Abstract
Previous studies have shown that intravenously applied bacteria can accumulate in tumors and lead to sporadic tumor regression. Recently, systemic administration of attenuated Salmonella typhimurium was demonstrated to generate no significant side effects in humans, but also no anti-tumor responses. We report the enhanced antitumor activity in preclinical mouse cancer models of non-virulent S. typhimurium engineered to synthesize the cytokine, Interleukin-18 (IL-18). IL-18-producing bacteria (but not control bacteria) inhibit the growth of primary subcutaneous tumors as well as pulmonary metastases in immunocompetent mice challenged with syngeneic multi-drug resistant clones of murine carcinoma cell lines, without overt toxicity to normal tissues. Anti-tumor activity was associated with increased accumulation of T-lymphocytes and NK cells in tumors, and massive infiltration of granulocytes, as well as increased intra-tumoral production of several cytokines. In summary, these findings provide evidence of promising preclinical anti-tumor activity of IL-18-expressing, attenuated S. typhimurium, suggesting a novel strategy for cancer immunotherapy.
Keywords: Salmonella, IL-18, cancer therapy, bacterial therapy, tumor targeting
Introduction
Although reports of using bacteria to treat tumors date as far back as 1893 (1), the significant toxicity associated with this treatment precluded widespread use (1–3) and has thus far been limited to use of Bacillus Calmette-Guerin (BCG) for the treatment of superficial bladder carcinomas (4, 5). Facultative anaerobic bacteria accumulate within tumors to concentrations of up to 109 colony-forming units per gram of tumor tissue (6, 7). The intra-tumoral accumulation of bacteria may be explained by a variety of proposed mechanisms, including access to nutrients released from necrotic cells in hypoxic areas of rapidly growing tumors, diminished bactericidal activity of macrophages and granulocytes due to lack of oxygen, suppression of the immune system in tumor regions, defective granulocyte chemotaxis due to tumor-derived cytokines such as TGF-β, and reduced anti-microbial antibodies as well as complement factors due to irregular vasculature and positive pressure within tumors. Thus, the intrinsic property of anaerobic bacteria to localize to tumors theoretically could be exploited for delivery of therapeutic molecules in a targeted fashion.
Among facultative anaerobic bacteria, Salmonella typhimurium has shown promise for development of microbial-based tumor therapies. S. typhimurium is a genetically tractable microbe, allowing genetic engineering. In preclinical animal models, intravenously (i.v.) injected Salmonella reportedly localize preferentially to solid tumors and proliferate at tumor sites (6, 7). Recently, a lipid A negative strain of S. typhimurium was used for phase I clinical trials and was found to be safe at doses up to 109 CFU per m2 body surface area (8, 9). Elimination of lipid A prevents production of endotoxin (lipopolysaccharide), making these microorganisms non-virulent. However, in clinical trials, lipid A-deficient Salmonella exhibited no antitumor activity.
Since S. typhimurium has an excellent safety profile in humans (7, 10), and accumulates in tumors in mouse models, we explored a strategy of engineering these non-virulent bacteria to express genes encoding secreted proteins with potential anti-tumor activity. S. typhimurium are able to synthesize functional cytokines at high levels (11), suggesting they could be exploited for in vivo production of cytokines in a tumor-targeted manner. Furthermore, their ability to grow anaerobically makes them an attractive delivery vehicle for the hypoxic regions of tumors, which tend to be resistant to radiation or chemotherapy. Moreover, from a safety perspective, bacterial delivery of cytokine genes may afford significant advantages over virus-based cancer gene therapies, including; (1) the possibility to immediately terminate treatment using antibiotics and (2) avoiding insertion of foreign genetic material into the host genome, which has been associated with insertional mutagenesis leading to leukemia in the case of retrovirus-based approaches (12).
In this report, we modified attenuated Salmonella to bear a plasmid vector allowing for stable expression of Interleukin-18 (IL-18). The cytokine IL-18 was originally described as an IFNγ-inducing factor (13), capable of not only enhancing cytokine production by T and NK cells, but also inducing their proliferation and cytolytic activity (14, 15). Furthermore, IL-18 leads to upregulation of MHC class I antigen expression and also favors the differentiation of CD4+ helper T cells toward the T helper 1 (Th1) subtype. In turn, these cells promote an anti-tumor responses mediated by NK cells, macrophages, and CD8+ T cells (16–18). Thus, through its direct and indirect activities on immune cells, IL-18 has broad effects on immune cell populations implicated in fighting malignancy. Besides these effects on the immune system, IL-18 also acts as a suppressor of angiogenesis by directly inhibiting fibroblast growth factor 2-induced endothelial cell proliferation (13, 19). Engineering tumor cells to produce IL-18 leads to reduced tumorigenicity (20), and systemic administration of the recombinant IL-18 protein causes considerable tumor inhibition (20, 21). Unfortunately, it has been shown that IL-18 administrated systemically causes serious side effects (22), thus precluding its clinical application. This toxicity problem could potentially be overcome with tumor-specific targeting.
As an initial step towards this goal, we report here the use of attenuated Salmonella typhimurium to express in vivo IL-18, showing evidence of anti-tumor activity in preclinical mouse cancer models and recruitment of immune and inflammatory cells into tumors, associated with enhanced intra-tumoral cytokine generation. The studies of IL-18 reported here thus extend our recent analysis of bacteria armed to express the cytokine LIGHT, a TNF-family member (24), and thus demonstrate the potential of using either Interleukin-family cytokines or TNF-family cytokines for this application.
Materials & Methods
Animals, bacterial strains, cell lines, and plasmid vectors
The cDNAs encoding enhanced green fluorescent protein (pEGFP, Clontech, Mountain View, California) or murine IL-18 with an Igκ leader sequence for directing secretion (27) were subcloned into pGEN206 (24). Protein expression of pGEN206-IL-18 was demonstrated by immunoblotting of 109 CFU Salmonella resuspended in Laemmli sample buffer or culture supernatants, using a monoclonal rat anti-mouse IL-18 antibody (MBL, Naka-ku, Nagoya, Japan). Additional details concerning procedures are found in our recent publication (24).
Mouse experiments
For experiments involving primary tumors, BALB/c mice (n=5) were injected subcutaneously (s.c.) with 105 CT26 colon carcinoma cells and treated 14 d later as indicated with either PBS or 5×106 CFU of Salmonella per mouse; mice were challenged with either 2.5×105 or 7.5×104 D2F2 breast carcinoma cells and treated i.v. with either PBS or Salmonella (5×106 CFU Salmonella per mouse) after 9, 14 and 19 d or 7d, respectively. Volumes of s.c. tumors were measured in 2 dimensions and calculated as length/2 × width2 or weighed. For the pulmonary metastasis model, BALB/c mice (n=8 per group) were injected i.v. with 5×104 D2F2 breast carcinoma cells and then treated i.v. with PBS, or Salmonella (5×106 CFU per mouse) after 6, 13 and 20 d. After 50 d, lungs were weighed and also examined for metastases assessing the percentage of lung surface covered by metastases as follows: 0=0%, 1≤20%, 2=20–50%, 3≥50%.
Immunohistochemistry, FACS analysis, and ELISA
Experiments were performed essentially as described previously (24), using fluorochrome-conjugated antibodies (BD, Franklin Lakes, New Jersey), including antibodies specific for the antigens Ly-6G, DX5, CD4 and CD3, andELISA kits for cytokines IL-1β, TNFα, IFNγ, GM-CSF (eBioscience, San Diego, California). Results represent amounts of cytokines per gram of protein (mean ± SD)
Statistics
Statistical significance was determined by 2-tailed Student's t test for most comparisons. Two-way ANOVA was used for time-course studies. The significance of metastases scores was determined by Mann-Whitney U test. Findings were regarded as significant, if P < 0.05.
Results
IL-18 expression by attenuated Salmonella
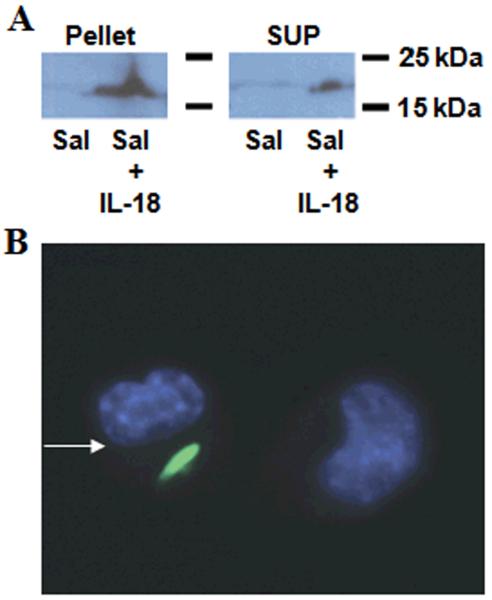
To engineer the attenuated S. typhimurium strain purI−/msbB− for expressing IL-18, we used the plasmid pGEN206, in which a human IL-18 cDNA fused to an N-terminal leader sequence for directing secretion was expressed under control of an ompC promoter. The engineered bacteria were grown in culture, recovered by centrifugation, and the resulting cell-containing pellet and cleared culture supernatant were tested for IL-18 protein by immunoblotting, revealing the presence of IL-18 in both cells and supernatant (Fig. 1a). The production of IL-18 under in vitro bacterial cultures is >50 pg/μg. Control Salmonella (transformed with empty plasmid) and their culture supernatants did not contain IL-18 protein.
Figure 1. IL-18 production by engineered Salmonella.
a, Expression of the 18 kD secretory IL-18 protein was demonstrated by immmunoblot analysis of cell lysates (“pellet”) or culture supernatants (“sup”) from Salmonella containing either empty vector (Sal) or the IL-18-expression plasmid (Sal+IL-18). Samples were normalized for total protein content. Results are representative of three independent experiments. b, The ability of Salmonella to infect CT-26 colon carcinoma cells in vitro was shown using GFP-expressing bacteria. Nuclear staining in blue was performed using Hoechst-33342 dye. The arrow points to an infected cell, which is shown in proximity to a non-infected cell.
Salmonella are non-obligate intracellular bacteria and it has been reported that they can infect many epithelial cancer cells (23). To explore whether the attenuated Salmonella strain employed here retains infective activity, we transformed the purI−/msbB− strain of S. typhimurium with a plasmid encoding Green Fluorescent Protein (GFP) and co-cultured bacteria with various murine carcinoma cell lines, before adding antibiotic gentamycin to kill extracellular bacteria. Cells were then fixed, stained with DNA-binding fluorochrome, and imaged. For all 6 of the carcinoma lines tested, attenuated Salmonella were able to infect the cancer cells, as revealed by the presence of GFP-emitting bodies in the cytosol (see Fig. 1b for representative example).
Salmonella-expressing IL-18 inhibit primary tumor growth in mice
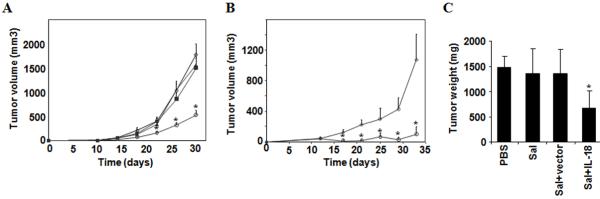
Recently, we showed that the attenuated strain of Salmonella used here accumulates in subcutaneous tumors in vivo in mice, using luciferase expressing bacteria in conjunction with bioluminescence imaging (24). We therefore performed studies in which control and IL-18-expressing Salmonella were compared for anti-tumor activity in mouse cancer models using syngeneic tumors in immunocompetent mice grown as subcutaneous tumors. For these experiments, mice were injected s.c. either with CT-26 colon (Fig. 2a) or D2F2 breast carcinoma cells (Fig. 2b). When tumors were visible, mice were treated i.v. with phosphate-buffered saline (PBS), control Salmonella, or Salmonella containing either control (empty) vector or vector expressing mature IL-18. Marked reduction of tumor growth was observed in those mice treated with Salmonella containing the IL-18-expression vector compared to the respective control groups (Figs. 2a and b). The suppression of tumor growth was also confirmed in experiments where tumor-bearing mice were treated for 26 days, then sacrificed, and tumors weighed (Fig. 2c). Again, IL-18-producing Salmonella demonstrated significant anti-tumor activity, unlike control Salmonella.
Figure 2. IL-18-expressing Salmonella inhibit subcutaneous tumor growth.
a, BALB/c mice (n=8) were challenged s.c. with 105 CT-26 colon carcinoma cells and treated with PBS (◆), empty Salmonella (■), Salmonella with empty vector (▲), or Salmonella with IL-18 vector (○) after 14 d, when tumors reached a visible size. Bar graphs indicate average tumor volumes estimated by external calipers (mean ± SE). Tumor volumes for Sal+IL-18 treated mice were significantly smaller compared to all other treatment groups. Asterisks indicate P<0.05. b, Mice were injected s.c. with 2.5×105 D2F2 breast carcinoma cells and treated i.v. with PBS (◆) or Salmonella with IL-18 vector (○) after 9, 14 and 19 d. Bar graphs indicate average tumor volumes (mean ± SD; n=5). Tumors in Sal+IL-18 treated mice were significantly smaller. Asterisks indicate P<0.05. c, A separate cohort of mice (n=5) was challenged with 7.5×104 cells and treated after 7d as above. After 26 d, mice were sacrificed and tumors were excised and weighed (mean ± SD). Asterisks indicates P<0.05 compared to the other treatment groups.
Though demonstrating potent anti-tumor activity, IL-18-expressing Salmonella did not cause greater systemic toxicity than the unmodified attenuated Salmonella strain. Histological analysis of multiple organs showed minimal changes that were comparable to the results obtained with control Salmonella that did not produce IL-18. For example, kidneys and lungs were completely normal, while spleen showed enlargement and diffuse inflammatory reactions that were similar in extent of severity in mice treated with either control of IL-18-producing Salmonella (Supplementary Fig. 1a). Liver tissue showed slight inflammation by polymorphonuclear cells and rare granulomatosis reactions, again with the extent of toxicity similar in mice treated with either control of IL-18-producing bacteria (Supplementary Fig. 1b). Thus, addition of IL-18 did not worsen the toxicity of Salmonella, which have been shown previously to be well tolerated in humans (8, 9). Grossly, the mice did not show decreased activity or grooming abnormalities until several weeks after Salmonella injections, when tumor burden increased, suggesting that the tumor rather than the therapy contributed to these signs of illness.
IL-18-expressing Salmonella induce massive leukocyte infiltration into tumors, and increase recruitment of NK and T cells
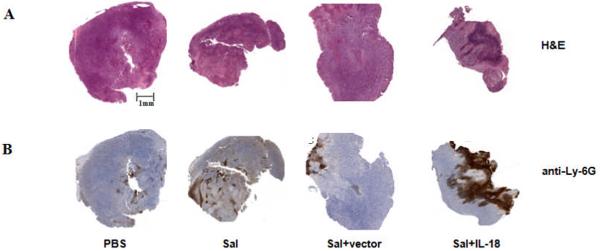
We examined the histology of subcutaneous tumors in mice treated with Salmonella (Fig. 3). By hematoxylin-eosin (H&E) staining, CT26 tumors derived from mice treated with PBS were comprised of monotonous accumulations of malignant epithelial cells with minimal stroma. Tumors from animals treated with control Salmonella or Salmonella containing only empty plasmid were similar, showing scan leukocyte infiltrates. By comparison, tumors from mice treated with IL-18-expressing Salmonella contained massive leukocyte infiltrates (Fig. 3a), comprised particularly of cells with morphological characteristics of granulocytes (Supplementary Fig. 2). Immunohistochemical staining with anti-Ly-6G antibody confirmed the abundant presence of granulocytes in these tumors (Fig. 3b). These histological data suggest that IL-18-expressing Salmonella have greatly enhanced capacity to induce host inflammatory responses to tumors.
Figure 3. IL-18-expressing Salmonella induce massive leukocyte infiltration into subcutaneous tumors.
Tumors derived from mice (n=3) challenged s.c. with CT-26 colon carcinoma cells were analyzed by H&E staining (a) or immunohistochemical staining with anti-Ly-6G (granulocyte) antibody (b). Mice were treated i.v. with PBS, Salmonella (Sal), Salmonella plus empty vector (Sal+vector) or Salmonella plus IL-18-bearing vector (Sal+IL-18). Tumors were excised at 19 days after bacterial treatment. Shown are representative images of the stained tissues.
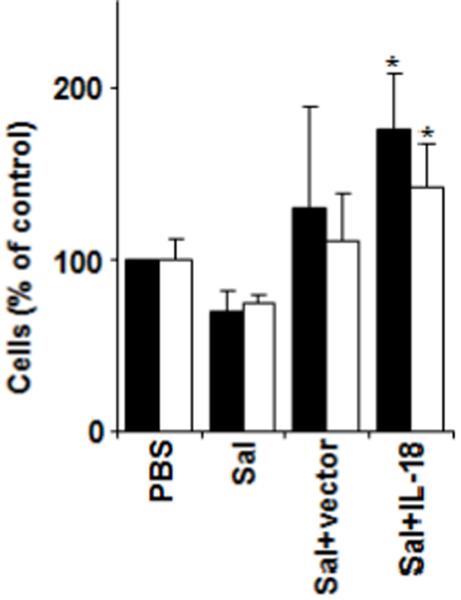
We also analyzed by fluorescence activated cell sorter (FACS) the numbers of DX5+ (NK cell marker), CD3+/CD8+, and CD3+/CD4+ T cells in tumors derived from CT26-bearing mice subjected to treatment with IL-18-expressing Salmonella or various controls. Compared to PBS, Salmonella, or Salmonella containing empty vector, tumors recovered from mice treated with IL-18-expressing Salmonella contained significantly more NK cells and CD4+ T cells (Fig. 4) but not CD8+ T cells (not shown).
Figure 4. Recruitment of leukocytes into tumors of mice treated with IL-18-expressing Salmonella.
BALB/c mice (n=5) were challenged s.c. with 7.5×104 CT-26 colon carcinoma cells. After 7d, mice were injected i.v. with PBS, Salmonella (Sal), Salmonella plus empty vector (Sal+vector) or Salmonella plus IL-18-bearing vector (Sal+IL-18). After 26 d, mice were sacrificed, tumors excised, and cells analyzed by FACS using anti-DX5 antibody for NK cells (black bars) or anti-CD3 and anti-CD4 antibodies for CD4+ T cells (white bars). Data represent change in absolute numbers of cells relative to PBS-treated control (mean ± SD). Asterisks indicate P<0.05 relative to PBS-treated control. Results are representative of three independent experiments.
IL-18-expressing Salmonella increase intratumoral cytokine expression
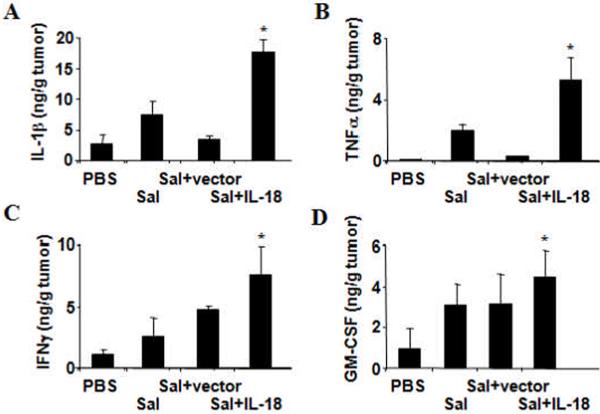
Because IL-18 is reported to induce expression of multiple cytokines (13, 16), we analyzed the intratumoral levels of various cytokines that IL-18 is known to induce, including IL-1β, TNFα, IFNγ and GM-CSF. For these experiments, subcutaneous CT26 tumors were harvested from mice at 19 days after initiation of bacterial therapy. Compared to mice treated with PBS, control Salmonella, or Salmonella containing empty vector, the IL-18-expressing Salmonella induced statistically greater accumulation of all cytokines tested in tumors (Fig. 5). The differences were most striking for IL-1β and TNFα, while only marginally higher for GM-CSF.
Figure 5. IL-18-expressing Salmonella induce increased intratumoral cytokine production.
CT-26 colon tumors from mice (n=3) were extracted after respective treatments and subject to ELISA for the expression of a, IL-1β. b, TNFα. c, IFNγ, or d, GM-CSF. Shown are quantities of the cytokines measured per gram of tumor tissue protein (mean ± SD), Asterisks indicate P<0.05, which were significantly different for comparisons of Sal-IL-18 against PBS, Sal, or Sal-vector.
IL-18-expressing Salmonella reduce tumor burden in a lung metastasis model
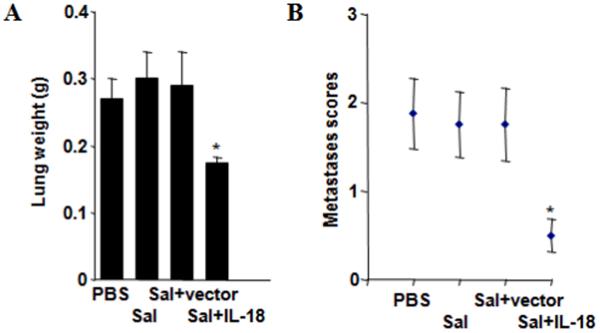
To explore the anti-tumor activity of IL-18-expressing Salmonella in a more stringent model, we induced lung metastases by iv injection of Lewis lung carcinoma cells into syngeneic, immunocompetent mice. Two methods of measuring tumor burden were employed: (a) lung weight; and (b) metastasis score, which measures the relative amount of the lung surface covered by tumors lesions on a 4-point scale (0–3). Compared to mice treated with PBS, control Salmonella, and Salmonella containing empty plasmid, the weight of lungs from IL-18-expressing Salmonella were markedly reduced, averaging almost half the weight of the other treatment groups and approaching the normal lung weight of ~0.15 grams (Fig. 6a). Metastasis scores were also substantially lower in mice treated with IL-18-expressing Salmonella (Fig. 6b). Thus, endowing Salmonella with the ability to produce IL-18 greatly enhances their anti-tumor activity, as evidenced by this lung metastasis model.
Figure 6. IL-18-expressing Salmonella demonstrate anti-tumor activity in lung metastasis model.
C57/Bl6 mice (n=8) were first injected i.v. with 5×104 Lewis lung carcinoma cells and then treated i.v. with PBS, empty Salmonella (Sal), Salmonella containing empty vector (Sal+vector), or Salmonella bearing the IL-18 plasmid (Sal+IL-18) after 6, 13 and 20 d. After 50 d, lungs were weighed (a) (mean ± SEM) (normal lung weight ~0.15 g) and (b) examined for metastases, assessing the percentage of lung surface covered by metastases: 0=0%, 1≤20%, 2=20–50%, 3≥50%. Asterisks indicate P<0.05. Results are representative of four similar experiments.
Discussion
In this report, we describe preclinical assessment of a novel bacteria-based experimental therapy for cancer, in which an attenuated strain of S. typhimurium is engineered to produce the cytokine IL-18. Endowing these non-virulent bacteria to produce mature IL-18 greatly increased their anti-tumor activity in immunocompetent mice, as determined in both subcutaneous primary tumor and lung metastasis models. The anti-tumor mechanism was associated with massive granulocyte infiltration into tumors, suggesting that IL-18 itself or other cytokines elaborated within tumors of mice treated with IL-18-expressing Salmonella are chemotactic for these leukocytes, promoting a host inflammatory response that inhibits tumor growth. Increased numbers of CD3+/CD4+ T cells and DX5+ NK cells were also found in tumors derived from mice treated with IL-18-producing Salmonella. Thus, by engineering Salmonella to express IL-18, a strong host reaction to tumors was induced, which included increased numbers of T cells and NK cells, and massive neutrophil infiltration. In contrast, inflammation of non-cancerous organs was limited principally to spleen, suggesting preferential targeting of the inflammatory response to tumors. In this regard, using the same mouse cancer models, we have previously shown that the attenuated S. typhimurium strain localizes predominantly to tumors (24), confirming several other studies that have documented tumor targeting of these facultative anaerobes (6, 7), and extending studies that have employed Salmonella that expressed IL-18 under a eukaryotic promoter in an oral vaccination protocol (28, 29).
We found that intratumoral levels of several cytokines previously reported to be induced by IL-18 were increased in tumors of mice treated with IL-18-expressing Salmonella. This observation suggests that IL-18 plays an amplifying role, inducing several cytokines within tumors, and thus bringing multiple immunostimulatory and inflammatory cytokines into play, presumably contributing to the anti-tumor activity. Individually, some cytokines may not be essential but in combination with others, they may make important contributors to the observed anti-tumor activity of IL-18-expressing Salmonella. Consequently, the mechanisms responsible for the anti-tumor activity of IL-18-expressing Salmonella are probably multifactoral, with the predominant mechanism possibly varying with the tumor involved, anatomical location (e.g. subcutaneous vs. pulmonary), mouse strain, and other variables. Further studies using mice genetically deficient in various cytokines could be employed to elucidate the relative contributions of individual cytokines to the host response to tumors in mice treated with IL-18-expressing Salmonella.
Taken together, our preclinical findings demonstrate the feasibility of a novel microbial-based approach for cancer immunotherapy involving engineering Salmonella typhimurium to express the cytokine IL-18. The studies of IL-18 reported here extend our recent analysis of bacteria armed to express the cytokine LIGHT, a TNF-family member (24), and thus demonstrate the potential of using either Interleukin-family cytokines or TNF-family cytokines for this application. The receptors for these two major classes of cytokines transduce signals by largely different mechanisms (25), and the immune cell populations recruited into the anti-tumor response may be only partially overlapping (24), but both types of cytokines show activity in the mouse cancer models employed here. Future studies involving other members of these families of cytokines, as well as other types of immunomodulatory proteins, should help to reveal the full repertoire of cytokines that might be appropriate to consider for cancer immunotherapies using engineered non-virulent Salmonella typhimurium. Ultimately, however, human clinical trials will be needed to test the utility of such approaches and the therapeutic index of specific types of cytokines, given the important genomics differences between rodent species and humans with respect to their repertoires of genes encoding cytokines and cytokine receptors (26).
Regardless of the relative merits of various cytokines for engineering non-virulent S. typhimurium, a bacteria-based strategy for de novo production of cytokines may have several advantages over other approaches. For example, virus-based cytokine gene delivery is difficult to terminate, given the general lack of anti-viral drugs that could stop viral infection if toxicity was encountered. Most of the currently used viruses also lack biological features that might preferentially target them to tumors. Furthermore, recombinant cytokines are typically expensive to produce and they also lack tumor-targeting features, often causing severe systemic inflammatory reactions that limit clinical use. In contrast, genetically engineered bacteria are inexpensively produced, can selectively target tumors, and are readily eradicated by antibiotic therapy in the event of toxicity. These advantages therefore warrant further preclinical evaluations of cytokine-producing bacteria as a novel microbial approach to cancer immunotherapy.
Supplementary Material
Acknowledgements
We thank Dr. Wei-Zen Wei for the D2F2 cell line, and acknowledge the generous support of the Austrian Academy of Sciences, APART Fellowship Program (M.L.) and the NIH (CA-69381) (J.C.R.).
References
- 1.Coley WB. The treatment of malignant tumors by repeated inoculations of erysipelas. With a report of ten original cases. Clin Orthop. 1893;262:3–11. [PubMed] [Google Scholar]
- 2.Coley W. Late results of the treatment of inoperable sarcoma by the mixed toxins of Erysipelas and Bacillus prodigosus. Am J Med Sci. 1906;131:375–430. [Google Scholar]
- 3.Coley W. Melanotic Cancer. Ann Surg. 1916;64:206–41. doi: 10.1097/00000658-191608000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Herr HW. Tumour progression and survival in patients with T1G3 bladder tumours: 15-year outcome. Br J Urol. 1997;80(5):762–5. doi: 10.1046/j.1464-410x.1997.00431.x. [DOI] [PubMed] [Google Scholar]
- 5.Jackson AM, Ivshina AV, Senko O, et al. Prognosis of intravesical bacillus Calmette-Guerin therapy for superficial bladder cancer by immunological urinary measurements: statistically weighted syndrome analysis. J Urol. 1998;159(3):1054–63. [PubMed] [Google Scholar]
- 6.Clairmont C, Lee KC, Pike J, et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J Infect Dis. 2000;181(6):1996–2002. doi: 10.1086/315497. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Spiess PJ, Kleiner DE. Antitumor effects in mice of the intravenous injection of attenuated Salmonella typhimurium. J Immunother. 2002;25(3):218–25. doi: 10.1097/01.CJI.0000014623.45316.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toso JF, Gill VJ, Hwu P, et al. Phase I study of the intravenous administration of attenuated Salmonella typhimurium to patients with metastatic melanoma. J Clin Oncol. 2002;20(1):142–52. doi: 10.1200/JCO.2002.20.1.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu YA, Shabahang S, Timiryasova TM, et al. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol. 2004;22(3):313–20. doi: 10.1038/nbt937. [DOI] [PubMed] [Google Scholar]
- 10.Thamm DH, Kurzman ID, King I, et al. Systemic administration of an attenuated, tumor-targeting Salmonella typhimurium to dogs with spontaneous neoplasia: phase I evaluation. Clin Cancer Res. 2005;11(13):4827–34. doi: 10.1158/1078-0432.CCR-04-2510. [DOI] [PubMed] [Google Scholar]
- 11.Carrier MJ, Chatfield SN, Dougan G, et al. Expression of human IL-1 beta in Salmonella typhimurium. A model system for the delivery of recombinant therapeutic proteins in vivo. J Immunol. 1992;148(4):1176–81. [PubMed] [Google Scholar]
- 12.Marwick C. FDA halts gene therapy trials after leukaemia case in France. Bmj. 2003;326(7382):181. doi: 10.1136/bmj.326.7382.181/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamura H, Tsutsi H, Komatsu T, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378(6552):88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- 14.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–50. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui H, Nakanishi K, Matsui K, et al. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157(9):3967–73. [PubMed] [Google Scholar]
- 16.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Annu Rev Immunol. 2001;19:423–74. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 17.Wigginton JM, Lee JK, Wiltrout TA, et al. Synergistic engagement of an ineffective endogenous anti-tumor immune response and induction of IFN-gamma and Fas-ligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J Immunol. 2002;169(8):4467–74. doi: 10.4049/jimmunol.169.8.4467. [DOI] [PubMed] [Google Scholar]
- 18.Osaki T, Hashimoto W, Gambotto A, et al. Potent antitumor effects mediated by local expression of the mature form of the interferon-gamma inducing factor, interleukin-18 (IL-18) Gene Ther. 1999;6(5):808–15. doi: 10.1038/sj.gt.3300908. [DOI] [PubMed] [Google Scholar]
- 19.Cao R, Farnebo J, Kurimoto M, Cao Y. Interleukin-18 acts as an angiogenesis and tumor suppressor. Faseb J. 1999;13(15):2195–202. doi: 10.1096/fasebj.13.15.2195. [DOI] [PubMed] [Google Scholar]
- 20.Micallef MJ, Tanimoto T, Kohno K, Ikeda M, Kurimoto M. Interleukin 18 induces the sequential activation of natural killer cells and cytotoxic T lymphocytes to protect syngeneic mice from transplantation with Meth A sarcoma. Cancer Res. 1997;57(20):4557–63. [PubMed] [Google Scholar]
- 21.Hashimoto W, Osaki T, Okamura H, et al. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas-Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163(2):583–9. [PubMed] [Google Scholar]
- 22.Woldbaek PR, Sande JB, Stromme TA, et al. Daily administration of interleukin-18 causes myocardial dysfunction in healthy mice. Am J Physiol Heart Circ Physiol. 2005;289(2):H708–14. doi: 10.1152/ajpheart.01179.2004. [DOI] [PubMed] [Google Scholar]
- 23.Zhao M, Yang M, Li XM, et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing Salmonella typhimurium. Proc Natl Acad Sci U S A. 2005;102(3):755–60. doi: 10.1073/pnas.0408422102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loeffler M, Le'negrate G, Krajewska M, Reed JC. Attenuated Salmonella engineered to produce human cytokine LIGHT inhibit tumor growth. Proc Natl Acad Sci U S A. 2007;104(31):12879–83. doi: 10.1073/pnas.0701959104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- 26.Reed JC, Doctor KS, Godzik A. The domains of apoptosis: a genomics perspective. Sci STKE. 2004;2004(239):re9. doi: 10.1126/stke.2392004re9. [DOI] [PubMed] [Google Scholar]
- 27.Luo Y, Zhou H, Mizutani M, et al. A DNA vaccine targeting Fos-related antigen 1 enhanced by IL-18 induces long-lived T-cell memory against tumor recurrence. Cancer Res. 2005;65(8):3419–27. doi: 10.1158/0008-5472.CAN-04-3120. [DOI] [PubMed] [Google Scholar]
- 28.Agorio C, Schreiber F, Sheppard M, et al. Live attenuated Salmonella as a vector for oral cytokine gene therapy in melanoma. J Gene Med. 2007 May;9(5):416–423. doi: 10.1002/jgm.1023. [DOI] [PubMed] [Google Scholar]
- 29.Luo Y, Zhou H, Mizutani M, Mizutani N, Reisfeld RA, Xiang R. Transcription factor Fos-related antigen 1 is an effective target for a breast cancer vaccine. Proc Natl Acad Sci U S A. 2003 Jul 22;100(15):8850–8855. doi: 10.1073/pnas.1033132100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.