Abstract
Background
Placental malaria causes fetal growth retardation (FGR), which has been linked epidemiologically to placental monocyte infiltrates. We investigated whether parasite or monocyte infiltrates were associated with placental hypoxia, as a potential mechanism underlying malarial FGR.
Methods
We studied the hypoxia markers hypoxia inducible factor (HIF)-1α, vascular endothelial growth factor (VEGF), placental growth factor, VEGF receptor 1 and its soluble form and VEGF receptor 2. We used real time PCR (in 59 women) to examine gene transcription, immunohistochemistry (in 30 women) to describe protein expression and laser capture microdissection (in 23 women) to examine syncytiotrophoblast-specific changes in gene expression. We compared gene and protein expression in relation to malaria infection, monocytes infiltrates and birth weight.
Results
we could not associate any hallmark of placental malaria with a transcription, expression or tissue distribution profile characteristic of a response to hypoxia but found higher HIF-1α (P=.0005) and lower VEGF levels (P=.0026) in the syncytiotrophoblast of malaria cases versus asymptomatic controls.
Conclusion
our data are inconsistent with a role for placental hypoxia in the pathogenesis of malaria-associated FGR. The laser capture microdissection study was small, but suggests that malaria affects syncytiotrophoblast gene transcription, and proposes novel potential mechanisms for placental malaria-associated FGR.
Keywords: Malaria, placenta, hypoxia, birth weight, growth restriction
INTRODUCTION
Placental malaria (PM) is characterised by the presence of malaria-infected erythrocytes in placental blood and is associated with both fetal and maternal mortality and morbidity [1, 2]. In particular, PM is a major preventable cause of low birth weight (LBW) and it is estimated that 200,000 children die as a result of PM-associated LBW every year [3]. In malaria endemic areas, LBW is mainly due to fetal growth restriction (FGR) rather than to pre-term delivery [1, 4] but causes of this FGR remain unknown.
Placental hypoxia is an important cause of FGR, as shown by studies of high altitude pregnancies (in which hypoxia is hypobaric in aetiology [5]) or pre-eclampsia (in which placental insufficiency causes tissue hypoxia [6]). To date, there is no published study on the role of placental hypoxia in PM-associated FGR despite clinical, pathological and biological similarities between PM and pre-eclampsia (reviewed in [7]), and a long-proposed pathogenic role for placental hypoxia in PM-associated FGR. It has been suggested that some histopathological characteristics of PM including thickening of basal membranes [8] and infiltration of monocytes in the intervillous spaces of the placenta (intervillositis) [9–11] could increase barriers to oxygen transport across the placenta [12]. It also has been postulated that the intervillous accumulation of inflammatory cells and infected erythrocytes could lead to placental and fetal hypoxia either by consumption of oxygen by the infiltrated cells or by decreasing blood perfusion and effective surface area for materno-fetal exchange [13, 14]. Such placental dysfunctions could directly affect fetal development and lead to FGR [15, 16]. Increased syncytial knotting, which is considered to be a physiological adaptation to hypoxia [17], has been reported in malaria-infected placentae [18].
Hypoxia alters cellular gene transcription profiles. A central hypoxia-responsive gene is the transcription factor Hypoxia Inducible Factor 1 (HIF-1), a heterodimer of inducible HIF-1α and constitutive HIF-1β, which controls cellular response to hypoxia. Under normoxic conditions, HIF-1α protein is degraded by O2-dependent ubiquitination and targeting to the proteasome [19–21]. In hypoxic tissues, HIF-1α protein is stabilised and can bind HIF-1β to form an active transcription factor that will alter the transcription of growth factor genes involved in regulation of angiogenesis [22]. In particular, hypoxia is associated with increased transcription of several molecules in placental tissue or cell lines, including vascular endothelial growth factor (VEGF) [23], Flt-1 (fems-like tyrosine kinase, or VEGFR1) [24] and its soluble form sFlt-1 [25], and decreased transcription of placental growth factor (PlGF) [26]. Expression of the proteins VEGFR2 (or fetal liver kinase-1/kinase insert domain-containing receptor (Flk-1/KDR)) [24, 27] and HIF-1α [28] is also up regulated under hypoxic conditions in placental tissue.
In order to test the hypothesis that placental hypoxia has a role in the pathogenesis of PM-associated FGR, we investigated whether placental malaria infection, intervillositis and LBW were associated with an expression profile characteristic of a response to hypoxia in normotensive pregnant women.
MATERIALS AND METHODS
1. Participant recruitment
This study was approved by the College of Medicine Research Committee at the University of Malawi. From November 2001 until April 2005, placental tissue samples were collected from women who delivered at Queen Elizabeth Central Hospital in Blantyre, Malawi, and who were participating in a study of interactions between HIV and PM. [29]. For the present study, samples were selected from HIV uninfected participants on the basis of normal Apgar scores at 1 and 5 minutes and the absence of maternal complications (other than placental malaria) such as hypertension, pre-eclampsia, chorioamnionitis or maternal anaemia. Placental histology was examined to classify eligible women into four groups: past malaria (malaria pigment deposits but no parasites; these women were not included in the present study), malaria uninfected, malaria parasites without pigment containing monocytes, and malaria parasites with pigment containing monocytes. A sample size of 60 was selected based on a previous study [30] in which highly significant differences in placental chemokine mRNA were found between groups stratified by malaria and monocyte densities. Within groups, women with the highest parasite or monocyte densities were selected from those with available samples.
2. Tissue sampling and handling
Placental tissue samples were taken near the centre of the placenta within 20 minutes post-delivery. One set of samples was snap-frozen in liquid nitrogen for RNA extraction, another set was embedded in OCT medium before being frozen at −80°C for laser capture microdissection and a last set was fixed in buffered formalin for immunohistochemistry and malaria infection grading.
3. Laboratory and pathology testing
Peripheral blood malaria infection was assessed on thick blood films stained with Field’s stain. Formalin-fixed placental biopsies were examined and presence and density of malaria parasitemia and presence of malaria pigment (hemozoin) were noted. In women with malaria, the density of intervillous space monocyte infiltrates was recorded, as described [9]. Biopsies were examined for chorioamnionitis as described [31]. Maternal hemoglobin concentration was determined by HemoCue hemoglobinometer (HemoCue, Ängelholm, Sweden). LBW was defined as a birth weight lower than 2500g.
4. Real time RT-PCR
Snap-frozen placental tissue was thawed in RNAlater-ICE (Ambion) according to supplier’s recommendations before being homogenized in Trizol RNA extraction reagent (Invitrogen) using a tissue homogeniser (Polytron). RNA extraction was conducted following supplier’s recommendations. The total cellular RNA obtained was resuspended in RNase-free water (Ambion) and kept at −80°C until use. RNA was reverse transcribed using Superscript III enzyme mix (InVitrogen) in a 20μL reaction volume following supplier’s recommendations. The cDNA was diluted 1 in 4 in DNase-free water and kept at −20°C.
Primers were either found in the literature or designed using Primer Express software and all were validated for use with SYBR Green chemistry on an ABI 7900HT real time PCR machine (all from Applied Biosystems). Data were analysed as previously described [32]. Briefly, RNA standards were synthesised for each gene and added to a pool of cellular RNA to be used as external standards. Up to three housekeeping genes validated for their use in placental comparative expression studies [33] were used as internal standards. They include the TATA box binding protein (TBP), the succinate dehydrogenase complex, subunit A (SDHA) and the tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein, zeta polypeptide (YWHAZ). Target gene signals were normalised to the geometric mean of the three housekeeping genes for accuracy and reliability as recommended by various authors [33, 34].
5. Laser capture microdissection
The syncytiotrophoblast layer of uninfected placentae (n=6) and P. falciparum-infected placentae with (n=10) or without (n=7) intervillositis was isolated using laser capture microdissection. These cases were selected on the basis of availability of additional placental samples, from within our group of 59 women studied at the whole tissue level. All available samples were used. Five to 7μm-thick tissue cryosections were immobilised on SuperFrost PLUS slides (Fisher) and air-dried for 10 min. before 10 min. of acetone treatment to fix the tissue. After re-hydration in DEPC-treated water, sections were lightly stained with methyl green (Sigma-Aldrich), and then dehydrated in ethanol and cleared in xylene. Slides were kept in a desiccator until capture. Capture was done by LMPC (Laser Microdissection and Pressure Catapulting) using a MicroBeam microscope (P.A.L.M. Microlaser Technologies). Captured material was catapulted directly into RNA extraction buffer (RLT buffer with β-mercaptoethanol, Qiagen) and RNA extraction was conducted using the RNeasy Micro Kit (Qiagen) according to the supplier recommendations. Purified RNA was eluted in RNase-free water (Ambion) and kept at −80°C until use.
6. Immunohistochemistry
Immunohistochemistry was conducted on 5μm-thick paraffin-embedded tissue sections immobilised on silanized slides. Depending on the target, epitope retrieval was achieved using either 10mM citrate buffer for KDR and VEGF or Target Retrieval Solution (Dako) for PlGF, HIF-1α and Flt-1. After quenching the endogenous peroxidase by 20 min. incubation in 0.5% H2O2 in water, sections were incubated for one hour with primary antibodies. Mouse monoclonal anti-HIF-1α (clone ESEE122 at 1/8000; Novus Biologicals), rabbit polyclonal anti-PlGF (CPP500 at 1/50; Cell Sciences), rabbit polyclonal anti-VEGF (sc-152 at 1/200), mouse monoclonal anti-KDR (sc-6251 at 1/100) or rabbit polyclonal anti-Flt-1 (sc-316 at 1/500), all from Santa Cruz were diluted in antibody diluent (S0809, Dako). After washing in PBS, biotinylated secondary antibodies were added for 30 minutes: polyclonal rabbit-anti-mouse (E0354 at 1/300 for KDR), polyclonal swine anti-goat, mouse, rabbit (E0453 at 1/300 for VEGF and PlGF) or polyclonal goat-anti-rabbit (E0432 at 1/300 for Flt-1), all from Dako. Biotinylated secondary (E0354 at 1/600) and tertiary (polyclonal swine anti-goat, mouse, rabbit; E0453 at 1/200) antibodies were used for HIF-1α detection. For different cell types, intensity and consistency of the staining was scored by two independent microscopists blinded for the clinical presentation (PB and CR) and averaged on 20 microscopic fields. Intensity scores ranged from 0 (no staining) to 3 (very intense staining) and consistency was scored from 0 (no cell stained) to 3 (more than 2/3 of the cells stained) [35].
7. Statistical tests
Numerical values were compared between two groups using Mann-Whitney’s test and between three groups using Kruskal-Wallis’ test. Immunohistochemistry scores (detailed above) were compared across groups using Mann-Whitney’s test. All analysis was done on Stata 8.0 software. P values of less than 0.05 were considered as significant.
RESULTS
1. Participants’ characteristics
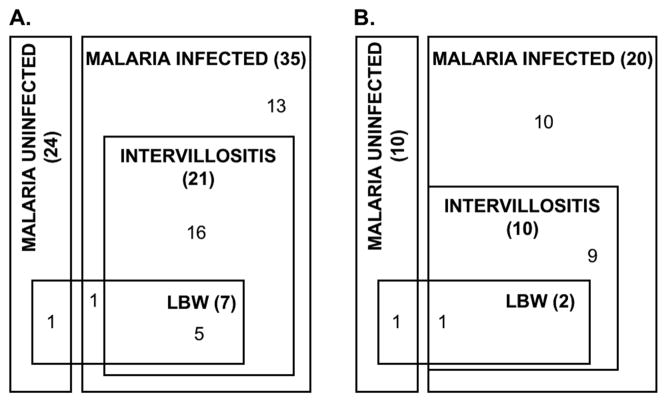
The characteristics of the participants whose samples were used in the RT-PCR analysis are summarised in table 1A. Thirty-five women with active placental malaria and 24 uninfected controls were included. Of women with placental malaria, 22/35 (63%) had intervillositis, and 8/35 (23%) had LBW babies. None of the uninfected women had intervillositis and only one delivered a LBW baby (figure 1A). Despite statistical differences in gestational age, these were ignored since all deliveries were within the normal range for term deliveries.
Table 1. Participants’ characteristics.
In the groups involved in real time RT-PCR study (A), gestational age differed whatever the classification criteria used (P≤.05) but stayed within the normal range for term deliveries. This difference was therefore ignored. Women who delivered LBW babies were of lower gravidity (P =.026) and were younger (P =.025) than those who delivered normal birth weight babies. Groups of participants involved in the immunohistochemistry (IHC) analysis (B) were comparable for all criteria (except birth weight for the group with LBW: P =.019). Values are medians with (in brackets) 25th and 75th centiles. Mann-Whitney’s test.
| A: RT-PCR | Control group | Malaria without monocytes | Malaria with monocytes | Normal birthweight | LBW |
|---|---|---|---|---|---|
| N | 24 | 14 | 21 | 52 | 7 |
| Age (years) | 22 (20–25.5) | 22.5 (21–28) | 21 (20–24) | 22 (20–26) | 20 (18–21) |
| Parity | 1.5 (1–3) | 2 (1–4) | 2 (1–3) | 2 (1–3) | 1 (1–2) |
| Maternal Hb (g/dL) | 11.5 (10.5–12.45) | 11.45 (10.4–12.5) | 11.2 (10.4–12) | 11.25 (10.4–12.4) | 11.3 (10.5–12.4) |
| Gestational age (weeks) | 40 (39–41) | 39 (39–40) | 39 (38.5–40) | 40 (39–40) | 39 (38–39) |
| Maternal weight (kg) | 62.5 (56–66.5) | 58.5 (55–63) | 58 (55–62) | 60.5 (55.25–64.75) | 58 (55–61) |
| Maternal height (cm) | 158 (153–159) | 157.5 (155–161) | 154.5 (152–157) | 156.5 (153.5–159) | 154 (152–161) |
| Birthweight (g) | 3100 (2825–3400) | 3135 (3000–3400) | 2925 (2550–3150) | 3100 (2900–3300) | 2500 (2400–2500) |
| B: IHC | Control group | Malaria without monocytes | Malaria with monocytes | Normal birthweight | LBW |
| N | 10 | 10 | 10 | 28 | 2 |
| Age (years) | 23 (22–26) | 23 (22–26) | 25 (21–28) | 23 (22–28) | 21 (21–23) |
| Parity | 2 (2–3) | 2 (1–3) | 2.5 (2–4) | 2 (1–3) | 2 (2–2) |
| Maternal Hb (g/dL) | 10.5 (9.4–11.4) | 12.5 (10.6–12.7) | 11.1 (10–11.2) | 11.2 (10.1–12.5) | 10.7 (10.5–10.7) |
| Gestational age (weeks) | 41 (40–42) | 40 (39–40) | 40 (39–41) | 40 (40–41) | 39 (35–39) |
| Maternal weight (kg) | 60 (56–73) | 56 (51–60) | 62 (57–64.5) | 59 (53–65) | 61 (56–61) |
| Maternal height (cm) | 153 (153–159) | 155 (151–157) | 155 (149.5–160) | 155 (151–159) | 161 (153–161) |
| Birthweight (g) | 3100 (3000–3400) | 3100 (2700–3590) | 2975 (2750–3300) | 3100 (2950–3590) | 2500 (2000–2500) |
Figure 1. Clinical characteristics of the participants.
A. Among the individuals recruited in the RT-PCR assays, 7 had a low birth weight (LBW): 5 malaria cases with intervillositis, 1 malaria case without intervillositis and 1 control. B. In the IHC assays, only 2 participants suffered from LBW (1 control and 1 malaria case). These individuals showed similar IHC profiles, even when LBW was defined as small for gestational age (n=7). Numbers refer to the number of samples from each overlapping group.
When malaria cases were separated on the basis of the presence/absence of intervillositis, babies from uninfected women and infected women without intervillositis had similar birth weights (P=.86) but the patients with intervillositis delivered significantly lighter babies than infected women without intervillositis (P=.027) and than uninfected women (P=.007).
2. Transcription profiles are not characteristic of a response to hypoxia
Quantitative real time RT-PCR was conducted to look for an increase in HIF-1α, VEGF, Flt-1 and sFlt-1 and a decrease in PlGF transcripts levels in malaria-infected samples, which would be a signature of tissue hypoxia. First, the quality of the extracted RNA was assessed by automated electrophoresis on RNA LabChipR (Bio-Rad). All the samples included in the study had a ribosomal RNA 28s/18s ratio higher than 1 (data not shown).
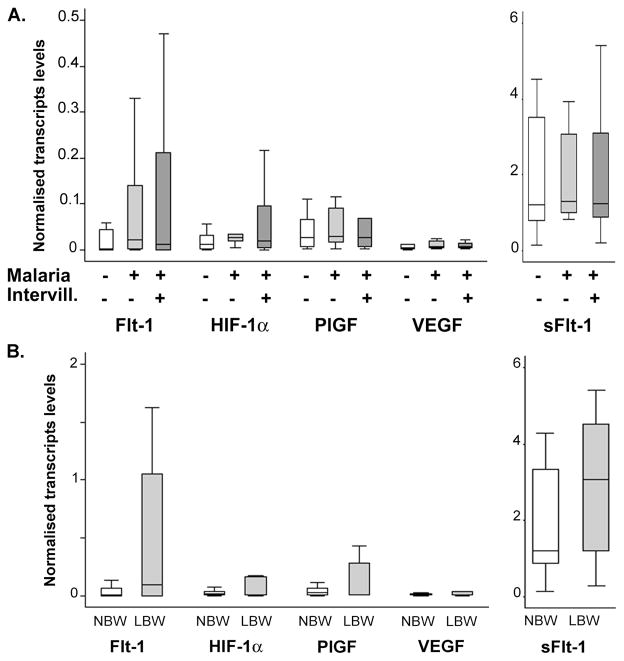
No difference was found in transcripts levels for any of the markers when comparing controls with malaria cases or between malaria cases with or without intervillositis (figure 2A). Samples were then classified according to other clinical or biological features of PM: intervillositis (regardless of malaria infection – data not shown) and LBW (figure 2B). Again, there was no difference in transcription profiles between groups for any classification criteria.
Figure 2. Transcription profiles of the different clinical groups.
The transcript levels of the different hypoxia markers were quantified in all participants using absolute quantitative real time RT-PCR and normalised to the expression of three placental housekeeping genes. A. The transcription profiles were similar between controls (open bars, n=24), malaria cases without intervillositis (light grey bars, n=14) and malaria complicated by intervillositis (dark grey bars, n=21). B. No difference in transcription profiles was found when comparing participants with normal birth weight (NBW, open bars, n=52) and patients with LBW (grey bars, n=7). P>.05 Mann-Whitney’s test. Bars represent median, 25th and 75th percentiles and the whiskers are 5th and 95th percentiles. Flt-1: VEGF receptor 1; HIF: Hypoxia-inducible factor; PlGF: Placental growth factor; VEGF: Vascular endothelial growth factor; sFlt-1: soluble Flt-1; Intervill.: intervillositis.
These results suggest that none of the clinical or biological features of PM is specifically associated with placental hypoxia.
3. Laser capture microdissection reveals differences in transcription profiles
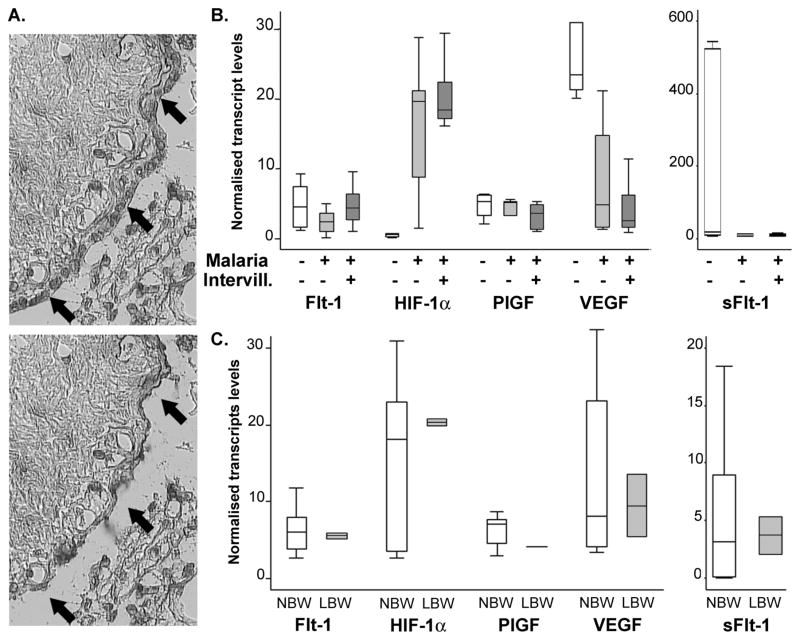
Since monocytes can produce some of the markers quantified [36, 37], the presence of intervillositis in only a portion of the samples is a potential bias when comparing groups. Using laser capture microdissection, we specifically addressed the transcription profile of the syncytiotrophoblast layer (figure 3A).
Figure 3. SCT-specific transcription profile using laser capture microdissection.
A. Syncytiotrophoblast was specifically captured from cryosections of placental tissue using laser capture microdissection and its transcription profile was determined by quantitative real time RT-PCR normalised to the expression of a placental housekeeping gene. B. Controls (open bars, n=6), malaria cases without intervillositis (light grey bars, n=7) and cases with monocyte infiltrates (dark grey bars, n=10) were compared. C. Participants with normal birth weight (NBW, open bars, n=21) and LBW cases (grey bars, n=2) had similar transcription profiles. P>.05 Mann-Whitney’s test. Bars represent median, 25th and 75th percentiles and the whiskers are 5th and 95th percentiles. Flt-1: VEGF receptor 1; HIF: Hypoxia-inducible factor; PlGF: Placental growth factor; VEGF: Vascular endothelial growth factor; sFlt-1: soluble Flt-1; Intervill.: intervillositis.
When controls were compared to malaria cases (regardless of the presence or absence of intervillositis), lower VEGF (P=.0026) and higher HIF-1α (P=.0005) transcripts levels in the SCT of infected placentae compared to control placentae were the only statistical differences noted. Differences in VEGF SCT levels did not reach significance (P=.49) when malaria cases were split according to the presence/absence of intervillositis (figure 3B). HIF-1α SCT levels were similar between malaria cases with or without intervillositis (P=.70) but higher than control placentae (P=.0001 and P=.0027 respectively). Classification of the samples on the basis of the presence/absence of intervillositis (regardless of malaria infection – data not shown) or birth weight (figure 3C) did not yield any statistical differences. Taken together, these data suggest that SCT transcription profile is not typical of a response to hypoxia but do indicate differences which were not detected when addressing placental tissue as a whole. Moreover, these differences appear to be associated with presence of malaria infection, and not specifically with intervillositis, as they were present whether monocytes infiltrates were detected or not.
4. Protein expression and tissue distribution is not characteristic of a response to hypoxia
To further validate these findings and look for a potential difference in protein expression and tissue distribution of the different hypoxia markers, we determined the protein expression profiles of the different samples using immunohistochemistry (IHC). Characteristics of the patients included in the IHC experiments are summarised in table 1B. Only 2 patients delivered LBW babies (figure 1B). All groups are comparable for every criterion (except the ones used for classification such as birth weight), whatever the classification used (P≥.057).
a. Both microscopists found similar patterns
We determined VEGF, Flt-1, KDR, PlGF and HIF-1α expression and tissue distribution for each placental tissue sample (figure 4). Both microscopists found the same expression and distribution profiles for each marker (data not shown). Results are provided in supplementary table 2.
Figure 4. Examples of immunohistochemistry staining.
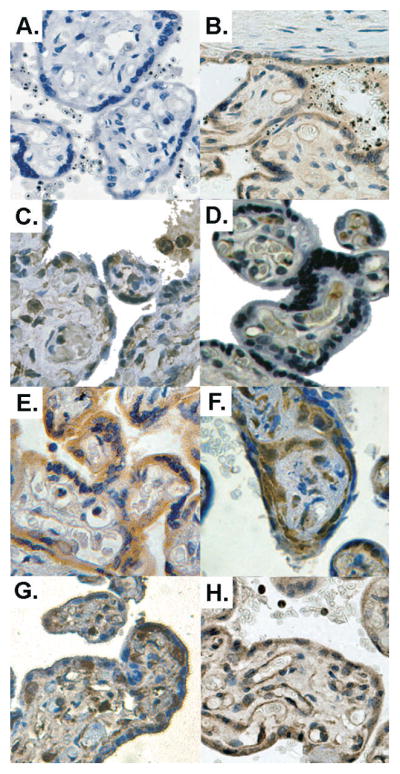
The expression and tissue distribution of the different hypoxia markers were assessed on fixed placental tissue sections. A. The specificity of the staining was checked by omitting the primary antibody. B. PlGF syncytial and endothelial staining of a malaria-infected placenta. C. Flt-1 positive cells are found in the syncytium, villus vessel walls and Hofbauer cells. D. KDR staining is mainly found in the villus vessels endothelium. In control placentas (E.), VEGF mainly stained the syncytium but malaria-infected placentas with intervillositis, (F.), showed significantly more frequent and intense staining of stromal cells. However, no difference was found for HIF-1α staining when uninfected placentas (G.) were compared with malaria-infected ones (H.), both showing syncytial and stromal staining (possibly Hofbauer cells). Original magnification: 200x. Colour balance, luminosity and contrast have been optimised.
b. Protein expression and tissue distribution patterns do not correspond to those reported for hypoxic tissue
When samples were classified into controls and malaria cases, no difference in consistency or intensity of staining was found for any of the markers in any cell type. No difference was found when malaria cases were split according to the presence/absence of intervillositis, or when samples were classified according to birth weight. However, when samples were classified according to the presence/absence of intervillositis, a more intense (P=.037) and consistent (P=.041) VEGF staining in the villous stroma was found in samples with intervillositis. However, these differences were not statistically significant after Bonferroni adjustment (P>.01). Staining patterns did not differ between these two groups for any other marker.
Taken together, transcription and expression profiles suggest that PM is not associated with placental hypoxia, and neither are LBW or intervillositis.
DISCUSSION
It has been long suggested that placental hypoxia could play a role in PM-associated FGR but direct evidence for such a role has been lacking. We tested this hypothesis by looking for molecular evidence of placental hypoxia in PM.
Cellular response to hypoxia is orchestrated by the transcription factor HIF-1, which modifies the expression of several genes, in particular angiogenic factors. Using a very reliable and sensitive quantitative real time RT-PCR approach, we could not associate any clinical or biological features of PM with a transcription profile characteristic of a molecular response to hypoxia when addressing the whole placental tissue.
The absence of transcriptional evidence for placental hypoxia in PM was further substantiated by the lack of association between the clinical and biological features of PM and expression patterns of hypoxia-specific markers as evidenced by immunohistochemistry. The staining patterns obtained were similar to those previously described [27, 28, 38, 39]. When we used a previously validated scoring process [35] to quantify the expression of these markers, we found no evidence for a hypoxia-specific expression pattern in any of the groups. Because only 2 samples were classified as LBW, we also used an alternative definition of LBW as a birth weight lower than the 5th centile of a normal foetal growth curve [40] to compare appropriate (n=32) and small (n=7) for gestational age infants and showed that they had similar IHC profiles (data not shown).
Taken together, these results strongly argue against a role for placental hypoxia in PM pathogenesis.
In contrast to a recent study that showed that PM was associated with an increase in sFlt-1 mRNA levels in normotensive primigravidae and in malaria-infected placentae with intervillositis compared with control placentae [41], we did not find any difference in sFlt-1 RNA levels between the different groups when addressing either the whole tissue or specifically the SCT. Differences in the recruitment criteria (we focused on normotensive women, and included all gravidities, rather than just primigravidae) and in the technical approaches used (the Flt-1 antibody we used only recognizes the membrane-bound form, unlike the ones the authors used which binds both membrane-bound and soluble forms) could explain these conflicting results. Moreover, each study was relatively small (around 60 women), and suffers from some limitations in power. Further investigation of the interactions between malaria and hypertension and pre-eclampsia in first pregnancies may be warranted.
Since all the samples in our cohort presented similar transcription and expression profiles, it was possible that they could all be hypoxic as a consequence of labour or tissue handling. However, since we included only placentae from children with a normal Apgar score, labour-induced acute hypoxia is unlikely. Moreover, since HIF-1α transcript levels have been shown to change very rapidly after the onset of cellular hypoxia, if all samples had suffered from hypoxia as a consequence of labour or tissue handling, all would have expressed similar levels of HIF-1α transcript. But we did find a difference in HIF-1α SCT transcript levels between malaria-infected placentae and controls, which further rules out the possibility of a general labour-induced hypoxia.
The increased HIF-1α transcript levels in the SCT of malaria-infected placentae could reflect local inflammation [42]. Indeed, hypoxia-induced HIF-1α up-regulation is thought to occur at the level of protein stability (post-transcriptional). Thus, higher HIF-1α transcript levels in the syncytium of malaria-infected placentae are likely to be a consequence of local inflammation that could be both the underlying cause of the molecular and protein changes noted, and the driving force behind malaria-associated LBW. Because similar HIF-1α transcripts levels were found in malaria cases in the presence or absence of monocyte infiltrates, which are markers of chronic inflammation, the increase in HIF-1α transcript levels might be due to a more acute inflammation or to the presence of infected erythrocytes. Testing of this hypothesis requires further study.
We could not associate any of the biological or clinical features of PM with a transcription or expression profile characteristic of a response to hypoxia. This argues against a role for placental hypoxia in PM-associated FGR pathogenesis. Although the role of placental hypoxia in the pathogenesis of FGR has been well described, particularly in animal models [43], it is not universally implicated in FGR. For example, in several studies there was no difference in the expression of VEGF between normal and FGR-affected placentae [44–46].
Our study does not rule out the possibility of fetal hypoxia, which could decrease fetal growth. Kingdom and Kaufmann described a situation leading to fetal hypoxia in the absence of placental hypoxia when the fetoplacental perfusion is inadequate. This is known as post-placental hypoxia [47]. However, one study did not find evidence of FGR-associated fetal hypoxia [47]. Thus, assessment of placental and fetal blood flow throughout pregnancy [48] combined with arterial gas analysis of cord blood appears to be essential to adequately address the impact of a potential fetal hypoxia on intra-uterine growth during PM.
Table 2. Immunohistochemistry scoring.
Intensity (Int.) and consistency (Cons.) of each staining is summarised for different cell types. Controls were compared to malaria cases with or without intervillositis. Also, participants with a normal birth weight were compared to those suffering from low birth weight (birth weight <2500g).
| Asymptomatic (n=10) | Malaria without intervillositis (n=10) | Malaria with intervillositis (n=10) | LBW (n=28) | Normal birth weight (n=2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Int. | Cons. | Int. | Cons. | Int. | Cons. | Int. | Cons. | Int. | Cons. | ||
| HIF-1α | Monocytes Neutrophils | + | ++ | ++ | ++ | ++ | +++ | + | + | ++ | ++ |
| Syncytium | ++ | +++ | ++ | +++ | ++ | +++ | ++ | +++ | ++ | +++ | |
| Villus stroma | − | − | − | − | − | − | − | − | − | − | |
| Villus vessel endothelium | ++ | +++ | ++ | ++ | ++ | +++ | ++ | ++ | ++ | +++ | |
| Villus vessel lumen | − | − | − | − | − | − | − | − | − | − | |
| Extravillous trophoblast | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| PlGF | Monocytes Neutrophils | ++ | ++ | ++ | +++ | ++ | +++ | +/− | +/− | + | + |
| Syncytium | ++ | +++ | ++ | ++ | ++ | +++ | + | + | ++ | ++ | |
| Villus stroma | − | − | − | − | − | − | − | − | − | − | |
| Villus vessel endothelium | ++ | +++ | ++ | +++ | ++ | +++ | ++ | ++ | ++ | +++ | |
| Villus vessel lumen | + | + | + | + | + | + | +/− | +/− | + | + | |
| Extravillous trophoblast | ++ | +++ | +++ | ++ | +++ | ++ | ++ | ++ | +++ | +++ | |
| G | Monocytes Neutrophils | ++ | ++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ |
| Syncytium | ++ | +++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ | +++ | |
| Villus stroma | +/− | +/− | +/− | +/− | + | + | − | − | + | + | |
| Villus vessel endothelium | ++ | ++ | + | + | ++ | ++ | + | + | + | ++ | |
| Villus vessel lumen | +/− | +/− | +/− | +/− | +/− | +/− | − | − | +/− | +/− | |
| Extravillous trophoblast | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Flt-1 | Monocytes Neutrophils | + | + | ++ | ++ | ++ | ++ | + | + | + | ++ |
| Syncytium | + | ++ | + | + | ++ | ++ | + | + | + | ++ | |
| Villus stroma | − | − | − | − | − | − | − | − | − | − | |
| Villus vessel endothelium | + | + | + | + | + | + | + | + | + | + | |
| Villus vessel lumen | +/− | +/− | +/− | +/− | +/− | +/− | − | − | +/− | +/− | |
| Extravillous trophoblast | ++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ | ++ | |
| KDR | Monocytes Neutrophils | − | − | − | − | − | − | − | − | − | − |
| Syncytium | − | − | − | − | − | − | − | − | − | − | |
| Villus stroma | + | + | + | + | + | + | + | +/− | + | + | |
| Villus vessel endothelium | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | |
| Villus vessel lumen | ++ | ++ | ++ | ++ | ++ | ++ | + | ++ | ++ | ++ | |
| Extravillous trophoblast | − | − | − | − | − | − | − | − | − | − | |
Scores are symbolised by − (score=0), +/− (score<0.1), + (0.1≤score≤1), ++ (1<score≤2) and +++ (score>2). The only statistically significant difference found between groups was a more intense (P=.037) and more consistent (P=.041) VEGF staining in the villous stroma of patients with intervillositis compared to placentae without intervillositis regardless of their malaria status (Mann-Whitney’s test). HIF: Hypoxia-inducible factor; PlGF: Placental growth factor; VEGF: Vascular endothelial growth factor; Flt-1: VEGF receptor 1; KDR: VEGF receptor 2.
Acknowledgments
Authors wish to thank the women who participated in the study and the medical staff at Queen Elizabeth Central Hospital in Blantyre, Malawi. This study was funded by The Wellcome Trust (Senior Fellowship to SJR) and the NHMRC. Francois Lacoste is acknowledged for his support.
Footnotes
The authors declare not to have any conflict of interest.
Part of this work has been presented at the IFPA 2006 (poster) and ICOPA XI (oral presentation) meetings.
References
- 1.Desai M, ter Kuile F, Nosten F, McGready R, Asamoa K, Brabin B, Newman RD. The global burden of malaria in pregnancy: what’s known and where are the gaps? Lancet Infectious Diseases. 2007;7:93–104. doi: 10.1016/S1473-3099(07)70021-X. [DOI] [PubMed] [Google Scholar]
- 2.Beeson JG, Duffy PE. The immunology and pathogenesis of malaria during pregnancy. Curr Top Microbiol Immunol. 2005;297:187–227. doi: 10.1007/3-540-29967-x_6. [DOI] [PubMed] [Google Scholar]
- 3.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64:28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 4.Guyatt HL, Snow RW. Impact of Malaria during Pregnancy on Low Birth Weight in Sub-Saharan Africa. Clin Microbiol Rev. 2004;17:760. doi: 10.1128/CMR.17.4.760-769.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamudio S. The placenta at high altitude. High Alt Med Biol. 2003;4:171–91. doi: 10.1089/152702903322022785. [DOI] [PubMed] [Google Scholar]
- 6.Soleymanlou N, Jurisica I, Nevo O, Ietta F, Zhang X, Zamudio S, Post M, Caniggia I. Molecular evidence of placental hypoxia in preeclampsia. J Clin Endocrinol Metab. 2005;90:4299–308. doi: 10.1210/jc.2005-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabin BJ, Johnson PM. Placental malaria and pre-eclampsia through the looking glass backwards? J Reprod Immunol. 2005;65:1–15. doi: 10.1016/j.jri.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ismail MR, Ordi J, Menendez C, Ventura PJ, Aponte JJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL. Placental pathology in malaria: a histological, immunohistochemical, and quantitative study. Hum Pathol. 2000;31:85–93. doi: 10.1016/s0046-8177(00)80203-8. [DOI] [PubMed] [Google Scholar]
- 9.Rogerson SJ, Pollina E, Getachew A, Tadesse E, Lema VM, Molyneux ME. Placental monocyte infiltrates in response to Plasmodium falciparum infection and their association with adverse pregnancy outcomes. Am J Trop Med Hyg. 2003;68:115–119. [PubMed] [Google Scholar]
- 10.Galbraith RM, Faulk WP, Galbraith GM, Holbrook TW, Bray RS. The human materno-foetal relationship in malaria: I. Identification of pigment and parasites in the placenta. Trans R Soc Trop Med Hyg. 1980;74:52–60. doi: 10.1016/0035-9203(80)90011-5. [DOI] [PubMed] [Google Scholar]
- 11.Ordi J, Ismail MR, Ventura PJ, Kahigwa E, Hirt R, Cardesa A, Alonso PL, Menendez C. Massive chronic intervillositis of the placenta associated with malarial infection. Am J Surg Path. 1998;22:1006–1011. doi: 10.1097/00000478-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Duffy PE. Immunity to malaria during pregnancy: different host, different parasite. In: Duffy PE, Fried M, editors. Malaria in pregnancy: deadly parasite, susceptible host. Washington: Taylor & Francis; 2001. pp. 71–126. [Google Scholar]
- 13.Kaushik A, Sharma VKS, Kumar R. Malaria placental infection and low birth weight babies. J Commun Dis. 1992;24:65–69. [PubMed] [Google Scholar]
- 14.Steketee RW, Wirima JJ, Hightower AW, Slutzker L, Heymann DL, Breman JG. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg. 1996;55:33–41. doi: 10.4269/ajtmh.1996.55.33. [DOI] [PubMed] [Google Scholar]
- 15.Garnham PCC. The placenta in malaria with special reference to reticulo-endothelial immunity. Trans R Soc Trop Med Hyg. 1938;32:13–35. [Google Scholar]
- 16.Watkinson M, Rushton DI. Plasmodial pigmentation of placentae and outcome of pregnancy in West African mothers. B Med J. 1983;287:251–254. doi: 10.1136/bmj.287.6387.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heazell AEP, Moll SJ, Jones CJP, Baker PN, Crocker IP. Formation of Syncytial Knots is Increased by Hyperoxia, Hypoxia and Reactive Oxygen Species. Placenta. 2007;28 (Suppl A):S33–40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 18.Crocker IP, Tanner OM, Myers JE, Bulmer JN, Walraven G, Baker PN. Syncytiotrophoblast Degradation and the Pathophysiology of the Malaria-infected Placenta. Placenta. 2004;25:273. doi: 10.1016/j.placenta.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Salceda S, Caro J. Hypoxia-inducible Factor 1alpha (HIF-1alpha) Protein Is Rapidly Degraded by the Ubiquitin-Proteasome System under Normoxic Conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem. 1997;272:22642. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- 20.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A. 1998;95:7987. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kallio PJ, Wilson WJ, O’Brien S, Makino Y, Poellinger L. Regulation of the Hypoxia-inducible Transcription Factor 1alpha by the Ubiquitin-Proteasome Pathway. J Biol Chem. 1999;274:6519. doi: 10.1074/jbc.274.10.6519. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–40. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- 23.Taylor CM, Stevens H, Anthony FW, Wheeler T. Influence of hypoxia on vascular endothelial growth factor and chorionic gonadotrophin production in the trophoblast-derived cell lines: JEG, JAr and BeWo. Placenta. 1997;18:451. doi: 10.1016/s0143-4004(97)80047-1. [DOI] [PubMed] [Google Scholar]
- 24.Trollmann R, Amann K, Schoof E, Beinder E, Wenzel D, Rascher W, Dotsch J. Hypoxia activates the human placental vascular endothelial growth factor system in vitro and in vivo: up-regulation of vascular endothelial growth factor in clinically relevant hypoxic ischemia in birth asphyxia. Am J Obstet Gynecol. 2003;188:517–23. doi: 10.1067/mob.2003.138. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Gu B, Zhang Y, Lewis DF, Wang Y. Hypoxia-induced increase in soluble Flt-1 production correlates with enhanced oxidative stress in trophoblast cells from the human placenta. Placenta. 2005;26:210. doi: 10.1016/j.placenta.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed A, Dunk C, Ahmad S, Khaliq A. Regulation of placental vascular endothelial growth factor (VEGF) and placenta growth factor (PIGF) and soluble Flt-1 by oxygen--a review. Placenta. 2000;21 (Suppl A):S16–24. doi: 10.1053/plac.1999.0524. [DOI] [PubMed] [Google Scholar]
- 27.Kumazaki K, Nakayama M, Suehara N, Wada Y. Expression of vascular endothelial growth factor, placental growth factor, and their receptors Flt-1 and KDR in human placenta under pathologic conditions. Hum Pathol. 2002;33:1069–77. doi: 10.1053/hupa.2002.129420. [DOI] [PubMed] [Google Scholar]
- 28.Rajakumar A, Conrad KP. Expression, Ontogeny, and Regulation of Hypoxia-Inducible Transcription Factors in the Human Placenta. Biology of Reproduction. 2000;63:559–69. doi: 10.1095/biolreprod63.2.559. [DOI] [PubMed] [Google Scholar]
- 29.Mwapasa V, Rogerson SJ, Molyneux ME, Abrams ET, Kamwendo DD, Lema VM, Tadesse E, Chaluluka E, Wilson PE, Meshnick SR. The Effect of Plasmodium falciparum Malaria on Peripheral and Placental HIV-1 RNA Concentrations in Pregnant Malawian Women. AIDS. 2004;18(7):1051–9. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 30.Abrams ET, Brown H, Chensue SW, Turner GD, Tadesse E, Lema VM, Molyneux ME, Rochford R, Meshnick SR, Rogerson SJ. Host Response to Malaria During Pregnancy: Placental Monocyte Recruitment Is Associated with Elevated beta Chemokine Expression. J Immunol. 2003;170:2759–2764. doi: 10.4049/jimmunol.170.5.2759. [DOI] [PubMed] [Google Scholar]
- 31.Abrams ET, Milner DA, Jr, Kwiek J, Mwapasa V, Kamwendo DD, Zeng D, Tadesse E, Lema VM, Molyneux ME, Rogerson SJ, Meshnick SR. Risk factors and mechanisms of preterm delivery in Malawi. Am J Reprod Immunol. 2004;52(2):174–83. doi: 10.1111/j.1600-0897.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 32.Boeuf P, Vigan-Womas I, Jublot D, Loizon S, Barale JC, Akanmori BD, Mercereau-Puijalon O, Behr C. CyProQuant-PCR: a real time RT-PCR technique for profiling human cytokines, based on external RNA standards, readily automatable for clinical use. BMC Immunol. 2005;6:5. doi: 10.1186/1471-2172-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta. 2005;26:601–7. doi: 10.1016/j.placenta.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Volm M, Koomagi R, Mattern J. Prognostic value of vascular endothelial growth factor and its receptor Flt-1 in squamous cell lung cancer. Int J Cancer. 1997;74:64–8. doi: 10.1002/(sici)1097-0215(19970220)74:1<64::aid-ijc11>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 36.Dirkx AEM, oude Egbrink MGA, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 37.Lewis C, Murdoch C. Macrophage Responses to Hypoxia: Implications for Tumor Progression and Anti-Cancer Therapies. Am J Pathol. 2005;167:627. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clark DE, Smith SK, Sharkey AM, Charnock-Jones DS. Localization of VEGF and expression of its receptors Flt and KDR in human placenta throughout pregnancy. Hum Reprod. 1996;11:1090–8. doi: 10.1093/oxfordjournals.humrep.a019303. [DOI] [PubMed] [Google Scholar]
- 39.Vuckovic M, Ponting J, Terman BI, Niketic V, Seif MW, Kumar S. Expression of the vascular endothelial growth factor receptor, KDR, in human placenta. J Anat. 1996;188:361–6. [PMC free article] [PubMed] [Google Scholar]
- 40.Kramer MS, Platt RW, Shi Wu Wen MB, Joseph KS, Allen A, Abrahamowicz M, Blondel B, Breart G. A New and Improved Population-Based Canadian Reference for Birth Weight for Gestational Age. Pediatrics. 2001;108:e35. doi: 10.1542/peds.108.2.e35. [DOI] [PubMed] [Google Scholar]
- 41.Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and Maternal/Fetal Conflict during Placental Malaria. PLoS Medicine. 2006;3:e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haddad JJ, Land SC. A non-hypoxic, ROS-sensitive pathway mediates TNF-alpha-dependent regulation of HIF-1alpha. FEBS Lett. 2001;505:269–74. doi: 10.1016/s0014-5793(01)02833-2. [DOI] [PubMed] [Google Scholar]
- 43.Regnault TRH, de Vrijer B, Galan HL, Wilkening RB, Battaglia FC, Meschia G. Development and Mechanisms of Fetal Hypoxia in Severe Fetal Growth Restriction. Placenta. 2006 doi: 10.1016/j.placenta.2006.06.007. In Press, Corrected Proof. [DOI] [PubMed] [Google Scholar]
- 44.Lyall F, Young A, Boswell F, Kingdom JC, Greer IA. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–76. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- 45.Gurel D, Ozer E, Altunyurt S, Guclu S, Demir N. Expression of IGR-IR and VEGF and Trophoblastic Proliferative Activity in Placentas from Pregnancies Complicated by IUGR. Pathol Res Pract. 2003;199:803. doi: 10.1078/0344-0338-00499. [DOI] [PubMed] [Google Scholar]
- 46.Tse JY, Lao TT, Chan CC, Chiu PM, Cheung AN. Expression of vascular endothelial growth factor in third-trimester placentas is not increased in growth-restricted fetuses. J Soc Gynecol Investig. 2001;8:77–82. [PubMed] [Google Scholar]
- 47.Kingdom JCP, Kaufmann P. Oxygen and placental villous development: Origins of fetal hypoxia. Placenta. 1997;18:613. doi: 10.1016/s0143-4004(97)90000-x. [DOI] [PubMed] [Google Scholar]
- 48.Arbeille P, Caries G, Tobal N, Herault S, Georgescus M, Bousquet F, Perrotin F. Fetal Flow Redistribution to the Brain in Response to Malaria Infection: Does Protection of the Fetus Against Malaria Develop Over Time? J Ultrasound Med. 2002;21:739–46. doi: 10.7863/jum.2002.21.7.739. [DOI] [PubMed] [Google Scholar]