Abstract
Purpose of review
To draw attention to recent work on the role of protein and the amount of protein needed with each meal to preserve skeletal muscle mass in ageing.
Recent findings
Ageing does not inevitably reduce the anabolic response to a high-quality protein meal. Ingestion of approximately 25–30g of protein per meal maximally stimulates muscle protein synthesis in both young and older individuals. However, muscle protein synthesis is blunted in elderly when protein and carbohydrate are coingested or when the quantity of protein is less than approximately 20g per meal. Supplementing regular mixed-nutrient meals with leucine may also enhance the muscle protein synthetic response in elders.
Summary
On the basis of recent work, we propose a novel and specific dietary approach to prevent or slow-muscle loss with ageing. Rather than recommending a large, global increase in the recommended dietary allowance (RDA) for protein for all elderly individuals, clinicians should stress the importance of ingesting a sufficient amount of protein with each meal. To maximize muscle protein synthesis while being cognizant of total energy intake, we propose a dietary plan that includes 25–30g of high quality protein per meal.
Keywords: aging, leucine, muscle protein synthesis, protein intake, sarcopenia
Introduction
Sarcopenia is a progressive, insidious process characterized by 3–8% reduction in lean muscle mass per decade after the age of 30 years. It is thought to affect 30% of individuals over 60 years of age and more than 50% of those over 80 years [1]. Research continues to focus on the mechanisms contributing to sarcopenia. This has included studies focusing on protein metabolism and cell signaling, voluntary or imposed reductions in physical activity, protein energy malnutrition and reduced anabolic efficiency to protein ingestion [2–4,5•]. Although protein synthesis and ultimately skeletal muscle mass are regulated by a host of factors, the fundamental prerequisite for muscle protein synthesis is dietary derived amino acids. The purpose of this review is to highlight recent research examining the role of protein in the prevention or management of sarcopenia and provide recommendations on the amount of protein needed with each meal to preserve skeletal muscle mass in ageing.
Ageing and the skeletal muscle protein synthesis response to feeding
Several studies have examined the acute and chronic effects of protein ingestion/supplementation on muscle protein anabolism in elders and the results have been well documented [4,5•,6–8,9••,10]. The majority of studies suggest that a moderate-to-large serving of protein or amino acids increases muscle protein synthesis similarly in both young and elderly [6,7,11–17]. Conversely, one recent study with a nice mix of signaling and metabolic measures but limited cohort (n = 4 per group) reported that 10 and 20g servings of essential amino acids were unable to stimulate muscle protein synthesis in older individuals to the same level as noted in the young [2].
Historically, the majority of protein metabolism studies have focused on the anabolic response to ingestion of protein or amino acids alone. However, when protein and carbohydrate are coingested, elderly respond with a diminished anabolic response compared with their younger counterparts. Volpi et al., [18] demonstrated that after ingestion of an amino acid glucose mixture, muscle protein synthesis increased in the young, but remained unchanged in the elderly. A subsequent study confirmed these results [19]. Although there is no evidence that coingestion of protein and fat negatively or differentially effects protein anabolism in young or elderly [9••,20], it appears that ageing may be associated with reduced anabolic efficiency in response to a carbohydrate-containing mixed nutrient meal. Fortunately, a modest bout of physical activity may be able to sensitize ageing muscle to subsequent nutritional stimuli. We have recently shown that 45min of treadmill walking in older individuals, the evening prior to an hyperinsulinemic–euglycemic clamp, restores the ability of insulin to stimulate muscle protein synthesis [4]. More recently, we demonstrated that ingestion of leucine-enriched essential amino acids stimulates muscle protein synthesis to a similar extent in young and elderly (although the response was delayed in the elderly) during the first 6 hours following exercise [21]. These data provide further supporting evidence that regular physical activity plays an important role in restoring or maintaining the normal protein anabolic response in the skeletal muscle of older individuals.
As mentioned, numerous studies support the notion that ingestion of a sufficient amount of amino acids or protein results in a similar increase in muscle protein synthesis in young and elderly [7,9••,22]. However, several recent studies have adopted a more practical approach and sought to examine the ability of protein-rich foods (e.g., milk) to stimulate protein anabolism. Although many of these studies have focused on a younger population [23,24], they are important as they more closely reflect responses to actual dietary practices and hence provide information on how meal choices may influence accrual of muscle mass and ultimately functional capacity. In one study directly comparing young and elderly, Symons et al. [9••] reported that a moderate 113g serving of an intact protein (that is, lean beef) contains sufficient amino acids (30g total; ~12g essential amino acids) to increase mixed muscle protein synthesis by 50% in both young and elderly men and women.
Should protein intake be increased in the elderly?
The adequacy of the recommended dietary allowance (RDA) for protein has been the subject of renewed debate over the past 12 months [25–30]. The current recommendation for protein intake for all men and women aged 19 years and older is 0.8g.kg−1.d−1. The recommendations were established by the Institute of Medicine and based on short-duration nitrogen balance studies in young adults [31,32]. Much of the recent commentary has argued that the current RDA for protein, while fulfilling the criteria as the ‘minimal daily average dietary intake level that meets the nutritional requirements of nearly all healthy individuals’, does not promote optimal health or protect elders from sarcopenic muscle loss [27,33,34,35••]. Although there is no consensus on the degree to which dietary protein needs change with advancing age, there is general agreement that additional longer term trials with defined health outcomes are needed to firmly establish the optimal protein intake range for various population groups [35••]. Although absolutely necessary, this process is certainly complicated by the host of mechanisms that contributing to sarcopenia. In the following sections, we argue that whereas a modest increase in protein intake beyond 0.8g.kg−1.d−1 may indeed be beneficial for some elders, there is a greater need to specifically examine the dose and distribution of protein across each meal.
An adequate amount of protein intake with each meal is essential
Although specialized supplementation with amino acid products may be beneficial or necessary in specific elderly or clinical populations [36,37], the most practical means of promoting skeletal muscle protein anabolism for the majority of older adults is to include a moderate serving of protein of high biological value during each meal. For example, an often-neglected aspect of daily protein intake that directly impacts net protein balance is the distribution of protein across three or more daily meals. For a 75 kg individual, the RDA represents 60g protein/day, or if distributed evenly across three meals, 20 g protein/meal. A 20 g serving of most animal or plant-based proteins contains 5–8 g of essential amino acids, which are primarily responsible for stimulating muscle protein synthesis [15]. This is an important issue because it has been recently shown that ageing is associated with an inability of skeletal muscle to respond to low doses (~7.5 g) of essential amino acids whereas higher doses (10–15 g) are capable of stimulating muscle protein synthesis to a similar extent as the young [7,38].
To examine the effect of protein dose on muscle protein anabolism using a protein-rich food, we recently demonstrated that ingestion of a large single 340 g serving of lean beef (90 g protein) in a cohort of healthy young and elderly individuals does not elicit a greater anabolic response than a serving one-third the size (Symons et al. [9], in review). Although this response may be influenced by physical activity (habitual and acute) and body size (lean muscle mass), the data suggests that, despite the additional protein and energy content, ingestion of more than 30 g of protein in a single meal may be an energetically inefficient means of stimulating muscle protein synthesis. Further, a recent study in healthy elders has shown that a large increase in the overall daily protein intake for 10 days did not improve protein anabolism and concerns were expressed over changes in glomerular filtration rate (GFR) [10].
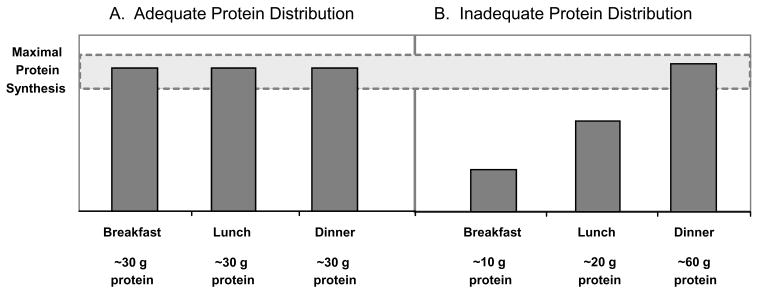
If we accept that 25–30 g of high quality protein (~10 g EAA) is necessary to maximally stimulate skeletal muscle protein synthesis, then it seems reasonable to suggest that ingestion of this amount of high-quality protein at each meal could be a useful strategy to maintain muscle mass in the elderly. In Fig. 1 Fig. 1, we provide a pictorial example of the proposed relationship between the amount of protein ingested per meal and the resultant anabolic response. The influence of factors such as physical activity notwithstanding, it is clear that the distribution of protein depicted in Fig. 1a, would result in a greater 24 h net protein balance than the protein distribution pattern depicted in Fig. 1b.
Figure 1. A pictorial example of the proposed relationship between the amount of protein ingested per meal and the resultant anabolic response gr1.
(a) Ingestion of 90 g of protein, distributed evenly over 3 meals. (b) Ingestion of 90 g of proteins unevenly distributed throughout the day. Stimulating muscle protein synthesis to a maximal extent during the meals shown in Figure 1A is more likely to provide a greater 24 h protein anabolic response than an unequal protein distribution.
Leucine supplementation to prevent sarcopenia
The rationale for the use of a nutritional supplement to slow or prevent sarcopenic muscle loss is based on the assumption that it will improve net muscle protein synthesis above that afforded by regular meals alone. In addition to this direct anabolic effect, the additional energy/nutrient content of a supplement should not interfere with the normal anabolic response to protein consumed as part of daily meals [39]. Many attempts have been made to combat muscle and strength loss in elderly via protein or amino acid supplementation see review by Milne et al., [40]. In some cases, protein–energy supplementation has been shown to be effective. However, other trials, particularly those involving acutely ill or frail patient populations, have been unsuccessful (11–13). In some instances protein supplementation increases satiety and simply replaces voluntary ingestion of regular menu items [39,41]. For others, cost, availability or medical complications such as dysphagia or impaired kidney function may limit the applicability or efficacy of protein supplementation regimens. In such instances, maximizing protein anabolism whereas minimizing the amount or volume of supplementation ingested would be desirable.
Leucine is an insulin secretagogue with well described effects on translation initiation and muscle protein synthesis [22,42–44]. Notably, leucine is a potent activator of the mammalian target of rapamycin (mTOR) nutrient and energy-sensing signaling pathway, in skeletal muscle. Increased insulin availability increases muscle protein synthesis by enhancing phosphorylation of Akt/PKB (an upstream regulator of mTOR), whereas increased leucine availability promotes the phosphorylation and activation of the downstream effectors of mTOR, 4E-BP1 and S6K1 [3,21]. With increasing age, muscle may become resistant to the stimulatory effects of normal postprandial concentrations of leucine [42]. Although such a deficit could contribute to reduced muscle protein anabolism and a loss of muscle mass, recent acute studies in both animals [42,45,46] and humans [47–49] suggest that the addition of supplemental leucine to normal mixed nutrient meals may improve or normalize muscle protein synthesis in aging muscle. Specifically, Rieu et al. [46] reported that in older rats, muscle protein synthesis following a normal mixed nutrient meal, was blunted compared with younger animals. However, the addition of supplemental leucine to the meal restored muscle protein synthesis to youthful levels in the older rats [46]. In a follow-up study in older humans, the addition of leucine to a mixed nutrient meal also resulted in a significant increase in muscle protein synthesis (0.053 ± 0.009%/h vs. 0.083 ± 0.008 %/h, P < 0.05) [47]. Pansarasa et al. [50] recently reported that amino acid supplementation in rats can prevent sarcopenia apparently through increased activation of the mTOR signaling pathway. These new findings are promising for the potential use of added leucine to regular meals in an effort to maximally stimulate muscle protein synthesis in the elderly.
Ageing and the interaction of physical activity and protein/nutrient intake
Elderly individuals are at increased risk of being physically incapacitated or placed on bed rest for an extended period. The loss of lean body mass is dramatically increased during inactivity and is driven by a chronic imbalance between muscle protein synthesis and breakdown and facilitated by decreased activation of the mTOR nutrient signaling pathway [51,52•,53]. In recent studies examining changes in protein synthesis and muscle mass in healthy adults subjected to bed rest, older individuals experienced an approximate three-fold greater loss of lean leg muscle mass compared with a cohort of younger individuals confined to bed for 28 days [12,52•]. In a medically compromised inpatient population, this could contribute to decreased immune function, impaired wound healing impact and delayed recovery following discharge [54]. The standard treatment modality for hospitalized elders is weight-bearing exercise [55,56]. Unfortunately, a substantial number of hospitalized or institutionalized elderly are severely limited in their ability to perform any physical activity [57]. In such instances, protein–energy supplementation represents one of the few viable alternatives.
The benefits of regular resistance exercise in elders have been well described. However, unlike younger individuals, the chronic additive or synergistic effects of combined protein supplementation and resistance exercise on muscle mass and function are less clear (37–39). In a recent study, 36 older men and women performed 12 week of resistance training in association with a lower (0.9 g protein kg−1.d−1) or higher protein (1.2 g protein kg−1.d−1) intake. There was general improvement in all outcome measures (e.g., strength, whole-body protein accretion and reduced fat mass), however, there were no significant differences between the lower-protein groups and higher-protein groups (38). Similarly, using a postexercise supplement, Andrews et al. (37) reported that differences in daily protein intake (1.35 vs. 0.72 g protein kg−1.d−1) did not differentially affect lean mass gains following a 12-week resistance exercise program in older men and women (60–69 years). Further studies designed to clarify this apparent age-related difference and identify key mechanistic event are needed.
Conclusion
Ageing is associated with a reduced ability to stimulate skeletal muscle protein synthesis in response to feeding, insulin, and resistance exercise. We propose a novel and specific dietary approach to. To prevent or slow sarcopenic muscle loss, clinicians should stress the importance of ingesting a sufficient amount of protein with each meal. To maximize muscle protein synthesis whereas being cognizant of total energy intake, we propose a dietary plan that includes 25–30 g of high quality protein per meal.
Acknowledgments
Supported by U.S. National Institute of Aging grant P30 AG024832 (UTMB Claude D. Pepper Older Americans Independence Center) and U.S. National Institute of Arthritis and Musculoskeletal and Skin Disease grant # R01 AR049877 (BBR). DPJ has received compensation for speaking and consulting engagements with the National Cattlemen’s Beef Association and the National Dairy Council.
References
Papers of particular interest, published within the annual period of review, have been highlighted as:
* of special interest
** of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 000–000).
- 1.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Cuthbertson D, Smith K, Babraj J, Leese G, Waddell T, Atherton P, Wackerhage H, Taylor PM, Rennie MJ. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 3.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Cadenas JG, Yoshizawa F, Volpi E, Rasmussen BB. Nutrient Signalling in the Regulation of Human Muscle Protein Synthesis. J Physiol. 2007 doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fujita S, Rasmussen BB, Cadenas J, Drummond MJ, Glynn EL, Sattler FR, Volpi E. Aerobic Exercise Overcomes the Age-Related Insulin Resistance of Muscle Protein Metabolism by Improving Endothelial Function Uand Akt/mTOR signaling. Diabetes. 2007 doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5 *.Campbell WW. Synergistic use of higher-protein diets or nutritional supplements with resistance training to counter sarcopenia. Nutr Rev. 2007;65:416–422. doi: 10.1111/j.1753-4887.2007.tb00320.x. This study highlights a potential age-related disparity in the synergystic effects of protein and exercise. [DOI] [PubMed] [Google Scholar]
- 6.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, Volpi E, Wolf SE, Aarsland A, Ferrando AA, Wolfe RR. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Short KR, Campbell WW, Volpi E, Wolfe RR. Role of dietary protein in the sarcopenia of aging. Am J Clin Nutr. 2008;87:1562S–1566S. doi: 10.1093/ajcn/87.5.1562S. [DOI] [PubMed] [Google Scholar]
- 9 **.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. This was the first study to demonstrate that a protein-rich meal (i.e., lean beef) stimulates muscle protein synthesis similarly in both young and elderly. [DOI] [PubMed] [Google Scholar]
- 10.Walrand S, Short KR, Bigelow ML, Sweatt A, Hutson SM, Nair KS. Functional impact of high protein intake on healthy elderly people. Am J Physiol Endocrinol Metab. 2008 doi: 10.1152/ajpendo.90536.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paddon-Jones D, Sheffield-Moore M, Aarsland A, Wolfe RR, Ferrando AA. Exogenous amino acids stimulate human muscle anabolism without interfering with the response to mixed meal ingestion. Am J Physiol Endocrinol Metab. 2005;288:E761–767. doi: 10.1152/ajpendo.00291.2004. [DOI] [PubMed] [Google Scholar]
- 12.Paddon-Jones D, Sheffield-Moore M, Urban RJ, Sanford AP, Aarsland A, Wolfe RR, Ferrando AA. Essential amino acid and carbohydrate supplementation ameliorates muscle protein loss in humans during 28 days bedrest. J Clin Endocrinol Metab. 2004;89:4351–4358. doi: 10.1210/jc.2003-032159. [DOI] [PubMed] [Google Scholar]
- 13.Paddon-Jones D, Wolfe RR, Ferrando AA. Amino acid supplementation for reversing bed rest and steroid myopathies. J Nutr. 2005;135:1809S–1812S. doi: 10.1093/jn/135.7.1809S. [DOI] [PubMed] [Google Scholar]
- 14.Volpi E, Ferrando AA, Yeckel CW, Tipton KD, Wolfe RR. Exogenous amino acids stimulate net muscle protein synthesis in the elderly. J Clin Invest. 1998;101:2000–2007. doi: 10.1172/JCI939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpi E, Lucidi P, Cruciani G, Monacchia F, Reboldi G, Brunetti P, Bolli GB, De Feo P. Contribution of amino acids and insulin to protein anabolism during meal absorption. Diabetes. 1996;45:1245–1252. doi: 10.2337/diab.45.9.1245. [DOI] [PubMed] [Google Scholar]
- 17.Volpi E, Mittendorfer B, Wolf SE, Wolfe RR. Oral amino acids stimulate muscle protein anabolism in the elderly despite higher first-pass splanchnic extraction. Am J Physiol Endocrinol Metab. 1999;277:E513–E520. doi: 10.1152/ajpendo.1999.277.3.E513. [DOI] [PubMed] [Google Scholar]
- 18.Volpi E, Mittendorfer B, Rasmussen BB, Wolfe RR. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillet C, Prod’homme M, Balage M, Gachon P, Giraudet C, Morin L, Grizard J, Boirie Y. Impaired anabolic response of muscle protein synthesis is associated with S6K1 dysregulation in elderly humans. Faseb J. 2004;18:1586–1587. doi: 10.1096/fj.03-1341fje. [DOI] [PubMed] [Google Scholar]
- 20.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc. 2006;38:667–674. doi: 10.1249/01.mss.0000210190.64458.25. [DOI] [PubMed] [Google Scholar]
- 21.Dreyer HC, Drummond MJ, Pennings B, Fujita S, Glynn EL, Chinkes DL, Dhanani S, Volpi E, Rasmussen BB. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koopman R, Verdijk L, Manders RJ, Gijsen AP, Gorselink M, Pijpers E, Wagenmakers AJ, van Loon LJ. Co-ingestion of protein and leucine stimulates muscle protein synthesis rates to the same extent in young and elderly lean men. Am J Clin Nutr. 2006;84:623–632. doi: 10.1093/ajcn/84.3.623. [DOI] [PubMed] [Google Scholar]
- 23.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–381. doi: 10.1093/ajcn/86.2.373. [DOI] [PubMed] [Google Scholar]
- 24.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 25.Campbell WW, Trappe TA, Wolfe RR, Evans WJ. The recommended dietary allowance for protein may not be adequate for older people to maintain skeletal muscle. J Gerontol A Biol Sci Med Sci. 2001;56:M373–380. doi: 10.1093/gerona/56.6.m373. [DOI] [PubMed] [Google Scholar]
- 26.Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475–482. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe RR, Miller SL. The recommended dietary allowance of protein: a misunderstood concept. Jama. 2008;299:2891–2893. doi: 10.1001/jama.299.24.2891. [DOI] [PubMed] [Google Scholar]
- 28.Leidy HJ, Carnell NS, Mattes RD, Campbell WW. Higher protein intake preserves lean mass and satiety with weight loss in pre-obese and obese women. Obesity (Silver Spring) 2007;15:421–429. doi: 10.1038/oby.2007.531. [DOI] [PubMed] [Google Scholar]
- 29.Thalacker-Mercer AE, Fleet JC, Craig BA, Carnell NS, Campbell WW. Inadequate protein intake affects skeletal muscle transcript profiles in older humans. Am J Clin Nutr. 2007;85:1344–1352. doi: 10.1093/ajcn/85.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Millward DJ, Layman DK, Tome D, Schaafsma G. Protein quality assessment: impact of expanding understanding of protein and amino acid needs for optimal health. Am J Clin Nutr. 2008;87:1576S–1581S. doi: 10.1093/ajcn/87.5.1576S. [DOI] [PubMed] [Google Scholar]
- 31.Rand WM, Pellett PL, Young VR. Meta-analysis of nitrogen balance studies for estimating protein requirements in healthy adults. Am J Clin Nutr. 2003;77:109–127. doi: 10.1093/ajcn/77.1.109. [DOI] [PubMed] [Google Scholar]
- 32.Trumbo P, Schlicker S, Yates AA, Poos M. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J Am Diet Assoc. 2002;102:1621–1630. doi: 10.1016/s0002-8223(02)90346-9. [DOI] [PubMed] [Google Scholar]
- 33.Bunker VW, Lawson MS, Stansfield MF, Clayton BE. Nitrogen balance studies in apparently healthy elderly people and those who are housebound. Br J Nutr. 1987;57:211–221. doi: 10.1079/bjn19870027. [DOI] [PubMed] [Google Scholar]
- 34.Pepersack T, Corretge M, Beyer I, Namias B, Andr S, Benoit F, Mergam A, Simonetti C. Examining the effect of intervention to nutritional problems of hospitalised elderly: a pilot project. J Nutr Health Aging. 2002;6:306–310. [PubMed] [Google Scholar]
- 35 **.Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–155. doi: 10.1093/ajcn/87.1.150. A large cohort study that suggests that dietary protein may be a modifiable risk factor to reduce the risk of sarcopenia. [DOI] [PubMed] [Google Scholar]
- 36.Thomas DR. Loss of skeletal muscle mass in aging: examining the relationship of starvation, sarcopenia and cachexia. Clin Nutr. 2007;26:389–399. doi: 10.1016/j.clnu.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Dillon EL, Volpi E, Wolfe RR, Sinha S, Sanford AP, Arrastia CD, Urban RJ, Casperson SL, Paddon-Jones D, Sheffield-Moore M. Amino acid metabolism and inflammatory burden in ovarian cancer patients undergoing intense oncological therapy. Clin Nutr. 2007;26:736–743. doi: 10.1016/j.clnu.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 39.Fiatarone Singh MA, Bernstein MA, Ryan AD, O’Neill EF, Clements KM, Evans WJ. The effect of oral nutritional supplements on habitual dietary quality and quantity in frail elders. J Nutr Health Aging. 2000;4:5–12. [PubMed] [Google Scholar]
- 40.Milne AC, Avenell A, Potter J. Meta-analysis: protein and energy supplementation in older people. Ann Intern Med. 2006;144:37–48. doi: 10.7326/0003-4819-144-1-200601030-00008. [DOI] [PubMed] [Google Scholar]
- 41.Pupovac J, Anderson GH. Dietary peptides induce satiety via cholecystokinin-A and peripheral opioid receptors in rats. J Nutr. 2002;132:2775–2780. doi: 10.1093/jn/132.9.2775. [DOI] [PubMed] [Google Scholar]
- 42.Dardevet D, Sornet C, Balage M, Grizard J. Stimulation of in vitro rat muscle protein synthesis by leucine decreases with age. J Nutr. 2000;130:2630–2635. doi: 10.1093/jn/130.11.2630. [DOI] [PubMed] [Google Scholar]
- 43.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 44.Koopman R, Wagenmakers AJ, Manders RJ, Zorenc AH, Senden JM, Gorselink M, Keizer HA, van Loon LJ. Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects. Am J Physiol Endocrinol Metab. 2005;288:E645–653. doi: 10.1152/ajpendo.00413.2004. [DOI] [PubMed] [Google Scholar]
- 45.Combaret L, Dardevet D, Rieu I, Pouch MN, Bechet D, Taillandier D, Grizard J, Attaix D. A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle. J Physiol. 2005;569:489–499. doi: 10.1113/jphysiol.2005.098004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rieu I, Sornet C, Bayle G, Prugnaud J, Pouyet C, Balage M, Papet I, Grizard J, Dardevet D. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J Nutr. 2003;133:1198–1205. doi: 10.1093/jn/133.4.1198. [DOI] [PubMed] [Google Scholar]
- 47.Rieu I, Balage M, Sornet C, Giraudet C, Pujos E, Grizard J, Mosoni L, Dardevet D. Leucine supplementation improves muscle protein synthesis in elderly men independently of hyperaminoacidaemia. J Physiol. 2006;575:305–315. doi: 10.1113/jphysiol.2006.110742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Layman DK. Role of leucine in protein metabolism during exercise and recovery. Can J Appl Physiol. 2002;27:646–663. doi: 10.1139/h02-038. [DOI] [PubMed] [Google Scholar]
- 49.Layman DK, Walker DA. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J Nutr. 2006;136:319S–323S. doi: 10.1093/jn/136.1.319S. [DOI] [PubMed] [Google Scholar]
- 50.Pansarasa O, Flati V, Corsetti G, Brocca L, Pasini E, D’Antona G. Oral amino acid supplementation counteracts age-induced sarcopenia in elderly rats. Am J Cardiol. 2008;101:35E–41E. doi: 10.1016/j.amjcard.2008.02.079. [DOI] [PubMed] [Google Scholar]
- 51.Dreyer HC, Glynn EL, Lujan HL, Fry CS, DiCarlo SE, Rasmussen BB. Chronic paraplegia-induced muscle atrophy downregulates the mTOR/S6K1 signaling pathway. J Appl Physiol. 2008;104:27–33. doi: 10.1152/japplphysiol.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52 *.Kortebein P, Ferrando AA, Lombeida J, Wolfe RR, Evans WJ. Effect of 10 Days of Bed Rest on Skeletal Muscle in Healthy Older Adults. JAMA. 2007;297:1772–1774. doi: 10.1001/jama.297.16.1772-b. The first controlled bedrest study in elders. Results indicate that muscle loss is greatly accelerated during periods of physical inactivity. [DOI] [PubMed] [Google Scholar]
- 53.Paddon-Jones D, Sheffield-Moore M, Cree MG, Hewlings SJ, Aarsland A, Wolfe RR, Ferrando AA. Atrophy and impaired muscle protein synthesis during prolonged inactivity and stress. J Clin Endocrinol Metab. 2006;91:4836–4841. doi: 10.1210/jc.2006-0651. [DOI] [PubMed] [Google Scholar]
- 54.Demling RH, DeSanti L. Involuntary weight loss and the nonhealing wound: the role of anabolic agents. Adv Wound Care. 1999;12:1–14. [PubMed] [Google Scholar]
- 55.Landefeld CS, Palmer RM, Kresevic DM, Fortinsky RH, Kowal J. A randomized trial of care in a hospital medical unit especially designed to improve the functional outcomes of acutely ill older patients. N Engl J Med. 1995;332:1338–1344. doi: 10.1056/NEJM199505183322006. [DOI] [PubMed] [Google Scholar]
- 56.Blocker WP., Jr Maintaining functional independence by mobilizing the aged. Geriatrics. 1992;47:42, 48–50. [PubMed] [Google Scholar]
- 57.Mahoney JE, Sager MA, Jalaluddin M. Use of an ambulation assistive device predicts functional decline associated with hospitalization. J Gerontol A Biol Sci Med Sci. 1999;54:M83–88. doi: 10.1093/gerona/54.2.m83. [DOI] [PubMed] [Google Scholar]