Abstract
Pulmonary hypertension (PH) is a common and life-threatening complication of pulmonary fibrosis. Estradiol (E2) is protective in experimental PH, and its non-estrogenic metabolite 2-methoxyestradiol (2ME) prevents the development and retards the progression of monocrotaline-induced PH in male and female rats. However, the effects of E2 and 2ME on pulmonary fibrosis and associated PH have not been examined. Therefore, we compared the growth-inhibitory effects of E2 and 2ME in human lung fibroblasts (hLFs) and pulmonary vascular smooth muscle cells (hPASMCs), and we investigated the effects of estrogen deficiency and 2ME on bleomycin-induced pulmonary fibrosis and PH. Intact and ovariectomized (OVX) female Sprague Dawley rats were administered intratracheally either saline or bleomycin (15 IU/kg), and a subset of OVX bleomycin-treated rats received 2ME (10 μg/kg/h) for 21 days. Estradiol had only limited inhibitory effects on growth in hPASMCs and no effect in hLFs, whereas 2ME exhibited strong and concentration-dependent (1−10 μM) antimitogenic effects in both cell types. Bleomycin caused lung injury/PH (significantly increased lung and right ventricle (RV) weights, RV peak systolic pressure (RVPSP), and RV/left ventricle+septum ratio (RV/LV+S); caused medial hypertrophy and adventitial widening of pulmonary arteries; induced marked focal/diffuse fibrosis with diffuse infiltration of inflammatory (ED1+) cells; and resulted in 30% mortality). OVX exacerbated the disease and increased mortality (to 75%); whereas 2ME tended to reduce mortality (55.5%) and in surviving animals reduced RVPSP and RV/LV+S ratio, and attenuated vascular remodeling, pulmonary inflammation and fibrosis. This study suggests that 2ME may have protective effects in bleomycin-induced PH and fibrosis. Further investigation of 2ME in pulmonary fibrosis and PH is warranted.
Keywords: Pulmonary fibrosis, pulmonary hypertension, estradiol, estradiol metabolites
INTRODUCTION
Pulmonary fibrosis is a penultimate consequence of chronic interstitial lung disease from various etiologies and it is characterized by a limited response to available therapies and poor prognosis. Pulmonary hypertension is common in patients with pulmonary fibrosis and its presence has a significant adverse impact on survival (Lettieri et al., 2006). Mortality rates for pulmonary fibrosis are increasing (34% in the last 15 years), and importantly the rate of increase is twice as high in women than men (Olson et al., 2007). However, little is known regarding the effects of gender and estrogens in development of lung fibrosis and associated pulmonary hypertension.
Among patients with systemic sclerosis (SSc, scleroderma), pulmonary hypertension is a serious complication and frequent cause of death (Proudman et al. 2007). Notably, in women with SSc menopause significantly increases the risk for development of disease (Scorza et al. 2002), whereas hormone replacement therapy prevents the development of PH (Beretta et al., 2006). It seems also that pregnancy and estrogens may influence the development of disease and never-pregnant women with SSc are at higher risk for developing PH (Arlett et al. 2002). Importantly, both estradiol and its non-estrogenic metabolite 2-methoxyestradiol (2ME) are present in high concentrations in women during the last trimester of pregnancy (Ball and Knuppen 1990; Tofovic et al., 2007), suggesting the notion that estrogens may attenuate the development and retard the progression of PH in patients with pulmonary fibrosis.
2ME is a major E2 metabolite which is the product of the sequential hydroxylation and methylation of E2 by the enzymes cytochrome P450 and catechol-O-methyltransferase. 2ME is not only a potent antimitogen in various cancer cells (Pribluda et al. 2000), but also inhibits proliferation of cardiovascular cells, including rat aortic smooth muscle cells, endothelial cells, cardiac fibroblasts and glomerular mesangial cells, and these effects are mediated by estrogen receptor-independent mechanisms (Dubey et al. 2004).
In vivo, 2ME retards the progression of PH in male rats (Tofovic et al., 2005a), and in female animals 2ME prevents the exacerbation of PH and eliminates the mortality in ovariectomized rats with MCT-induced PH (Tofovic et al., 2006). These beneficial effects are associated with marked inhibition of vascular remodeling and inflammation. 2ME also reduces acute lung injury and attenuates the development of PH and pulmonary vascular remodeling induced by the rodentocide alpha-naphthylthiourea (Tofovic et al., 2005b), and in the constricted-aorta rat model, 2ME inhibits pressure-independent vascular remodeling (Tofovic et al., 2003), Furthermore, 2ME and its metabolic precursor 2-hydroxyestradiol have direct (pressure-independent) inhibitory effects of cardiac remodeling and fibrosis in rats with isoproterenol-induced cardiac hypertrophy (Tofovic et al. 2008).
Because of the aforementioned considerations, we hypothesized that 2ME has protective effects and that estrogen deficiency exacerbates pulmonary fibrosis and associated pulmonary hypertension. To test our hypothesis we used bleomycin-induced lung fibrosis and pulmonary hypertension rat model. Although this model exhibits some features of human form of interstitial pulmonary fibrosis, it also has clear limitations (Gharaee-Kermani et al., 2005) that should be taken in consideration when assessing the clinical relevance of therapeutic responses in this model. The results of the present study suggest that 2ME is antimitogenic in human lung fibroblasts and pulmonary artery vascular smooth muscle cells and has beneficial effects in experimental pulmonary fibrosis and associated pulmonary hypertension.
MATERIALS AND METHODS
1. Growth inhibitory effects of estradiol and 2ME
Cryopreserved human pulmonary artery smooth muscle cells (hPASMCs; Cascade Biologics, Inc.) were cultured in M231 supplemented with 10% FCS and 2 mM glutamax (Invitrogen). Cryopreserved human lung fibroblast (hLFs; Cell Applications, Inc., San Diego, CA) were cultured in DMEM (Invitrogen) supplemented with 10% FCS and 2 mM glutamax. Cells were plated in a 75 cm2 culture flask (Falcon) and incubated at 37° C with 5% CO2. Twenty four-hours later, cells were rinsed and provided fresh medium and thereafter fresh medium was provided every three days.
For cell proliferation assay, hPASMCs and hLFs (5,000 cells/well) were grown in 24-well tissue culture plates (Nunc). The growth of starved hPASMCs was stimulated with 2.5% FCS and Smooth Muscle Cell Growth Supplement (Cascade Biologics, Inc) (containing human basic fibroblast growth factor (bFGF 2 ng/ml), human epidermal growth factor (0.5 ng/ml), heparin (5 ng/ml), insulin (5 μg/ml) and BSA (0.2 μg/ml)), and in the presence or absence of the tested agents. For hLFs, cells were treated with M200 (phenol red free) containing 2.5% FCS and 1% Low Serum Growth Supplement (LSGS; Cascade Biologics) (containing hydrocortisone (0.5 μg/ml), human epidermal growth factor, (5ng/ml), bFGF (1.5 ng/ml) and heparin (5 μg/ml) and bFGF (6 ng/ml)), in the presence or absence of the tested agents. The cells were detached with 0.025% trypsin/EDTA (Sigma) at 5−7 days. The Quick Cell Proliferation Assay Kit (BioVision Research Products, Mountain View, CA) was used for quantification of cell proliferation and viability. Briefly, the essay is based on the cleavage of the tetrazolium salt WST-1 to formazan by cellular mitochondrial dehydrogenase. The activity of dehydrogenase correlates with cell proliferation, and formation of formazan dye is quantified by multi-well spectrophotometer by measuring the absorbance of the dye at 440 nm.
2. Animal studies
Forty-three female Sprague Dawley rats weighing 200−250 g were obtained from Charles River Laboratories (Wilmington, MA). Animals were fed rodent chow (Pro Lab RHM 3000 rodent diet, PMI Nutrition, Inc, St Louis, MO), had free access to water, and were housed at 22° C, 12-hour light cycle, and 55% relative humidity. All experiments were carried out in accordance with the University of Pittsburgh Institutional guidelines for animal welfare, and the Animal Care and Use Committee approved experimental protocols.
A subset of animals (n=27) underwent bilateral ovariectomy using the flank approach, whereas the remaining animals (n=16) were sham operated. To confirm that the ovaries were successfully removed, uterus weight was measured at autopsy. To produce pulmonary fibrosis, both intact and OVX animals were randomly assigned to receive under halothane anesthesia either an intratracheal injection of bleomycin (Sigma, St Louis, MO; 5 mg/kg/0.3 ml saline; Bleo and OVX-Bleo groups) or 0.3 ml saline (Control and OVX groups). A subset of animals (n=9) were implanted ALZET osmotic pumps (2ML-4, Durect Corporation, Cupertino, CA) delivering 2ME (10 μg/kg/h; Steraloids, Inc., Newport, RI). The effects of 2ME on bleomycin disposition are unknown, and it is possible that 2ME interferes with bleomycin disposition. The half-life of bleomycin in rodents ranges between 30 minutes and two hours (Lazo and Pham, 1984). Therefore, to avoid possible interference with bleomycin disposition, pumps were implanted 8 hours after bleomycin administration.
3. Acute Hemodynamic measurements
Twenty-one days after administration of bleomycin, animals underwent surgery and were instrumented for measurement of right ventricular peak systolic pressure (RVPSP) as described previously (Tofovic et al., 2005a, 2006). Briefly, rats were anesthetized with Inactin (90 mg/kg i.p.), and a PE-240 polyethylene catheter was inserted into the trachea to facilitate breathing. A PE-50 catheter was inserted into the left carotid artery and connected to a digital blood pressure analyzer (BPA, Micro-Med. Inc., Louisville, KY) for continuous measurements of systolic, diastolic and mean arterial blood pressures and heart rate. The rats were then mechanically ventilated (Harvard Rodent Ventilator, Model 683, Harvard Apparatus, MA) using constant respiratory rate (50/min) and tidal volume (1.0 ml). Next, the thorax was opened, and the right heart was punctured with a 23-gauge needle attached to a PE-50 line and Heart Performance Analyzer (HPA-200 τ, Micro-Med. Inc., Louisville, KY). After a 30-minute stabilization period, RVPSP was recorded for 20 minutes at 1-minute intervals.
4. Morphometric measurements and immunohistochemical studies
Animals were euthanatized by anesthetic overdose and heart and lungs were dissected and weighed. The ratios of wet weights of heart and lung to body weight (BW) were calculated (H/BW and LV/BW, respectively). The right ventricle (RV) free wall was separated from the left ventricle and the septum (LV+S) to determine the wet weight, the RV to body weight ratio (RV/BW), the LV+S to body weight ratio (LV+S/BW), and the RV to LV+S weight ratio (RV/LV+S, Fulton Index).
The lungs were removed from the chest cavity en block with the trachea and perfused via the trachea with a fixative solution (10% neutral-buffered formalin) at a pressure of 25 cm H2O. Perfused tissue samples were immersed in the fixative for 24 to 72 hours for subsequent light microscopy and immunohistochemistry. Tissue samples were embedded in paraffin blocks for light microscopy. Five-micron serial tissue sections from formalin-fixed, paraffin-embedded lungs were dewaxed and stained with H&E and Masson's trichrome for histological and morphometric assessment (to identify inflammatory cells, connective tissue and collagen deposition). Lung tissues sections were examined by light microscopy and were scored in blinded fashion.
The number of interstitial monocytes/macrophages was studied using a polyclonal anti-ED1 antibody (Serotec, Raleigh, NC). This antibody specifically stains positive the cytoplasm of alveolar and interstitial macrophages. Nonspecific staining was assessed by replacing the primary antibody with affinity-purified, nonimmune, rabbit IgG (R & D Systems). Sections were washed and developed further according to the directions of the manufacturer (Dako, Carpentaria, CA) using an LSAB2 kit, which contained a second antibody linked to avidin and peroxidase-conjugated to biotin.
To study vascular remodeling of small pulmonary arteries, arterial wall smooth muscle cells were stained using a mouse monoclonal anti-smooth muscle-alpha actin antibody at a dilution of 1/100 ( Lab Vision, Fremont, CA). The measurements of media thickness, and media and adventitia surface were conducted using an Image Analyzing System (Diagnostic Instruments, Inc., Sterling Heights, MI) that included SPOT RT Camera installed on NIKON Eclipse 50 light microscope and specialized computer software program (SPOT Software, Version 4.1). Measurements were done (×20 magnification) on ten cross sectioned pulmonary artery branches with 50−250-micron diameters. The peripheral lung fields were located at approximately equal distances from the pleural lining. Only vessels with an approximately circular profile (cross-sectional cuts) were studied. For each blood vessel, two rectangular diameters and their four respective media were measured. The media % index was calculated as 2 × media/diameter × 100 and is presented as average of four media measurements for a single vessel. Since bleomycin induced a significant widening of the adventitia, corrected media index was calculated using the diameter that includes only media + lumen measurement. For each blood vessel, two rectangular diameters and their four respective media were measured, and averages of four individual values of media thickness and media % index were calculated.
Collagen fibrosis was assessed on Mason's Trichrome stained lung sections by the same image analyzing system on 10 low-power random fields (×10) using the region area measurement option and expressed as percent from a calibrated 1,000,000 μ2 microscopic field area.
5. Statistical Analysis
Statistical analyses were performed using the Number Cruncher Statistical software program (Kaysville, Utah). Group comparisons were performed by a one- or two- factor analysis of variance (1F-, 2-F ANOVA), followed by post-hoc comparison using the Fisher=s LSD test. The probability value of p<0.05 was considered statistically significant. All data are presented as mean ± S.E.M.
RESULTS
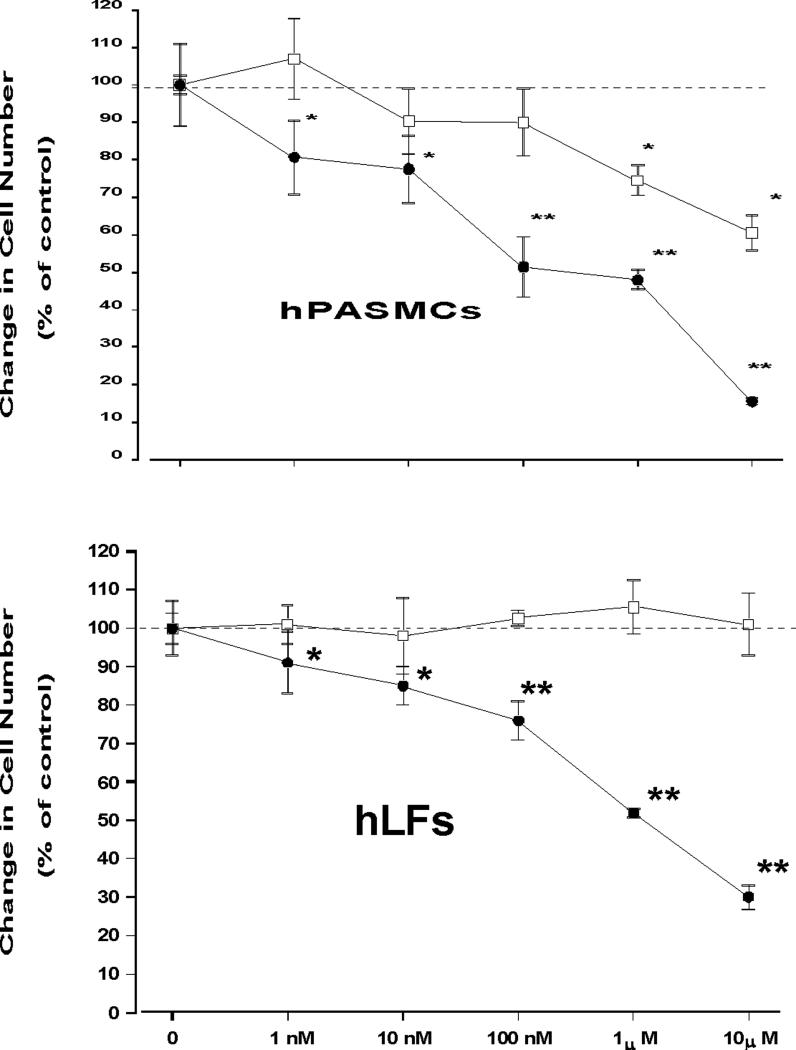
The growth inhibitory effects of estradiol and 2ME in human pulmonary artery smooth muscle cells (hPASMCs) and human lung fibroblasts (hLFs) are presented in Figure 1. In hPASMCs at physiological (1−10 nM) and low pharmacological (100 nM) concentrations, E2 had no effects on cell growth stimulated by 2.5% FCS and growth factor-supplement, but exhibited modest (−20 and −40%) antimitogenic effects at high concentrations (1 and 10 μM; Figure 1A). No effects on hLF growth were detected when cells were exposed for 5 days to increasing concentrations (1 nM-10 μM) of E2 (Figure 1B). In contrast, 2ME exhibited strong and concentration-dependent antimitogenic effects in both hPASMCs and hLFs.
Figure 1.
Effects of increasing concentrations of 17β-estradiol (E2;□) and 2-methoxyestradiol (2ME;•) on cell growth in human pulmonary artery vascular smooth muscle cells (hPASMC) and human lung fibroblasts (hLF). The results are presented as the percent change from control. Values for each data point represent mean ± SEM from 2−3 separate experiments conducted in quadruplicate. p<0.05, * - vs. control treated with FCS alone; l ** - vs. Cont & E2
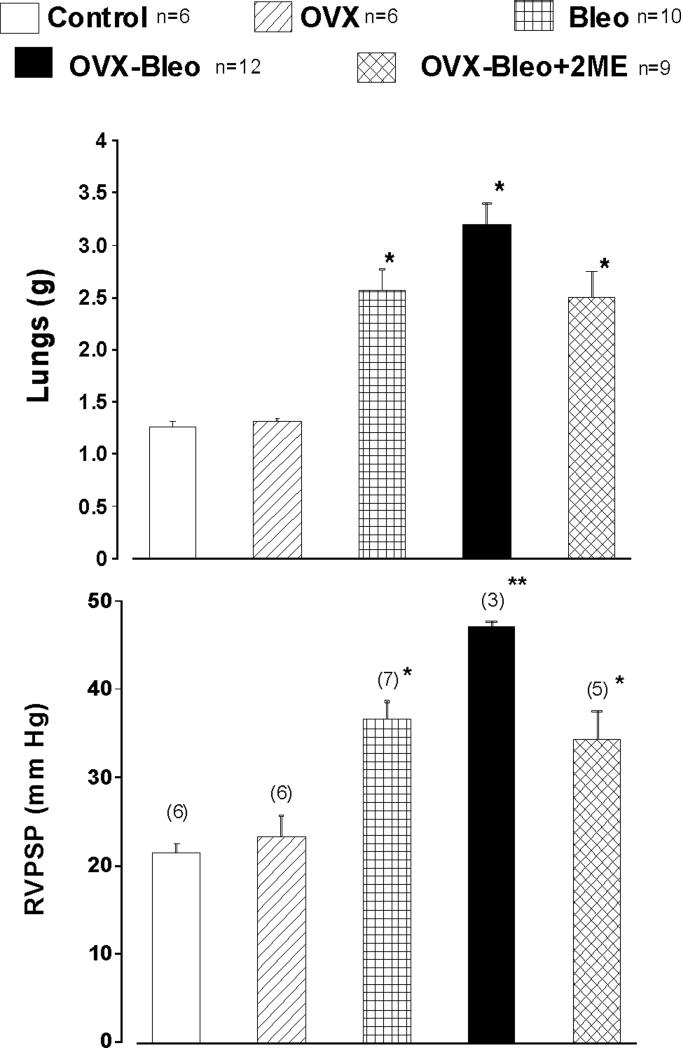
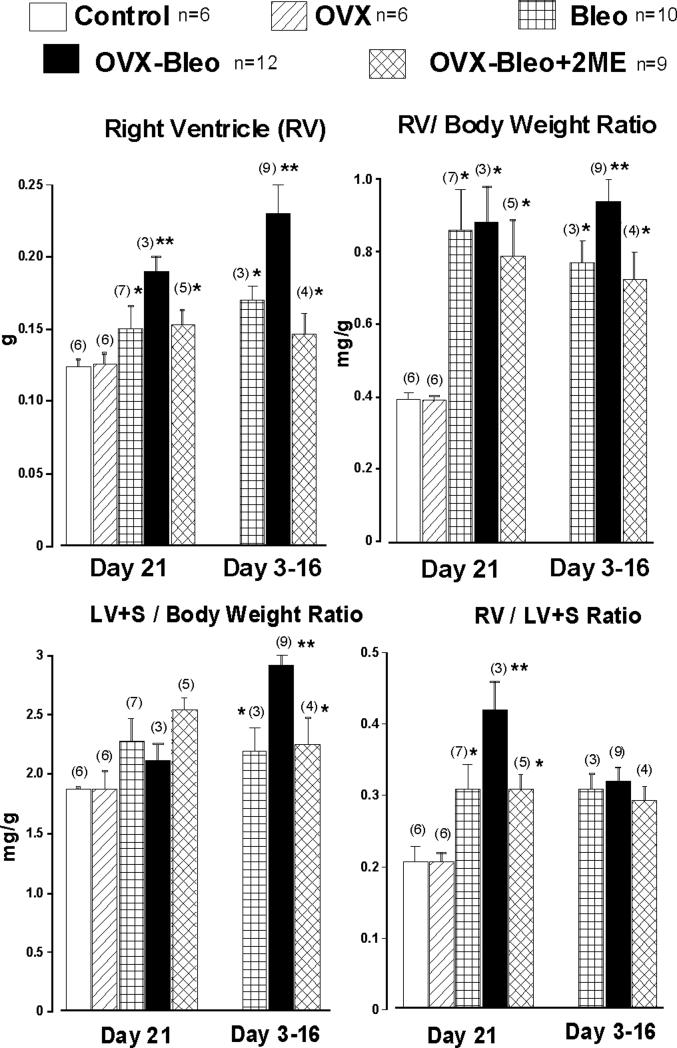
The data examining the effects of 2ME on bleomycin-induced pulmonary hypertension and fibrosis in estrogen-deficient rats are presented in Figures 2- 7 and Table 1. The intratracheal instillation of bleomycin induced severe lung injury, substantial weight loss and premature deaths that occurred between days 3 and 16 of the experiment. The body weight of rats that received saline intratracheally increased with time, and weight gain was significantly greater in OVX animals (Day 21: 317±11 and 346±7g, Cont and OVX group, respectively). In animals receiving bleomycin, there was no body weight gain during the 3-week study period (data not shown), and there was no significant difference in body weight among the three diseased experimental groups (Bleo, OVX-Bleo and OVX-Bleo-2ME). Bleomycin increased lung weight, and animals exposed to bleomycin had a doubling of lung weight compared to animals that received saline intratracheally. Importantly, ovariectomy further increased lung weight, and 2ME prevented further increases in lung weight in OVX-Bleo rats (Figure 2 upper panel, OVX-Bleo+2ME group). The differences in lung weight correlated with signs of microvascular leakage and severity of fibrosis (infra vide). Intratracheal instillation of bleomycin induced pulmonary hypertension, ovariectomy further increased the RVPSP (Figure 2, lower panel) and treatment with 2ME prevented further increases in RVPSP in OVX animals with intratracheally instilled bleomycin (OVX-Bleo vs. OVX-Bleo+2ME group, p<0.05, Fisher post hoc comparison). Bleomycin-treated animals that died prematurely developed both right and left ventricular hypertrophy and OVX-Bleo group had the most severe cardiac hypertrophy (Figure 3, Day 3−16). Twenty-one days into treatments, the bleomycin-induced isolated RV hypertrophy (i.e., Fulton index, RV+S/RV ratio) was exacerbated in diseased OVX animals, and 2ME reduced the RV hypertrophy in OVX-Bleo rats (Figure 3, Day 21).
Figure 2.
Lung weight (A) and right ventricular peak systolic pressure, RVPSP (B) in intact (Control) and ovariectomized (OVX) female rats, intact and OVX animals with bleomycin-induced pulmonary hypertension and fibrosis (Bleo and OVX-Bleo group, respectively), and OVX-Bleo rats treated with 2-methoxyestradiol (OVX-Bleo+2ME); Fisher LSD test for post-hoc comparison: * - p<0.05 vs. respective control (Cont or OVX) ** - p<0.05 vs. all other groups.
Table 1.
Vascular remodeling in control in ovariectomized rats with bleomycin-induced pulmonary fibrosis and hypertension
| Group | Days into Experiment |
Diameter Range |
Vessels Size |
Media Thickness |
Media Area (μ2) |
Adventitia Area (μ2) |
Wall Area (μ2) |
Wall to Lumen |
Media to Lumen |
|---|---|---|---|---|---|---|---|---|---|
| Control N=6 |
21 |
53−189 |
103.2 ±6.0 |
11.0 ±0.6 |
2900 ±346 |
1612 ±207 |
4512 ±468 |
124 ±10.9 |
80.1 ±7.6 |
| OVX n=6 |
21 |
52−191 |
100.2 ±4.7 |
10.4 ±0.5 |
2744 ±284 |
1247 ±123 |
3999 ±397 |
100.7 ±8.6 |
69.9 ±7.0 |
| Bleomycin n=7 |
21 |
54−296 |
124.8 * ±9.6 |
21.5 * ±1.6 |
7533 * ±1282 |
2191 * ±353 |
9725 * ±1590 |
304 * ±40 |
235 * ±34 |
| OVX-Bleo N=3 |
21 |
51−299 |
139.7 ** ±16.7 |
30.3 ** ±5.0 |
11666 ** ±2859 |
2856 ** ±710 |
14522 ** ±3430 |
461 ** ±93 |
378 ** ±82 |
| OVX-Bleo+2ME N=5 |
21 |
57−201 |
116.0 ± 8.7 |
15.9 ±2.8 |
5468 ±1723 |
1616 ±369 |
7076 ±2031 |
167 *** ±33 |
125 *** ±43 |
| Bleo n=3 |
3−16 |
68−226 |
154.7 * ±14.7 |
21.0 * ±2.5 |
7597 * ±1511 |
2966 * ±481 |
10563 * ±1924 |
167 * ±40 |
122 * ±29 |
| OVX-Bleo N=8 |
3−16 |
71−299 |
161.2 * ±13.2 |
21.7 * ±3.2 |
10100 * ±2751 |
5105 * ±962 |
15206 * ±3357 |
186 * ±30 |
121 * ±23 |
| OVX-Bleo+2ME N=4 |
3−16 |
58−286 |
135.1 * ±11.8 |
22.1 * ±3.1 |
7652 * ±1856 |
2520 ±423 |
9752* ±2193 |
218 * ±63 |
127 * ±32 |
1F-Anova, p<0.05
- vs Control
- vs all other groups
- vs Bleomycin
Figure 3.
Right ventricle (RV) free wall wet weight, left ventricle plus septum (LV+S) weight and LV/ LV+S ratio in intact (Control) and ovariectomized (OVX) female rats, intact and OVX female animals with bleomycin-induced pulmonary hypertension and fibrosis (Bleo and OVX-Bleo group, respectively) and OVX-Bleo rats treated with 2-methoxyestardiol (OVX-Bleo+2ME). Fisher LSD test for post-hoc comparison: * - p<0.05 vs. respective control (Cont or OVX); ** - p<0.05 vs. all other groups.
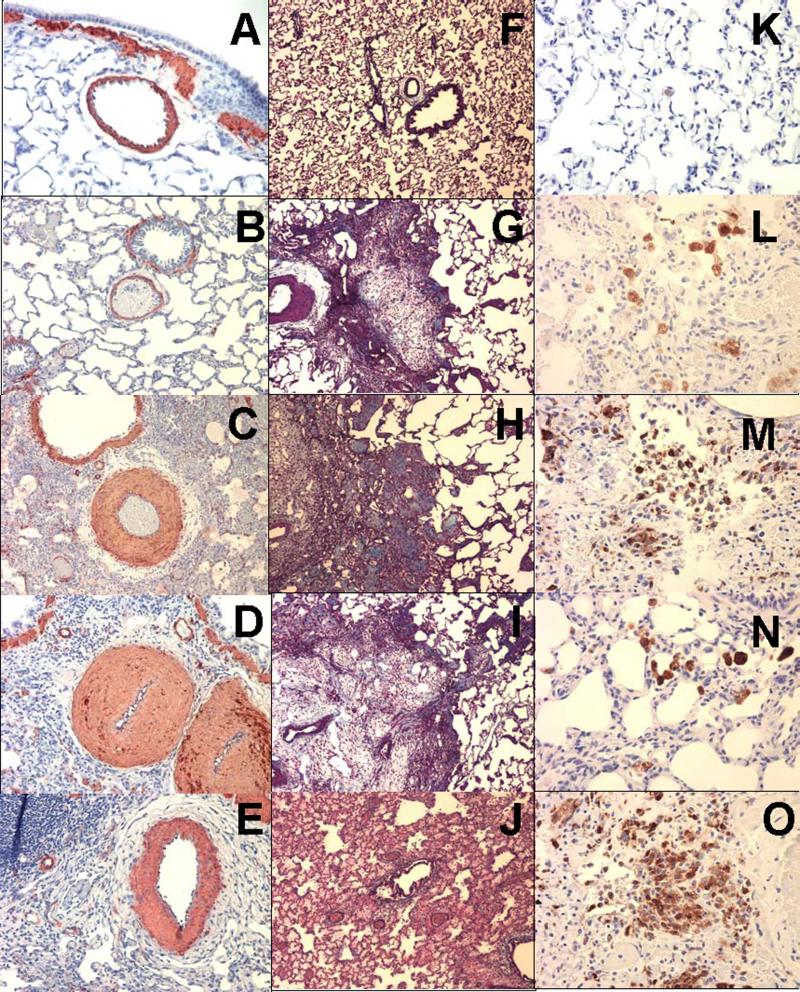
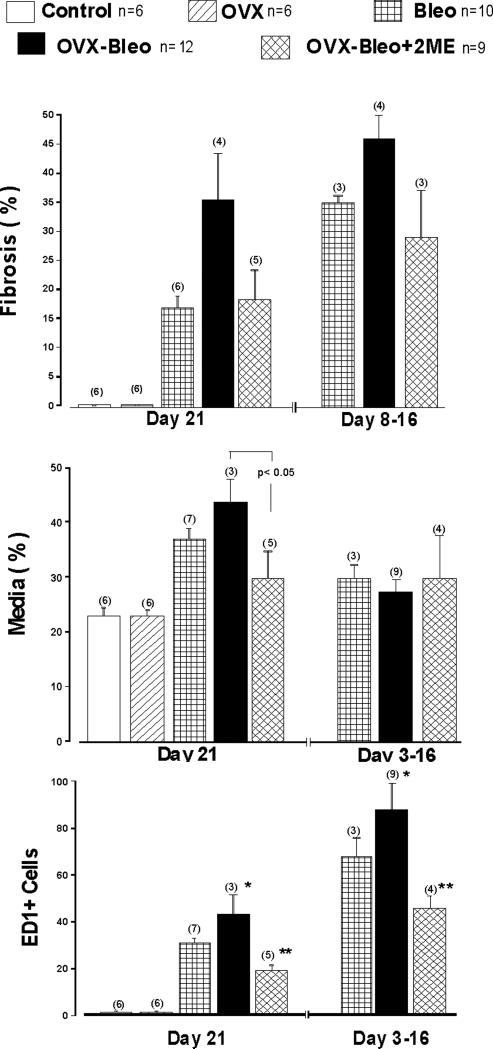
Animals that died in the first week had no fibrosis, but rather expressed marked congestion, edema, and inflammatory-cell infiltration (Figure 5J), and these alterations were multifocal in nature in intact females treated with Bleo. More severe changes were detected in OVX-Bleo rats, and these animals had diffuse (rather than multifocal) exudative, inflammatory and proliferative reactions. Animals that died between Day 8 and Day 16 had significant interstitial fibrosis, and the Bleo and Ovx-Bleo groups did not differ in regard to the extension of fibrosis (Figure 4A). In contrast, in animals that survived, Masson-trichrome staining of formalin-fixed tissue revealed greater and denser amounts of collagen deposition in lungs from Ovx-Bleo rats compared to Bleo group (Day 21; Figure 4A, Figures 5 G & H). Treatment with 2ME eliminated the exacerbation of fibrosis due to ovariectomy (Figures 4A and 4I). In fibrotic areas significant number of cells stained positive for smooth muscle α-actin indicating myofibroblastic proliferation and semi-quantitative assessment suggested reduced myofibroblastic proliferation in animals treated with 2ME.
Figure 5.
Small size pulmonary arteries (alpha actin, × 20) in rat lung from Control (A), OVX (B), beomycin (C), OVX-belomycin (D) and OVX-Bleo+ 2ME groups (E); Pulmonary fibrosis (Trichrome-Mason, × 10) at day 21 in rats from OVX (F), Bleo (G), OVX-Bleo (H) and OVX-Bleo+2ME group (I); Severe exudation and absence of fibrosis in OVX-Bleo rat that died at day 6 (J); Inflammatory cells (ED1+; × 40) at day 21 in rats from OVX (K), Bleo (L), OVX-Bleo group (M) and OVX-Bleo+2ME group (N) and severe inflammation in OVX-Bleo rat that died at day 13 (O).
Figure 4.
[A] Pulmonary fibrosis (Trichrome-Mason); [B] Media hypertrophy (Alpha Actin); and [C] Activated monocytes/macrophages (ED1+ cells) in the lungs of intact (Cont) and ovariectomizted (OVX) controls, intact and OVX rats with bleomycin (Bleo)-induced pulmonary fibrosis and hypertension (Bleo and OVX-Bleo groups), and OVX-Bleo rats treated with 2-methoxyestradiol (OVX-Bleo+2ME); 1F-Anova, p<0.001; Fisher LSD test for post-hoc comparison: p< 0.05, * - vs. Cont, OVX and OVX-Bleo+2ME, ** - vs. versus all other groups.
Bleomycin-induced pulmonary fibrosis and hypertension detected on Day 21 were accompanied by significant vascular remodeling (Figure 4B, Table 1), and ovariectomy increased medial hypertrophy and adventitial widening (Table 1). In OVX-Bleo rats, treatment with 2ME markedly inhibited vascular remodeling, and OVX-Bleo+2ME rats had lesser vascular remodeling and adventitial widening than even intact diseased animals (Bleo group; Table 1).
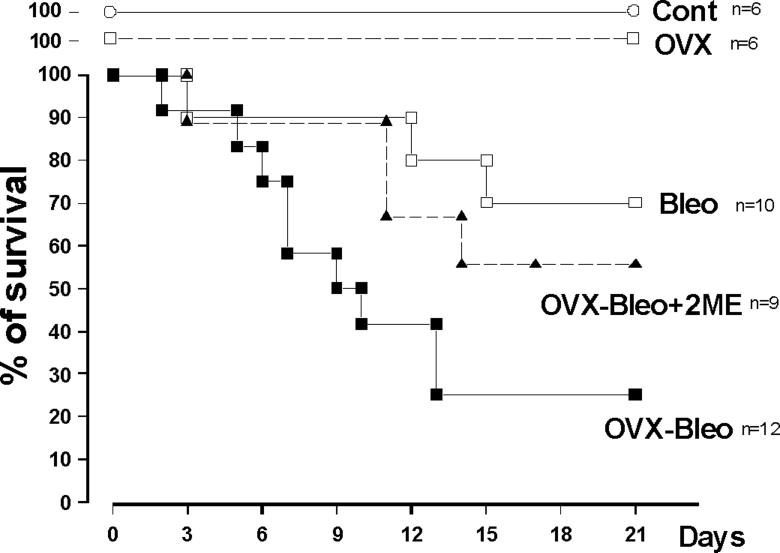
Intratracheal instillation of bleomycin and the subsequent lung injury were also associated with significant inflammatory responses as evidenced by the presence of a large number of ED1+ cells (Figures 4C and 5L). The inflammatory response was even more pronounced in animals that died prematurely. Importantly, OVX exacerbated inflammation in all animals, 2ME markedly reduced the number of ED1+ cells, and the intensity of inflammation in OVX-Bleo+2ME group was even smaller than intact females treated with bleomycin (Bleo group). Finally, the more severe lung injury (fibrosis, inflammation, PH) in the OVX-Bleo group was associated with increased mortality, and treatment with 2ME tended to reduce the augmented mortality due to ovariectomy (Figure 6).
Figure 6.
Survival in intact and OVX female rats with bleomycin-induced pulmonary hypertension and fibrosis (Bleo and OVX-BLeo group), OVX-Bleo rats treated with 2-methoxyestradiol (OVX-Bleo+2ME) and respective controls (Cont and OVX group).
DISCUSSION
Bleomycin induces dose-dependent lung parenchymal injury and fibrosis in both humans and animals. In rodents, bleomycin-induced lung fibrosis exhibits certain features of the interstitial pulmonary fibrosis (IPF) in humans, and it is the most commonly used model to study the pathogenesis and treatment of IPF. Even so, it also has clear limitations (the rapidity of its development, its self-limiting nature, and the severity of the associated inflammation) that should be considered when discussing the clinical relevance of therapeutic interventions in this model (Gharaee-Kermani et al., 2005).
An important finding of the present study is that estradiol and its downstream metabolite 2ME differ significantly in regard to their antimitigenic effects in cell lines involved in pulmonary fibrosis and vascular remodeling, i.e., in hLFs and hPASMCs. The present study shows that estradiol has no effects on growth of hLFs in concentrations up to 10 μM. To our knowledge, this is the first investigation of E2 in adult hLFs. Previous studies in fetal lung fibroblasts demonstrate an antimitogenic effect of estradiol only at high pharmacological concentrations (>5 μM) (Kondo et al., 1983). Also, previous studies of cellular growth in fibroblasts from various origins reveal both stimulatory and inhibitory properties of E2 (Dubey et al. 2004; Tomaszewski J et al., 2003). The variable effects of E2 may reflect the phenotypic heterogeneity among fibroblasts, including their differentiation (particularly during growth and repair) into myofibroblasts.
Our results also show that estradiol has only modest antimitogenic effects in hPASMCs. This is in contrast to the well established antimitogenic effects of E2 in vascular smooth muscle cells from systemic arteries (Dubey et al, 2004; Espinosa et al., 1996). Nonetheless, it is plausible that E2 may have different anti-remodeling effects on systemic and pulmonary blood vessels that phylogenetically are from different origins. Along these lines, E2 stimulates proliferation of rat PASMCs in cell culture, has no effect on growth in intact canine pulmonary artery segments, and stimulates growth of PASMCs in segments when the endothelium is removed (Farhat et al., 1992).
In contrast to E2, 2ME in a concentration-dependent manner inhibits growth of hLFs and hPASMCs. This is not surprising since 2ME is a more potent antimitogen than E2 in cardiac fibroblasts, vascular smooth muscle cells and glomerular mesangial cells (Dubey et al., 2004). In the order of potency 2ME>2HE>E2, E2 and its metabolites inhibit the proliferation of hepatic stellate cells and collagen synthesis by these fibroblast-like cells (Liu et al., 2004). Hepatic stellate cells, together with vascular smooth muscle cells, cardiac fibroblasts, and glomerular mesangial cells belong to the pericyte family. During growth or tissue injury and repair, these cells display properties of myofibroblasts and are the most important source for collagen, fibronectin, and other extracellular matrix proteins. It is notable that in bleomycin-treated animals in fibrotic areas, a significant number of cells stained positive for smooth muscle α-actin, indicating myofibroblastic proliferation. Importantly, the present study suggests (using semi-quantitative assessment) reduced myofibroblastic proliferation in animals treated with 2ME.
The present study demonstrates that ovariectomy exacerbates pulmonary fibrosis and pulmonary vascular remodeling and hypertension, suggesting that E2 is protective in pulmonary hypertension due to lung fibrosis. These effects are consistent with previous reports in pulmonary hypertensive rats (Farhat et al. 1993, Tofovic et al. 2006). The effects of ovariectomy on pulmonary fibrosis are also in accordance with previous studies showing that ovariectomy increases lung collagen, airway smooth muscle thickening and cardiac hypertrophy in wild-type females and aggravates the pathology in relaxin-gene knockout female mice (Lekgabe et al., 2006) and that ovariectomy has fibrogenic effects, whereas E2 suppresses fibrotic responses in models of liver fibrosis (Liu et al., 2004; Yasuda et al., 1999). However, the present study contradicts a recent report that OVX ameliorates and exogenous E2 exacerbates bleomycin-induced pulmonary fibrosis in female Fisher rats (Gharaee-Kermani et al., 2005b). The reason for the discrepancy between the two studies is unknown, but there are several factors that may account for the different outcomes. First, there is significant strain difference among rodents with regard to sensitivity to pneumotoxins. Thus, compared to Sprague Dawley (SD) rats, Fisher rats are less sensitive (mortality) to the pneumotoxin monocrotaline and they develop a less severe inflammatory and vascular remodeling response (Pan et al., 1993). Notably, the present study shows that bleomycin produces a 3−9 times greater inflammatory response (i.e., fold increase in ED1+ cells) in SD rats than in intact and OVX female Fisher rats (Gharaee-Kermani et al., 2005b). Second, in OVX Fisher rats, there are significant plasma levels of E2 (that are twice higher than levels in SD rats), bleomycin augments the levels of E2, and E2 supplementation in Fisher rats produces plasma levels of E2 only observed in Sprague Dawley rats during pregnancy. Notably, high physiological concentrations of E2 enhance the activity of the pro-inflammatory transcription factor NFkB (Hirano et al., 2007), and the differences in baseline inflammatory response together with high levels of E2 may be the reason that in Fisher rats E2 is pro-inflammatory rather than anti-inflammatory (Straub, 2007).
In the present study bleomycin induced influx of macrophages into injured lungs, and ovariectomy exacerbated the inflammatory response in the lungs injured by bleomycin. This is not surprising because estrogen deficiency is associated with increases in cell adhesion molecule expression (Speyer et al., 2005), E2 inhibits the expression of cell adhesion molecules in macrophages (Frazier-Jessen et al., 1995), and ovariectomy increases whereas E2 suppresses adherence of monocytes in estrogen-deficient rats (Kurokawa et al., 2007).
Treatment with 2ME reduces both early and late inflammatory responses in diseased, estrogen-deficient animals. This remarkable effect of 2ME on inflammatory cells (Figure 5N) is consistent with our previous studies in male and OVX female rats with MCT-induced PH (Tofovic et al., 2005a, 2006). Although the mechanism of the effect of 2ME on lung macrophages is unclear, there is cumulating evidence that 2ME may have anti-inflammatory properties. Along these lines, in vivo when used in doses (30−300 μg/kg/day) similar to that (240μg/kg/day) used in the present study, 2ME inhibits the adherence of monocytes to endothelium in estrogen deificient rats (Kurokawa et al., 2007), attenuates the basal or TNF-alpha induced migration of inflammatory cells (Issekutz et al., 2008) and reduces the expression of inflammatory transcription factor NFkB (Zhang et al., 2007). In vitro, 2ME reduces TNF-alpha induced increases in monocyte chemoattracting protein 1 (Seeger and Mueck, 2006), and inhibits posttranscriptional activity of NFκB (Takata et al., 2005). Interestingly, 2ME not only triggers a significant decrease in cytoskeletal/structural genes (tubulin, actin), but also down-regulates the expression of genes that regulate the activity of TNF-alpha, neutrophil activation and chemotaxis (Chauhan et al., 2003). These findings raise the possibility that in addition to antimitogenic effects, 2ME may have anti-inflammatory properties. This would also suggest that the anti-inflammatory effects of E2 may be mediated, at least in part, by its downstream metabolite, and that the inhibition of the inflammatory response may also explain the antifibrotic effects of 2ME. Sufficient evidence exists to suggest that alveolar macrophages play an important role in pulmonary fibrosis. During bleomycin-induced lung injury alveolar macrophages produce large quantities of the pro-fibrotic cytokine TGF-β and fibronectin (Khalil et al., 1993).
In the present study, 2ME significantly inhibited cardiac remodeling and this effect was most likely due to reduced pulmonary hypertension, However, it is worth mentioning that 2-ME may have direct effects on RV function and remodeling. In this regard, 2ME inhibits proliferation of cardiac fibroblasts (Dubey et al., 2004) in vitro, and in vivo has direct, pressure-independent, anti-fibrotic and anti-remodeling effects in both right and left ventricles in rats with isoproterenoil-induced cardiac hypertrophy (Tofovic et al. 2008). This suggests that the overall beneficial effects of 2ME may extend beyond its effects on elevated pressure and remodeling in pulmonary arteries, and may be due in part to its direct anti-remodeling effects in the right ventricle. Further studies are needed to explore this possibility.
In summary, this study suggests that, in contrast to estradiol, 2ME is antimitogen in cells involved in pulmonary vascular remodeling and fibrosis. The presented data suggest strong anti-inflammatory, anti-fibrotic and anti-remodeling effects of 2ME in a model of pulmonary fibrosis and hypertension and warrant further investigation of the role of female hormones and their metabolites and analogs in pulmonary fibrosis and associated pulmonary hypertension.
Acknowledgments
This work was supported by NIH grant # HL080560-01A and presented in part at American Thoracic Society International Conference in San Diego, May 2006.
ABBREVATIONS
- PH
Pulmonary hypertension
- E2
17β-estradiol
- 2ME
2-methoxyestradiol
- OVX
ovariectomy
- RV
right ventricle
- RVPSP
right ventricular peak systolic pressure
- LV+S
left ventricle plus septum
- ANTU
alpha-naphthylthiourea
- hLFs
human lung fibroblasts
- hPASMCs
human pulmonary artery smooth muscle cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Artlett CM, Rasheed M, Russo-Stieglitz KE, Sawaya HH, Jimenez SA. Influence of prior pregnancies on disease course and cause of death in systemic sclerosis. Ann. Rheum. Dis. 2002;61:346–350. doi: 10.1136/ard.61.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball P, Knuppen R. Formation, metabolism and physiological significance of catechol estrogens. Am. J. Obstet. Gynecol. 1990;163:2163–2170. doi: 10.1016/0002-9378(90)90558-o. [DOI] [PubMed] [Google Scholar]
- Beretta I, Caronni M, Origgi L, Ponti A, Santaniello A, Scrorza R. Hormone replacement therapy may prevent the development of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Scand. J. Rheumatol. 2006;35:468–471. doi: 10.1080/03009740600844498. [DOI] [PubMed] [Google Scholar]
- Chauhan D, Li G, A D, Hideshima T, Richardson P, Podar K, Mitsiades N, Mitsiades C, Li C, Kin RS, Munshi N, Chen LB, Wong W, Anderson KC. Identification of genes regulated by 2-methoxyestradiol (2ME2) in multiple myeloma cell using oligonucleotide arrays. Blood. 2003;101:3606–3614. doi: 10.1182/blood-2002-10-3146. [DOI] [PubMed] [Google Scholar]
- Dubey RK, Tofovic SP, Jackson EK. Cardiovascular pharmacology of estradiol metabolites. J. Pharmacol. Exp. Ther. 2004;308:403–409. doi: 10.1124/jpet.103.058057. [DOI] [PubMed] [Google Scholar]
- Espinosa E, Oemar BS, Fisher TF. 17beta-Estradiol and smooth muscle cell proliferation in aortic cells of male and female rats. Biochem. Biophys. Res. Commun. 1996;221:8–14. doi: 10.1006/bbrc.1996.0535. [DOI] [PubMed] [Google Scholar]
- Farhat MY, Vargas R, Dingaan B, Ramwell PW. In vitro effects of oestradiol on thymidine uptake in pulmonary vascular smooth muscle cell: role of the endothelium. Br. J. Pharmacol. 1992;107:678–683. doi: 10.1111/j.1476-5381.1992.tb14506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhat MY, Chen MF, Bhatti T, Iqbal A, Cathapermani S, Ramwell PW. Protection of oestradiol against the development of cardiovascular changes associated with monocrotaline pulmonary hypertension in rats. Br. J. Pharmacol. 1993;110:719–723. doi: 10.1111/j.1476-5381.1993.tb13871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier-Jessen MR, Kovac EJ. Estrogen modulation of JE/monocyte chemoattractant protein-1 mRNA expression in murine macrophages. J. Immunol. 1995;154:1838–1845. [PubMed] [Google Scholar]
- Gharaee-Kermani M, Ullenbruch M, Phan SH. Animal models of pulmonary fibrosis. Methods. Mol. Med. 2005a;117:251–259. doi: 10.1385/1-59259-940-0:251. [DOI] [PubMed] [Google Scholar]
- Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-base differences in bleomycin-induced pulmonary hypertension. Am. J. Pathology. 2005b;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Furutama D, Hanafusa T. Physiologically high concentrations of 17b-estradiol enhance NF-kB activity in human T cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R1465–R1471. doi: 10.1152/ajpregu.00778.2006. [DOI] [PubMed] [Google Scholar]
- Issekutz AC, Sapru K. Modulation of adjuvant arthritis in the rat by 2-methoxyestardiol: An effect independent of anti-angiogenic action. Int Immunopharmacology. 2008;8:708–716. doi: 10.1016/j.intimp.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Khalil N, Whitman C, Zuo L, Danielpour D, Breenberg A. Regulation of alveolar macrophage transforming growth factor-β secretion by corticosteroids in bleomycin-induced pulmonary inflammation in the rat. J. Clin. Invest. 1993;92:1812–1818. doi: 10.1172/JCI116771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Kasuga H, Noumura T. Effects of various steroids on in vitro lifespan and cell growth of human fetal lung fibroblasts (WI-38). Mech. Ageing. Dev. 1983;21:335–344. doi: 10.1016/0047-6374(83)90050-7. [DOI] [PubMed] [Google Scholar]
- Kurokawa A, Azuma K, Mita T, Toyofuku Y, Fujitani Y, Hirose T, Iwabuchi K, Ogawa H, Taked S, Kawamori R, Watada H. 2-Methoxyestradiol reduces monocytes adhesion to aortic endothelial cells in ovariectomized rats. Endocrinology. J. 2007;54:1027–1031. doi: 10.1507/endocrj.k07e-034. [DOI] [PubMed] [Google Scholar]
- Lazo JS, Pham ET. Pulmonary fate of [3H] bleomycin A2 in mice. J. Pharmacol. Exp. Ther. 1984;228:13–18. [PubMed] [Google Scholar]
- Lekgabe ED, Royce SG, Hewitson TD, Tang MLK, Zhao C, Moore XL, Tregear GW, Bathgate RAD, Du X-J, Samuel CS. The effects of relaxin and estrogen deficiency on collagen deposition and hypertrophy of nonreproductive organs. Endocrinology. 2006;147:5575–5583. doi: 10.1210/en.2006-0533. [DOI] [PubMed] [Google Scholar]
- Lettieri CJ, Nathan SD, Barnett S, Ahmad S, Shorr AF. Prevalence and outcomes of pulmonary arterial hypertension in idiopathic pulmonary fibrosis. Chest. 2006;129:746–752. doi: 10.1378/chest.129.3.746. [DOI] [PubMed] [Google Scholar]
- Liu Q-H, Li D-G, Huang X, Zong C-H, Xu Q-F, Lu H-M. Suppressive effects of 17bestradiol on hepatic fibrosis in CCI4-induced rat model. World. J. Gastroenterol. 2004;10:1315–1320. doi: 10.3748/wjg.v10.i9.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson AL, Swigrils JJ, Lezotte DC, Norris JM, Wilson CG, Brown K. Mortality from pulmonary fibrosis increased in the United States from 1992 to 2003. Am. J. Resp. Crit. Care. Med. 2003;176:227–284. doi: 10.1164/rccm.200701-044OC. [DOI] [PubMed] [Google Scholar]
- Pan LC, Wilson DW, Segall HJ. Strain differences in the response of Fisher 344 and Sprague-Dawley rats to monocrotaline induced pulmonary vascular disease. Toxicology. 1993;79:21–35. doi: 10.1016/0300-483x(93)90203-5. [DOI] [PubMed] [Google Scholar]
- Pribluda VS, Gubish ER, Lavallee TM, Treston A, Swartz GM, Green SJ. 2-Methoxyestradiol: an endogenous antiangiogenic and antiproliferative drug candidate. Cancer & Metastasis Reviews. 2000;19:173–179. doi: 10.1023/a:1026543018478. [DOI] [PubMed] [Google Scholar]
- Proudman SM, Stevens WM, J. Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37:485–494. doi: 10.1111/j.1445-5994.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- Scorza R, Caronni M, Bazzi S, Nador F, Beretta L, Antonioli R, Origgi L, Ponti A, Marchini M, Vanoli M. Post-menopause is the main risk factor for developing isolated pulmonary hypertension in systemic sclerosis. Ann. N. Y. Acad. Sci. 2002;996:238–246. doi: 10.1111/j.1749-6632.2002.tb04221.x. [DOI] [PubMed] [Google Scholar]
- Seeger H, Mueck AO. Effects of estradiol, tamoxifen and 2-methoxyestradiol on TNF-α induced changes of biochemical markers for growth and invasion of human breast cancer cells. J. Menaupose. 2006;13:15–17. doi: 10.1016/j.lfs.2005.07.042. [DOI] [PubMed] [Google Scholar]
- Speyer CL, Rancilio NJ, Mcclintock SD, Crawford JD, Gao H, Sarma JV, Ward PA. Regulatory effects of estrogen on acute lung inflammation in mice. Am. J. Pysiol. Cell. Physiol. 2005;288:C881–C990. doi: 10.1152/ajpcell.00467.2004. [DOI] [PubMed] [Google Scholar]
- Straub RH. The complex role of estrogens in inflammation. Endocrine. Reviews. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- Takata H, Tomizawa K, Matsushita M, Matsui H. 2-Methoxyestradiol enhances p53 protein transduction therapy-associated inhibition of the proliferative oral cancer cells through suppression of NFkB activity. Acta. Med. Okayama. 2004;58:181–187. doi: 10.18926/AMO/32086. [DOI] [PubMed] [Google Scholar]
- Tofovic SP, Jackson EK, Mady H. 2-Methoxyestradiol attenuates hypertension and cardiac and vascular remodeling in constricted aorta rat model. J Am Soc Nephrol. 2003;14:620A. [Abstract] [Google Scholar]
- Tofovic SP, Eman S, Mady H, Jackson EK, Melhem M. Estradiol metabolites attenuates monocrotaline-induced pulmonary hypertension in rats. J. Cardiovasc. Pharmacol. 2005a;46:430–437. doi: 10.1097/01.fjc.0000175878.32920.17. [DOI] [PubMed] [Google Scholar]
- Tofovic SP, Jackson EK, Zhang X. 2-Methoxyestradiol attenuates pulmonary hypertension induced by α-naphthylthiourea. FASEB J. 2005b;19:A1333. [Abstract] [Google Scholar]
- Tofovic SP, Zhang X, Jackson EK, Dacic S, Petrusevska G. 2-Methoxyestradiol mediates the protective effects of estradiol in monocrotaline-induced pulmonary hypertension. Vascr Pharmacol. 2006;45:358–367. doi: 10.1016/j.vph.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Tofovic SP, Jackson EK, Tofoski G. Dysregulated estradiol metabolism in preeclampsia. Physiologist. 2007;50(21):10–17. [Abstract] [Google Scholar]
- Tofovic SP, Zhang X, Zhu H, Jackson EK, Rafikova O, Petrusevska G. 2-Ethoxyestradiol is antimitogenic and attenuates monocrotaline-induced pulmonary hypertension and vascular remodeling. Vasc. Pharmacol. 2008;48:174–183. doi: 10.1016/j.vph.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Tomaszewski J, Adamiak A, Skorupski P, Rzeski W, Rechberger T. Effects of 17 beta-estradiol and phytoestrogen daidzein on the proliferation of pubocervial fascia and skin fibroblast derived from women suffering from stress urinary incontinence. Ginekologia Polska. 2003;74:1410–1414. [PubMed] [Google Scholar]
- Yasuda M, Schimizu I, Shiba M, Ito S. Suppressive effects of estradiol on dimethylnitrosamine-induced fibrosis of the liver in rats. Hepathology. 1999;29:719–727. doi: 10.1002/hep.510290307. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jia Y, Jackson EK, Tofovic SP. 2-Methoxyestradiol and 2-ethoxyestradiol retard the progression of renal disease in aged, obese, diabetic ZSF1 rats. J. Cardiovasc. Pharmacol. 2007;49:56–63. doi: 10.1097/FJC.0b013e31802cb88e. [DOI] [PubMed] [Google Scholar]