Abstract
Toll like receptors (TLRs) activate signals that are critically involved in innate immune responses and that contribute to the initiation of adaptive immune responses. Resveratrol (trans-3, 5,4-trihydroxystilbene), a polyphenol found in red grapes and in several other plant sources, is an effective chemopreventive agent in cutaneous chemical carcinogenesis. In this study, we investigated whether TLR4 was required for the chemopreventive action of resveratrol in DMBA skin carcinogenesis. For this purpose, mice with normal and deficient TLR4 function were compared when pretreated with resveratrol and then subjected to a DMBA-induced skin carcinogenesis protocol. There were fewer tumors/group (p<0.001) in resveratrol treated TLR4 competent C3H/HeN mice than in TLR4 deficient C3H/HeJ mice. In addition, the size of tumors in C3H/HeN mice was reduced in vivo and their survival in vitro was inhibited by resveratrol to a significantly greater extent than in C3H/HeJ mice. Resveratrol inhibited angiogenesis to a much greater extent in the TLR4 competent mice than in TLR4 deficient mice. IFN-γ and IL-12 levels were also increased in TLR4 competent mice compared to TLR4 deficient mice, and TLR4 competent C3H/HeN mice exhibited a greater increase in the cell-mediated immune response to DMBA. The results of this study indicate that TLR4 is an important mediator of resveratrol chemoprevention in DMBA skin tumorigenesis.
Keywords: Toll-like receptor 4 (TLR4); Resveratrol; Skin Carcinogenesis; 7,12-dimethylbenz(a)anthracene (DMBA); Chemoprevention
Introduction
Toll-like receptors (TLRs) contribute to host defenses by recognizing invading pathogens and activating potentially beneficial antimicrobial inflammatory and adaptive immune responses (1). One of these receptors, TLR4, is important because it recognizes lipopolysaccharide present in the cell wall of gram negative bacteria. Recent studies have shown that a variety of nonbacterial agonists such as taxol (2), fibronectin (3, 4), and heat shock protein 60 (5) can also initiate TLR4 signaling, suggesting that it is important for non-infectious diseases as well. In this regard, TLR4 has been shown to play an important role in cancer. In a two-stage chemical carcinogenesis mouse model, the presence of a functional TLR4 inhibited lung carcinogenesis (6). In addition, we have shown that TLR4 activation has a protective effect in DMBA cutaneous carcinogenesis (7).
TLRs trigger the secretion of critical cytokines such as IL-1, IL-6 and IL-12 by dendritic cells, and thus provide a link between innate immunity and adaptive immune responses (1). TLRs can also directly stimulate the proliferation of CD4+ and CD8+ T-cells and can reverse the suppressive function of regulatory T-cells. In the skin, TLR 1, 2, 3, 4, 5 and 9 have been identified on keratinocytes (8) and TLR 1, 2, 4, 5, 6, 8 and 10 have been found on epidermal Langerhans cells (9).
Resveratrol (trans-3, 5, 4-trihydroxystilbene), a phytoalexin found in red grapes and in several other plant sources, has a number of health benefits in vitro and in various animal models (10). It has anti-microbial and anti-inflammatory effects, and it promotes cardiovascular health, protects against neurodegenerative diseases, prolongs life and prevents cancer in several different organ systems. Resveratrol has been observed to enhance immune responses in mice by promoting production of Th1 cytokines such as IL-2 and IFN-γ, and by enhancing lymphocyte proliferation and IL-12 production (11). It also effectively suppresses the function of regulatory T-cells and inhibits TGF-β synthesis (12). In the skin, resveratrol inhibits the induction of cutaneous tumors in mice subjected to both polyaromatic hydrocarbon (PAH) and UV radiation cutaneous tumorigenesis (13, 14).
Since resveratrol and TLR4 are both effective at preventing DMBA skin carcinogenesis, we investigated whether the two might be linked. For this purpose, the chemopreventive activity of resveratrol in DMBA-induced skin carcinogenesis was compared in mice with normal and deficient TLR4 function. TLR4 is normal in C3H/HeN mice; C3H/HeJ mice, on the other hand, have a mutation in the TLR4 gene that results in a TLR4 signaling pathway that functions poorly. Our results indicate that resveratrol is not an effective agent for prevention of DMBA-induced skin tumorigenesis in mice that have deficient TLR4 function and therefore that the TLR4 signaling pathway is necessary for resveratrol to mediate its protective effects against the induction of chemically-induced skin tumors.
MATERIALS & METHODS
Animals and Reagents
Female C3H/HeN mice were purchased from Charles River Laboratories (Boston, MA). Female C3H/HeJ mice were obtained from the Jackson Laboratory (Bar Harbor, Maine). All mice used for experiments were 6–8 weeks of age. All animal procedures were performed according to NIH guidelines under protocols approved by the Institute Animal Care and Use Committee of the University of Alabama at Birmingham. Resveratrol, MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and dimethylbenz(a)anthracene (DMBA) were purchased from Sigma Chemical Co. (St. Louis, MO). Streptavidin PE-Cy5, anti-CD31 (platelet/endothelial cell adhesion molecule), anti-IL-12 and anti-IFN-γ were purchased from Pharmingen (San Diego, CA).
Skin tumorigenesis
A standard DMBA complete carcinogenesis protocol was employed for this study (7). DMBA (400 nmol, w/v in acetone) was painted weekly on the shaved and depilated dorsal skin of C3H/HeN and C3H/HeJ mice (15 mice/panel). For chemoprevention studies, resveratrol was applied topically to the skin (10µmol/mouse) one-hour prior to DMBA treatment. Mice were evaluated weekly for tumors. Only tumors that had attained a size of one mm or greater and were present for two weeks or longer were counted.
Tumor cell proliferation assays
The proliferative potential of tumor cells obtained from C3H/HeJ and C3H/HeN mice was assessed using the MTT assay as described previously (15). Tumor cells were cultured in vitro (15). Briefly, <2 mm3 sized tumor fragments were seeded into culture plates and cultured in RPMI 1640 supplemented with 10% fetal bovine serum, 1% L-glutamine, 1% MEM nonessential amino acids, and 1% antibiotic/penicillin-streptomycin solution. When the cultures had reached ~80% confluence, the adherent cells were detached with 0.1% trypsin/0.05% EDTA (Life Technologies) and used for subsequent passage. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2. Cells were harvested and seeded in triplicate into 96-well plates at a density of 2 × 104 per well. To determine the effect of resveratrol on cell proliferation, cell lines were treated with resveratrol (0–100µM) for 1, 2, and 3 days and then subjected to an MTT proliferation assay as described earlier (15). The MTT solution was added to each well followed by incubation for 2 hours at 37°C. After incubation, DMSO was added to each well. Spectrophotometric absorbance of each sample was measured at 540 nm using a microplate reader (Bio-Rad, Hercules, CA).
Preparation of tissue lysates
Back skin samples were scraped of subcutaneous fat and washed with PBS. Both skin and tumors collected from at least 3 mice were homogenized in ice-cold lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 20 mM NaF, 100 mM Na3VO4, 0.5% NP-40, 1% Triton X-100, 1 mM PMSF, pH 7.4) with freshly added protease inhibitor cocktail (Protease Inhibitor Cocktail Sigma). The homogenate was then centrifuged at 14,000g for 25 min at 4°C and the supernatant (total cell lysate) was collected, aliquoted and stored at −80°C (7). The protein content in the lysates was measured by DC Bio-Rad assay as per the manufacturer's protocol.
ELISA
Cytokines IFN-γ and IL-12 in skin lysates were measured by ELISA as described earlier (7) using antibodies from BD pharmingen. VEGF was measured in tumor lysates by enzyme linked immunosorbent assay (ELISA) using commercial kit from Invitrogen (Carlsbad, CA) according to manufacturer’s instructions.
Sensitization and elicitation of contact hypersensitivity (CHS) with dimethylbenz(a)anthracene (DMBA)
C3H/HeN and C3H/HeJ mice were sensitized with 100µl of 0.1% DMBA (w/v) in acetone on their shaved and depilated abdomen on day 0 (16). Resveratrol was applied topically on the skin (10 µmol/mouse) 3h and 24h before sensitization. DMBA sensitized mice which were not treated with resveratrol served as positive controls. The mice were challenged on the ear after five days with 20µl of 0.1% DMBA (w/v) in acetone. CHS was measured immediately prior to challenge and every 24 hours thereafter for 5 days.
RNA extraction and semi-quantitative RT-PCRs
The total RNA was extracted from the mouse tumor samples using Trizol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, tumor samples were homogenized with Trizol reagent using a Teflon homogenizer (Fisher Scientific, Pittsburgh, PA). The homogenate was centrifuged at 12,000 g for 10 min at 4°C. The supernatant was then collected in a fresh tube and mixed with chloroform (0.2 ml/ml trizol). The aqueous phase was separated by centrifugation at 12,000 g. RNA was precipitated by adding 0.5 ml of isopropanol/ml trizol. The sample was shaken, incubated for 10 min at room temperature, and centrifuged again as described previously. The precipitate was washed with 75% ethanol in DEPC–treated water, centrifuged and dissolved in DEPC-treated water and stored at −80°C. The concentration of total RNA was determined by measuring the absorbance at 260 nm using a Biorad Smart Spec spectrophotometer.
mRNA expression of MMPs in tumor samples was determined using RT-PCR. For mRNA quantification, cDNA was synthesized using 1 µg RNA through a reverse transcription reaction (iScript cDNA Synthesis kit, Bio-Rad, Hercules, CA). As controls, RNA samples without reverse transcription were subjected to PCR to exclude DNA contamination. The primers used were as follows: MMP-2 Sense 5’–GAGATCTGCAAACAGGACA-3’; Anti-sense 5’-TTACCTGAAGCTGGAGAACC-3’; MMP-9 Sense 5’-CGACGAGTTGTGGTCGCTGG-3’; Anti-sense 5’-GCACTGAAGAATGATCTAAG-3’; GAPDH Sense 5’-AATGGTGAAGGTCGGTGTGAAC -3’; Anti-sense 5’-GAAGATGGTGATGGGCTTCC -3’. PCR amplification was performed for 35 cycles of 1 min denaturation at 94 °C, 1 min annealing at 60°C and 2 min extension at 72°C (17). PCR products were separated by agarose electrophoresis and results were recorded using a gel documentation system (Bio-Rad, Hercules, CA). The density of bands was measured using Quantity One software (Bio-Rad). The relative expression level of MMPs for each sample was calculated as the density of metallo protease/density of GAPDH. The values shown in the figure are arbitrary. In each case, the values of control group (DMBA treated group of C3H HeJ ) was taken as "1" and comparison was then made with densitometry values obtained with other groups.
Quantitative Real time PCR
The mRNA expression of VEGF, MMP-2 and MMP-9 from tumor samples was also determined using real-time PCR. For the mRNA quantification, cDNA was synthesized as described above. Using iQ™ SYBR Green Master Mix (Biorad), cDNA was amplified using real-time PCR with a Bio-Rad MyiQ thermocycler and SYBR Green detection system (Bio-Rad). Samples were run in triplicate to ensure amplification integrity. The primers used were described else where (18,19). The standard PCR conditions were 95°C for 10 min and then 40 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s. The expression levels of genes were normalized to the expression level of the GAPDH mRNA in each sample. The 2−ΔΔCT method was used to compare gene expression levels between samples.
Assessment of expression of vascular endothelial cell antigen, CD31
Immunostaining for CD31 was done on frozen tumor sections from C3H/HeJ and C3H/HeN mice as described earlier (7). Briefly, frozen sections (5 µm thick) were fixed in cold acetone and nonspecific binding sites were blocked by immersing the sections in Tris-HCl buffer containing 5% goat serum and bovine serum albumin (0.5% w/v). The sections were then incubated with monoclonal antibodies specific for CD31 for 1 h. Antibody binding was detected by subsequent incubation of sections with streptavidin-phycoerythrin-Cy5 secondary antibody for 1 h. After washing, the sections were counterstained with Hoechst 33342 which stains nuclei. The intensity of the staining was evaluated using a microscope equipped for immunofluorescence analysis. Images were captured using identical exposure and brightness/contrast settings. Digitized images were analyzed using Image Pro software and relative fluorescence intensity was measured with relative intensity of control skin taken as 1. Images from at least five different samples were viewed and analysed using a 20x objective.
Statistical Analysis
Statistical analysis of tumor data was done at the termination of the experiment. The differences between experimental groups for CHS and cytokine ELISAs were analyzed using the Student’s t-test. The ANOVA test was employed to test for statistically significant differences between groups for analysis of the tumors per group and the percentage of mice with tumors followed by pair-wise tests using Scheffe’s adjustment for multiple tests. In all cases, a p<0.05 was considered significant.
Results
Resveratrol prevents skin tumors in mice with normal TLR4 function but not in TLR 4 deficient mice
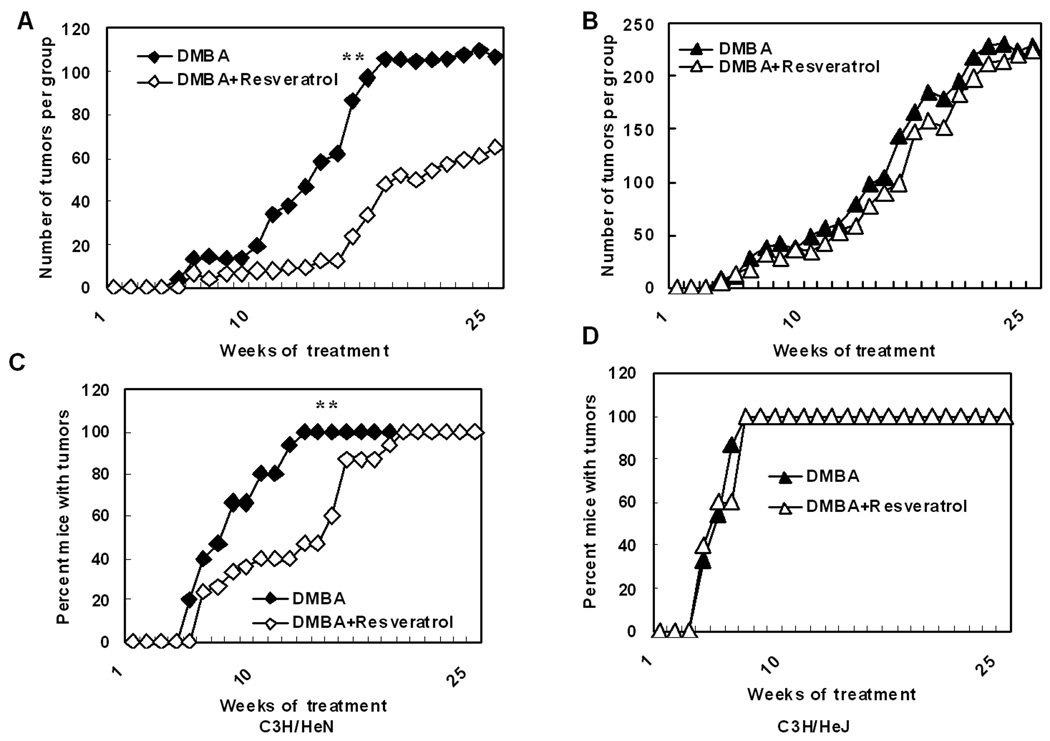
A series of experiments was conducted in which separate panels of C3H/HeN mice were treated with either resveratrol topically or vehicle prior to each DMBA treatment. In C3H/HeN mice, treatment with resveratrol resulted in significantly fewer tumors (p<0.001) at the termination of the experiment at 25 weeks than in mice treated with the vehicle (Fig. 1A). Only after 20 weeks did 100% of resveratrol treated mice develop tumors whereas it took only thirteen weeks for 100% of control mice to develop them (Fig. 1C). However, when C3H/HeJ mice were pretreated with resveratrol, the compound was ineffective at reducing the total number of tumors (Fig. 1B) or the percentage of mice with tumors (Fig. 1D) compared to vehicle pretreated controls. Previously, we have shown that TLR4 deficient C3H/HeJ mice when subjected to a DMBA skin tumorigenesis protocol develop more tumors than C3H/HeN mice with functional TLR4 (7) and that was confirmed in this experiment (Fig. 1).
Figure 1.
Resveratrol suppresses DMBA induced tumor formation in a TLR4 dependent manner. TLR4 deficient C3H/HeJ mice and TLR normal C3H/HeN mice were subjected to a DMBA complete cutaneous carcinogenesis protocol (15 mice/panel) as described in the Methods section. The number of tumors per group was plotted as a function of the number of weeks on the test. There were significantly fewer tumors in C3H/HeN mice (**p<0.001) after treatment with resveratrol but there was no inhibition of tumor formation in C3H/HeJ mice (A&B).The percentage of mice with tumors was plotted as a function of the number of weeks on the test. There were significantly lower percentage of C3H/HeN mice (**p<0.001) with tumors after treatment with resveratrol compared to C3H/HeJ mice (C&D).
Resveratrol was also effective at controlling the growth of tumors in C3H/HeN mice (p<0.05), but was less so in C3H/HeJ mice (Figure 2A and 2B). C3H/HeN mice that were treated with resveratrol had a 78% reduction in the volume per tumor compared to mice that were treated with vehicle. In contrast, resveratrol-induced tumor volume was inhibited by only 53% in C3H/HeJ mice. The difference in resveratrol-induced suppression of tumor volume was statistically significant (p<0.05) in C3H/HeN mice.
Figure 2.
Resveratrol reduces the growth of DMBA-induced tumors in a TLR4 dependent fashion. (A) The tumor volume was measured at various times after the initiation of treatment in C3H/HeN mice, and was significantly less in the resveratrol group than in the vehicle treated panel (*p<0.05). (B) The tumor volume was measured at various times after the initiation of treatment in C3H/HeJ mice, and was not significantly different in the resveratrol group than in the vehicle treated panel. (C, D and E) Tumor cells were obtained from DMBA-induced tumors from C3H/HeN or C3H/HeJ mice and were subjected to an MTT assay after 1, 2 or 3 days of resveratrol treatment as described in Methods section. The proliferative potential of tumor cells is expressed in terms of absorbance of each sample. The effect of resveratrol on the proliferative capacity was higher in tumor cells obtained from C3H/HeN mice when compared with tumor cells derived from C3H/HeJ mice. Results are the mean ± SD of triplicate cultures and each experiment was repeated three times. The Graph is representative of one of them.
TLR-4 proficient cancer cell line is more sensitive to resveratrol than TLR-4 deficient tumor cell line
To further study the effect of resveratrol on the growth behavior of the DMBA-induced tumors, tumor specimens were obtained from both C3H/HeJ and C3H/HeN mice. Tumor fragments <2 mm3 were seeded onto culture plates and cultured as described in the Methods section. To determine their capacity to proliferate, cell lines were treated with resveratrol for 1, 2, and 3 days and subjected to an MTT proliferation assay (Fig. 2C, D and E). In all samples tested, resveratrol inhibited the proliferative capacity of tumors in cell lines obtained from DMBA treated C3H/HeN mice to a greater extent than that of cell lines from C3H/HeJ mice (p<0.05).
Resveratrol inhibits angiogenesis in DMBA carcinogenesis through TLR4 signaling
Because angiogenesis has been associated with the growth and invasiveness of DMBA-induced tumors (17), we were interested in determining whether resveratrol had an effect on the tumor vasculature and on factors that influence angiogenesis, and, if so, whether TLR4 was required. For this purpose, we selected the endothelial cell marker CD31 (17) and the angiogenesis factors vascular endothelial growth factor (VEGF), matrix metalloproteinase-2 (MMP-2) and matrix metalloproteinase-9 (MMP-9) (20). Resveratrol treatment decreased CD31 staining in C3H/HeN mice, but did not do so in C3H/HeJ mice (Fig. 3).
Figure 3.
Resveratrol reduces CD31 expression in tumors from DMBA treated mice. Samples of tumors and normal skin from C3H HeN and C3H HeJ mice were examined for CD31 fluorescence staining. DMBA-induced tumors from C3H/HeJ mice had higher levels of CD31 positive staining than tumors induced in C3H HeN mice. Upon resveratrol treatment the CD31 expression in C3H HeN mice was greatly reduced while in C3H HeJ mice there was no effect of resveratrol on CD31 expression. (A) Representative photomicrographs are shown from experiments conducted in tumor/skin samples from at least five mice in each group. Identical patterns were observed in all of the tumors examined. CD31-positive staining is indicated by bright pink fluorescence (Bar = 100 µm). (B) Graph showing CD31 intensity from all the groups from five different samples. *P<0.05.
We also observed that following treatment with resveratrol, VEGF (p<0.05) levels in tumor cell lysates were significantly decreased in C3H/HeN mice. Resveratrol did not inhibit VEGF expression in C3H/HeJ mice as analyzed by ELISA and real time PCR (Fig. 4A and B). When mRNA expression of MMP-2, and MMP-9 was analyzed in tumor samples of these mice using RT-PCR and Real time PCR, resveratrol treatment significantly decreased MMP-2 and -9 expression in C3H/HeN mice but had no significant effect in C3H/HeJ mice (p<0.05) (Fig. 4C and 4D).
Figure 4.
Resveratrol inhibition of angiogenesis in DMBA treated mice is dependent on TLR4. (A)VEGF was measured in tumor lysates from mice placed on a DMBA cutaneous carcinogenesis protocol for 25 weeks after resveratrol treatment. TLR4 deficient C3H/HeJ mice showed significantly higher levels of VEGF in their tumor lysates as compared to TLR4 normal C3H/HeN mice as evaluated by ELISA. Upon resveratrol treatment there was significant decrease in VEGF in C3H HeN mice than C3H HeJ mice indicating TLR4 is important in resveratrol mediated decrease of angiogenic factors like VEGF. The results are expressed as ng of VEGF per milligram protein. (B) C3H HeJ mice express higher levels of mRNA of VEGF in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the VEGF levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of VEGF was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH. (C) Resveratrol also effectively inhibited MMP-2 and -9 mRNA expressions in DMBA induced tumors in C3H/HeN mice but did not do so in C3H/HeJ mice. Densitometry of MMP-2 and -9 expressions in tumors can be seen as the values under each band. The values shown are arbitrary. In each case, the values for control group (DMBA treated C3H HeJ group) was taken as "1" and comparison was then made with densitometry values obtained with other groups. Each experiment was repeated three times. (*p<0.05). The figure is representative of one of them. (D) C3H HeJ mice express higher levels of mRNA of MMP-2 and MMP-9 in DMBA treated tumors compared to C3HHeN mice as evaluated by Real Time PCR. Upon resveratrol treatment there was no change in mRNA levels of C3H HeJ mice while the MMP levels in C3H HeN mice had greatly reduced. RNA was isolated and mRNA expression of MMP-2 and MMP-9 was determined using real-time PCR with SYBR Green as the fluorescent dye as described in Materials and Methods. The results are presented as the relative expression of the individual mRNA with normalization to GAPDH.
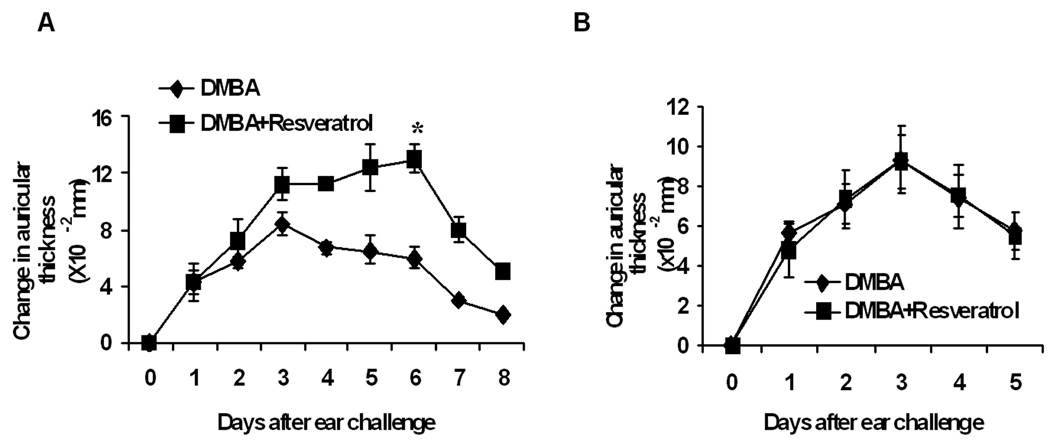
Resveratrol increases the contact hypersensitivity response to DMBA in mice with functional TLR4
In C3H/HeN mice, topical application of DMBA results in cell-mediated immunity to that compound that is due to the generation of antigen specific CD8+ T-cells that produce IFN-γ (16,21,22). The cell-mediated immune response to DMBA protects against skin tumor development by that compound (21, 23). To determine whether resveratrol influences the cell-mediated immune response to DMBA and, if so, whether it does so through TLR4 signaling pathways, panels of C3H/HeN and C3H/HeJ mice were pretreated with resveratrol and sensitized by epicutaneous application of DMBA as described in the Methods section. Resveratrol significantly increased the contact hypersensitivity response in C3H/HeN mice compared to the vehicle control (Fig. 5A). However, resveratrol pretreatment produced no increase in CHS in C3H/HeJ mice compared to the vehicle pre-treated DMBA sensitized control group (Fig. 5B). Effector T-cells for contact hypersensitivity to DMBA have been shown to produce IFN-γ (21), and resveratrol is known to increase the production of IFN-γ (11, 12). To determine the effects of resveratrol on IFN-γ levels after DMBA carcinogenesis in C3H/HeN and C3H/HeJ mice, skin samples were collected from the two strains after they had been on the DMBA skin tumorigenesis protocol for 25 weeks (Fig. 6A). Skin lysates from mice that had been subjected to the DMBA cutaneous carcinogenesis protocol were examined for IFN-γ by ELISA. Skin from resveratrol treated C3H/HeN mice had more than double the amount of IFN-γ than vehicle treated controls (p<0.05). In contrast, there was no significant difference in IFN-γ in resveratrol treated C3H/HeJ mice compared to control mice.
Figure 5.
Effect of resveratrol on DMBA contact hypersensitivity (CHS) in TLR4 deficient C3H/HeJ and C3H/HeN mice. (A) C3H/HeN mice were pretreated with resveratrol and sensitized with DMBA and ear challenged five days later as described in the Methods section. The CHS response was significantly higher in resveratrol pre-treated C3H/HeN mice than in vehicle pretreated C3H/HeN mice (*p<0.05). (B) C3H/HeJ mice were pretreated with resveratrol and sensitized with DMBA and ear challenged five days later as described in the Methods section. The CHS response was not significantly different in resveratrol pre-treated C3H/HeJ mice than in vehicle pretreated C3H/HeJ mice (*p>0.05). Results are the mean ± SD with 3 mice per group and each experiment was repeated twice with similar results. The graph is representative of one of them (*p<0.05).
Figure 6.
IFN-γ and IL-12 production in skin lysates from C3H/HeN and C3H/HeJ mice after having been subjected to a DMBA cutaneous carcinogenesis protocol for 25 weeks with or without resveratrol treatment. (A) Skin lysates from resveratrol treated C3H/HeN mice showed higher levels of IFN-γ than vehicle pretreated C3H/HeN, whereas IFN-γ levels in skin lysates from resveratrol pretreated C3H/HeJ were not increased (*p<0.05). (B) Skin lysates from resveratrol treated C3H/HeN mice showed higher levels of IL-12 than vehicle pretreated C3H/HeN, whereas IL-12 levels in skin lysates from resveratrol pretreated C3H/HeJ were decreased compared to vehicle pretreated C3H/HeJ mice (*p<0.05). Results are the mean ± SD of panels containing 4 mice per group. Each experiment was repeated twice with similar results.
Resveratrol increases IL-12 after DMBA carcinogenesis in C3H/HeN mice with a functional TLR4
The effect of resveratrol treatment on IL-12 levels in these two strains of mice was examined, since resveratrol is also known to increase the production of IL-12 (11). Skin samples were collected from C3H/HeN and C3H/HeJ mice after having been on the DMBA skin tumorigenesis protocol for 25 weeks. In C3H/HeN mice treated with resveratrol the amount of IL-12 increased (Fig. 6B). In contrast, resveratrol pretreatment resulted in decreased levels of IL-12 levels in the skin of C3H/HeJ mice. Thus, the findings of this experiment suggest that resveratrol effects on IL-12 production are mediated at least in part through TLR4 signaling pathways.
Discussion
It has previously been demonstrated that resveratrol inhibits UV radiation and polyaromatic hydrocarbon skin carcinogenesis in mice (24–26). With respect to UV carcinogenesis, there is evidence to suggest that changes in cell cycle regulatory events (25) and in survivin (27) are produced by resveratrol and that influences UV-induced skin tumor development. There is no information on the role of immune system in skin cancer prevention by resveratrol. In this study, we have found that resveratrol prevents the growth and development of DMBA-induced skin tumors through activation of the TLR4 signaling pathway. Resveratrol, which protected against skin tumor development in TLR4 proficient mice, was not effective when TLR4 signaling was deficient. Moreover, resveratrol-induced signaling through TLR4 was observed to have multiple actions that may have an impact on chemical carcinogen-induced skin tumor development. It had direct anti-proliferative effects, stimulated IL-12 production, inhibited angiogenesis and augmented the cell-mediated immune response to DMBA, the compound that caused the tumors. Our results are thus consistent with the hypothesis that resveratrol-induced TLR4 signaling initiates an orderly sequence of events in which increased amounts of IL-12 are produced, which in turn enhance immune responses and inhibit the production of factors involved in tumor angiogenesis.
In humans, most cutaneous squamous cell carcinomas are caused by excessive exposure to ultraviolet radiation. However, polyaromatic hydrocarbon-induced skin cancers have been observed in individuals exposed to these agents in the occupational setting. Individuals at risk for polyaromatic hydrocarbon skin cancers include workers in tar distillation, shale extraction and creosote industries (28–38). Cutaneous chemical carcinogenesis has also been used extensively as a model to investigate the mechanisms by which skin cancer develops, its genetics and, as in this report, methods for its chemoprevention (39–43).
The findings with respect to resveratrol are analogous to other studies we have performed examining the role of TLR4 in cutaneous chemical carcinogenesis (7). We have previously shown that significantly fewer DMBA-induced tumors develop in C3H/HeN mice that have normal TLR4 function than in C3H/HeJ mice in which TLR4 is defective. That observation was confirmed in these studies (see Fig. 1). The decreased number of tumors in C3H/HeN mice was associated with increased levels of IFN-γ, which acts both as a mediator of cell-mediated immunity and as an anti-angiogenesis factor (7).
In previous studies, we have shown that the cell-mediated immune response to DMBA that occurs in some strains of mice (22, 23), serves to protect those animals against the carcinogenic activities of that compound (23). We have also found that TLR4 determines the type of effector T-cell that is generated following topical application of hapten (15). Specifically, an intact TLR4 signaling pathway results primarily in IFN-γ-producing effector T-cells whereas a defect in that pathway leads to the generation of IL-17 producing effector T-cells. Although both are effective at producing DMBA contact hypersensitivity, only the IFN-γ producing T-cells confer resistance to DMBA tumorigenesis (7).
TLR4 has protective effects in other systems as well. For example, breast cancer patients who carry a non-functional TLR4 allele relapse more rapidly than those with a normal TLR4 allele (44). In addition, Caucasian populations with a defective TLR4 allele are at increased risk for Helicobacter pylori gastritis and cancer (45). In contrast to those studies in which TLR4 signaling pathways had a beneficial effect, other tumor models have found that TLR4 activation is a risk factor for tumors. TLR4 is over-expressed in human and murine inflammation-associated colorectal neoplasia, and TLR4-deficient mice were protected from colon carcinogenesis (46). TLR4 signaling has also been found to augment prostate cancer development (47). Thus, the chemopreventive effects of TLR4 may depend on the tissue of origin of the tumor or on the etiologic agent producing the tumor.
We found that one of the downstream effects of resveratrol-induced TLR4 signaling was an augmentation in the production of IL-12. IL-12 has been shown to cure or improve the survival of tumor-bearing mice by enhancing in vivo antitumor immune responses in a number of different tumor models (48). Initially described as an immune response modifier through its ability to stimulate the generation of IFN-γ-producing Th1-cells, IL-12 is now known to have multiple phenotypic traits which could contribute to its antitumor activities. In addition to enhancing in vivo antitumor immune responses, IL-12 has been observed to inhibit tumor angiogenesis (49). The anti-angiogenesis effects of IL-12 are mediated in part through inhibition of VEGF and matrix metalloproteinase, which are necessary for remodeling of the extracellular matrix (52). In our experiments, fewer CD31+ cells were observed in the tumors of resveratrol pretreated C3H/HeN mice and VEGF, MMP-2 and MMP-9 were all reduced, consistent with a negative effect on tumor angiogenesis. Inhibition of angiogenesis was dependent on TLR4 signaling because the effects of resveratrol on angiogenesis did not occur in TLR4 deficient mice. Although we have not shown that the effects on angiogenesis were a direct consequence of IL-12, it is reasonable to speculate that this may be the case. In this report we have shown that resveratrol not only acts by inhibiting the proliferation of tumor cells directly via TLR4, it also bolster the immune system via TLR4 to produce IL-12 which in turn results in activation of T-cells to produce IFN-γ resulting in tumor inhibition.
IL-12 is an important mediator of other chemopreventive agents as well. Epigallocatechin-3-gallate (EGCG), a polyphenolic compound found in green tea, and silymarin, a flavanoid present in milk thistle, both protect mice against the carcinogenic effects of ultraviolet radiation in mice. Similar to the effect of resveratrol, pretreatment with EGCG and silymarin augments IL-12 production and inhibits angiogenesis (50). Thus, increased production of IL-12 may be a feature common to multiple chemopreventive agents and could therefore be employed as a biomarker to screen for additional chemopreventive agents for skin cancer. Whether EGCG and silymarin require TLR4 ligation for their chemopreventive activities in skin cancer is unknown at this time. Resveratrol, through its interactions with TLR4, was found to have a direct anti-proliferative effect on the cutaneous tumor cells. Previous studies have shown that TLR4 is expressed by keratinocytes and cell lines derived from epithelial tumors (51). In vitro treatment of mouse keratinocytes and the RTE2 cell line with LPS, a TLR4 agonist, resulted in inhibition of cell proliferation. The effect on cell proliferation was dependent on NF-κB, but was independent of nitric oxide and TNF-α. Resveratrol, at concentrations as low as 25 µM, also has been shown to limit the proliferation of keratinocytes in vitro in a time and concentration dependent manner. From our in vitro data it seems that functional TLR4 is important for resveratrol mediated tumor killing. We therefore concluded that TLR4 activation is important for resveratrol-induced chemoprevention of DMBA-induced skin tumorigenesis. It has been reported previously that TLR-4 activates proapoptotic pathways by activation of caspase 8 and Fas ligand, which in turn results in cell death (52, 53).
Myeloid-differentiation factor 88 (MyD88) plays a critical role in mediating signaling by TLR4 as well as that of several of the other TLRs. When ligands bind to TLRs, the intracellular TIR domain of these receptors associates with MyD88, which eventuates in the activation of the NF-κB and AP-1 signaling pathways (54). TLR4 signaling can also proceed through an MyD88-independent process in which the TIR domain-containing adaptor inducing IFN-β (TRIF) leads to type I interferons as well as NF-κB (54). Because TLR4 is involved in both MyD88-dependent and –independent pathways, it will be of interest to determine which is activated by resveratrol to produce its protective effects.
Our findings have important implications for resveratrol chemoprevention in humans. Oral dose of Resveratrol (25mg/70kg body weight dissolved in beverage) has been reported to be administered in humans (55). Approximately 10% of the Caucasian population has an A-G mutation at position +896 of the TLR4 gene. This SNP renders individuals less responsive to TLR4 signaling and increases their susceptibility to gram negative sepsis, malaria, and gastric cancer due to Helicobacter pylori (47). If our observations in mice can be extended to humans, then those individuals who have a polymorphism in the TLR4 gene that renders them less responsive to TLR4 agonists may be resistant to the beneficial effects of resveratrol.
Acknowledgments
Grant Support: NIH Grants P30 AR050948 (CAE), P30CA13148, Veterans Administration Merit Review Award 18-103-02 (CAE) and a grant from the Department of Defense (CAE).
Abbreviations
- DMBA
7,12-dimethylbenz(a)anthracene
- MTT
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- TLR4
toll-like receptor-4
- VEGF
vascular endothelial growth factor
- DC
dendritic cells
- MMP
matrix metalloproteinase
- CHS
contact hypersensitivity
References
- 1.Takeda K, Kaisho T, Akira S. Annu Rev Immunol. 2003;21:335–376. doi: 10.1146/annurev.immunol.21.120601.141126. [DOI] [PubMed] [Google Scholar]
- 2.Byrd-Liefer CA, Block EF, Takeda K, Akira S, Ding A. Eur J Immunol. 2001;31:2448–2457. doi: 10.1002/1521-4141(200108)31:8<2448::aid-immu2448>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Okamura Y, Watari M, Jerud ES, Young DW, Ishizaka ST, Rose J, Chow JC, Strauss JF., III The extra domain A of fibronectin activates Toll-like receptor 4. J Biol Chem. 2001;276:10229–10233. doi: 10.1074/jbc.M100099200. [DOI] [PubMed] [Google Scholar]
- 4.Termeer C, Benedix F, Sleeman J, Fieber C, Voith U, Ahrens T, Miyake K, Freudenberg M, Galanos C, Simon JC. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohashi K, Burkart V, Flohé S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the toll-like receptor-4 complex. J Immunol. 2000;164:558–561. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 6.Bauer AK, Dixon D, DeGraff LM, Bauer AK, Dixon D, DeGraff LM, Cho HY, Walker CR, Malkinson AM, Kleeberger SR. Toll-like receptor 4 in butylated hydroxytoluene-induced mouse pulmonary inflammation and tumorigenesis. J Natl Cancer Inst. 2005;97:1778–1781. doi: 10.1093/jnci/dji403. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf N, Nasti TH, Long JA, Naseemuddin M, Lucas AP, Xu H, Elmets CA. Protective Role of Toll-like receptor 4 during the initiation stage of cutaneous chemical carcinogenesis. Cancer Res. 2008;68:615–622. doi: 10.1158/0008-5472.CAN-07-5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McInturff JE, Modlin RL, Kim J. The role of toll-like receptors in the pathogenesis and treatment of dermatological disease. J Invest Dermatol. 2005;125:1–8. doi: 10.1111/j.0022-202X.2004.23459.x. [DOI] [PubMed] [Google Scholar]
- 9.Rozis G, Benlahrech A, Duraisingham S, Gotch F, Patterson S. Human Langerhans' cells and dermal-type dendritic cells generated from CD34 stem cells express different toll-like receptors and secrete different cytokines in response to toll-like receptor ligands. Immunology. 2008;124:329–338. doi: 10.1111/j.1365-2567.2007.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng YH, Zhou WL, Wu QL, Li XY, Zhao WM, Zou JP. Low dose of resveratrol enhanced immune response of mice. Acta Pharmacol Sin. 2002;23:893–897. [PubMed] [Google Scholar]
- 12.Yang Y, Paik JH, Cho D, Cho JA, Kim CW. Resveratrol induces the suppression of tumor-derived CD4+CD25+ regulatory T cells. Int Immunopharmacol. 2008;8:542–547. doi: 10.1016/j.intimp.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 13.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 14.Afaq F, Adhami VM, Ahmad N, Mukhtar H. Botanical antioxidants for chemoprevention of photocarcinogenesis. Front. Biosci. 2002;7:d784–d792. doi: 10.2741/afaq. [DOI] [PubMed] [Google Scholar]
- 15.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 16.Klemme JC, Mukhtar H, Elmets CA. Induction of contact hypersensitivity to dimethylbenz(a)anthracene and benzo(a)pyrene in C3H/HeN mice. Cancer Res. 1987;47:6074–6078. [PubMed] [Google Scholar]
- 17.Ishihara Y, Nishikawa T, Iijima H, Matsunaga K. Expression of matrix metalloproteinase, tissue inihibitors of metalloproteinase and adhesion molecules in silicotic mice with lung tumor metastasis. Toxicology Lett. 2003;142:71–75. doi: 10.1016/s0378-4274(02)00486-1. [DOI] [PubMed] [Google Scholar]
- 18.Wu J, Liu J, Waalkes MP, Cheng M, Li L, Li C, Yang Q. High Dietary Fat Exacerbates Arsenic-Induced Liver Fibrosis in Mice. Experimental Biology and Medicine. 2008;233:377–384. doi: 10.3181/0710-RM-269. [DOI] [PubMed] [Google Scholar]
- 19.Shan S, Robson ND, Cao Y, Qiao T, Li CY, Kontos CD, Garcia-Blanco M, Dewhirst MW. Responses of vascular endothelial cells to angiogenic signaling are important for tumor cell survival. FASEB J. 2004;18 doi: 10.1096/fj.03-0765fje. 326-238. [DOI] [PubMed] [Google Scholar]
- 20.Risau W. Differentiation of endothelium. FASEB J. 1995;9:926–933. [PubMed] [Google Scholar]
- 21.Yusuf N, Nasti TH, Katiyar SK, Jacobs MK, Seibert MD, Ginsburg AC, Timares L, Xu H, Elmets CA. Antagonistic roles of CD4+ and CD8+ T-cells in 7,12 dimethylbenz(a)anthracene cutaneous carcinogenesis. Cancer Res. 2008;68:3924–3930. doi: 10.1158/0008-5472.CAN-07-3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson C, Hehr A, Robbins R, Hasan R, Athar M, Mukhtar H, Elmets CA. Metabolic requirements for induction of contact hypersensitivity to immunotoxic polyaromatic hydrocarbons. J Immunol. 1995;155:3530–3537. [PubMed] [Google Scholar]
- 23.Elmets CA, Athar M, Tubesing KA, Rothaupt D, Xu H, Mukhtar H. Susceptibility to the biological effects of polyaromatic hydrocarbons is influenced by genes of the major histocompatibility complex. Proc Natl Acad Sci U S A. 1998;95:14915–14919. doi: 10.1073/pnas.95.25.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athar M, Back JH, Tang X, Kim KH, Kopelovich L, Bickers DR, Kim AL. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol Appl Pharmacol. 2007;224:274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aziz MH, Reagan-Shaw S, Wu J, Longley BJ, Ahmad N. Chemoprevention of skin cancer by grape constituent resveratrol: relevance to human disease? FASEB J. 2005;19:1193–1195. doi: 10.1096/fj.04-3582fje. [DOI] [PubMed] [Google Scholar]
- 26.Reagan-Shaw S, Afaq F, Aziz MH, Ahmad N. Modulations of critical cell cycle regulatory events during chemoprevention of ultraviolet B-mediated responses by resveratrol in SKH-1 hairless mouse skin. Oncogene. 2004;23:5151–5160. doi: 10.1038/sj.onc.1207666. [DOI] [PubMed] [Google Scholar]
- 27.Aziz MH, Afaq F, Ahmad N. Prevention of Ultraviolet-B Radiation Damage by Resveratrol in Mouse Skin Is Mediated via Modulation in Survivin. Photochem Photobiol. 2005;81:25–31. doi: 10.1562/2004-08-13-RA-274. [DOI] [PubMed] [Google Scholar]
- 28.Boutwell RK, Bosch DK. The carcinogenicity of creosote oil: Its role in the induction of skin tumors in mice. Cancer Res. 1958;18:1171–1175. [PubMed] [Google Scholar]
- 29.Friedmann PS, Wilkinson M. Occupational Dermatoses. In: Bolognia JL, Jorizzo JL, Rapini RP, Horn TD, Mascaro JM, Saurat J-H, Mancini AJ, Salasche SJ, Stingl G, editors. Dermatology. London: Mosby; 2007. [Google Scholar]
- 30.Letzel S, Drexler H. Occupationally related tumors in tar refinery workers. J Amer Acad Dermatol. 1998;39:712–720. doi: 10.1016/s0190-9622(98)70043-x. [DOI] [PubMed] [Google Scholar]
- 31.Cookson HA. Epithelioma of the skin after prolonged exposure to creosote. Br Med J. 1924;1:368. doi: 10.1136/bmj.1.3296.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emmett EA. Occupational skin cancer: a review. J Occup Med. 1975;17:44–49. doi: 10.1097/00043764-197501000-00011. [DOI] [PubMed] [Google Scholar]
- 33.O'Donovan WJ. Epitheliomatous ulceration among tar workers. Br J Dermatol. 1920;32:215–228. [Google Scholar]
- 34.O'Donovan WJ. Epitheliomatous ulceration among tar workers. Br J Dermatol. 1920;32:245–252. [Google Scholar]
- 35.Pott P. In Cancer Scroti. Dublin: James Williams; 1775. Chirurgical works of Percivall Pott; p. 403. [Google Scholar]
- 36.Boffetta P, Jourenkova N, Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes Control. 1997;8:444–472. doi: 10.1023/a:1018465507029. [DOI] [PubMed] [Google Scholar]
- 37.Fisher REW. Occupational skin cancer in a group of tar workers. Arch Ind Hyg Occup Med. 1953;7:12–18. [PubMed] [Google Scholar]
- 38.Hueper WC, Payne WW. Carcinogenic studies on petroleum asphalt, cooling oil and coal tar. Arch Pathol. 1960;70:372–384. [PubMed] [Google Scholar]
- 39.DiGiovanni J. Multistage carcinogenesis in mouse skin. Pharmacol Ther. 1992;54:63–128. doi: 10.1016/0163-7258(92)90051-z. [DOI] [PubMed] [Google Scholar]
- 40.DiGiovanni J. Genetic factors controlling responsiveness to skin tumor promotion in mice. Prog Clin Biol Res. 1995;391:195–212. [PubMed] [Google Scholar]
- 41.Angel JM, DiGiovanni J. Genetics of skin tumor promotion. Prog Exp Tumor Res. 1999;35:143–157. doi: 10.1159/000062010. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Mukhtar H. Chemoprevention of Skin Cancer: Current Status and Future Prospects. Cancer Metast Rev. 2002;21:363–380. doi: 10.1023/a:1021275330385. [DOI] [PubMed] [Google Scholar]
- 43.Bickers DR, Athar M. Novel approaches to chemoprevention of skin cancer. J Dermatol. 2000;27:691–695. doi: 10.1111/j.1346-8138.2000.tb02259.x. [DOI] [PubMed] [Google Scholar]
- 44.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 45.Hold GL, Rabkin CS, Chow WH, Hold GL, Rabkin CS, Chow WH, Smith MG, Gammon MD, Risch HA, Vaughan TL, McColl KE, Lissowska J, Zatonski W, et al. A functional polymorphism of toll-like receptor 4 gene increases risk of gastric carcinoma and its precursors. Gastroenterology. 2007;132:905–912. doi: 10.1053/j.gastro.2006.12.026. [DOI] [PubMed] [Google Scholar]
- 46.Fukata M, Chen A, Vamadevan AS, Cohen J, Breglio K, Krishnareddy S, Hsu D, Xu R, Harpaz N, Dannenberg AJ, Subbaramaiah K, Cooper HS, Itzkowitz SH, et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology. 2007;133:1869–1881. doi: 10.1053/j.gastro.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen YC, Giovannucci E, Lazarus R, Kraft P, Ketkar S, Hunter DJ. Sequence variants of Toll-like receptor 4 and susceptibility to prostate cancer. Cancer Res. 2005;65:11771–11778. doi: 10.1158/0008-5472.CAN-05-2078. [DOI] [PubMed] [Google Scholar]
- 48.Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, Parmiani G, Anichini A. Interleukin-12: biological properties and clinical application. Clin Cancer Res. 2007;13:4677–4685. doi: 10.1158/1078-0432.CCR-07-0776. [DOI] [PubMed] [Google Scholar]
- 49.Katiyar SK. UV-induced immune suppression and photocarcinogenesis: chemoprevention by dietary botanical agents. Cancer Lett. 2007;255:1–11. doi: 10.1016/j.canlet.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Müller-Decker K, Manegold G, Butz H, Hinz DE, Hüttner D, Richter KH, Tremmel M, Weissflog R, Marks F. Inhibition of cell proliferation by bacterial lipopolysaccharides in TLR4-positive epithelial cells: independence of nitric oxide and cytokine release. J Invest Dermatol. 2005;124:553–561. doi: 10.1111/j.0022-202X.2004.23598.x. [DOI] [PubMed] [Google Scholar]
- 51.Coughlin CM, Salhany KE, Wysocka M, Aruga E, Kurzawa H, Chang AE, Hunter CA, Fox JC, Trinchieri G, Lee WM. Interleukin-12 and interleukin-18 synergistically induce murine tumor regression which involves inhibition of angiogenesis. J Clin Invest. 1998;101:1441–1452. doi: 10.1172/JCI1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lehner M, Bailo M, Stachel D, Roesler W, Parolini O, Holter W. Caspase-8 dependent apoptosis induction in malignant myeloid cells by TLR stimulation in the presence of IFN-alpha. Leuk Res. 2007;12:1729–1735. doi: 10.1016/j.leukres.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Tano T, Okamoto M, Oshikawa T, Ahmed SU, Sasai A, Sato M. Induction of apoptosis in human head and neck cancer cell lines by an active component of OK-432 through p53-independent pathway via toll-like receptor (TLR) 4 signaling. Gan To Kagaku Ryoho. 2005;32:1562–1564. [PubMed] [Google Scholar]
- 54.Akazawa T, Masuda H, Saeki Y, Matsumoto M, Takeda K, Tsujimura K, Kuzushima K, Takahashi T, Azuma I, Akira S, Toyoshima K, Seya T. Adjuvant-mediated tumor regression and tumor-specific cytotoxic response are impaired in MyD88-deficient mice. Cancer Res. 2004;64:757–764. doi: 10.1158/0008-5472.can-03-1518. [DOI] [PubMed] [Google Scholar]
- 55.Soleas GJ, Angelini M, Grass L, Diamandis EP, Goldberg DM. Absorption of trans-resveratrol in rats. Methods Enzymol. 2001;335:145–154. doi: 10.1016/s0076-6879(01)35239-4. [DOI] [PubMed] [Google Scholar]