Abstract
Pulmonary CMV infection (CMVI) and disease (CMVD) is associated with reduced long-term survival post-lung transplantation, however, the specific biologic mechanisms remain unclear. We have demonstrated a role of CC chemokines during lung allograft dysfunction. Based on these findings, we hypothesized that pulmonary CMV upregulates the expression of multiple CC chemokines that leads to allograft dysfunction and decreased long-term survival.
We performed a nested case control study in lung transplant recipients to investigate alterations in CC chemokine biology during pulmonary CMV. Levels of CC chemokines were measured in bronchoalveolar lavage fluid (BALF) from recipients with CMVI (n = 33), CMVD (n = 6), and in healthy lung transplant controls (n = 33). We found a trend toward increased levels of MIP-1α/CCL3 during pulmonary CMVI. Levels of MCP-1/CCL2 and RANTES/CCL5 were significantly elevated during pulmonary CMV. Interestingly, elevated levels of CCL3 in BALF were protective with regards to survival. Importantly, elevated levels of CCL2 in BALF predicted the development of BOS, while elevated levels of CCL5 in BALF predicted an increase in mortality post-lung transplant.
Altered levels of specific CC chemokines during pulmonary CMV are associated with future clinical outcomes. These results suggest a possible utility of BALF CC chemokines as biomarkers for guiding risk assessment during pulmonary CMV post-lung transplantation.
Keywords: Bronchiolitis obliterans syndrome, chemokines, cytomegalovirus (CMV), lung transplantation, survival
Introduction
Lung transplantation is only a treatment and not a cure for end-stage pulmonary disorders due to both infectious and noninfectious complications. With regard to infectious complications, cytomegalovirus (CMV) remains the most common opportunistic pathogen (1). CMV post-lung transplantation can be divided into CMV infection (CMVI) and CMV disease (CMVD). CMVI is viral replication without notable organ disease. This viral shedding can be detected in bronchoalveolar lavage fluid (BALF), blood or other body fluids (2). CMVD is defined by histologic evidence of tissue invasion that results in organ damage (2). While CMVD can manifest as symptomatic viremia, bone marrow suppression, hepatitis, gastroenteritis and colitis, CMV pneumonitis is the most common presentation post-lung transplantation (3).
In addition to ‘direct’ allograft injury due to uncontrolled viral replication, CMV also has ‘indirect’ effects leading to long-term adverse sequelae (4). For instance, CMVI is considered a ‘potential’ and CMVD a ‘probable’ risk factor for bronchiolitis obliterans syndrome (BOS), the most common cause of mortality post-lung transplantation (5). The indirect mechanism by which pulmonary CMV may promote BOS and decrease long-term survival post-lung transplantation remains to be elucidated.
CC chemokines are potent recruiters of inflammatory cells and have been shown to be critical in mediating allograft dysfunction across all solid organ transplantations (6–8). In addition, we and others have demonstrated that increased levels of CCL2 and CCL5 in BALF post-lung transplantation are associated with the continuum of acute-to-chronic lung allograft rejection (9–11). Based on these findings we hypothesized that pulmonary CMV upregulates these specific immune response CC chemokines, which perpetuate allograft inflammation, ultimately leading to lung allograft dysfunction and increased mortality.
This study evaluates the ability of pulmonary CMV to up-regulate the production of CC chemokines, which predict the future development of BOS and mortality among lung transplant recipients.
Materials and Methods
Patient selection and study definitions
With Institutional Review Board approval and informed written consent, patients undergoing lung transplantation between June 1, 1992 and May 31, 2000 were prospectively enrolled into an observational cohort to investigate mechanisms of allograft dysfunction with the collection of BALF for subsequent research analysis. Patients from this cohort were eligible for this nested case control study if they had at least one BALF specimen available in our research laboratory. We were able to capture at least one BALF specimen from 93% of all patients transplanted during this time period.
For the purpose of this study, pulmonary CMVI was defined as CMV detection in BALF by shell vial (early antigen detection), pp65 antigen, or culture, without CMV cytopathologic changes on cytology or transbronchial biopsy (TBBx), in an asymptomatic lung transplant recipient undergoing surveillance bronchoscopy. Pulmonary CMVD was defined by the detection of CMV inclusions in BALF cytology or on biopsy in a symptomatic lung transplant recipient with radiographic allograft infiltrates. A healthy BALF sample was defined as one collected from an asymptomatic lung transplant recipient undergoing surveillance bronchoscopy without evidence of acute cellular rejection (ACR) (grade ≥A1), BOS or infection/colonization. Infection/colonization was defined as any positive BALF culture, as determined by the clinical microbiology laboratory. ACR was diagnosed by histologic examination of (TBBx) specimens according to standard criteria (12,13).
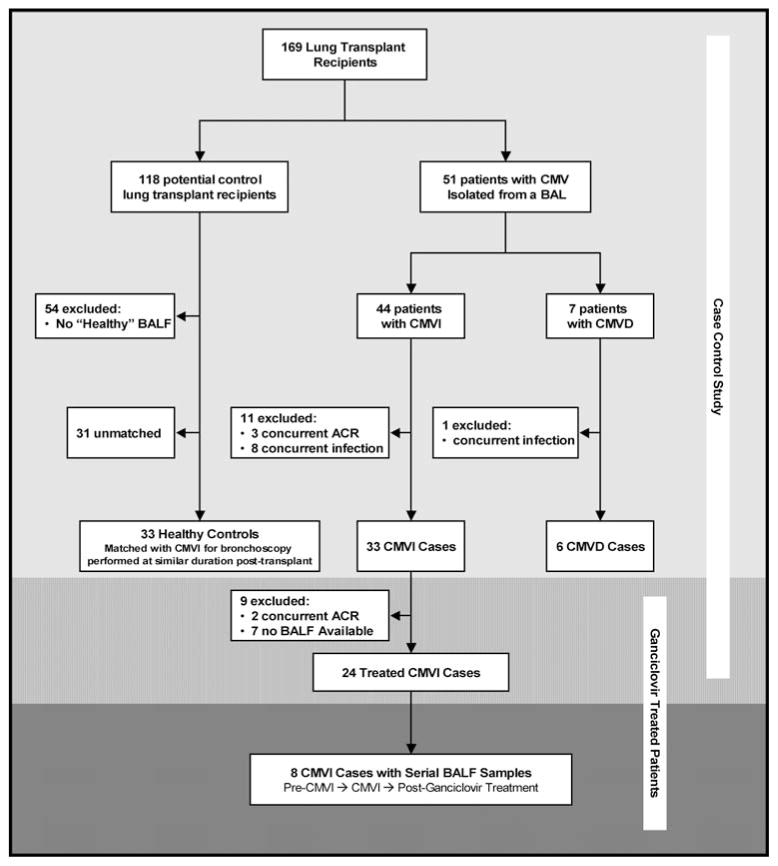
Patients with pulmonary CMVI, without concurrent ACR (grade ≥ A1), BOS or other infection/colonization were selected as CMVI cases. None of these CMVI cases went on to develop CMVD. Patients with pulmonary CMVD without concurrent ACR (grade ≥ A1), BOS or other infection/colonization, were selected as CMVD cases. Lung transplant patients who never developed pulmonary CMV (CMVI or CMVD) were potential controls. Potential controls without a ‘healthy’ BALF sample were excluded. More specifically, we excluded potential control BALF samples with concurrent ACR (grade ≥ A1), BOS or infection/colonization. Healthy control subjects were selected based on a healthy BALF sample collected at a duration post-lung transplant similar to CMVI BALF samples and matched in a 1 to 1 manner (Figure 1).
Figure 1. Algorithm for subject selection in this nested case control study.
BALF samples with concurrent rejection or infection/colonization other than CMV were excluded. CMVI cases (n = 33) were compared to healthy lung transplant control patients (n = 33) that were matched 1:1 based on a BALF sample collected at a similar duration post-transplantation. Unmatched CMVD cases (n = 6) were also included in the study and compared separately. The shaded areas represent pulmonary CMV patients treated with a full course of i.v. ganciclovir. Of the CMVI cases (n = 33), 24 had a post-treatment sample (medium shaded area) and 8 had both a pre-CMVI sample and a post-ganciclovir sample (dark shaded area).
All lung transplant recipients with pulmonary CMV were treated with i.v. ganciclovir 5 mg/kg every 12 h for 5 days followed by 5 mg/kg/day and completed a 21-day course. A subset of CMVI cases had serial BALF samples available for analysis. A posttreatment BALF sample, without concurrent ACR or non-CMV infection/colonization, was available for 24 CMVI cases (Figure 1, medium shaded area). Both a pre-CMVI sample and a posttreatment sample, without concurrent infection or rejection, were available for eight CMVI cases (Figure 1, dark shaded area).
Clinical data collection
The data base for the lung transplant cohort included demographic characteristics, survival data and clinical information corresponding to each bronchoscopy. Specifically, bronchoscopy derived data included BAL microbiology, cytology and the (TBBx) pathology results. For this study, we diagnosed ACR (ISHLT grade A1 or greater) by (TBBx). Episodes of clinically significant ACR were defined as ISHLT grade A2 or greater or grade A1 that was symptomatic, all of which were treated with methylprednisolone 1 g i.v. for 3 days. Asymptomatic grade A1 ACR was not treated. A cumulative acute rejection score was calculated by adding the sum of A grades of each rejection episode as previously described (14). The presence of BOS was determined according to standard International Society for Heart and Lung Transplant definitions (5,15). Briefly, an unexplained decrease in the forced expiratory volume in 1 s (FEV1) by 20% or more from the peak posttransplant value was defined as BOS (5,15). The date and cause of death were obtained from the medical record and included in the data base. Data up to 2 years post-bronchoscopy classified as either CMVI, CMVD or healthy, and up to May 31 2000 were considered for this study.
Immunosuppressive and CMV prophylaxis protocols
All patients received maintenance immune suppression consisting of cyclosporine or tacrolimus, azathioprine or mycophenolate mofetil and corticosteroids tapered down over the first 6 months posttransplantation to 10 mg/day. All recipients with positive serology for CMV and those with serologic-positive donors were routinely prophylaxed for CMV with 12 weeks of i.v. ganciclovir 5 mg/kg/day.
Posttransplant surveillance protocol
Lung transplant recipients were followed according to a standard protocol. Briefly, this protocol included clinical visits weekly for the first 3 weeks, then at 6 weeks, and then at 3, 6, 9 and 12 months. Subsequently, they were seen every 4 months for the second year and thereafter annually. Routine surveillance bronchoscopy with bronchoalveolar lavage (BAL) and TBBx was preformed at 6 weeks and at 3, 6, 9 and 12 months post-lung transplantation. Bronchoscopy was also preformed at times when infection or rejection was suspected.
BALF specimen collection and processing
Attempts were made to collect BALF specimens for research analysis at the time of each lung transplant bronchoscopy and BALF processing was performed as previously described (16). Briefly, a flexible bronchoscope was wedged into a sub-segmental bronchus in either the lingula, right middle lobe or a predetermined region of interest based on radiographic findings. A BAL was done by instilling a total of 240 mL of isotonic saline in 60 mL aliquots. After each aliquot, BALF was retrieved using low-pressure suction and the fluid fractions were pooled. BALF samples were split equally into clinical and research samples. After centrifugation of the research sample to remove cells, the supernatant was collected for storage at −80°C until used for ELISA. For this study, to avoid any influences of rejection or infection/colonization on BALF chemokine levels, we excluded any BALF sample with concurrent ACR (grade ≥ A1), BOS or infection/colonization other than CMV.
MCP-1/CCL2, MIP-1α/CCL3, MIP-1β/CCL4 and RANTES/CCL5 ELISAs
Human CCL2, CCL3, CCL4 and CCL5 protein levels from the unconcentrated BALF samples were quantified using DuoSet ELISA development kits (R&D Systems, Minneapolis, MN). Levels were not adjusted for internal or external markers because there is no available method for accurate determination of the dilution factor. The lowest detectable limits for CCL2, CCL3, CCL4 and CCL5 were determined to be 12, 14, 15 and 12 pg/mL, respectively.
Immunohistochemistry
Immunohistochemistry was performed on paraffin embedded slides for the localization of CC chemokine ligands and their receptors as previously described (17). Specifically, after deparaffinization and steam bath antigen retrieval in citrate buffer (pH 6.0), endogenous perioxidase was quenched with 3% hydrogen peroxide in 50% methanol for 15 min. Non-specific binding was minimized by incubation in either 3% normal rabbit serum (for anti-CCL5) or 3% normal horse serum (for all other antibodies) for 30 min. Endogenous biotin was blocked with an avidin/biotin blocking kit according to the manufacturer specifications (Vector Laboratory, Inc., Burlingame, CA). Slides were then incubated overnight at 4°C with murine anti-human CCL2 antibody (R&D clone 23002), goat polyclonal anti-human CCL5 antibody (R&D), murine anti-human CCR1 antibody (R&D clone 53504), murine anti-human CCR2 antibody (R&D clone 48607), murine anti-human CCR5 antibody (R&D clone 45523) or the appropriate isotype control IgG. Specific labeling was detected with a biotin conjugated rabbit anti-goat or horse anti-murine secondary antibody and application of horseradish peroxidase–bound avidin–biotin from Vectastain ABC kits (Vector), followed by development with DAB solution (Vector).
Statistical analysis
Baseline characteristics of the study population are expressed as means ± SD unless otherwise noted. The hypotheses tested were ordered according to predetermined levels of importance. To account for multiple comparisons, each hypothesis level is treated as a ‘gatekeeper’ so that the next level is tested only if the null hypothesis for the gatekeeper test has been rejected. This approach controls for type 1 error at the 0.05 level as previously described (18). The predetermined order of hypotheses tested included the comparison of CCL5 levels in the healthy and CMVI groups followed by the comparison of CCL2 between healthy and CMVI groups. These tests were followed by comparisons of CCL2 and CCL5 between healthy and CMVD groups, then by inter-group comparisons of CCL3 and CCL4, chemokines that share receptors with CCL5. All other measures and scenarios tested were considered exploratory and reported p-values were unadjusted.
The paired Wilcoxon test was used to compare BALF CC chemokine levels between CMVI cases and matched healthy controls as well as for the comparison of pre-CMVI CC chemokine levels to those during CMVI and post-ganciclovir treatment. Comparisons of BALF CC chemokine levels between unmatched groups (CMVD vs. healthy, CMVD vs. CMVI, and treated CMVI vs. healthy) were performed using the unpaired Wilcoxon test. Data were displayed using a Box plot summary. The plot’s horizontal line represents the median, the box encompasses the 25th to 75th percentile and the error bars encompass the 10th to the 90th percentile. The comparison of acute rejection rates (episodes/month) and cumulative acute rejection scores was carried out using Poisson regression.
To assess the effect of CMVI and CMVD on subsequent clinical outcomes, Kaplan–Meier curves for survival, freedom from BOS and freedom from death due to BOS were constructed and compared using the log-rank test. More specifically, for comparisons of freedom from death due to BOS, all deaths due to causes other than BOS were censored. Cox Proportional Hazards regression models were constructed to assess the impact of BALF CC chemokine levels on the development of BOS and on mortality among study patients.
Results
Study patient selection
A total of 182 transplants (172 lung and 10 heart-lung) were performed during the study period. The study cohort consisted of 169 transplant recipients with at least one BALF sample captured for research purposes. A pure population of pulmonary CMVI cases (n = 33) was matched with healthy lung transplant controls (n = 33) based on a BALF sample collected at a similar duration posttransplant. In addition, we included a pure population of pulmonary CMVD cases (n = 6) (Figure 1). CMVI was first identified in BALF at a median of 135 days posttransplantation (range, 21–1110 days). The BALF used for comparison from healthy lung transplant controls was performed at a median of 149 days posttransplantation (range, 16–1115 days). CMVD occurred at a median of 136 days (range, 48–546). The mean number of bronchoscopies performed was similar between groups (Healthy 5.3 ± 3.9, CMVI 5.8 ± 2.3, CMVD 4.8 ± 1.5). The baseline characteristics of the study groups were similar (Table 1).
Table 1.
Base line characteristics of subjects in each study group
| Patient characteristics | Healthy (N = 33) | CMV infection (N = 33) | CMV disease (N = 6) |
|---|---|---|---|
| Age, y (SD) | |||
| Age at transplant | 53 (±7) | 53 (±7) | 57 (±4) |
| Age at bronchoscopy | 54 (±7) | 54 (±8) | 58 (±3) |
| Sex, n (%) | |||
| Female | 17 (52) | 19 (58) | 4 (67) |
| Male | 16 (48) | 14 (42) | 2 (23) |
| Race, n (%) | |||
| White | 31 (94) | 31 (94) | 5 (83) |
| Black | 2 (6) | 1 (3) | 1 (17) |
| Other | – | 1 (3) | – |
| Transplant indication, n (%) | |||
| COPD | 26 (79) | 20 (61) | 4 (66) |
| Alpha-1 ATD | 2 (6) | 6 (18) | 1 (17) |
| Sarcoid | 2 (6) | 2 (6) | – |
| IPF | 2 (6) | 3 (9) | 1 (17) |
| Other | 1 (3) | 2 (6) | – |
| Type of transplant, n (%) | |||
| Single | 24 (73) | 32 (97) | 6 (100) |
| Double | 8 (24) | 1 (3) | – |
| Heart-lung | 1 (3) | – | – |
| CMV serology, n (%) | |||
| D+/R− | 4 (12) | 13 (39) | 2 (33) |
| D−/R− | 3 (9) | 0 | 0 |
| Days posttransplant | |||
| Median | 149 | 135 | 136 |
| Range | 16–1115 | 21–1110 | 48–546 |
CC chemokine alterations in BALF during CMVI and CMVD
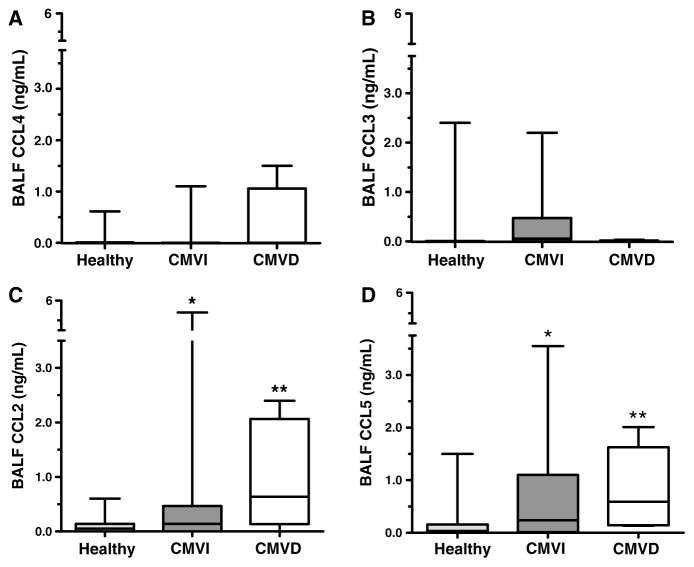
We determined if multiple immune response CC chemokines are elevated during pulmonary CMVI and CMVD. Neither CCL3 nor CCL4 BALF protein levels were increased significantly during pulmonary CMV compared to healthy lung transplant recipients (Figure 2A, B). However, there was a trend toward increased CCL3 protein levels during pulmonary CMVI (Figure 2B). Notably, the protein levels of CCL2 and CCL5 in BALF were significantly elevated during both CMVI and CMVD, compared to healthy lung transplant controls (Figure 2C, D).
Figure 2. BALF CC chemokine protein levels by ELISA among healthy lung transplant controls (n = 33), CMVI cases (n = 33) and CMVD cases (n = 6).
Data are expressed as box plots with median and intra-quartile range. (A) MIP1-β/CCL4 and (B) MIP1-α/CCL3 protein levels in BALF are not significantly increased during CMVI or CMVD, although there is a trend for increased CCL3 during CMVI compared to healthy controls (p = 0.11). (C) MCP-1/CCL2 and (D) RANTES/CCL5 BALF protein levels are significantly increased during both CMVI and CMVD. *p ≤ 0.05 (CMVI vs. healthy); **p ≤ 0.01 (CMVD vs. healthy).
Immunolocalization of CC chemokine ligands and receptors during pulmonary CMVI and CMVD
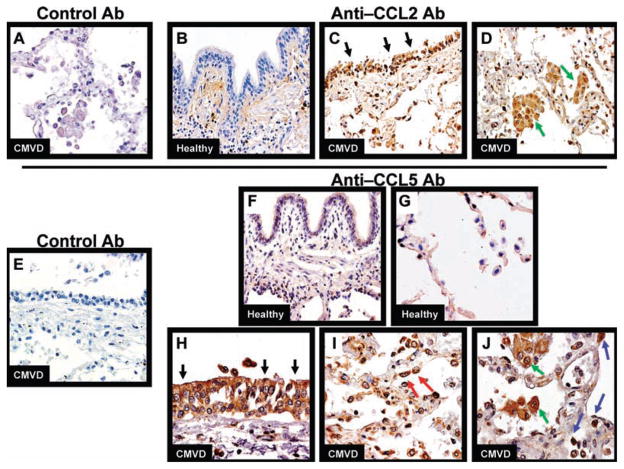
Finding BALF protein levels of CCL2 and CCL5 are increased during CMVI and CMVD, we performed immunohistochemistry (IHC) to identify the cells responsible for the production of these chemokines during CMVI and CMVD. We found CCL2 protein expression by bronchial epithelial cells and alveolar macrophages (AM) during CMVD (Figure 3C, D) and CMVI (not shown). Conversely, we found only occasional CCL2 protein expression by random mononuclear cells in healthy control biopsies (Figure 3B). CCL5 protein expression localized to bronchial epithelial cells, type II pneumocytes as identified by their location and cuboidal morphology, AM, and mononuclear cells during both CMVD (Figure 3H–J) and CMVI (not shown). There was virtually no CCL5 protein expression by these cell types in our healthy control biopsies (Figure 3F, G). There was no significant staining using the appropriate control Ab for CCL2 (Figure 3A) or CCL5 (Figure 3E).
Figure 3. Representative immunohistochemistry for CCL2, CCL5 and isotype control antibodies on lung allograft biopsy tissue.
(A) Lung biopsy from pulmonary CMVD demonstrates no specific staining with murine isotype control antibody. (B) Lung biopsy from healthy lung transplant recipient demonstrating that CCL2 protein only localized to rare mononuclear cells and no epithelial cells. Lung biopsy from pulmonary CMVD demonstrating CCL2 protein localized to (C) bronchial epithelial cells (black arrows) and (D) alveolar macrophages (AM) (green arrows). (E) Lung biopsy from pulmonary CMVD demonstrates no specific staining with goat isotype control antibody. Lung biopsy from healthy lung transplant recipient demonstrating no CCL5 protein localization to (F) mononuclear cells or bronchial epithelial cells, and (G) pneumocytes or AM. Lung biopsy from pulmonary CMVD showing CCL5 localizes to (H) bronchial epithelial cells (black arrows), (I) type II pneumocytes (red arrows), (J) AM (green arrows) and mononuclear cells (blue arrows). Panels A–G were photographed at 200× original magnification. Panels H–J were photographed at 400× original magnification.
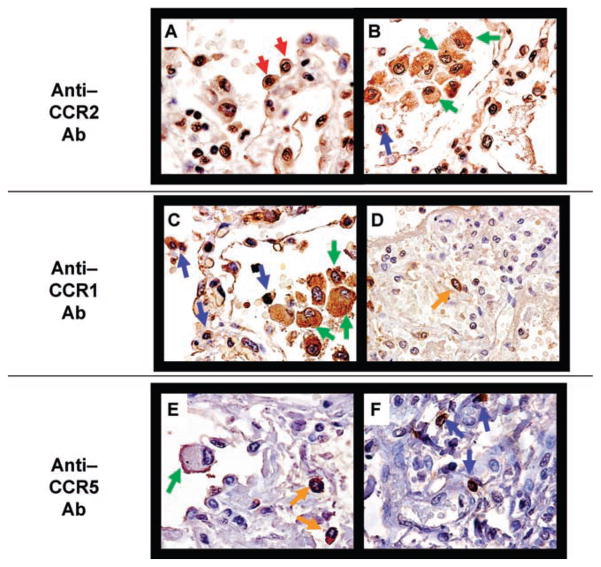
CC chemokine receptors were also evaluated by IHC in CMVI (n = 5) and CMVD (n = 5) patients. Specifically, we stained for CCR2, the major receptor for CCL2, and CCR1 and CCR5, the major receptors for CCL5. CCR2 protein expression localized to type II pneumocytes, AM and other mononuclear cells during CMVD (Figure 4A, B) and CMVI (not shown). CCR1 and CCR5 protein expression localized to AM, other mononuclear cells and mast cells during CMVD (Figure 4C–E) and CMVI (not shown).
Figure 4. Representative immunohistochemistry for CCR1, CCR2 and CCR5 on lung allograft biopsy tissue.
Pulmonary CMVD biopsy specimen showing CCR2 localization to (A) type II pneumocytes (red arrows) and (B) alveolar macrophages (AM) (green arrows) and other mononuclear cells (blue arrows). Pulmonary CMVD biopsy specimen showing CCR1 expression predominantly by (C) AM (green arrows), other mononuclear cells (blue arrows) and (D) interstitial mast cells (orange arrows). Pulmonary CMVD biopsy specimen showing CCR5 localization to (E) AM (green arrows), interstitial mast cells (orange arrows) and (F) other mononuclear cells (blue arrows). All images were photographed at 400× original magnification.
CCL5 remains elevated in BALF samples after treatment for pulmonary CMVI
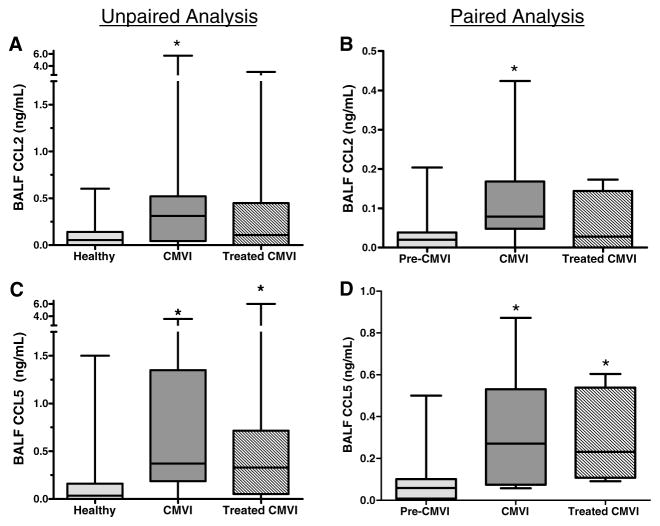
We determined if CCL2 and CCL5 remained elevated after ganciclovir treatment for pulmonary CMVI. Among the 33 CMVI cases, 24 patients had a posttreatment sample, without concurrent ACR (grade ≥ A1) or infection/colonization. Among these cases, posttreatment BALF levels of CCL2 were not different than healthy control levels (Figure 5A). However, posttreatment BALF levels of CCL5 remained persistently elevated compared to healthy controls (Figure 5C).
Figure 5. BALF CC chemokine protein levels by ELISA in treated CMVI patients.
(A) Among CMVI cases with a post-treatment BALF sample (n = 24), CCL2 levels in post-treated CMVI samples were not significantly different than levels among healthy controls (n = 33) or during CMVI. (B) Among CMVI cases with both a pre-CMVI sample and a post-treatment sample (n = 8), CCL2 levels were elevated during CMVI, but not in post-treated CMVI compared to pre-CMVI levels. (C) Among CMVI cases with a post-treatment BALF sample (n = 24), CCL5 protein levels in post-treatment CMVI BALF samples were elevated compared to healthy controls and not different than CCL5 levels during CMVI. (D) Among CMVI cases with both a pre-CMVI sample and a post-treatment sample (n = 8), CCL5 protein levels in BALF were elevated during CMVI and remained persistently elevated in post-treatment CMVI samples as compared to pre-CMVI levels. *p ≤ 0.05 compared to healthy or pre-CMVI.
Confirmatory studies were performed using eight available lung transplant recipients that had serial BALF samples including both a pre-CMVI sample and a post-ganciclovir treatment sample, without concurrent ACR (grade ≥ A1), BOS or infection/colonization other than CMV. Similar to our case-control data, we did not find significant elevation of CCL3 or CCL4 during pulmonary CMV or posttreatment with ganciclovir (data not shown). CCL2 levels were elevated during CMVI but not post-ganciclovir treatment (Figure 5B). CCL5 levels were elevated during CMVI and post-ganciclovir treatment (Figure 5D).
Pulmonary CMV and acute cellular rejection
The rate of ACR (grade ≥ A1) prior to the matched bronchoscopy was 0.18 ± 0.24, 0.11 ± 0.16 and 0.05 ± 0.12 episodes/month in the healthy control, CMV infection, and CMV disease groups, respectively (p = 0.27). The rate of clinically significant ACR (grade ≥ A2 and symptomatic A1) prior to the matched bronchoscopy was 0.11 ± 0.18, 0.08 ± 0.15 and 0.05 ± 0.12 episodes/month, respectively (p = 0.43). The mean cumulative AR score prior to the matched bronchoscopy was 1.4 ± 2.0, 1.0 ± 1.3 and 0.3 ± 0.6, respectively (p = 0.28). The mean cumulative AR score for the duration of the study (before and after matched bronchoscopy) was 2.8 ± 2.1, 2.0 ± 2.1 and 0.8 ± 1.0, respectively (p = 0.13).
Pulmonary CMV is associated with a reduction in freedom from BOS and freedom from death due to BOS post-lung transplant
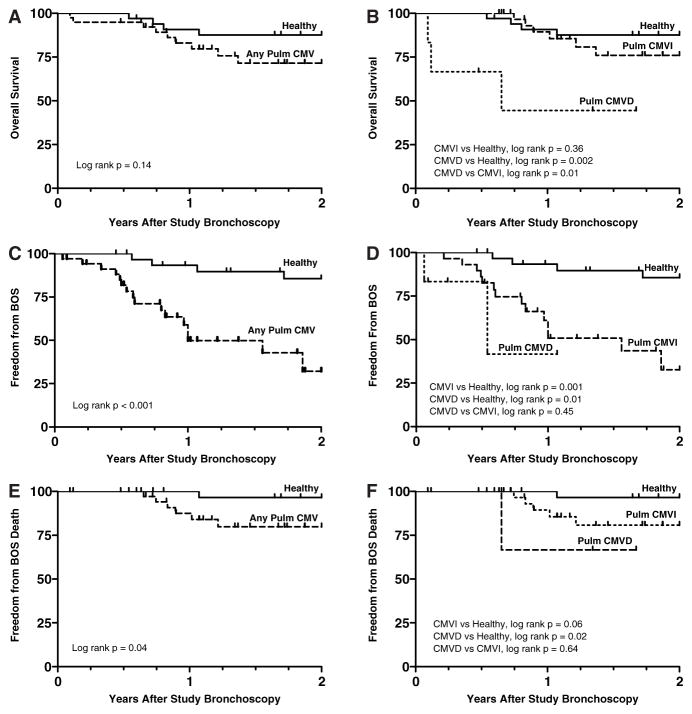
There was a trend toward reduced overall survival during the 2-year follow-up period among pulmonary CMV cases compared to healthy controls (Figure 6A). With CMVI and CMVD cases considered separately, overall long-term survival for CMVI was not significantly different from healthy controls. However, cases with CMVD had a lower probability of survival compared to both healthy controls and CMVI cases (Figure 6B).
Figure 6. Kaplan–Meier curves comparing overall survival, freedom form BOS and freedom from death due to BOS after study bronchoscopy between healthy lung transplant recipient and pulmonary CMV groups.
(A) There is a nonsignificant trend for increased mortality in patients with pulmonary CMV compared to the healthy lung transplant control group. (B) The overall survival for the CMVI group is not significantly different than the healthy group. The overall survival for the CMVD group is significantly worse than the healthy and the CMVI groups. (C) Pulmonary CMV is associated with a reduced freedom from BOS compared to healthy controls. (D) When considered separately, both CMVI and CMVD are associated with a significant reduction in freedom from BOS compared to the healthy group. (E) There is reduced freedom from death specifically due to BOS in pulmonary CMV patients compared to the healthy group. (F) When considered separately, differences in freedom from death due to BOS trend toward significance for pulmonary CMVI (p = 0.06) and are significant for pulmonary CMVD compared to the healthy group.
Pulmonary CMV, including both CMVI and CMVD cases had a reduced probability of freedom from BOS after the study bronchoscopy compared to healthy controls (Figure 6C, D). Additionally, pulmonary CMV was associated with a reduced freedom from death due to BOS, compared to healthy lung transplant controls (Figure 6E). Furthermore, there was a trend for pulmonary CMVI (p = 0.06) and a significant association for pulmonary CMVD with a reduced freedom from death due to BOS, compared to healthy lung transplant controls (Figure 6F).
BALF CC chemokine levels have a significant impact on subsequent clinical outcomes
We constructed Cox regression models to test the impact of specific CC chemokine levels on overall mortality and upon the development of BOS among all patients. Based on elevated BALF protein levels during pulmonary CMV, we included levels of CCL2, CCL3 and CCL5 as continuous variables in these models. In the model for risk of development of BOS, BALF levels of CCL3 had no significant impact. However, elevated BALF levels of CCL5 trended for an increased risk of BOS (p = 0.08) and elevated BALF levels of CCL2 significantly predicted an increase risk of BOS (Table 2). In the model for risk of mortality, elevated BALF levels of CCL3 were associated with a significantly reduced risk of mortality, while BALF levels of CCL2 did not predict a change in mortality (Table 3). However, elevated BALF levels of CCL5 were associated with a significant increased risk of mortality (Table 3).
Table 2.
Impact of BALF CC chemokine concentration on the development of BOS post-lung transplantation
| BOS |
|||
|---|---|---|---|
| RR | 95% CI | p-Value | |
| BALF CCL5 (1 ng/mL) | 1.78 | 0.93–3.35 | 0.08 |
| BALF CCL2 (1 ng/mL) | 1.66 | 1.09–2.30 | 0.02 |
| BALF CCL3 (1 ng/mL) | 0.80 | 0.27–1.95 | 0.64 |
Table 3.
Impact of BALF CC chemokine concentration on mortality post-lung transplantation
| Mortality |
|||
|---|---|---|---|
| RR | 95% CI | p-Value | |
| BALF CCL5 (1 ng/mL) | 5.45 | 1.76–16.6 | 0.005 |
| BALF CCL2 (1 ng/mL) | 0.61 | 0.16–1.4 | 0.29 |
| BALF CCL3 (1 ng/mL) | 0.01 | 0.00–0.11 | 0.001 |
Discussion
Monti et al. in a small study, demonstrated elevated levels of CCL5 in BALF were associated with CMV pneumonitis post-lung transplantation (19). We chose to evaluate multiple-CC chemokines (CCL2, CCL3, CCL4 and CCL5) during pulmonary CMV based on studies demonstrating their importance in human and animal solid organ allograft dysfunction (10,11,20,21). Our novel data expands upon the study by Monti et al. by demonstrating that more than one CC chemokine are elevated during both pulmonary CMVI and CMVD. Specifically, we found a strong trend for increased CCL3 and significant elevations of CCL2 and CCL5 in BALF during pulmonary CMV. We have also determined the cellular sources of CCL2, which are bronchial epithelial cells and AM, whereas CCL5 sources are bronchial and type II epithelial cells, AM and other mononuclear cells. These patterns and levels of CC chemokine expression during pulmonary CMV are similar to what we have previously found during human BOS (11) and suggests that pulmonary CMV may be priming the lung allograft for chronic rejection.
Finding elevations of CCL2 and CCL5 in BALF during pulmonary CMV led to the evaluation of their corresponding receptors. CCR2 is the only receptor for CCL2 and both CCR1 and CCR5 are the major receptors for CCL5 (22). CCR2 was expressed on AM, mononuclear cells and cells determined to be type II pneumocytes based on morphologic characteristics. Previously, we have shown that mononuclear cells expressing CCR2 are phenotypically profibrotic and are involved in the fibro-obliteration of allograft airways during BOS (11). This insinuates that CMV creates a pro-fibrotic milieu by increasing the expression CCL2 that sequesters CCR2 expressing pro-fibrotic mononuclear cells in the lung allograft. We suspect, as was shown for A549 cells, type II pneumocytes expressing CCR2 are interacting with CCL2 augmenting CCL5 expression (23). CCL5 then recruits mononuclear cells and mast cells expressing CCR1 and CCR5, which have been shown to potentiate allograft rejection (20,24,25).
To determine whether pulmonary CMV could have long-term effects on CC chemokine biology, even after aggressive anti-viral treatment, we performed subgroup analyses on the pulmonary CMVI population. All pulmonary CMVI cases were treated with a full course of ganciclovir. Available posttreatment BALF samples demonstrated that CCL2 returned to pre-CMV levels, yet CCL5 remained persistently elevated. A conflicting report has demonstrated that ganciclovir was able to reduce CCL5 levels in BALF (19). The different findings in these studies likely have to do with timing of BALF collection for CCL5 measurement and sample size. Specifically, our study analyzed BALF samples collected after completion of therapy while the latter study performed a follow-up bronchoscopy during therapy and only included two patients (19).
Interestingly, we did find persistent CMVI (P-CMVI) in 12 of the 24 posttreatment BALF samples. Ganciclovir treatment is known to be ineffective at eradicating CMV from reservoirs of latent infection and CMV replication commonly resumes after cessation of therapy (21). Importantly, only CCL5 and not CCL2 levels were elevated in post-ganciclovir treatment BALF samples irrespective of whether or not there was evidence of P-CMVI (data not shown). We presume that after ganciclovir treatment, there is a low-grade CMV replication in the allograft that is below a threshold level required to stimulate the persistent release of CCL2, yet enough to stimulate the persistent release of CCL5. The persistent elevation of CCL5 levels in BALF would suggest that even a low-grade CMV infection (possibly even below the capability of clinical detection) is enough to create a pro-inflammatory microenvironment that may eventually lead to increased mortality.
None of the patients in this study died as a direct result of CMV. However, we did find a trend and a significant reduction in long-term survival for pulmonary CMVI and CMVD groups, respectively. We also found a significant reduction in freedom from BOS and death due to BOS in patients with pulmonary CMV. Similarly, previous studies have shown associations between CMV and allograft dysfunction and mortality (26–40). Collectively, these studies demonstrate that in addition to the ability of CMV to cause direct allograft injury, CMV causes indirect effects including late allograft dysfunction and reduced long-term survival.
We have previously demonstrated roles for CCL2 and CCL5 in the pathogenesis of acute and chronic allograft dysfunction. Reynaud-Gaubert et al. have also described that the upregulation of CCL2 and CCL5 in BALF predicts the development of BOS post-lung transplantation (9). Finding that pulmonary CMV is associated with elevated BALF CC chemokine levels, we explored an association between CC chemokines during pulmonary CMV and subsequent clinical outcomes. Consistent with previous studies, we found that elevated levels of CCL2 in the BALF were associated with an increased risk of BOS (11). Interestingly, elevated BALF CCL3 was associated with a survival advantage, but had no significant effect on the development of BOS. The expression of CCL3 is known to be important in containing viral infections through its ability to recruit interferon-expressing leukocytes (41–43). In addition, CCL3 expression has been shown to be important in limiting pulmonary graft-versus-host-disease (44). Thus, increased expression of CCL3 should be protective during pulmonary CMV. In contrast, CCL5 expression during CMV clearly is detrimental. Elevated levels of CCL5 in the BALF strongly trended for an increased risk of BOS and were significantly predictive of mortality in the future. These links between CCL2 and CCL5 and the development of BOS and increased mortality is consistent with multiple animal models of allograft dysfunction (10,11,20,22,24).
Others have advocated that treated pulmonary CMV does not cause BOS and showed that pulmonary CMVD typically occurred after an episode of ACR, possibly due to augmented immune suppression (45). The authors argued that any association between pulmonary CMV and BOS is more likely explained by the effects of ACR (45). In this study, we did not find that rates of ACR or cumulative acute rejection scores were significantly different between pulmonary CMV patients and healthy lung transplant controls, and therefore, differences in outcomes between groups are not explained by ACR.
We focused our investigations on pulmonary CMV because of previous descriptions of an association with the development of BOS. However, we acknowledge that elevated CC chemokine levels may not be unique to pulmonary CMV infections. HHV-6 is known to induce CCL2 production by macrophages and CCL5 production by endothelial cells (46,47). HHV-6 has also been linked with the development of BOS (48). Furthermore, a recent report describes that CMV infection is frequently associated with concurrent HHV-6 and HHV-7 antigenemia in lung transplant recipients (49). Unfortunately, during the era of this study, the potential significance of HHV-6/7 was not yet recognized, and no screening for these infections was performed within our cohort.
There is increasing evidence that primary graft dysfunction (PGD) may impart a lasting fingerprint upon chemokine and cytokine profiles in survivors that may affect longer-term outcomes (50,51). While we did not evaluate for PGD in these patients, the CMVI and healthy control BALF samples were matched for the duration posttransplant. Assuming that PGD does not influence the development of CMVI, differences in CC chemokine levels between groups should not be the result of PGD. Furthermore, in the eight cases where serial BALF samples were available, levels of CCL2 and CCL5 were significantly lower prior to the development of CMVI. This supports our conclusion that CMVI directly causes alterations in CC chemokine profiles.
In conclusion, we have demonstrated an association between elevated BALF protein levels of CCL2 and CCL5 and pulmonary CMV. In addition, these chemokines seem to be working in a parallel, nonredundant fashion during pulmonary CMV. Moreover, specific patterns of CC chemokine expression during pulmonary CMV appear to be predictive of the development of BOS and mortality. While in clinical practice, we do not always find pure pulmonary CMV in the absence of concurrent ACR or other infection, this study represents a step toward elucidating the mechanism responsible for the indirect effects of pulmonary CMV. These data support the notion that indefinite CMV prophylaxis may reduce the incidence of BOS and decrease mortality post-lung transplantation. Furthermore, elevated BALF levels of CCL2 and CCL5 during pulmonary CMV could potentially serve as useful biomarkers of future poor outcomes, thus guiding a tiered approach to immune suppression, a hypothesis that ultimately needs to be tested in a prospective clinical trial.
Acknowledgments
This work was supported, in part, by grants from the National Institutes of Health (HL080206 and HL086491 to J.A.B.; P50HL67665 to M.P.K. and R.M.S.; CA87879, P50CA90388, and HL66027 to R.M.S.; HL087186 and AR055075 to M.P.K.).
References
- 1.Pereyra F, Rubin RH. Prevention and treatment of cytomegalovirus infection in solid organ transplant recipients. Curr Opin Infect Dis. 2004;17:357–361. doi: 10.1097/01.qco.0000136933.67920.dd. [DOI] [PubMed] [Google Scholar]
- 2.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 3.Ruttmann E, Geltner C, Bucher B, et al. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81:1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. [DOI] [PubMed] [Google Scholar]
- 4.Reinke P, Prosch S, Kern F, Volk HD. Mechanisms of human cytomegalovirus (HCMV) (re)activation and its impact on organ transplant patients. Transpl Infect Dis. 1999;1:157–164. doi: 10.1034/j.1399-3062.1999.010304.x. [DOI] [PubMed] [Google Scholar]
- 5.Estenne M, Maurer JR, Boehler A, et al. Bronchiolitis obliterans syndrome 2001: An update of the diagnostic criteria. J Heart Lung Transplant. 2002;21:297–310. doi: 10.1016/s1053-2498(02)00398-4. [DOI] [PubMed] [Google Scholar]
- 6.Grandaliano G, Gesualdo L, Ranieri E, Monno R, Stallone G, Schena FP. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation. 1997;63:414–420. doi: 10.1097/00007890-199702150-00015. [DOI] [PubMed] [Google Scholar]
- 7.Ruster M, Sperschneider H, Funfstuck R, Stein G, Grone HJ. Differential expression of beta-chemokines MCP-1 and RANTES and their receptors CCR1, CCR2, CCR5 in acute rejection and chronic allograft nephropathy of human renal allografts. Clin Nephrol. 2004;61:30–39. doi: 10.5414/cnp61030. [DOI] [PubMed] [Google Scholar]
- 8.Yun JJ, Whiting D, Fischbein MP, et al. Combined blockade of the chemokine receptors CCR1 and CCR5 attenuates chronic rejection. Circulation. 2004;109:932–937. doi: 10.1161/01.CIR.0000112595.65972.8A. [DOI] [PubMed] [Google Scholar]
- 9.Reynaud-Gaubert M, Marin V, Thirion X, et al. Upregulation of chemokines in bronchoalveolar lavage fluid as a predictive marker of post-transplant airway obliteration. J Heart Lung Transplant. 2002;21:721–730. doi: 10.1016/s1053-2498(02)00392-3. [DOI] [PubMed] [Google Scholar]
- 10.Belperio JA, Burdick MD, Keane MP, et al. The role of the CC chemokine, RANTES, in acute lung allograft rejection. J Immunol. 2000;165:461–472. doi: 10.4049/jimmunol.165.1.461. [DOI] [PubMed] [Google Scholar]
- 11.Belperio JA, Keane MP, Burdick MD, et al. Critical role for the chemokine MCP-1/CCR2 in the pathogenesis of bronchiolitis obliterans syndrome. J Clin Invest. 2001;108:547–556. doi: 10.1172/JCI12214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry GJ, Brunt EM, Chamberlain D, et al. A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: Lung Rejection Study Group. The International Society for Heart Transplantation. J Heart Transplant. 1990;9:593–601. [PubMed] [Google Scholar]
- 13.Yousem SA, Berry GJ, Cagle PT, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15(1 Pt 1):1–15. [PubMed] [Google Scholar]
- 14.Palmer SM, Burch LH, Davis RD, et al. The role of innate immunity in acute allograft rejection after lung transplantation. Am J Respir Crit Care Med. 2003;168:628–632. doi: 10.1164/rccm.200303-447OC. [DOI] [PubMed] [Google Scholar]
- 15.Cooper JD, Billingham M, Egan T, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts. International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 16.Belperio JA, Keane MP, Burdick MD, et al. Role of CXCR2/CXCR2 ligands in vascular remodeling during bronchiolitis obliterans syndrome. J Clin Invest. 2005;115:1150–1162. doi: 10.1172/JCI24233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kao J, Kobashigawa J, Fishbein MC, et al. Elevated serum levels of the CXCR3 chemokine ITAC are associated with the development of transplant coronary artery disease. Circulation. 2003;107:1958–1961. doi: 10.1161/01.CIR.0000069270.16498.75. [DOI] [PubMed] [Google Scholar]
- 18.Dmitrienko A, Molenberghs G, Chuang-Stein C, Offen W. Analysis of clinical trials using Sas: A Practical Guide SAS. 2005 [Google Scholar]
- 19.Monti G, Magnan A, Fattal M, et al. Intrapulmonary production of RANTES during rejection and CMV pneumonitis after lung transplantation. Transplantation. 1996;61:1757–1762. doi: 10.1097/00007890-199606270-00016. [DOI] [PubMed] [Google Scholar]
- 20.Gao W, Faia KL, Csizmadia V, et al. Beneficial effects of targeting CCR5 in allograft recipients. Transplantation. 2001;72:1199–1205. doi: 10.1097/00007890-200110150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Fischereder M, Luckow B, Hocher B, et al. CC chemokine receptor 5 and renal-transplant survival. Lancet. 2001;357:1758–1761. doi: 10.1016/s0140-6736(00)04898-4. [DOI] [PubMed] [Google Scholar]
- 22.Luster AD. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 23.Abonyo BO, Lebby KD, Tonry JH, Ahmad M, Heiman AS. Modulation of eotaxin-3 (CCL26) in alveolar type II epithelial cells. Cytokine. 2006;36:237–244. doi: 10.1016/j.cyto.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Fairchild RL, VanBuskirk AM, Kondo T, Wakely ME, Orosz CG. Expression of chemokine genes during rejection and long-term acceptance of cardiac allografts. Transplantation. 1997;63:1807–1812. doi: 10.1097/00007890-199706270-00018. [DOI] [PubMed] [Google Scholar]
- 25.Yousem SA. The potential role of mast cells in lung allograft rejection. Hum Pathol. 1997;28:179–182. doi: 10.1016/s0046-8177(97)90103-9. [DOI] [PubMed] [Google Scholar]
- 26.Helantera I, Koskinen P, Finne P, et al. Persistent cytomegalovirus infection in kidney allografts is associated with inferior graft function and survival. Transpl Int. 2006;19:893–900. doi: 10.1111/j.1432-2277.2006.00364.x. [DOI] [PubMed] [Google Scholar]
- 27.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalovirus infection and disease on long-term recipient and kidney graft survival. Kidney Int. 2004;66:329–337. doi: 10.1111/j.1523-1755.2004.00735.x. [DOI] [PubMed] [Google Scholar]
- 28.Arnold JC, Portmann BC, O’Grady JG, Naoumov NV, Alexander GJ, Williams R. Cytomegalovirus infection persists in the liver graft in the vanishing bile duct syndrome. Hepatology. 1992;16:285–292. doi: 10.1002/hep.1840160202. [DOI] [PubMed] [Google Scholar]
- 29.Lautenschlager I, Hockerstedt K, Jalanko H, et al. Persistent cytomegalovirus in liver allografts with chronic rejection. Hepatology. 1997;25:190–194. doi: 10.1053/jhep.1997.v25.pm0008985289. [DOI] [PubMed] [Google Scholar]
- 30.Falagas ME, Snydman DR, Griffith J, Ruthazer R, Werner BG. Effect of cytomegalovirus infection status on first-year mortality rates among orthotopic liver transplant recipients. The Boston Center for Liver Transplantation CMVIG study group. Ann Intern Med. 1997;126:275–279. doi: 10.7326/0003-4819-126-4-199702150-00003. [DOI] [PubMed] [Google Scholar]
- 31.de Otero J, Gavalda J, Murio E, et al. Cytomegalovirus disease as a risk factor for graft loss and death after orthotopic liver transplantation. Clin Infect Dis. 1998;26:865–870. doi: 10.1086/513949. [DOI] [PubMed] [Google Scholar]
- 32.Danziger-Isakov LA, DelaMorena M, Hayashi RJ, et al. Cytomegalovirus viremia associated with death or retransplantation in pediatric lung-transplant recipients. Transplantation. 2003;75:1538–1543. doi: 10.1097/01.TP.0000061607.07985.BD. [DOI] [PubMed] [Google Scholar]
- 33.Koskinen PK, Nieminen MS, Krogerus LA, et al. Cytomegalovirus infection and accelerated cardiac allograft vasculopathy in human cardiac allografts. J Heart Lung Transplant. 1993;12:724–729. [PubMed] [Google Scholar]
- 34.Lemstrom KB, Koskinen PK, Bruning JH, Bruggeman CA, Lautenschlager IT, Hayry PJ. Effect of ganciclovir prophylaxis on cytomegalovirus-enhanced allograft arteriosclerosis. Transpl Int. 1994;7(Suppl 1):S383–384. doi: 10.1111/j.1432-2277.1994.tb01398.x. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin K, Wu C, Fick G, Muirhead N, Hollomby D, Jevnikar A. Cytomegalovirus seromismatching increases the risk of acute renal allograft rejection. Transplantation. 2002;74:813–816. doi: 10.1097/00007890-200209270-00014. [DOI] [PubMed] [Google Scholar]
- 36.Sageda S, Nordal KP, Hartmann A, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transpl. 2002;2:850–856. doi: 10.1034/j.1600-6143.2002.20907.x. [DOI] [PubMed] [Google Scholar]
- 37.Duncan SR, Paradis IL, Yousem SA, et al. Sequelae of cytomegalovirus pulmonary infections in lung allograft recipients. Am Rev Respir Dis. 1992;146:1419–1425. doi: 10.1164/ajrccm/146.6.1419. [DOI] [PubMed] [Google Scholar]
- 38.Duncan AJ, Dummer JS, Paradis IL, et al. Cytomegalovirus infection and survival in lung transplant recipients. J Heart Lung Transplant. 1991;10(5 Pt 1):638–644. discussion 645–636. [PubMed] [Google Scholar]
- 39.Bando K, Paradis IL, Komatsu K, et al. Analysis of time-dependent risks for infection, rejection, and death after pulmonary transplantation. J Thorac Cardiovasc Surg. 1995;109:49–57. doi: 10.1016/s0022-5223(95)70419-1. discussion 57–49. [DOI] [PubMed] [Google Scholar]
- 40.Kroshus TJ, Kshettry VR, Savik K, John R, Hertz MI, Bolman RM., 3rd Risk factors for the development of bronchiolitis obliterans syndrome after lung transplantation. J Thorac Cardiovasc Surg. 1997;114:195–202. doi: 10.1016/S0022-5223(97)70144-2. [DOI] [PubMed] [Google Scholar]
- 41.Cook DN, Beck MA, Coffman TM, et al. Requirement of MIP-1 alpha for an inflammatory response to viral infection. Science. 1995;269:1583–1585. doi: 10.1126/science.7667639. [DOI] [PubMed] [Google Scholar]
- 42.Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. The chemokine macrophage-inflammatory protein-1 alpha and its receptor CCR1 control pulmonary inflammation and antiviral host defense in paramyxovirus infection. J Immunol. 2000;165:2677–2682. doi: 10.4049/jimmunol.165.5.2677. [DOI] [PubMed] [Google Scholar]
- 43.Salazar-Mather TP, Hamilton TA, Biron CA. A chemokine-to-cytokine-to-chemokine cascade critical in antiviral defense. J Clin Invest. 2000;105:985–993. doi: 10.1172/JCI9232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Panoskaltsis-Mortari A, Hermanson JR, Taras E, Wangensteen OD, Serody JS, Blazar BR. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood. 2003;101:3714–3721. doi: 10.1182/blood-2002-08-2465. [DOI] [PubMed] [Google Scholar]
- 45.Tamm M, Aboyoun CL, Chhajed PN, Rainer S, Malouf MA, Glanville AR. Treated cytomegalovirus pneumonia is not associated with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med. 2004;170:1120–1123. doi: 10.1164/rccm.200310-1405OC. [DOI] [PubMed] [Google Scholar]
- 46.Arena A, Stassi G, Speranza A, Iannello D, Mastroeni P. Modulatory effect of HHV-6 on MCP-1 production by human monocytes. New Microbiol. 2002;25:335–340. [PubMed] [Google Scholar]
- 47.Caruso A, Favilli F, Rotola A, et al. Human herpesvirus-6 modulates RANTES production in primary human endothelial cell cultures. J Med Virol. 2003;70:451–458. doi: 10.1002/jmv.10416. [DOI] [PubMed] [Google Scholar]
- 48.Neurohr C, Huppmann P, Leuchte H, et al. Human herpesvirus 6 in bronchalveolar lavage fluid after lung transplantation: A risk factor for bronchiolitis obliterans syndrome? Am J Transplant. 2005;5:2982–2991. doi: 10.1111/j.1600-6143.2005.01103.x. [DOI] [PubMed] [Google Scholar]
- 49.Lehto JT, Halme M, Tukiainen P, Harjula A, Sipponen J, Lauten-schlager I. Human herpesvirus-6 and -7 after lung and heart-lung transplantation. J Heart Lung Transplant. 2007;26:41–47. doi: 10.1016/j.healun.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Belperio JA, Keane MP, Burdick MD, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol. 2005;175:6931–6939. doi: 10.4049/jimmunol.175.10.6931. [DOI] [PubMed] [Google Scholar]
- 51.Christie JD, Kotloff RM, Ahya VN, et al. The effect of primary graft dysfunction on survival after lung transplantation. Am J Respir Crit Care Med. 2005;171:1312–1316. doi: 10.1164/rccm.200409-1243OC. [DOI] [PMC free article] [PubMed] [Google Scholar]