Abstract
Objective
To determine whether the variable nucleotide tandem repeat polymorphism in intron 2 of the interleukin-1 receptor antagonist gene is associated with lung injury in children with community-acquired pneumonia.
Design
A prospective cohort of children diagnosed with community-acquired pneumonia.
Setting
Two pediatric hospitals.
Patients
Eight hundred fifty pediatric patients with community-acquired pneumonia were enrolled.
Interventions
Genotyping of the variable nucleotide tandem repeat polymorphism in intron 2 of the interleukin-1 receptor antagonist gene was performed on DNA isolated from whole blood.
Measurements and Main Results
The requirement for positive pressure ventilation or the diagnosis of acute lung injury or acute respiratory distress syndrome were the main outcomes of the study. Children (14 days–19 yrs) with community-acquired pneumonia (850) were enrolled; analysis was limited to African American (515) and Caucasian (232) patients. Of the 82 patients requiring positive pressure ventilation, 44 were diagnosed with acute lung injury or acute respiratory distress syndrome. Multivariate logistic regression analyses indicated that children without a copy of the A1 allele of the variable nucleotide tandem repeat polymorphism in intron 2 of the interleukin-1 receptor antagonist gene were more likely to need positive pressure ventilation compared to those with one or two copies of this allele (odds ratio = 2.65, confidence interval, 1.02– 6.90). In addition, the absence of the A1 allele also appeared to be associated with the development of community-acquired pneumonia–induced acute lung injury/acute respiratory distress syndrome (odds ratio = 3.1, confidence interval, 0.99 –9.67).
Conclusions
In children with community-acquired pneumonia, absence of the A1 allele at the interleukin-1 receptor antagonist intron 2 polymorphic site is associated with increased risk for more severe lung injury, as measured by the need for positive pressure ventilation or the development of acute lung injury or acute respiratory distress syndrome. Conversely, presence of the A1 allele is associated with decreased risk for more severe lung injury in this patient population.
Keywords: interleukin-1 receptor antagonist, genetic polymorphisms, pediatrics, lung injury, acute respiratory distress syndrome
Community-acquired pneumonia (CAP) is one of the most common pediatric infections with a prevalence of 34–40 cases per 1000 in Europe and North America. In third world countries, it is more common, more severe, and the most prevalent cause of death in children (1). In the United States, CAP also results in a significant number of pediatric admissions to hospitals with over 120,000 infant hospitalizations from 1997 to 1999 (2). CAP-induced respiratory failure accounts for a significant number of admissions to pediatric intensive care units and is also one of the most common causes of acute lung injury (ALI) in the pediatric population (3). Thus, respiratory failure because of CAP is a significant problem in the pediatric population and poses a substantial economic and social burden (4, 5).
Lung injury resulting in respiratory failure is the primary morbidity associated with CAP. The mechanisms underlying and influencing lung injury are complex and involve a variety of molecular and cellular processes that may be influenced by genetic factors. A number of studies suggest that an imbalance of the normal mechanisms controlling these processes may result in an overzealous or persistent inflammatory response resulting in an increased risk for the progression of mild CAP-induced lung injury to more severe injury (6–9). The variability in individual responses to pneumonia may in part be the consequence of variability in genes coding for inflammatory mediators.
One of the first proinflammatory cytokines activated in response to CAP is interleukin (IL)-1β. IL-1β induces the expression of cytokines and chemokines involved in the inflammatory response. IL-1β activity is inhibited by interleukin-1 receptor antagonist (IL-1ra), a naturally occurring inhibitor of IL-1β that lacks agonist activity but competitively binds to the IL-1 receptor thereby blocking IL-1β effects (10). A genetic polymorphism in the IL-1ra gene (also called IL-1RN) consisting of a variable number of tandem repeats in intron 2 is associated both with variability in circulating levels of IL-1ra and IL-1β and with a number of acute and chronic inflammatory processes. This polymorphic site has five alleles designated A1-A5, however most studies have examined only the most common alleles, A1 and A2. The IL-1ra A1 and A2 alleles have been associated with variable transcription rates and circulating levels of both IL-1ra and IL-1β (11–13), and several studies have demonstrated association of this polymorphism with a number of diseases in which inflammation plays an important role (10, 14 –16).
IL-1ra and IL-1β are both involved in the inflammatory response induced by CAP and the subsequent lung injury, which in some cases progresses to ALI and acute respiratory distress syndrome (ARDS). IL-1ra and IL-1β are elevated in bronchoalveolar lavage and pulmonary edema fluid from adult patients with early ALI or ARDS (17–19); similar studies have not been performed in children. Interestingly, IL-1β activity has been shown to be important for the activation of lung fibroblasts early after ALI becomes apparent and appears to be involved in the fibroproliferative response seen with ALI/ARDS (19). As IL-1β activity is inhibited by IL-1ra, variability in IL-1ra could impact the development of ALI/ARDS. These observations together with the documented associations of the intron 2 variable number of tandem repeats polymorphism in the IL-1ra gene with inflammatory diseases and with variability in IL-1ra and IL-1β levels support the hypothesis that the intron 2 polymorphism in the IL-1ra gene might be associated with more severe lung injury in children with CAP. The purpose of this study was to determine whether the polymorphism in intron 2 of the IL1-ra gene was associated with positive pressure ventilation (PPV) or ALI/ARDS in children with CAP.
METHODS
Study Design
A prospective cohort of patients with CAP was enrolled at two large tertiary care, university affiliated, hospitals in large cities (Le Bonheur Children’s Medical Center or Children’s Memorial Hospital) between March 2004 and August 2006. The institutional review boards from each institution approved the study.
Inclusion Criteria
All children presenting to the emergency department, or directly admitted to the general pediatric ward, or intensive care unit (during the work week or on weekends), with the primary or secondary diagnosis of CAP and with a complete blood count obtained as part of standard medical care were screened. Patients and/or their guardians were approached or called for enrollment within 48 hrs of diagnosis. Less than 1% of those approached refused consent. Criteria for CAP was defined by previously published guidelines (20); an acute illness (<14 days of symptoms), the presence of a new chest radiographic infiltrate or consolidation confirmed by a radiologist (blinded to the patient genotype), and clinical features compatible with pneumonia. The clinical features required were one of the following three: fever >37.8°C, hypothermia <36°C, peripheral caucasian blood count >10,000/μL or <4,500/μL or >15% immature neutrophils; and two of the following three: tachypnea (respiratory rate >2 standard deviations from the mean for age), dyspnea, or hypoxemia (pulse oximetry ≤94% on room air on initial evaluation without a known mixing heart lesion).
Exclusion Criteria
Exclusion criteria included: 1) Patients with any severe immunodeficiency including acquired immune deficiency syndrome as defined by the Centers for Disease Control (21); 2) Patients with a malignancy or history of malignancy; 3) Patients receiving treatment with corticosteroids equivalent to prednisolone >20 mg/day for more than 14 days; 4) Patients receiving immunosuppression after organ transplantation; 5) Patients on cyclosporine, cyclophosphamide or azathioprine; 6) Patients from chronic care facilities or hospitalized within the past 30 days.
Data Collection
Data collected from the medical records included date of birth, gender, race, date of diagnosis, comorbid conditions, presenting signs, symptoms, and findings on physical exam, peripheral caucasian blood count and differential, chest radiograph findings, oxygen requirement and duration of oxygen therapy, Pao2, Fio2, whether or not PPV, either invasive (administered via an endotracheal tube or tracheostomy) or noninvasive (administered via nasal prongs or face mask) was required. Oxygen delivered by low or high flow via nasal cannula was not considered PPV. The 1994 American European Consensus Conference definition of ALI or ARDS (Pao2/Fio2 <300, ALI; or Pao2/Fio2 <200, ARDS; with acute onset of bilateral infiltrates on chest radiographs as determined by a radiologist blinded to the genotypes, and without evidence of left atrial hypertension) (22) was used to identify patients with ALI or ARDS.
DNA Extraction and Genotypic Analysis
Whole blood was obtained from left over specimens from complete blood counts. DNA was extracted directly from whole blood samples using the Genomic DNA Purification Kit (Promega, Madison, WI). The genotypic analysis was performed in a blinded fashion; that is, the analysis was performed without knowledge of any clinical data. All samples were analyzed at Le Bonheur Children’s Medical Center. The IL-1ra polymorphism was analyzed as described by Tarlow, et al. (23). Five alleles were observed: Allele 1 (A1) is 410 base pairs (bp)/4 repeats, A2 is 240bp/2 repeats, A3 is 500bp/5 repeats, A4 is 325bp/3 repeats, and A5 595bp/6 repeats. Two individuals independently assessed the results from the analyses and assigned genotypes. To assess reproducibility at least 10% of the samples were analyzed a second time for each polymorphism and gave the same result. Genotypic frequencies of the polymorphism did not deviate from Hardy Weinberg equilibrium.
Statistical Evaluation
The multiallelic IL-1ra polymorphism was tested for Hardy-Weinberg equilibrium with the exact test using GenePop online software (24). The statistical calculations, including multivariate logistic analyses, were performed using the SAS 9.1. Unless otherwise stated, results are expressed as mean ± SD. Odds ratios are reported as odds ratios (95% confidence intervals). The statistical significance of differences in continuous variables were calculated using Student’s t-test or Mann Whitney test, and for categorical variables with Fisher’s exact test. The significance of trends was assessed using Chi-square analysis. Logistic regression modeling was used for multivariate analyses. All reported p values are two-tailed with a value of <0.05 considered significant. Baseline variables with a p < 0.10 remained in the multivariate logistic regression model.
RESULTS
Demographics
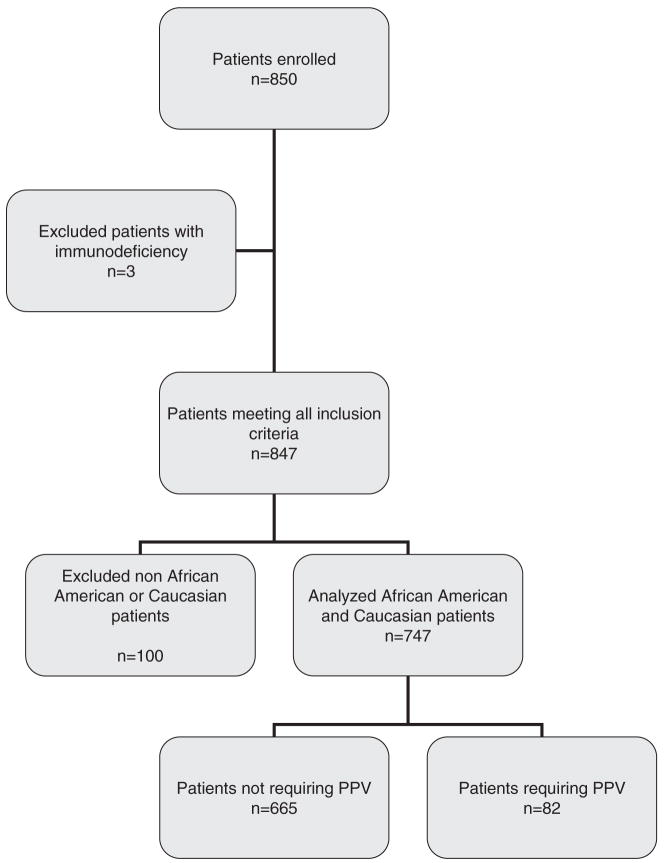
A total of 850 children with CAP were enrolled in the study (Fig. 1). Three patients were found to have an immunodeficiency and were excluded from genetic analysis. The median age of the remaining 847 patients was 2.1 yrs (range 14 days–19 yrs) (Table 1). Pathogens were identified in 320% of the children and are listed in Table 1. Eleven percent of the children required PPV and 6% met the criteria for ALI/ARDS. There were very few deaths (<1%) in this patient population. The racial/ethnic distribution was predominantly African American (61%) and Caucasian (27%). Further analyses were limited to these two groups (Fig. 1) as the number of children with CAP in the other groups was too small to adequately evaluate.
Figure 1.
Flow diagram of patient selection; PPV, positive pressure ventilation.
Table 1.
Demographics of patients
| Patient Characteristics | |
|---|---|
| Age, median (range), yrs | 2.1 (14 days–19yrs) |
| Gender, n (%) | |
| Male | 456 (54) |
| Female | 391 (46) |
| Ethnicity/Racea, n (%) | |
| Caucasian | 232 (27) |
| African American | 515 (61) |
| Hispanic | 68 (8) |
| Asian | 12 (1) |
| Other and unknown | 20 (2) |
| Identified pathogensb, n | |
| Bacterial | 80 |
| Viral | 65 |
| Bacterial and viral | 20 |
| Yeast | 6 |
| Unknown | 676 |
| Positive pressure ventilation, n (%) | 96 (11) |
| ALI/ARDS, n (%) | 49 (6) |
| Mortality, n (%) | 5 (1) |
Self-reported
Pathogens identified by culture of blood, deep tracheal suctioning sample, or pleural fluid, or direct fluorescent antibody from a nasal swab.
ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
Characteristics of patients in the study population (limited to African Americans and Caucasians) requiring PPV compared with those who did not are shown in Table 2. Comorbid conditions at enrollment which were considered risk factors for the need for PPV included asthma, chronic lung disease (CLD, which was defined as a history of bronchopulmonary dysplasia) and cystic fibrosis.
Table 2.
Comparison of characteristics of CAP patients treated with or without positive pressure ventilation
| Patient Characteristics |
||
|---|---|---|
| No PPV (n = 665) | PPV (n = 82) | |
| Age, median (range), yrs | 2.1 (14 days–19 yrs) | 1.4 (25 days–17 yrs) |
| Gender, n (%) | ||
| Male | 358 (54) | 48 (58) |
| Female | 307 (46) | 34 (42) |
| Race, n (%) | ||
| Caucasian | 197 (30) | 35 (42) |
| African American | 468 (70) | 47 (58)a |
| Comorbid conditions, n (%) | ||
| Asthma | 121 (18.2) | 5 (6.0)a |
| Chronic lung disease | 20 (3.0) | 13 (5.7)a |
| Cystic fibrosis | 2 (0.3) | 1 (1.2) |
p < 0.05 compared to frequency seen in patients without PPV (analyzed by chi-square test)
CAP, community-acquired pneumonia; PPV, positive pressure ventilation.
Frequency of IL-1ra Alleles
The frequencies of IL-1ra genotypes for the study population are shown in Table 3. Both the Caucasian and African American populations separately and combined were in Hardy-Weinberg equilibrium for this polymorphic site. The frequencies of the IL-1ra A1-A4 alleles in the Caucasian and African American patients are 0.720, 0.254, 0.019, and 0.006 or 0.913, 0.057, 0.009, and 0.021, respectively; no children in these two groups had the A5 allele. The genotypes and allele frequencies for the A1-A4 alleles differ between African Americans and Caucasians with CAP and are similar to previously reported frequencies in healthy individuals of each race (23, 25–27).
Table 3.
Frequency of IL-1ra genotypes in African American and Caucasian children with community-acquired pneumonia
| Genotype, n (%) | Caucasian, n (%) | African American, n (%) | Combined, n (%) |
|---|---|---|---|
| A1/A1 | 121 (52.2) | 428 (83.6) | 549 (73.8) |
| A1/A2 | 83 (35.8) | 50 (9.8) | 133 (17.9) |
| A2/A2 | 16 (6.9) | 3 (0.6) | 19 (2.6) |
| A1/A3 | 6 (2.6) | 9 (1.8) | 15 (2.0) |
| A1/A4 | 3 (1.3) | 20 (3.9) | 23 (3.1) |
| A2/A3 | 3 (1.3) | 0 | 3 (0.4) |
| A2/A4 | 0 | 2 (0.4) | 2 (0.3) |
IL-1ra genotype could not be determined in three African American patients. There were no African American or Caucasian children enrolled with the A5 allele in the IL-1ra gene.
IL-1ra, interleukin-1 receptor antagonist.
Frequency of IL-1ra Alleles in Patients Requiring Positive Pressure Ventilation
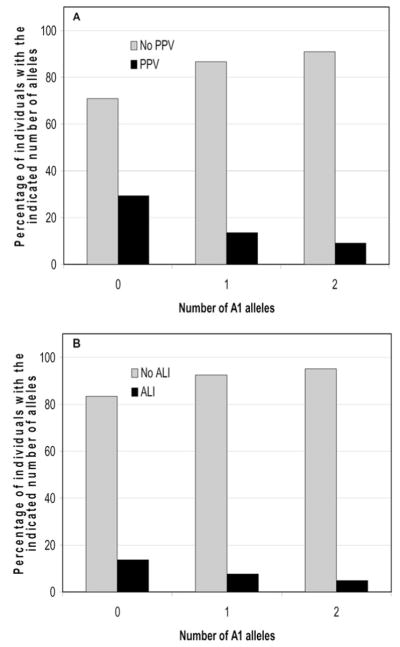
Genotype frequencies of children who required PPV compared with those that did not is shown in Table 4. The genotype of the IL-ra polymorphic site is expressed as the presence of 2 (A1/A1), 1 (A1/A2, A1/A3, A1/A4), or 0 (A2/A2, A2/A3, A2/A4) copies of the A1 allele. As shown in Table 4 the frequency of genotypes without an A1 allele was 8.6% in patients requiring PPV and only 2.6% in patients without PPV (p = 0.003, by Chi-square analysis comparing zero copies with both one and two copies combined) indicating that absence of the A1 allele was associated with PPV and that presence of the A1 allele was associated with protection from the need for PPV. Interestingly, the prevalence of PPV in patients with no copies of the A1 allele was 29% (7 of 24) compared with 14% (23 of 171) in patients with one copy and 9% (50 of 549) in patients with two copies of the A1 allele (Fig. 2A). Trend analysis indicates that the need for PPV decreases with increasing number of A1 alleles (p = 0.002 by Cochran-Armitage trend test).
Table 4.
Comparison of frequencies of interleukin-1 receptor antagonist alleles in children with community-acquired pneumonia treated with or without positive pressure ventilation
| Patients with or without PPV |
||
|---|---|---|
| Copies of the A1 Allele | No PPV, n (%) | PPVa, n (%) |
| 2, n = 549 | 499 (75.1) | 50 (63.0) |
| 1, n = 171 | 148 (22.3) | 23 (28.4) |
| 0, n = 24 | 17 (2.6) | 7 (8.6)b |
Of the three patients who could not be genotyped (see Table 3), two were treated with positive pressure ventilation
p = 0.003 by chi-square analysis.
PPV, Positive Pressure Ventilation.
Figure 2.
Increasing number of A1 alleles is associated with increased prevalence of positive pressure ventilation or acute lung injury/acute respiratory distress syndrome. A, percentage of individuals with 0, 1, or 2 A1 alleles who did or did not require positive pressure ventilation. (p = 0.002 by Cochran-Armitage trend test). B, percentage of individuals with 0, 1, or 2 A1 alleles with or without acute lung injury/acute respiratory distress syndrome. (p = 0.017 by Cochran-Armitage trend test). PPV, positive pressure ventilation.
The association of the absence of the A1 allele with an increased prevalence of PPV persisted when patients with pulmonary comorbidities (asthma, CLD, cystic fibrosis) were excluded from the analysis (p = 0.028, by Chi-square analysis comparing zero copies with both one and two copies combined, data not shown). When Caucasians and African Americans were analyzed separately the increased prevalence of the need for PPV seen for patients with no copies of the IL-1ra A1 allele was also observed but did not reach significance (possibly due to the decrease in numbers when the cohort is stratified into two groups). In the African American population, the prevalence of PPV in patients with no copies of the A1 allele was 40% (2 of 5) compared with 8.5% (43 of 507) in patients with one or two copies of the A1 allele (p = 0.058, by Fisher’s exact test). In the Caucasian patient population, which had fewer patients, the association was still present with 26% (5 of 19) of the patients without the A1 allele requiring PPV compared with 14.1% (30 of 213) in patients with one or two copies of the A1 allele (p = 0.153).
To determine whether association of the A1 allele with protection from PPV remained after other factors such as race and pulmonary comorbidities were considered, SAS PROC LOGISTIC was applied to perform multivariate logistic regression analysis on the combined African American and Caucasian study population. Baseline variables included in the model were race, CLD, and asthma. As shown in Table 5, this analysis demonstrated that the absence of the A1 allele remained a statistically significant predictor of the patient’s need for PPV (odds ratios = 2.65, confidence interval, 1.02–6.90). (Race [p = 0.019], CLD [p < 0.001], and absence of asthma [p = 0.009] were significant independent covariates). Further, the number of copies of A1 and A2 were compared to determine their association with the need for PPV. Children without a copy of the A1 allele were more likely to require PPV compared with children with either one or two copies of A1 (odds ratio 2.41 and 2.92, respectively, Table 5).
Table 5.
Odds ratios of the number of copies of the interleukin-1 receptor antagonist A1 allele and the need for positive pressure ventilation
| Copies of the A1 Allele | Odds Ratio | Confidence Limits | p |
|---|---|---|---|
| 0 vs. 1 & 2 | 2.65 | 1.02–6.90 | 0.046 |
| 0 vs. 1 | 2.41 | 0.87–6.63 | 0.089 |
| 0 vs. 2 | 2.92 | 1.09–7.81 | 0.033 |
| 0 & 1 vs. 2 | 1.88 | 1.01–3.51 | 0.047 |
| 1 vs. 2 | 1.21 | 0.69–2.14 | 0.507 |
Frequency of IL-1ra Alleles in Patients with ALI/ARDS
Forty-four of the children met consensus criteria for either ALI or ARDS. As shown in Table 6 the frequency of genotypes without an A1 allele was 9.1% in children with ALI/ARDS and only 2.9% in children without ALI/ARDS (p = 0.023, by Chi-square analysis comparing zero copies with both one and two copies combined) indicating that absence of the A1 allele was associated with ALI/ARDS and that presence of the A1 allele was associated with protection from ALI/ARDS. Interestingly, the prevalence of ALI/ARDS in children with no copies of the A1 allele was 17% (4 of 24) compared with 8% (13 of 171) in children with one copy and 5% (27 of 549) in children with two copies of the A1 allele (Fig. 2B). This trend was statistically significant (p = 0.017 by Cochran-Armitage trend test). Multivariate logistic regression analysis was performed using the baseline variables described above. Analysis indicated that association of the absence of the A1 allele with increased prevalence of ALI/ARDS remained after accounting for other factors using the multivariate model (Table 7). (The absence of asthma [p = 0.0413] was the only significant covariate in this analysis.)
Table 6.
Comparison of frequencies of interleukin-1 receptor antagonist alleles in children with community-acquired pneumonia–induced ALI/ARDS
| Presence or absence of ALI/ARDS |
||
|---|---|---|
| Copies of the A1 allele | No ALI/ARDS, n (%) | ALI/ARDS, n (%) |
| 2, n = 549 | 522 (74.6) | 27 (61.4) |
| 1, n = 171 | 158 (22.6) | 13 (29.5) |
| 0, n = 24 | 20 (2.9) | 4 (9.1)a |
p = 0.023 by chi-square analysis.
ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
Table 7.
Odds ratios of the number of copies of the interleukin-1 receptor antagonist A1 allele and the presence of ALI/ARDS
| Copies of the A1 Allele | Odds Ratio | Confidence Limits | p |
|---|---|---|---|
| 0 vs. 1 & 2 | 3.10 | 0.99–9.67 | 0.052 |
| 0 vs. 1 | 2.52 | 0.74–8.61 | 0.140 |
| 0 vs. 2 | 3.80 | 1.20–12.02 | 0.023 |
| 0 & 1 vs. 2 | 2.40 | 1.16–4.94 | 0.018 |
| 1 vs. 2 | 1.51 | 0.75–3.02 | 0.246 |
ALI, acute lung injury; ARDS, acute respiratory distress syndrome.
DISCUSSION
Studies examining CAP-associated lung injury and respiratory failure in children are limited and conclusions are often inferred from adult and neonatal studies. To our knowledge, this is the first evaluation of the influence of genetic polymorphisms on the clinical course of CAP in children. Analysis of genetic variants has the potential to help identify children whose genetic makeup puts them at increased risk of developing more severe CAP-induced lung injury, indicated by the need for PPV and the development of ALI/ARDS. Such data may also allow for a better understanding of the development of more severe lung injury in children and may provide insight into newer individualized therapies based on the patients’ genetic predispositions.
This study demonstrates an association between the absence of the IL-1ra A1 allele in children with CAP and the subsequent development of more severe lung injury as indicated by the need for PPV and the development of ALI/ARDS. Multivariate logistic regression indicates that this association remains even after adjustment for conditions (such as race, asthma, and CLD) which may contribute to severity of lung injury are considered. This observation suggests that the absence of the A1 allele is a predictor of severe lung injury indicated by the need for PPV and the development of ALI/ARDS. Conversely, the presence of the A1 allele appears to be protective, decreasing the risk of developing more severe lung injury. There is some indication that there may be a dose-related response to the presence of the A1 allele. Specifically, a comparison of children who carried one copy of the A1 allele with those with no copies of the A1 allele resulted in a lower odds ratio, 2.41 and 2.52 for PPV and ALI/ARDS, respectively, whereas the comparison of children with two copies of the A1 allele with those with no copies resulted in odds ratios of 2.92 and 3.80 for PPV and ARDS, respectively (Tables 5 and 7).
The mechanism by which the presence of the IL-1ra A1 allele decreases the risk of the development of more severe lung injury is unclear. We speculate that this finding may be related to a difference in the level of IL-1β or IL-1β responsiveness in patients with the A1 allele of IL-1ra compared with those with copies of the A2-A4 alleles. A number of studies have suggested that the IL-1ra A1 allele is associated with lower circulating levels of IL-1β when compared with the A2 allele (13, 28, 29) although other studies have demonstrated conflicting data (11, 12, 30, 31). One possible explanation for our results is that the A1 allele is protective because the inflammatory response in these individuals is more moderate than that seen in individuals with the A2, and possibly A3 and A4 alleles. (The influence of the A3 and A4 alleles on the inflammatory response has been largely ignored because of their low frequencies in the population.) Studies examining the influence of the A1 or A2 allele on the inflammatory response are complicated by the fact that other members of the IL-1 gene family, including IL-1β, are located in the same area of chromosome 2 as the IL-1ra gene (32, 33). Consequently, the protective effect of IL-1ra A1 allele may be due to it being in linkage disequilibrium with other polymorphisms in either the IL-1ra or IL-1β genes or possibly even in other IL-1 family genes present in that area.
In addition to proinflammatory effects, IL-1β appears to be specifically involved with lung injury and repair. IL-1β appears to promote repair of alveolar epithelium (17) but also to stimulate fibroblast proliferation and activation resulting in deposition of extracellular matrix proteins which may be involved in fibrotic lung injury (19). Certainly, genetic variations that influence IL-1β levels or activity may impact the severity of lung disease by influencing IL-1β effects on lung pathology.
Several limitations of this study should be discussed. First, it is likely that the IL-1ra polymorphism analyzed in this study is not the causative site but rather acts as a marker linked to another polymorphism or combination of polymorphisms that are responsible for the observed association. However, this does not negate the possible usefulness of this polymorphism as a marker. Furthermore, this study examined a single polymorphism and there are other polymorphisms within the IL-1ra and IL-1β genes that may influence the levels of either IL-1ra or IL-1β (34) and presumably would also be associated with the degree of lung injury. However, for this study we chose this particular polymorphism because it is perhaps the best studied of the polymorphisms in the IL-1ra gene and it is the polymorphism for which the most evidence exists that the genetic variation is associated with a variety of diseases in which inflammation is a major component of the disease. Further analysis of other polymorphisms within genes in this region is currently underway, as is enrollment of patients of other ethnicities for analysis when adequate numbers are obtained. This study was also limited by the use of discarded blood samples, which likely increased enrollment but eliminated the possibility of meaningful measurements of IL-1ra or IL-1 β blood or plasma levels. Future studies are planned to include measurements of biomarkers such as IL-1ra and IL-1β. Another limitation of the study is the heterogeneity of our population in regards to age, comorbid conditions, and the causative pathogens. We elected before the initiation of the study to include patients with other pulmonary disorders in part because it is likely that many genetic variations that influence lung injury will do so regardless of the presence of other disease states. Supporting this hypothesis was the finding that when patients with pulmonary-related comorbid conditions were eliminated from the analysis, the association of the IL-1ra A1 allele with protection from need for PPV remained significant (see results section). Another heterogeneous aspect of our population was the causative pathogens for CAP. Such pathogens were identified in less than 25% of our study population. In this study, as in most cases with pediatric CAP, tests to identify the causative pathogen were not routinely performed, particularly for children who were less ill. In many other studies of patients with CAP the causative agent is also unknown (9, 35, 36). Finally, this study has not been replicated in a separate cohort, however, the National Cancer Institute–National Human Genome Research Institute Working Group on Replication in Association Studies has stated that such data still provide valuable information if the study has been carefully designed and described and the need for replication acknowledged (37).
CONCLUSION
In conclusion, this study demonstrates that in children with CAP the absence of the A1 allele at the IL-1ra intron 2 polymorphic site is associated with an increased risk for the need for PPV or the development of ALI/ARDS. This suggests that children with CAP who have the IL-1ra A1 allele have a decreased risk for the development of lung injury and respiratory failure, including development of ALI/ARDS. Future studies will be required to verify these findings and possibly to extend analyses to genotypes of other genes. Identification of genotypes associated with need for PPV or ALI/ARDS may allow for more targeted therapies, elucidation or confirmation of the underlying pathophysiology, or identification of novel drug targets.
Acknowledgments
We would like to thank Qing Zhang for excellent technical assistance.
Supported, in part, by research grant R21 HD47670 (MWQ, MKD) from the NICHD and M01 RR00048 from the National Center for Research Resources, NIH.
Footnotes
The authors have not declared any potential conflicts of interest.
References
- 1.McIntosh K. Community-acquired pneumonia in children. N Engl J Med. 2002;346:429–437. doi: 10.1056/NEJMra011994. [DOI] [PubMed] [Google Scholar]
- 2.Leader S, Kohlhase K. Respiratory syncytial virus-coded pediatric hospitalizations, 1997 to 1999. Pediatr Infect Dis J. 2002;21:629–632. doi: 10.1097/00006454-200207000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Flori HR, Glidden DV, Rutherford GW, et al. Pediatric acute lung injury: Prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 4.Shudy M, de Almeida ML, Ly S, et al. Impact of pediatric critical illness and injury on families: A systematic literature review. Pediatrics. 2006;118(Suppl 3):S203–S218. doi: 10.1542/peds.2006-0951B. [DOI] [PubMed] [Google Scholar]
- 5.Shoham Y, Dagan R, Givon-Lavi N, et al. Community-acquired pneumonia in children: Quantifying the burden on patients and their families including decrease in quality of life. Pediatrics. 2005;115:1213–1219. doi: 10.1542/peds.2004-1285. [DOI] [PubMed] [Google Scholar]
- 6.Wunderink RG, Waterer GW. Genetics of community-acquired pneumonia. Semin Respir Crit Care Med. 2005;26:553–562. doi: 10.1055/s-2005-925522. [DOI] [PubMed] [Google Scholar]
- 7.Gallagher PM, Lowe G, Fitzgerald T, et al. Association of IL-10 polymorphism with severity of illness in community acquired pneumonia. Thorax. 2003;58:154–156. doi: 10.1136/thorax.58.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marshall RP, Webb S, Bellingan GJ, et al. Angiotensin converting enzyme insertion/deletion polymorphism is associated with susceptibility and outcome in acute respiratory distress syndrome. Am J Respir Crit Care Med. 2002;166:646–650. doi: 10.1164/rccm.2108086. [DOI] [PubMed] [Google Scholar]
- 9.Waterer GW, Quasney MW, Cantor RM, et al. Septic shock and respiratory failure in community-acquired pneumonia have different TNF polymorphism associations. Am J Respir Crit Care Med. 2001;163:1599–1604. doi: 10.1164/ajrccm.163.7.2011088. [DOI] [PubMed] [Google Scholar]
- 10.Arend WP. The balance between IL-1 and IL-1Ra in disease. Cytokine Growth Factor Rev. 2002;13:323–340. doi: 10.1016/s1359-6101(02)00020-5. [DOI] [PubMed] [Google Scholar]
- 11.Danis VA, Millington M, Hyland VJ, et al. Cytokine production by normal human monocytes: Inter-subject variation and relationship to an IL-1 receptor antagonist (IL-1Ra) gene polymorphism. Clin Exp Immunol. 1995;99:303–310. doi: 10.1111/j.1365-2249.1995.tb05549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurme M, Santtila S. IL-1 receptor antagonist (IL-1Ra) plasma levels are co-ordinately regulated by both IL-1Ra and IL-1beta genes. Eur J Immunol. 1998;28:2598–2602. doi: 10.1002/(SICI)1521-4141(199808)28:08<2598::AID-IMMU2598>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Santtila S, Savinainen K, Hurme M. Presence of the IL-1RA allele 2 (IL1RN*2) is associated with enhanced IL-1beta production in vitro. Scand J Immunol. 1998;47:195–198. doi: 10.1046/j.1365-3083.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 14.Arnalich F, Lopez-Maderuelo D, Codoceo R, et al. Interleukin-1 receptor antagonist gene polymorphism and mortality in patients with severe sepsis. Clin Exp Immunol. 2002;127:331–336. doi: 10.1046/j.1365-2249.2002.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El-Omar EM, Carrington M, Chow WH, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 16.Witkin SS, Gerber S, Ledger WJ. Influence of interleukin-1 receptor antagonist gene polymorphism on disease. Clin Infect Dis. 2002;34:204–209. doi: 10.1086/338261. [DOI] [PubMed] [Google Scholar]
- 17.Geiser T, Atabai K, Jarreau PH, et al. Pulmonary edema fluid from patients with acute lung injury augments in vitro alveolar epithelial repair by an IL-1beta-dependent mechanism. Am J Respir Crit Care Med. 2001;163:1384–1388. doi: 10.1164/ajrccm.163.6.2006131. [DOI] [PubMed] [Google Scholar]
- 18.Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164:1896–1903. doi: 10.1164/ajrccm.164.10.2104013. [DOI] [PubMed] [Google Scholar]
- 19.Olman MA, White KE, Ware LB, et al. Pulmonary edema fluid from patients with early lung injury stimulates fibroblast proliferation through IL-1 beta-induced IL-6 expression. J Immunol. 2004;172:2668–2677. doi: 10.4049/jimmunol.172.4.2668. [DOI] [PubMed] [Google Scholar]
- 20.Chow AW, Hall CB, Klein JO, et al. Evaluation of new anti-infective drugs for the treatment of respiratory tract infections. Infectious Diseases Society of America and the Food and Drug Administration. Clin Infect Dis. 1992;15(Suppl 1):S62–S88. doi: 10.1093/clind/15.Supplement_1.S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro K, Ward J, Slutsker L, et al. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41(RR17):1–19. [PubMed] [Google Scholar]
- 22.Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 23.Tarlow JK, Blakemore AI, Lennard A, et al. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Hum Genet. 1993;91:403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- 24.Raymond M, Rousset F. GENEPOP (version 1.2): Population genetics software for exact tests and ecumenicism. J Heredity. 1995;86:248–249. [Google Scholar]
- 25.Mwantembe O, Gaillard MC, Barkhuizen M, et al. Ethnic differences in allelic associations of the interleukin-1 gene cluster in South African patients with inflammatory bowel disease (IBD) and in control individuals. Immunogenetics. 2001;52:249–254. doi: 10.1007/s002510000265. [DOI] [PubMed] [Google Scholar]
- 26.Rider LG, Artlett CM, Foster CB, et al. Polymorphisms in the IL-1 receptor antagonist gene VNTR are possible risk factors for juvenile idiopathic inflammatory myopathies. Clin Exp Immunol. 2000;121:47–52. doi: 10.1046/j.1365-2249.2000.01266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabaleta J, Camargo MC, Piazuelo MB, et al. Association of interleukin-1beta gene polymorphisms with precancerous gastric lesions in African Americans and Caucasians. Am J Gastroenterol. 2006;101:163–171. doi: 10.1111/j.1572-0241.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson RJ, Patel P, Llewelyn M, et al. Influence of polymorphism in the genes for the interleukin (IL)-1 receptor antagonist and IL-1beta on tuberculosis. J Exp Med. 1999;189:1863–1874. doi: 10.1084/jem.189.12.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Witkin SS, Vardhana S, Yih M, et al. Polymorphism in intron 2 of the fetal interleukin-1 receptor antagonist genotype influences midtrimester amniotic fluid concentrations of interleukin-1beta and interleukin-1 receptor antagonist and pregnancy outcome. Am J Obstet Gynecol. 2003;189:1413–1417. doi: 10.1067/s0002-9378(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 30.Andus T, Daig R, Vogl D, et al. Imbalance of the interleukin 1 system in colonic mucosa—association with intestinal inflammation and interleukin 1 receptor antagonist [corrected] genotype 2. Gut. 1997;41:651–657. doi: 10.1136/gut.41.5.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tountas NA, Casini-Raggi V, Yang H, et al. Functional and ethnic association of allele 2 of the interleukin-1 receptor antagonist gene in ulcerative colitis. Gastroenterology. 1999;117:806–813. doi: 10.1016/s0016-5085(99)70338-0. [DOI] [PubMed] [Google Scholar]
- 32.Nicklin MJ, Barton JL, Nguyen M, et al. A sequence-based map of the nine genes of the human interleukin-1 cluster. Genomics. 2002;79:718–725. doi: 10.1006/geno.2002.6751. [DOI] [PubMed] [Google Scholar]
- 33.Taylor SL, Renshaw BR, Garka KE, et al. Genomic organization of the interleukin-1 locus. Genomics. 2002;79:726–733. doi: 10.1006/geno.2002.6752. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Wilkins LM, Aziz N, et al. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 35.Chalasani NP, Valdecanas MA, Gopal AK, et al. Clinical utility of blood cultures in adult patients with community-acquired pneumonia without defined underlying risks. Chest. 1995;108:932–936. doi: 10.1378/chest.108.4.932. [DOI] [PubMed] [Google Scholar]
- 36.Michelow IC, Olsen K, Lozano J, et al. Epidemiology and clinical characteristics of community-acquired pneumonia in hospitalized children. Pediatrics. 2004;113:701–707. doi: 10.1542/peds.113.4.701. [DOI] [PubMed] [Google Scholar]
- 37.Chanock SJ, Manolio T, Boehnke M, et al. Replicating genotype-phenotype associations. Nature. 2007;447:655–660. doi: 10.1038/447655a. [DOI] [PubMed] [Google Scholar]