Abstract
Sex-associated hormones such as estradiol, testosterone and progesterone have all been shown to modulate immune responses, which can result in differential disease outcomes between males and females, as well as between pregnant and nonpregnant females. Most parasitic diseases, including leishmaniasis, usually result in more severe disease in males compared with females. This review highlights our current knowledge concerning the role of sex hormones in modulating leishmaniasis in both clinical settings and experimental disease models.
Keywords: Leishmaniasis, Sex-associated hormones, Immune responses
Introduction
Leishmaniasis represents a broad spectrum of diseases caused by obligate intracellular parasites belonging to the genus Leishmania. Leishmania infections are vector borne, spread by the bite of the female phlebotomine sandfly. Twenty species of Leishmania are pathogenic in human hosts, carried by 30 known species of sandflies. Depending on the infective species of Leishmania and on host factors, 3 major disease manifestations can occur: cutaneous, mucocutaneous and visceral [1].
Cutaneous leishmaniasis (CL) manifests as skin ulcerations, usually on exposed areas of the body, such as the face, arms, and legs [2]. These ulcers often self-resolve in Old World forms of the disease, but New World infections often cause chronic, nonhealing lesions. In individuals with impaired cell-mediated immune responses, a more severe form of CL can occur – diffuse CL. Diffuse CL is characterized by disseminated lesions that resemble lepromatous leprosy. This form does not spontaneously heal and often relapses following treatments. Mucocutaneous leishmaniasis (ML) causes extensive tissue destruction of the oral, nasal, and pharyngeal mucosae, resulting in extreme disfigurement of infected individuals. Visceral leishmaniasis (VL) is the most severe form of the disease and is often fatal if left untreated. VL causes profound hepatosplenomegaly due to widespread dissemination of parasites throughout the reticuloendothelial system [1].
Leishmaniasis is endemic in intertropical regions of the world where sandfly vectors thrive. Over 350 million people are at risk worldwide in 88 countries spanning 4 continents. In total 12 million people are infected with leishmaniasis, and 2 million new cases occur each year (1.5 million cutaneous and mucocutaneous, and 0.5 million visceral) [1]. Ninety percent of CL cases occur in Afghanistan, Brazil, Iran, Peru, Saudi Arabia and Syria, and 90% of ML cases occur in Bolivia, Brazil and Peru [2]. The majority of VL cases are found in poor areas of Bangladesh, India, Nepal, Sudan and Brazil [1]. It is officially estimated that 59,000 people die each year from VL [3], but this number is probably a gross underestimate [1].
Leishmaniasis represents a major global health problem. Case of leishmaniasis continue to rise each year due to environmental risk factors such as migration, urbanization and deforestation, and individual risk factors such as malnutrition and immunosuppression. There has been a sharp increase in the number of VL cases worldwide due to HIV/Leishmania coinfections. Currently there is no vaccine available, and the treatment regimens are less than ideal due to severe side effects, high costs and emerging resistance [1]. Therefore, further research that will lead to a better understanding of the immune responses involved in leishmaniasis is critically important for the development of future prevention and treatment strategies.
Immunology of Leishmaniasis
Leishmania species are capable of parasitizing cells of the mononuclear phagocyte lineage [4], dendritic cells [5] and neutrophils [6], although their definitive host cell is the tissue macrophage. Immunity against Leishmania is primarily mediated by T cells, and a substantial body of research exists to support a paradigm in which type 1 T helper cell (Th1) responses are associated with protective immunity and disease resolution, whereas type 2 T helper cell (Th2) responses are associated with susceptibility and disease exacerbation [7, 8]. Clinically, individuals who have successfully resolved infection exhibit strong delayed-type hypersensitivity (DTH) reactions, while individuals with nonhealing disease exhibit negative DTH reactions [4].
During protective responses, IL-12 secretion by antigen-presenting cells and phagocytes stimulates naïve CD4+. T cells to differentiate into IFN-γ-producing Th1 effector cells [8, 9]. This may occur independently or in conjunction with other cytokines such as IL-1α [10], IL-18 [11], IL-23 [12] and/or IL-27 [13]. In addition to its role in stimulating the adaptive immune response, IL-12 can also act on the innate immune system by inducing natural killer (NK) cells to secrete IFN-γ early during infection [9]. Although NK cells do serve as early sources of IFN-γ and may be involved in the initial development of Th1 responses, they are not required for ultimate disease resolution [14, 15]. IFN-γ is critical in the protective response against leishmaniasis because it stimulates macrophage activation and the production of nitric oxide (NO) via expression of inducible NO synthase, enabling macrophages to effectively kill internalized parasites and infected hosts to resolve the disease [16, 17]. This leishmanicidal activity of IFN-γ can be enhanced by TNF [18] and CD40/CD40L interactions [19–22].
In contrast, experimental animal models and individuals in whom Th2 responses predominate experience severe disease. Although it was initially thought that IL-4 was required for the development of Th2 responses and the differentiation of naïve CD4+ T cells into Th2 cells, further research has shown that IL-4 knockout mice are capable of mounting Th2 responses [23, 24], and type 2 dendritic cells can induce the in vitro development of Th2 cells in the absence of IL-4 [25], suggesting that IL-4 is not required for the initiation of Th2 responses and other mediators influence the development of Th2 responses [26]. In fact, IL-4 may be protective during visceral disease caused by Leishmania donovani [27, 28] and, under certain circumstances, is capable of directing Th1 responses [29]. While IL-4 may be capable of downregulating IFN-γ production and macrophage activation during experimental disease [30, 31], it appears that a failure to produce IL-12 and the inability to mount a Th1 response are more critical in determining disease susceptibility [26], In addition to IL-4, other cytokines that have been shown to play a role in dampening Th1 leishmanicidal responses and driving disease progression and exacerbation include the Th2 cytokines IL-10 [32], IL-13 [33] and the anti-inflammatory cytokine TGF-β [34]. Another factor that leads to disease exacerbation is antibody production. In experimental disease models, antibodies are not protective and actually mediate susceptibility to infection [35]. This corroborates the clinical finding that patients with nonhealing disease exhibit high antibody titers, while those who are healing or have cured disease have low antibody levels [36].
Sex Hormones and Immunity
Sex hormones such as androgens, estrogens and progestins can directly interact with cells of the immune system, thus impacting the development of immune responses. Macrophages and lymphocytes, the two major cell types involved in the disease outcome of leishmaniasis, both possess receptors for sex hormones. Macrophages and T cells possess androgen [37–39], estrogen [37, 40] and progesterone [41, 42] receptors. Based on these data, it is not surprising that gender can affect the outcome of various diseases, including leishmaniasis.
Evidence has indicated that both testosterone, the major circulating androgen in men, and progesterone, a hormone associated with the maintenance of pregnancy, are immunosuppressive. Both hormones reduce NK cell activity [43–46], impair macrophage production of TNF and NO [47–49], and suppress NFκB signal transduction, which is involved in mediating proinflammatory cytokine production [50]. Both hormones can also promote the production of Th2-associated anti-inflammatory cytokines such as IL-10 [47] and IL-4 [51]. Progesterone has been shown to act through the protein progesterone-induced blocking factor (PIBF). PIBF has been shown to activate STAT6, which positively regulates IL-4 production, while inhibiting STAT4, the transcription factor responsible for IL-12 production [52]. The anti-inflammatory properties of progesterone prevent the development of Th1 responses that could result in fetal abortion [42].
In contrast to testosterone and progesterone, estrogen, or 17β-estradiol (E2), is considered a proinflammatory mediator. Reports concerning the effect of E2 on NK cells are conflicting. A study using human NK cells showed that E2 enhances NK cell cytotoxicity [53], while reports using murine NK cells found that E2 reduces NK cell numbers [54] and inhibits NK cell cytotoxicity [55]. E2 has been shown to stimulate the production of the proinflammatory cytokine TNF [56, 57], is known to directly interact with the IFN-γ promoter [58], and has been shown to enhance antigen-specific CD4+ T cell responses [59]. The ability of E2 to drive proinflammatory, Th1-associated immune responses and of testosterone to inhibit them may help to explain why females have a higher incidence of autoimmune disorders [60] and a lower incidence of parasitic infections [61].
Sex Hormones and Leishmaniasis
Clinical Disease
As a general trend, males tend to be more susceptible to leishmaniasis than females, although there are exceptions. The vast majority of clinically based reports in the literature are epidemiological analyses or case studies that do not focus on the underlying mechanisms that render males more susceptible to infection. Nevertheless, the epidemiological analyses do indicate that most often. males are more susceptible to CL, ML and VL.
A human study done in Campeche, Mexico, found that in a population of patients exhibiting Chiclero’s ulcers caused by L. mexicana, only 10% of the infected population was female. The resistance of female subjects correlated with higher levels of GM-CSF [62], which is a growth factor involved in macrophage and dendritic cell differentiation that has also been shown to improve CL treatments in conjunction with standard antimonial therapy [63]. Another human epidemiological study of a population in a deforested region of Colombia found that while gender was not a factor in acquiring infection with L. panamensis, males were at greater risk of developing more severe ML as a result of the infection [64]. As an exception to the general trend, one study in Kabul, Afghanistan, found that females were actually more susceptible to lesions and scars than men as a result of L. tropica infection [65]. This difference in results could represent genetic differences between the parasites responsible for New World (L. mexicana) and Old World (L. tropica) CL.
Both New World and Old World forms of VL show a strong association between gender and disease incidence and severity, with males being more likely to be infected and having more severe clinical presentations. A canine VL study in the endemic city of Split, Croatia, found that 19% of male dogs were seropositive for L. infatum, whereas only 9% of female dogs tested seropositive [66]. Studies of VL in humans have also exhibited a similar trend for L. chagasi infections in Brazil [67] and L. donovani infections in Somalia [68]. However, the report in Somalia and others attribute the higher prevalence of male infection to gender-associated differences in exposure and a higher proportion of male patients seeking medical care. While these factors probably do play a role in the gender differences seen in clinical cases, a great deal of experimental evidence suggests that modulation of infection by sex hormones is also relevant to the outcome of the disease.
Experimental Disease Models
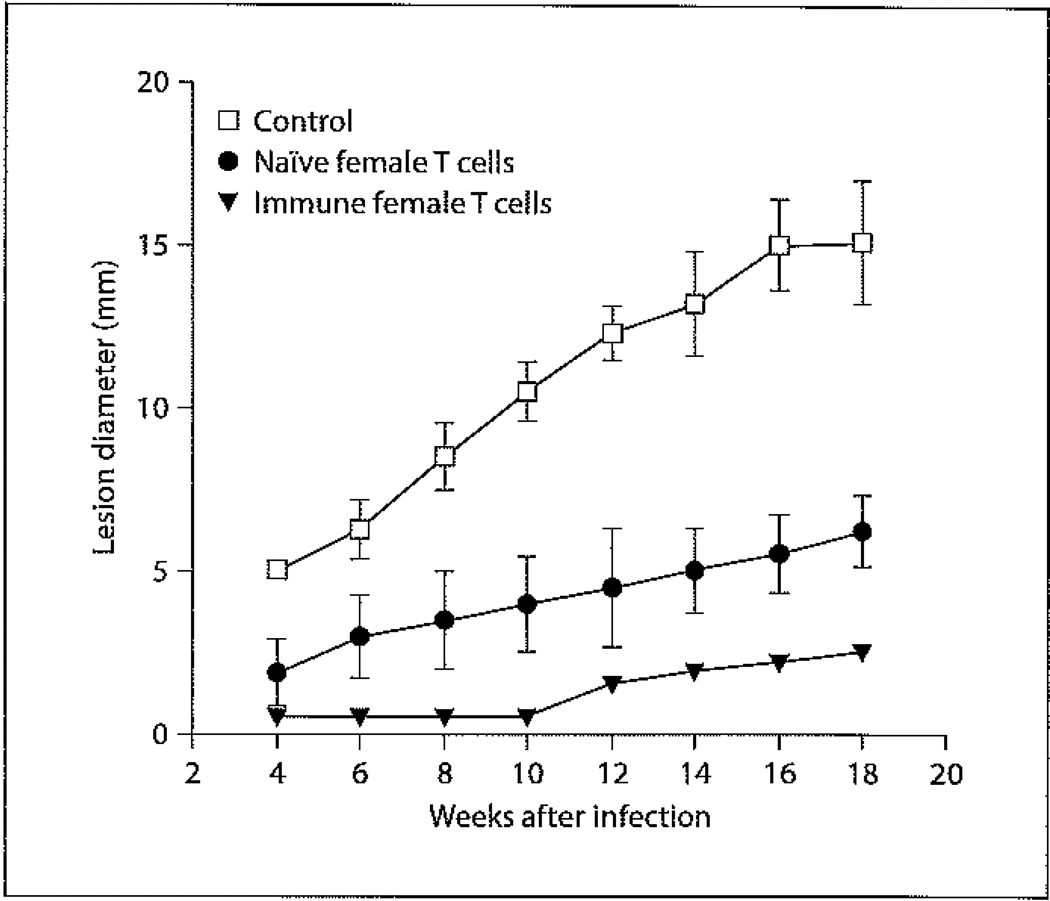
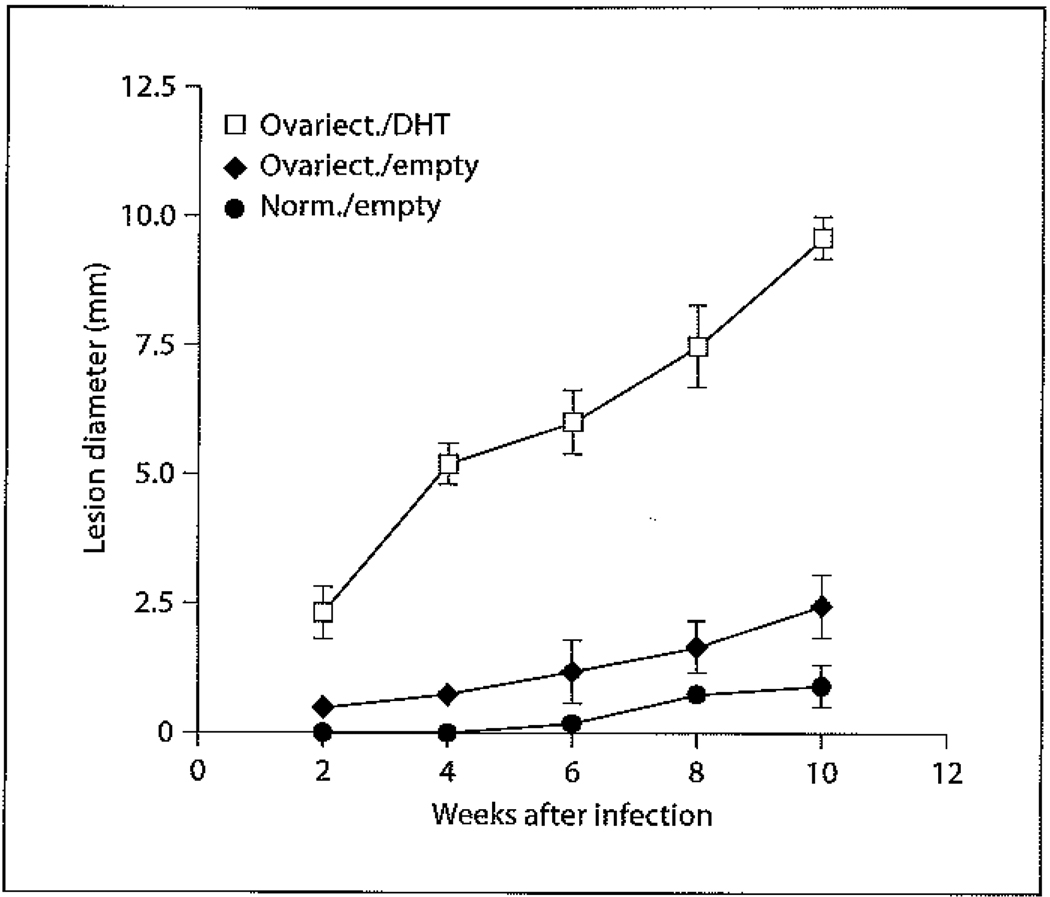
Numerous reports have focused on studying the basis of gender differences exhibited in the immune responses of DBA/2 mice to CL. Interestingly, male and female DBA/2 mice exhibit different disease manifestations depending on the infective species. Following inoculation with L. major, both male and female DBA/2 mice form lesions. However, unlike the male counterparts, female mice do not resolve their lesions. In contrast, female DBA/2 mice infected with L. mexicana are highly resistant to disease and often do not exhibit lesions following high-dose inoculations, whereas male mice are highly susceptible and form chronic lesions upon low-dose infection [69]. This difference in experimental gender-based susceptibility mirrors the aforementioned clinical situation exhibited in humans suffering from Chiclero’s ulcer due to L. mexicana in Mexico [62] versus L. tropica in Afghanistan [65]. The experimental difference is also most likely due to genetic differences between Leishmania species causing New World and Old World CL. Further research exploring the basis behind female DBA/2 resistance against L. mexicana has shown that female mice preferentially express and secrete more IFN-γ during infection compared to male mice [70, 71]. This difference in IFN-γ expression occurred despite the fact that there were no differences in the expression [70] or secretion [71] of IL-4 or IL-5 between male and female mice. However, female mice did exhibit a DTH response indicative of a Th1 response which was absent in males, and male mice exhibited high levels of Th2-associated IgG1 antibodies that were not detectable in female mice [71]. The importance of IFN-γ in mediating resistance was confirmed by showing that treatment of female mice with neutralizing IFN-γ antibodies resulted in lesion growth and progression equivalent to that of male mice [70], and treatment of male mice with intralesional injections of IFN-γ significantly inhibited lesion progression [71]. The resistance of female DBA/2 mice appears to be T cell dependent, as the transfer of immune female T cells into naïve male DBA/2 mice renders them highly resistant to infection (fig. 1). Perhaps even more interesting is the fact that the transfer of naïve female T cells into male mice prior to infection confers significant (albeit reduced in comparison to the transfer of immune T cells) resistance (fig. 1). To address whether male sex hormones could be involved in disease exacerbation, lesion size was compared in normal female mice, ovariectomized female mice, and ovariectomized female mice treated with 5α-dihydroxy testosterone (DHT) (fig. 2). While ovariectomizing female mice alone had little effect on lesion size, treatment of ovariectomized female mice with DHT significantly increased lesion size to levels comparable to those seen in male mice (fig. 2). These data suggest that male sex hormones can directly drive disease exacerbation. Although no published studies have been performed to date, studies on the effects of E2 treatment on castrated male mice could provide insight into whether female sex hormones are capable of directly inhibiting lesion growth.
Fig. 1.
Effect of adoptive transfer of female T cells from DBA/2 mice into naïve male DBA/2 mice on disease progression in subsequent infection. Male mice were either adoptively transferred intraperitoneally with 1 million naïve female T cells isolated from the spleen (closed circles), 1 million immune T cells (closed triangles), or received no T cells (control) (open squares). Male mice were then infected with 5 × 106 L. mexicana amastigotes 15 days after transfer. Immune T cells were generated by infecting female mice with 5 × 106 L. mexicana amastigotes and then isolating T cells from the spleen 20 weeks after infection. Data are expressed as mean lesion diameter ± standard error.
Fig. 2.
Effect of DHT on disease progression in L.-mexicana-infected female DBA/2 mice. Following general anesthesia, ovariectomy was performed as described previously [72]. Two weeks later, Silastic capsules containing 7 mg of DHT were implanted subcutaneously into the dorsal region just below the neck through small subcutaneous incisions (open squares). Control sham ovariectomized (closed diamonds) and intact (closed circles) female mice were similarly implanted with empty Silastic capsules. The incisions were closed using Michel Clips (7.5 × 1.75 mm), and 2 weeks later the mice were infected with 5 × 106 L. mexicana amastigotes subcutaneously into the shaven rump. All animals were individually ear marked, and disease progression was monitored in individual mice by measuring lesion diameter every 2 weeks up to week 10. Data are expressed as mean lesion diameter ± standard error.
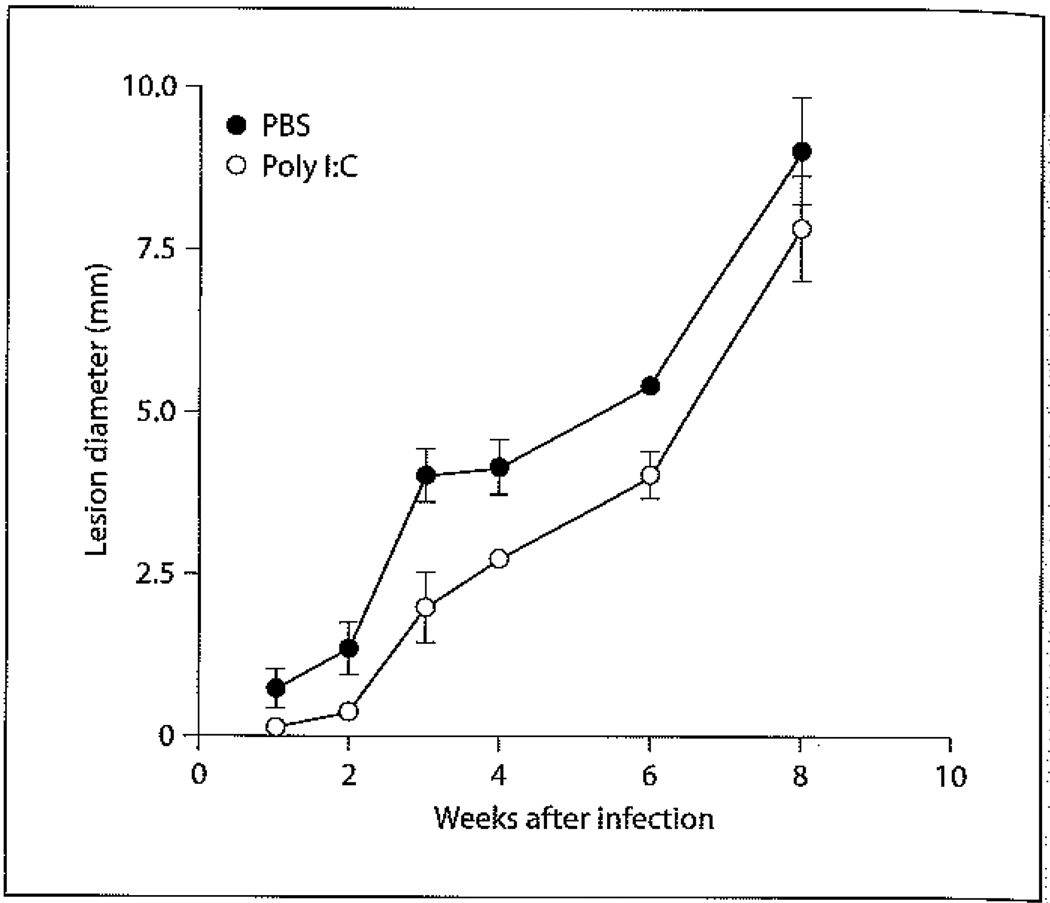
While the above studies implicate a role for sex hormones in the modulation of adaptive immunity against L. mexicana in DBA/2 mice, further studies were undertaken to determine whether sex hormones play a role in shaping innate immune responses. Using poly I:C as a stimulant it was found that male DBA/2 mice do not have an impairment in NK function (fig. 3). Bone marrow-derived macrophages (BMDMs) from female DBA/2 mice were found to be more resistant to in vitro L. mexicana infection than male BMDMs. E2 treatment of both male and female BMDMs enhances parasite killing and NO production without an increase in the proinflammatory cytokine production of IL-6, IL-12 or TNF [74]. These data indicate that E2 may enhance parasite killing by directly regulating NO production in DBA/2 mice. To determine if E2 could enhance parasite killing on other murine genetic backgrounds, similar studies were performed on male and female BMDMs derived from C57BL/6 mice. It was found that E2 was only capable of enhancing the leishmanicidal activity of female BMDMs and was associated with higher levels of IFN-γRα expression, IL-12, IL-6 and NO production. It was further shown that E2-treated BMDMs from PI3Kγ knockout mice were less efficient in killing L. mexicana than E2-treated BMDMs from wild-type counterparts, despite producing similar levels of IL-6, IL-12, TNF and NO [75]. These data show that E2 has differential effects on macrophage leishmanicidal activity between male and female C57BL/6 mice, and that while the PI3Kγ pathway is not required for E2-induced proinflammatory cytokine and NO production in C57BL/6 macrophages, it may play a role in parasite clearance.
Fig. 3.
Effect of poly I:C treatment on the course of L. mexicana infection in male DBA/2 mice. Male DBA/2 mice received intraperitoneal injections of 100 µg of poly I:C 18 h and 50 µg 2 h before infection with 5 × 106 L. mexicana amastigotes into the back rump followed by daily intraperitoneal injections of 50 µg of poly I:C up to 4 weeks as described before (open circles) [73]. Control mice received daily intraperitoneal injections of sterile PBS (closed circles). Disease progression was monitored as described above. Data are expressed as mean lesion diameter ± standard error.
Other experimental disease models using C57BL/6 mice have studied the effect of pregnancy in the disease resolution of female mice infected with L. major. These studies sought to determine whether the tendency to mount Th2 responses during pregnancy could render normally resistant C57BL/6 female mice susceptible to disease with L. major. These studies found that when compared to nonpregnant infected mice, pregnant infected mice had higher parasite burdens associated with a reduction in IFN-γ levels and increases in IL-4, IL-5 and IL-10 production [76–78]. These studies show that the predilection towards Th2 cell responses during pregnancy can impair normal Th1 responses against parasitic infections. However, whether progesterone, the primary hormone associated with pregnancy, is responsible for the downregulation of Th1 responses remains to be studied. Interestingly, another study on pregnancy showed that pregnant hamsters infected with cutaneous L. (Viannia) panamensis had lower parasite burdens associated with enhanced NO production by macrophages [79]. These disparate outcomes between disease models could be the result of differences in parasite and host genetics.
Another hamster study showed that male hamsters infected with L. (Viannia) panamensis were more susceptible to infection, exhibited larger and more severe lesions and had higher parasite burdens in draining lymph nodes compared to females. More severe disease in males was associated with increases in Th2-associated IL-4 and IL-10, and anti-inflammatory TGF-β production [80]. In this disease model, the role of specific sex hormones in disease exacerbation/resistance needs to be further investigated.
Male hamsters infected with L. donovani are also more susceptible to disease and have higher parasite burdens than the female counterparts. Treatment with exogenous testosterone enhanced disease in both male and female animals, while treatment with E2 suppressed infection in both genders. Castration reduced parasite levels, while ovariectomy promoted disease [81]. This study demonstrates that male sex hormones are directly involved in disease exacerbation in VL in hamsters, while the female sex hormone E2 mediates protection. Further in vitro studies have shown that testosterone treatment of L.-donovani-infected BMDMs results in an increase in initial parasite uptake [82], and modulation of apoptosis. The precise role of testosterone in mediating apoptosis is unclear, as the literature presents conflicting reports in which one study demonstrates the inhibition of apoptosis via testosterone [83] and another reports increased apoptotic activity in cells treated with testosterone [84].
Concluding Remarks
Our understanding of the role that sex hormones play in immunity against experimental Leishmania infections has dramatically increased over the past 10 years. Experimental models have shown that sex hormones can directly influence disease resistance or susceptibility. These studies have also shown that key immunological factors such as cytokines or NO can be differentially modulated as a result of gender or pregnancy. These experimental studies lay the foundation to better understand the variations exhibited during clinical disease. A better understanding of gender-dependent differences in human disease will allow for the design and development of more efficacious treatments and vaccination programs that will take gender differences into account.
References
- 1.Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 2.WHO programmes and Projects. http://www.who.int/leishmaniasis/en/
- 3.Geneva: World Health Organization; World Health Organization: The World Health Report. 2002:192–197.
- 4.Alexander J, Russell D. The interaction of Leishmania species with macrophages. Adv Parasitol. 1992;31:175–254. doi: 10.1016/s0065-308x(08)60022-6. [DOI] [PubMed] [Google Scholar]
- 5.Moll H, Fuchs H, Blank C, Rollinghoff M. Langerhans cells transport Leishmania major from infected skin to the draining lymph node for presentation to antigen-specific T cells. Eur J Immunol. 1993;23:1595–1601. doi: 10.1002/eji.1830230730. [DOI] [PubMed] [Google Scholar]
- 6.van Zandbergen G, Klinger M, Mueller A, Dannenberg S, Gebert A, Solbach W, Laskay T. Cutting edge: neutrophil granulocyte serves as a vector for Leishmania entry into macrophages. J Immunol. 2004;173:6521–6525. doi: 10.4049/jimmunol.173.11.6521. [DOI] [PubMed] [Google Scholar]
- 7.Alexander J, Satoskar AR, Russell DG. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. doi: 10.1242/jcs.112.18.2993. [DOI] [PubMed] [Google Scholar]
- 8.Alexander J, Bryson K. T helper (h)1/Th2 and Leishmania: paradox rather than paradigm. Immunol Lett. 2005;99:17–23. doi: 10.1016/j.imlet.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 10.von Stebut E, Ehrchen JM, Belkaid Y, Kostka SL, Molle K, Knop J, Sunderkotter C, Udey MC. Interleukin 1alpha promotes Th1 differentiation and inhibits disease progression in Leishmania major-susceptible BALB/c mice. J Exp Med. 2003;198:191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu D, TrajKovic V, Hunter D, Leung BP, Schulz K, Gracie JA, McInnes IB, Liew FY. IL-18 induces the differentiation of Th1 or Th2 cells depending upon cytokine milieu and genetic background. Eur J Immunol. 2000;30:3147–3156. doi: 10.1002/1521-4141(200011)30:11<3147::AID-IMMU3147>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 12.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 13.Artis D, Villarin A, Silverman M, He W, Thornton EM, Mu S, Summer S, Covey TM, Huang E, Yoshida H, Koretzky G, Goldschmidt M, Wu GD, de Sauvage F, Miller HR, Saris CJ, Scott P, Hunter CA. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 14.Wakil AE, Wang ZE, Ryan JC, Fowell DJ, Locksley RM. Interferon gamma derived from CD4(+) T cells is sufficient to mediate T helper cell type 1 development. J Exp Med. 1998;188:1651–1656. doi: 10.1084/jem.188.9.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Satoskar AR, Stamm LM, Zhang X, Okano M, David JR, Terhorst C, Wang B. Mice lacking NK cells develop an efficient Th1 response and control cutaneous Leishmania major infection. J Immunol. 1999;162:6747–6754. [PubMed] [Google Scholar]
- 16.Cunningham AC. Parasite adaptive mechanisms in infection by Leishmania. Exp Mol Pathol. 2002;72:132–141. doi: 10.1006/exmp.2002.2418. [DOI] [PubMed] [Google Scholar]
- 17.Liew FY, Xu D, Chan WL. Immune effector mechanism in parasitic infections. Immunol Lett. 1999;65:101–104. doi: 10.1016/s0165-2478(98)00131-x. [DOI] [PubMed] [Google Scholar]
- 18.Bogdan C, Moll H, Solbach W, Rollinghoff M. Tumor necrosis factor alpha in combination with interferon gamma, but not with interleukin-4, activates murine macrophages for elimination of Leishmania major amastigotes. Eur J Immunol. 1990;20:1131–1135. doi: 10.1002/eji.1830200528. [DOI] [PubMed] [Google Scholar]
- 19.Heinzel FP, Rerko RM, Hujer AM. Underproduction of interleukin-12 in susceptible mice during progressive leishmaniasis is due to decreased CD40 activity. Cell Immunol. 1998;184:124–142. doi: 10.1006/cimm.1998.1267. [DOI] [PubMed] [Google Scholar]
- 20.Soong L, Xu JC, Grewal IS, Kima P, Sun J, Longley BJ, Jr, Ruddle NH, McMahon-Pratt D, Flavell RA. Disruption of CD40-CD40 ligand interactions results in a enhanced susceptibility to Leishmania amazonensis infection. Immunity. 1996;4:263–273. doi: 10.1016/s1074-7613(00)80434-3. [DOI] [PubMed] [Google Scholar]
- 21.Campbell KA, Ovendale PJ, Kennedy MK, Fanslow WC, Reed SG, Maliszewski CR. CD40 ligand is required for protective cell-mediated immunity to Leishmania major. Immunity. 1996;4:286–289. doi: 10.1016/s1074-7613(00)80436-7. [DOI] [PubMed] [Google Scholar]
- 22.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 23.Noben-Trauth N, Kropf P, Muller I. Susceptibility to Leishmania major infection in interleukin-4-deficient mice. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 24.Mohrs M, Holscher C, Brombacher F. Interleukin-4 receptor alpha-deficient BALB/c mice show an unimpaired T helper 2 polarization in response to Leishmania major infection. Infect Immun. 2000;68:1773–1780. doi: 10.1128/iai.68.4.1773-1780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 26.Sacks D, Anderson C. Re-examination of the immunosuppressive mechanisms mediating non-cure of Leishmania infection in mice. Immunol Rev. 2004;201:225–228. doi: 10.1111/j.0105-2896.2004.00185.x. [DOI] [PubMed] [Google Scholar]
- 27.Alexander J, Carter KC, Al-Fasi N, Satoskar A, Brombrocher F. Endogenous IL-4 is necessary for effective drug therapy against visceral leishmaniasis. Eur J Immunol. 2000;30:2935–2943. doi: 10.1002/1521-4141(200010)30:10<2935::AID-IMMU2935>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 28.Stager S, Alexander J, Carter KC, Brombacher F, Kay PM. Both interleukin-4 (IL-4) and IL-4 receptor alpha signaling contribute to the development of hepatic granulomas with optimal antileishmanial activity. Infect Immun. 2003;71:4804–4807. doi: 10.1128/IAI.71.8.4804-4807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biedermann T, Zimmermann S, Himmelrich H, Gumy A, Egeter O, Sakrauski AK, Seegmuller I, Voigt H, Launois P, Levine AD, Wagner H, Heeg K, Louis JA, Rocken M. IL-4 instructs TH1 responses and resistance to Leishmania major in susceptible BALB/c mice. Nat Immunol. 2001;2:1054–1060. doi: 10.1038/ni725. [DOI] [PubMed] [Google Scholar]
- 30.Chatelain R, Varkila K, Coffman RL. IL-4 induces a Th2 response in Leishmania major-infected mice. J Immunol. 1992;148:1182–1187. [PubMed] [Google Scholar]
- 31.Kopf M, Brombacher F, Kohler G, Kienzle G, Widmann KH, Lefrang K, Humbor C, Ledermann B, Solbach W. IL-4-deficient Balb/c mice resist infection with Leishmania major. J Exp Med. 1996;184:1127–1136. doi: 10.1084/jem.184.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kane MM, Mosser DM. The role of IL-10 in promoting disease progression in leishmaniasis. J Immunol. 2001;166:1141–1147. doi: 10.4049/jimmunol.166.2.1141. [DOI] [PubMed] [Google Scholar]
- 33.Matthews DJ, Emson CL, McKenzie GJ, Jolin HE, Blackwell JM, McKenzie AN. IL-13 is a susceptibility factor for Leishmania major infection. J Immunol. 2000;164:1458–1462. doi: 10.4049/jimmunol.164.3.1458. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Hunter CA, Farrell JP. Anti-TGF-beta treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162:974–979. [PubMed] [Google Scholar]
- 35.Kima PE, Constant SL, Hannum L, Colmenares M, Lee KS, Haberman AM, Shlomchik MJ, McMahon-Pratt D. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmanisis. J Exp Med. 2000;191:1063–1067. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turk JL, Bryceson ADM. Immunological phenomena in leprosy and related diseases. Adv Immunol. 1971;13:209–266. doi: 10.1016/s0065-2776(08)60185-6. [DOI] [PubMed] [Google Scholar]
- 37.Cutolo M, Accardo S, Villaggio B, Barone A, Sulli A, Coviello DA, Carabbio C, Felli L, Miceli D, Farruggio R, Carruba G, Castagnetta L. Androgen and estrogen receptors are present in primary cultures of human synovial macrophages. J Clin Endorcrinol Metab. 1996;81:820–827. doi: 10.1210/jcem.81.2.8636310. [DOI] [PubMed] [Google Scholar]
- 38.Wunderlich F, Benten WP, Lieberherr M, Guo Z, Stamm O, Wrehlke C, Sekeris CE, Mossman H. Testosterone signaling in T cells and macrophages. Steroids. 2002;67:535–538. doi: 10.1016/s0039-128x(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 39.Benten WP, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossman H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999;13:123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- 40.Danel L, Souweine G, Monier JC, Saez S. Specific estrogen binding sites in human lymphoid cells and thymic cells. J Steroid Biochem. 1983;18:559–563. doi: 10.1016/0022-4731(83)90131-0. [DOI] [PubMed] [Google Scholar]
- 41.Jones LA, Anthony JP, Henrique FL, Lyons RE, Nickdel MB, Carter KC, Alexander J, Roberts CW. Toll-like receptor-4-mediated macrophage activation is differentially regulated by progesterone via the glucocorticoid and progesterone receptors. Immunology. 2008;125:59–69. doi: 10.1111/j.1365-2567.2008.02820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Piccinni MP, Scaletti C, Maggi E, Romagnani S. Role of hormone-controlled Th1- and Th2-type cytokines in successful pregnancy. J Neuroimmunol. 2000;109:30–33. doi: 10.1016/s0165-5728(00)00299-x. [DOI] [PubMed] [Google Scholar]
- 43.Hou J, Zheng WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. 1988;10:15–22. doi: 10.1016/0192-0561(88)90145-2. [DOI] [PubMed] [Google Scholar]
- 44.Baley JE, Schacter BZ. Mechanisms of diminished natural killer cell activity in pregnant women and neonates. J Immunol. 1985;134:3042–3048. [PubMed] [Google Scholar]
- 45.Furukawa K, Itoh K, Okamura K, Kumagai K, Suzuki M. Changes in NK cell activity during the estrous cycle and pregnancy in mice. J Reprod Immunol. 1984;6:353–363. doi: 10.1016/0165-0378(84)90045-7. [DOI] [PubMed] [Google Scholar]
- 46.Toder V, Nebel L, Elrad H, Blank M, Durdana A, Gleicher N. Studies of natural killer cells in pregnancy. 2. The immunoregulatory effect of pregnancy substances. J Clin Lab Immunol. 1984;14:129–133. [PubMed] [Google Scholar]
- 47.D’Agostino P, Milano S, Barbera C, Di Bella G, La Rosa M, Ferlazzo V, Farruggio R, Miceli DM, Miele M, Castagnetta L, Cillari E. Sex hormones modulate inflammatory mediators produced by macrophages. Ann NY Acad Sci. 1999;876:426–429. doi: 10.1111/j.1749-6632.1999.tb07667.x. [DOI] [PubMed] [Google Scholar]
- 48.Miller L, Alley EW, Murphy WJ, Russell SW, Hunt JS. Progesterone inhibits inducible nitric oxide synthase gene expression and nitric oxide production in murine macrophages. J Leukoc Biol. 1996;59:442–450. doi: 10.1002/jlb.59.3.442. [DOI] [PubMed] [Google Scholar]
- 49.Miller L, Hunt JS. Regulation of TNF-alpha production in activated mouse macrophages by progesterone. J Immunol. 1998;160:5098–5104. [PubMed] [Google Scholar]
- 50.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 51.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognar S, Parronchi P, Manetti R, Annunziato F, Livi C, Romagnani S, Maggi E. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane expression in established Th1 cell clones. J Immunol. 1995;155:128–133. [PubMed] [Google Scholar]
- 52.Szekeres-Barthos J, Polgar B, Kozma N, Miko E, Par G, Szereday L, Barakonyi A, Palkovics T, Papp O, Varga P. Progesterone-dependent immunomodulation. Chem Immunol Allergy. 2005;89:118–125. doi: 10.1159/000087953. [DOI] [PubMed] [Google Scholar]
- 53.Sorachi K, Kumagai S, Sugita M, Yodoi J, Imura H. Enhancing effect of 17 beta-estradiol on human NK cell activity. Immunol Lett. 1993;36:31–35. doi: 10.1016/0165-2478(93)90065-a. [DOI] [PubMed] [Google Scholar]
- 54.Seaman WE, Blackman MA, Gindhart TD, Roubinian JR, Loeb JM, Talal N. Beta-estradiol reduces natural killer cells in mice. J Immunol. 1978;121:2193–2198. [PubMed] [Google Scholar]
- 55.Nilsson N, Carlsten H. Estrogen induces suppression of natural killer cell cytotoxicity and augmentation of polyclonal B cell activation. Cell Immunol. 1994;158:131–139. doi: 10.1006/cimm.1994.1262. [DOI] [PubMed] [Google Scholar]
- 56.Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- 57.Zuckerman SH, Bryan-Poole N, Evans GF, Short L, Glasebrook AL. In vivo modulation of marine serum tumour necrosis factor and interleukin-6 levels during endotoxemia by oestrogen agonists and antagonists. Immunology. 1995;86:18–24. [PMC free article] [PubMed] [Google Scholar]
- 58.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- 59.Maret A, Coudert JD, Garidou L, Foucras G, Gourdy P, Krust A, Dupont S, Chambon P, Druet P, Bayard F, Guery JC. Estradiol enhances primary antigen-specific CD4 T cell responses and Th1 development in vivo, Essential role of estrogen receptor alpha expression in hematopoietic cells. Eur J Immunol. 2003;33:512–521. doi: 10.1002/immu.200310027. [DOI] [PubMed] [Google Scholar]
- 60.O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 61.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lezama-Davila CM, Oghumu S, Satoskar AR, Isaac-Marquez AP. Sex-associated susceptibility in humans with chiclero’s ulcer: resistance in females is associated with increased serum-levels of GM-CSF. Scand J Immunol. 2007;65:210–211. doi: 10.1111/j.1365-3083.2006.01887.x. [DOI] [PubMed] [Google Scholar]
- 63.Almeida RP, Brito J, Machado PL, De Jesus AR, Schriefer A, Guimaraes LH, Carvalho EM. Successful treatment of refractory cutaneous leishmaniasis with GM-CSF and antimonials. Am J Trop Med Hyg. 2005;73:79–81. [PubMed] [Google Scholar]
- 64.Munoz G, Davies CR. Leishmania panamensis transmission in the domestic environment: the results of a prospective epidemiological survey in Santander, Columbia. Biomedica. 2006;26 suppl 1:131–144. [PubMed] [Google Scholar]
- 65.Reithinger R, Mohsen M, Aadil K, Sidiqi M, Erasmus P, Coleman PG. Anthroponotic cutaneous leishmaniasis, Kabul, Afghanistan. Emerg Infect Dis. 2003;9:727–729. doi: 10.3201/eid0906.030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zivicnjak T, Martinkovic F, Marinculic A, Mrljak V, Kucer N, Matijatko V, Mihaljevic Z, Baric-Rafaj R. A seroepidemiologic survey of canine visceral leishmaniosis among apparently healthy dogs in Croatia. Vet Parasitol. 2005;131:35–43. doi: 10.1016/j.vetpar.2005.04.036. [DOI] [PubMed] [Google Scholar]
- 67.Jeronimo SM, Duggal P, Braz RF, Cheng C, Monteiro GR, Nascimento ET, Martins DR, Karplus TM, Ximenes MF, Oliveira CC, Pinheiro VG, Pereira W, Peralta JM, Sousa J, Medeiros IM, Pearsoni RD, Burns TL, Pugh EW, Wilson ME. An emerging peri-urban pattern of infection with Leishmania chagasi, the protozoan causing visceral leishmaniasis in northeast Brazil. Scand J Infect Dis. 2004;36:443–449. doi: 10.1080/00365540410020451. [DOI] [PubMed] [Google Scholar]
- 68.Shiddo SA, Aden Mohamed A, Akuffo HO, Mohamud KA, Herzi AA, Herzi Mohamed H, Huldt G, Nilsson LA, Ouchterlony O, Thorstensson R. Visceral leishmaniasis in Somalia: prevalence of markers of infection and disease manifestations in a village in an endemic area. Trans R Soc Trop Med Hyg. 1995;89:361–365. doi: 10.1016/0035-9203(95)90008-x. [DOI] [PubMed] [Google Scholar]
- 69.Alexander J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology. 1988;96:297–302. doi: 10.1017/s0031182000058303. [DOI] [PubMed] [Google Scholar]
- 70.Satoskar A, Alexander J. Sex-determined susceptibility and differential IFN-gamma and TNF-alpha mRNA expression in DBA/2 mice infected with Leishmania mexicana. Immunology. 1995;84:1–4. [PMC free article] [PubMed] [Google Scholar]
- 71.Satoskar A, Al-Quassi HH, Alexander J. Sex-determined resistance against Leishmania mexicana is associated with the preferential induction of a Th1-like response and IFN-gamma production by female but not male DBA/2 mice. Immunol Cell Biol. 1998;76:159–166. doi: 10.1046/j.1440-1711.1998.00730.x. [DOI] [PubMed] [Google Scholar]
- 72.Kittas C, Henry L. Effect of sex hormones on the response of mice to infection with Toxoplasma godii. Br J Exp Pathol. 1980;61:590–600. [PMC free article] [PubMed] [Google Scholar]
- 73.Laskay T, Rollinghoff M, Solbach W. Natural killer cells participate in the early defense against Leishmania major infection in mice. Eur J Immunol. 1993;23:2237–2241. doi: 10.1002/eji.1830230928. [DOI] [PubMed] [Google Scholar]
- 74.Lezama-Davila CM, Isaac-Marquez AP, Barbi J, Oghumu S, Satoskar AR. 17beta-estradiol increases Leishmania mexicana killing in macrophages from DBA/2 mice by enhancing production of nitric oxide but not pro-inflammatory cytokines. Am J Trop Med Hyg. 2007;76:1125–1127. [PMC free article] [PubMed] [Google Scholar]
- 75.Lezama-Davila CM, Isaac-Marquez AP, Barbi J, Cummings HE, Lu B, Satoskar AR. Role of phosphatidylinositol-3-kinase-gamma (PI3Kgamma)-mediated pathway in 17beta-estradiol-induced killing of L. mexicana in macrophages from C57BL/6 mice. Immunol Cell Biol. 2008;86:539–543. doi: 10.1038/icb.2008.39. [DOI] [PubMed] [Google Scholar]
- 76.Krishnan L, Guilbert LJ, Russell AS, Wegmann TG, Mosmann TR, Belosevic M. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection and causes decreased antigen-specific IFN-gamma response and increased production of T helper 2 cytokines. J Immunol. 1996;156:644–652. [PubMed] [Google Scholar]
- 77.Arinola OG, Louis J, Tacchini-Cottier F, Aseffa A, Salimonu LS. Interleukin-4 (IL-4) and interferon gamma (IFN-gamma) in pregnant C57BL/6 mice infected with L. major at different gestational periods. West Afr J Med. 2004;23:202–207. doi: 10.4314/wajm.v23i3.28121. [DOI] [PubMed] [Google Scholar]
- 78.Arinola OG, Louis JS, Tacchini-Cottier F, Aseffa A, Salimonu LS. Pregnancy impairs resistance of C57BL/6 mice to Leishmania major infection. Afr J Med Med Sci. 2005;34:65–70. [PubMed] [Google Scholar]
- 79.Osorio Y, Bonilla DL, Peniche AG, Melby PC, Travi BL. Pregnancy enhances the innate immune response in experimental cutaneous leishmaniasis through hormone-modulated nitric oxide production. J Leukoc Biol. 2008;83:1413–1422. doi: 10.1189/jlb.0207130. [DOI] [PubMed] [Google Scholar]
- 80.Travi BL, Osorio Y, Melby PC, Chandrasekar B, Arteaga L, Saravia NG. Gender is a major determinant of the clinical evolution and immune response in hamsters infected with Leishmanai spp. Infect Immun. 2002;70:2288–2296. doi: 10.1128/IAI.70.5.2288-2296.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anuradha Pal R, Katiyar JC. Sex-influenced population kinetics of Leishmania donovani in hamsters. Indian J Exp Biol. 1990;28:876–879. [PubMed] [Google Scholar]
- 82.Yin G, Guo Z, Yin L, Zhao J, Qiao Z, Frank W. Effect of testosterone on Leishmania donovani infection levels of murine bone marrow derived-macrophages. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1998;16:251–255. [PubMed] [Google Scholar]
- 83.Qiao X, Guo Z, Yin G, Yin L, Zhao J, Wunderlich F. Testosterone inhibits apoptosis of Leishmania donovani-infected macrophages. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1999;17:21–24. [PubMed] [Google Scholar]
- 84.Liu L, Benten WP, Wang L, Hao X, Li Q, Zhang H, Guo D, Wang Y, Wunderlich F, Qiao Z. Modulation of Leishmania donovani infection and cell viability by testosterone in bone marrow-derived macrophages: signaling via surface binding sites. Steroids. 2005;70:604–614. doi: 10.1016/j.steroids.2005.02.020. [DOI] [PubMed] [Google Scholar]