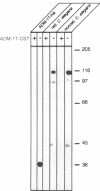
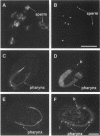
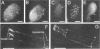
Abstract
A search was carried out for homologues of possible fusogenic proteins to study their function in a genetically tractable animal. The isolation, molecular, and cellular characterization of the Caenorhabditis elegans adm-1 gene (a disintegrin and metalloprotease domain) are described. A glycoprotein analogous to viral fusion proteins has been identified on the surface of guinea pig sperm (PH-30/fertilin) and is implicated in sperm-egg fusion. adm-1 is the first reported invertebrate gene related to PH-30 and a family of proteins containing snake venom disintegrin- and metalloprotease-like domains. ADM-1 shows a domain organization identical to PH-30. It contains prepro, metalloprotease, disintegrin, cysteine rich with putative fusion peptide, epidermal growth factor-like repeat, transmembrane, and cytoplasmic domains. Antibodies which recognize ADM-1 protein in immunoblots were generated. Using immunofluorescence and in situ hybridization, the products of adm-1 have been detected in specific cells during different stages of development. The localization of ADM-1 to the plasma membrane of embryonic cells and to the sheath cells of sensory organs suggests a function in cell adhesion. ADM-1 expression in the hypodermis, pharynx, vulva, and mature sperm is consistent with a putative role in somatic and gamete cell fusions.
Full text
PDF


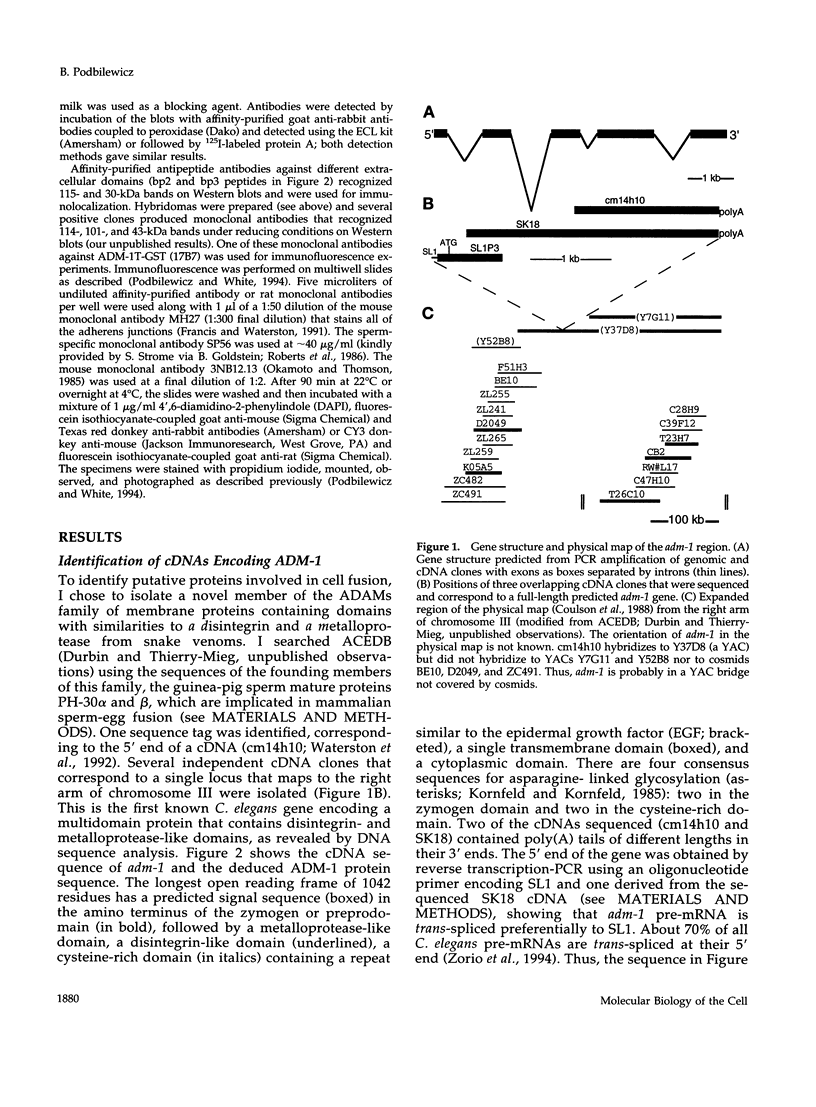





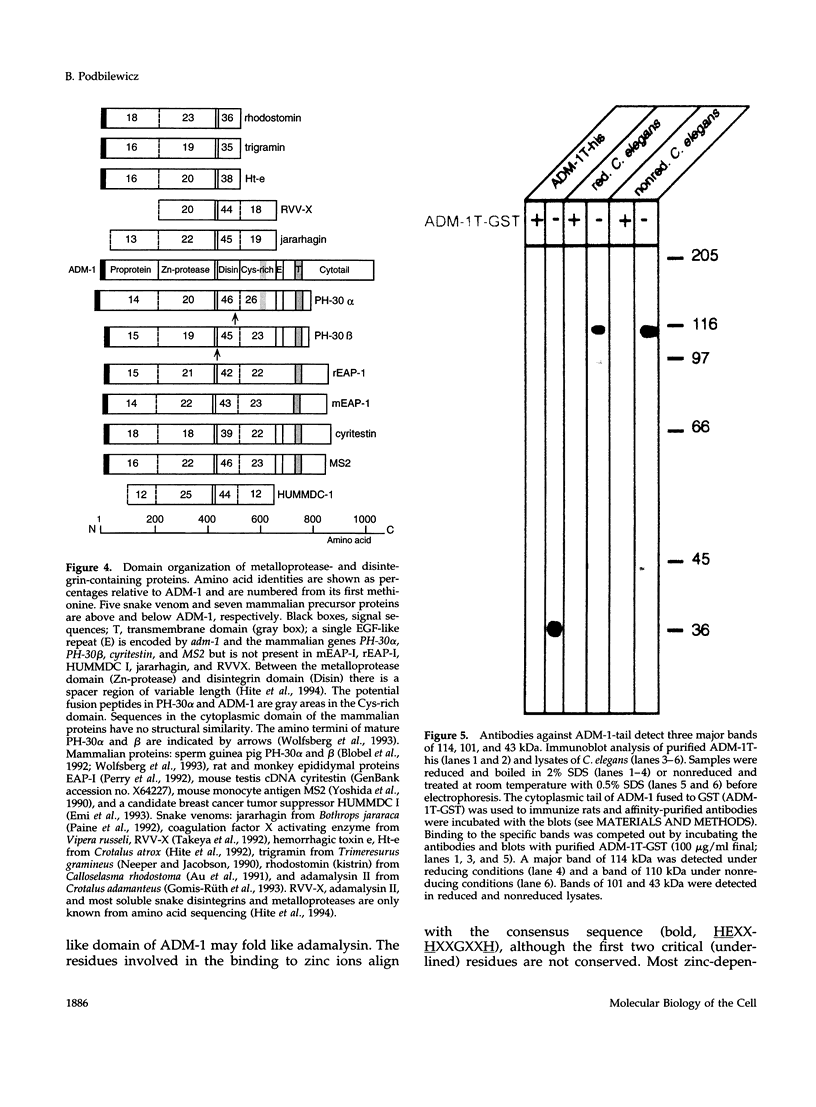
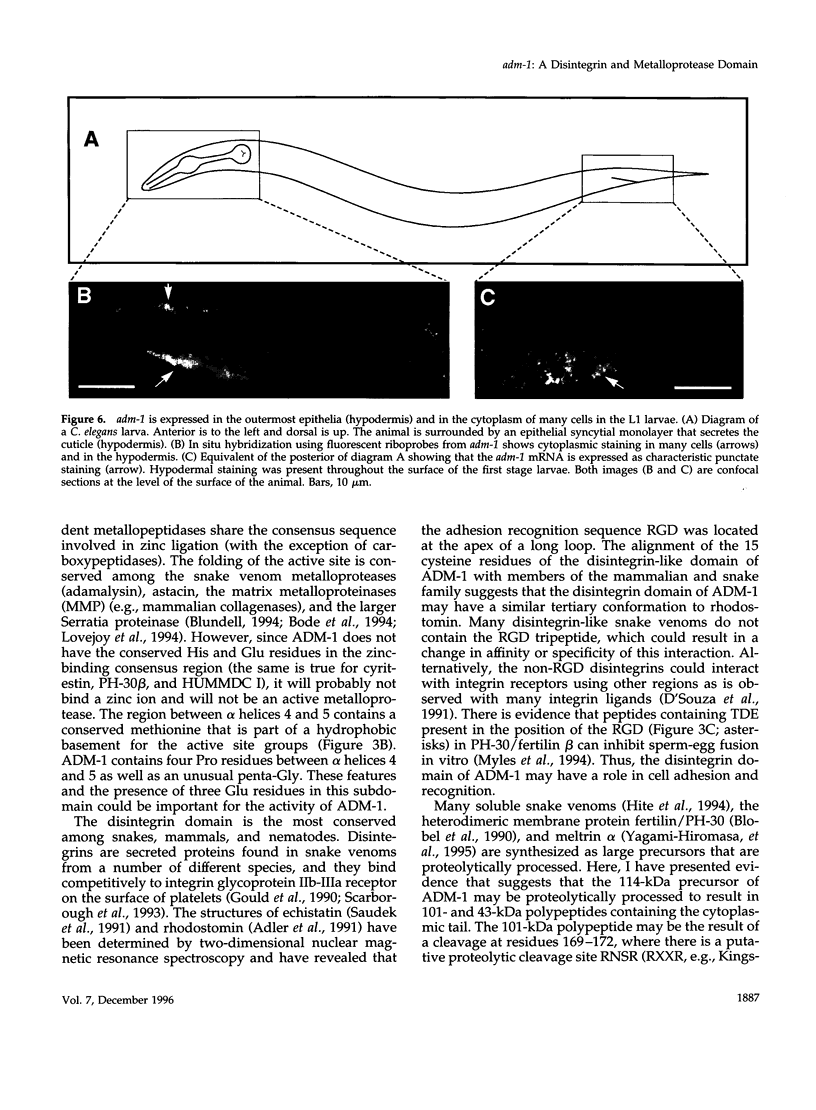
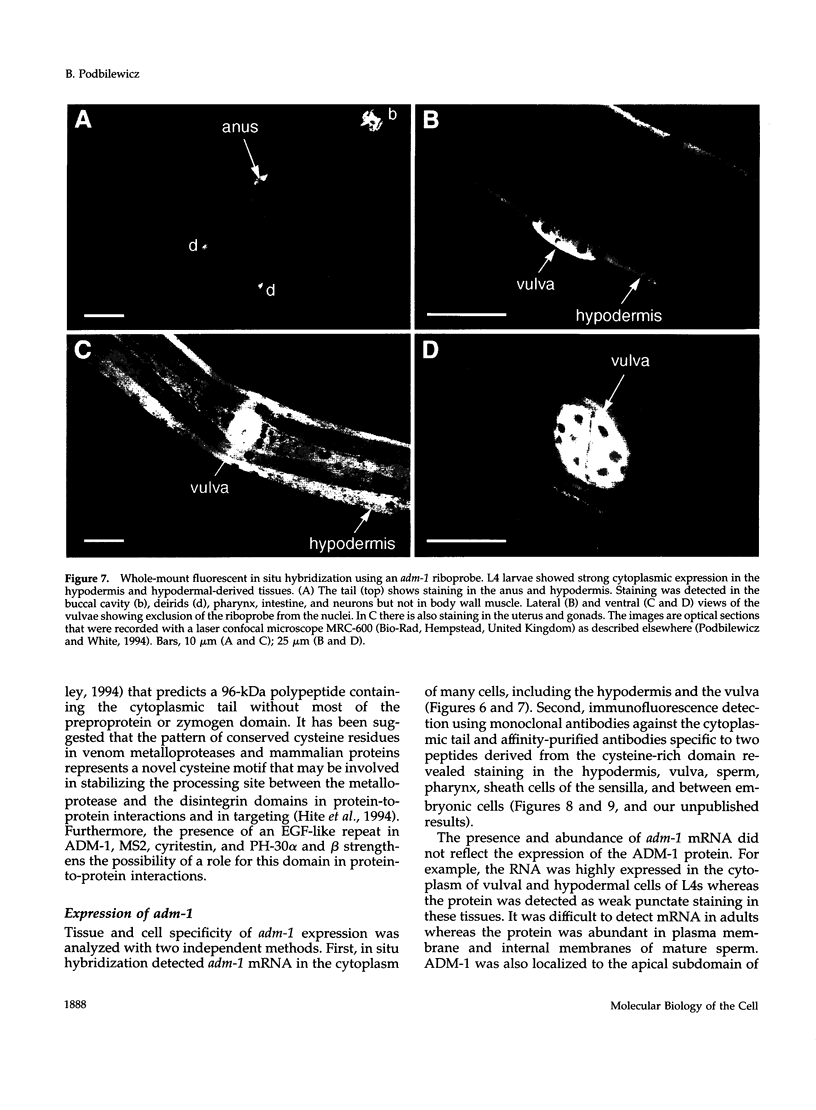




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler M., Lazarus R. A., Dennis M. S., Wagner G. Solution structure of kistrin, a potent platelet aggregation inhibitor and GP IIb-IIIa antagonist. Science. 1991 Jul 26;253(5018):445–448. doi: 10.1126/science.1862345. [DOI] [PubMed] [Google Scholar]
- Albertson D. G., Fishpool R. M., Birchall P. S. Fluorescence in situ hybridization for the detection of DNA and RNA. Methods Cell Biol. 1995;48:339–364. doi: 10.1016/s0091-679x(08)61395-3. [DOI] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Au L. C., Huang Y. B., Huang T. F., Teh G. W., Lin H. H., Choo K. B. A common precursor for a putative hemorrhagic protein and rhodostomin, a platelet aggregation inhibitor of the venom of Calloselasma rhodostoma: molecular cloning and sequence analysis. Biochem Biophys Res Commun. 1991 Dec 16;181(2):585–593. doi: 10.1016/0006-291x(91)91230-a. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Myles D. G., Primakoff P., White J. M. Proteolytic processing of a protein involved in sperm-egg fusion correlates with acquisition of fertilization competence. J Cell Biol. 1990 Jul;111(1):69–78. doi: 10.1083/jcb.111.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Blundell T. L. Metalloproteinase superfamilies and drug design. Nat Struct Biol. 1994 Feb;1(2):73–75. doi: 10.1038/nsb0294-73. [DOI] [PubMed] [Google Scholar]
- Bode W., Reinemer P., Huber R., Kleine T., Schnierer S., Tschesche H. The X-ray crystal structure of the catalytic domain of human neutrophil collagenase inhibited by a substrate analogue reveals the essentials for catalysis and specificity. EMBO J. 1994 Mar 15;13(6):1263–1269. doi: 10.1002/j.1460-2075.1994.tb06378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr C. M., Kim P. S. Flu virus invasion: halfway there. Science. 1994 Oct 14;266(5183):234–236. doi: 10.1126/science.7939658. [DOI] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Cross J. C., Werb Z., Fisher S. J. Implantation and the placenta: key pieces of the development puzzle. Science. 1994 Dec 2;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Edgar L. G., McGhee J. D. Embryonic expression of a gut-specific esterase in Caenorhabditis elegans. Dev Biol. 1986 Mar;114(1):109–118. doi: 10.1016/0012-1606(86)90387-8. [DOI] [PubMed] [Google Scholar]
- Emi M., Katagiri T., Harada Y., Saito H., Inazawa J., Ito I., Kasumi F., Nakamura Y. A novel metalloprotease/disintegrin-like gene at 17q21.3 is somatically rearranged in two primary breast cancers. Nat Genet. 1993 Oct;5(2):151–157. doi: 10.1038/ng1093-151. [DOI] [PubMed] [Google Scholar]
- Francis R., Waterston R. H. Muscle cell attachment in Caenorhabditis elegans. J Cell Biol. 1991 Aug;114(3):465–479. doi: 10.1083/jcb.114.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomis-Rüth F. X., Kress L. F., Bode W. First structure of a snake venom metalloproteinase: a prototype for matrix metalloproteinases/collagenases. EMBO J. 1993 Nov;12(11):4151–4157. doi: 10.1002/j.1460-2075.1993.tb06099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould R. J., Polokoff M. A., Friedman P. A., Huang T. F., Holt J. C., Cook J. J., Niewiarowski S. Disintegrins: a family of integrin inhibitory proteins from viper venoms. Proc Soc Exp Biol Med. 1990 Nov;195(2):168–171. doi: 10.3181/00379727-195-43129b. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Hite L. A., Jia L. G., Bjarnason J. B., Fox J. W. cDNA sequences for four snake venom metalloproteinases: structure, classification, and their relationship to mammalian reproductive proteins. Arch Biochem Biophys. 1994 Jan;308(1):182–191. doi: 10.1006/abbi.1994.1026. [DOI] [PubMed] [Google Scholar]
- Hite L. A., Shannon J. D., Bjarnason J. B., Fox J. W. Sequence of a cDNA clone encoding the zinc metalloproteinase hemorrhagic toxin e from Crotalus atrox: evidence for signal, zymogen, and disintegrin-like structures. Biochemistry. 1992 Jul 14;31(27):6203–6211. doi: 10.1021/bi00142a005. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Lander A. D. Contact and adhesive specificities in the associations, migrations, and targeting of cells and axons. Cell. 1992 Jan 24;68(2):303–322. doi: 10.1016/0092-8674(92)90472-o. [DOI] [PubMed] [Google Scholar]
- Kingsley D. M. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994 Jan;8(2):133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Fukuzawa H., Shimada T., Saito T., Matsuda Y. Primary structure and expression of a gamete lytic enzyme in Chlamydomonas reinhardtii: similarity of functional domains to matrix metalloproteases. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4693–4697. doi: 10.1073/pnas.89.10.4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kuwabara P. E., Okkema P. G., Kimble J. tra-2 encodes a membrane protein and may mediate cell communication in the Caenorhabditis elegans sex determination pathway. Mol Biol Cell. 1992 Apr;3(4):461–473. doi: 10.1091/mbc.3.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littler E., Stuart A. D., Chee M. S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature. 1992 Jul 9;358(6382):160–162. doi: 10.1038/358160a0. [DOI] [PubMed] [Google Scholar]
- Lovejoy B., Cleasby A., Hassell A. M., Longley K., Luther M. A., Weigl D., McGeehan G., McElroy A. B., Drewry D., Lambert M. H. Structure of the catalytic domain of fibroblast collagenase complexed with an inhibitor. Science. 1994 Jan 21;263(5145):375–377. doi: 10.1126/science.8278810. [DOI] [PubMed] [Google Scholar]
- Mundy D. I., Strittmatter W. J. Requirement for metalloendoprotease in exocytosis: evidence in mast cells and adrenal chromaffin cells. Cell. 1985 Mar;40(3):645–656. doi: 10.1016/0092-8674(85)90213-2. [DOI] [PubMed] [Google Scholar]
- Myles D. G., Kimmel L. H., Blobel C. P., White J. M., Primakoff P. Identification of a binding site in the disintegrin domain of fertilin required for sperm-egg fusion. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4195–4198. doi: 10.1073/pnas.91.10.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles D. G. Molecular mechanisms of sperm-egg membrane binding and fusion in mammals. Dev Biol. 1993 Jul;158(1):35–45. doi: 10.1006/dbio.1993.1166. [DOI] [PubMed] [Google Scholar]
- Neeper M. P., Jacobson M. A. Sequence of a cDNA encoding the platelet aggregation inhibitor trigramin. Nucleic Acids Res. 1990 Jul 25;18(14):4255–4255. doi: 10.1093/nar/18.14.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Thomson J. N. Monoclonal antibodies which distinguish certain classes of neuronal and supporting cells in the nervous tissue of the nematode Caenorhabditis elegans. J Neurosci. 1985 Mar;5(3):643–653. doi: 10.1523/JNEUROSCI.05-03-00643.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine M. J., Desmond H. P., Theakston R. D., Crampton J. M. Purification, cloning, and molecular characterization of a high molecular weight hemorrhagic metalloprotease, jararhagin, from Bothrops jararaca venom. Insights into the disintegrin gene family. J Biol Chem. 1992 Nov 15;267(32):22869–22876. [PubMed] [Google Scholar]
- Palazzolo M. J., Hamilton B. A., Ding D. L., Martin C. H., Mead D. A., Mierendorf R. C., Raghavan K. V., Meyerowitz E. M., Lipshitz H. D. Phage lambda cDNA cloning vectors for subtractive hybridization, fusion-protein synthesis and Cre-loxP automatic plasmid subcloning. Gene. 1990 Mar 30;88(1):25–36. doi: 10.1016/0378-1119(90)90056-w. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A. C., Jones R., Barker P. J., Hall L. A mammalian epididymal protein with remarkable sequence similarity to snake venom haemorrhagic peptides. Biochem J. 1992 Sep 15;286(Pt 3):671–675. doi: 10.1042/bj2860671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podbilewicz B., White J. G. Cell fusions in the developing epithelial of C. elegans. Dev Biol. 1994 Feb;161(2):408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Primakoff P., Hyatt H., Tredick-Kline J. Identification and purification of a sperm surface protein with a potential role in sperm-egg membrane fusion. J Cell Biol. 1987 Jan;104(1):141–149. doi: 10.1083/jcb.104.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryer N. K., Wuestehube L. J., Schekman R. Vesicle-mediated protein sorting. Annu Rev Biochem. 1992;61:471–516. doi: 10.1146/annurev.bi.61.070192.002351. [DOI] [PubMed] [Google Scholar]
- Roberts T. M., Pavalko F. M., Ward S. Membrane and cytoplasmic proteins are transported in the same organelle complex during nematode spermatogenesis. J Cell Biol. 1986 May;102(5):1787–1796. doi: 10.1083/jcb.102.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J. L., Farach H. A., Jr, Strittmatter W. J., Lennarz W. J. Evidence for involvement of metalloendoproteases in a step in sea urchin gamete fusion. J Cell Biol. 1988 Aug;107(2):539–544. doi: 10.1083/jcb.107.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist T. A., Kimble J. Molecular cloning and transcript analysis of fem-3, a sex-determination gene in Caenorhabditis elegans. Genes Dev. 1988 May;2(5):606–616. doi: 10.1101/gad.2.5.606. [DOI] [PubMed] [Google Scholar]
- Rothman J. E. Mechanisms of intracellular protein transport. Nature. 1994 Nov 3;372(6501):55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Saudek V., Atkinson R. A., Pelton J. T. Three-dimensional structure of echistatin, the smallest active RGD protein. Biochemistry. 1991 Jul 30;30(30):7369–7372. doi: 10.1021/bi00244a003. [DOI] [PubMed] [Google Scholar]
- Scarborough R. M., Rose J. W., Naughton M. A., Phillips D. R., Nannizzi L., Arfsten A., Campbell A. M., Charo I. F. Characterization of the integrin specificities of disintegrins isolated from American pit viper venoms. J Biol Chem. 1993 Jan 15;268(2):1058–1065. [PubMed] [Google Scholar]
- Seger R., Krebs E. G. The MAPK signaling cascade. FASEB J. 1995 Jun;9(9):726–735. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Sonnhammer E. L., Durbin R. A workbench for large-scale sequence homology analysis. Comput Appl Biosci. 1994 Jun;10(3):301–307. doi: 10.1093/bioinformatics/10.3.301. [DOI] [PubMed] [Google Scholar]
- Staden R. Staden: comparing sequences. Methods Mol Biol. 1994;25:155–170. doi: 10.1385/0-89603-276-0:155. [DOI] [PubMed] [Google Scholar]
- Stegmann T., Doms R. W., Helenius A. Protein-mediated membrane fusion. Annu Rev Biophys Biophys Chem. 1989;18:187–211. doi: 10.1146/annurev.bb.18.060189.001155. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Horvitz H. R. Post-embryonic cell lineages of the nematode, Caenorhabditis elegans. Dev Biol. 1977 Mar;56(1):110–156. doi: 10.1016/0012-1606(77)90158-0. [DOI] [PubMed] [Google Scholar]
- Sulston J. E., Schierenberg E., White J. G., Thomson J. N. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983 Nov;100(1):64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Takeya H., Nishida S., Miyata T., Kawada S., Saisaka Y., Morita T., Iwanaga S. Coagulation factor X activating enzyme from Russell's viper venom (RVV-X). A novel metalloproteinase with disintegrin (platelet aggregation inhibitor)-like and C-type lectin-like domains. J Biol Chem. 1992 Jul 15;267(20):14109–14117. [PubMed] [Google Scholar]
- Trueheart J., Fink G. R. The yeast cell fusion protein FUS1 is O-glycosylated and spans the plasma membrane. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9916–9920. doi: 10.1073/pnas.86.24.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart H. E., Birkedal-Hansen H. The cysteine switch: a principle of regulation of metalloproteinase activity with potential applicability to the entire matrix metalloproteinase gene family. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5578–5582. doi: 10.1073/pnas.87.14.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S., Thomson N., White J. G., Brenner S. Electron microscopical reconstruction of the anterior sensory anatomy of the nematode Caenorhabditis elegans.?2UU. J Comp Neurol. 1975 Apr 1;160(3):313–337. doi: 10.1002/cne.901600305. [DOI] [PubMed] [Google Scholar]
- Waterston R., Martin C., Craxton M., Huynh C., Coulson A., Hillier L., Durbin R., Green P., Shownkeen R., Halloran N. A survey of expressed genes in Caenorhabditis elegans. Nat Genet. 1992 May;1(2):114–123. doi: 10.1038/ng0592-114. [DOI] [PubMed] [Google Scholar]
- Weskamp G., Blobel C. P. A family of cellular proteins related to snake venom disintegrins. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2748–2751. doi: 10.1073/pnas.91.7.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Viral and cellular membrane fusion proteins. Annu Rev Physiol. 1990;52:675–697. doi: 10.1146/annurev.ph.52.030190.003331. [DOI] [PubMed] [Google Scholar]
- Wolfsberg T. G., Bazan J. F., Blobel C. P., Myles D. G., Primakoff P., White J. M. The precursor region of a protein active in sperm-egg fusion contains a metalloprotease and a disintegrin domain: structural, functional, and evolutionary implications. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10783–10787. doi: 10.1073/pnas.90.22.10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfsberg T. G., Straight P. D., Gerena R. L., Huovila A. P., Primakoff P., Myles D. G., White J. M. ADAM, a widely distributed and developmentally regulated gene family encoding membrane proteins with a disintegrin and metalloprotease domain. Dev Biol. 1995 May;169(1):378–383. doi: 10.1006/dbio.1995.1152. [DOI] [PubMed] [Google Scholar]
- Yagami-Hiromasa T., Sato T., Kurisaki T., Kamijo K., Nabeshima Y., Fujisawa-Sehara A. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995 Oct 19;377(6550):652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Setoguchi M., Higuchi Y., Akizuki S., Yamamoto S. Molecular cloning of cDNA encoding MS2 antigen, a novel cell surface antigen strongly expressed in murine monocytic lineage. Int Immunol. 1990;2(6):585–591. doi: 10.1093/intimm/2.6.585. [DOI] [PubMed] [Google Scholar]
- Zorio D. A., Cheng N. N., Blumenthal T., Spieth J. Operons as a common form of chromosomal organization in C. elegans. Nature. 1994 Nov 17;372(6503):270–272. doi: 10.1038/372270a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]