Abstract
The Hsp90 multichaperone complex has important roles in the development and progression of malignant transformation. Several small-molecule inhibitors of Hsp90 of diverse chemotypes have shown potent antitumor activity in a wide-range of malignancies, and are currently in clinical or late-stage preclinical investigation. This review intends to update the reader on advances made over the past two years in the clinical development of Hsp90 inhibitors in advanced cancers. It will refer to the two 17-AAG formulations, tanespimycin and IPI-504, and to synthetic small molecules, among which are the purine-scaffold Hsp90 inhibitor CNF2024/BIIB021, the isoxazole derivative VER-52296/NVP-AUY922, and the carbazol-4-one benzamide derivative SNX-5422, and will present our current knowledge on their clinical performance.
Introduction
The Hsp90 superchaperone complex has wide-ranging functions that result from the ability of this sophisticated machinery to assist in the folding and function of a variety of oncogenic ‘client proteins’ [1,2]. In this sense, multiple proteins involved in cell-specific oncogenic processes have been shown to be tightly regulated by the binding of the Hsp90 machinery. These include BCR-ABL in the chronic myelogenous leukemia (CML) [3], nucleophosmin-anaplastic lymphoma kinase (NPM–ALK) in lymphomas [4], mutated FLT3 in acute myeloid leukemia [5], EGFR harboring kinase mutations in nonsmall cell lung cancer (NSCLC) [6], the zeta-associated protein of 70 kDa (ZAP-70) as expressed in patients with aggressive chronic lymphocytic leukemia (CLL) [7], mutant B-Raf in melanoma [8], human epidermal growth factor receptor 2 (HER2) in HER2-overexpressing breast cancer [9], mutant c-Kit in gastrointestinal stromal tumors (GIST) [10], and activated Akt in small cell lung carcinoma [11], to list a few. It is now accepted that at the phenotypic level, the Hsp90 machinery serves as a biochemical buffer for the numerous cancer-specific lesions that are characteristic of diverse tumors. Pharmacologic inhibition of Hsp90 by structurally diverse small molecules destabilizes the cancer cell's aberrant protein subset, leading to protein degradation by the 26S proteasome [12]. Selective depletion of the cancer cell's malignancy driving molecules results in growth arrest, apoptosis, and renders cells vulnerable to the actions of chemotherapeutic interventions that otherwise afford limited benefit [1,2]. Moreover, cancer cells are selectively sensitive to pharmacologic Hsp90 inhibitors, and administration of these agents to multiple cancer animal models results in significant antitumor effects associated mostly with little or no target-associated toxicities [1,2,13].
The successful validation of Hsp90 as a target in cancer through the use of pharmacologic agents has catalyzed the development of these small-molecule tools into anticancer therapeutics [14]. This review will focus on advances made over the past two years in the clinical translation and development of several Hsp90 inhibitor chemotypes.
Geldanamycin-based Hsp90 inhibitors
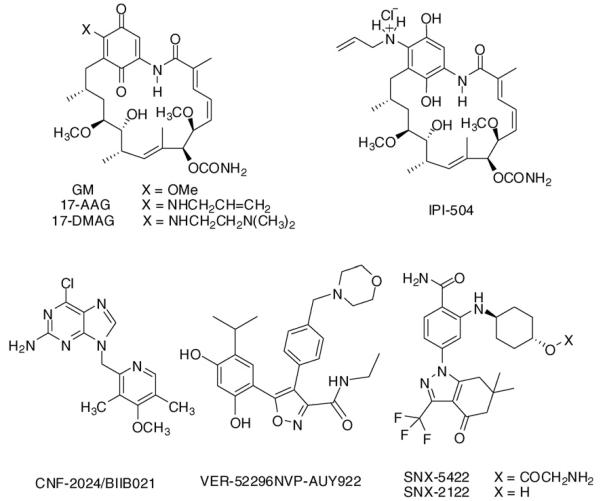
The first Hsp90 inhibitor to enter clinic was the geldanamycin (GM) derivative 17-allylamino-17-desmethoxygeldanamycin (17-AAG) (Figure 1). Initial clinical evaluation of 17-AAG was of limited success, with hints of activity demonstrated in melanoma, where stable disease (SD) was reported [14]. Improvements in this drug's formulation and delivery have led to more encouraging results in several difficult-to-treat patient populations. Kosan Biosciences has developed both a Cremophorcontaining formulation and an injectable suspension formulation of 17-AAG (tanespimycin, KOS-953). At the 2007 Annual Meeting of the American Society of Hematology (ASH), results of a Phase Ib dose-escalating trial that evaluated tanespimycin with bortezomib in patients with relapsed, refractory multiple myeloma were reported [15]. Dose escalations in the entire trial ranged from 100 to 340 mg/m2 for tanespimycin, and from 0.7 to 1.3 mg/m2 for bortezomib. In the bortezomib-naive group, the overall response rate (complete, partial, and minor responses) was 47% (9 out of 19 evaluable patients), including 2 complete responses (CRs), 1 near-CR, 2 partial responses (PRs), and 4 minor responses (MRs). In the bortezomib-pretreated group, the overall response rate was 47% (7 out of 15 evaluable patients; 1 CR, 2 PR, and 4 MR). In the bortezomib-refractory group, the overall response rate was 17% (3 out of 18 evaluable patients; 3 PR). In an interesting twist, neuropathy cases, a common side effect seen with bortezomid, were fewer in the combination studies than in bortezomid alone, suggesting a possible neuroprotective effect of tanespimycin. Although the mechanism of this effect has not been yet elucidated, it may relate to induction of Hsp70, a chaperone with antiapototic and misfolding-protective abilities, by Hsp90 inhibitors [16]. Kosan has been granted orphan drug status for tanespimycin in multiple myeloma in the US and in Europe (http://www.kosan.com/clinical-programs.html).
Figure 1.
Chemical structure of several Hsp90 inhibitors currently in clinical evaluation in patients with advanced cancers.
Tanespimycin has also recently demonstrated promising antitumor activity and tolerability in a Phase II trial in patients with HER2-positive metastatic breast cancer, when administered in combination with trastuzumab (Herceptin®) in patients whose disease progressed following treatment with trastuzumab [17]. Twenty-five patients were enrolled onto four tanespimycin dose levels: 225 mg/m2 (n = 4), 300 mg/m2 (n = 3), 375 mg/m2 (n = 8), and 450 mg/m2 (n = 10). On subsequent weeks, trastuzumab 2 mg/kg was administered over 30 min, followed by tanespimycin. One patient with trastuzumab-refractory HER2-positive breast cancer had a confirmed PR by Response Evaluation Criteria in Solid Tumors (RECIST) criteria. Three additional patients with HER2 amplified breast cancer had tumor regressions of 25, 22, and 21%. Overall, the noted antitumor activity was one PR, four MR, and four SD. Interestingly, tumor regressions were seen only in patients with HER2-positive metastatic breast cancer. An update of the trial was presented at the 2008 ASCO meeting, with a reported 24% response rate and a clinical benefit rate of 57% [18]. Recently, Bristol-Myers Squibb has agreed to acquire Kosan, and continue the development of tanespimycin in MM and metastatic breast cancer (http://newsroom.bms.com/article_display.cfm?article_id=5336).
In a different approach, Infinity Pharmaceuticals has developed a reduced form of 17-AAG, retaspimycin, also known as IPI-504 (Figure 1), that, when isolated as a hydrochloride salt, is water soluble [19]. Data from Infinity's open-label, dose-escalation Phase I clinical trial of retaspimycin hydrochloride in patients with metastatic and/or unresectable GIST were presented at the American Society of Clinical Oncology (ASCO) 2008 Annual Meeting [20]. Although no RECIST-defined responses were observed, 29 of 37 evaluated patients (78%) had a best response of stable disease. Expansion of the twice-weekly IPI-504 for two weeks with one week of treatment at 400 mg/m2 cohort is ongoing. Preliminary data from the Phase I portion of Infinity's open-label Phase I/II clinical trial of retaspimycin hydrochloride in patients with advanced metastatic NSCLC were presented at the AACR-NCI-EORTC International Conference in October 2007 [21]. Preliminary evidence of biological activity was reported in a heavily pretreated population of patients. In seven of nine evaluable patients, disease stabilization by RECIST was achieved over at least one cycle of administration. One patient with a mutation in EGFR and prior history of progression on targeted kinase inhibitors experienced SD for more than six months.
A second-generation GM-derivative, 17-dimethylaminoethylamino-17-desmethoxygeldanamycin (17-DMAG) also known as Alvespimycin and KOS-1022 has also entered Phase I clinical testing (Figure 1). Promising early results were reported in a Phase I trial of 17-DMAG in patients with chemotherapy refractory acute myelogenous leukemia in which 3 of 17 patients had a CR to therapy [22]. Owing to an unfavorable overall toxicity profile, however, Kosan has halted Alvespimycin's clinical development in March 2008.
Although these results are proof for the successful targeting of Hsp90 in clinical settings, several issues intrinsic to the chemical structure of 17-AAG have precluded fulfilling the maximal potential of this target in cancer [23]. The molecule contains a benzoquinone moiety that has been made accountable for the observed elevation of liver enzymes and liver toxicity in clinical settings. Additionally, expression of P-glycoprotein (P-gp) or loss or mutation of the NQO1 gene that is required for the bio-reduction of 17-AAG to the more potent hydroquinone have been proposed as mechanisms of de novo or acquired resistance to 17-AAG. For reasons not yet clearly understood, 17-AAG is also less potent in several tumor types sensitive to other Hsp90 inhibitor chemotypes (reviewed in [23]).
Synthetic Hsp90 inhibitors
Novel synthetic Hsp90 inhibitors based on diverse chemical scaffolds have been developed, and several are currently undergoing Phase I/II clinical evaluation in cancers. These are reported to generally have an improved pharmacologic profile when compared to 17-AAG, especially with regard to their availability through synthesis, evasion of multidrug resistance (MDR)-mediated efflux, metabolic stability, water solubility and ease of administration, and retained biological activity over a wider panel of tumors [24,25]. The first synthetic Hsp90 inhibitor to enter clinic is CNF2024/BIIB021, an Hsp90 inhibitor developed initially by Conforma Therapeutics (currently Biogen Idec) based on the purine-scaffold discovered by investigators at Memorial Sloan-Kettering Cancer Center through structure-based design (Figure 1) [24]. There are currently several ongoing Phase I trial studies of oral CNF2024/BIIB021 in advanced solid tumors, lymphomas, B cell CLL, and in HER2-advanced breast cancer, alone or in combination with trastuzumab. Results of the Phase I trials were recently released at the ASCO meeting, 2008 [26]. CNF2024/BIIB021 appeared to be well tolerated, and a dose of 800 mg twice weekly appeared as maximally tolerated. Biological activity was observed with significant induction of Hsp70 and inhibition of the HER2 bio-marker extracellular domain in solid tumors. In CLL, 1 patient at 25 mg had a 39% reduction in lymph nodes, whereas in solid tumors, 11 of 16 patients (68%) reported stable disease. A Phase II evaluation of CNF2024/BIIB021 in patients with GIST was started in March 2008, in which pharmacodynamic assessment of tumor response by 18FDG-Positron Emission Topography has been implemented (http://www.clinicaltrials.gov/).
A second synthetic Hsp90 inhibitor to enter clinic is VER-52296/NVP-AUY922 currently in development by Novartis [27]. The lead compound that resulted in AUY922 was a pyrazole scaffold derivative identified through a highthroughput screening effort by investigators at the Royal Cancer Institute, and further developed in collaboration with Vernalis [25]. A Phase I–II trial of intravenously administered AUY922 is currently open (http://www.clinicaltrials.gov/), with the Phase I portion of the trial recruiting patients with several types of cancer, whereas the Phase II portion is limited to patients with either HER2 positive or ER positive locally advanced or metastatic breast cancer.
SNX-5422 is a small-molecule Hsp90 inhibitor based on the 6,7-dihydro-indazol-4-one scaffold (Figure 1) [28]. This agent developed by Serenex, converts to SNX-2122, which is the active Hsp90 inhibitor form (Figure 1). A Phase I trial of orally administered SNX-5422 mesylate opened in late May 2007 in patients with refractory solid tumor malignancies. The results of a Phase I dose-escalation study on the safety and pharmacokinetics of SNX-5422 were disclosed at the ASCO 2008 meeting [29]. The agent was well tolerated at low doses and is currently enrolling at 21 mg/m2. In March 2008, Pfizer Inc has announced that it has entered into an agreement to acquire Serenex, Inc (http://pfizer.com/news/press_releases/pfizer_press_releases.jsp).
STA-9090, developed by Synta Pharmaceuticals is claimed to have a chemical structure unrelated to the ansamycin family of Hsp90 inhibitors, such as 17-AAG. STA-9090 is currently enrolling patients in two dose-ranging Phase I clinical trials in solid tumors. The trials are open-label studies in patients with solid tumors and are designed to identify the maximum tolerated dose of STA-9090 based on a twice-a-week or once-a-week intravenous dosing schedule (http://www.syntapharma.com/PrdHsp90.aspx).
Conclusions
In conclusion, several Hsp90 inhibitors have now entered clinical evaluation. One lesson from these trials is that the more we have learned how to administer these agents, the better the clinical outcomes have become. It is now expected that through new formulations of 17-AAG and through the novel synthetic Hsp90 inhibitors, also orally administrable, it will be possible to more favorably modulate the schedule and dose these inhibitors are given. It is the hope that these advances will increase the efficacy with which we can deliver these agents to patients, with the promise of better clinical outcomes in a wider range of cancers to follow.
Acknowledgements
This work was supported partly by the Susan G. Komen Breast Cancer Foundation, the SynCure Cancer Research Foundation, the Clinical & Translational Science Center of Weill Cornel Medical College, Geoffrey Beene Cancer Research Center of Memorial Sloan-Kettering Cancer Center (MSKCC), the Byrne Fund of MSKCC, the Manhasset Women's Coalition Against Breast Cancer and by generous donations from the Mr William H Goodwin and Mrs Alice Goodwin and the Commonwealth Cancer Foundation for Research and The Experimental Therapeutics Center of MSKCC.
References
- 1.Workman P, Burrows F, Neckers L, Rosen N. Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci. 2007;1113:202–216. doi: 10.1196/annals.1391.012. See annotation to Ref. [2] [DOI] [PubMed] [Google Scholar]
- 2.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. Refs. [1] and [2] are good introductory reviews on aspects regarding the biological activity of small-molecule Hsp90 inhibitors and the selectivity of tumors toward pharmacologic Hsp90 interference. [DOI] [PubMed] [Google Scholar]
- 3.Peng C, Li D, Li S. Heat shock protein 90: a potential therapeutic target in leukemic progenitor and stem cells harboring mutant BCR-ABL resistant to kinase inhibitors. Cell Cycle. 2007;6:2227–2231. doi: 10.4161/cc.6.18.4722. [DOI] [PubMed] [Google Scholar]
- 4.Bonvini P, Gastaldi T, Falini B, Rosolen A. Nucleophosmin–anaplastic lymphoma kinase (NPM–ALK), a novel Hsp90-client tyrosine kinase: down-regulation of NPM-ALK expression and tyrosine phosphorylation in ALK(+) CD30(+) lymphoma cells by the Hsp90 antagonist 17-allylamino, 17-demethoxygeldanamycin. Cancer Res. 2002;62:1559–1566. [PubMed] [Google Scholar]
- 5.Knapper S. FLT3 inhibition in acute myeloid leukaemia. Br J Haematol. 2007;138:687–699. doi: 10.1111/j.1365-2141.2007.06700.x. [DOI] [PubMed] [Google Scholar]
- 6.Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–6408. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- 7.Castro JE, Prada CE, Loria O, Kamal A, Chen L, Burrows FJ, Kipps TJ. ZAP-70 is a novel conditional heat shock protein 90 (Hsp90) client: inhibition of Hsp90 leads to ZAP-70 degradation, apoptosis, and impaired signaling in chronic lymphocytic leukemia. Blood. 2005;106:2506–2512. doi: 10.1182/blood-2005-03-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerji U, Affolter A, Judson I, Marais R, Workman P. BRAF and NRAS mutations in melanoma: potential relationships to clinical response to HSP90 inhibitors. Mol Cancer Ther. 2008;7:737–739. doi: 10.1158/1535-7163.MCT-08-0145. [DOI] [PubMed] [Google Scholar]
- 9.Citri A, Kochupurakkal BS, Yarden Y. The Achilles heel of ErbB-2/HER2: regulation by the Hsp90 chaperone machine and potential for pharmacological intervention. Cell Cycle. 2004;1:51–60. [PubMed] [Google Scholar]
- 10.Bauer S, Yu LK, Demetri GD, Fletcher JA. Heat shock protein 90 inhibition in imatinib-resistant gastrointestinal stromal tumor. Cancer Res. 2006;66:9153–9161. doi: 10.1158/0008-5472.CAN-06-0165. [DOI] [PubMed] [Google Scholar]
- 11.Rodina A, Vilenchik M, Moulick K, Aguirre J, Kim J, Chiang A, Litz J, Clement CC, Kang Y, She Y, et al. Selective compounds define Hsp90 as a major inhibitor of apoptosis in small-cell lung cancer. Nat Chem Biol. 2007;3:498–507. doi: 10.1038/nchembio.2007.10. [DOI] [PubMed] [Google Scholar]
- 12.Sepp-Lorenzino L, Ma Z, Lebwohl DE, Vinitsky A, Rosen N. Herbimycin A induces the 20 S proteasome- and ubiquitin-dependent degradation of receptor tyrosine kinases. J Biol Chem. 1995;270:16580–16587. doi: 10.1074/jbc.270.28.16580. [DOI] [PubMed] [Google Scholar]
- 13.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 14.Solit DB, Chiosis G. Development and application of Hsp90 inhibitors. Drug Discov Today. 2008;13:38–43. doi: 10.1016/j.drudis.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 15.Richardson PG, Chanan-Khan A, Lonial S, Krishman A, Carroll M, Cropp GF, Kersey K, Abitar M, Johnson RG, Hannah AL, et al. Tanespimycin (T) + bortezomib (BZ) in multiple myeloma (MM): confirmation of the recommended dose using a novel formulation. ASH Annual Meeting Abstracts. 2007;110:1165. Describes the first significant clinical outcomes in the combination trial of an Hsp90 inhibitor with bortezomid in multiple myeloma. [Google Scholar]
- 16.Whitesell L, Bagatell R, Falsey R. The stress response: implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- 17.Modi S, Stopeck AT, Gordon MS, Mendelson D, Solit DB, Bagatell R, Ma W, Wheler J, Rosen N, Norton L, et al. Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol. 2007;25:5410–5417. doi: 10.1200/JCO.2007.11.7960. This is the first major report on a significant clinical outcome in patients with HER2-overexpressing cancers observed with an Hsp90 inhibitor in combination with trastuzumab. [DOI] [PubMed] [Google Scholar]
- 18.Modi S, Sugarman S, Stopeck A, Linden H, Ma W, Kersey K, Johnson RG, Rosen N, Hannah AL, Hudis CA. Phase II trial of the Hsp90 inhibitor tanespimycin (Tan) + trastuzumab (T) in patients (pts) with HER2-positive metastatic breast cancer (MBC) J Clin Oncol. 2008 May 20;26(suppl) abstr 1027. [Google Scholar]
- 19.Sydor JR, Normant E, Pien CS, Porter JR, Ge J, Grenier L, Pak RH, Ali JA, Dembski MS, Hudak J, et al. Development of 17-allylamino-17-demethoxygeldanamycin hydroquinone hydrochloride (IPI-504), an anti-cancer agent directed against Hsp90. Proc Natl Acad Sci U S A. 2006;103:17408–17413. doi: 10.1073/pnas.0608372103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner AJ, Morgan JA, Chugh R, Rosen LS, George S, Gordon MS, Devine CM, Van den Abbeele AD, Grayzel D, Demetri GD. Inhibition of heat shock protein 90 (Hsp90) with the novel agent IPI-504 in metastatic GIST following failure of tyrosine kinase inhibitors (TKIs) or other sarcomas: clinical results from phase I trial. J Clin Oncol. 2008 May 20;26(suppl) abstr 10503. Describes promising clinical activity of an Hsp90 inhibitor in relapsed, refractory gastrointestinal stromal tumors. [Google Scholar]
- 21.Sequist LV, Jänne PA, Walker J, Sweeney J, Grayzel D, Lynch TJ. Phase I/II trial of the novel Hsp90 inhibitor, IPI-504, in patients with relapsed and/or refractory stage IIIB or stage IV nonsmall cell lung cancer stratified by EGFR mutation status; AACRNCI-EORTC Molecular Targets and Cancer Therapeutics Meeting; San Francisco, CA. October 22-26: 2007; abstract B79. [Google Scholar]
- 22.Lancet J, Baer MR, Gojo I, Burton M, Quinn M, Tighe SM, Bhalla K, Kersey K, Wells S, Zhong Z, et al. Phase 1, pharmacokinetic (PK) and pharmacodynamic (PD) study of intravenous alvespimycin (KOS-1022) in patients with refractory hematological malignancies. Blood (ASH Annual Meeting Abstracts) 2006;108 abstract 1961. Described the first significant recorded responses in chemotherapy refractory AML observed in clinic with an Hsp90 inhibitor. [Google Scholar]
- 23.Chiosis G, Caldas Lopes E, Solit D. Heat shock protein-90 inhibitors: a chronicle from geldanamycin to today's agents. Curr Opin Investig Drugs. 2006;7:534–541. [PubMed] [Google Scholar]
- 24.Chiosis G, Kang Y, Sun W. Discovery and development of purine-scaffold Hsp90 inhibitors. Expert Opin Drug Discov. 2008;3:99–114. doi: 10.1517/17460441.3.1.99. Gives a historical overview on the discovery and clinical translation of purine-scaffold Hsp90 inhibitors. [DOI] [PubMed] [Google Scholar]
- 25.McDonald E, Jones K, Brough PA, Drysdale MJ, Workman P. Discovery and development of pyrazole-scaffold Hsp90 inhibitors. Curr Top Med Chem. 2006;6:1193–1203. doi: 10.2174/156802606777812086. Gives a historical overview on the discovery and preclinical development of pyrazole and oxazole-scaffold Hsp90 inhibitors. [DOI] [PubMed] [Google Scholar]
- 26.Elfiky A, Saif MW, Beeram M, O'Brien S, Lammanna N, Castro JE, Woodworth J, Perea R, Storgard C, Von Hoff DD. BIIB021, an oral, synthetic non-ansamycin Hsp90 inhibitor: Phase I experience. J Clin Oncol. 2008 May 20;26(suppl) abstr 2503. Gives an initial clinical report on the Phase I experience with the first synthetic Hsp90 inhibitor to enter clinical evaluation. [Google Scholar]
- 27.Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall F, et al. NVPAUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res. 2008;68:2850–2860. doi: 10.1158/0008-5472.CAN-07-5256. [DOI] [PubMed] [Google Scholar]
- 28.Hall SE. Discovery and pre-clinical profile of SNX-5422: an orally active Hsp90 inhibitor in phase 1 trials for solid and hematological tumors; Proceedings of the 99th Annual Meeting of the American Association for Cancer Research; San Diego, CA. April 12-16: 2008; abstract #2449. [Google Scholar]
- 29.Bryson JC, Infante JR, Ramanathan RK, Jones SF, Von Hoff DD, Burris HA., III A Phase 1 dose-escalation study of the safety and pharmacokinetics (PK) of the oral Hsp90 inhibitor SNX-5422. J Clin Oncol. 2008 May 20;26(suppl) abstr 14613. [Google Scholar]