Abstract
Purpose. To determine whether transplantation of Schwann cells (SCs) overexpressing different isoforms of fibroblast growth factor 2 (FGF-2) combined with manual stimulation (MS) of vibrissal muscles improves recovery after facial nerve transection in adult rat. Procedures. Transected facial nerves were entubulated with collagen alone or collagen plus naïve SCs or transfected SCs. Half of the rats received daily MS. Collateral branching was quantified from motoneuron counts after retrograde labeling from 3 facial nerve branches. Quality assessment of endplate reinnervation was combined with video-based vibrissal function analysis. Results. There was no difference in the extent of collateral axonal branching. The proportion of polyinnervated motor endplates for either naïve SCs or FGF-2 over-expressing SCs was identical. Postoperative MS also failed to improve recovery. Conclusions. Neither FGF-2 isoform changed the extent of collateral branching or polyinnervation of motor endplates; furthermore, this motoneuron response could not be overridden by MS.
1. Introduction
It has been well established that artificial nerve grafts filled with naïve Schwann cells (SCs) from neonatal [1, 2] and adult rats [3–5] stimulate peripheral nerve fiber regeneration. The growth-promoting effect is thought to occur as a result of the SCs ability to provide basal membrane components and to secrete a range of trophic factors including fibroblast growth factor-2 (FGF-2) [6, 7].
In the central nervous system (CNS), FGF-2 could cause demyelination [8] and it is also known to protect different neuronal cell types from damage-induced death [9]. One example is the developing and adult nigrostriatal system, where applied exogenous FGF-2 promotes survival and neurite outgrowth of dopaminergic neurons and protects them from neurotoxin-induced death in vitro and in vivo [10].
In the peripheral nervous system (PNS) especially FGF-2 appears to be a particularly potent promoter of regeneration [11]. For example, entrapment of FGF-2 in synthetic nerve guidance channels enhances the growth of myelinated and unmyelinated axons across long gaps [12, 13], which are a major challenge in peripheral nerve repair. Furthermore, transgenic mice overexpressing FGF-2 have considerably faster regeneration of peripheral axons and remyelination compared to wildtypes [14]. As another example, genetically modified SC overexpressing the 21- and 23-kD-FGF-2 isoforms (FGF-221/23kD) have turned out to be a useful tool to bridge long gaps (15 mm) after sciatic nerve injury, a mixed peripheral nerve [15].
Our group has addressed the problems inherent in PNS regeneration using transection and suture of the infratemporal portion of the facial nerve in rats [16]. One of our major recent findings was that brief manual stimulation (MS; 5 minutes per day, 5 days per week for 2 months) of the whisker pad muscles can over-ride the functional deficit after facial nerve injury and end-to-end anastomosis and restore normal whisking. Manual stimulation neither reduces branching at the injury site nor, consequently, restores myotopic organization in the facial nucleus. Rather, manual stimulation appears to affect the periphery by shifting the balance from abnormal polyinnervation of the motor end-plates to the more normal monoinnervated state [17].
Given the effectiveness of manual stimulation in over-riding the detrimental effects of collateral branching and its resultant polyinnervation of motor endplates after facial nerve injury, we asked whether a combinatorial therapy would also improve function. Since the different FGF-2 isoforms (FGF-218kD and FGF-221/23kD) promote encouraging effects after injury in other peripheral nerves, namely, the sciatic [15, 18], we first asked here whether these isoforms would also benefit the outcome after facial nerve injury.
Then we asked whether FGF-2 treatment combined with manual stimulation might also influence an outcome. We, therefore, compared the extent of sprouting, degree of polyinnervation, and whisking function after facial nerve transection followed by entubulation of both ends into a silicone tube filled thereafter either with collagen type I [19], or with collagen plus SCs genetically modified to overexpress the low- (18-kD) or high- (21/23-kD) molecular-weight isoforms of FGF-2 [20] in animals either receiving MS or not.
2. Materials and Methods
2.1. Animal Groups and Overview of Experiments
144 female adult (175–200 g) rats (Sprague-Dawley, Harlan-Winkelmann, Borchen, Germany) divided into one intact and 8 experimental groups were used. Animals were kept on standard laboratory food (Ssniff, D-59494 Soest, Germany) and tap water ad libitum with an artificial light-dark cycle of 12 hours light on, 12 hours off. All experiments were conducted in accordance with the “German Law for Animals Protection” and were approved by the local animal care committee (Bezirksregierung Köln, Az. 23.203.2-K35,13/95). Normal, intact rats were used while all animals in the 8 experimental groups had their right facial nerves transected and were treated as follows (n = 16 in each):
Group 1: intact rats,
Group 2: entubulation into a conduit containing collagen type I,
Group 3: entubulation into a conduit containing (naïve) nonmanipulated neonatal Schwann cells (SCs);
Group 4: entubulation into a conduit containing SCs overexpressing FGF-218kD (SC-FGF-218kD);
Group 5: entubulation into a conduit containing SCs overexpressing FGF221/23kD (SC-FGF221/23kD);
Group 6: as for group 2 + manual stimulation (MS) of the right whisker pad muscles
Group 7: as for group 3 + manual stimulation (MS)
Group 8: as for group 4 + MS
Group 9: as for group 5 + MS
At 2 months postoperatively we quantified (i) vibrissal motor performance, (ii) the degree of axonal branching, and (iii) the pattern of motor endplate reinnervation. Data from each experimental group (groups 2–9) were compared to those from intact rats (group 1). Vibrissal motor performance was analysed in all rats using video-based motion analysis (Table 1) whereupon half (n = 8 per group) of the animals in all groups were used to quantify collateral axonal branching using triple retrograde neuronal labeling (Table 2). The remaining rats (n = 8 per group) were used to determine the proportion of mono- and polyinnervated motor endplates (Table 3) in the ipsilateral levator labii superioris muscle using immunocytochemistry.
Table 1.
Estimation of vibrissae function recovery. Vibrissae performance in unoperated rats (Group 1: Intact), in rats after transection of the right facial nerve followed by entubulation with collagen only (Group 2: Collagen), with collagen plus neonatal Schwann cells (Group 3: Collagen + naive SCs), or with collagen plus genetically modified SCs (Groups 4 and 5). In groups 6–9 entubulations were followed by manual mechanical stimulation (MS) of the vibrissal muscles.
| Group of animals | Frequency (in Hz) | Angle at maximal protraction (in degrees) | Amplitude (in degrees) | Angular velocity during protraction (in degrees/sec) |
|---|---|---|---|---|
| (1) Intact SD rats | 6.0 ± 0.9 | 60 ± 15 | 47 ± 18 | 635 ± 395 |
| (2) Collagen only | 5.4 ± 1.2 | 93 ± 16* | 15 ± 9* | 213 ± 44* |
| (3) Collagen + neonatal, naive SCs | 6.0 ± 0.7 | 90 ± 8* | 16 ± 9* | 140 ± 90* |
| (4) Collagen + SC-FGF-218kD | 6.0 ± 0.5 | 91 ± 8* | 19 ± 4* | 238 ± 164* |
| (5) Collagen + SC-FGF-221/23kD | 6.0 ± 0.6 | 96 ± 10* | 11 ± 4* | 109 ± 62* |
| (6) Collagen only + MS | 5.8 ± 1.6 | 93 ± 19* | 20 ± 8* | 295 ± 78* |
| (7) Collagen + neonatal, naive SCs + MS | 7.4 ± 1.3 | 97 ± 18* | 16 ± 9* | 238 ± 54* |
| (8) Collagen + SC-FGF-218kD + MS | 6.4 ± 0.9 | 96 ± 12* | 15 ± 8* | 240 ± 96* |
| (9) Collagen + SC-FGF-221/23kD + MS | 6.2 ± 0.6 | 96 ± 16* | 19 ± 7* | 307 ± 98* |
*Significant differences of group mean values (ANOVA and post-hoc Tukey's test, P < .05) from intact rats.
Table 2.
Projection patterns of facial motoneurons. Number of motoneurons with axons in the zygomatic, buccal, or marginal mandibular branches of the facial nerve of intact rats (Group 1: Intact), in rats after transection of the right facial nerve followed by entubulation with collagen only (Group 2: Collagen), with collagen plus naive SC's (Group 3: Collagen+SC's), or with collagen plus genetically modified SC's (groups 4 and 5). In groups 6–9 entubulations were followed by manual mechanical stimulation (MS) of the vibrissal muscles.
| Group of animals | Neurons projecting only into the zygomatic nerve (Dil-only) | Neurons projecting into the zygomatic and buccal nerves (Dil+FG) | Neurons projecting into the zygomatic and marginal mandibular nerves (Dil+FB) | All Dil labeled neurons projecting into the zygomatic nerve (Dil, DiI+FG, DiI+FB) | Neurons projecting only into the buccal nerve (FG-only) | Neurons projecting only into the marginal mandibular nerve (FB-only) | Total number of neurons projecting into zygomatic buccal and marginal mandibular nerves (Dil-only, FG-only, FB-only) |
|---|---|---|---|---|---|---|---|
| (1) Intact SD rats | 291 ± 35 | — | — | 291 ± 35 | 1406 ± 214 | 349 ± 78 | 2046 ± 327 |
| 100% | 0% | 0% | 100% | ||||
| (2) Collagen only | 323 ± 67 | 202 ± 58* | 161 ± 60* | 696 ± 192* | 2387 ± 256* | 1976 ± 243* | 5059 ± 691* |
| 46% | 29% | 25% | 100% | ||||
| (3) Collagen + naïve Schwann cells (SC's) | 372 ± 88 | 174 ± 66* | 138 ± 73* | 684 ± 215* | 2040 ± 443* | 1754 ± 509* | 4478 ± 1167* |
| 54% | 25% | 21% | 100% | ||||
| (4) Collagen + SC-FGF-218KD | 234 ± 68 | 130 ± 54* | 83 ± 41* | 447 ± 162* | 2062 ± 296* | 1883 ± 184* | 4392 ± 642* |
| 52% | 29% | 19% | 100% | ||||
| (5) Collagen + SC-FGF-221/23KD | 269 ± 115 | 151 ± 70* | 110 ± 52* | 532 ± 232* | 2304 ± 363* | 1918 ± 334* | 4754 ± 929* |
| 50% | 28% | 22% | 100% | ||||
| (6) Collagen only + MS | 322 ± 68 | 184 ± 86* | 128 ± 43* | 634 ± 197* | 2243 ± 423* | 1784 ± 523* | 4670 ± 1143* |
| 51% | 29% | 20% | 100% | ||||
| (7) Collagen + naïve SC's + MS | 321 ± 87 | 186 ± 36* | 168 ± 78* | 675 ± 201* | 2190 ± 543* | 1752 ± 309* | 4617 ± 1053* |
| 48% | 28% | 24% | 100% | ||||
| (8) Collagen + SC-FGF-218KD + MS | 252 ± 91 | 330 ± 93* | 177 ± 45* | 759 ± 229* | 2215 ± 204* | 1965 ± 492* | 4939 ± 925* |
| 33% | 43% | 24% | 100% | ||||
| (9) Collagen + SC-FGF-221/23KD + MS | 303 ± 69 | 333 ± 96* | 195 ± 48* | 831 ± 213* | 2280 ± 306* | 1899 ± 723* | 5010 ± 1242* |
| 36% | 40% | 24% | 100% | ||||
*Significant differences of group mean values (ANOVA and post-hoc Tukey's test, P < .05) from intact rats.
Table 3.
Quality of target muscle reinnervation. Reinnervation pattern of the levator labii superioris motor-end plates in intact rats, in rats after transection of the right facial nery followed by:- entubulation with collagen only, with collagen plus naive SC's, or with collagen plus genetically modified SC's. In group 6–9 entubulations were followed by manual mechanical stimulation (MS) of the vibrissal muscles. Motor endplates were classified a monoinnervated, polyinnervated, or noninnervated according to the number of beta-tubulin-immunoreactive axons that crossed th boundaries of the end-plate).
| Group of animals | Monoinnervated motor end-plates (percent) | Polyinnervated motor end-plates (percent) | Noninnervated motor end-plates (percent) | Total number of motor end-plates examined |
|---|---|---|---|---|
| 1. Intact SD rats | 100 ± 0.0 | 0.0 | 0.0 | 1633 ± 143 |
| 2. Collagen only | 44 ± 8.0* | 46 ± 10* | 10 ± 3.0* | 1521 ± 162 |
| 3. Collagen +neonatal, naive SC's | 42 ± 8.0* | 49 ± 4.0* | 9.0 ± 2.0* | 1404 ± 108 |
| 4. Collagen + SC-FGF-218kD | 49 ± 7.0* | 45 ± 12* | 6.0 ± 1.0* | 1464 ± 159 |
| 5. Collagen + SC-FGF-221/23kD | 48 ± 16* | 47 ± 15* | 5.0 ± 4.0* | 1602 ± 258 |
| 6. Collagen only + MS | 47 ± 9.0* | 51 ± 10* | 2.0 ± 1.0* | 1362 ± 303 |
| 7. Collagen + neonatal, naive SC's + MS | 40 ± 8.0* | 48 ± 10* | 12 ± 3.0* | 1512 ± 102 |
| 8. Collagen + SC-FGF-218kD + MS | 41 ± 3.0* | 49 ± 11* | 10 ± 1.0* | 1413 ± 141 |
| 9. Collagen + SC-FGF-221/23kD + MS | 51 ± 8.0* | 42 ± 10* | 7 ± 3.0* | 1602 ± 150 |
*Significant differences of group mean values (ANOVA and post-hoc Tukey's test, P < .05 from intact rats.
2.2. Surgery
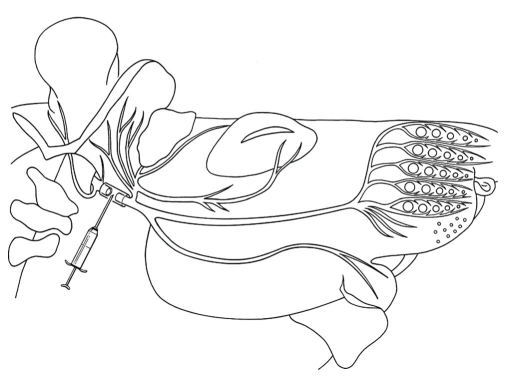
All surgery was performed unilateraly under an operating microscope and with surgical anesthesia (ketamin/xylazin (100 mg Ketanest and 5 mg Rompun® per kg body weight; IP) as already described [21]. The main trunk of the facial nerve was transected and the two stumps inserted into a silicone precision tube with an inner diameter of 1.47 mm and an outer diameter of 1.96 mm (Aromando Medizintechnik, Cat. Nr. 602-235, Düsseldorf). The interstump distance measured 5 mm (Figure 1). The empty space between the proximal and distal nerve stumps with a volume of approximately 8 mm3 (5 mm × 1,47/22 mm × π) was filled carefully with the following.
Figure 1.
Surgical procedure: entubulation of the facial nerve trunk after transection (adapted from [21]).
10 μL collagen type I for groups 2 and 6;
10 μL taken from a gel consisting of 66 μL collagen type I (Serva, Cat. No. 47254) plus 33 μL of a suspension containing naïve nonmanipulated SC for groups 3 and 7;
10 μL taken from a gel consisting of 66 μL collagen type I plus 33 μL of a suspension containing genetically modified SCs overexpressing FGF-218kD for groups 4 and 8;
10 μL taken from a gel consisting of 66 μL collagen type I plus 33 μL of a suspension containing genetically modified SCs overexpressing FGF-221/23kD for groups 5 and 9.
2.3. Collagen and Schwann Cell Preparation
The collagen gel was obtained from a stock solution (100 μg/mL, Serva, Heidelberg, Germany, Cat. No. 47254) which was mixed with ×10 phosphate buffered saline (PBS) and 0.1 M NaOH till the pH reached 7.4. Thereafter, 106 μL of this pure collagen gel were mixed with 54 μL collagen (ratio 2 : 1) which contained a suspension of Schwann cells so that a total of 160 μL gel (collagen plus SC) was obtained. This volume was then divided in 16 tubes (for 16 rats) each containing 10 μL, that is, the portion for each animal. To polymerize in a 3-dimensional gel, the collagen/SC mixture was left at 37°C for 2 hours [22, 23]. Each animal received approximately 0.2 × 106 cells.
Primary physiological Schwann cells were prepared as described earlier [24]. In brief, sciatic nerves were dissected from neonatal Sprague-Dawley rats. After enzymatic dissociation and primary seeding, most of contaminating fibroblasts were erased by addition of arabinoside C (1 mM) for 2 days and subsequent removing form the cultures using α-Thy1 antibody-coupled magnetic beads (Dynabeads, Dynal, Denmark). The protocol resulted in about 99% pure naïve SCs cultures.
Overexpression of FGF-2-isoforms was induced by transfection of SCs using Metafectene (Biontex, Germany) [25], transfection rate approximately 20%, and plasmid constructs with FGF-218kD isoform [26] or FGF-221/23kD isoforms in pCI-neo [20].
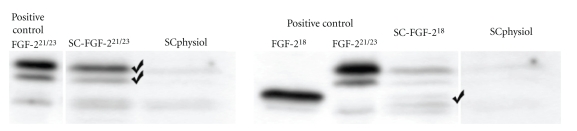
All SC populations transplanted in this study were freshly prepared and transfected. As described earlier [15], transfected SCs were positively selected for their coexpressed resistance to Geneticin (G418) and successful transfection was monitored in Western blot analysis with regard to FGF-2 isoform overexpression in the different cell groups (Figure 2): nontransfected SCs (SCphysiol), SCs overexpressing either 18kD-FGF-2 (SC-FGF-218) or 21/23-kD-FGF-2 (SC-FGF-221/23). Transfected cells were grown to confluence and withdrawn from fetal calf serum prior to transplantation. SCs were detached from culture flasks and stored on ice. Directly prior to implantation of the silicon nerve guides, SCs were resuspended in the collagen solution (see above) and injected in the sterile silicone tubes used.
Figure 2.
Western Blot analysis of Schwann cell populations prior to transplantation: nontransfected SCs, SCphysiol, show less amount of FGF-2 isoforms (18kD, 21kD, and 23kD) as SCs genetically modified to overexpress FGF-2-18kD, SC-FGF-218, or FGF-2-21/23kD, SC-FGF-221/23. As positive controls served cell lysates from FGF-2 isoform overexpressing PC12 cells.
Transplanted SCs overexpressing FGF-2-18kD or FGF2-21/23kD increase the amount of these proteins inside a peripheral nerve gap after entubulation surgery. Using the ELISA technique we previously found higher concentrations of free FGF-2 isoforms for at least one week after entubulation. The amounts of free FGF-2 one week after surgery were (1) nonmanipulated neonatal Schwann cells (SC): 0.56 ng/μL; (2) SC overexpressing the FGF-2-18kD: 0.90 ng/μL; (3) SC overexpressing the FGF-2-21/23kD: 0.66 ng/μL [20].
2.4. Identification of SC Grafts
Identification of SCs after transplantation could be achieved by cell surface labeling prior to grafting [27]. Exemplarily, prelabeling was performed by the incubation of SCs with PKH26-GL cell linker (Sigma, Germany). After sacrifice, tissue was fixed (4% paraformaldehyde), cryo-preserved (30% sucrose), and cryo-embedded. Transplanted SCs were identified by their red fluorescence using a BX60 microscope (Leica, Germany) in longitudinal cryo sections of regenerated peripheral nerve tissue (Figure 4).
Figure 4.
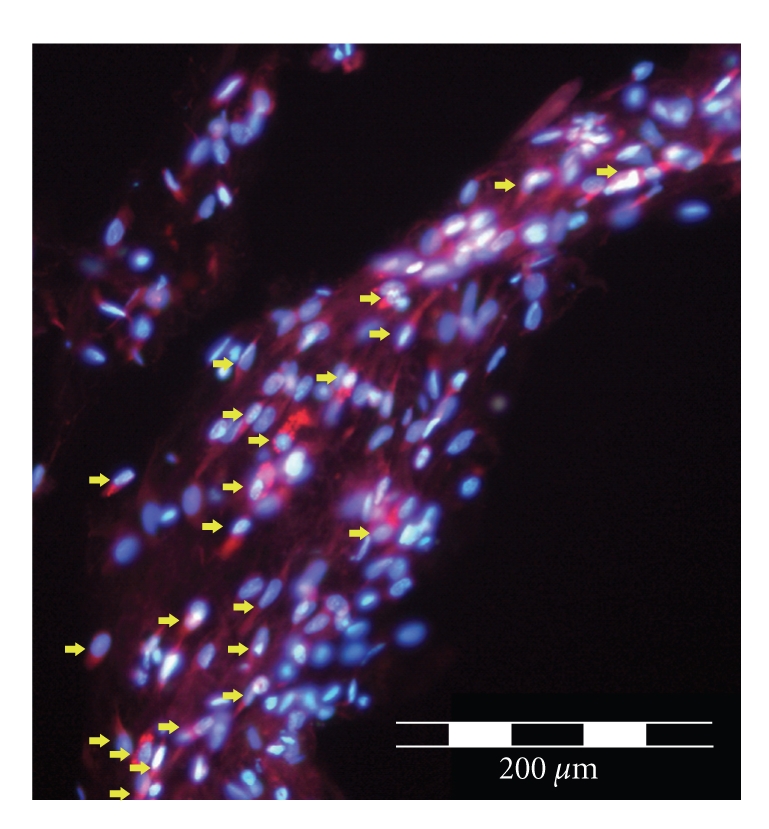
Depicts exemplarily red-fluorescent PKH26-GL cell surface staining of transplanted SCs (red arrows, costained with DAPI in blue) in a longitudinal cryostat section through the regrown nerve in a silicone tube 3 weeks post-operation.
2.5. Manual Mechanical Stimulation of the Vibrissal Muscles
As previously described, manual mechanical stimulation was initiated one day after surgery [17]. To mimick natural active vibrissal movements during whisking, groups 6–9 received daily gentle rhythmic forward stroking by hand, while letting the whiskers retract backwards unassisted, of the right vibrissae and whisker pad muscles (5 min/day, 5 days/week for 2 months; figure not shown, see [17]).
2.6. Analysis of Vibrissal Motor Performance
Animals were videotaped for 3–5 minutes when undertaking active exploratory sweeps [28]. We evaluated (i) protraction (forward vibrissal movement); (ii) whisking frequency, (cycles of protraction and retraction, that is, backward movement); (iii) amplitude (difference between maximal retraction; maximal protraction and (iv) angular velocity (degrees per second). Measurements for all rats were performed by three independent observers (S.K. Angelova, D. Bösel, D. Felder) blinded to the treatment.
2.7. Estimation of Axonal Branching by Triple Retrograde Labeling
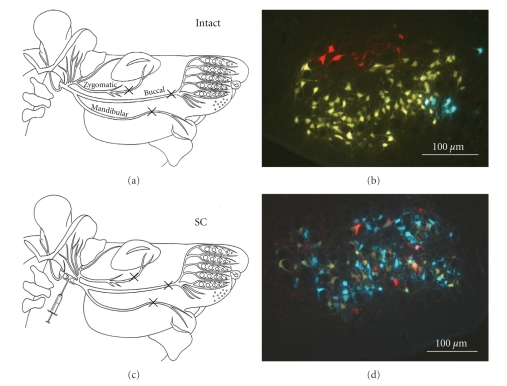
Application of Fluorescencent Tracers. The protocol followed our previously described method [21]. Briefly, one day after videotaping animals were anesthetized and crystals of DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindo-carbocyanine perchlorate; Molecular Probes, The Netherlands), Fluoro-Gold (FG; Fluorochrome Inc., Denver, Colo, USA) and Fast Blue (FB; EMS-Chemie GmbH, Groß-Umstadt, Germany) instilled, respectively, into the zygomatic, buccal, and marginal mandibular branches of the facial nerve (Figure 3). Ten days later, animals were terminally anaesthetized and transcardially perfused (4% formaldehyde in 0.1 M phosphate buffer, pH 7.4). Brainstems were serially sectioned (vibratome, frontal, 50 μm).
Figure 3.
Myotopic organization of the facial nucleus and collateral axonal branching as estimated by the pattern of retrograde labeling. In intact animals, simultaneous application of DiI (red), FG (yellow), and FB (blue) to the zygomatic, buccal and mandibular nerve branches, respectively, labels distinct subnuclei with no overlap (a), (b). Two months after entubulation with collagen or collagen plus SC the myotopic organization is lost irrespective whether the animals received MS (c), (d).
Fluorescence microscopy. Sections were observed using an epifluorescence microscope (Axioskop 50; Zeiss Oberkochen, Germany) fitted with a custom-made band pass-filter set (AHF Analysentechnik, Tübingen, Germany) to eliminate fluorescence cross-talk between tracers. Separate color images of retrogradely labeled facial motoneurons were generated (CCD Video Camera System: Optronics DEI-470, Goleta, Calif, USA) and combined using image analysis software Optimas 6.5. (Optimas Corporation, Bothell, Wash, USA). All cells stained by DiIonly, FGonly, FBonly, as well as those double-stained (i.e., DiI+FG or DiI+FB) were counted using an optical dissector technique [29]. The counts therefore allowed us to quantify the degree (index) of axonal branching (i.e., sum of the percentages given in the third and fourth column in Table 2).
Double-labeled motoneuronal perikary with FG+FG (in green colour) was not counted. This was a pity, because since FG and FB were both simultaneously visualized with the same UV epi-fluorescence excitation filter (Zeiss, Filter set 01), such a measurement would be rather easy. However, earlier own work has shown that the blue emission of FB obscured the yellow emission of FG. In other words, the number of FB+FG-labeled perikarya would be always lower than its real value [30].
2.8. Analysis of Target Muscle Reinnervation
As described previously, we quantified the ratio of monoinnervated to poly-innervated motor endplates in the levator labii superioris muscle [31]. Cryoprotected muscle tissue was cut on a cryostat (longitudinal; 30 μm thick sections) and sections immunostained (rabbit polyclonal antibody against neuronal class III β-tubulin; Covance, Richmond, Calif, USA, No. PRB-435P, 1 : 1000 and Cy3-conjugated antirabbit IgG; 1 : 400; Sigma as a secondary antibody). The boundaries of the motor end-plates were visualized using acetylcholine receptor staining (Alexa Fluor 488-conjugated alpha-bungarotoxin; Molecular Probes, 1 : 500).
2.9. Statistical Evaluation
Data were analyzed using one-way ANOVA with post-hoc Tukey test and significance level of 0.05 (Statistica 6.0 software; StatSoft, Tulsa, OK, USA).
3. Results
3.1. Identification of SCs
Examination of tissue after sacrifice showed that transplanted neonatal SC integrated well into regenerating peripheral nerve tissue (Figure 4).
3.2. Manual Stimulation of Vibrissal Muscles Does Not Promote Recovery of Whisking after Grafting Naïve SCs or Those Overexpressing FGF-218kD or FGF-221/23kD
We first examined the effects of MS on the return of whisking function. As described by us and others, normal animals explore the environment by coordinated sweeps of individual vibrissae (whisking) with a frequency of about 6 Hz [28]. Vibrissal movements occur via muscle contractions which produce active rostral protraction and, to a lesser extent, active caudal retraction; that is, some of the backward movement is passive. The amplitude of the movement from maximum protraction to maximum retraction was approximately 50° (Table 1, first row).
Compared to normal animals, vibrissal motion was poor in rats receiving entubulation alone (i.e., no manual stimulation) of the facial nerve in collagen (group 2) or collagen plus naïve SC or plus SC-FGF-218kD or SC-FGF-221/23kD (groups 3–5) with the amplitude and angular velocity being reduced by about 70% (Table 1). Furthermore, MS following entubulation in collagen (group 6) or collagen + naïve and FGF-overexpressing SC (groups 7–9) also failed to improve whisking of the vibrissae (Table 1).
3.3. A High Degree of Collateral Axonal Branching Occurs Regardless of Postoperative Treatment
As previously described by us and others, in intact animals, motoneurons with axons entering the zygomatic, buccal or marginal mandibular ramus (Figure 3(a)) were myotopically organized in the dorsal, lateral, and intermediate facial subnuclei, respectively [16]. No double- or triple-labeled motoneurons were observed because intact motoneurons send only one unbranched axon into each of the facialis rami (Figure 3(b)). Thus, for intact animals the index of axonal branching in the facial nerve trunk, calculated by examining the zygomatic motoneurons, was 0% (Table 2).
After facial nerve cut and entubulation with collagen or collagen plus SCs but without MS (Figure 3(c)), myotopic organization into specific subnuclei was no longer observed, that is, all retrogradely labeled motoneurons were scattered throughout the facial nucleus (Figure 3(d)). A similar lack of myotopy was observed when manual stimulation was combined with entubulation with collagen or collagen plus naïve SCs or those overexpressing FGF-218kD or FGF-221/23kD. The lack of myotopy was presumably due to individual transected axons sprouting to produce numerous collateral branches [31] which grew into different facial nerve rami, persisted in the long-term and retrogradely transported the three fluorescent dyes to their parent motoneurons in the facial nucleus.
We also commonly observed double and triple labeling of motoneurons (Figure 3(d)). Multiple labeling of individual motoneurons can be explained by injury-induced collateral axonal branches projecting into different facial nerve rami (i.e., in this experiment, the ones that were labeled, namely, the ramus zygomaticus, ramus buccalis or ramus marginalis mandibulae). Such branches would have retrogradely transported two or three fluorescent dyes simultaneously to the parent perikarya. The final observation is that based on elevated motoneuron numbers in the facial nucleus for all experimental animals, each of the individual facial nerve rami contained axons or axonal branches of more motoneurons than in intact animals.
Retrograde tracing also revealed that transection and entubulation of the facial nerve significantly changed the fiber composition of the different rami (Table 2). In intact rats (Group 1), the total number of single-labeled (i.e., DiI-only + FG-only + FB-only) cells in the facial nucleus was about 2000 but increased to 4000–5000 following entubulation with collagen, with neonatal SC and with SCs overexpressing FGF-218kD or FGF-221/23kD isoforms (last column in Table 1). A similar scenario was observed following MS in groups 6–9 in which the total numbers of single-labeled cells were significantly increased compared to those in intact rats (Table 1).
High numbers of single-labeled motoneurons presumably arose as follows. Axons in individual rami do not branch in intact animals, and therefore retrograde labeling from specific rami (zygomatic, buccal, and marginal mandibular) yields consistent motoneuron numbers with little variation (Table 2). The elevated numbers of motoneurons after labeling the buccal (FG-only) and marginal mandibular (FB-only), but not the zygomatic, branches (Table 2) indicate that other axons must have sprouted and entered these rami. The sources of these additional axons were presumably the two other branches of the facial nerve (posterior auricular and cervical). Thus, while not labeled in intact animals, such axons must presumably have also sprouted into the zygomatic, buccal, and marginal mandibular rami and, therefore, have become labelled (Table 2).
Across all treatment groups, the index of axonal branching (i.e., sum of the percentages of DiI+FG and DiI+FB retrogradely labeled perikarya in the third and fourth column of Table 2) was 46–67% and none of the procedures significantly influenced the projection patterns (Figure 3(d)). Thus, to summarise, there was a complete lack of myotopic organization, increased total numbers of projecting motoneurons and a consistently elevated degree of axonal branching regardless of whether the animals were subjected to any of the paradigms or not.
3.4. Polyinnervation of the Motor Endplates Persists Regardless of Postoperative Treatment
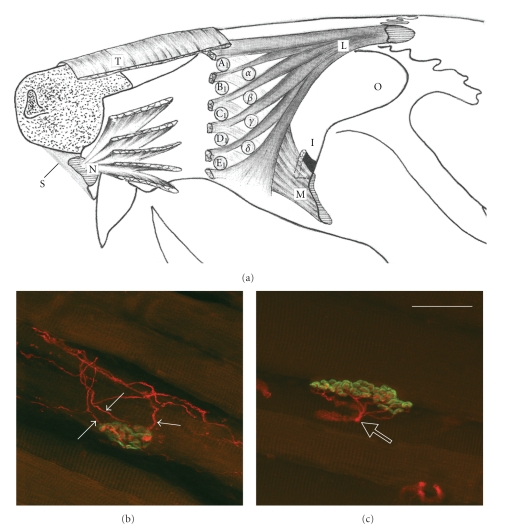
Reinnervation of individual skeletal target muscle fibers revealed that in contrast to intact animals, in which all motor endplates were monoinnervated, nerve transection followed by treatments with collagen, naïve SCs or those overexpressing FGF-218kD or FGF-221/23kD resulted in 45–49% of the motor endplates being polyinnervated, that is, innervated by two or more axons (groups 2–5; Figure 5; Table 3). Furthermore, manual stimulation did not reduce the degree of polyinnervation (groups 6–9; 42–51%, Table 3).
Figure 5.
(a) Schematic drawing of the extrinsic vibrissae muscles. α-δ: the four caudal hair follicles, the muscles slings of which “straddle” the five vibrissae rows (A)–(E); T–m. transversus nasi; L–m. levator labii superioris; N–m. nasalis; M–m. maxilolabialis; O–orbit; S–septum intermusculare. (b) and (c) are the superimposed stacks of confocal images of end-plates in LLS muscles of intact and surgically treated rats visualized by staining of the motor end-plates with Alexa Fluor 488 α-bungarotoxin (green fluorescence) and immunostaining of the intramuscular axons for neuronal class III β-tubulin (Cy3 red fluorescence). (b) and (c) show examples of a polyinnervated and a monoinnervated end-plate, respectively. Three axonal branches (arrows in (b)) reach the boundaries of the polyinnervated end-plate delineated by the alpha-bungarotoxin staining. In contrast, the monoinnervated end-plate is reached by a single axon (empty arrow in (c)) with several preterminal rami. In both examples, the whole end-plates are within the stack of confocal images. Scale bar shown in (c) indicates 125 μm (adopted from [31]).
4. Discussion
Here we show that regardless of the post-operative treatment following facial nerve injury, the index of collateral branching at the lesion site remained high (46–67%), the proportion of polyinnervated motor endplates in the musculature rose from 0% to 42–51% and the amplitude of vibrissal whisking remained low, ranging from 25% to 30% of that in intact animals. Thus, although axonal sprouting occurred, it appeared to be invariant and occurred to a similar extent for each of the treatment groups and none was functionally useful. Furthermore, in contrast to previous findings [17], daily MS of whiskerpad muscles failed to “harness” the regenerative sprouting to deliver improved postoperative recovery of function.
Growth of lesioned peripheral axons is vigorously supported by a large number of trophic factors expressed locally (review in [32]). Within an earlier experimental set we tested the hypothesis that neutralization of diffusable neurotrophic factors at the lesion site in rats could reduce the collateral branching of transected axons and thus improve quality of reinnervation [33]. We inserted both ends of the transected facial nerve into a silicon tube containing collagen gel with neutralizing concentrations of antibodies to several growth factors including FGF-2. Two months later, retrograde labeling was used to estimate the proportion of motoneurons with branched axons projecting into the three major branches of the facial nerve. Neutralizing concentration of anti-FGF-2 significantly reduced the index of collateral axonal branching to 22% [33]. Thus, by manipulating the local environment using neutralizing antibody to FGF2, we achieved a strong reduction in collateral axonal branching from the proximal stump and a significant improvement of the reinnervation quality in several groups of rats. In the same animals, however, the function of the reinnervated vibrissae muscles remained as poor as in nontreated injured animals. As a potential reason for the ineffectiveness of the treatment we identified the well-known posttransectional polyneuronal innervation of the motor endplates, a phenomenon which was not directly manipulated in our experiments. These results raise questions of fundamental importance with regard to the mechanisms limiting functional recovery and to the perspectives for identifying new efficient treatment strategies [31].
So far we have no evidence that the observed pattern of reinnervation and recovery of vibrissae whisking after treatment with collagen, naïve SCs, or those overexpressing FGF-218kD or FGF-221/23kD represent an endpoint, and not just a delayed recovery. This is why we cannot exclude that an improvement might take palce in these animals. However, based on several long term clinical data [34] and own observations [16, Chapter 3.1.5.] we anticipate exactly the opposite. The persisting axonal misguidance and poly-innervation of muscles (in contrast to the events during embryonic development, there occurs no retrieval of excessive axonal terminals from the NMJ in adults) cause a wasting palsy (paresis and atrophy of the muscles) and progressive functional deterioration. Anyway, to clear this issue definitely, new experiments with longer postoperative survival periods have to be performed.
Within the CNS, transplantation of supportive cells, such as Schwann cells, significantly increases the extent of regeneration within the bands of Büngner—long chains Schwann cells of cells, which bridge the interfragmentary gap and form guiding channels for the regenerating branches on their way to the target(s) [2, 35, 36]. Schwann cell transplantation is used within the PNS to bridge larger nerve gaps that axons lacking physical and trophic support are not able to cross [20]. Similar to autologous peripheral nerve grafts, transplanted SCs in synthetic nerve bridges provide regrowing peripheral axons with supportive environmental conditions to overcome large nerve defects [37]. A further advantage is that synthetic bridges avoid the problem of removing nerve tissue for transplantation, which in turn would induce its own defect. In particular, SCs have been considered to be useful because of the large range of neurotrophic factors that they naturally secrete [17, 38]. Synthetic nerve bridges can be constructed in a number of ways [39]. The efficacy of bridges containing neurotrophic factors alone is limited by short half-life. Schwann cells, however, could in addition to secreting their own range of growth factors be genetically engineered exvivo to induce long-term release of selected neurotrophic factors in a site-directed manner [40].
Indeed, we have previously demonstrated that transplantation of genetically modified SCs overexpressing FGF-2 into a large gap (15 mm) in the rat sciatic nerve supported functional recovery [20]. However, the type of recovery was depended on the FGF-2 isoform. Whereas sensory recovery was accelerated by FGF-221/23kD, motor recovery seemed only to be influenced by FGF-218kD [11]. Additionally, it has recently been demonstrated that FGF-2 deficient mice show a faster sensory recovery after sciatic nerve crush injury [41]. However, synergistic beneficial effects on promoting axonal regrowth could be demonstrated for FGF-221/23kD, and not FGF-218kD, gene therapy after sciatic nerve entubulation surgery followed by motor-enriched rehabilitation, such as voluntary wheel running [20]. Today there is no clear evidence if motor axonal regeneration and functional recovery of a mixed peripheral nerve could be supported by any FGF-2 isoform alone.
In contrast, we have shown here, for the purely motor facial nerve, that neither FGF-2 isoform, delivered via an ex vivo gene therapy approach, increased the amount of regeneration, as measured by the extent of sprouting, nor enhanced functional recovery. Thus for both FGF-2 isoforms, the index of collateral axonal branching, that is, sum of the percentages of DiI+FG (third column, Table 2) plus DiI+FB (fourth column, Table 2) labeled neurons was 48% and 50%, respectively, and did not differ significantly from collagen alone (54%) or naïve SCs (46%). The present data thus suggest that although FGF-2 supports bridging of the 5 mm gap, this growth factor does not improve the accuracy of regrowth of facial nerve axons over and above that induced by collagen alone or naïve SCs.
Interestingly, the extent of collateral branching at the lesion site in the current study may be somewhat less than that induced by facial nerve transection and end-to-end re-anastomosis (67% in [17], that is, without the ~5 mm gap inherent during the silicone tube grafting procedure. This indicates that the extent of collateral axonal branching at the lesion site is somehow limited when the gap is large. One possible reason could be found in the lack of a suture-caused tight apposition between the regrowing neurites and the approximated Büngner's bands of the distal fragment undergoing Walerian degeneration.
Whereas it is evident that neither FGF-2 isoform confers an advantage for bridging large defects in a pure motor nerve like the facial nerve, FGF-221/23kD is clearly advantageous within the mixed sciatic nerve: functional recovery is improved to differing degrees by the different FGF-2 isoforms [20]. Furthermore, synergistic beneficial effects of FGF-221/23kD gene therapy with regard to promoted axonal growth was demonstrated for sciatic nerve entubulation surgery followed by motor-enriched rehabilitation, such as voluntary wheel running [18]. The reasons for the difference in outcomes for the sciatic and facial nerve are unknown but could include differences in the types of readouts and assessments naturally associated with two such functionally different nerves. In addition, depending on different stimuli applied, differences in target tissue signaling after reconstructive surgery could have accounted for the different outcome in the discussed models: denervated muscles have been shown to produce short-range diffusible sprouting stimuli [42, 43]. Various growth factors have been identified as possible candidates for this role [44]; described in what follows). Their amount is inversely proportional on muscle activity [45, 46]. A future set of experiments would be needed to determine if ex vivo gene therapy with other single or combined growth factors could beneficially affect the outcome of facial nerve entubulation repair.
It is puzzling why a gap of only 5 mm, which is after all bridged successfully and located far from the target, would hamper the effects of manual stimulation of the vibrissal muscles. What is the reason for the lack of reduced polyinnervation of the motor end-plates? We do not know that so far, but will try to answer this question using the time course of target reinnervation. Let us assume that mechanical stimulation “simulates” sufficient muscle activity and normal (i.e., nonincreased) local production of growth factors only for a limited period of time, immediately after the nerve lesion. This would allow the regrowing axons to reach their motor targets slowly, accurately and with less intramuscular terminal sprouting. After a 5 mm nerve gap, this time schedule cannot be followed anymore: the regrowing axons need more time to bridge the gap. The synchronously increased production of trophic factors urges them to grow rapidly, with more intramuscular sprouts. Thus it seems that though relative short, the gap of 5 mm is a serious hurdle for the effects of manual mechanical stimulation [47].
In summary, we show that although a gap of 5 mm in the facial nerve lesion paradigm can be successfully bridged by entubulation, in terms of axonal sprouting, regeneration across the gap is the same regardless of whether SCs are present and also regardless of whether they overexpress FGF-218kD/FGF-221/23kD. Comparison with end-to-end suturing as a repair technique shows that the amount of sprouting might be reduced, the larger the gap, possibly due to the lack of support and increased guidance errors. Furthermore, manual stimulation, which is able to restore normal function if the gap is small, fails to do so for any of the treatments involving entubulation in which the gap is long. We conclude that although entubulation of a transected peripheral (facial) nerve in suspension of Schwann cells whcih overexpress FGF-218kD/FGF-221/23kD supports the axon regeneration through a large (5 mm) gap, the procedure induces excessive collateral branching at the lesion site and massive terminal sprouting in the mimic muscles. Furthermore, under these circumstances, the therapeutic procedure of manual stimulation of denervated muscles had no beneficial effect.
Acknowledgments
The present study was supported by the Jean-Uhrmacher Foundation, Köln Fortune Programm, and the DFG (AN 331/3-1, AN 331/5-1). S. A. Dunlop is a Senior Research Fellow (National Health & Medical Research Council, Australia; Grant ID: 513700). The skillful assistance of D. Bösel, D. Felder, K. Glück, N. Lange J. Rahn and L. Wilken is highly appreciated. We are very grateful to M. Wesemann, K. Kuhlemann, Y. Haile, and H. Streich for the excellent technical help.
References
- 1.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Engineering. 2000;6(2):119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 2.Mosahebi A, Fuller P, Wiberg M, Terenghi G. Effect of allogeneic schwann cell transplantation on peripheral nerve regeneration. Experimental Neurology. 2002;173(2):213–223. doi: 10.1006/exnr.2001.7846. [DOI] [PubMed] [Google Scholar]
- 3.Ansselin AD, Fink T, Davey DF. Peripheral nerve regeneration through nerve guides seeded with adult Schwann cells. Neuropathology and Applied Neurobiology. 1997;23(5):387–398. [PubMed] [Google Scholar]
- 4.Guenard V, Aebischer P, Bunge RP. The astrocyte inhibition of peripheral nerve regeneration is reversed by Schwann cells. Experimental Neurology. 1994;126(1):44–60. doi: 10.1006/exnr.1994.1041. [DOI] [PubMed] [Google Scholar]
- 5.Lago N, Casas C, Muir EM, Rogers J, Navarro X. Effects of Schwann cell transplants in an experimental nerve amputee model. Restorative Neurology and Neuroscience. 2009;27(1):67–78. doi: 10.3233/RNN-2009-0462. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y-S, Hsieh C-L, Tsai C-C, et al. Peripheral nerve regeneration using silicone rubber chambers filled with collagen, laminin and fibronectin. Biomaterials. 2000;21(15):1541–1547. doi: 10.1016/s0142-9612(00)00028-4. [DOI] [PubMed] [Google Scholar]
- 7.Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. Journal of the Peripheral Nervous System. 2007;12(2):65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 8.Butt AM, Dinsdale J. Fibroblast growth factor 2 induces loss of adult oligodendrocytes and myelin in vivo. Experimental Neurology. 2005;192(1):125–133. doi: 10.1016/j.expneurol.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Dono R. Fibroblast growth factors as regulators of central nervous system development and function. American Journal of Physiology. 2003;284(4):R867–R881. doi: 10.1152/ajpregu.00533.2002. [DOI] [PubMed] [Google Scholar]
- 10.Grothe C, Timmer M. The physiological and pharmacological role of basic fibroblast growth factor in the dopaminergic nigrostriatal system. Brain Research Reviews. 2007;54(1):80–91. doi: 10.1016/j.brainresrev.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Grothe C, Haastert K, Jungnickel J. Physiological function and putative therapeutic impact of the FGF-2 system in peripheral nerve regeneration-Lessons from in vivo studies in mice and rats. Brain Research Reviews. 2006;51(2):293–299. doi: 10.1016/j.brainresrev.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Aebischer P, Salessiotis AN, Winn SR. Basic fibroblast growth factor released from synthetic guidance channels facilitates peripheral nerve regeneration across long nerve gaps. Journal of Neuroscience Research. 1989;23(3):282–289. doi: 10.1002/jnr.490230306. [DOI] [PubMed] [Google Scholar]
- 13.Danielsen N, Pettmann B, Vahlsing HL, Manthorpe M, Varon S. Fibroblast growth factor effects on peripheral nerve regeneration in a silicone chamber model. Journal of Neuroscience Research. 1988;20(3):320–330. doi: 10.1002/jnr.490200306. [DOI] [PubMed] [Google Scholar]
- 14.Jungnickel J, Haase K, Konitzer J, Timmer M, Grothe C. Faster nerve regeneration after sciatic nerve injury in mice over-expressing basic fibroblast growth factor. Journal of Neurobiology. 2006;66(9):940–948. doi: 10.1002/neu.20265. [DOI] [PubMed] [Google Scholar]
- 15.Timmer M, Robben S, Muller-Ostermeyer F, Nikkhah G, Grothe C. Axonal regeneration across long gaps in silicone chambers filled with Schwann cells overexpressing high molecular weight FGF-2. Cell Transplantation. 2003;12(3):265–277. doi: 10.3727/000000003108746821. [DOI] [PubMed] [Google Scholar]
- 16.Angelov DN, Guntinas-Lichius O, Wewetzer K, Neiss WF, Streppel M. Axonal branching and recovery of coordinated muscle activity after transection of the facial nerve in adult rats. Advances in Anatomy Embryology and Cell Biology. 2005;180:1–130. [PubMed] [Google Scholar]
- 17.Angelov DN, Ceynowa M, Guntinas-Lichius O, et al. Mechanical stimulation of paralyzed vibrissal muscles following facial nerve injury in adult rat promotes full recovery of whisking. Neurobiology of Disease. 2007;26(1):229–242. doi: 10.1016/j.nbd.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Haastert K, Ying Z, Grothe C, Gomez-Pinilla F. The effects of FGF-2 gene therapy combined with voluntary exercise on axonal regeneration across peripheral nerve gaps. Neuroscience Letters. 2008;443(3):179–183. doi: 10.1016/j.neulet.2008.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keilhoff G, Prätsch F, Wolf G, Fansa H. Bridging extra large defects of peripheral nerves: possibilities and limitations of alternative biological grafts from acellular muscle and Schwann cells. Tissue Engineering. 2005;11(7-8):1004–1014. doi: 10.1089/ten.2005.11.1004. [DOI] [PubMed] [Google Scholar]
- 20.Haastert K, Lipokatic E, Fischer M, Timmer M, Grothe C. Differentially promoted peripheral nerve regeneration by grafted Schwann cells over-expressing different FGF-2 isoforms. Neurobiology of Disease. 2006;21(1):138–153. doi: 10.1016/j.nbd.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Dohm S, Streppel M, Guntinas-Lichius O, et al. Local application of extracellular matrix proteins fails to reduce the number of axonal branches after varying reconstructive surgery on rat facial nerve. Restorative Neurology and Neuroscience. 2000;16(2):117–126. [PubMed] [Google Scholar]
- 22.Guidry C, Grinnell F. Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. Journal of Cell Biology. 1987;104(4):1097–1103. doi: 10.1083/jcb.104.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mauch C, Hatamochi A, Scharffetter K, Krieg T. Regulation of collagen synthesis in fibroblasts within a three-dimensional collagen gel. Experimental Cell Research. 1988;178(2):493–503. doi: 10.1016/0014-4827(88)90417-x. [DOI] [PubMed] [Google Scholar]
- 24.Haastert K, Grosskreutz J, Jaeckel M, et al. Rat embryonic motoneurons in long-term co-culture with Schwann cells—a system to investigate motoneuron diseases on a cellular level in vitro. Journal of Neuroscience Methods. 2005;142(2):275–284. doi: 10.1016/j.jneumeth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Mauritz C, Grothe C, Haastert K. Comparative study of cell culture and purification methods to obtain highly enriched cultures of proliferating adult rat Schwann cells. Journal of Neuroscience Research. 2004;77(3):453–461. doi: 10.1002/jnr.20166. [DOI] [PubMed] [Google Scholar]
- 26.Muller-Ostermeyer F, Claus P, Grothe C. Distinctive effects of rat fibroblast growth factor-2 isoforms on PC12 and Schwann cells. Growth Factors. 2001;19(3):175–191. doi: 10.3109/08977190109001085. [DOI] [PubMed] [Google Scholar]
- 27.Verdu E, Navarro X, Gudino-Cabrera G, et al. Olfactory bulb ensheathing cells enhance peripheral nerve regeneration. NeuroReport. 1999;10(5):1097–1101. doi: 10.1097/00001756-199904060-00035. [DOI] [PubMed] [Google Scholar]
- 28.Tomov TL, Guntinas-Lichius O, Grosheva M, et al. An example of neural plasticity evoked by putative behavioral demand and early use of vibrissal hairs after facial nerve transection. Experimental Neurology. 2002;178(2):207–218. doi: 10.1006/exnr.2002.8040. [DOI] [PubMed] [Google Scholar]
- 29.Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R. Thompson. Journal of Microscopy. 1986;143:3–45. [PubMed] [Google Scholar]
- 30.Popratiloff AS, Neiss WF, Skouras E, Streppel M, Guntinas-Lichius O, Angelov DN. Evaluation of muscle re-innervation employing pre- and post-axotomy injections of fluorescent retrograde tracers. Brain Research Bulletin. 2001;54(1):115–123. doi: 10.1016/s0361-9230(00)00411-1. [DOI] [PubMed] [Google Scholar]
- 31.Guntinas-Lichius O, Irintchev A, Streppel M, et al. Factors limiting motor recovery after facial nerve transection in the rat: combined structural and functional analyses. European Journal of Neuroscience. 2005;21(2):391–402. doi: 10.1111/j.1460-9568.2005.03877.x. [DOI] [PubMed] [Google Scholar]
- 32.Boyd JG, Gordon T. Neurotrophic factors and their receptors in axonal regeneration and functional recovery after peripheral nerve injury. Molecular Neurobiology. 2003;27(3):277–323. doi: 10.1385/MN:27:3:277. [DOI] [PubMed] [Google Scholar]
- 33.Streppel M, Azzolin N, Dohm S, et al. Focal application of neutralizing antibodies to soluble neurotrophic factors reduces collateral axonal branching after peripheral nerve lesion. European Journal of Neuroscience. 2002;15(8):1327–1342. doi: 10.1046/j.1460-9568.2002.01971.x. [DOI] [PubMed] [Google Scholar]
- 34.Greulich M. Anchoring the nasolabial fold. In: Beurskens CH, van Gelder RS, Heymans PG, Manni JJ, Nicolai JPA, editors. The Facial Palsies. Utrecht, The Netherlands: Lemma Publishers; 2005. pp. 235–243. [Google Scholar]
- 35.Büngner O. Ueber die Degenerations- und Regenerationsvorgänge am Nerven nach Verletzungen. Beiträge zur Pathologischen Anatomie. 1891;10:21–393. [Google Scholar]
- 36.Golden KL, Pearse DD, Blits B, et al. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Experimental Neurology. 2007;207(2):203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lundborg G. Alternatives to autologous nerve grafts. Handchirurgie Mikrochirurgie Plastische Chirurgie. 2004;36(1):1–7. doi: 10.1055/s-2004-820870. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annual Review of Biomedical Engineering. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 39.Chalfoun CT, Wirth GA, Evans GRD. Tissue engineered nerve constructs: where do we stand? Journal of Cellular and Molecular Medicine. 2006;10(2):309–317. doi: 10.1111/j.1582-4934.2006.tb00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haastert K, Grothe C. Gene therapy in peripheral nerve reconstruction approaches. Current Gene Therapy. 2007;7(3):221–228. doi: 10.2174/156652307780859035. [DOI] [PubMed] [Google Scholar]
- 41.Jungnickel J, Haastert K, Grzybek M, et al. Mice lacking basic fibroblast growth factor showed faster sensory recovery. doi: 10.1016/j.expneurol.2009.06.003. Experimental Neurology. In press. [DOI] [PubMed] [Google Scholar]
- 42.Pockett S, Slack JR. Source of the stimulus for nerve terminal sprouting in partially denervated muscle. Neuroscience. 1982;7(12):3173–3176. doi: 10.1016/0306-4522(82)90239-1. [DOI] [PubMed] [Google Scholar]
- 43.Slack JR, Pockett S. Terminal sprouting of motoneurones is a local response to a local stimulus. Brain Research. 1981;217(2):368–374. doi: 10.1016/0006-8993(81)90013-5. [DOI] [PubMed] [Google Scholar]
- 44.Sendtner M. Neurotrophic factors: effects in modulating properties of the neuromuscular endplate. Cytokine and Growth Factor Reviews. 1998;9(1):1–7. doi: 10.1016/s1359-6101(97)00033-6. [DOI] [PubMed] [Google Scholar]
- 45.Brown MC, Ironton R. Motor neurone sprouting induced by prolonged tetrodotoxin block of nerve action potentials. Nature. 1977;265(5593):459–461. doi: 10.1038/265459a0. [DOI] [PubMed] [Google Scholar]
- 46.Brown MC, Holland RL, Hopkins WG, Keynes RJ. An assessment of the spread of the signal for terminal sprouting within and between muscles. Brain Research. 1981;210(1-2):145–151. doi: 10.1016/0006-8993(81)90891-x. [DOI] [PubMed] [Google Scholar]
- 47.Grosheva M, Arnhold S, Guntinas-Lichius O, et al. Bone marrow-derived mesenchymal stem cell transplantation does not improve quality of muscle reinnervation or recovery of motor function after facial nerve transection in rats. Biological Chemistry. 2008;389(7):873–888. doi: 10.1515/BC.2008.100. [DOI] [PubMed] [Google Scholar]