Abstract
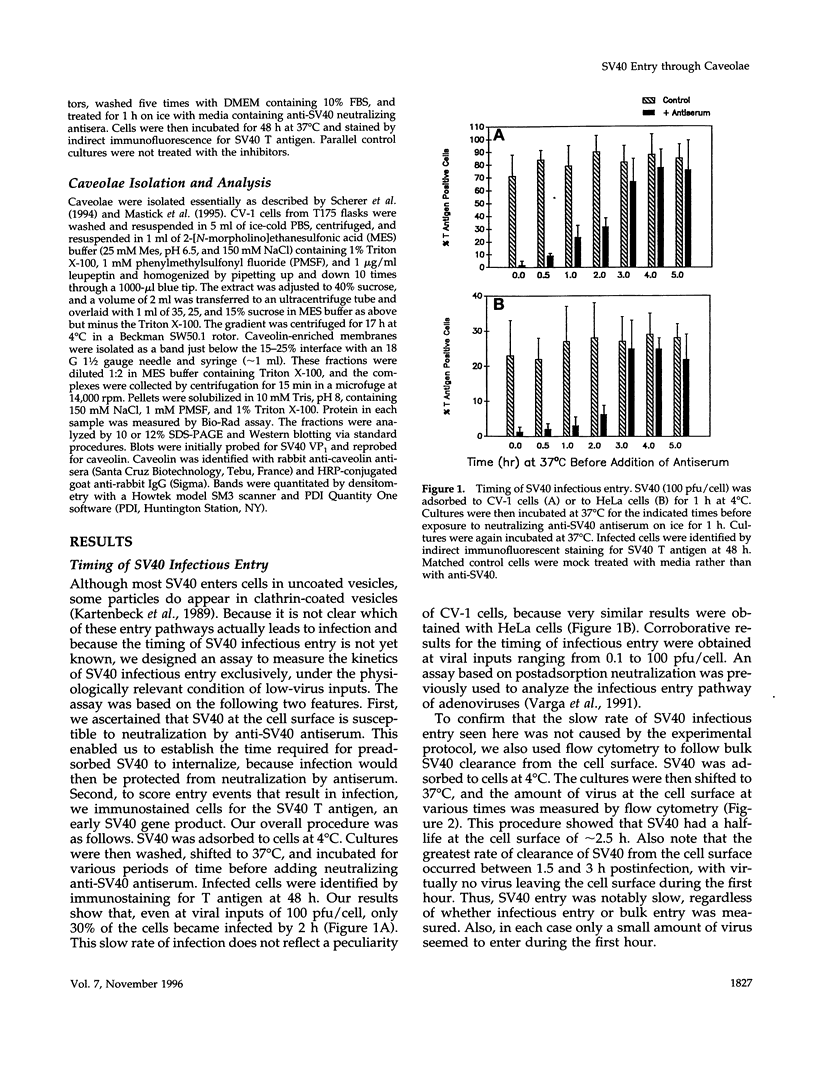
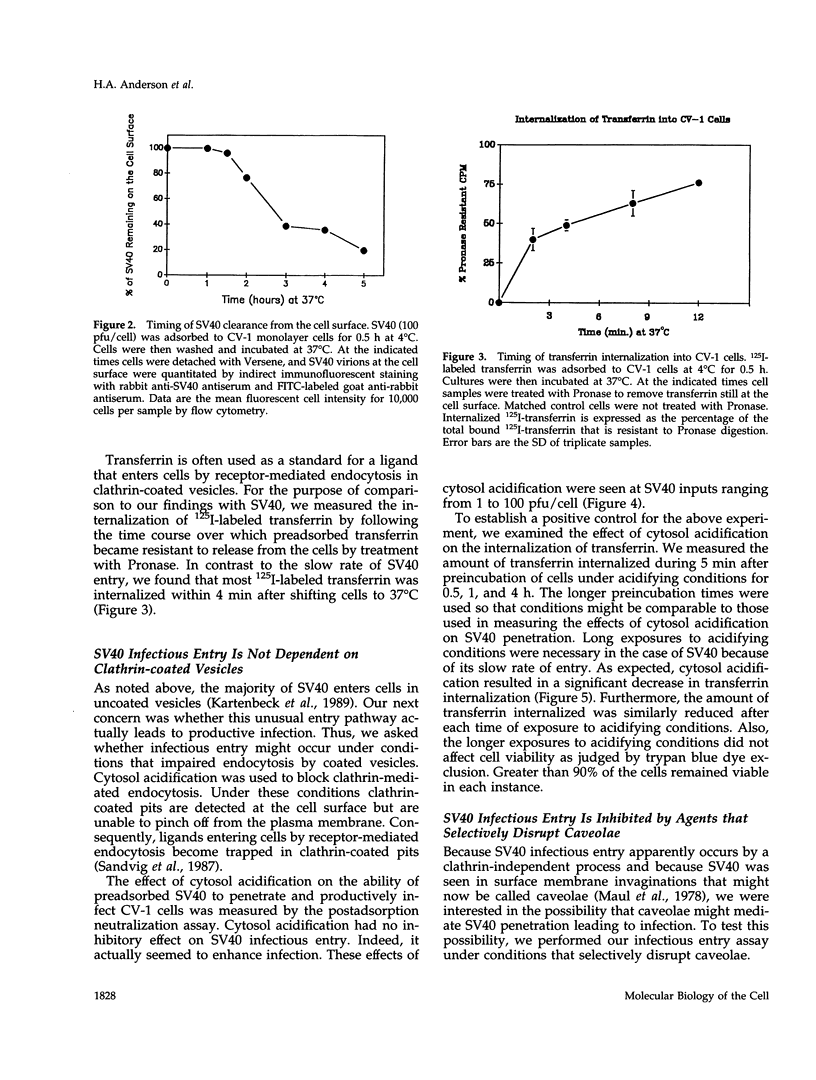
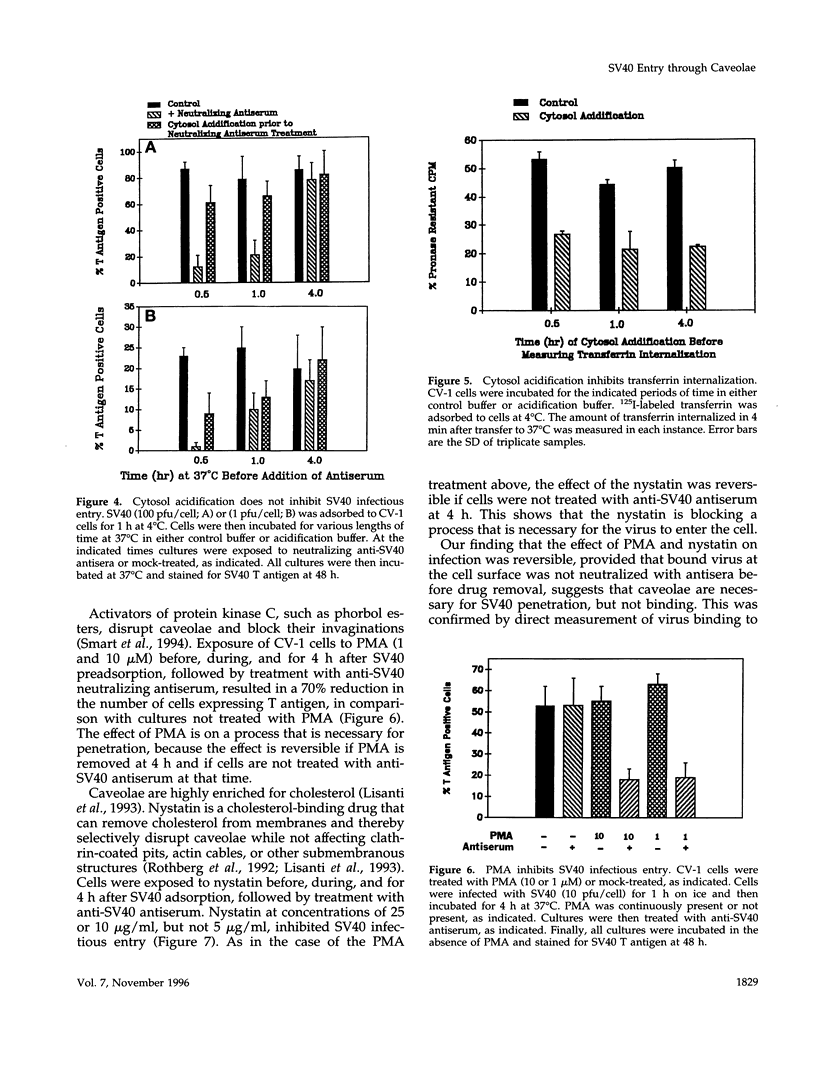
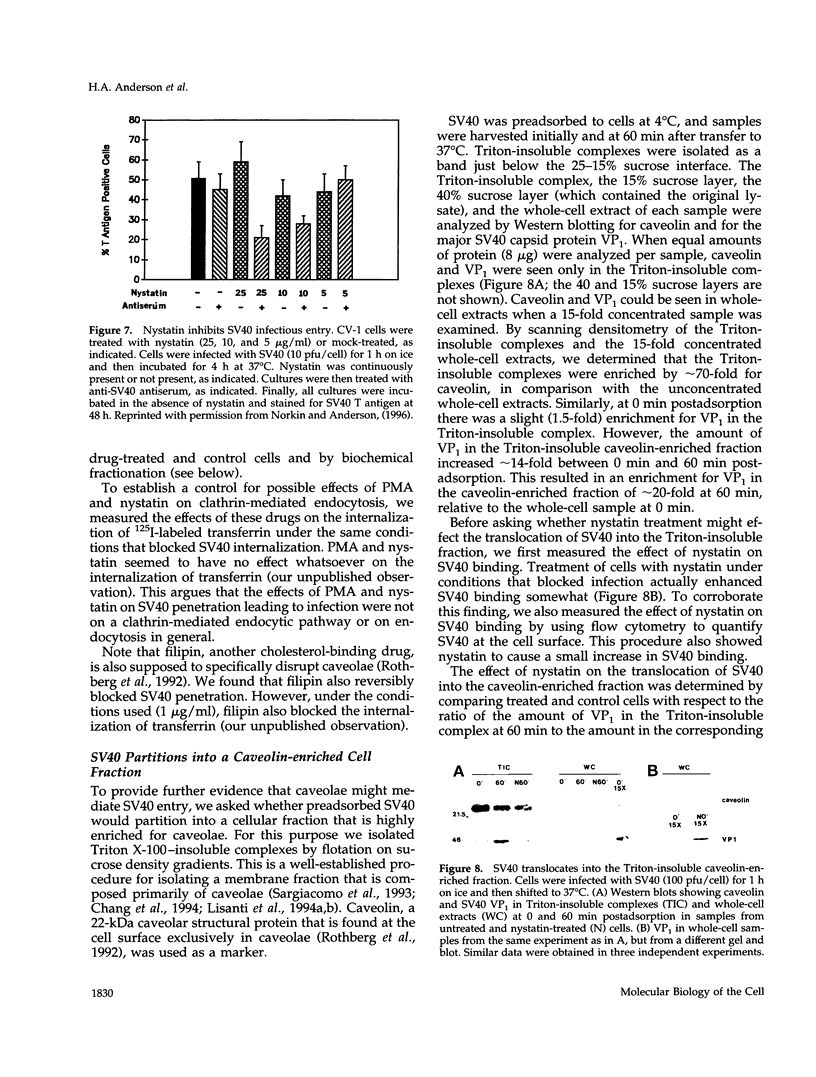
Simian virus 40 (SV40) entry leading to infection occurred only after the virus was at the cell surface for 1.5 to 2 h. SV40 infectious entry was not sensitive to cytosol acidification, a treatment that blocks endocytosis via clathrin-coated vesicles. Instead, SV40 infectious entry was blocked by treating cells with the phorbol ester PMA or nystatin, which selectively disrupts caveolae. In control experiments, transferrin internalization was sensitive to cytosol acidification but was not sensitive to PMA or nystatin. Also, absorbed transferrin entered cells within minutes. Finally, bound SV40 translocated to caveolin-enriched membrane complexes isolated by a Triton X-100 insolubility protocol. Treatment with nystatin did not impair SV40 binding but did block the partitioning of virus into the caveolin-enriched complexes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Caveolae: where incoming and outgoing messengers meet. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10909–10913. doi: 10.1073/pnas.90.23.10909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. G. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993 Mar;3(3):69–72. doi: 10.1016/0962-8924(93)90065-9. [DOI] [PubMed] [Google Scholar]
- Atwood W. J., Norkin L. C. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989 Oct;63(10):4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S., Turner H. Infectious entry pathway for canine parvovirus. Virology. 1992 Feb;186(2):368–376. doi: 10.1016/0042-6822(92)90002-7. [DOI] [PubMed] [Google Scholar]
- Breau W. C., Atwood W. J., Norkin L. C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992 Apr;66(4):2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bundgaard M., Hagman P., Crone C. The three-dimensional organization of plasmalemmal vesicular profiles in the endothelium of rat heart capillaries. Microvasc Res. 1983 May;25(3):358–368. doi: 10.1016/0026-2862(83)90025-0. [DOI] [PubMed] [Google Scholar]
- Chang W. J., Ying Y. S., Rothberg K. G., Hooper N. M., Turner A. J., Gambliel H. A., De Gunzburg J., Mumby S. M., Gilman A. G., Anderson R. G. Purification and characterization of smooth muscle cell caveolae. J Cell Biol. 1994 Jul;126(1):127–138. doi: 10.1083/jcb.126.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciechanover A., Schwartz A. L., Dautry-Varsat A., Lodish H. F. Kinetics of internalization and recycling of transferrin and the transferrin receptor in a human hepatoma cell line. Effect of lysosomotropic agents. J Biol Chem. 1983 Aug 25;258(16):9681–9689. [PubMed] [Google Scholar]
- Dangoria N. S., Breau W. C., Anderson H. A., Cishek D. M., Norkin L. C. Extracellular simian virus 40 induces an ERK/MAP kinase-independent signalling pathway that activates primary response genes and promotes virus entry. J Gen Virol. 1996 Sep;77(Pt 9):2173–2182. doi: 10.1099/0022-1317-77-9-2173. [DOI] [PubMed] [Google Scholar]
- Dupree P., Parton R. G., Raposo G., Kurzchalia T. V., Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993 Apr;12(4):1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding H., Dimitrov D. S., Manischewitz J., Broder C. C., Robinson J., Fabian S., Littman D. R., Lapham C. K. Phorbol ester-induced down modulation of tailless CD4 receptors requires prior binding of gp120 and suggests a role for accessory molecules. J Virol. 1995 Oct;69(10):6140–6148. doi: 10.1128/jvi.69.10.6140-6148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Lai C. J. Preparation of simian virus 40 and its DNA. Methods Enzymol. 1979;58:404–412. doi: 10.1016/s0076-6879(79)58155-5. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994 Jul;4(7):231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Vidugiriene J., Tang Z., Hermanowski-Vosatka A., Tu Y. H., Cook R. F., Sargiacomo M. Characterization of caveolin-rich membrane domains isolated from an endothelial-rich source: implications for human disease. J Cell Biol. 1994 Jul;126(1):111–126. doi: 10.1083/jcb.126.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Tang Z. L., Sargiacomo M. Caveolin forms a hetero-oligomeric protein complex that interacts with an apical GPI-linked protein: implications for the biogenesis of caveolae. J Cell Biol. 1993 Nov;123(3):595–604. doi: 10.1083/jcb.123.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay R. L., Consigli R. A. Early events in polyoma virus infection: attachment, penetration, and nuclear entry. J Virol. 1976 Aug;19(2):620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Adsorptive endocytosis of Semliki Forest virus. J Mol Biol. 1980 Sep 25;142(3):439–454. doi: 10.1016/0022-2836(80)90281-8. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastick C. C., Brady M. J., Saltiel A. R. Insulin stimulates the tyrosine phosphorylation of caveolin. J Cell Biol. 1995 Jun;129(6):1523–1531. doi: 10.1083/jcb.129.6.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maul G. G., Rovera G., Vorbrodt A., Abramczuk J. Membrane fusion as a mechanism of simian virus 40 entry into different cellular compartments. J Virol. 1978 Dec;28(3):936–944. doi: 10.1128/jvi.28.3.936-944.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. S., Fuller A. O. Cell-specific kinetics and efficiency of herpes simplex virus type 1 entry are determined by two distinct phases of attachment. Virology. 1994 Feb;198(2):690–702. doi: 10.1006/viro.1994.1081. [DOI] [PubMed] [Google Scholar]
- Milici A. J., Watrous N. E., Stukenbrok H., Palade G. E. Transcytosis of albumin in capillary endothelium. J Cell Biol. 1987 Dec;105(6 Pt 1):2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo C., James G. L., Smart E. J., Anderson R. G. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996 May 17;271(20):11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Moldovan N. I., Heltianu C., Simionescu N., Simionescu M. Ultrastructural evidence of differential solubility in Triton X-100 of endothelial vesicles and plasma membrane. Exp Cell Res. 1995 Jul;219(1):309–313. doi: 10.1006/excr.1995.1233. [DOI] [PubMed] [Google Scholar]
- Montesano R., Roth J., Robert A., Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982 Apr 15;296(5858):651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Anderson H. A. Multiple stages of virus-receptor interactions as shown by simian virus 40. Adv Exp Med Biol. 1996;408:159–167. doi: 10.1007/978-1-4613-0415-9_18. [DOI] [PubMed] [Google Scholar]
- Norkin L. C., Einck K. H. Cell killing by Simian virus 40: protective effect of chloroquine. Antimicrob Agents Chemother. 1978 Dec;14(6):930–932. doi: 10.1128/aac.14.6.930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkin L. C. Virus receptors: implications for pathogenesis and the design of antiviral agents. Clin Microbiol Rev. 1995 Apr;8(2):293–315. doi: 10.1128/cmr.8.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Ying Y. S., Kolhouse J. F., Kamen B. A., Anderson R. G. The glycophospholipid-linked folate receptor internalizes folate without entering the clathrin-coated pit endocytic pathway. J Cell Biol. 1990 Mar;110(3):637–649. doi: 10.1083/jcb.110.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., Olsnes S., Petersen O. W., van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987 Aug;105(2):679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K., van Deurs B. Endocytosis without clathrin. Trends Cell Biol. 1994 Aug;4(8):275–277. doi: 10.1016/0962-8924(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Sargiacomo M., Sudol M., Tang Z., Lisanti M. P. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993 Aug;122(4):789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer P. E., Lisanti M. P., Baldini G., Sargiacomo M., Mastick C. C., Lodish H. F. Induction of caveolin during adipogenesis and association of GLUT4 with caveolin-rich vesicles. J Cell Biol. 1994 Dec;127(5):1233–1243. doi: 10.1083/jcb.127.5.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Liu J., Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995 Jun 16;270(24):14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P., Pinney E., Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994 Dec;127(5):1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs N. J. Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact? J Cell Sci. 1988 Jul;90(Pt 3):341–348. doi: 10.1242/jcs.90.3.341. [DOI] [PubMed] [Google Scholar]
- Simionescu M., Simionescu N., Palade G. E. Differentiated microdomains on the luminal surface of capillary endothelium: distribution of lectin receptors. J Cell Biol. 1982 Aug;94(2):406–413. doi: 10.1083/jcb.94.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotte J. P., Bierman E. L. Movement of plasma-membrane sterols to the endoplasmic reticulum in cultured cells. Biochem J. 1987 Nov 15;248(1):237–242. doi: 10.1042/bj2480237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E. J., Foster D. C., Ying Y. S., Kamen B. A., Anderson R. G. Protein kinase C activators inhibit receptor-mediated potocytosis by preventing internalization of caveolae. J Cell Biol. 1994 Feb;124(3):307–313. doi: 10.1083/jcb.124.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Mineo C., Anderson R. G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Collawn J. F., Hopkins C. R. Signal-dependent membrane protein trafficking in the endocytic pathway. Annu Rev Cell Biol. 1993;9:129–161. doi: 10.1146/annurev.cb.09.110193.001021. [DOI] [PubMed] [Google Scholar]
- Varga M. J., Weibull C., Everitt E. Infectious entry pathway of adenovirus type 2. J Virol. 1991 Nov;65(11):6061–6070. doi: 10.1128/jvi.65.11.6061-6070.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham T. J., Mathias P., Cheresh D. A., Nemerow G. R. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993 Apr 23;73(2):309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]



