N=21; age=69.7) vs. NC; clinic-based group | MMSE 26.6±1.4; memory performance > 1 S.D. below NC | Encoding:visually presented words(event-related) with old/new recognition test | Similar to controls | Group template space, voxel-based whole brain SPM analysis | Successful “Hits” vs. Fixation (encoding trials sorted based on recognition performance) | Magnitude/extent composite (SPM) | Hyperactivation of left hippocampus | N.R. |
NC = cognitively intact “normal” controls
N.R. = not reported
Three studies have now demonstrated greater MTL activation in MCI patients compared to controls. We used the associative face-name encoding paradigm described above to compare MTL activation in very mild MCI, AD, and controls (Dickerson et al., 2005b). Compared with controls, MCI subjects showed a greater extent of hippocampal activation and a trend toward greater entorhinal activation (Figure 2). This group of MCI subjects was very mildly impaired based on Clinical Dementia Rating (CDR) (Morris et al., 1997) ratings, MMSE, and neuropsychological data, as well as fMRI memory task performance (which was similar to controls). Furthermore, there was minimal atrophy of the hippocampal formation or entorhinal cortex in this MCI group. The AD patients had smaller MTL volumes and a lesser degree of activation in these regions, and performed below controls on the post-scan memory test. Across all the subjects in the three groups, post-scan memory task performance correlated with extent of activation in both the entorhinal cortex and hippocampus.
Figure 2.
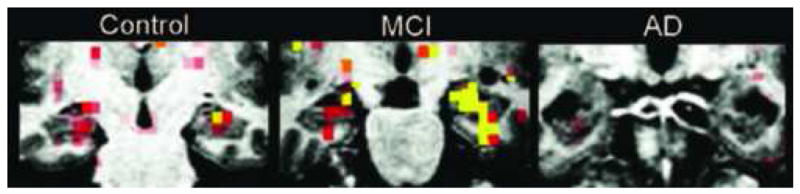
A phase of compensatory hyperactivation appears to occur in the medial temporal lobe (MTL) in very mild mild cognitive impairment, prior to AD dementia. Representative single subjects from each group, showing normal memory-related MTL activation measured with fMRI in Normal Older Controls, hyperactivation and very mild atrophy in MCI, and hypoactivation and more prominent atrophy in mild AD (Dickerson et al., 2005b). Color scale indicates p values of greater significance over threshold from red to orange to yellow. Figure used with permission. (WE NEED TO REQUEST PERMISSION FROM NeuroRx, Elsevier—Dickerson BC, July 2007)
Using a visual object encoding paradigm, Hamalainen et al. found that MCI subjects had greater activation (than controls) of caudal hippocampal formation, parahippocampal gyrus, and fusiform cortex (Hamalainen et al., 2006). Based on MMSE and neuropsychological data, the MCI subjects in this study were on the relatively more impaired end of the MCI spectrum (although CDR-SB was still mildly impaired), yet the group performed the fMRI memory paradigm relatively well—better than the AD group—although not as well as controls. In the first event-related subsequent memory study of MCI, Kircher et al. used an item-based task with words and found that MCI subjects activated rostral left hippocampal and surrounding cortical regions to a greater degree than controls (Kircher et al., 2007). MMSE scores from these MCI participants suggested that the group was at the more impaired end of the MCI spectrum, but neuropsychological data indicated milder impairment—in fact, delayed verbal recall scores were minimally impaired relative to controls, with scores for the MCI participants ranging as high as 14 items freely recalled after a 20 minute delay in this 15-item test. In addition, the MCI participants performed similarly to controls on the fMRI memory paradigm.
The variability in fMRI data from MCI subjects probably relates, at least in part, to the complex relationships between the severity of the subjects’ clinical impairment and to their ability to perform the memory task employed as the fMRI paradigm. This hypothesis is based primarily on data from our initial fMRI study of MCI, which was a within-group investigation of individuals who spanned a broad range of MCI, the common feature of which was an overall CDR rating of 0.5 (Dickerson et al., 2004), as well as a more recent investigation separating the MCI spectrum into two subgroups, one on the milder end and one on the more impaired end (this investigation (Celone et al., 2006) is discussed below). Because we explicitly sought to study a broad range of impairment within the spectrum of MCI, we did not require subjects to perform below a particular cutoff on neuropsychologic memory tests, and thus some subjects were included who performed relatively well on neuropsychological testing, despite symptoms of memory impairment in daily life.
Thirty-two subjects with a total CDR rating of 0.5 and CDR Sum of Boxes (CDR-SB) scores ranging from 0.5 – 3.0 were studied. We focused on analysis of functional activation in the novel vs. familiar scene-encoding paradigm in the hippocampal formation and the parahippocampal gyrus, and the volumes of these regions were also quantified. As expected, there was an inverse linear relationship between the degree of clinical impairment (CDR-SB) and volume of MTL ROIs, most significant in the left hippocampus, such that subjects with greater clinical impairment had smaller hippocampal volumes. Interestingly, however, there was a direct linear relationship between the degree of clinical impairment and the extent (number of voxels activated) of fMRI activation bilaterally in both the hippocampus and parahippocampus, such that subjects with greater clinical impairment had a relatively larger extent of MTL activation. This “paradoxical” relationship was still apparent after correction for volume, and a multivariate analysis showed that greater clinical impairment (as measured by the CDR-SB) was associated with older age, increased extent of activation in the right parahippocampal gyrus, and decreased volume of the left hippocampus. Furthermore, similarly to the data from the face-name paradigm described above, better performance on the post-scan recognition memory task correlated with greater MTL activation and larger volume.
Based on these data, we believe that discrepant findings on memory-related MTL activation in the MCI literature may potentially be explained, at least in part, by differences in the level of clinical impairment and in performance on the fMRI memory task between the subject groups. From the descriptions of the clinical data from the studies above (and detailed in the Table), it is clear that these clinical-behavioral measures are not necessarily completely correlated with each other—that is, some samples of MCI subjects may appear relatively more impaired from certain clinical measures (e.g., symptom-based measures such as CDR), less impaired on other clinical measures (e.g., neuropsychological performance), and may or may not be able to perform the fMRI behavioral task used for activation at a level comparable to controls. Further research is needed to clarify these relationships, a deeper understanding of which is critical to our interpretation of imaging data.
There are also a number of other factors that likely contribute to variability in MTL activation, which may vary between studies in a manner that could also explain some of the discrepancies, including differences in the memory tasks themselves (e.g., encoding vs. retrieval; visual vs. verbal material; paired-associate vs. item-based memory, etc.), differences in analysis methods (i.e., voxel-based whole-brain vs. focused region-of-interest approaches) and dependent variables used to determine level of activation (i.e., magnitude vs. extent of activation vs. voxel-based measures that take both magnitude and extent into account), and differences in age, education, and apolipoprotein E genotype (Dickerson et al., 2005b). Aside from the details of the studies, the table highlights the broad scope of methodologic variability that makes it difficult to compare fMRI studies of MCI. Additional research will be critical to the further elucidation of the contributions of these and other factors to variability in fMRI measures, if such measures are to be translated into biomarkers for clinical trials. It will be important to study structure-function relationships to better understand the relationships between anatomic abnormalities (e.g., hippocampal and entorhinal atrophy, as well as neocortical atrophy) and functional hypo- and hyperactivation. Multimodal investigations including positron emission tomography (PET) measures of metabolism and pathology will likely be helpful to ensure that patients have abnormalities consistent with AD, as well as to determine relationships between the localization and severity of these abnormalities and alterations in functional activation. Longitudinal studies—involving both clinical and imaging follow-up data—will probably shed a great deal of light on the variety of contributors to fMRI abnormalities in MCI. Finally, a multi-center study in which investigators agree on the use of a particular set of behavioral task paradigms and data collection methods would potentially enable these issues to be further clarified, and would enable data analysis methods to be compared (Friedman et al., 2007). In all of these future investigations, we believe it is important to provide detailed demographic, clinical, neuropsychological, and behavioral performance data to help clarify the similarities or differences between samples of MCI subjects in fMRI studies. In addition, it may be helpful to report hippocampal volumes as well.
Despite all the caveats, there is replicated evidence to support the hypothesis that there may be a phase of increased MTL activation in MCI. This increase, which also may be present in cognitively intact carriers of the APOE-ε4 allele (for review, see (Wierenga and Bondi, 2007)), may represent an attempted compensatory response to AD neuropathology, given that some MCI individuals with smaller hippocampal volume perform similarly on memory tasks to MCI individuals with larger hippocampal volume but have relatively greater MTL activation (Dickerson et al., 2004; Hamalainen et al., 2006) (Figure 3). Additional studies employing event-related fMRI paradigms (Sperling et al., 2003a; Dickerson et al., 2007a; Kircher et al., 2007) will be very helpful in determining whether increased MTL activation in MCI patients is specifically associated with successful memory, as opposed to a general effect that is present regardless of success (possibly indicating increased effort). It is possible that MTL hyperactivation reflects cholinergic or other neurotransmitter upregulation in MCI patients (DeKosky et al., 2002). Alternatively, increased regional brain activation may be a marker of the pathophysiologic process of AD itself, such as aberrant sprouting of cholinergic fibers (Hashimoto and Masliah, 2003) or inefficiency in synaptic transmission (Stern et al., 2004). It is important, however, to acknowledge that multiple non-neural factors may confound the interpretation of changes in the hemodynamic response measured by BOLD fMRI, such as age- and disease-related changes in neurovascular coupling (Buckner et al., 2000; D’Esposito et al., 2003), AD-specific alterations in vascular physiology (Mueggler et al., 2002), and resting hypoperfusion and metabolism in MCI and AD (El Fakhri et al., 2003), which may result in an amplified BOLD fMRI signal during activation (Davis et al., 1998; Cohen et al., 2002). Further research to determine the specificity of hyperactivation with respect to particular brain regions and behavioral conditions will be valuable to better characterize this phenomenon.
Figure 3.
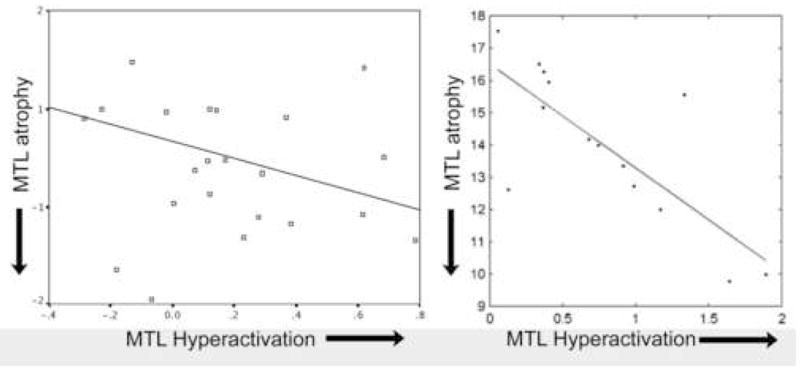
A greater degree of hyperactivation of MTL regions is present in MCI individuals with a greater degree of MTL (hippocampal) atrophy, supporting the possible compensatory role of hyperactivation for AD pathology. Data shown here are taken from two separate studies, Dickerson et al. (Dickerson et al., 2004), left, and Hamalainen et al (Hamalainen et al., 2006), right. Analyses were performed differently in these studies so quantitative values on axes are not directly comparable, but represent conceptually similar measures (both are p<0.05).
A number of authors have hypothesized that MTL and other cortical hyperactivation during the performance of memory and other cognitive tasks may play, at least in part, a compensatory role for neuropathologic abnormalities in MCI/mild AD (Becker et al., 1996; Backman et al., 1999; Stern et al., 2000). “Compensation” is typically defined as greater regional brain activity (hyperactivation) in an MCI/AD group in the setting of task performance accuracy that is similar to that of a matched control group. Regional hyperactivation may involve greater magnitude of activity in brain regions typically active during performance of the task (when performed by controls), or the recruitment of additional brain regions not normally engaged by controls. However, it is also clear that greater task difficulty may provoke similar alterations in regional brain activity in healthy individuals (Gur et al., 1988; Grasby et al., 1994; Grady, 1996; Rypma and D’Esposito, 1999). It is challenging to know to what degree MCI/AD groups find memory tasks to be “more difficult” than they would in the absence of disease. This has led some investigators to attempt to match task difficulty between MCI/AD patients and controls (Stern et al., 2000). It is also possible that different cognitive strategies during memory task performance (e.g., semantic elaborative encoding strategies vs. visualization strategies) may contribute to differences in the recruitment of particular brain regions (Kirchhoff and Buckner, 2006), and that this may vary between patient and control groups. Further work in this area, including longitudinal studies in MCI/AD patients, ideally including detailed behavioral measures of reaction time as well as accuracy and possibly self report of task difficulty, will be important to better clarify the situations in which activity increases can be reasonably interpreted as compensatory for brain disease.
Despite these caveats, we believe that the accumulating evidence indicates that task-related regional brain hyperactivation may be a universal neural response to insult, as it occurs in sleep deprivation (Drummond et al., 2000), aging (Cabeza et al., 2002), and a variety of neuropsychiatric disorders and conditions, including AD/MCI, Huntington’s disease (Rosas et al., 2004), Parkinson’s disease (Monchi et al., 2004), cerebrovascular disease (Carey et al., 2002; Johansen-Berg et al., 2002), multiple sclerosis (Reddy et al., 2000; Morgen et al., 2004), traumatic brain injury (McAllister et al., 1999), HIV (Ernst et al., 2002), alcoholism (Desmond et al., 2003), schizophrenia (Callicott et al., 2003). In many of these studies, task-related regional brain hyperactivation was associated with the relative preservation of performance on the task, suggesting that hyperactivation may be serving, at least in part, a compensatory role for neurologic insult. The evidence discussed above also indicates that increased MTL activation can be seen in MCI in the setting of minimal MTL atrophy (Dickerson et al., 2005b), which provides in vivo support for laboratory and animal data suggesting that physiologic alterations may precede significant structural abnormalities very early in the course of a neurodegenerative disease such as AD (Selkoe, 2002; Walsh and Selkoe, 2004) and may represent inefficient neural circuit function (Stern et al., 2004). Thus, fMRI may provide a means to detect changes in human memory circuit function that underlie the earliest symptoms of AD, and may be useful in identifying groups of subjects at high risk for future cognitive decline prior to a diagnosis of AD.
MTL hyperactivation as a predictive biomarker in MCI
In the original study of the spectrum of MCI, we showed that the subgroup of individuals who converted to AD dementia within 2.5 years of clinical follow-up after scanning were those with the greatest MTL hyperactivation when originally scanned (Dickerson et al., 2004). We recently extended this study to include about 4 years of follow-up after scanning in 25 of the subjects in the original sample. Over the follow-up interval, subjects demonstrated a wide range of cognitive decline, with some showing no change and others progressing to dementia (change in CDR-SB ranged from 0 to 4.5). The degree of cognitive decline was predicted by hippocampal activation at the time of baseline scanning, with greater hippocampal activation predicting greater decline (p<0.05) (Figure 4). This finding was present even after controlling for baseline degree of impairment (CDR-SB), age, education, and hippocampal volume. These data suggest that fMRI may provide a physiologic imaging biomarker useful for identifying the subgroup of MCI individuals at highest risk of cognitive decline for potential inclusion in disease-modifying clinical trials.
Figure 4.
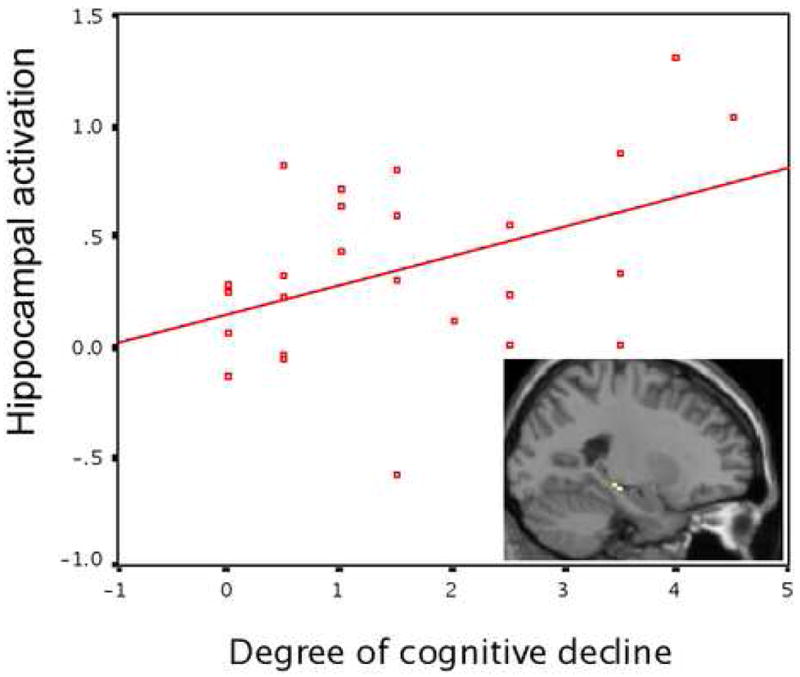
Memory-related MTL activation as a predictive quantitative imaging biomarker. In a group of MCI patients, hippocampal activation at baseline (y axis) predicts degree of cognitive decline (change in CDR-SB, x axis) over four years after scanning (Miller et al., 2006) (p<0.05).
If, in fact, the “inverse U-shaped curve” of hyperactivation that we hypothesize takes place early in the course of prodromal AD (at the clinical stage of MCI) is confirmed by future longitudinal studies, then the use of fMRI as a physiologic imaging biomarker will have to grapple with the problem of “pseudonormalization” of activation when individuals with MCI demonstrate progressive decline that results in the loss of hyperactivation. It may be possible to use a combination of clinical (e.g., CDR Sum-of-Boxes), neuropsychologic (e.g., memory tasks), and anatomic (e.g., hippocampal and/or entorhinal volume) measures to assist in the determination of where an individual is along the inverse U-shaped curve of MTL activation. That is, moderate hyperactivation in the setting of minimal clinical and memory impairment and relatively little MTL atrophy would be consistent with the upgoing phase of the hyperactivation curve while the same level of hyperactivation in the setting of more prominent clinical and memory impairment and MTL atrophy would be consistent with the downgoing phase of the curve. In the end, it will be critical to perform longitudinal studies to determine whether this model of the physiologic, anatomic, and behavioral progression of MCI is supported by trajectories in individuals and groups of subjects.
Links between task-related brain function abnormalities and altered resting brain activity
Recent fMRI studies are beginning to reveal a link between disease-related hemodynamic alterations and the well-described resting perfusion/metabolic abnormalities in AD. Hypoperfusion/metabolism is typically seen with nuclear medical imaging techniques (such as FDG-PET or SPECT) in lateral temporoparietal and posterior cingulate and precuneus cortical regions in AD patients during the “resting” state. The medial parietal/posterior cingulate cortex, along with medial frontal and lateral parietal regions, appear to compose a “default mode” network that is more active when individuals are not engaged in particular tasks, and which is thought to play a role in vigilance, readiness, or monitoring—these regions “deactivate” (BOLD signal amplitude falls below baseline) during cognitive task performance (Raichle et al., 2001). Several recent studies in AD patients have demonstrated alterations in the de-activation and functional connectivity of these regions, suggesting that this “default mode” network is disrupted by the disease (Lustig et al., 2003; Greicius et al., 2004; Celone et al., 2006; Rombouts et al., 2006). It appears that the MTL may be an important node of this network (Greicius et al., 2004; Vincent et al., 2006), which has been hypothesized as playing a role in memory function (Greicius et al., 2004; Buckner et al., 2005), but further study will be needed to determine the roles that MTL regions may play in relation to the neocortical nodes of the network during specific memory activities (e.g., encoding vs. retrieval) (Daselaar et al., 2004). Tantalizing evidence has also emerged recently that substantial overlap is present between these “default mode” areas and the localization of PET amyloid tracer binding (Buckner et al., 2005); exploration of the relationships between pathologic markers and memory-related MTL activation will be a fertile area for future investigation.
Functional connectivity of the MTL and neocortical nodes of the larger scale memory network
As more is learned about the task-related function of default mode brain regions, it is becoming clear that at least some of these regions—including the precuneus—play important roles in encoding that are predictive of subsequent memory. That is, the deactivation of the precuneus, presumably at the same time that the hippocampus activates, is critical for information to be encoded in a manner that leads to successful retrieval (Daselaar et al., 2004). Other default-mode brain regions, such as lateral parietal cortex, are important for the correct recognition of previously encountered stimuli (Wagner et al., 2005).
We recently used independent component analysis to identify brain regions with temporally synchronous brain activity during the face-name associative memory paradigm described above (Celone et al., 2006). The study included cognitively intact older adults, a very mildly impaired MCI group, a more impaired MCI group, and an AD group. We found a strong reciprocal relationship between the degree of memory-related MTL activation and the deactivation of precuneus and lateral parietal regions, elements of the default-mode network. Furthermore, we found evidence supporting the non-linear trajectory of functional abnormalities across the spectrum of MCI, with the very mildly impaired MCI subjects showing MTL hyperactivation and precuneus “hyper-deactivation,” while the more impaired MCI subjects and AD patients demonstrated MTL hypoactivation and hypo-deactivation (Figure 5). These findings suggest that the pathophysiologic process of AD disrupts the functioning of multiple nodes of the MTL-neocortical memory network, as well as the coordinated activity between these nodes, which may be related to pathology within the nodes (Arnold et al., 1991) or the deafferenting of parietal cortex in the setting of MTL pathology (Meguro et al., 1999).
Figure 5.
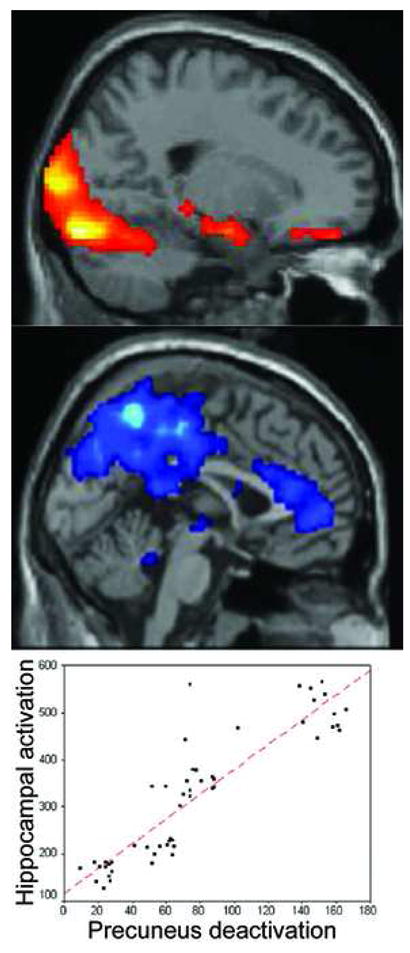
Reciprocal MTL-medial parietal memory network. MTL is hyperactivated in very mildly impaired MCI individuals compared to controls (top), while precuneus is hyper-deactivated (middle). Across a group including cognitively intact, MCI, and AD individuals, the degree of hippocampal activation (x axis) correlates with the degree of precuneus deactivation (y axis) (Celone et al., 2006) (p<0.0001).
Conclusions
FMRI is a particularly attractive method for studying cognitive task-related patterns of brain activation in MCI and AD. Despite the relative infancy of the field, there have already been a number of promising fMRI studies in AD, MCI, and related disorders which highlight the potential uses of fMRI in both basic and clinical spheres of investigation. FMRI may provide novel insights into the neural correlates of memory and other cognitive abilities, and how they are altered in AD and MCI. Finally, fMRI measures hold promise for multiple clinical applications, including early detection and differential diagnosis, predicting future change in clinical status or cognitive performance, and as a marker of alterations in brain physiology related to potential therapeutic agents (Dickerson, 2006). The greatest potential of fMRI likely lies in the study of very early AD, at the point of subtle neuronal dysfunction. However, a number of challenges remain.
Since a definitive diagnosis of AD and related neurodegenerative diseases can only be made at autopsy, neuroimaging studies of these disorders face challenges related to clinicopathologic heterogeneity; this is particularly true for MCI. Although all patients with AD progress through some form of an MCI phase prior to dementia, the converse is not true. That is, some patients who fulfill MCI criteria may have non-AD disease states (Petersen et al., 2006). Furthermore, the rate at which individuals with MCI decline within this diagnostic category and ultimately develop dementia may vary considerably. Thus, although prodromal AD may be identifiable as MCI clinically (Grundman et al., 2004), it is important to recognize the heterogeneity present within this clinical construct. Continued efforts to further refine clinical diagnostic (Petersen, 2004) and staging methods (Daly et al., 2000) should help improve our understanding of the relationships between the characteristics of individuals with MCI and imaging data. Thus, while the data reviewed above indicates that fMRI is sensitive to clinical diagnosis, symptom severity, and memory performance abilities, the discrepancies highlight the need for further fMRI research in the context of rigorous clinical assessment, longitudinal follow-up, and ideally multimodal imaging (i.e., volumetric structural MRI, perfusion measures, and nuclear medicine studies of metabolism and pathology).
Acknowledgments
Supported by grants from the NIA (K23-AG22509), the NINDS (K23-NS02189), and the Beeson Scholars in Aging Program (American Federation of Aging Research).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- Backman L, Andersson JL, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- Becker JT, Mintun MA, Aleva K, Wiseman MB, Nichols T, DeKosky ST. Compensatory reallocation of brain resources supporting verbal episodic memory in Alzheimer’s disease. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol (Berl) 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Sanders AL, Raichle ME, Morris JC. Functional brain imaging of young, nondemented, and demented older adults. J Cogn Neurosci. 2000;12(Suppl 2):24–34. doi: 10.1162/089892900564046. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Snyder AZ, Shannon BJ, LaRossa G, Sachs R, Fotenos AF, Sheline YI, Klunk WE, Mathis CA, Morris JC, Mintun MA. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. doi: 10.1176/appi.ajp.160.12.2209. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–788. doi: 10.1093/brain/awf091. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen ER, Ugurbil K, Kim SG. Effect of basal conditions on the magnitude and dynamics of the blood oxygenation level-dependent fMRI response. J Cereb Blood Flow Metab. 2002;22:1042–1053. doi: 10.1097/00004647-200209000-00002. [DOI] [PubMed] [Google Scholar]
- Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology. 2004;63:1155–1162. doi: 10.1212/01.wnl.0000140626.48118.0a. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Alzheimer’s disease. N Engl J Med. 2004;351:56–67. doi: 10.1056/NEJMra040223. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Deouell LY, Gazzaley A. Alterations in the BOLD fMRI signal with ageing and disease: a challenge for neuroimaging. Nat Rev Neurosci. 2003;4:863–872. doi: 10.1038/nrn1246. [DOI] [PubMed] [Google Scholar]
- Daly E, Zaitchik D, Copeland M, Schmahmann J, Gunther J, Albert M. Predicting conversion to Alzheimer disease using standardized clinical information. Arch Neurol. 2000;57:675–680. doi: 10.1001/archneur.57.5.675. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Prince SE, Cabeza R. When less means more: deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95:1834–1839. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward to move forward: early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Ikonomovic MD, Styren SD, Beckett L, Wisniewski S, Bennett DA, Cochran EJ, Kordower JH, Mufson EJ. Upregulation of choline acetyltransferase activity in hippocampus and frontal cortex of elderly subjects with mild cognitive impairment. Ann Neurol. 2002;51:145–155. doi: 10.1002/ana.10069. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Dickerson BC. Functional magnetic resonance imaging of cholinergic modulation in mild cognitive impairment. Curr Opin Psychiatry. 2006;19:299–306. doi: 10.1097/01.yco.0000218602.25346.c6. [DOI] [PubMed] [Google Scholar]
- Dickerson BC. Advances in Functional Magnetic Resonance Imaging: Technology and Clinical Applications. Neurotherapeutics. 2007;4:360–370. doi: 10.1016/j.nurt.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Sperling RA. Neuroimaging Biomarkers for Clinical Trials of Disease-Modifying Therapies in Alzheimer’s Disease. Neurorx. 2005;2:348–360. doi: 10.1602/neurorx.2.2.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Miller S, Fenstermacher E, Greve D, Dale A, Albert M, Sperling R. Regional brain activity during encoding that predicts successful free recall vs. recognition: An event-related fMRI study. Society for Neuroscience meeting abstracts 2005a [Google Scholar]
- Dickerson BC, Miller SL, Greve DN, Dale AM, Albert MS, Schacter DL, Sperling RA. Prefrontal-hippocampal-fusiform activity during encoding predicts intraindividual differences in free recall ability: An event-related functional-anatomic MRI study. Hippocampus. 2007a doi: 10.1002/hipo.20338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005b;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Rosas HD, Sperling RA, Atri A, Wright CI, Grodstein F, Blacker D, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer’s disease (AD): A high-throughput in vivo MRI-based quantitative biomarker; Massachusetts Alzheimer’s Disease Research Center 20th Annual Scientific Poster Symposium; Boston, MA. 2007b. [Google Scholar]
- Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delacourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O’Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- El Fakhri G, Kijewski MF, Johnson KA, Syrkin G, Killiany RJ, Becker JA, Zimmerman RE, Albert MS. MRI-guided SPECT perfusion measures and volumetric MRI in prodromal Alzheimer disease. Arch Neurol. 2003;60:1066–1072. doi: 10.1001/archneur.60.8.1066. [DOI] [PubMed] [Google Scholar]
- Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59:1343–1349. doi: 10.1212/01.wnl.0000031811.45569.b0. [DOI] [PubMed] [Google Scholar]
- Friedman L, Stern H, Brown GG, Mathalon DH, Turner J, Glover GH, Gollub RL, Lauriello J, Lim KO, Cannon T, Greve DN, Bockholt HJ, Belger A, Mueller B, Doty MJ, He J, Wells W, Smyth P, Pieper S, Kim S, Kubicki M, Vangel M, Potkin SG. Test-retest and between-site reliability in a multicenter fMRI study. Hum Brain Mapp. 2007 doi: 10.1002/hbm.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer’s disease. J Neurosci. 1996;16:4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady CL. Age-related changes in cortical blood flow activation during perception and memory. Ann N Y Acad Sci. 1996;777:14–21. doi: 10.1111/j.1749-6632.1996.tb34396.x. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Simpson J, Fletcher PC, Frackowiak RS, Dolan RJ. A graded task approach to the functional mapping of brain areas implicated in auditory-verbal memory. Brain. 1994;117 (Pt 6):1271–1282. doi: 10.1093/brain/117.6.1271. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, Van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Arch Neurol. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE, Skolnick BE, Resnick SM, Silver FL, Chawluk J, Muenz L, Obrist WD, Reivich M. Effects of task difficulty on regional cerebral blood flow: relationships with anxiety and performance. Psychophysiology. 1988;25:392–399. doi: 10.1111/j.1469-8986.1988.tb01874.x. [DOI] [PubMed] [Google Scholar]
- Hamalainen A, Pihlajamaki M, Tanila H, Hanninen T, Niskanen E, Tervo S, Karjalainen PA, Vanninen RL, Soininen H. Increased fMRI responses during encoding in mild cognitive impairment. Neurobiol Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Masliah E. Cycles of aberrant synaptic sprouting and neurodegeneration in Alzheimer’s and dementia with Lewy bodies. Neurochem Res. 2003;28:1743–1756. doi: 10.1023/a:1026073324672. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science. 1984;225:1168–1170. doi: 10.1126/science.6474172. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–2742. doi: 10.1093/brain/awf282. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42:980–989. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Schmitz TW, Moritz CH, Meyerand ME, Rowley HA, Alexander AL, Hansen KW, Gleason CE, Carlsson CM, Ries ML, Asthana S, Chen K, Reiman EM, Alexander GE. Activation of brain regions vulnerable to Alzheimer’s disease: the effect of mild cognitive impairment. Neurobiol Aging. 2006;27:1604–1612. doi: 10.1016/j.neurobiolaging.2005.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T, Knopman D, Liu H. Dissociation of regional activation in mild AD during visual encoding: a functional MRI study. Neurology. 2001;57:812–816. doi: 10.1212/wnl.57.5.812. [DOI] [PubMed] [Google Scholar]
- Kircher T, Weis S, Freymann K, Erb M, Jessen F, Grodd W, Heun R, Leube DT. Hippocampal activation in MCI patients is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007 doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhoff BA, Buckner RL. Functional-anatomic correlates of individual differences in memory. Neuron. 2006;51:263–274. doi: 10.1016/j.neuron.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, Mufson EJ. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. [PubMed] [Google Scholar]
- Kukull WA, Bowen JD. Dementia epidemiology. Med Clin North Am. 2002;86:573–590. doi: 10.1016/s0025-7125(02)00010-x. [DOI] [PubMed] [Google Scholar]
- Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100:14504–14509. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Witter MP. FMRI of visual encoding: reproducibility of activation. Hum Brain Mapp. 2000;9:156–164. doi: 10.1002/(SICI)1097-0193(200003)9:3<156::AID-HBM4>3.0.CO;2-Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61:500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Halpern EF, Kramer TS, Chang Y, Goff DC, Rauch SL, Kennedy DN, Gollub RL. Test-retest reliability of a functional MRI working memory paradigm in normal and schizophrenic subjects. Am J Psychiatry. 2001;158:955–958. doi: 10.1176/appi.ajp.158.6.955. [DOI] [PubMed] [Google Scholar]
- McAllister TW, Saykin AJ, Flashman LA, Sparling MB, Johnson SC, Guerin SJ, Mamourian AC, Weaver JB, Yanofsky N. Brain activation during working memory 1 month after mild traumatic brain injury: a functional MRI study. Neurology. 1999;53:1300–1308. doi: 10.1212/wnl.53.6.1300. [DOI] [PubMed] [Google Scholar]
- Meguro K, Blaizot X, Kondoh Y, Le Mestric C, Baron JC, Chavoix C. Neocortical and hippocampal glucose hypometabolism following neurotoxic lesions of the entorhinal and perirhinal cortices in the non-human primate as shown by PET. Implications for Alzheimer’s disease. Brain. 1999;122 (Pt 8):1519–1531. doi: 10.1093/brain/122.8.1519. [DOI] [PubMed] [Google Scholar]
- Miller S, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in MCI predicts subsequent cognitive decline. International Conference on Alzheimer’s Disease. 2006 doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson’s disease. J Neurosci. 2004;24:702–710. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgen K, Kadom N, Sawaki L, Tessitore A, Ohayon J, McFarland H, Frank J, Martin R, Cohen LG. Training-dependent plasticity in patients with multiple sclerosis. Brain. 2004;127:2506–2517. doi: 10.1093/brain/awh266. [DOI] [PubMed] [Google Scholar]
- Morris JC, Ernesto C, Schafer K, Coats M, Leon S, Sano M, Thal LJ, Woodbury P. Clinical dementia rating training and reliability in multicenter studies: the Alzheimer’s Disease Cooperative Study experience. Neurology. 1997;48:1508–1510. doi: 10.1212/wnl.48.6.1508. [DOI] [PubMed] [Google Scholar]
- Mueggler T, Sturchler-Pierrat C, Baumann D, Rausch M, Staufenbiel M, Rudin M. Compromised hemodynamic response in amyloid precursor protein transgenic mice. J Neurosci. 2002;22:7218–7224. doi: 10.1523/JNEUROSCI.22-16-07218.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- Petrella JR, Krishnan S, Slavin MJ, Tran TT, Murty L, Doraiswamy PM. Mild cognitive impairment: evaluation with 4-T functional MR imaging. Radiology. 2006;240:177–186. doi: 10.1148/radiol.2401050739. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer’s disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy H, Narayanan S, Arnoutelis R, Jenkinson M, Antel J, Matthews PM, Arnold DL. Evidence for adaptive functional changes in the cerebral cortex with axonal injury from multiple sclerosis. Brain. 2000;123 (Pt 11):2314–2320. doi: 10.1093/brain/123.11.2314. [DOI] [PubMed] [Google Scholar]
- Rombouts SA, Barkhof F, Veltman DJ, Machielsen WC, Witter MP, Bierlaagh MA, Lazeron RH, Valk J, Scheltens P. Functional MR imaging in Alzheimer’s disease during memory encoding. AJNR Am J Neuroradiol. 2000;21:1869–1875. [PMC free article] [PubMed] [Google Scholar]
- Rombouts SARB, Goekoop R, J SC, Barkhof F, Scheltens P. Delayed rather than decreased BOLD response as a marker for early Alzheimer’s disease. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Rombouts SARB, Barkhof F, Goekoop R, J SC, Scheltens P. Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Hum Brain Mapp. 2006 doi: 10.1002/hbm.20160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas HD, Feigin AS, Hersch SM. Using advances in neuroimaging to detect, understand, and monitor disease progression in Huntington’s disease. NeuroRx. 2004;1:263–272. doi: 10.1602/neurorx.1.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M. The roles of prefrontal brain regions in components of working memory: effects of memory load and individual differences. Proc Natl Acad Sci U S A. 1999;96:6558–6563. doi: 10.1073/pnas.96.11.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selkoe DJ. Alzheimer’s disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Small SA, Nava AS, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Circuit mechanisms underlying memory encoding and retrieval in the long axis of the hippocampal formation. Nat Neurosci. 2001;4:442–449. doi: 10.1038/86115. [DOI] [PubMed] [Google Scholar]
- Sperling R, Chua E, Cocchiarella A, Rand-Giovannetti E, Poldrack R, Schacter DL, Albert M. Putting names to faces: successful encoding of associative memories activates the anterior hippocampal formation. Neuroimage. 2003a;20:1400–1410. doi: 10.1016/S1053-8119(03)00391-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R, Greve D, Dale A, Killiany R, Holmes J, Rosas HD, Cocchiarella A, Firth P, Rosen B, Lake S, Lange N, Routledge C, Albert M. Functional MRI detection of pharmacologically induced memory impairment. Proc Natl Acad Sci U S A. 2002;99:455–460. doi: 10.1073/pnas.012467899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003b;74:44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern EA, Bacskai BJ, Hickey GA, Attenello FJ, Lombardo JA, Hyman BT. Cortical synaptic integration in vivo is disrupted by amyloid-beta plaques. J Neurosci. 2004;24:4535–4540. doi: 10.1523/JNEUROSCI.0462-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Moeller JR, Anderson KE, Luber B, Zubin NR, DiMauro AA, Park A, Campbell CE, Marder K, Bell K, Van Heertum R, Sackeim HA. Different brain networks mediate task performance in normal aging and AD: defining compensation. Neurology. 2000;55:1291–1297. doi: 10.1212/wnl.55.9.1291. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Res. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Res. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Res. 1975b;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer’s disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000;911:254–274. doi: 10.1111/j.1749-6632.2000.tb06731.x. [DOI] [PubMed] [Google Scholar]
- Vincent JL, Snyder AZ, Fox MD, Shannon BJ, Andrews JR, Raichle ME, Buckner RL. Coherent spontaneous activity identifies a hippocampal-parietal memory network. J Neurophysiol. 2006;96:3517–3531. doi: 10.1152/jn.00048.2006. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Walsh DM, Selkoe DJ. Deciphering the molecular basis of memory failure in Alzheimer’s disease. Neuron. 2004;44:181–193. doi: 10.1016/j.neuron.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Wierenga CE, Bondi MW. Use of functional magnetic resonance imaging in the early identification of Alzheimer’s disease. Neuropsychol Rev. 2007;17:127–143. doi: 10.1007/s11065-007-9025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeineh MM, Engel SA, Thompson PM, Bookheimer SY. Dynamics of the hippocampus during encoding and retrieval of face-name pairs. Science. 2003;299:577–580. doi: 10.1126/science.1077775. [DOI] [PubMed] [Google Scholar]


