Abstract
This review examines current approaches available for articular cartilage repair, not only in terms of their regeneration potential, but also as a function of immunologic response. Autogenic repair techniques, including osteochondral plug transplantation, chondrocyte implantation, and microfracture, are the most widely accepted clinical treatment options due to the lack of immunogenic reactions, but only moderate graft success rates have been reported. Although suspended allogenic chondrocytes are shown to evoke an immune response upon implantation, allogenic osteochondral plugs and tissue-engineered grafts using allogenic chondrocytes exhibit a tolerable immunogenic response. Additionally, these repair techniques produce neotissue with success rates approaching those of currently available autogenic repair techniques, while simultaneously obviating their major hindrance of donor tissue scarcity. To date, limited research has been performed with xenogenic tissue, although several studies demonstrate the potential for its long-term success. This article focuses on the various treatment options for cartilage repair and their associated success rates and immunologic responses.
Introduction
Articular cartilage degeneration onset by either acute traumatic injury or a degenerative joint disease, such as osteoarthritis, results in the formation of unhealthy tissue with inferior structural and mechanical properties.1 Hyaline articular cartilage is a differentiated tissue distinct from most tissues because of its vascular deficiency and has only limited self-regenerative ability.2,3 This ability to self-repair has been shown to be somewhat modulated by the size, location, and depth of cartilage lesions.4 Pure chondral lesions that do not penetrate the underlying subchondral bone have been shown unable to self-repair spontaneously, while full-thickness defects have been shown to undergo only a transient healing response.4,5 Analogously, some researchers believe that cartilage lesions less than 3 mm in diameter self-repair with hyaline-like cartilage.6–9 In contrast, it is widely accepted that larger defects are replaced with fibrous cartilage possessing different structure and composition compared to normal cartilage with accompanying inferior mechanical properties.10,11
Numerous strategies have been employed to repair cartilage defects with an end goal of filling the defect with tissue having biochemical and biomechanical properties approximating surrounding native tissue. Such clinical and experimental efforts include subchondral drilling (e.g., microfracture technique),12 osteochondral graft transplantation,13–18 suspended chondrocyte implantation,19,20 and tissue-engineered grafting.21–23 A number of studies have investigated these treatment options, and varying degrees of efficacy and immune responses in clinical trials and in vivo animal experiments have been reported. Though several methodological variations may be attributed to these differences, controversy and uncertainty remain with respect to the best available treatment option.
Organ transplantation has been investigated extensively, yet the process by which rejection occurs is only moderately understood. Traditionally, transplantation of a graft from a genetically disparate donor causes an acute immune response in the host due to the detection of donor antigen-presenting cells (APCs).24,25 This detection sets in motion a series of concurrent cascade events that eventually lead to graft rejection. The first stage of rejection includes the recognition and sensitization phase, whereby T lymphocytes (both CD4+ and CD8+) recognize alloantigen and respond immediately by undergoing proliferation and activation. Activation occurs through the interaction of the host T cell with a donor APC that expresses an appropriate antigenic ligand on its major histocompatibility complex (MHC) receptor in addition to a required costimulator signal.26 Simultaneously, a population of nonlymphoid passenger leukocytes migrates from the graft tissue to the host's lymphoid organs whereby they stimulate the host's immune system. During migration, these passenger leukocytes undergo maturation from immature dendritic cells to mature APCs that activate an array of T lymphocytes, including CD4+, CD8+, and naïve T cells. The versatility in stimulating the host's immune response of these mature cells has led to their naming of “professional APCs.”27
The proliferation and activation of T lymphocytes plays a major role in signaling the second phase of immune rejection involving effector immune responses that reject the graft, known as the effector stage. Activated T cells secrete various cytokines (i.e., interleukin-2, interferon-gamma, tissue necrosis factor-beta etc., which rapidly enhance the immune system's response by recruiting a variety of other host immune cells and inducing increased expression of MHC class I and class II molecules by donor cells.24–27 Interleukin-2 is also critical in the generation of cytotoxic T lymphocytes that attack APCs, while IFN-γ also promotes the influx of macrophages into the graft and their later destructive activation. Finally, TNF-β has a direct, cytotoxic effect on graft cells.
Articular cartilage's avascularity has led to an assertion that the tissue is immunoprivileged, whereby a body's immune system is limited in its ability to detect and reject implanted tissue. However, many researchers have shown that both chondrocytes and their embedded extracellular matrix (ECM) contain antigens that can be immunogenic.28,29 Chondrocytes have been found to contain MHC class II antigens, which during transplantation could react with T cells and elicit a cell-mediated immune response as described above.20,30 Additionally, chondrocytes are known to be susceptible to attack by natural killer cells,31–33 and various components of the matrix itself, including collagen types II, IX, and XI and proteoglycan core proteins, have been shown to have antigenic properties.34–38 However, when cartilage tissue is intact, chondrocytes are protected and separated from contact with both natural killer and T cells by the ECM. Thus, an efferent and afferent block to the immune system is believed to impart the immunoprivileged nature of intact cartilage.28
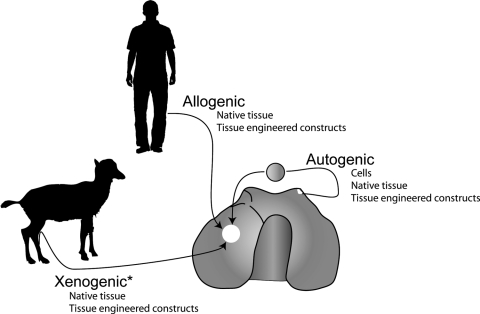
The objective of this review is to illustrate the vast array of articular cartilage repair techniques, both clinical and experimental, and their respective immunological, histological, biochemical, and biomechanical efficacies and responses. This review includes a description of autogenic, allogenic, and xenogenic approaches to tissue repair and tissue repair strategies available for each tissue source (Fig. 1). Throughout this review, the terms “autogenic” and “autologous” are used interchangeably to denote tissue originating from the same individual. Additionally, cartilage defects are defined as follows: (i) a partial-thickness cartilage defect is a defect that does not fully penetrate through the cartilage layer, (ii) a full-thickness defect penetrates through the entire cartilage thickness but not into the underlying subchondral bone, and (iii) an osteochondral defect penetrates through the entire cartilage thickness and into the underlying bone.
FIG. 1.
Successful approaches to articular cartilage defect repair. Autogenic sources have shown success using isolated chondrocytes, native tissue autografts, and tissue-engineered constructs. Clinically, allogenic cell sources have only been successful when surrounded by an ECM in native tissue grafts and tissue-engineered construct. While xenogenic tissue has yet to be used clinically, based on this review of animal studies there exists a possibility to successfully repair cartilage defects using tissue grafts or tissue-engineered constructs, but not isolated cells. Asterisk (*) denotes approaches not currently used in clinical practice.
Autogenic
Autogenic tissue has been used to repair cartilage defects using several techniques. Such tissue transplantation options include osteochondral plug transplantation and single cell suspensions of either terminally differentiated chondrocytes or undifferentiated bone marrow–derived mesenchymal stem cells (MSCs). Though these techniques afford repair advantages insofar as the lack of disease transmission or elicitation of an immune response, severe limitations exist, such as tissue scarcity and requirement of additional invasive surgeries that may give rise to donor-site morbidity, thus preventing their widespread usage.
Autografts
Osteochondral autografts used experimentally and clinically in the repair of damaged articular cartilage have historically been harvested from non-weight-bearing locations and transplanted into defect sites. Satisfactory clinical results, as defined by resumption of normal preinjury activity levels, have been reported in long-term studies16 with accompanied good graft–host tissue integration. These results have proved encouraging, but the major drawbacks have caused researchers to consider this technique a last-resort solution when other techniques are too complex or prove inadequate. Several approaches to circumvent the limitations of donor tissue availability required to fill large osteochondral defects have been implemented, including the use of undersized grafts. Recently, a 6-mm-diameter autologous osteochondral plug was transplanted into a 10-mm-diameter defect in an adult sheep model to assess the ability of large lesions to be treated using a plug not completely filling the defect.14 Results indicated a superior ability to regenerate tissue ingrowth compared to untreated defects or autologous cancellous bone graft treatment, but this neotissue was fibrocartilaginous in nature. This effect could be attributed to the lack of contact between graft and surrounding native cartilage, which causes the application of additional stresses to an undersized graft upon weight bearing.
Similar treatment options that have been employed to thwart issues of contour nonuniformity and tissue scarcity involve tissue mosaicplasty. Autologous mosaicplasty involves the use of multiple, small-sized cylindrical osteochondral grafts harvested from non-weight-bearing areas transplanted in a mosaic-like fashion into a large osteochondral defect.39 Advantages of this procedure include the survival of transplanted tissue and a smoother contour imparted to the transplantation site. Hangody and Fules15 and Hangody et al.39 reviewed various mosaicplasty studies and report that after 3, 4, and 5 years, mosaicplasty provided significantly better clinical results than other cartilage resurfacing techniques, including Pridie drilling, abrasion arthroplasty, and microfracture repair. Clinical trials have shown the effectiveness of this technique in comparison to autologous chondrocyte implantation (ACI).40 Evaluation of patients revealed that a complete clinical recovery, as assessed by the Lysholm Knee Scoring Scale,41 was detected in 88% of patients treated with mosaicplasty, while only a 68% success rate was seen in patients with ACI. Additionally, long-term examination of the mosaicplasty technique revealed good/excellent scores for resurfacing treatment of the femoral condyle (92%), tibia (87%), patella and/or trochlea (79%), and talus (94%).42 However, morbidity was observed in 3% of donor sites, which were consistently filled with fibrocartilage in all patients of the study. Bentley et al.43 presented contradicting evidence that suggests that chondrocyte implantation significantly outperformed mosaicplasty in the treatment of large, heterogeneous lesions in terms of both functional assessment and arthroscopy. Many proponents of mosaicplasty point to its inherent benefit in obtaining small, self-healing cartilage specimens to repair large defects. However, while it has been postulated that small cartilage defects can self-repair with hyaline-like cartilage, the intentional removal of healthy native tissue could be a long-term concern for this technique as fibrocartilage tissue possesses inferior properties that can degenerate into a permanent defect. Most studies investigating mosaicplasty to date have not included long-term follow-up to address the prevailing theory that with time, defects, no matter their size, will be filled with fibrocartilage tissue with inferior properties and will degenerate into a permanent defect.
Autologous chondrocyte implantation
First described by Peterson et al. in 1984,44 ACI is a widely used repair technique in both the United States and Europe. Known as the Carticel® procedure by Genzyme Corporation (Cambridge, MA), it was the first cell-based articular cartilage repair technique to be granted approval by the U.S. Food and Drug Administration (FDA) for clinical use in the United States.45 ACI is a repair technique that harvests thin slices of cartilage from minor weight-bearing areas of the knee. Enzymatic digestion of these cartilage slices yields chondrocytes that are subsequently expanded in vitro for several weeks. When a sufficient number of cells to fill the articular cartilage defect have been obtained, chondrocytes are implanted into the defect and secured with a periosteal patch.46 Though this is an attractive treatment due to the chondrocytes being autologous in nature as no immune responses have been reported in ACI treatment of focal defects in either animal or clinical usage, its success remains inconclusive.
Clinical use of ACI began in 1987 in Sweden and has been described extensively in several reports.19,46–48 Since its inception, studies have reported upward of 92% success in treatment of isolated femoral condylar lesions with good adherence of neotissue to underlying bone and adjacent cartilage tissue.49 Long-term investigation of this technique revealed that 89% of patients scored a good/excellent clinical score after 2 years, and grafts were 100% durable during the remaining 9 years.50 Clinical scores include ratings of defect repair, integration with surrounding tissue, and macroscopic appearance as parameters for repair assessment. Further, mechanical assessment of repair tissue 5 years postimplantation indicated that indentation stiffness values measuring 90% of the surrounding native tissue and 66% of graft biopsy samples were consistent with hyaline tissue. A similar study assessed mechanical integrity of ACI grafts in 30 patients and found indentation stiffness reached 62% of the surrounding native tissue.51 However, only 53% of patients graded their repair as good/excellent 1 year posttreatment. Magnetic resonance imaging results showed proteoglycan replenishment, but the lower stiffness values indicated fibrous tissue repair, suggesting the conflicting appropriateness of various endpoint analyses.
In spite of its success, the ACI technique is not without its skeptics.52–54 Horas et al.55 compared ACI with autologous osteochondral graft transplantation in 40 patients monitored over a 2-year period. At the conclusion of the clinical trial, tissue recovery with chondrocyte implantation occurred at a slower rate than osteochondral transplantation. Additionally, biopsy specimens of chondrocyte implantation grafts were consisted primarily of fibrocartilage with small, localized hyaline-like regions near the subchondral bone. These repair tissues were in contrast to the homogeneous hyaline appearance of transplanted osteochondral grafts. Other studies have compared results of ACI with microfracture surgery.56,57 While both treatments demonstrated short-term benefits, enhanced structural properties were only fleeting56 as long-term evaluation showed that inferior tissue was present in both treatments.57 At 2 years posttreatment, only one failure was observed for microfracture treatment and two failures for ACI. However, neither graft remained stable, whereby nine failures occurred in each treatment 5 years postoperatively.57 Further investigation into matrix repair after ACI treatment revealed that 10–15 months after treatment, repair tissue underwent proteoglycan replenishment but lacked the preferential collagen alignment seen in normal cartilage essential to maintaining the tissue's functional mechanical properties.54 Finally, a recent study reviewed adverse events involving 294 patients who received the Carticel procedure between 1996 and 2003.45 In this report, the most common adverse events reported were graft failure (25%), graft delamination (22%), and tissue hypertrophy (18%), occurring at a median time point of 240 days. These data agree with previous reports of adverse events occurring in approximately 3.5% of administered Carticel procedures,58 as these adverse events studied occurred in an overall population of 7500 operations (3.8%).45
The apparent large variability and disparity displayed in ACI treatment studies can possibly be explained by several well-documented limitations in study designs that influence treatment outcomes. These include the use of controlled rehabilitation regimens,59,60 patient age,61 joint alignment postoperatively,49 and surgical history.62 It is also worth noting that most clinical trials have used the original techniques of Peterson et al.44 and Brittberg et al.46 employing the patient's own serum as part of the culture medium for expansion of chondrocytes in the ACI procedure. However, the Carticel procedure utilizes fetal bovine serum in lieu of the patient's own serum for chondrocyte proliferation. Genzyme Corporation reports that trace amounts of bovine-derived proteins may be present in the Carticel product, and that this procedure should not be employed in patients with a hypersensitivity to materials of bovine origin.63 In animal studies, there are no consistencies in serum usage between studies as some utilize autologous serum, while others use bovine-derived serum. In future ACI studies, as well as for all in vivo cartilage repair studies, physicians should control these design factors as they have major influences on graft success and can severely impact the implicit power of study conclusions.
Tissue-engineered constructs
Inherent disadvantages with ACI, including the use of periosteum, led researchers to begin modifying the procedure by combining natural and synthetic biomaterials to both secure the implanted chondrocytes in the defect site and enhance their chondrogenic potential.64–66 Several studies have reported both experimental and clinical successes of collagen bilayer scaffolds, known as matrix-associated ACI (MACI).67,68 In an ovine model,68 MACI contributed to short-term significant histological improvements though biomechanical properties remained inferior. When used in a long-term clinical trial,67 MACI resulted in 72% of patients reporting improved joint function out to 5 years posttransplant.
Polylactic/glycolic acid (PLGA)27 and polylactic acid (PLA)69 and grafts have been studied in both experimental and clinical trials with moderate success. A porcine model was used to investigate autologous chondrocytes seeded into a biphasic scaffold of PLGA and a mixture of PLGA and β-tricalcium phosphate.21 Results showed that the autologous cell-seeded biphasic construct had significantly higher mean gross morphological and histological scores than unseeded constructs 6 months after transplantation. Further, under creep indentation, seeded constructs achieved similar stiffness values but larger permeability values compared to native tissue, while inferior stiffness and permeability values were reported for unseeded controls. The increased permeability of seeded constructs is representative of the inability of matrix to trap fluid within the construct. Therefore, while similar equilibrium stiffness is attained, inferior matrix remains compared to native tissue.
Several patented products for cartilage regeneration exist that utilize autologous chondrocytes seeded with or without polymer matrices. ChondroCelect® (Tigenix, Leuven, Germany) is a modified ACI procedure using a chondrocyte subpopulation thought to possess specific molecular markers that predict the formation of hyaline-type tissue. BioSeed®-C (Biotissue Technologies, Freiburg, Germany) is a tissue-engineered cartilage graft available for use in Europe formed by seeding autologous chondrocytes in fibrin and a polymer-based scaffold of PLGA and polydioxanone. A recent 40-patient, 2-year clinical study reported that the implantation of BioSeed-C into osteochondral defects led to good integration with surrounding tissue, significant improvement in clinical scores resulting from the formation of both fibrocartilage and hyaline-like cartilage, and improved knee-related quality of life.70 NeoCart® (Histogenics, Waltham, MA) is another autogenic implant currently undergoing phase I FDA trials that uses patient's own chondrocytes seeded in a collagen matrix. Neotissue is formed through in vitro culture in a patented, high-pressure bioreactor and reimplanted into the defect site. Hyalograft-C is yet another modified ACI graft that uses hyaluronan as a dual-purpose scaffold material. This material has been shown to provide initial structural integrity as the cellularized graft produces its own matrix followed by the emission of chondrocyte-specific informational cues in the form of small hyaluronan oligomers in latter stages as the scaffold degrades.71 Recent clinical trials have shown Hyalograft-C to be effective out to 24 months postimplantation, with over 90% of patients presenting with significantly improved clinical scores.72,73 Other patented autogenic cartilage repair products used clinically in Europe, such as CaReS® (Arthro Kinetics, Esslingen, Germany) and Chondrokin® (Orthogen, Dusseldorf, Germany), employ autologous cells in combination with other materials, such as a gel-like collagen matrix (CaReS) or collagen fleece (Chondrokin), to grow replacement tissue.
Mesenchymal stem cells
Bone marrow–derived MSCs offer another possible solution toward articular cartilage defect repair. In a procedure known as microfracture surgery, these pluripotent, autologous cells are believed to be recruited to the defect site by making multiple holes in the subchondral bone with an arthroscopic awl to elicit the formation of a blood clot and stimulate the body's natural healing cascade.12,74–78 After migration and filling of the defect site, bone marrow stromal cells differentiate into fibrocartilage cells. Presently, this surgical procedure is the most commonly used clinical treatment of articular cartilage condylar lesions,57 with long-term results indicating reduced pain and statistically significant improvements in joint function.79 However, results of this technique have been mixed as reports suggest neotissue growth occurs without collagen type II, and the surrounding matrix can be damaged by MSC expression of nitric oxide synthase.80 Further, histological evaluation of failed microfracture surgical grafts shows fibroblastic cells and fibrocartilaginous tissue formation with a moderate detection of collagen type I and only a weak presence of collagen type II and proteoglycans.81 Despite this procedure's clinical results, its use has been hindered further by the plethora of limitations for candidates of microfracture surgery. These limitations include partial-thickness defects, axial misalignment in the knee, requirement of a rigorous rehabilitation program, an inability to use the opposite leg for weight bearing up to 12 weeks posttreatment, and age greater than 45 years.78 Collectively, microfracture surgery may represent a moderately successful cartilage repair technique for a small population of individuals with cartilage defects, thereby creating the need for other more widely applicable repair techniques with higher success rates.
Animal studies have also been performed with autologous MSCs in conjunction with biodegradable scaffolds.82–84 One noteworthy study by Zhou et al.85 investigated the implantation of a PLGA scaffold seeded with autologous chondrogenically differentiated porcine MSCs into non-weight-bearing osteochondral defects of hybrid pigs. After 6 months, neotissue contained hyaline-like cartilage atop cancellous bone with a compressive modulus 80% of native values and GAG composition similar to surrounding native tissue. Further, the defect was found to contain implanted MSCs present in both the engineered tissue and underlying subchondral bone, illustrating the ability of these cells to further differentiate into chondrocytes and osteoblasts in an articular cartilage microenvironment. Similar autologous MSC results have been demonstrated for cell-seeded collagen gels implanted in a rabbit model in which large, full-thickness defects of weight-bearing regions of the knee were repaired with hyaline-like cartilage and underlying subchondral bone.86 The use of MSCs in this manner has advantages over ACI because (i) a less invasive procedure is required to obtain MSCs than native chondrocytes, (ii) limited or reduced donor-site morbidity is associated with cell acquisition, (iii) only one surgical procedure on the damaged joint is required, and (iv) MSCs have a greater proliferative capacity than chondrocytes.87–89
Recently, MSCs have been shown to modulate MHC-mediated immune responses through several modalities.90,91 First, it appears that MSCs can inhibit T cell proliferation by both cell-to-cell contact and through the release of soluble factors.91 While only minimally understood, human MSCs express cytokines, such as transforming growth factor-β1 and indoleamine 2,3 dioxygenase, that suppress the response of allogenic leukocytes in vitro, even in the presence of normally inflammatory cytokines such as IFN-γ.90 These findings could be an additional benefit in the use of MSCs in future cartilage repair techniques; however, caution must be heeded when transplanting cells after chondrogenic differentiation. It has been well documented that in addition to cartilage-specific markers such as collagen type II and proteoglycans, chondrogenic differentiation can induce fibroblastic92,93 and hypertrophic92,94 features such as collagen type I expression and matrix metalloproteinase 13 and collagen type X expression, respectively. Therefore, when using MSCs as a cell source for cartilage repair, it is of utmost importance to ensure the phenotypic stability before implantation for the safety of the patient and long-term survival of the implanted graft.
Allogenic
Due to the many practical limitations associated with autogenic tissue use, investigators have turned to allogenic tissue as a possible means to generate successful cartilage tissue grafts. It has long been thought that the lack of vasculature in articular cartilage contributes to an immunologically privileged transplantation site that would allow implantation of cells from various sources without the risk of rejection.95 For this reason, allogenic tissue has been investigated for use in cartilage defect repair under the hypothesis that allogenic grafts can approximate autogenic graft success. Allogenic grafts, then, would be preferable due to the need for only one surgical procedure and their potential availability to satisfy the growing clinical demand.
Allografts
Osteochondral allograft transplantation is similar to the autologous approach in that it involves transplanting a bilayer bone–cartilage graft, but here the tissue is of cadaveric origin. Clinical results of osteochondral allograft transplantation have demonstrated a high success rate (60–95%) as defined by graft survival and good/excellent patient evaluations (Table 1). In early clinical trials examining the usefulness of fresh osteochondral allografts, Meyers et al.17 reported a clinical success rate of 78% in 40 osteochondral allograft transplantations at various locations within the knee (range 2–10 years). Success was defined by a lack of joint pain, unlimited joint function, and increased range of motion. Although this treatment was successful for a variety of cartilage defect indications, such as lesions of the femoral condyle and osteochondritis dissecans, the authors found that patients with unicompartmental traumatic arthritis had a success rate of only 30%. Other clinical trials demonstrated that allograft implantation was unsuitable for bipolar lesion repairs, with 50% of grafts failing 6 years after treatment compared to an 84% success rate for unipolar grafts.96,97 Here, a bipolar graft is defined as the transplantation of tissue on both articulating surfaces within a joint compartment (e.g., resurfacing the patella and trochlea). Long-term follow-ups of patients receiving fresh osteochondral allografts revealed allograft survival rates of 95%, 80%, and 65% at 5, 10, and 15 years, respectively.98 Similarly, allograft survival rates of 75%, 64%, and 63% have been reported in large allograft studies at 5, 10, and 14 years, respectively, after transplantation and that the failure rate of allografts was increased in bipolar lesions as well as older patients (age 60 years and above).99 Smaller clinical assessments of fresh osteochondral allografts have revealed similar positive results over a relatively short assessment time. A 2-year study of 17 patients and a separate 12-patient study lasting 5 years had similar clinical results, whereby successful grafts were reported in 71% and 67% of patients, respectively.100 Analogously, a 20-patient study revealed 75% of transplanted allografts resulted in good/excellent scores and minimal arthrosis out to 7 years.101 While allografting of condylar lesions has met with fair success, it has shown to be a salvage operation aimed at young, active patients with isolated patellofemoral articular cartilage disease in whom previous procedures have failed.
Table 1.
Outcomes of Clinical Osteochondral Allograft Procedures
| Authors | Year | Site of defect | Number of patients | Preservation | Mean follow-up time (years) | Success rate |
|---|---|---|---|---|---|---|
| Meyers et al.17 | 1989 | Knee | 31 | Fresh | 3.5 | 78% good/excellent scores |
| Convery et al.100 | 1991 | Knee | 12 | Fresh | 5.3 | 67% good/excellent scores |
| Convery et al.100 | 1991 | Knee | 17 | Fresh | 2 | 71% good/excellent scores |
| Beaver et al.99 | 1992 | Knee | 92 | Fresh | 5 | 75% survival |
| 10 | 64% survival | |||||
| 14 | 63% survival | |||||
| Garrett140 | 1994 | Femoral condyle | 17 | Fresh | 3.5 | 94% good/excellent scores |
| Flynn et al.108 | 1994 | Femoral condyle | 17 | Fresh-frozen | 4.2 | 70% good/excellent scores |
| Ghazavi et al.141 | 1997 | Knee | 126 | Fresh | 7.5 | 85% survival |
| Bakay et al.107 | 1998 | Knee | 33 | Cryopreserved | 1.6 | 67% good/excellent scores |
| Chu et al.96 | 1999 | Knee | 55 | Fresh | 6.2 | 76% good/excellent scores |
| Friedlaender et al.106 | 1999 | Knee/elbow | 29 | Cryopreserved | 10.6 | 79% good/excellent scores |
| Gross et al.98 | 2005 | Knee | 60 | Fresh | 5 | 95% survival |
| 10 | 80% survival | |||||
| 15 | 65% survival | |||||
| Jamali et al.101 | 2005 | Patellofemoral | 20 | Fresh | 7.8 | 75% good/excellent scores |
| Emmerson et al.142 | 2007 | Femoral condyle | 65 | Fresh | 7.7 | 72.3% good/excellent scores |
Animal studies investigating the use of osteochondral allografts with a range of animal models have reported varying degrees of success. Early work by Langer and Gross102 implanted chondrocytes maintained within their ECM in the subcutaneous dorsum of both inbred rat and unrelated rabbit models. Recipients did not experience significant leukocyte migration into the defect site, a characteristic closely attributed to a cell-mediated immune response, nor did they possess any cytotoxic humoral antibodies associated with the allograft, indicating that cartilage grafts of chondrocytes maintained within their matrix are relatively nonimmunogenic. Similar results were observed in other animal studies involving rabbits13 and dogs103 that showed allograft transplantation led to at least an 80% success rate, as assessed by complete filling of the defect with cartilaginous ECM. Additionally, no differences in biochemical composition and biomechanical functionality were found compared to fresh autografts and surrounding native tissue. Stevenson et al.104 report potentially conflicting results when leukocyte antigen-matched and antigen-mismatched allografts were transplanted in a dog model. After 11 months, antigen-mismatched allografts contained significantly less articular cartilage than either sham operations or antigen-matched allografts, with increased presence of inflammatory mononuclear cells. This immune response was mitigated by cryopreserving allografts before harvest, but this process led to significant cell death and biochemically and histologically inferior grafts.
Due to limited availability of fresh cadaveric tissue, several clinical studies have been performed assessing the efficacy and viability of frozen and cryopreserved osteochondral allografts.105–108 Clinical results showed that 67% and 70% of patients reported good/excellent scores after transplantation of cryopreserved107 and frozen108 allografts, respectively, out to 4 years. Compared to fresh allografts, frozen allografts offer many advantages, including greater tissue availability, a longer time period for infectious disease testing, easier size matching, and a reduction in graft immunogenicity.107,108 It is believed that the decreased immunogenicity due to the lack of cell viability was a result of the freezing procedure.109,110 Specifically, the freezing process has been shown to reduce the viability of chondrocytes, eliminating more than 95% from the articular cartilage portion of osteochondral grafts, which subsequently leads to deterioration of cartilage matrix molecules.95,111
Special care and treatment of allografts have been shown to be of paramount importance in limiting immunogenic reactions to osteochondral allografts. A common preparation step for osteochondral allografts is a high-pressure wash between harvest and transplantation to remove allogenic APCs. It is worth noting that in all the above clinical studies, pressure washes were administered to allografts before implantation, and no tissue typing, blood grouping, or immunosuppressive therapies were used. In fresh allograft studies, transplantation occurred 2–7 days after the death of the donor to permit a sufficient testing time for diseases such as HIV, hepatitis, and syphilis, while frozen allografts provided ample testing time for potential disease transmission.
Chondrocyte implantation
In addition to osteochondral replacement, allogenic chondrocytes have been used in a procedure similar to ACI to repair cartilage defects. A wide body of evidence exists demonstrating that allogenic transplantation of isolated chondrocytes elicits an immune response that gradually destroys the resulting cartilage tissue.112–115 Specifically, it has been shown in a rabbit model that cytotoxic humoral antibodies were identified in animals that received transplanted chondrocytes.102 These grafts were immunogenic as assessed by leukocyte migration assays, indicating the presence of a cell-mediated immunologic reaction. A separate study involved the transplantation of isolated rabbit chondrocytes into the anterior tibial compartment, resulting in a rapid immune response after only 10 days.116 In contrast, when these same chondrocytes were allowed to produce a matrix atop decalcified bone before implantation, the genesis of tissue repair was observed at 10 days, and only a minimal immune response was detected out to 6 months.
Other work has investigated the role the immune system plays toward detection and rejection of allogenic chondrocytes. To ensure detection of implanted chondrocytes by the host's immune system, Romaniuk et al.20 implanted allogenic chondrocytes into posterior tibial muscles immediately after enzymatic digestion and assessed repair tissue out to 12 weeks. Cartilage neotissue nodules formed immediately, but they were slowly destroyed over time by large infiltrations of mononuclear lymphoid cells. Specifically, macrophages accumulated around the chondrocytes immediately after transplantation, while later stages of cartilage destruction showed large populations of natural killer and cytotoxic/suppressor T cells.
Tissue-engineered constructs
Due to the relative success of transplanted osteochondral allografts and the simultaneous failure of transplanted isolated allogenic chondrocytes, tissue engineering of neocartilage using allogenic chondrocytes with natural and synthetic scaffolds has been investigated as an alternative for the repair of cartilage defects (Table 2). To date, most in vivo experimental studies have utilized natural and synthetic scaffolds in small-animal models, that is, rabbit and rat. Natural scaffolds, such as collagen and agarose, have been successfully used in both animal models for allogenic transplantation.87,117–121 In the rabbit, cell-seeded collagen allografts contained a majority of collagen type II, had a success rate of 80% out to 24 weeks with no sign of immunologic reactions,121 and possessed similar compressive stiffness values to native tissue after 12 weeks of implantation that persisted out to 48 weeks.122 Thus, neotissue with stiffness values approaching native values accompanied by hyaline-like histological scores indicated successful repair of the induced cartilage defects. Masuoka et al.117 used allogenic chondrocytes cultured in a variant of a type I collagen scaffold, known as an atelocollagen honeycomb sponge, for treatment of osteochondral defects in the rabbit. Analysis at 3 months revealed hyaline cartilage neotissue exhibiting collagen type II staining with no signs of tissue degeneration or immunologic rejection. In rats, collagen-seeded allografts filled with hyaline neocartilage after 2 weeks with a small degree of inflammatory cell infiltration. This infiltration had almost disappeared by 12 weeks, and no signs of inflammatory infiltration were present at 1 year posttransplantation. Transplantation of rabbit chondrocytes in an agarose gel after in vitro matrix accumulation resulted in no graft rejections or immune cell infiltrations after 18 months.119 Additionally, 47% of the grafts revealed a neotissue that had morphology approximating native tissue. In these studies, it is postulated that three-dimensional in vitro growth led to chondrocyte phenotype maintenance and matrix production that shielded chondrocytes from a host-mediated immune response after implantation.
Table 2.
Immune Responses of Tissue-Engineered Constructs with Allogenic Chondrocytes
| Authors | Year | Species | Tissue engineering modality | Follow-up time (months) | Immunogenic results |
|---|---|---|---|---|---|
| Itay et al.128 | 1987 | Rooster | Thrombin scaffold | 6 | No signs of rejection |
| Wakitani et al.121 | 1989 | Rabbit | Collagen gel | 5.5 | No monocytes detected out to 8 weeks |
| Kawabe and Yoshinao127 | 1991 | Rabbit | Cell-seeded disc | Rejected due to cell-mediated toxicity and humoral response | |
| Noguchi et al.118 | 1994 | Rat | Collagen gel | 12 | After 3 months, no signs of rejection |
| Freed et al.123 | 1994 | Rabbit | PGA scaffold | 6 | No signs of rejection |
| Sams and Nixon129 and Sams et al.130 | 1995 | Horse | Collagen scaffold | 8 | No signs of rejection |
| Wakitani et al.122 | 1998 | Rabbit | Collagen gel | 11 | Stiffness similar to native after 12 weeks |
| Rahfoth et al.119 | 1998 | Rabbit | Agarose gel | 18 | No immune cells discovered |
| Schreiber et al.124 | 1999 | Rabbit | PGA mesh | 24 | Low level of mononuclear cells detected |
| Perka et al.125 | 2000 | Rabbit | PGA-PLA scaffold | 2.8 | Mononuclear cells present at 4 weeks, disappeared out to 12 weeks |
| Fragonas et al.22 | 2000 | Rabbit | Alginate gel | 6 | No signs of rejection |
| Tanaka et al.120 | 2005 | Rabbit | Collagen gel + β-tricalcium phosphate biphasic graft | 6.9 | No immune response assessed |
| Masuoka et al.117 | 2005 | Rabbit | Atelocollagen honeycomb sponge | 3 | No signs of rejection |
| Shangkai et al.126 | 2007 | Rabbit | Silk fibroin sponge | 2.8 | No signs of rejection |
The use of synthetic biomaterials combined with allogenic chondrocytes has also been explored for tissue repair with considerable success. Freed et al.123 used rabbit chondrocytes seeded onto polyglycolic acid (PGA) scaffolds for transplantation into full-thickness defects. Before implantation, cell-seeded scaffolds were cultured for 3–4 weeks to allow the formation of a small amount of neotissue. At 1 month posttransplant, a moderate number of lymphocytes were present in transplanted joints, which disappeared after 6 months. The nonimmunogenicity of the implanted construct was attributed to the surface antigens of allogenic chondrocytes being shielded and sequestered by cartilage matrix deposited during in vitro culture. In a similar study, allogenic rabbit chondrocytes were seeded in PGA meshes before implantation in osteochondral defects.124 Similarly, a low level of mononuclear cells was detected in regions of the underlying bone of treated defects. These cells, however, did not disappear over time as this cell infiltration was maintained throughout the 24-month study. This persistence of mononuclear cells was attributed to the allogenic cell population because lymphocytes were absent from defects treated with unseeded scaffolds. It is worth noting that this mild immune response appeared benign, as no evidence of graft resorption or rejection was observed, and neotissue maintenance was preserved over the entire 2-year observation period.
Other studies combining natural and synthetic scaffolds with allogenic chondrocytes affirm previous successes of allogenic cartilage tissue engineering. In one study, rabbit chondrocyte-fibrin gels were embedded within a PGA–lactic acid polymer fleece.125 Treated defects were filled with hyaline-like cartilage tissue, and while a large number of mononuclear cells were observed around the defect 4 weeks after surgery, none of these cells infiltrated into the newly formed cartilage. The mild immune response at 4 weeks was attributed to polymer scaffold resorption, which was complete by 12 weeks when no immune response was observed. Additional evidence of successful allogenic cartilage tissue engineering was provided in a study in which the effectiveness of allogenic transplantation of silk fibroin sponges seeded with rabbit chondrocytes after bioreactor culture was investigated.126 Results indicated that 12 weeks after transplantation, hyaline-like tissue ingrowth had occurred with no signs of an immune response.
While an overwhelming number of studies have indicated that allogenic chondrocytes used in conjunction with a wide variety of natural and synthetic scaffolds are effective in treating articular cartilage defects without eliciting an immune response, one study by Kawabe and Yoshinao127 has put forth conflicting evidence. In this study, rabbit growth plate chondrocytes were cultured for 10 days in a scaffold-free system, eventually generating 200-μm-thick neotissue discs. After in vitro culture, these constructs were implanted with a fibrin clot for attachment in the defect site. These grafts produced satisfactory articular cartilage tissue out to 3 weeks, but all grafts were replaced by fibrous tissue by 24 weeks. Moreover, this fibrous tissue replacement coincided with an increase in mononuclear cells throughout the graft, including an accumulation of lymphocytes around chondrocytes from 2 to 12 weeks, with a peak at 3 weeks; authors concluded that the observed immune response was both cell mediated and humoral. It remains unclear the quantity of matrix accumulation present after 10 days of culture before implantation, as only slight safranin-O staining was observed immediately after implantation. The low accumulation may be an indicator for the observed chronic cell infiltration, and had more time been given during in vitro culture for the accumulation of additional ECM, the immune response may have been mitigated. Nevertheless, even when chondrocytes were surrounded by a small amount of ECM when implanted, a vigorous immune response occurred.
Although most animal studies have been performed in the rabbit model, there exists a small body of evidence of allogenic cartilage tissue engineering in other animal models with moderate success. Thrombin scaffolds seeded with embryonic chondrocytes were implanted into osteochondral defects of White Leghorn roosters.128 Resulting neocartilage integrated well with surrounding matrix out to 6 months posttransplant, while no signs of immunogenic rejection were observed. Sams and Nixon129 and Sams et al.130 used foal chondrocytes seeded in a collagen scaffold for use in large (15 mm) cartilage defects in horses. Grafts contained significant aggrecan deposition 8 months after transplantation but only a small amount of collagen type II. Additionally, no cellular infiltrates were present indicating the absence of an immune response.
Another technique utilized allogenic chondrogenically differentiated MSCs seeded in a hyaluronic acid–based gel to resurface caprine articular cartilage.131 Results showed fibrocartilage tissue production 3 months after implantation that contained blood cell infiltrates and signs of mild immunologic rejection. In contrast, autogenic MSC-derived chondrocytes produced hyaline cartilage neotissue without any signs of an immunologic reaction. The authors indicate that the inherent heterogeneity of chondrogenically induced MSC-derived cells may contain a small subpopulation of noncartilage cells that do not produce a cartilage ECM coat. Without the ECM imparting immunoprivileged protection, the allogenic cells are subject to eliciting an immunogenic reaction.
The fate of transplanted allografts has been investigated for over three decades and remains a matter of debate. Though a few animal studies indicate that there may be a host immune response after implantation of an osteochondral allograft causing tissue rejection, a large amount of clinical evidence exists to the contrary. Allogenic chondrocytes that have been isolated from their native surrounding matrix are immunogenic and contribute to an inflammatory immune response and tissue resorption, but this immunogenicity is tempered and chondrocytes remain viable when transplanted within their dense surrounding matrix of a tissue graft or engineered construct. More specifically, the presence of ECM is believed to form a protective barrier around the chondrocytes, which blocks both the infiltration of host immune cells into the graft as well as the escape of immunogenic chondrocytes out of the graft.28,102,123
Xenogenic
Another option in addressing limitations of autogenic tissue use in cartilage repair involves the use of xenogenic chondrocytes. To date, only a small number of researchers have explored these cells as a viable alternative to traditional repair techniques, but successes with allogenic chondrocytes surrounded by ECM have quelled some original fears from researchers regarding major immunologic tissue rejection. The highly limited availability of healthy human cartilage for allogenic use bolsters the potential excitement and keen interest associated with xenogenic chondrocyte usage.
Osiecka-Iwan et al.132 investigated the implantation of rat chondrocytes in intramuscular rabbit tissue to assess the humoral immune response of xenogenic chondrocyte implantation. Cartilage transplants were rejected when assessed 2 weeks postimplantation, and the implant site was marked by the presence of nonviable chondrocytes and a matrix devoid of proteoglycans. Additionally, the implant site was completely invaded by macrophages and a small number of giant foreign body cells. The implantation of xenogenic chondrocytes in this model differs dramatically from implantation into a joint defect, as the intramuscular implant site is strongly vascularized, which guarantees the detection of xenogenic cells by the host's immune system. While a primarily humoral response was observed in this model, the authors concede that implantation into a joint surface defect would likely elicit a different host immune response. However, this finding indicates a potential inflammatory reaction should a vascularized source be subjected to unshielded xenogenic cells.
Xenotransplantation of chondrocytes into a cartilage defect as opposed to an intramuscular site is a more realistic model for assessing immune responses and the neotissue growth potential. One such study examined pig chondrocyte implanted into induced human cartilage defects (cadaveric explants) in an in vitro model.23 After 12 weeks, immunohistochemical analysis of neotissue, which filled 36% of the induced defect, indicated the presence of proteoglycans and both collagen types I and II. While this model illustrates xenogenic chondrocytes' ability to regenerate a neotissue of hyaline-like biochemical properties, a major limitation was that in the in vitro model, no assessment of an immune response was possible for the isolated xenogenic chondrocytes and resulting xenotransplant. An improvement in this method was performed by Ramallal et al.,3 whereby they implanted pig chondrocytes into osteochondral defects of adult rabbits in vivo. Results 24 weeks after surgery revealed the presence of hyaline-like repair tissue. Moreover, this neotissue was found to have been produced by the xenotransplanted pig chondrocytes and not by the native rabbit tissue, as pig cells were distributed throughout the newly synthesized cartilage. The absence of inflammatory cells or other external inflammatory signs at the defect site indicates the lack of a host immune response against the implanted graft. These results indicate a potential role for xenotransplantation, though a complete battery of immunogenicity parameters was not investigated, including specific antibody reactivity against pig cells.
In another experimental model, osteochondral repair tissue in rabbits was compared using a PLA scaffold seeded with rabbit allogenic chondrocytes, MSCs, and fibroblasts and undifferentiated human umbilical cord blood (hUCB) stem cells. After 12 weeks, all four groups produced repair tissue of varying hyaline and fibrous tissue characteristics. Allogenic chondrocytes and MSCs produced hyaline-like tissue, allogenic fibroblasts produced complete fibrous tissue, while xenogenic hUCB cells produced a predominant amount of fibrous tissue with scattered chondrocytes and a thin layer of hyaline-like cartilage. In terms of an immunologic response, no lymphocyte infiltration was observed in the repaired graft tissue for any treatment group, but lymphocyte infiltration was observed in the synovial tissue for only the hUCB cell group most likely associated with the difference in species.
Xenogenic transplants of rabbit chondrocytes embedded in a fibrin glue scaffold were implanted in goats and formed a cartilage matrix 52 weeks posttransplantation with a matrix consisting of 75% collagen type II.133 At the conclusion of the study, xenotransplants produced fibrous tissue similar to tissue produced in control, unfilled defects. Mild signs of synovitis were observed in some specimens and were also observed in several sham operations, indicating that an inflammatory response could be attributed to the transplantation procedure, and not the presence of xenogenic chondrocytes.
Decellularization of osteochondral xenografts has been hypothesized as a means of repairing cartilage defects while avoiding a potential immune response associated with xenogenic chondrocytes. von Rechenberg et al.18 reported exciting results using a photooxidation technique for decellularization of bovine osteochondral grafts with subsequent xenotransplantation into osteochondral ovine defects. Previous in vitro studies demonstrated that photooxidation of cartilage produced a tissue containing only nonviable chondrocytes and a mechanically stronger ECM, due to the photooxidation's ability to affect the aromatic and sulfur-containing amino acids of cartilage's collagen fibrils.134,135 When implanted in vivo into a sheep model, photooxidized bovine transplants outperformed untreated xenotransplants, untreated autografts, and both treated and untreated allografts as assessed by histological and gross morphological scoring. Additionally, fewer cystic lesions were observed for photooxidized xenografts than in all other groups, indicating a tight fit between host and graft tissue. A simultaneous reduction in mononuclear and plasma cell infiltration was observed in the treated xenotransplant group after 6 months. It is of note that while native chondrocyte recruitment occurred in treated xenografts, the middle zone remained acellular even out to 6 months. This photooxidation process was demonstrated to reduce the immunogenic properties of osteochondral grafts and inhibit graft bone resorption, thus yielding a possible treatment that may enable nonviable osteochondral xenotransplants or engineered xenografts to be used clinically in cartilage defect repairs.
Discussion
Attempts to bypass articular cartilage's limited ability to self-repair have led researchers to consider a wide array of tissue sources and repair techniques to achieve tissue similar to native surrounding tissue. These approaches exhibit technical complexities of varying degree that must be overcome before acceptance as the best clinical treatment option (Table 3). Autogenic tissue has been thoroughly investigated because it avoids any potential for immune rejection or disease transmission, but results have proved inconsistent. Beneficial results have been obtained using autogenic osteochondral plug replacement and mosaicplasty, with success rates of >70% occurring for each treatment modality. The ACI technique has been approved by FDA for clinical use and has contributed to moderately successful short-term graft repair, while long-term results may be less promising. These autogenic treatments, however, have many limitations preventing widespread use, chief among which is the requirement of a cartilage donor site from a non-weight-bearing portion of the knee. As stated previously, it has been suggested that a critical-size defect exists for cartilage (generally accepted as a defect measuring less than 3 mm in diameter) below which a natural healing response occurs whereby the defect is filled with normal hyaline tissue. Mounting evidence has arisen counter to this argument, stating that any size defect, especially one penetrating into the subchondral bone, repairs with a fibrous tissue that is inferior to normal cartilage.136 With time, this fibrocartilage repair tissue degenerates and the defect becomes permanent, frequently hastening further degeneration at the site of injury.22,119,124 MSC usage, while advantageous over other autogenic treatments due to the reduced donor-site morbidity, contributes to a large amount of fibrocartilaginous tissue with inferior properties. Evidence, therefore, indicates that all current autogenic treatment techniques have major practical and functional hurdles associated with them, leading researchers to consider alternate tissue sources and strategies.
Table 3.
Limitations Associated with Current Cartilage Repair Techniques
| |
Hindrance to technique |
||||
|---|---|---|---|---|---|
| Donor-site morbidity | Multiple surgical sites | Tissue scarcity | Immune response | Disease transmission | |
| Autogenic | |||||
| Osteochondral plug transplantation | +++ | ++ | +++ | − | − |
| Mosaicplasty | ++ | +++ | ++ | − | − |
| Chondrocyte implantation | ++ | ++ | ++ | − | − |
| Chondrocyte-seeded scaffolds | ++ | ++ | ++ | − | − |
| Microfracture | − | − | − | − | − |
| Mesenchymal stem cell implantation | + | + | + | − | − |
| Allogenic | |||||
| Allograft transplantation | − | − | ++ | − | + |
| Chondrocyte implantation | − | − | ++ | +++ | + |
| Tissue-engineered constructs | − | − | ++ | + | + |
| Xenogenic | |||||
| Chondrocyte implantation | − | − | − | ++ | ++ |
| Chondrocyte-seeded scaffolds | − | − | − | ++ | ++ |
Hindrance level: −, none; +, minor; ++, moderate; +++, major.
Many of the same treatment strategies have been employed with allogenic and, to a lesser degree, xenogenic tissue, and have led to encouraging results. Specifically, osteochondral allografts and a wide range of engineered cartilage grafts using allogenic chondrocytes have shown high success rates in both clinical and experimental studies. An original concern in using allogenic tissue was an immune response to the graft by the host, but this issue has been thoroughly investigated and very few immune responses and their associated setbacks have been documented. On the contrary, implantation of suspended allogenic chondrocytes has led to rapid immune responses in host tissue with subsequent graft rejection and resorption. Together, these findings lead to the theory that allogenic chondrocytes are indeed immunogenic, but an ECM acts to form a protective barrier around the chondrocytes, thereby shielding this immunogenicity and enabling grafts with sufficient matrix accumulation to resist immune rejection. Nonetheless, future studies are needed to fully understand and optimize exactly how much and what type of matrix accumulation is necessary to impede this immunogenicity. These results are encouraging in that allogenic tissue can be used to produce cartilage repair tissue, either as osteochondral plugs or through various tissue engineering strategies, with similar success rates as autogenic therapies and similar biochemical and biomechanical functionality as native tissue.
While results of experimental studies presented in Table 2 reveal a muted immune response using allogenic chondrocytes with natural and synthetic scaffolds, we would be remiss to not comment on a potential implicit bias and confounding effect in using small-animal models for immune response studies, particularly the rabbit. These results may be due to some degree of inbreeding present in many species of rabbits and hens used as laboratory study animals.29 Should allograft transplants be performed between closely related animals, an absence of monocyte cell infiltration, and accompanying downstream immunologic response, may be inaccurately attributed to an immunoprivileged response. This explanation can be challenged because it is known that skin transplants exchanged between members of a specific rabbit colony are immunologically rejected.137 Additional evidence supporting this position is the success of allografted osteochondral plugs in both human clinical experience and a multitude of animal trials. Nevertheless, to adequately compare allogenic and xenogenic results to the wide body of clinical data supporting autogenic repair techniques, further experimental investigations into potential cartilage repair techniques should focus on a wider range of larger animal models with more clinical relevance, such as the goat.138,139
A wide range of treatment techniques are available for the treatment of articular cartilage defects. In vivo clinical and experimental studies coupled with in vitro animal studies have made great strides toward providing improved treatment techniques. Further, this research has defined the immunologic response against chondrocytes when implanted in the joint space and has illustrated various modalities to mitigate this response. Though advances in tissue grafting have been achieved, certain limitations remain. Most investigated techniques are still only successful in a younger patient (<45 years), and are only successful in unipolar graft replacement. Therefore, further research should continue along both the allogenic and xenogenic path, pursuing the techniques that best reproduce neotissue similar to healthy, native tissue. The breadth of endpoint analyses should also be widened, and more emphasis should be placed on graft biomechanical and biochemical functionality in conjunction with histology, immunogenicity, and clinical scoring.
Because autologous repair techniques avoid immunological concerns, they possess logistical and biological limitations that impede their widespread use. The major concern in implementing alternate sources for transplantation has been the associated immunogenicity inherent in allogenic and xenogenic cells and tissue. As shown here, however, a large amount of clinical and experimental evidence exists suggesting that an immune response is elicited when isolated allogenic and xenogenic cells are implanted into cartilage defects in the absence of any ECM. When chondrocytes are implanted while contained within their ECM, the immune response is significantly reduced, as the ECM acts to protect and shield the antigenicity of the cells and prevents the host's immune response from mounting an attack for rejection. This exciting finding opens the door for an increase in research toward allogenic and xenogenic repair techniques that can finally aid in solving the major clinical problem of defective articular cartilage.
Acknowledgments
The authors would like to gratefully acknowledge funding support from NIAMS R01 AR053286 and NIAMS R01 AR047839.
Disclosure Statement
No competing financial interests exist.
References
- 1.Buckwalter J.A. Articular cartilage injuries. Clin Orthop Relat Res. 2002;21:402. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Mauck R.L. Nicoll S.B. Seyhan S.L. Ateshian G.A. Hung C.T. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 3.Ramallal M. Maneiro E. Lopez E. Fuentes-Boquete I. Lopez-Armada M.J. Fernandez-Sueiro J.L. Galdo F. de Toro F.J. Blanco F.J. Xeno-implantation of pig chondrocytes into rabbit to treat localized articular cartilage defects: an animal model. Wound Repair Regen. 2004;12:337. doi: 10.1111/j.1067-1927.2004.012309.x. [DOI] [PubMed] [Google Scholar]
- 4.Mankin H.J. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460. [PubMed] [Google Scholar]
- 5.Hunziker E.B. Articular cartilage repair: are the intrinsic biological constraints undermining this process insuperable? Osteoarthritis Cartilage. 1999;7:15. doi: 10.1053/joca.1998.0159. [DOI] [PubMed] [Google Scholar]
- 6.Hangody L. Feczko P. Bartha L. Bodo G. Kish G. Mosaicplasty for the treatment of articular defects of the knee and ankle. Clin Orthop Relat Res. 2001;S328:391. doi: 10.1097/00003086-200110001-00030. [DOI] [PubMed] [Google Scholar]
- 7.Hangody L. Kish G. Karpati Z. Szerb I. Eberhardt R. Treatment of osteochondritis dissecans of the talus: use of the mosaicplasty technique—a preliminary report. Foot Ankle Int. 1997;18:628. doi: 10.1177/107110079701801005. [DOI] [PubMed] [Google Scholar]
- 8.Matsusue Y. Yamamuro T. Hama H. Arthroscopic multiple osteochondral transplantation to the chondral defect in the knee associated with anterior cruciate ligament disruption. Arthroscopy. 1993;9:318. doi: 10.1016/s0749-8063(05)80428-1. [DOI] [PubMed] [Google Scholar]
- 9.Shapiro F. Koide S. Glimcher M.J. Cell origin and differentiation in the repair of full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1993;75:532. doi: 10.2106/00004623-199304000-00009. [DOI] [PubMed] [Google Scholar]
- 10.Hurtig M.B. Fretz P.B. Doige C.E. Schnurr D.L. Effects of lesion size and location on equine articular cartilage repair. Can J Vet Res. 1988;52:137. [PMC free article] [PubMed] [Google Scholar]
- 11.Convery F.R. Akeson W.H. Keown G.H. The repair of large osteochondral defects. An experimental study in horses. Clin Orthop Relat Res. 1972;82:253. [PubMed] [Google Scholar]
- 12.Blevins F.T. Steadman J.R. Rodrigo J.J. Silliman J. Treatment of articular cartilage defects in athletes: an analysis of functional outcome and lesion appearance. Orthopedics. 1998;21:761. doi: 10.3928/0147-7447-19980701-05. [DOI] [PubMed] [Google Scholar]
- 13.Aston J.E. Bentley G. Repair of articular surfaces by allografts of articular and growth-plate cartilage. J Bone Joint Surg Br. 1986;68:29. doi: 10.1302/0301-620X.68B1.3941138. [DOI] [PubMed] [Google Scholar]
- 14.Burks R.T. Greis P.E. Arnoczky S.P. Scher C. The use of a single osteochondral autograft plug in the treatment of a large osteochondral lesion in the femoral condyle: an experimental study in sheep. Am J Sports Med. 2006;34:247. doi: 10.1177/0363546505279914. [DOI] [PubMed] [Google Scholar]
- 15.Hangody L. Fules P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 16.Marcacci M. Kon E. Zaffagnini S. Visani A. Use of autologous grafts for reconstruction of osteochondral defects of the knee. Orthopedics. 1999;22:595. doi: 10.3928/0147-7447-19990601-09. [DOI] [PubMed] [Google Scholar]
- 17.Meyers M.H. Akeson W. Convery F.R. Resurfacing of the knee with fresh osteochondral allograft. J Bone Joint Surg Am. 1989;71:704. [PubMed] [Google Scholar]
- 18.von Rechenberg B. Akens M.K. Nadler D. Bittmann P. Zlinszky K. Kutter A. Poole A.R. Auer J.A. Changes in subchondral bone in cartilage resurfacing—an experimental study in sheep using different types of osteochondral grafts. Osteoarthritis Cartilage. 2003;11:265. doi: 10.1016/s1063-4584(03)00006-2. [DOI] [PubMed] [Google Scholar]
- 19.Brittberg M. Peterson L. Sjogren-Jansson E. Tallheden T. Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A Suppl 3:109. doi: 10.2106/00004623-200300003-00017. [DOI] [PubMed] [Google Scholar]
- 20.Romaniuk A. Malejczyk J. Kubicka U. Hyc A. Olszewski W.L. Moskalewski S. Rejection of cartilage formed by transplanted allogeneic chondrocytes: evaluation with monoclonal antibodies. Transpl Immunol. 1995;3:251. doi: 10.1016/0966-3274(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 21.Jiang C.C. Chiang H. Liao C.J. Lin Y.J. Kuo T.F. Shieh C.S. Huang Y.Y. Tuan R.S. Repair of porcine articular cartilage defect with a biphasic osteochondral composite. J Orthop Res. 2007;25:1277. doi: 10.1002/jor.20442. [DOI] [PubMed] [Google Scholar]
- 22.Fragonas E. Valente M. Pozzi-Mucelli M. Toffanin R. Rizzo R. Silvestri F. Vittur F. Articular cartilage repair in rabbits by using suspensions of allogenic chondrocytes in alginate. Biomaterials. 2000;21:795. doi: 10.1016/s0142-9612(99)00241-0. [DOI] [PubMed] [Google Scholar]
- 23.Fuentes-Boquete I. Lopez-Armada M.J. Maneiro E. Fernandez-Sueiro J.L. Carames B. Galdo F. de Toro F.J. Blanco F.J. Pig chondrocyte xenoimplants for human chondral defect repair: an in vitro model. Wound Repair Regen. 2004;12:444. doi: 10.1111/j.1067-1927.2004.012412.x. [DOI] [PubMed] [Google Scholar]
- 24.Hale D.A. Basic transplantation immunology. Surg Clin North Am. 2006;86:1103. doi: 10.1016/j.suc.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Pietra B.A. Transplantation immunology 2003: simplified approach. Pediatr Clin North Am. 2003;50:1233. doi: 10.1016/s0031-3955(03)00119-6. [DOI] [PubMed] [Google Scholar]
- 26.Goldsby R.A. Kindt T.J. Osborne B.A. Kuby J. Fifth edition. New York: W.H. Freeman and Company; 2003. Immunology. [Google Scholar]
- 27.Trivedi H.L. Immunobiology of rejection and adaptation. Transplant Proc. 2007;39:647. doi: 10.1016/j.transproceed.2007.01.047. [DOI] [PubMed] [Google Scholar]
- 28.Bolano L. Kopta J.A. The immunology of bone and cartilage transplantation. Orthopedics. 1991;14:987. doi: 10.3928/0147-7447-19910901-10. [DOI] [PubMed] [Google Scholar]
- 29.Moskalewski S. Hyc A. Osiecka-Iwan A. Immune response by host after allogeneic chondrocyte transplant to the cartilage. Microsc Res Tech. 2002;58:3. doi: 10.1002/jemt.10110. [DOI] [PubMed] [Google Scholar]
- 30.Lance E.M. Immunological reactivity towards chondrocytes in rat and man: relevance to autoimmune arthritis. Immunol Lett. 1989;21:63. doi: 10.1016/0165-2478(89)90013-8. [DOI] [PubMed] [Google Scholar]
- 31.Malejczyk J. Natural anti-chondrocyte cytotoxicity of normal human peripheral blood mononuclear cells. Clin Immunol Immunopathol. 1989;50:42. doi: 10.1016/0090-1229(89)90220-1. [DOI] [PubMed] [Google Scholar]
- 32.Malejczyk J. Kaminski M.J. Malejczyk M. Majewski S. Natural cell-mediated cytotoxic activity against isolated chondrocytes in the mouse. Clin Exp Immunol. 1985;59:110. [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaga K.M. Kimura L.H. Plymyer M.R. Glant T.T. Lance E.M. Differentiation antigens of human articular chondrocytes and their tissue distribution as assessed by monoclonal antibodies. J Autoimmun. 1994;7:203. doi: 10.1006/jaut.1994.1016. [DOI] [PubMed] [Google Scholar]
- 34.Bujia J. Alsalameh S. Naumann A. Wilmes E. Sittinger M. Burmester G.R. Humoral immune response against minor collagens type IX and XI in patients with cartilage graft resorption after reconstructive surgery. Ann Rheum Dis. 1994;53:229. doi: 10.1136/ard.53.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dayer E. Mathai L. Glant T.T. Mikecz K. Poole A.R. Cartilage proteoglycan-induced arthritis in BALB/c mice. Antibodies that recognize human and mouse cartilage proteoglycan and can cause depletion of cartilage proteoglycan with little or no synovitis. Arthritis Rheum. 1990;33:1394. doi: 10.1002/art.1780330912. [DOI] [PubMed] [Google Scholar]
- 36.Glant T.T. Buzas E.I. Finnegan A. Negroiu G. Cs-Szabo G. Mikecz K. Critical roles of glycosaminoglycan side chains of cartilage proteoglycan (aggrecan) in antigen recognition and presentation. J Immunol. 1998;160:3812. [PubMed] [Google Scholar]
- 37.Takagi T. Jasin H.E. Interactions between anticollagen antibodies and chondrocytes. Arthritis Rheum. 1992;35:224. doi: 10.1002/art.1780350217. [DOI] [PubMed] [Google Scholar]
- 38.Yablon I.G. Cooperband S. Covall D. Matrix antigens in allografts. The humoral response. Clin Orthop Relat Res. 1982;243:168. [PubMed] [Google Scholar]
- 39.Hangody L. Kish G. Karpati Z. Szerb I. Udvarhelyi I. Arthroscopic autogenous osteochondral mosaicplasty for the treatment of femoral condylar articular defects. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1997;5:262. doi: 10.1007/s001670050061. [DOI] [PubMed] [Google Scholar]
- 40.Dozin B. Malpeli M. Cancedda R. Bruzzi P. Calcagno S. Molfetta L. Priano F. Kon E. Marcacci M. Comparative evaluation of autologous chondrocyte implantation and mosaicplasty: a multicentered randomized clinical trial. Clin J Sport Med. 2005;15:220. doi: 10.1097/01.jsm.0000171882.66432.80. [DOI] [PubMed] [Google Scholar]
- 41.Lysholm J. Gillquist J. Evaluation of knee ligament surgery results with special emphasis on use of a scoring scale. Am J Sports Med. 1982;10:150. doi: 10.1177/036354658201000306. [DOI] [PubMed] [Google Scholar]
- 42.Szerb I. Hangody L. Duska Z. Kaposi N.P. Mosaicplasty: long-term follow-up. Bull Hosp Jt Dis. 2005;63:54. [PubMed] [Google Scholar]
- 43.Bentley G. Biant L.C. Carrington R.W. Akmal M. Goldberg A. Williams A.M. Skinner J.A. Pringle J. A prospective, randomised comparison of autologous chondrocyte implantation versus mosaicplasty for osteochondral defects in the knee. J Bone Joint Surg Br. 2003;85:223. doi: 10.1302/0301-620x.85b2.13543. [DOI] [PubMed] [Google Scholar]
- 44.Peterson L. Menche D. Grande D. Klein M. Burmester G. Pugh J. Pitman M. Chondrocyte transplantation—an experimental model in the rabbit. Trans Orthop Res Soc. 1984;9:218. [Google Scholar]
- 45.Wood J.J. Malek M.A. Frassica F.J. Polder J.A. Mohan A.K. Bloom E.T. Braun M.M. Cote T.R. Autologous cultured chondrocytes: adverse events reported to the United States Food and Drug Administration. J Bone Joint Surg Am. 2006;88:503. doi: 10.2106/JBJS.E.00103. [DOI] [PubMed] [Google Scholar]
- 46.Brittberg M. Lindahl A. Nilsson A. Ohlsson C. Isaksson O. Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331:889. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 47.Mandelbaum B.R. Browne J.E. Fu F. Micheli L. Mosely J.B., Jr. Erggelet C. Minas T. Peterson L. Articular cartilage lesions of the knee. Am J Sports Med. 1998;26:853. doi: 10.1177/03635465980260062201. [DOI] [PubMed] [Google Scholar]
- 48.Gillogly S.D. Voight M. Blackburn T. Treatment of articular cartilage defects of the knee with autologous chondrocyte implantation. J Orthop Sports Phys Ther. 1998;28:241. doi: 10.2519/jospt.1998.28.4.241. [DOI] [PubMed] [Google Scholar]
- 49.Peterson L. Minas T. Brittberg M. Nilsson A. Sjogren-Jansson E. Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;212:374. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 50.Peterson L. Brittberg M. Kiviranta I. Akerlund E.L. Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am J Sports Med. 2002;30:2. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 51.Vasara A.I. Nieminen M.T. Jurvelin J.S. Peterson L. Lindahl A. Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005;233:433. doi: 10.1097/01.blo.0000150567.00022.2e. [DOI] [PubMed] [Google Scholar]
- 52.Grigolo B. Roseti L. de Franceschi L. Piacentini A. Cattini L. Manfredini M. Faccini R. Facchini A. Molecular and immunohistological characterization of human cartilage two years following autologous cell transplantation. J Bone Joint Surg Am. 2005;87:46. doi: 10.2106/JBJS.C.01685. [DOI] [PubMed] [Google Scholar]
- 53.Hunziker E.B. Articular cartilage repair: basic science and clinical progress. A review of the current status and prospects. Osteoarthritis Cartilage. 2002;10:432. doi: 10.1053/joca.2002.0801. [DOI] [PubMed] [Google Scholar]
- 54.Kurkijarvi J.E. Mattila L. Ojala R.O. Vasara A.I. Jurvelin J.S. Kiviranta I. Nieminen M.T. Evaluation of cartilage repair in the distal femur after autologous chondrocyte transplantation using T2 relaxation time and dGEMRIC. Osteoarthritis Cartilage. 2007;15:372. doi: 10.1016/j.joca.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Horas U. Pelinkovic D. Herr G. Aigner T. Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A:185. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 56.Saris D.B. Vanlauwe J. Victor J. Haspl M. Bohnsack M. Fortems Y. Vandekerckhove B. Almqvist K.F. Claes T. Handelberg F. Lagae K. van der Bauwhede J. Vandenneucker H. Yang K.G. Jelic M. Verdonk R. Veulemans N. Bellemans J. Luyten F.P. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am J Sports Med. 2008;36:235. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 57.Knutsen G. Drogset J.O. Engebretsen L. Grontvedt T. Isaksen V. Ludvigsen T.C. Roberts S. Solheim E. Strand T. Johansen O. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89:2105. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]
- 58.Genzyme. Cambridge, MA: Genzyme Corporation; 2003. Biosurgery 2002 Annual Report. [Google Scholar]
- 59.Reinold M.M. Wilk K.E. Macrina L.C. Dugas J.R. Cain E.L. Current concepts in the rehabilitation following articular cartilage repair procedures in the knee. J Orthop Sports Phys Ther. 2006;36:774. doi: 10.2519/jospt.2006.2228. [DOI] [PubMed] [Google Scholar]
- 60.Hambly K. Bobic V. Wondrasch B. van Assche D. Marlovits S. Autologous chondrocyte implantation postoperative care and rehabilitation: science and practice. Am J Sports Med. 2006;34:1020. doi: 10.1177/0363546505281918. [DOI] [PubMed] [Google Scholar]
- 61.Giannoni P. Pagano A. Maggi E. Arbico R. Randazzo N. Grandizio M. Cancedda R. Dozin B. Autologous chondrocyte implantation (ACI) for aged patients: development of the proper cell expansion conditions for possible therapeutic applications. Osteoarthritis Cartilage. 2005;13:589. doi: 10.1016/j.joca.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Mithofer K. Peterson L. Mandelbaum B.R. Minas T. Articular cartilage repair in soccer players with autologous chondrocyte transplantation: functional outcome and return to competition. Am J Sports Med. 2005;33:1639. doi: 10.1177/0363546505275647. [DOI] [PubMed] [Google Scholar]
- 63.Genzyme. Cambridge, MA: Genzyme Corporation; 2007. Carticel product label, revision K. [Google Scholar]
- 64.de Franceschi L. Grigolo B. Roseti L. Facchini A. Fini M. Giavaresi G. Tschon M. Giardino R. Transplantation of chondrocytes seeded on collagen-based scaffold in cartilage defects in rabbits. J Biomed Mater Res A. 2005;75:612. doi: 10.1002/jbm.a.30471. [DOI] [PubMed] [Google Scholar]
- 65.Ito Y. Adach N. Nakamae A. Yanada S. Ochi M. Transplantation of tissue-engineered osteochondral plug using cultured chondrocytes and interconnected porous calcium hydroxyapatite ceramic cylindrical plugs to treat osteochondral defects in a rabbit model. Artif Organs. 2008;32:36. doi: 10.1111/j.1525-1594.2007.00456.x. [DOI] [PubMed] [Google Scholar]
- 66.Bartlett W. Skinner J.A. Gooding C.R. Carrington R.W. Flanagan A.M. Briggs T.W. Bentley G. Autologous chondrocyte implantation versus matrix-induced autologous chondrocyte implantation for osteochondral defects of the knee: a prospective, randomised study. J Bone Joint Surg Br. 2005;87:640. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 67.Behrens P. Bitter T. Kurz B. Russlies M. Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)—5-year follow-up. Knee. 2006;13:194. doi: 10.1016/j.knee.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Jones C.W. Willers C. Keogh A. Smolinski D. Fick D. Yates P.J. Kirk T.B. Zheng M.H. Matrix-induced autologous chondrocyte implantation in sheep: objective assessments including confocal arthroscopy. J Orthop Res. 2008;26:292. doi: 10.1002/jor.20502. [DOI] [PubMed] [Google Scholar]
- 69.Dounchis J.S. Bae W.C. Chen A.C. Sah R.L. Coutts R.D. Amiel D. Cartilage repair with autogenic perichondrium cell and polylactic acid grafts. Clin Orthop Relat Res. 2000;248:377. doi: 10.1097/00003086-200008000-00033. [DOI] [PubMed] [Google Scholar]
- 70.Ossendorf C. Kaps C. Kreuz P.C. Burmester G.R. Sittinger M. Erggelet C. Treatment of posttraumatic and focal osteoarthritic cartilage defects of the knee with autologous polymer-based three-dimensional chondrocyte grafts: 2-year clinical results. Arthritis Res Ther. 2007;9:R41. doi: 10.1186/ar2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tognana E. Borrione A. de Luca C. Pavesio A. Hyalograft C: hyaluronan-based scaffolds in tissue-engineered cartilage. Cells Tissues Organs. 2007;186:97. doi: 10.1159/000102539. [DOI] [PubMed] [Google Scholar]
- 72.Gobbi A. Kon E. Berruto M. Francisco R. Filardo G. Marcacci M. Patellofemoral full-thickness chondral defects treated with Hyalograft-C: a clinical, arthroscopic, and histologic review. Am J Sports Med. 2006;34:1763. doi: 10.1177/0363546506288853. [DOI] [PubMed] [Google Scholar]
- 73.Marcacci M. Berruto M. Brocchetta D. Delcogliano A. Ghinelli D. Gobbi A. Kon E. Pederzini L. Rosa D. Sacchetti G.L. Stefani G. Zanasi S. Articular cartilage engineering with Hyalograft C: 3-year clinical results. Clin Orthop Relat Res. 2005;96:435. doi: 10.1097/01.blo.0000165737.87628.5b. [DOI] [PubMed] [Google Scholar]
- 74.Steadman J.R. Rodkey W.G. Singleton S.B. Briggs K.K. Microfracture technique for full-thickness chondral defects: technique and clinical results. Oper Tech Orthop. 1997;7:300. [Google Scholar]
- 75.Gill T.J. McCulloch P.C. Glasson S.S. Blanchet T. Morris E.A. Chondral defect repair after the microfracture procedure: a nonhuman primate model. Am J Sports Med. 2005;33:680. doi: 10.1177/0363546504271744. [DOI] [PubMed] [Google Scholar]
- 76.Steadman J.R. Miller B.S. Karas S.G. Schlegel T.F. Briggs K.K. Hawkins R.J. The microfracture technique in the treatment of full-thickness chondral lesions of the knee in National Football League players. J Knee Surg. 2003;16:83. [PubMed] [Google Scholar]
- 77.Knutsen G. Engebretsen L. Ludvigsen T.C. Drogset J.O. Grontvedt T. Solheim E. Strand T. Roberts S. Isaksen V. Johansen O. Autologous chondrocyte implantation compared with microfracture in the knee. A randomized trial. J Bone Joint Surg Am. 2004;86-A:455. doi: 10.2106/00004623-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 78.Steadman J.R. Rodkey W.G. Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin Orthop Relat Res. 2001;S362:391. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 79.Steadman J.R. Briggs K.K. Rodrigo J.J. Kocher M.S. Gill T.J. Rodkey W.G. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19:477. doi: 10.1053/jars.2003.50112. [DOI] [PubMed] [Google Scholar]
- 80.Robinson D. Nevo Z. Articular cartilage chondrocytes are more advantageous for generating hyaline-like cartilage than mesenchymal cells isolated from microfracture repairs. Cell Tissue Bank. 2001;2:23. doi: 10.1023/A:1011570628357. [DOI] [PubMed] [Google Scholar]
- 81.Nehrer S. Spector M. Minas T. Histologic analysis of tissue after failed cartilage repair procedures. Clin Orthop Relat Res. 1999;149:365. doi: 10.1097/00003086-199908000-00020. [DOI] [PubMed] [Google Scholar]
- 82.Yanai T. Ishii T. Chang F. Ochiai N. Repair of large full-thickness articular cartilage defects in the rabbit: the effects of joint distraction and autologous bone-marrow-derived mesenchymal cell transplantation. J Bone Joint Surg Br. 2005;87:721. doi: 10.1302/0301-620X.87B5.15542. [DOI] [PubMed] [Google Scholar]
- 83.Johnstone B. Yoo J.U. Autologous mesenchymal progenitor cells in articular cartilage repair. Clin Orthop Relat Res. 1999;S156:367. doi: 10.1097/00003086-199910001-00017. [DOI] [PubMed] [Google Scholar]
- 84.Oshima Y. Watanabe N. Matsuda K. Takai S. Kawata M. Kubo T. Fate of transplanted bone-marrow-derived mesenchymal cells during osteochondral repair using transgenic rats to simulate autologous transplantation. Osteoarthritis Cartilage. 2004;12:811. doi: 10.1016/j.joca.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 85.Zhou G. Liu W. Cui L. Wang X. Liu T. Cao Y. Repair of porcine articular osteochondral defects in non-weightbearing areas with autologous bone marrow stromal cells. Tissue Eng. 2006;12:3209. doi: 10.1089/ten.2006.12.3209. [DOI] [PubMed] [Google Scholar]
- 86.Wakitani S. Yamamoto T. Response of the donor and recipient cells in mesenchymal cell transplantation to cartilage defect. Microsc Res Tech. 2002;58:14. doi: 10.1002/jemt.10111. [DOI] [PubMed] [Google Scholar]
- 87.Wakitani S. Goto T. Pineda S.J. Young R.G. Mansour J.M. Caplan A.I. Goldberg V.M. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 88.Wakitani S. Nawata M. Tensho K. Okabe T. Machida H. Ohgushi H. Repair of articular cartilage defects in the patello-femoral joint with autologous bone marrow mesenchymal cell transplantation: three case reports involving nine defects in five knees. J Tissue Eng Regen Med. 2007;1:74. doi: 10.1002/term.8. [DOI] [PubMed] [Google Scholar]
- 89.Wakitani S. Imoto K. Yamamoto T. Saito M. Murata N. Yoneda M. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage. 2002;10:199. doi: 10.1053/joca.2001.0504. [DOI] [PubMed] [Google Scholar]
- 90.Ryan J.M. Barry F. Murphy J.M. Mahon B.P. Interferon-gamma does not break, but promotes the immunosuppressive capacity of adult human mesenchymal stem cells. Clin Exp Immunol. 2007;149:353. doi: 10.1111/j.1365-2249.2007.03422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zappia E. Casazza S. Pedemonte E. Benvenuto F. Bonanni I. Gerdoni E. Giunti D. Ceravolo A. Cazzanti F. Frassoni F. Mancardi G. Uccelli A. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 92.Pelttari K. Steck E. Richter W. The use of mesenchymal stem cells for chondrogenesis. Injury. 2008;39 Suppl 1:S58. doi: 10.1016/j.injury.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 93.Steck E. Bertram H. Abel R. Chen B. Winter A. Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 94.Nelea V. Luo L. Demers C.N. Antoniou J. Petit A. Lerouge S. Wertheimer M.R. Mwale F. Selective inhibition of type X collagen expression in human mesenchymal stem cell differentiation on polymer substrates surface-modified by glow discharge plasma. J Biomed Mater Res A. 2005;75:216. doi: 10.1002/jbm.a.30402. [DOI] [PubMed] [Google Scholar]
- 95.Gortz S. Bugbee W.D. Allografts in articular cartilage repair. J Bone Joint Surg Am. 2006;88:1374. doi: 10.2106/00004623-200606000-00030. [DOI] [PubMed] [Google Scholar]
- 96.Chu C.R. Convery F.R. Akeson W.H. Meyers M. Amiel D. Articular cartilage transplantation. Clinical results in the knee. Clin Orthop Relat Res. 1999;159:360. [PubMed] [Google Scholar]
- 97.Zukor D.J. Oakeshott R.D. Gross A.E. Osteochondral allograft reconstruction of the knee. Part 2: experience with successful and failed fresh osteochondral allografts. Am J Knee Surg. 1989;2:182. [Google Scholar]
- 98.Gross A.E. Shasha N. Aubin P. Long-term followup of the use of fresh osteochondral allografts for posttraumatic knee defects. Clin Orthop Relat Res. 2005;79:435. doi: 10.1097/01.blo.0000165845.21735.05. [DOI] [PubMed] [Google Scholar]
- 99.Beaver R.J. Mahomed M. Backstein D. Davis A. Zukor D.J. Gross A.E. Fresh osteochondral allografts for post-traumatic defects in the knee. A survivorship analysis. J Bone Joint Surg Br. 1992;74:105. doi: 10.1302/0301-620X.74B1.1732235. [DOI] [PubMed] [Google Scholar]
- 100.Convery F.R. Meyers M.H. Akeson W.H. Fresh osteochondral allografting of the femoral condyle. Clin Orthop Relat Res. 1991;139:273. [PubMed] [Google Scholar]
- 101.Jamali A.A. Emmerson B.C. Chung C. Convery F.R. Bugbee W.D. Fresh osteochondral allografts. Clin Orthop Relat Res. 2005;176:437. [PubMed] [Google Scholar]
- 102.Langer F. Gross A.E. Immunogenicity of allograft articular cartilage. J Bone Joint Surg Am. 1974;56:297. [PubMed] [Google Scholar]
- 103.Glenn R.E., Jr. McCarty E.C. Potter H.G. Juliao S.F. Gordon J.D. Spindler K.P. Comparison of fresh osteochondral autografts and allografts: a canine model. Am J Sports Med. 2006;34:1084. doi: 10.1177/0363546505284846. [DOI] [PubMed] [Google Scholar]
- 104.Stevenson S. Dannucci G.A. Sharkey N.A. Pool R.R. The fate of articular cartilage after transplantation of fresh and cryopreserved tissue-antigen-matched and mismatched osteochondral allografts in dogs. J Bone Joint Surg Am. 1989;71:1297. [PubMed] [Google Scholar]
- 105.Davidson P.A. Rivenburgh D.W. Dawson P.E. Rozin R. Clinical, histologic, and radiographic outcomes of distal femoral resurfacing with hypothermically stored osteoarticular allografts. Am J Sports Med. 2007;35:1082. doi: 10.1177/0363546507299529. [DOI] [PubMed] [Google Scholar]
- 106.Friedlaender G.E. Strong D.M. Tomford W.W. Mankin H.J. Long-term follow-up of patients with osteochondral allografts. A correlation between immunologic responses and clinical outcome. Orthop Clin North Am. 1999;30:583. doi: 10.1016/s0030-5898(05)70111-5. [DOI] [PubMed] [Google Scholar]
- 107.Bakay A. Csonge L. Papp G. Fekete L. Osteochondral resurfacing of the knee joint with allograft. Clinical analysis of 33 cases. Int Orthop. 1998;22:277. doi: 10.1007/s002640050260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Flynn J.M. Springfield D.S. Mankin H.J. Osteoarticular allografts to treat distal femoral osteonecrosis. Clin Orthop Relat Res. 1994;38:303. [PubMed] [Google Scholar]
- 109.Elves M.W. Humoral immune response to allografts of bone. Int Arch Allergy Appl Immunol. 1974;47:708. doi: 10.1159/000231262. [DOI] [PubMed] [Google Scholar]
- 110.Friedlaender G.E. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin Orthop Relat Res. 1983;58:164. [PubMed] [Google Scholar]
- 111.Enneking W.F. Campanacci D.A. Retrieved human allografts: a clinicopathological study. J Bone Joint Surg Am. 2001;83-A:971. [PubMed] [Google Scholar]
- 112.Kaminski M.J. Kaminska G. Moskalewski S. Species differences in the ability of isolated epiphyseal chondrocytes to hypertrophy after transplantation into the wall of the Syrian hamster cheek pouch. Folia Biol (Krakow) 1980;28:27. [PubMed] [Google Scholar]
- 113.Ksiazek T. Moskalewski S. Studies on bone formation by cartilage reconstructed by isolated epiphyseal chondrocytes, transplanted syngeneically or across known histocompatibility barriers in mice. Clin Orthop Relat Res. 1983;233:177. [PubMed] [Google Scholar]
- 114.Malejczyk J. Osiecka A. Hyc A. Moskalewski S. Effect of immunosuppression on rejection of cartilage formed by transplanted allogeneic rib chondrocytes in mice. Clin Orthop Relat Res. 1991;266:269. [PubMed] [Google Scholar]
- 115.Malejczyk J. Moskalewski S. Effect of immunosuppression on survival and growth of cartilage produced by transplanted allogeneic epiphyseal chondrocytes. Clin Orthop Relat Res. 1988;292:232. [PubMed] [Google Scholar]
- 116.Green W.T., Jr. Articular cartilage repair. Behavior of rabbit chondrocytes during tissue culture and subsequent allografting. Clin Orthop Relat Res. 1977;237:124. [PubMed] [Google Scholar]
- 117.Masuoka K. Asazuma T. Ishihara M. Sato M. Hattori H. Ishihara M. Yoshihara Y. Matsui T. Takase B. Kikuchi M. Nemoto K. Tissue engineering of articular cartilage using an allograft of cultured chondrocytes in a membrane-sealed atelocollagen honeycomb-shaped scaffold (ACHMS scaffold) J Biomed Mater Res B Appl Biomater. 2005;75:177. doi: 10.1002/jbm.b.30284. [DOI] [PubMed] [Google Scholar]
- 118.Noguchi T. Oka M. Fujino M. Neo M. Yamamuro T. Repair of osteochondral defects with grafts of cultured chondrocytes. Comparison of allografts and isografts. Clin Orthop Relat Res. 1994;251:302. [PubMed] [Google Scholar]
- 119.Rahfoth B. Weisser J. Sternkopf F. Aigner T. von der Mark K. Brauer R. Transplantation of allograft chondrocytes embedded in agarose gel into cartilage defects of rabbits. Osteoarthritis Cartilage. 1998;6:50. doi: 10.1053/joca.1997.0092. [DOI] [PubMed] [Google Scholar]
- 120.Tanaka T. Komaki H. Chazono M. Fujii K. Use of a biphasic graft constructed with chondrocytes overlying a beta-tricalcium phosphate block in the treatment of rabbit osteochondral defects. Tissue Eng. 2005;11:331. doi: 10.1089/ten.2005.11.331. [DOI] [PubMed] [Google Scholar]
- 121.Wakitani S. Kimura T. Hirooka A. Ochi T. Yoneda M. Yasui N. Owaki H. Ono K. Repair of rabbit articular surfaces with allograft chondrocytes embedded in collagen gel. J Bone Joint Surg Br. 1989;71:74. doi: 10.1302/0301-620X.71B1.2915011. [DOI] [PubMed] [Google Scholar]
- 122.Wakitani S. Goto T. Young R.G. Mansour J.M. Goldberg V.M. Caplan A.I. Repair of large full-thickness articular cartilage defects with allograft articular chondrocytes embedded in a collagen gel. Tissue Eng. 1998;4:429. doi: 10.1089/ten.1998.4.429. [DOI] [PubMed] [Google Scholar]
- 123.Freed L.E. Grande D.A. Lingbin Z. Emmanual J. Marquis J.C. Langer R. Joint resurfacing using allograft chondrocytes and synthetic biodegradable polymer scaffolds. J Biomed Mater Res. 1994;28:891. doi: 10.1002/jbm.820280808. [DOI] [PubMed] [Google Scholar]
- 124.Schreiber R.E. Ilten-Kirby B.M. Dunkelman N.S. Symons K.T. Rekettye L.M. Willoughby J. Ratcliffe A. Repair of osteochondral defects with allogeneic tissue engineered cartilage implants. Clin Orthop Relat Res. 1999;S382:367. doi: 10.1097/00003086-199910001-00037. [DOI] [PubMed] [Google Scholar]
- 125.Perka C. Sittinger M. Schultz O. Spitzer R.S. Schlenzka D. Burmester G.R. Tissue engineered cartilage repair using cryopreserved and noncryopreserved chondrocytes. Clin Orthop Relat Res. 2000;245:378. doi: 10.1097/00003086-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 126.Shangkai C. Naohide T. Koji Y. Yasuji H. Masaaki N. Tomohiro T. Yasushi T. Transplantation of allogeneic chondrocytes cultured in fibroin sponge and stirring chamber to promote cartilage regeneration. Tissue Eng. 2007;13:483. doi: 10.1089/ten.2006.0181. [DOI] [PubMed] [Google Scholar]
- 127.Kawabe N. Yoshinao M. The repair of full-thickness articular cartilage defects. Immune responses to reparative tissue formed by allogeneic growth plate chondrocyte implants. Clin Orthop Relat Res. 1991;279:268. [PubMed] [Google Scholar]
- 128.Itay S. Abramovici A. Nevo Z. Use of cultured embryonal chick epiphyseal chondrocytes as grafts for defects in chick articular cartilage. Clin Orthop Relat Res. 1987;284:220. [PubMed] [Google Scholar]
- 129.Sams A.E. Nixon A.J. Chondrocyte-laden collagen scaffolds for resurfacing extensive articular cartilage defects. Osteoarthritis Cartilage. 1995;3:47. doi: 10.1016/s1063-4584(05)80037-8. [DOI] [PubMed] [Google Scholar]
- 130.Sams A.E. Minor R.R. Wootton J.A. Mohammed H. Nixon A.J. Local and remote matrix responses to chondrocyte-laden collagen scaffold implantation in extensive articular cartilage defects. Osteoarthritis Cartilage. 1995;3:61. doi: 10.1016/s1063-4584(05)80038-x. [DOI] [PubMed] [Google Scholar]
- 131.Butnariu-Ephrat M. Robinson D. Mendes D.G. Halperin N. Nevo Z. Resurfacing of goat articular cartilage by chondrocytes derived from bone marrow. Clin Orthop Relat Res. 1996;234:330. doi: 10.1097/00003086-199609000-00031. [DOI] [PubMed] [Google Scholar]
- 132.Osiecka-Iwan A. Hyc A. Jozwiak J. Komar A. Niderla J. Moskalewski S. Transplants of rat chondrocytes evoke strong humoral response against chondrocyte-associated antigen in rabbits. Cell Transplant. 2003;12:389. doi: 10.3727/000000003108746939. [DOI] [PubMed] [Google Scholar]
- 133.van Susante J.L. Buma P. Schuman L. Homminga G.N. van den Berg W.B. Veth R.P. Resurfacing potential of heterologous chondrocytes suspended in fibrin glue in large full-thickness defects of femoral articular cartilage: an experimental study in the goat. Biomaterials. 1999;20:1167. doi: 10.1016/s0142-9612(97)00190-7. [DOI] [PubMed] [Google Scholar]
- 134.Akens M.K. von Rechenberg B. Bittmann P. Nadler D. Zlinszky K. Auer J.A. Long term in-vivo studies of a photo-oxidized bovine osteochondral transplant in sheep. BMC Musculoskelet Disord. 2001;2:9. doi: 10.1186/1471-2474-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Akens M.K. von Rechenberg B. Bittmann P. Nadler D. Zlinszky K. Auer J.A. In vitro studies of a photo-oxidized bovine articular cartilage. J Vet Med A Physiol Pathol Clin Med. 2002;49:39. doi: 10.1046/j.1439-0442.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 136.Feczko P. Hangody L. Varga J. Bartha L. Dioszegi Z. Bodo G. Kendik Z. Modis L. Experimental results of donor site filling for autologous osteochondral mosaicplasty. Arthroscopy. 2003;19:755. doi: 10.1016/s0749-8063(03)00402-x. [DOI] [PubMed] [Google Scholar]
- 137.Chesterman P.J. Smith A.U. Homotransplantation of articular cartilage and isolated chondrocytes. An experimental study in rabbits. J Bone Joint Surg Br. 1968;50:184. [PubMed] [Google Scholar]
- 138.Jackson D. Halbrecht J. Proctor C. van Sickle D. Simon T. Assessment of donor cell and matrix survival in fresh articular cartilage allografts in a goat model. J Orthop Res. 1996;14:255. doi: 10.1002/jor.1100140214. [DOI] [PubMed] [Google Scholar]
- 139.Lane J. Tontz W.J. Ball S. Massie J. Chen A. Bae W. Amiel M. Sah R. Amiel D. A morphologic, biochemical, and biomechanical assessment of short-term effects of osteochondral autograft plug transfer in an animal model. Arthroscopy. 2001;17:856. doi: 10.1016/s0749-8063(01)90010-6. [DOI] [PubMed] [Google Scholar]
- 140.Garret J.C. Fresh osteochondral allografts for treatment of articular cartilage defects in osteochondritis dissecans of the lateral femoral condyle in adults. Clin Orthop Rel Res. 1994;33:303. [PubMed] [Google Scholar]
- 141.Ghazavi M.T. Pritzker K.P. Davis A.M. Gross A.E. Fresh osteochondral allografts for post-traumatic osteochondral defects of the knee. J Bone Joint Surg Br. 1997;79:1008. doi: 10.1302/0301-620x.79b6.7534. [DOI] [PubMed] [Google Scholar]
- 142.Emmerson B.C. Gortz S. Jamali A.A. Chung C. Amiel D. Bugbee W.D. Fresh osteochondral allografting in the treatment of osteochondritis dissecans of the femoral condyle. Am J Sports Med. 2007;35:907. doi: 10.1177/0363546507299932. [DOI] [PubMed] [Google Scholar]