Abstract
Objective
Mice with Col9a1 gene inactivation (Col9a1-/-) prematurely develop osteoarthritis and intervertebral disc degeneration. In this study, we investigate Col9a1-/- mice for functional and symptomatic changes that may associate with these pathologies.
Methods
Col9a1-/- and wildtype mice were investigated for reflexes, functional impairments, (beam walking, pole climbing, wire-hang, grip-strength), sensorimotor skills (rotarod), mechanical sensitivity (von Frey hair) and thermal sensitivity (hot plate/tail flick). Gait was also analyzed to determine velocity, stride frequency, symmetry, percentage stance times, stride lengths, and step widths. Post-mortem, serum was analyzed for hyaluronan, and knees and spines were graded histologically for degeneration.
Results
Col9a1-/- mice had compensatory gait changes, increased mechanical sensitivity, and impaired physical ability. Col9a1-/- mice ambulated with gaits characterized by increased percentage stance times and shorter stride lengths. These mice also had heightened mechanical sensitivity and were deficient in contact righting, wire-hang, rotarod, and pole climbing. Male-Col9a1-/- mice had the highest mean serum hyaluronan levels and strong histological evidence of cartilage erosion. Intervertebral disc degeneration was also detected, with Col9a1-/- mice having increased incidence of disc tears.
Conclusions
These data describe a Col9a1-/- behavioral phenotype characterized by altered gait, increased mechanical sensitivity, and impaired function. These gait and functional differences suggest Col9a1-/- mice select locomotive behaviors that limit joint loads. The nature and magnitude of behavioral changes were largest in males, which also had the greatest evidence of knee degeneration. These findings suggest that Col9a1-/- mice present behavioral changes consistent with anatomic signs of osteoarthritis and intervertebral disc degeneration.
Introduction
Osteoarthritis (OA) and degenerative disc disease (DDD) are common musculoskeletal disorders, and as chronic conditions, both have large economic costs (1). Clinically, OA and DDD associate with joint pain, loss of function, and decreased quality of patient life. Genetic predisposition to musculoskeletal diseases has been suggested as a determinant of individual risk (2, 3), and extracellular matrix mutations have been linked with premature onset of OA and DDD (4-10). Type IX collagen is a heterotrimeric collagen that associates with type II collagen fibrils and contains domains suited to promote extracellular matrix cohesion (11). Type IX collagen mutations are hypothesized to weaken cartilaginous tissues (8). Mice with inactivation of the Col9a1 gene, hereto referred to as Col9a1-/- mice, do not form functional type IX collagen molecules (12) and develop spontaneous, premature cartilage degeneration (as early as 3 months) in the intervertebral disc (IVD), knee, and temporomandibular joint that worsens with age (up to 12 months) (12-14). It is not known, however, if type IX collagen deletion associates with functional or symptomatic changes characteristic of OA or DDD.
The objective of this study was to evaluate Col9a1-/- mice for functional and symptomatic measures, towards the goal of determining a Col9a1-/- mouse behavioral phenotype indicative of OA or DDD. Mice of advanced age (9-11 months) were studied to represent an age of histological evidence of OA and DDD (12-14). Functional tests were selected to measure physical capabilities that could be impaired due to OA or DDD. Tests include reflexes, posture, strength, coordination, balance, sensorimotor skills, and gait. Symptomatic pain was assessed through mechanical and thermal withdrawal thresholds. Histological evidence of knee cartilage and IVD degeneration was evaluated as well as serum hyaluronan (HA), an OA-related biomarker (15). Finally, functional and symptomatic measures were compared to the prevalence and severity of OA and DDD. Data show that Col9a1-/- mice have significant functional deficiencies and increased mechanical sensitivity. The pattern of behavioral changes suggest a relationship to OA- and DDD-like degeneration observed in mutant mice, such that the Col9a1-/- mouse model may provide the potential to study interventions and their effects on the behavioral features of OA and DDD.
Materials and Methods
Wildtype (WT) and Col9a1-/- mice (C57BL/6) were obtained from a colony bred at Harvard Medical School (Dr. BR Olsen), originally developed by Fassler and coworkers (12). Mice were genotyped, bred, and housed at Duke University as described previously (13). At 9 months of age, male- and female-Col9a1-/- and WT mice were transferred to the Mouse Behavioral and Neuroendocrine Analysis Core Facility (n=5 per sex-genotype). Mice were evaluated for coordination, gait, and sensitivity through the following tests: 1) reflexes, posture, and righting; 2) balance beam; 3) wire-hang; 4) grip-strength; 5) gait; 6) accelerating rotarod; 7) constant-speed rotarod; 8) mechanical sensitivity; and 9) thermal sensitivity. Test order is dictated by numbers and were separated by a minimum of 1 day.
Neuromuscular Screen
Animals were weighed and screened for reflexive behaviors by bringing a cotton swab into contact with the whiskers, eye lashes, and pinna; twitch or withdrawal indicated normal reflexes (16). Postural ability was determined by insuring a mouse could maintain posture when an observation cage was displaced horizontally or vertically. Righting was assessed in a contact righting tube; a mouse was placed on its back and its ability to regain footing was scored as normal, delayed (1-5 sec), or impaired (>5 sec). Hind- and fore-limb grip-strengths were determined on an automated meter (maximum force applied as the mouse is removed, 3 trials; Stoelting, Wood Dale, IL). Coordination and balance were assessed by recording wire-hang duration on a 3-mm diameter wire and time to climb up, down, or across a fabric-lined pole (2 cm diameter, 43 cm long). Sensorimotor skills were studied on an accelerating and constant-speed rotarod (4-40 rpm/5 min, 16 rpm/5 min - successive days; Stoelting). Rotarod latencies were time to fall or passive rotation (4 trials per protocol, 5 min maximum test length; Stoelting).
Gait
Subjects were placed in a custom-built acrylic gait arena with transparent floor and sides (27″ × 3.5″, camera set to record 15″). Underneath the arena, a mirror oriented at 45° allowed for recording in the sagittal and ventral planes. Multiple unprompted and prompted trials were recorded for each animal. In unprompted trials, a mouse explored the arena with no external stimulus (20-30 minutes). In prompted trials, movements were induced by brushing the animal's hind quarters with a cotton swab (5-10 minutes). All trials were recorded at 200 frames per second (1.5-4.0 secs of recorded data, Phantom V4.2; Vision Research, Wayne, NJ). Video frame (time) and spatial position of the nose, tail, foot-strike and toe-off events were determined by tracking nose, tail, and foot positions in DLTdataviewer2 (17). Since steady-state gait was required for determination of gait parameters, 16 trials with velocity fluctuations greater than 10% about the mean were excluded from statistical models. In total, 60 trials were analyzed for unprompted gait (14 male-WT, 13 male-Col9a1-/-, 15 female-WT and 18 female-Col9a1-/- trials measured from 5 mice in each sex-genotype), and 39 trials were analyzed for prompted gait (11 male-WT, 9 male-Col9a1-/-, 9 female-WT and 10 female-Col9a1-/- trials measured from 5 mice in each sex-genotype). The quantified gait parameters were velocity, percentage stance time (percentage of stride time a limb is in ground contact), stride length, step width (distance between left and right foot in the hind- or fore-limb pair orthogonal to the animal's midline), stride frequency, and symmetry (time between left and right foot-strikes for the hind- or fore-limb pair divided by the time between two left foot-strikes in the same limb pair).
Mechanical Sensitivity
Mice were acclimated to a wire-bottom cage and the von Frey hair testing procedure over 3 days. Using a protocol detailed by Fuchs and coworkers (18), withdrawal frequencies to a series of von Frey hairs (2.83, 3.22, 3.61, 3.84, 4.08, 4.17, 4.31, 4.56; Stoelting) were recorded over 8 trials (4 per hind-paw, applications separated by 1 minute for each subject). Hairs were applied in ascending order with application occurring normal to the hind paw's plantar surface (1-2 secs). Two graders detected the presence a positive response (paw flick, lick or vocalization). Positive response frequencies were then plotted against each hair's bending force and fit to a sigmoid function to determine the force at 50% likelihood of postitive response (50% withdrawal threshold).
Thermal Sensitivity
A mouse was placed on a hot plate (52 ± 1°C; Columbus Instruments, Columbus, OH); paw withdrawal latency was recorded. The mouse was then gently restrained in a towel, and heat was applied to the tail base via a radiant light source (Columbus Instruments); tail withdrawal latency was recorded. This sequence, hot-plate followed by tail-flick, was repeated at 0, 15, 30, 60, 90, 120, and 240 minutes. Heat exposure in each trial did not exceed 30 sec.
Serum HA Concentrations
Prior work has described serum HA concentration changes in human OA subjects (15, 19) and a mouse joint pathology model (20). To investigate serum HA changes in a model of spontaneous cartilage wear, sera were obtained from WT and Col9a1-/- blood collected via retro-orbital bleed after euthanasia. Serum HA was quantified using a commercially available ELISA (#029-001; Corgenix Inc., Westminster, CO). Briefly, HA reference and mouse serum, diluted 1:100, were incubated in microwells coated with HA binding protein. Serum HA concentrations were determined against a standard curve prepared from reference solutions via colorimetric absorbance readings. The intra- and inter-assay coefficients of variation were 4.2% and 6.3%, respectively.
Histology
Following euthanasia, mouse tissue was stored at -80°C for 2 months. Samples were thawed, and spines and both knees were dissected, fixed in 10% neutral buffered formalin for 48 hours, decalcified in formic acid, and embedded in paraffin using routine methods (13).
To evaluate Col9a1-/- spine degeneration as previously described (13), a histological processing and grading scheme was employed (13, 21). Spines (n=20) were sectioned in the sagittal plane (∼7 μm thick) with representative sections selected every 140 μm (6 per spine). Alternate sections were stained with hematoxylin-eosin or Safranin O-fast green. Images were acquired for two lumbar discs of each stained section; these were randomized, and two blinded graders evaluated endplate and IVD regions using a scheme described by Boos and coworkers (21). IVD degeneration was scored for tears/cleft formations (ordinal rank range: 0-4), chondrocyte proliferation (0-6), mucous degeneration (0-4), cell death (0-4), and granular changes (0-4). Vertebral endplate changes were scored for cracks/tears (ordinal rank range: 0-4), cell proliferation (0-4), cartilage disorganization (0-4), microfracture (0-2), new bone formation (0-2), and bony sclerosis (0-2). Lower rank indicates less evidence of degeneration; descriptions of the severity observed at each rank are described by Boyd and coworkers (13). Scores in each category were compared for inter-observer reliability; when percentage agreement was below 70%, consensus was reached between the blinded graders (13). Thereby, category grades were established by averaging (>70% agreement) or taking consensus (<70% agreement) for each graded image.
To evaluate Col9a1-/- knee degeneration as previously described (14), a knee histological processing and grading scheme was employed (14, 22, 23). Knees (n=40, 2 per animal) were sectioned in the sagittal plane (∼8 μm). Sections from the medial and lateral cartilage load bearing regions were stained with Safranin O-fast green. A single section representing the most severe evidence of lesion formation was selected for each compartment. These images were randomized, and two blinded graders evaluated the tibial and femoral cartilage using a modified Mankin scheme (22, 24). Degeneration was scored in cartilage structure (ordinal rank range: 0-11), tidemark duplication (0-3), loss of Safranin O staining (0-8), fibrocartilage formation (0-2), chondrocyte cloning above the tidemark (0-2), presence of hypertrophic chondrocytes below the tidemark (0-2), and subchondral bone thickness (0-2). Lower rank indicates less evidence of degeneration; descriptions of the severity observed at each rank are described by Furman and coworkers (22). Scores for each image were averaged between graders to obtain a separate grade for the tibial and femoral cartilage in each joint's medial or lateral compartment.
Statistics
Continuous data were analyzed with full-factorial, two-factor analysis of variance (ANOVA) treating genotype and sex as factors, with the following exceptions: percentage stance time, stride frequency, stride length, rotarod latencies, and HA data. Percentage stance time, stride frequency, and stride length co-vary with velocity; these data were analyzed for deviations from expected values using a full-factorial generalized linear model (GLM) that incorporated a linear dependence on velocity. Similarly, rotarod data are dependent upon learning; these data were analyzed with a GLM that incorporated a linear dependence on trial number. HA data were not normally distributed; a log transformation was performed, with normality of the transformed data verified by a Kolmogorov-Smirnov test, prior to performing a full-factorial, two-factor ANOVA. Providing significance in an ANOVA or GLM, post-hoc Tukey's HSD tests were performed to detect inter-group differences. Ordinal data sets (contact righting and histological scores) were analyzed via full-factorial ordinal logistic regression, treating genotype and sex as factors, with post-hoc Kruskal-Wallis median test to detect inter-group differences for sex-genotype when appropriate.
Results
While males were larger than females, no significant difference in weight was observed in genotype (Table 1). With the exception of righting, Col9a1-/- reflexes were normal as mutant mice responded to light touch and maintained posture (data not shown). Righting delays were observed in Col9a1-/- mice (p<0.001), with delays observed in females and impairments observed in males. Col9a1-/- mice also had increased pole climbing up latency (p<0.001); latencies for climbing down or across were not significant. Col9a1-/- mice had decreased wire-hang latencies (p<0.001) and appeared to have poor coordination in gripping the wire with both the hind- and fore-limbs simultaneously. Finally, Col9a1-/- mice grasped the automated grip-strength meter with more force than WTs with both their fore- and hind-limbs (p<0.05).
Table 1. Neuromuscular and Sensory Data.
Data are presented as mean plus or minus standard deviation (n=5 for each sex-genotype) Statistical significance is delineated as follows: * p < 0.05, male vs. female, ** p < 0.05, Col9a1-/- vs. wildtype, and *** p < 0.001, Col9a1-/- vs. wildtype.
| Male | Female | |||
|---|---|---|---|---|
| Wildtype | Col9a1-/- | Wildtype | Col9a1-/- | |
| Weight (grams) | 33.4 ± 2.2* | 35.5 ± 4.6* | 28.4 ± 3.0 | 26.3 ± 1.3 |
| Righting (median score) | Normal | Impaired*** | Normal | Delay*** |
| Wire hang (sec) | 42.8 ± 4.9 | 11.4 ± 3.5*** | 37.9 ± 6.1 | 11.1 ± 3.7*** |
| Fore limb grip strength (grams-force) | 38.7 ± 2.6 | 51.2 ± 3.4** | 44.3 ± 3.4 | 50.5 ± 4.9** |
| Hind limb grip strength (grams-force) | 15.6 ± 1.6 | 23.9 ± 2.6** | 21.1 ± 0.9 | 25.8 ± 3.2** |
| Pole climbing down (sec) | 11.4 ± 2.3 | 11.8 ± 1.5 | 9.3 ± 0.9 | 13.4 ± 3.7 |
| Pole climbing up (sec) | 12.8 ± 2.7 | 26.1 ± 1.9*** | 9.7 ± 0.9 | 18.9 ± 5.3*** |
| Pole walking (sec) | 17.7 ± 3.9 | 19.6 ± 1.7 | 14.8 ± 3.6 | 16.0 ± 1.9 |
| 50% Withdrawal Threshold (Mechanical, grams-force) | 2.3 ± 1.3 | 1.0 ± 0.7** | 2.2 ± 1.5 | 1.7 ± 0.6** |
| Latency to Paw Flick (Thermal, sec) | 6.2 ± 1.0 | 6.5 ± 0.6 | 6.5 ± 0.2 | 6.9 ± 0.7 |
| Latency to Tail Flick (Themral, sec) | 4.3 ± 0.3 | 4.6 ± 0.5 | 4.7 ± 0.3 | 4.2 ± 0.7 |
Col9a1-/- mice had heightened sensitivity to mechanical stimuli, as demonstrated by a decreased 50% withdrawal threshold (p<0.05, Table 1). In particular, the threshold for male-Col9a1-/- mice was half that of male-WTs. No genotype differences were discerned in thermal sensitivity.
In sensorimotor assessments, Col9a1-/- mice under-performed WTs (Figure 1). Deficiencies were particularly notable in males, where rotarod latencies were shortened for male-Col9a1-/- mice relative to male-WTs in both the accelerating and constant-speed paradigms (p-values<0.01). Female-Col9a1-/- mice fell from the accelerating rotarod sooner than female-WTs (p<0.05), but not for constant-speed trials.
Figure 1. Col9a1-/- mice have deficient sensorimotor skills (rotarod).
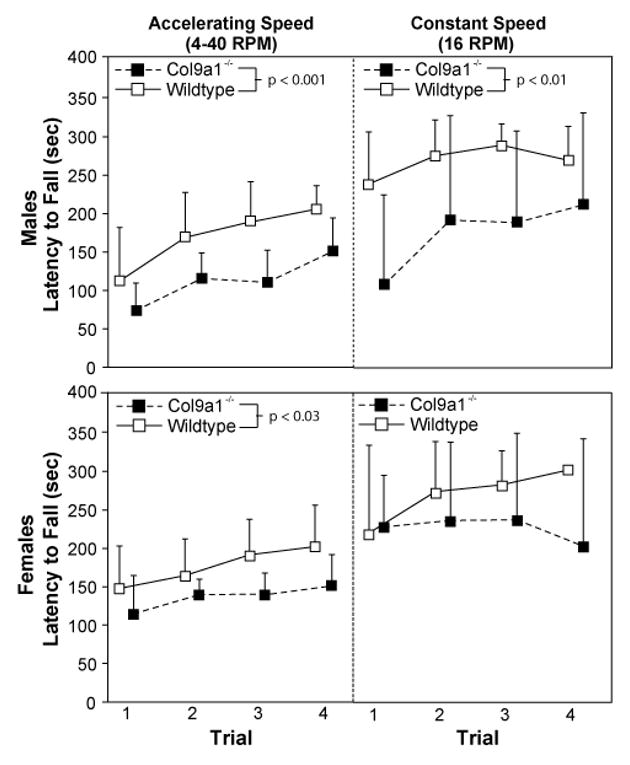
In rotarod tests, Col9a1-/- mice under-performed wildtype (WT) controls. In accelerating trials, Col9a1-/- mice fell from the rotarod sooner than their sex-matched WT controls (male-Col9a1-/- vs. male-WT: p<0.001; female-Col9a1-/- vs. female-WT: p<0.03). Although latencies to fall from the accelerating rotarod for female-Col9a1-/- mice were lower than for female-WTs, differences in constant-speed trials were only significant in males (male-Col9a1-/- vs. male-WT: p<0.01). Data are presented as mean plus standard deviation (n=5 for each sex-genotype); the female-WT constant-speed trial 4 does not have a standard deviation as all female-WTs remained on the rod for the 5 min limit.
In unprompted gait, Col9a1-/- mice locomoted at slower velocities than sex-matched WTs (p<0.001; Figure 2). Moreover, at a given velocity, Col9a1-/- mice used higher hind-limb percentage stance times (p<0.001). Col9a1-/- mice also differed in foot placement, using shorter stride lengths (p<0.001), wider hind-limb step widths (p<0.001), and narrower fore-limb step widths (p<0.01); these Col9a1-/--WT differences were largest in male mice. Due to velocity dependence, hind-limb percentage stance time and stride length are presented as deviations from expected values in Figure 2. Hind-limb percentage stance times ranged from 52-73% for WTs and 56-84% for Col9a1-/- mice, and stride lengths ranged from 4.4-8.0 cm for WTs and 3.8-7.2 cm for Col9a1-/- mice.
Figure 2. Col9a1-/- mice use an altered gait during unprompted trials.
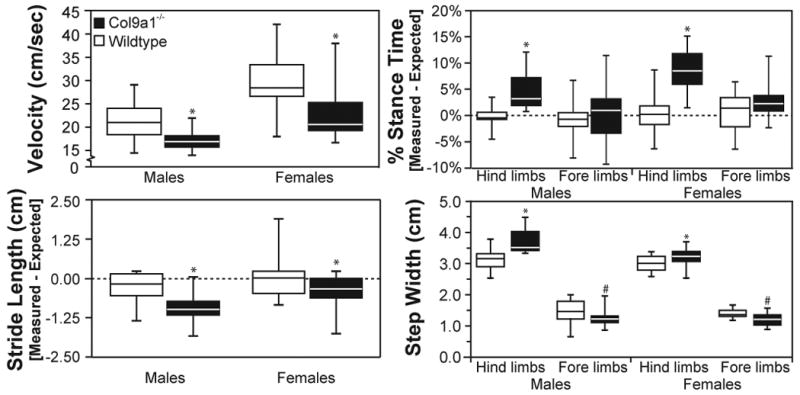
In gait trials where mice were freely exploring an open arena (unprompted trial), Col9a1-/- mice had statistically significant differences in velocity, hind-limb percentage stance time, stride length, and step widths. Both male and female-Col9a1-/- mice locomoted at slower speeds (* p<0.001) and used higher hind-limb percentage stance times (* p<0.001) than WT controls. Col9a1-/- mice also had differences in stride geometries, using shorter stride lengths (* p<0.001), wider hind-limb step widths (* p<0.001), and narrower fore-limb step widths (# p<0.01). Data are presented as quartile box blots of 14 male-WT, 13 male-Col9a1-/-, 15 female-WT, and 18 female-Col9a1-/- trials measured from 5 mice in each sex-genotype.
In prompted gait, velocity increased in all mice, and genotype differences were less apparent than those observed in unprompted trials (compare Figure 2 and 3). Significant differences were not observed in velocity (Figure 3); however, Col9a1-/- mice used higher hind-limb percentage stance times at a given velocity (p<0.05). Hind-limb percentage stance times decreased due to increased velocity, ranging from 39-64% for WTs and 42-71% for Col9a1-/- mice. Stride lengths increased due to increased velocity, ranging from 5.5-8.6 cm for WT and 5.8-8.6 cm for Col9a1-/- mice (non-significant). Male-Col9a1-/- mice also differed in fore-limb foot placement, using narrower fore-limb step widths (p<0.001). Genotype differences in stride frequency and fore-limb percentage stance times were not observed for unprompted or prompted trials; moreover, gaits were largely symmetric [data not shown].
Figure 3. Gait differences for Col9a1-/- mice exist in prompted trials, but are less extensive than in unprompted trials.
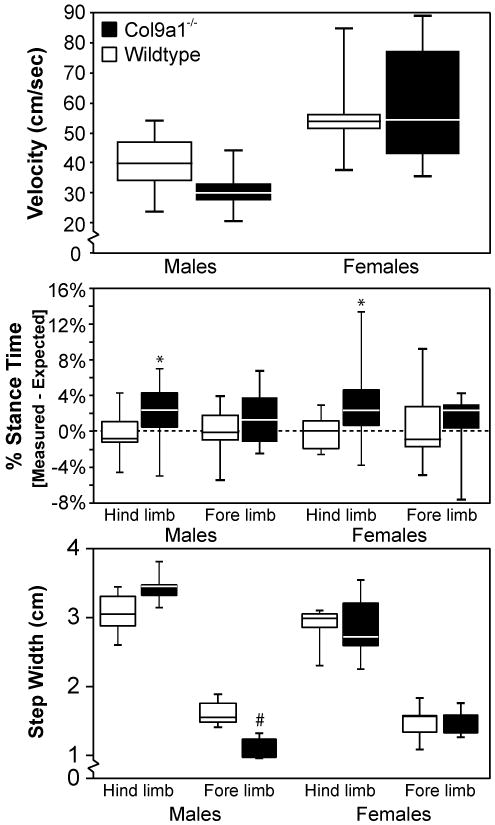
In prompted gait trials, mice were startled into movement by brushing their hind quarters with a swab. When prompting this movement, most gait differences between Col9a1-/- mice and wildtype (WT) controls diminished relative to those observed in unprompted trials (See Figure 2). For velocity, male-Col9a1-/- mice tended to locomote at slower velocities than male-WTs; however, these differences were not statistically significant. Col9a1-/- mice did use higher hind-limb percentage stance times (* p<0.02), and mutant males used narrower fore-limb step widths than WT controls (# p<0.001). When comparing these data with the unprompted results, differences in prompted trials were smaller in magnitude and less significant. Data are presented as quartile box blots of 11 male-WT, 9 male-Col9a1-/-, 9 female-WT, and 10 female-Col9a1-/- trials measured from 5 mice in each sex-genotype.
Serum HA differences were not observed (p=0.11). However, serum HA concentrations were greatest in male-Col9a1-/- mice (888±378 ng/mL, mean±SD), followed by male-WTs (510±105 ng/mL), female-WTs (665±426 ng/mL), and female-Col9a1-/- mice (605±130 ng/mL).
Col9a1-/- mice had more signs of IVD tears relative to WTs (p<0.001, Supplemental Table 1). Col9a1-/- discs graded as “not present (score=0, 8%),” “rare (score=1, 57%),” “moderate (score=2, 33%),” or “abundant (score=3, 2%)” for evidence of IVD tears, while WT discs graded as “not present (score=0, 33%),” “rare (score=1, 60%),” or “moderate (score=2, 7%).” In addition, female-Col9a1-/- mice had higher scores in IVD cell proliferation relative to female-WTs (p<0.05). Female-Col9a1-/- discs scored as “no evidence (score=0, 33%),” “increased cell density (score=1, 47%),” “connection of two cells (score=2, 13%),” or “small-sized clones (score=3, 7%),” while female-WT discs were “no evidence (score=0, 73%)” or “increased cell density (score=1, 27%)”. While significant, cell proliferation scores were relatively low on the grading scale (maximum score=6) (13, 21). Significant differences were not observed in the other categories. IVD histology is consistent with an in-depth report of spine degeneration in Col9a1-/- mice (13).
Knee scores indicate structural changes occurring through the cartilage depth (Figure 4); this observation was associated with a significant difference in medial compartment structure score between male-Col9a1-/- mice and all other groups (p<0.01, Table 2). The male-Col9a1-/- median cartilage structure score for medial-femoral and medial-tibial cartilage was “fibrillation or erosion extending through the tidemark (score=10)”. No other group's median cartilage structure score in the medial compartment rose above “superficial fibrillation, under half of surface (score=2).” Structural changes were less in the lateral compartment, but differences were significant between both male- and female-Col9a1-/- mice and their respective WTs (p<0.05). Col9a1-/- mice had a wide range of structural scores for lateral compartment cartilage, with median scores tending to be larger in femoral cartilage. Trends toward chondrocyte cloning in the non-calcified cartilage and increased incidence of hypertrophic chondrocytes below the tidemark existed (0.05<p≤0.10), but near-significance may be attributed to cartilage erosion (Supplemental Table 2). Knee histology is consistent with an in-depth report of knee degeneration in Col9a1-/- mice (14).
Figure 4. Representative Histology Images for the Knee Structural Scores Observed.
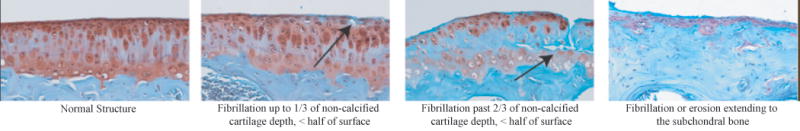
Representative images for the range of structural changes observed for the knee are shown for Safranin O-Fast Green stained sections. Levels of degeneration increase from left to right. Higher levels of structural changes associated with degeneration were more common in Col9a1-/- mice relative the WTs (See Table 2).
Table 2. Structural Changes in Knee Cartilage.
The frequency of observation for each ordinal rank subcategory is presented for the femoral-medial (FM), tibial-medial (TM), femoral-lateral (FL), and tibial-lateral (TL) cartilage. For knee grades, a single section, representing the most significant lesion for each surface-compartment, was graded for each mouse/knee. * indicates ranks are significantly higher relative to the sex-genotype control in the specific surface-compartment (p<0.05).
| Co19a1-/--Male | Wildtype-Male | Col9a1-/--Female | Wildtype-Female | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Changes in Knee Cartilage Structure (Ordinal Rank) | FM* | TM* | FL* | TL* | FM | TM | FL | TL | FM | TM | FL* | TL* | FM | TM | FL | TL |
| Normal (0) | - | - | 1 | 1 | - | 3 | 1 | 2 | 2 | 5 | 1 | 2 | - | 1 | - | 2 |
| Undulating surface (1) | 1 | 3 | 1 | 5 | 7 | 2 | 5 | 1 | 7 | 3 | 2 | 4 | 3 | 5 | 4 | 4 |
| Surface fibrillation, < half of surface (2) | 1 | - | 1 | 1 | 2 | 1 | - | 4 | 1 | 2 | - | - | 4 | 2 | 3 | 2 |
| Surface fibrillation, > half of surface (3) | - | - | 1 | 1 | - | 1 | 2 | 1 | - | - | 3 | - | - | 1 | 1 | 1 |
| Fibrillation up to 1/3 of non-calcified cartilage depth, < half of surface (4) | - | - | 2 | - | 1 | 2 | 1 | 1 | - | - | 2 | - | 2 | 1 | 1 | 1 |
| Fibrillation up to 1/3 of non-calcified cartilage depth, > half of surface (5) | - | - | - | - | - | 1 | - | - | - | - | - | - | - | - | 1 | - |
| Fibrillation up to 2/3 of non-calcified cartilage depth, < half of surface (6) | - | - | 1 | - | - | - | 1 | - | - | - | 1 | - | 1 | - | - | - |
| Fibrillation up to 2/3 of non-calcified cartilage depth, > half of surface (7) | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | - |
| Fibrillation past 2/3 of non-calcified cartilage depth, < half of surface (8) | 1 | 1 | 2 | - | - | - | - | 1 | - | - | 1 | 1 | - | - | - | - |
| Fibrillation past 2/3 of non-calcified cartilage depth, > half of surface (9) | - | - | - | - | - | - | - | - | - | - | - | 2 | - | - | - | - |
| Fibrillation or erosion extending through the tidemark (10) | 2 | 3 | 1 | 1 | - | - | - | - | - | - | - | 1 | - | - | - | - |
| Fibrillation or erosion extending to the subchondral bone (11) | 5 | 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
Discussion
Our goal was to evaluate Col9a1-/- mice for an array of functional and symptomatic measures that may be characteristic of OA and DDD. This study's data clearly identify behavioral characteristics of pain and functional loss in Col9a1-/- mice. Col9a1-/- mice have delayed righting, decreased sensorimotor skills, and altered gait. These effects occur in concert with increased mechanical, but not necessarily thermal, sensitivity. Moreover, cartilage degeneration was observed for Col9a1-/- mice, as mutant mice had elevated levels of knee and IVD structural changes. Some or all of these functional and symptomatic differences may be due to OA- and DDD-like pathologies in these same animals.
Severe cartilage degeneration is known to occur in Col9a1-/- mice at 9-11 months. We selected this age because we anticipated behaviors associating with OA and DDD would be large at this age. It is not yet known how behavioral changes correlate to developing pathology; however, this study provides a basis from which these parameters can be selected for longitudinal studies.
Quantification of gait in Col9a1-/- mice is reported here for the first time. Col9a1-/- mice presented with gaits characteristic of compensatory changes to reduce peak joint forces. Col9a1-/- mice selected slower velocities in unprompted trials; and at these speeds, Col9a1-/- mice used higher hind-limb percentage stance times, shorter stride lengths, and different step widths. Since stride frequencies were similar between Col9a1-/- and WT mice, the higher hind-limb percentage stance times observed were due to increased stance times. Bilateral increases in hind-limb stance time reduce the periods where a single hind-limb must support weight alone and represent both a relative and absolute increase in the time available to generate force. While force was not directly measured in this study, higher bilateral hind-limb percentage stance times do tend to correspond with lower peak ground reaction forces for symmetric gaits (25, 26). Similarly, slower velocities and shorter stride lengths correspond with decreased peak forces (27-29). While the mechanics of quadruped and biped gaits differ substantially, percentage stance time increases, slower velocities, shorter stride lengths, and increased step widths have also been observed in human OA subjects (30-32). Combined, these data indicate that gait can measure the functional consequences of OA in both quadruped animal models and in the clinic.
Some of the observed Col9a1-/--WT gait differences were attenuated upon prompting locomotion. As expected, velocities in prompted trials were higher than unprompted trials, but differences in velocity and other gait abnormalities between Col9a1-/- mice and WTs were either lost or reduced in magnitude. These data indicate that, when stressed, Col9a1-/- mice can achieve velocities and gaits more comparable to WTs; however, when voluntarily exploring, the Col9a1-/- gaits show substantial differences from WTs. As such, our gait data likely describe locomotion compensation, not functional inability.
Altered neuromuscular skills were also observed for Col9a1-/- mice. The pattern of changes in sex-genotype are similar in neuromuscular tests to that of gait. Male-Col9a1-/- mice presented with the greatest impairments in contact righting, quickest latencies in accelerating and constant-speed rotarod tests, largest wire-hang differences, and increased latencies in climbing up a pole. Some similar effects were observed for female-Col9a1-/- mice compared to female-WTs, although the significance and magnitude were less than that of males. Since pole climbing, wire-hang, and rotarod tests are strenuous tasks with a likelihood of generating large joint loads, these tasks may amplify the effects of joint-loading induced pain in Col9a1-/- mice.
Wire-hang times, but not grip-strength forces, were higher for Col9a1-/- mice. Combined, these data do not necessarily indicate a Col9a1-/- strength deficiency. Experimental observations from the wire-hang tests noted Col9a1-/- mice had difficulty coordinating the hind- and fore-limb pairs simultaneously -- a coordination behavior that was not required in the grip-strength test. This coordination deficiency is likely a contributor to the apparent conflict in strength data; however, muscle atrophy resultant from decreased activity and contributions of hand, elbow, foot, and ankle degeneration can not be definitely ruled out in this study.
Mechanical allodynia has been observed in models of knee pathology (33-36), facet joint pathology (37), and radiculopathy and nerve root constriction (38, 39). These studies (conducted in the rat) induce disease characteristics through chemical or surgical insults. Thus, pathology occurs acutely with post-procedural inflammation, but offers the advantage of pre-disorder and contralateral comparisons. For Col9a1-/- mice, changes occur spontaneously and progress over months, and thus, pre-disorder controls are biased by age. WTs do offer a sham-like control, but it remains challenging to discern whether heightened mechanical sensitivity for the Col9a1-/- mice, or even gait and neuromuscular deficits, are driven by knee degeneration, spine degeneration, synergistic combinations from multiple pathologies, or the type IX collagen knockout itself. Nonetheless, Col9a1-/- mice did present with significant signs of mechanical allodynia, which have been similarly described in other knee and spine pathology models.
While heightened mechanical sensitivity was observed, changes in thermal sensitivity were not. Currently, it is not known how joint nociceptors are affected by degenerative changes that occur in the Col9a1-/- model. Aδ fibers, which conduct sharp pain information, may be sensitized by local changes in pressure and mechanics associated with joint degeneration. Conversely, C fibers may be relatively unaffected due to the lack of a chemical insult and inflammatory response in this non-inflammatory model. Continued investigations are necessary to explore these relationships.
As with many genetic models of human pathology, the effects of gene inactivation are not confined to a single anatomical area; as such, the behavioral phenotype observed may result from ubiquitous cartilage degeneration or from other effects of a type IX collagen deficiency (40, 41). In this manner, the broad profile of joint pathology observed in this model differs from that observed in the human, in which a single anatomical site or IVD level may exhibit pathology. As multiple factors are known or purported contributors to OA - including loading history, genetics, inflammatory factors, or obesity - the recognized utility of the Col9a1-/- mouse model is in the study of genetic background as a contributor to arthritis amongst other joint pathologies. It should be recognized, however, that defects in type IX collagen have been widely linked to the premature onset of IVD pathology (2), although not OA, such that this mouse gene mutation model may be of particular relevance to human disease.
Of the other known effects of type IX collagen deletion, inner ear malformations in the Organ of the Corti (40, 41) may affect some of the parameters measured. If the inner ear is affected by the genetic alteration, animal balance may also be affected with unknown contributions to the observed functional deficiencies, particularly decreased sensorimotor skills and altered gait. In protocol development for these same animals, startle was observed in all Col9a1-/- and WT mice to a 100 dB pulse (data not shown). While this test insures that subjects were not deaf, it does not verify normal inner ear structure or that balance and coordination were unaffected by an inner ear malformation. These possibilities further underscore the challenge of separating behavioral effects in ubiquitous knockout models where several pathologies may occur.
Like behavioral assessments, serum HA data are advantageous in that they may be tracked longitudinally in the same research animal. However, serum HA was not statistically significant in this model, despite trends in the predicted direction. Thus, while serum HA provided additional information, behavioral parameters were more likely to detect differences in Col9a1-/- mice.
We observed consistent and large sex-associated differences in knee degeneration. The reasons for the greater OA severity in male-Col9a1-/- are unknown, but prior work has detailed cartilage degeneration occurs at a higher incidence in male mice relative to females in both spontaneous and induced models (42-47). These sex-associated differences in mice are in contrast to that of human epidemiology and other OA animal models (46). Carlsen and coworkers (48) found male-Col9a1-/- mice crossed against a DBA/1 background had more severe “stress induced arthritis” than DBA/1-WTs; “stress induced arthritis” is not observed in DBA/1 females, castrated males, or males housed individually (49). It should be noted, however, that pathologic changes described in Carlsen and coworkers (48) are evidence of joint swelling, and the DBA/1 model varies substantially from the histological changes associated with a non-inflammatory joint pathology described herein and in prior publications (12, 14).
The results of this study present new evidence for a detectable behavioral phenotype in Col9a1-/- mice which includes functional impairment and increased mechanical sensitivity. Many of these detected differences are coincident with cartilage degeneration; in the current study, deficiencies in rotarod, pole climbing, and gait parameters were largest in male-Col9a1-/- mice, which also had the highest degree of knee OA and serum HA. In females, where histological and serum HA differences between WT and Col9a1-/- mice were lower in magnitude, lesser differences in gait and other functional parameters were observed. The detected differences appear to point toward protective behaviors in Col9a1-/- mice, suggesting that Col9a1-/- mice choose locomotion patterns that limit peak joint forces and behaviors that reduce induced pain sensation. In future work, these measures may help track signs and symptoms as degeneration progresses and serve useful for evaluating the efficacy of therapeutic interventions for musculoskeletal disorders.
Supplementary Material
The frequency of observation for each ordinal rank subcategory is presented. Three sections of two lumbar discs were graded for each mouse (n=30, 2 lumbar disc × 3 sections × 5 animals per sex-genotype). * indicates ranks are significantly higher relative to the sex-genotype control (p<0.05).
The frequency of observation for each ordinal rank subcategory is presented for the femoral-medial (FM), tibial-medial (TM), femoral-lateral (FL), and tibial-lateral (TL) cartilage. For knee grades, a single section, representing the most significant lesion for each surface-compartment, was graded for each mouse/knee (n=10, 2 knees per animal).
Acknowledgments
Thank you to B. R. Olsen and Y. Li for sharing the Col9a1-/- model, A. Blount and H. A. Leddy for histology assistance, R. W. Nightingale for video equipment, and S. Johnson and L. Jing for veterinary care and genotyping. This publication was made possible by NIH support AR047442, AR050245, AR051672, T32EB001630 (KD Allen), and F32AR056190 (KD Allen), and by Arthritis Foundation support (H. I. Duggan Investigator Award, TM Griffin).
References
- 1.The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal, and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. [Google Scholar]
- 2.Battie MC, Videman T, Parent E. Lumbar disc degeneration: epidemiology and genetic influences. Spine. 2004;29(23):2679–90. doi: 10.1097/01.brs.0000146457.83240.eb. [DOI] [PubMed] [Google Scholar]
- 3.Bierma-Zeinstra SM, Koes BW. Risk factors and prognostic factors of hip and knee osteoarthritis. Nat Clin Pract Rheumatol. 2007;3(2):78–85. doi: 10.1038/ncprheum0423. [DOI] [PubMed] [Google Scholar]
- 4.Reginato AM, Olsen BR. The role of structural genes in the pathogenesis of osteoarthritic disorders. Arthritis Res. 2002;4(6):337–45. doi: 10.1186/ar595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alizadeh BZ, Njajou OT, Bijkerk C, Meulenbelt I, Wildt SC, Hofman A, et al. Evidence for a role of the genomic region of the gene encoding for the alpha1 chain of type IX collagen (COL9A1) in hip osteoarthritis: A population-based study. Arthritis Rheum. 2005;52(5):1437–42. doi: 10.1002/art.21020. [DOI] [PubMed] [Google Scholar]
- 6.Ikeda T, Mabuchi A, Fukuda A, Kawakami A, Ryo Y, Yamamoto S, et al. Association analysis of single nucleotide polymorphisms in cartilage-specific collagen genes with knee and hip osteoarthritis in the Japanese population. J Bone Miner Res. 2002;17(7):1290–6. doi: 10.1359/jbmr.2002.17.7.1290. [DOI] [PubMed] [Google Scholar]
- 7.Paassilta P, Lohiniva J, Goring HH, Perala M, Raina SS, Karppinen J, et al. Identification of a novel common genetic risk factor for lumbar disk disease. Jama. 2001;285(14):1843–9. doi: 10.1001/jama.285.14.1843. [DOI] [PubMed] [Google Scholar]
- 8.Jim JJ, Noponen-Hietala N, Cheung KM, Ott J, Karppinen J, Sahraravand A, et al. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine. 2005;30(24):2735–42. doi: 10.1097/01.brs.0000190828.85331.ef. [DOI] [PubMed] [Google Scholar]
- 9.Higashino K, Matsui Y, Yagi S, Takata Y, Goto T, Sakai T, et al. The alpha2 type IX collagen tryptophan polymorphism is associated with the severity of disc degeneration in younger patients with herniated nucleus pulposus of the lumbar spine. Int Orthop. 2007;31(1):107–11. doi: 10.1007/s00264-006-0117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seki S, Kawaguchi Y, Mori M, Mio F, Chiba K, Mikami Y, et al. Association study of COL9A2 with lumbar disc disease in the Japanese population. J Hum Genet. 2006;51(12):1063–7. doi: 10.1007/s10038-006-0062-9. [DOI] [PubMed] [Google Scholar]
- 11.Eyre DR. Collagens and cartilage matrix homeostasis. Clin Orthop Relat Res. 2004;(427 Suppl):S118–22. doi: 10.1097/01.blo.0000144855.48640.b9. [DOI] [PubMed] [Google Scholar]
- 12.Fassler R, Schnegelsberg PN, Dausman J, Shinya T, Muragaki Y, McCarthy MT, et al. Mice lacking alpha 1 (IX) collagen develop noninflammatory degenerative joint disease. Proc Natl Acad Sci U S A. 1994;91(11):5070–4. doi: 10.1073/pnas.91.11.5070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyd LM, Richardson WJ, Allen KD, Flahiff C, Jing L, Li Y, et al. Early-onset degeneration of the intervertebral disc and vertebral end plate in mice deficient in type IX collagen. Arthritis Rheum. 2008;58(1):164–71. doi: 10.1002/art.23231. [DOI] [PubMed] [Google Scholar]
- 14.Hu K, Xu L, Cao L, Flahiff CM, Brussiau J, Ho K, et al. Pathogenesis of osteoarthritis-like changes in the joints of mice deficient in type IX collagen. Arthritis Rheum. 2006;54(9):2891–900. doi: 10.1002/art.22040. [DOI] [PubMed] [Google Scholar]
- 15.Kraus VB. Biomarkers in osteoarthritis. Curr Opin Rheumatol. 2005;17(5):641–6. doi: 10.1097/01.bor.0000174195.15421.17. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguiz RM, Wetsel WC. Assessments of Cognitive Deficits in Mutant Mice. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. Boca Raton, FL: CRC Press; 2006. pp. 223–82. [PubMed] [Google Scholar]
- 17.Hedrick T, Reinschmidt C. DLTdataviewer2, Custom MATLAB software. http://www.unc.edu/∼thedrick/software1.html.
- 18.Fuchs PN, Roza C, Sora I, Uhl G, Raja SN. Characterization of mechanical withdrawal responses and effects of mu-, delta- and kappa-opioid agonists in normal and mu-opioid receptor knockout mice. Brain Res. 1999;821(2):480–6. doi: 10.1016/s0006-8993(99)01060-4. [DOI] [PubMed] [Google Scholar]
- 19.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52(1):105–11. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 20.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis following intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum. 2008;58(3):744–53. doi: 10.1002/art.23288. [DOI] [PubMed] [Google Scholar]
- 21.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27(23):2631–44. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25(5):578–92. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 23.Xu L, Flahiff CM, Waldman BA, Wu D, Olsen BR, Setton LA, et al. Osteoarthritis-like changes and decreased mechanical function of articular cartilage in the joints of mice with the chondrodysplasia gene (cho) Arthritis Rheum. 2003;48(9):2509–18. doi: 10.1002/art.11233. [DOI] [PubMed] [Google Scholar]
- 24.Carlson CS, Guilak F, Vail TP, Gardin JF, Kraus VB. Synovial fluid biomarker levels predict articular cartilage damage following complete medial meniscectomy in the canine knee. J Orthop Res. 2002;20(1):92–100. doi: 10.1016/S0736-0266(01)00066-3. [DOI] [PubMed] [Google Scholar]
- 25.Witte TH, Knill K, Wilson AM. Determination of peak vertical ground reaction force from duty factor in the horse (Equus caballus) J Exp Biol. 2004;207(Pt 21):3639–48. doi: 10.1242/jeb.01182. [DOI] [PubMed] [Google Scholar]
- 26.Alexander RM. Principles of Animal Locomotion. Princeton, NJ: Princeton University Press; 2003. [Google Scholar]
- 27.Jordan K, Challis JH, Newell KM. Walking speed influences on gait cycle variability. Gait Posture. 2007;26(1):128–34. doi: 10.1016/j.gaitpost.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Khumsap S, Clayton HM, Lanovaz JL, Bouchey M. Effect of walking velocity on forelimb kinematics and kinetics. Equine Vet J Suppl. 2002;(34):325–9. doi: 10.1111/j.2042-3306.2002.tb05441.x. [DOI] [PubMed] [Google Scholar]
- 29.Derrick TR, Hamill J, Caldwell GE. Energy absorption of impacts during running at various stride lengths. Med Sci Sports Exerc. 1998;30(1):128–35. doi: 10.1097/00005768-199801000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Chen CP, Chen MJ, Pei YC, Lew HL, Wong PY, Tang SF. Sagittal plane loading response during gait in different age groups and in people with knee osteoarthritis. Am J Phys Med Rehabil. 2003;82(4):307–12. doi: 10.1097/01.PHM.0000056987.33630.56. [DOI] [PubMed] [Google Scholar]
- 31.Cheing GL, Hui-Chan CW. The motor dysfunction of patients with knee osteoarthritis in a Chinese population. Arthritis Rheum. 2001;45(1):62–8. doi: 10.1002/1529-0131(200102)45:1<62::AID-ANR85>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 32.Lee TH, Tsuchida T, Kitahara H, Moriya H. Gait analysis before and after unilateral total knee arthroplasty. Study using a linear regression model of normal controls -- women without arthropathy. J Orthop Sci. 1999;4(1):13–21. doi: 10.1007/s007760050068. [DOI] [PubMed] [Google Scholar]
- 33.Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene-related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett. 2005;388(2):75–80. doi: 10.1016/j.neulet.2005.06.044. [DOI] [PubMed] [Google Scholar]
- 34.Bove SE, Laemont KD, Brooker RM, Osborn MN, Sanchez BM, Guzman RE, et al. Surgically induced osteoarthritis in the rat results in the development of both osteoarthritis-like joint pain and secondary hyperalgesia. Osteoarthritis Cartilage. 2006;14(10):1041–8. doi: 10.1016/j.joca.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 35.McDougall JJ, Watkins L, Li Z. Vasoactive intestinal peptide (VIP) is a modulator of joint pain in a rat model of osteoarthritis. Pain. 2006;123(1-2):98–105. doi: 10.1016/j.pain.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Beyreuther B, Callizot N, Stohr T. Antinociceptive efficacy of lacosamide in the monosodium iodoacetate rat model for osteoarthritis pain. Arthritis Res Ther. 2007;9(1):R14. doi: 10.1186/ar2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tachihara H, Kikuchi S, Konno S, Sekiguchi M. Does facet joint inflammation induce radiculopathy?: an investigation using a rat model of lumbar facet joint inflammation. Spine. 2007;32(4):406–12. doi: 10.1097/01.brs.0000255094.08805.2f. [DOI] [PubMed] [Google Scholar]
- 38.Winkelstein BA, Weinstein JN, DeLeo JA. The role of mechanical deformation in lumbar radiculopathy: an in vivo model. Spine. 2002;27(1):27–33. doi: 10.1097/00007632-200201010-00009. [DOI] [PubMed] [Google Scholar]
- 39.Murata Y, Onda A, Rydevik B, Takahashi I, Takahashi K, Olmarker K. Changes in pain behavior and histologic changes caused by application of tumor necrosis factor-alpha to the dorsal root ganglion in rats. Spine. 2006;31(5):530–5. doi: 10.1097/01.brs.0000201260.10082.23. [DOI] [PubMed] [Google Scholar]
- 40.Asamura K, Abe S, Imamura Y, Aszodi A, Suzuki N, Hashimoto S, et al. Type IX collagen is crucial for normal hearing. Neuroscience. 2005;132(2):493–500. doi: 10.1016/j.neuroscience.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki N, Asamura K, Kikuchi Y, Takumi Y, Abe S, Imamura Y, et al. Type IX collagen knock-out mouse shows progressive hearing loss. Neurosci Res. 2005;51(3):293–8. doi: 10.1016/j.neures.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Silberberg M, Silberberg R. Role of sex hormone in the pathogenesis of osteoarthrosis of mice. Lab Invest. 1963;12:285–9. [PubMed] [Google Scholar]
- 43.Sokoloff L. Natural history of degenerative joint disease in small laboratory animals. I. Pathological anatomy of degenerative joint disease in mice. AMA Arch Pathol. 1956;62(2):118–28. [PubMed] [Google Scholar]
- 44.Ma HL, Blanchet TJ, Peluso D, Hopkins B, Morris EA, Glasson SS. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007;15(6):695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 45.van Osch GJ, van der Kraan PM, Vitters EL, Blankevoort L, van den Berg WB. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage. 1993;1(3):171–7. doi: 10.1016/s1063-4584(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 46.Sniekers YH, Weinans H, Bierma-Zeinstra SM, van Leeuwen JP, van Osch GJ. Animal models for osteoarthritis: the effect of ovariectomy and estrogen treatment - a systematic approach. Osteoarthritis Cartilage. 2008;16(5):533–41. doi: 10.1016/j.joca.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 47.Walton M. Degenerative joint disease in the mouse knee; histological observations. J Pathol. 1977;123(2):109–22. doi: 10.1002/path.1711230207. [DOI] [PubMed] [Google Scholar]
- 48.Carlsen S, Nandakumar KS, Holmdahl R. Type IX collagen deficiency enhances the binding of cartilage-specific antibodies and arthritis severity. Arthritis Res Ther. 2006;8(4):R102. doi: 10.1186/ar1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Holmdahl R, Jansson L, Andersson M, Jonsson R. Genetic, hormonal and behavioural influence on spontaneously developing arthritis in normal mice. Clin Exp Immunol. 1992;88(3):467–72. doi: 10.1111/j.1365-2249.1992.tb06473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The frequency of observation for each ordinal rank subcategory is presented. Three sections of two lumbar discs were graded for each mouse (n=30, 2 lumbar disc × 3 sections × 5 animals per sex-genotype). * indicates ranks are significantly higher relative to the sex-genotype control (p<0.05).
The frequency of observation for each ordinal rank subcategory is presented for the femoral-medial (FM), tibial-medial (TM), femoral-lateral (FL), and tibial-lateral (TL) cartilage. For knee grades, a single section, representing the most significant lesion for each surface-compartment, was graded for each mouse/knee (n=10, 2 knees per animal).


