Abstract
Vaccination is one of the most effective methods used for protecting the public against infectious disease. Vaccines can be segregated into two general categories; replicating vaccines (i.e., live, attenuated vaccines) and non-replicating vaccines (e.g., inactivated or subunit vaccines). It has been assumed that live attenuated vaccines are superior to non-replicating vaccines in terms of the quality of the antiviral immune response, the level of protective immunity, and the duration of protective immunity. Although this a prevalent viewpoint within the field, there are several exceptions to the rule. Here, we will explore the historical literature in which some of these conclusions have been based, including “Experiments of Nature” and describe examples of the efficacy of replicating vaccines compared to their non-replicating counterparts. By building a better understanding of how successful vaccines work, we hope to develop better “next-generation” vaccines as well as new vaccines against HIV – a pathogen of global importance for which no licensed vaccine currently exists.
Keywords: Immunological memory, T cell, B cell, antibody, vaccine, virus
1. Introduction
The remarkable success of vaccination against a wide spectrum of human pathogens represents one of the great achievements in medicine. In this regard, there is no doubt that live vaccines have played a critical role in controlling many human diseases. The world's first vaccine was developed against smallpox by Edward Jenner (Jenner, 1798; Jenner, 1799; Jenner, 1800) and this breakthrough eventually led to the eradication of natural smallpox (Fenner et al., 1988). Live vaccines have been important in controlling other pathogens including polio (Sabin vaccine, introduced in 1961) and yellow fever virus (introduced in 1936). However, these important advances have not come without a price; smallpox vaccination of the general public resulted in 1–8 deaths per million vaccinations between the 1940's through the 1980's (Kretzschmar et al., 2006). Routine vaccination of civilians was therefore discontinued worldwide in 1980 when the World Health Organization confirmed the global eradication of smallpox. Likewise, routine vaccination with the live oral polio vaccine (OPV) resulted in an average of 9 cases of vaccine-associated paralytic poliomyelitis (VAPP) each year from 1961–1989 in the US (Alexander et al., 2004). More recently, OPV vaccination campaigns in Nigeria have also resulted in at least 69 cases of VAPP (CDC, 2007). With these safety concerns, the US replaced the live polio vaccine entirely with the inactivated polio vaccine (IPV) in 2000 and this has led to the complete elimination of VAPP (Alexander et al., 2004). The current yellow fever vaccines (yellow fever 17D or 17DD strains) result in 1 to 2 deaths per million doses administered (Kitchener, 2004; Lindsey et al., 2008; Struchiner et al., 2004) including fatalities among young, otherwise healthy adults (Doblas et al., 2006; Gerasimon and Lowry, 2005; Vasconcelos et al., 2001). Although the yellow fever vaccine has been described as one of the safest vaccines ever developed, it has been contraindicated in infants since the 1960's due to high rates of encephalitis (Monath, 2004; Sencer et al., 1966) and viscerotropic disease (with ∼50% mortality) occurs in the elderly at an alarming rate of 1 per 50,000 doses (Barrett et al., 2007; CDC, 2005b; Lindsey et al., 2008).
Safety concerns explain why some live attenuated vaccines are eventually replaced by non-replicating or inactivated vaccines (Figure 1). In general, this represents an evolutionary process; prior to the development of a specific vaccine, people developed immunity by natural infection with wild-type circulating pathogens. In some cases, the infection and disease outcome could be modified by the route of exposure or the age of exposure. Prior to the development of the smallpox vaccine by Edward Jenner, a type of immunization described as “variolation” was practiced. This procedure involved inoculation of a patient's skin with smallpox (variola virus), which resulted in only 0.5-1% mortality in comparison with the natural route of exposure via the respiratory route, which resulted in approximately 30% mortality. Age at the time of infection can play a substantial role in disease outcome. Prior to licensure of the current varicella zoster virus (VZV, i.e., chickenpox) vaccine, it was not uncommon for parents to expose their young children to other VZV-infected children on purpose in order for them to be infected with the virus at a younger age when the disease severity is much less than what is typically observed during primary VZV infection as an older adolescent or as an adult. Induction of immunity through natural infection is often first replaced by vaccine-mediated immunity derived from live, attenuated vaccines. This approach, in turn, may later be replaced by the use of an inactivated or subunit vaccine – especially if there are common (or even rare) severe adverse events (AEs) associated with the original live vaccine. This process may be dictated to a large degree by the spread and severity of the disease itself and the means used to treat it. When smallpox was endemic, the one in a million chance of vaccine-associated death was small compared to the 30% mortality of the disease itself. However, once smallpox was eradicated, the risk:benefit ratio changed sharply. The rare but sometimes severe AEs associated with smallpox vaccination overshadowed its protective use in the absence of an outbreak situation and this resulted in the interest to develop a second generation tissue culture-based vaccine as well as a third-generation non-replicating vaccine based on Modified Vaccinia Ankara (MVA). Likewise, once polio was no longer endemic in the US, the risk of live virus vaccination became higher than the risk of the disease itself – resulting in the shift from using the live attenuated oral Sabin vaccine to the injected Salk vaccine comprised of inactivated virus. The evolution from natural infection to live attenuated vaccines to inactivated vaccines is by no means a universal process. In some cases, such as the Hepatitis B vaccine, the safety and efficacy of the subunit vaccine allowed its routine use without a live attenuated intermediate vaccine being pursued. In contrast, the remarkably high safety profile of the live attenuated measles-mumps-rubella (MMR) vaccine (Amanna and Slifka, 2005) indicates that it is unlikely to be replaced by an inactivated vaccine formulation any time soon.
Figure 1.
Evolution of Vaccines. Prior to the introduction of vaccines, immunity developed following natural infection with a specific viral pathogen. Live attenuated vaccines often are developed from the wild-type pathogen after selecting for less virulent strains that are able to infect the host and elicit antiviral immunity, but with greatly reduced disease severity. In cases in which live attenuated vaccines have rare but serious adverse events, further refinement of the vaccine is provided by developing an inactivated formulation for immunization. This may consist of a whole virus vaccine that has been inactivated by formaldehyde (e.g., IPV), use of a non-replicating strain of virus (e.g., MVA), or replacement with a subunit vaccine consisting of only one or a few protective antigens (e.g., Hepatitis B surface antigen). The risk of morbidity or mortality is reduced as the immunogenic insult is modified from natural infection to attenuated infection, to immunization with an inactivated or non-replicating antigen. In some cases, such as in the development of the Hepatitis B vaccine, the intermediate step involving the development of an attenuated vaccine may be omitted if a non-replicating vaccine provides effective immunity.
Abbreviations: OPV; live oral polio vaccine, IPV; inactivated polio vaccine, MVA; Modified Vaccinia Ankura
There remains considerable controversy over the efficacy and use of live vaccines versus inactivated or subunit vaccines. One concern is whether inactivated vaccines can stimulate protective mucosal immunity. A study of 527 infants given the oral polio vaccine (OPV) or IPV followed by OPV demonstrated that partial mucosal immunity was observed after vaccination with IPV (Laassri et al., 2005). Following IPV immunization, there was a 35-56% reduction in the number of infants who shed virus (depending on the serotype) following infection with OPV. Of the IPV-immunized infants who shed virus, the fecal titers were much lower (reduced by 75%). One caveat to this study however, is that two doses of IPV may not have provided the full immunity that is observed after the standard 3-dose schedule. Although IPV may not be as effective as prior OPV immunization in preventing/reducing fecal virus shedding, it is administered by either the intramuscular or subcutaneous routes and is therefore not expected to induce substantial levels of mucosal immunity. However, because IPV blocks systemic spread of the virus in the infected host, it is still 96% effective at protecting against paralyitc poliomyelitis (Melnick et al., 1961). An alternative model for examining mucosal responses is presented by cholera vaccines. The most widely used vaccine is Dukoral®, an inactivated orally administered formulation shown to be effective at preventing severe diarrhea in large field trials in Bangladesh (Clemens et al., 1986; Clemens et al., 1990). In contrast, a live attenuated oral cholera vaccine (Orochol®) showed no significant protection in a large field trial in Indonesia (Richie et al., 2000). Together, these results demonstrate that inactivated vaccines can elicit at least some degree of mucosal immunity and provide protection against mucosal pathogens. Moreover, the comparison between inactivated polio and inactivated cholera vaccines suggest that the route of vaccination (intramuscular vs. oral route, respectively) may also play a role in inducing protective immunity at mucosal sites.
Although most current vaccines induce antibody-dependent immunity (Plotkin, 2008; Siegrist, 2008), many vaccinologists still believe that a live virus vaccine is needed to induce strong T cell responses and that strong T cell responses are required for protective immunity. This assumption may be based on historical studies involving human genetic disorders, often described as, “Experiments of Nature” in which affected individuals with deficiencies in either T cell or B cell immunity were studied in terms of their susceptibility to viral and bacterial diseases. In addition, early failures of inactivated vaccines appear to have provided further proof of the superiority of live vaccines over inactivated vaccines but there were often caveats to these early studies that may change their modern interpretation. In the following sections, we will describe some recent changes to our understanding of the mechanisms of vaccine-mediated immunity, compare and contrast live attenuated vaccines versus inactivated vaccines and discuss the future development of new antiviral vaccines.
2. Experiments of Nature
2.1 Genetic deficiencies in humoral immunity
Patients with genetic immune deficiencies have been described as, “Experiments of Nature” since they provide substantial insight into the relative roles of humoral and cell-mediated immunity against infectious disease. In 1952, Bruton published the first case of agammaglobulinemia, in which he described an 8-year-old patient who completely lacked the gamma globulin fraction in serum (i.e., antibody deficient) (Bruton, 1952). This young patient presented with persistent bacterial infections, with at least 19 cases of clinical sepsis documented over a 4-year period. The patient proved unable to mount a successful antibody response against both the diphtheria and typhoid vaccines. While treatment with antibiotics led to temporary reprieves, longer-term relief was only achieved after the administration of human serum globulin at monthly intervals.
The observation that agammaglobulinemia led to repeated bacterial infections was striking evidence for antibody playing a primary role in battling bacterial disease. However, even in this initial report there was substantial evidence indicating that immunoglobulin deficiency could greatly affect the resolution of viral disease. Although the patient had an uneventful recovery from varicella (chicken pox) infection at approximately 4-years of age, he developed herpes zoster (i.e., shingles) only 5 years later, suggesting an impaired ability to control viral latency. His response to natural measles infection was also complicated by pneumonia. Considering that such complications occur in only about 1-6% of measles cases in industrialized nations (Cutts, 1993), this is also suggestive of an inadequate antiviral immune response. Perhaps most striking was the patient's inability to develop protective immunity against repeated infection by the mumps virus. Generally, a single infection by mumps confers lifelong immunity [page 140 in (CDC, 2005a)](Amanna et al., 2007). In sharp contrast, this patient contracted three clinically defined cases of mumps in less than four years. As noted by Bruton: “The author observed him only the third time when he had an illness clinically identical with epidemic parotitis [mumps], a history of exposure to epidemic parotitis and a white and differential blood count consistent with that disease.” This indicates that although the child was eventually able to resolve mumps infection, the resulting T cell memory was clearly inadequate for protecting him against reinfection in the absence of an antiviral antibody response.
After the initial description by Bruton, Good and Zak published an influential study further detailing patients with defects in gamma globulin synthesis (Good and Zak, 1956). The authors noted that, “It may be that some virus infections are handled well by these patients while others, in which circulating antibody or gamma globulin play a vital role in recovery, are handled with lesser facility”. Despite this viewpoint shared by the authors themselves, many immunologists continue to cite these “Experiments of Nature” as evidence that the humoral immune response is necessary for resolution of bacterial infections, but largely dispensable for the clearance of viral infections. A review by Sanna and Burton (Sanna and Burton, 2000) expands on the perspective that antibodies play a larger role in viral resolution than many realize. Although some believe that patients with agammaglobulinemia live relatively healthy lives despite this form of immune deficiency, this is not supported by the literature. One multi-center study describing 96 agammaglobulinemic patients and approximately 1,200 patient-years noted that 50% of these patients present with symptoms of greatly increased vulnerability to infection by 8 months of age, 75% by age 12 months, and 90% have immunological problems by the age of 18 months (Lederman and Winkelstein, 1985). Decreased morbidity and mortality among agammaglobulinemic patients is likely due to the protective efficacy of gamma globulin replacement therapy, which has been the standard of care for the last several decades (Sanna and Burton, 2000). Initial immunoglobulin replacement therapy consisted of intramuscular injections of purified antibody, which limited doses to only ∼100 mg/kg/month. During this period of time (circa 1950-1980 in the United States), patients still commonly presented with complications from a number of viral diseases including cytomegalovirus, adenovirus, enterovirus, herpes simplex virus and varicella zoster virus (Lederman and Winkelstein, 1985). Live viral vaccines appear to pose a particularly high risk for this immunocompromised population. Despite having a relatively intact T cell compartment (Lederman and Winkelstein, 1985) two cases of vaccine-associated poliomyelitis from the live oral polio vaccine occurred among 96 agammaglobulinemic patients, as well as one case of vaccinia necrosum (a life-threatening infection) following smallpox vaccination. During the study, 16 of 96 patients (17%) died from infectious disease at a mean age of 16.9 ± 7.5 years and 8/16 (50%) of the deaths were directly attributable to viral infections. Only after the introduction of more potent formulations of intravenous immunoglobulin (administered at 400 mg/kg/month) in the 1970-1980s have many of these viral infections finally been controlled (Eibl, 2008; Skull and Kemp, 1996).
2.2 Genetic deficiencies in cellular immunity
Severe combined immunodeficiencies (SCID) represent a group of genetic disorders that include at least 8 forms of disease that are characterized by problems in T cell differentiation as well as variable degrees of abnormal development of other lymphocyte lineages including B cells, NK cells, or in rare instances, myeloid cells (Fischer, 2000). About 20% of SCID patients lack mature T cells and B cells, but retain a functional NK cell compartment. Another 50-60% of SCID patients lack mature T cells and NK cells but retain normal B cell development, and approximately 20% of SCID patients present with adenosine deaminase deficiency which results in a severe loss of T cells, NK cells, and B cells (Fischer, 2000). Clinical presentation is relatively uniform and diagnosis of SCID occurs at a mean of 6.59 months of age (Fischer, 2000). Interestingly, this is also about the age that maternal antibodies reach their lowest levels (Ukkonen et al., 1984) and is similar to the age that agammaglobulinemic patients begin to present with symptoms (Lederman and Winkelstein, 1985). The most common health issues at the time of SCID diagnosis include oral candidiasis, respiratory syncytial virus, parainfluenza 3 or Pneumocystis carinii pneumonia, adenovirus infection, gram-negative sepsis, persistent diarrhea and failure to thrive (Fischer, 2000). Viral infections represent <5% of infections observed in SCID patients just prior to bone marrow transplantation (Bortin and Rimm, 1977), indicating that similar to agammaglobulinemic patients, bacterial and fungal infections represent the main source of opportunistic infection. If untreated, SCID patients do not typically survive beyond the first year of life (Buckley et al., 1997; Stephan et al., 1993). Unlike agammaglobulinemia, which can be treated by reconstituting the humoral response by IVIG, SCID patients require bone marrow transplantation in order to reconstitute a functioning immune system. Because SCID is a much more severe disease that is more difficult to treat than agammaglobulinemia, it is thought that cellular immunity is more important than humoral immunity in terms of protection against infectious disease. This is not necessarily a fair comparison, however, because agammaglobulinemia represents a defect in only one arm of the immune system whereas SCID deficiency results in a defect in both T cell- and B cell-mediated immunity.
2.3 Efficacy of passive immunotherapy
Passive immunotherapy refers to the administration of serum antibodies, purified immunoglobulin preparations, or monoclonal antibodies that contain protective levels of antibody of a known specificity. As early as 1890, passive immunotherapy was used as a treatment for diphtheria (Wesselhoeft, 1936), and was commonly administered to patients in the pre-antibiotic era from the 1920's through the 1940's for treating bacterial pneumonia, meningitis, measles, scarlet fever, and whooping cough (Bordetella pertussis) [reviewed in (Casadevall, 1996; Casadevall, 1999; Casadevall and Scharff, 1995; Eibl, 2008; Skerrett, 2001; Zeitlin et al., 2000)]. Though initial use often focused on toxin-mediated diseases (e.g., tetanus and diphtheria), the administration of convalescent serum in treating viral diseases such as measles was recorded as early as 1907 (Keller and Stiehm, 2000). In 1945, Janeway detailed the use of concentrated γ -globulin in the prevention and attenuation of measles in children (Janeway, 1945). As might be expected, both the timing and the dose of γ-globulin related to efficacy of the treatment. Out of 1,024 cases of measles exposure, 36% of those given γ-globulin within 0-2 days showed clinical symptoms of infection, whereas 48.4% contracted measles if γ-globulin administration was delayed until 6-8 days post-exposure. The dose of γ-globulin used in prophylactic therapy was critical; 67% of patients given approximately 0.01 ml/kg showed clinical signs of measles, whereas only 16% of patients presented with symptoms of measles if the dose was increased to 0.06 ml/kg. One review of postexposure prophylaxis against measles indicates that the variability of anti-measles titers in immunoglobulin preparations will likewise have a profound impact on efficacy, with the post-exposure incidence of measles increasing from 17% to 57% as the measles titer in the γ-globulin decreased from 33 IU/ml to 16 IU/ml (Endo et al., 2001). This may be a concern for future preparations of immunoglobulin produced almost entirely from vaccinated populations, since the live attenuated vaccine appears to elicit antibody titers that are about 10-fold lower than that achieved following natural measles infection (Itoh et al., 2002).
Smallpox represents one viral disease in which convalescent serum has demonstrated dramatic prophylactic and therapeutic potential (Keller and Stiehm, 2000). Anecdotal evidence of passive immunotherapy against smallpox dates back to as early as 1893 (Couzi and Kircher, 1941). Vaccinia-immune gamma-globulin (VIG) was also used as smallpox prophylaxis during a 1953 outbreak in India (Kempe et al., 1961). In this study involving 705 smallpox contacts, the administration of low dose VIG (10 mL/adult, intramuscularly) in addition to immediate smallpox vaccination, reduced the number of smallpox cases by 70% over that achieved through post-exposure vaccination alone (Kempe et al., 1961). Likewise, administration of a similar volume of vaccinia-specific antibody of animal origin (in addition to vaccination) resulted in 0/13 (0%) cases of smallpox in close contacts compared to 13/29 (45%) cases of smallpox in a control group of contacts that received post-exposure vaccination alone (Marennikova, 1962). Smallpox convalescent serum is known to have a neutralizing titer that is 10 to 20 times greater than that obtained from vaccinia-immune individuals, which the authors surmised would likely offer increased protection against disease if it was available (Kempe et al., 1961). Interestingly, in a classic study published 20 years earlier (Couzi and Kircher, 1941), the utility of smallpox convalescent serum therapy was tested during a smallpox epidemic in Morocco. During the first 15 days of the outbreak, 3 out of 10 (30%) patients died of smallpox. In an effort to reduce the death toll of the ensuing outbreak, serum immunotherapy was initiated. Convalescent serum was collected at early time points from recovered smallpox patients and administered to new cases daily until the illness resolved. When treatment was switched to serotherapy for the following 250 cases (with 75 cases listed as intensely suppurative or hemorrhagic at the initiation of therapy) all of the patients recovered, dropping the mortality rate from 30% to 0% - a remarkable difference in clinical outcome. The success of this study is most likely due to two factors that are critical to passive immunotherapy; the antibody composition must have high specific neutralizing activity and treatment must be started as early as possible after exposure or disease onset (Slifka and Hanifin, 2004; Whitton et al., 2004).
As indicated with measles virus, the timing of antibody-mediated therapy is crucial in determining disease outcome for many viral infections – with early administration correlating with greatly increased efficacy (Dubois et al., 2005). For example, RSV-specific immunotherapy is effective at preventing initial infection and a monoclonal antibody is used to protect premature infants from RSV infection (American Academy of Pediatrics, 2003; Sawyer, 2000), but passive immunotherapy shows little or no clinical efficacy in treating children who have already contracted severe RSV infection (Skerrett, 2001). Varicella-zoster immune globulin is most effective if administered within 4 days after exposure, but may still reduce disease symptoms if administered up to 10 days following exposure (Keller and Stiehm, 2000). Junin virus, the causative agent of Argentine hemorrhagic fever, normally causes 15-30% mortality but if convalescent serum is administered within 8 days of disease symptom onset, the mortality rate drops to <1% (Enria and Barrera Oro, 2002; Enria et al., 2008; Harrison et al., 1999). The proven efficacy of passive immunotherapy in the treatment of a variety of virus infections, coupled with a revisitation of the “Experiments of Nature” indicates a much broader role for antibodies in the prevention and resolution viral disease than previously believed.
3. Early failures of inactivated vaccines
3.1 Smallpox
There is continuing debate over the relative merits of using live vaccines versus inactivated vaccines (Meldrum, 1999). One of the main concerns is whether an inactivated vaccine can achieve the protective efficacy and duration of immunity that is afforded by a live attenuated vaccine. Some of this concern stems from initial failed or suboptimal attempts to develop inactivated versions of successful live vaccines, such as the smallpox vaccine. In order to decrease the risk associated with live vaccination, early attempts were made to develop an inactivated vaccine for use in humans. In one study, rabbits immunized with inactivated vaccinia mounted robust antiviral antibody responses but showed an increased incidence of fever after lethal challenge with rabbitpox whereas animals that received live vaccination showed no signs of fever after challenge (Boulter et al., 1971). This result has led to the conclusion that inactivated orthopoxvirus vaccines provide little to no protection [page 154 in (Fenner et al., 1988)]. Despite the noted differences in fever, this study still demonstrated that 51/54 (94%) of the animals that received the inactivated vaccine formulation survived challenge whereas only 7/28 (25%) of unvaccinated animals survived. Moreover, the three vaccinated rabbits that died following challenge had also received the lowest dose of vaccine (Boulter et al., 1971), indicating that 100% survival was attained with an optimal vaccine dosage. This suggests that some older studies that have been described as vaccine failures were actually reasonably successful, depending on one's definition of protective immunity (i.e., onset of fever upon challenge or survival from lethal infection).
In terms of human smallpox vaccine trials using inactivated vaccinia virus, initial results were not as promising as that observed with traditional live smallpox vaccination. In one study using UV-inactivated virus injected subcutaneously (Kaplan et al., 1962), neutralizing antibody titers were observed in only about half of vaccinees (19/37 subjects = 51%), and the low neutralizing activity was often near the limit of detection. Secondary challenge with live vaccinia virus by scarification indicated that only 8/25 (32%) of the subjects vaccinated with UV-inactivated virus showed a modified skin reaction indicative of antiviral immunity. However, no adjuvant was used in the vaccine formulation and so it is not surprising that the immune responses were low and variable after vaccination. A follow-up study involving intramuscular injection of inactivated vaccinia virus at a 50-fold higher concentration than the earlier study showed that 10/10 previously vaccinated subjects mounted an increased neutralizing antibody response (Kaplan, 1962). Again, no adjuvant was used in the vaccine formulation and in this case, only subjects with pre-existing immunity were vaccinated and this, along with the higher antigen dose, may have contributed to the improved immunogenicity observed in these studies. Similar results were found in an independent study (Giurca et al., 1976); vaccination of previously immunized subjects with UV- or formalin-inactivated vaccinia virus by scarification resulted in a cutaneous response in 75% of the vaccinees and an increase in antibody titers. In contrast, primary vaccination by scarification of naïve subjects did not result in a cutaneous reaction (0/7 subjects, 0%) whereas 5/7 (71%) of the primary vaccinees developed detectable antiviral antibody responses following this route of immunization. Overall, the early studies on inactivated smallpox vaccines varied considerably in terms of the antigen preparation, method of inactivation, route of vaccination, and the assessment of efficacy and this, along with problems of incomplete inactivation of live virus in some of the studies, resulted in highly conflicting results (Turner et al., 1970). Moreover, the high success rate of traditional live smallpox vaccination, coupled with the eradication of naturally occurring smallpox in 1980, reduced the efforts to develop inactivated smallpox vaccines for many years.
3.2 Yellow fever
Yellow fever represents a viral disease in which inactivated vaccines were initially under investigation, only to be replaced by an effective live attenuated vaccine in the mid 1930's (Monath et al., 2008). In 1936, a series of studies on inactivated yellow fever virus (YFV) in which heat, UV and formaldehyde were utilized as inactivating agents indicated that when the vaccine preparations were administered to rhesus macaques, no antigenicity could be demonstrated (Gordon and Hughes, 1935). Again, this is not surprising since these studies were performed before the introduction of modern adjuvants (Manmohan, 2007), which would have greatly limited the immunogenicity of these early vaccine formulations. Interestingly, an independent group described protection against yellow fever using a phenol-glycerin inactivated YFV vaccine (Hindle, 1928). This crude vaccine, prepared from infected spleen and liver tissue protected 5/6 monkeys (83% protection) from lethal YFV infection whereas 4/4 (100%) of control monkeys died following challenge. Results with this vaccine in human subjects were considered promising, though immunogenicity was described as irregular (Pettit, 1931). Further attempts at refining and optimizing an inactivated YFV vaccine however, were abandoned following the development of cost-effective live attenuated yellow fever strains that were highly effective at protecting against natural YFV infection (Monath et al., 2008). These early attempts at developing an inactivated YFV vaccine were left behind as failures and this perception has persisted despite signs of early success. At present, there is no alternative to live YFV vaccination because it has been assumed that the current vaccine is adequately safe. However, as noted in one review on flavivirus vaccines (Pugachev et al., 2005), “Because of the availability of effective 17D vaccine, development of alternative yellow fever vaccines has not been pursued. In the light of recent adverse events, this may change”. Efforts are now underway to develop new and improved YFV vaccines with a better safety profile.
3.3 Measles
One concern with inactivated vaccines is the potential to exacerbate, rather than prevent, disease. One prototypical case is inactivated measles virus (IMV). Early developments in attenuated measles vaccines showed promising results, but the initial Edmonston B strains demonstrated significant side effects including fever and rash [page 123 in (CDC, 2005a)]. In an effort to develop a better-tolerated vaccine, a formaldehyde-inactivated vaccine was tested. Children given the IMV vaccine demonstrated seroconversion (Krugman et al., 1965). However, upon exposure to wild-type measles virus, an atypical form of disease was observed (Cutts, 1996). This atypical measles often included fever, muscle pain and a hemorrhagic or vesicular rash accompanied by lung inflammation. Annunziatio et. al. suggested that the underlying mechanism was a failure of IMV recipients to mount a successful antibody response against hemolysin (i.e., F component), a virulence factor involved in cell-to-cell spread of the virus (Annunziato et al., 1982). The authors also hypothesized that the manner of inactivation (i.e. formaldehyde treatment) may have led to the destruction of the hemolysin's antigenic properties, leading to an imbalanced response primarily against the hemagglutinin protein.
3.4 Respiratory Syncytial Virus
Similar to the inactivated measles vaccine, the original formaldehyde-fixed vaccine against respiratory syncytial virus (RSV) appeared to enhance disease following natural infection with RSV. RSV represents the most common and important cause of lower respiratory tract disease among children worldwide (Shay et al., 1999). In the 1960's, researchers developed a formaldehyde-inactivated RSV (FIRSV) vaccine (Kim et al., 1969). The vaccine itself was well tolerated, but severe complications were observed after exposure to wild type RSV. In one study, 80% of RSV vaccinees required hospitalization (compared with only 5% of influenza vaccinated controls) following exposure to RSV, with symptoms including pneumonia and bronchiolitis (Kim et al., 1969). Two fatalities occurred in the FIRSV-immunized group, which was unexpected since RSV infection is not typically lethal. Contemporary studies of the FIRSV vaccine in monkeys and mice have suggested that the formaldehyde-fixed vaccine primes a strong Th2 response (De Swart et al., 2002; Waris et al., 1996) leading to pulmonary eosinophilia and enhanced inflammation following wild-type virus infection. It is thought that formaldehyde treatment of RSV destroys or diminishes key neutralizing epitopes, leading to the altered immune response observed following wild type challenge (Moghaddam et al., 2006; Murphy and Walsh, 1988). Recently, Delgado et. al. expanded on this hypothesis, demonstrating that FIRSV was able to elicit antibodies to key antigenic determinants, but the quality of these antibodies (i.e., antibody avidity) was poor due to the lack of toll-like receptor (TLR) co-stimulation (Delgado et al., 2009), and that appropriate use of adjuvants may be able to remedy the FIRSV vaccine. Interestingly, in this same report, the authors were able to stimulate a protective immune response with UV-inactivated RSV even in the absence of TLR stimulation, but exacerbated disease was observed when a formaldehyde-inactivated vaccine formulation was used. This indicates that the method used to inactivate a virus is a critical aspect of vaccine design and poor immunological outcome is not necessarily associated with virus inactivation per se, but may instead be due to improper or deleterious methods of inactivation. This aspect of vaccinology has received little attention and there is growing frustration with the lack of available alternatives to formaldehyde for use in vaccine development (Brown, 1993).
4. Mechanisms of vaccine-mediated immunity
4.1 Role of antibody and T cells during primary infection versus secondary infection
Genetic or antibody-mediated depletion of T cells during primary infection has demonstrated how important T cells are for the induction of antiviral immunity in many animal models. However, similar to the “Experiments of Nature” in which human patients lack T cell function due to genetic deficiencies, it is important to note that effective antibody responses can be directly affected by T cell deficiency. For instance, if a mouse lacks the ability to mount an antibody response, then antiviral T cells may be able to compensate for the loss of this arm of the adaptive immune response during control of a viral infection (Belyakov et al., 2003). In contrast, if T cell responses are deficient, then this leads not only to a direct impact on T cell immunity but also impacts the humoral immune response as well. This is because development of an effective, high affinity virus-specific antibody response is mainly T cell-dependent and without sufficient T cell help, antibody responses are greatly reduced in terms of both quantity and quality (Whitton et al., 2004).
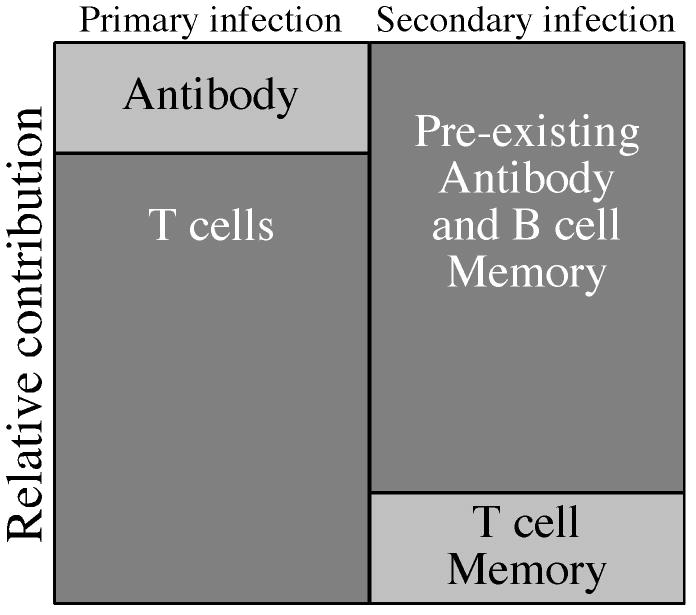
It is generally agreed that T cells are critical for resolving primary viral infection (Slifka, 2004; Whitton et al., 2004). As shown in Figure 2, we propose that T cells play the predominant role during primary viral infection of an immunologically naïve host because T cells are required not only for the initial control of the infection but also for the induction of an effective antiviral antibody response. The roles are typically reversed, however, during vaccine-mediated immunity against secondary infection. In an immune host, pre-existing neutralizing antibodies represent the first line of defense against re-infection. Since pre-existing antibody appears to be maintained by long-lived plasma cells, memory T cells would not be required for the maintenance of serological memory. This may explain why antibody responses following smallpox vaccination do not correlate with T cell memory (Hammarlund et al., 2003) and are maintained with a half-life of approximately 92 years (Amanna et al., 2007) despite virus-specific T cell memory declining with a half-life of 8-15 years (Crotty et al., 2003; Hammarlund et al., 2003). If an invading virus is neutralized before it is able to infect its first target cell and replicate, then there is a reduced dependence on pre-existing T cell memory because the infection is blocked before it can begin. Alternatively, even if pre-existing antibody is unable to completely block initial infection then circulating antibodies (in addition to memory B cells) would still likely slow the dissemination of virus and allow time for the host to mount a new antiviral T cell response from the naïve T cell repertoire even in the absence of a pre-existing T cell memory compartment. It is possible that the role of antibody in determining vaccine-associated immunity is not relevant to all viral pathogens but this theory represents a testable hypothesis and is supported by data emerging from studies involving unrelated viruses including monkeypox and yellow fever virus.
Figure 2.
Relative contributions of humoral and cellular immunity during primary or secondary infection. During primary viral infection, antiviral T cell responses are critical for reducing viral replication in addition to contributing to the development of an effective antibody response. Primary T cell-dependent antibody responses are mounted during the course of infection and take time to undergo immunoglobulin class-switching and somatic hypermutation as they provide assistance to virus-specific T cells in resolving the infection. Following recovery from primary infection (or vaccination), persisting virus-specific antibody represents the first line of defense against secondary infection. If secondary infection does occur, then circulating antibodies and presumably memory B cells that proliferate and differentiate into antibody secreting cells will reduce virus dissemination and allow time for the development of an antiviral T cell response. Memory B cells are highly efficient at presenting specific antigen (Lanzavecchia, 1985) and therefore may also be involved with more rapid and efficient presentation to T cells as well. Pre-existing T cell memory will also play a role in protection against secondary infection. However, even if T cell memory has declined or is lost, the long-term maintenance of antiviral antibody responses will suppress virus replication until a new virus-specific T cell response is mounted from the naïve repertoire.
4.2 Role of antibody and T cell-mediated immunity against monkeypox
Monkeypox is an orthopoxvirus that is closely related to smallpox. Although the 5-10% mortality rate associated with monkeypox is less than the 30% mortality rate associated with variola major, it is still considered a serious human pathogen and a potential bioterrorist threat. There has been considerable debate over the correlates of protective immunity against orthopoxviruses and in an elegant study by Edgehill-Smith et al. (Edghill-Smith et al., 2005) we have learned the mechanisms of vaccine-mediated protection. In these studies, rhesus macaques (RM) that had received smallpox vaccination (i.e., vaccinia virus) were depleted of CD4+ T cells or CD8+ T cells at the time of lethal monkeypox challenge. Depletion of either memory T cell population had little or no effect on the protective immunity observed in the vaccinated animals. This indicated that memory T cells were not necessary for control of the monkeypox infection and that humoral immunity may be the important arm of the protective immune response. To examine this question further, CD20+ B cells were depleted at the time of vaccination to reduce or eliminate vaccine-mediated neutralizing antiviral antibody responses. Following this depletion procedure, ¾ of the B cell-depleted animals died following monkeypox challenge. Incidentally, the only vaccinated animal that survived the monkeypox challenge was also the only one that had been able to mount a detectable antiviral antibody response prior to challenge. These results were in stark contrast to the lack of clinical effect when T cells were depleted and this demonstrated that antiviral antibodies were necessary for protective immunity against monkeypox. To determine if neutralizing antibody could be sufficient for protection against lethal monkeypox infection, vaccinia immune globulin (VIG) was administered to unvaccinated RM prior to virus challenge. Despite the development of skin lesions following challenge, the animals survived the normally lethal viral infection. Together, these studies demonstrated that antibody responses were both necessary and sufficient for vaccine-mediated protective immunity against lethal challenge with a virulent orthopoxvirus.
4.3 Protective immunity against yellow fever
The importance of T cell memory in vaccine-mediated protection against flavivirus infection is central to the development of new and improved vaccines against this family of viruses. A recent study utilizing closely related chimeric flaviviviruses has shed new light on this question (Monath et al., 2002). The ChimeriVax® vaccines use a recombinant yellow fever virus (YFV) vector in which the envelope and pre-membrane genes are deleted and replaced by those of a different flavivirus, thus forming a chimeric virus vaccine. Because of concerns that pre-existing immunity to the yellow fever vector might inhibit the induction of JEV-specific immunity following vaccination with ChimeriVax-JE, the authors conducted a randomized double-blind clinical trial in which flavivirus-naïve subjects or YFV-immune subjects were vaccinated with ChimeriVax-JE or YFV-17D (YF-VAX®). Vaccination of naïve subjects with either of these live virus vaccines resulted in readily detectable viremia (Monath et al., 2002). In contrast, YFV-17D booster vaccination of YFV-17D-immune subjects (with verified pre-existing YFV-specific neutralizing antibody titers) resulted in no detectable viremia, thus demonstrating the strong protective immunity elicited by this vaccine. However, when YFV-immune subjects were infected/vaccinated with ChimeriVax-JE, the subjects became viremic and the area under the curve (AUC) measurements indicated that the magnitude and duration of viremia was similar or slightly higher than that observed in flavivirus-naïve subjects. This is an important and intriguing study because it shows that although live YFV vaccination can fully protect against homologous YFV challenge, it is essentially incapable of preventing infection and dissemination of ChimeriVax-JE, a virus that is identical to YFV except for the expression of the JEV envelope and pre-membrane proteins. Vaccination with nonstructural proteins such as YFV NS1 can be protective in animal models (Schlesinger et al., 1986), and the majority of the antiviral T cell response in humans is targeted to nonstructural proteins (Co et al., 2002). However, the study described here (Monath et al., 2002) clearly shows that the combined CD4+ and CD8+ T cell memory response against 8/10 viral proteins – including all of the nonstructural proteins of YFV, are not capable of blocking viral replication and dissemination in YFV-17D-immune subjects. Since pre-existing T cell memory did not reduce viral load upon secondary challenge, this indicates that antiviral antibody responses are likely to be necessary for vaccine-mediated protection. Interestingly, in a study published in 1931 (Sawyer, 1931), Wilbur Sawyer demonstrated that injection of convalescent serum (at concentrations as low as 1-2 mL serum/Kg) from yellow fever immune human subjects completely protected non-human primates from lethal yellow fever infection. In some cases, the treated animals were protected from any sign of illness or fever despite being challenged with a lethal dose of yellow fever virus. Although these studies were published over 70 years apart, together they demonstrate that antiviral antibody is likely to be both necessary (Monath et al., 2002) and sufficient (Sawyer, 1931) for protective immunity against yellow fever.
4.4 HIV and Failure of the STEP trial
Despite the many successes in antiviral vaccine development, perhaps the most disappointing failure has been the inability to develop an effective HIV vaccine. Intensive efforts at vaccine development spanning over a quarter of a century have yet to come to fruition (Steinbrook, 2007). The latest setback comes from the failure of the Merck HIV vaccine, known as the STEP trial (Buchbinder et al., 2008; Lu, 2008; Moore et al., 2008; Sekaly, 2008; Steinbrook, 2007; Watkins et al., 2008). Unlike most traditional vaccines that elicit neutralizing antibody responses in addition to T cell immunity, the Merck vaccine was based on the development of HIV-specific T cell immunity alone. This was accomplished by a 3-dose vaccination regimen with a trivalent vaccine utilizing recombinant serotype 5 adenovirus (Ad5) vectors expressing one of three HIV antigens; Gag, Pol, and Nef (Priddy et al., 2008). Since neutralizing antibody is the only mechanism that can technically block infection, the goal of this vaccine was not to stop infection but to limit viral replication and subsequent transmission by reducing viremia (Watkins et al., 2008). This T cell-focused vaccine approach was reasonably immunogenic and elicited detectable HIV-specific T cell responses in up to 77% of vaccinees (McElrath et al., 2008). T cell memory was also maintained for at least 78 weeks regardless of pre-existing immunity to the Ad5 vector (Priddy et al., 2008). Despite this somewhat promising immunogenicity data on the development of HIV-specific memory T cells, the Phase 2b efficacy trial was halted when an interim analysis by the data and safety monitoring board concluded that the vaccine did not prevent infection nor did it reduce viremia, the main objective of the trial. Moreover, there were more cases of HIV infection in the vaccinated group (49 HIV infections) compared to the placebo group (33 HIV infections), suggesting that the vaccine may have increased susceptibility to HIV infection. Although there is the suggestion that activation of vector-specific (i.e., adenovirus-specific) CD4+ T cells or induction of HIV-specific CD4+ T cells may be involved with the apparent increase in HIV susceptibility, with such a small number of cases to compare it is difficult to determine if these represent significant differences in susceptibility or only coincidence.
It is often difficult to predict the outcome of a clinical trial, especially in the case of a complex pathogen such as HIV. However, T cell-mediated vaccines analogous to the one used in the STEP trial failed to demonstrate efficacy in rhesus macaques challenged with simian immunodeficiency virus (SIV) (Watkins et al., 2008). In addition, there were 6 cases of HIV infection identified among 236 vaccine recipients during the Phase I trial (Priddy et al., 2008). Although no cases of HIV occurred in the placebo group, the number of controls (n = 21) was too few to be sufficiently powered for statistical significance to be made. Nevertheless, both the preclinical studies and the Phase I trial indicated that the STEP trial was unlikely to succeed. This does not mean that an HIV vaccine is impossible. A recently developed recombinant rhesus cytomegalovirus vector-based vaccine encoding Env, Gag, Rev-Tat-Nef protected approximately one-third of animals from repeated low-dose SIV challenge (Hansen et al., 2009). It is unclear if protection was mediated solely by T cells or the combination of T cells and neutralizing antibodies. Moreover, previous studies have shown that neutralizing antibody alone can be effective at protecting non-human primates from SIV infection and reducing viral burden (Baba et al., 2000; Haigwood et al., 2004; Hessell et al., 2009; Johnson et al., 2009; Mascola et al., 2000; Nishimura et al., 2002; Parren et al., 2001). These studies provide proof-of-principle that an HIV vaccine may be possible in the future. However, most would agree that development of an effective HIV vaccine will require one that elicits strong, broadly reactive neutralizing antibodies in combination with robust polyfunctional HIV-specific T cell responses in order to have the greatest likelihood of success.
4.5 Success of HPV vaccines
Similar to HIV, there was considerable skepticism over the chances of developing an effective vaccine against human papilloma virus (HPV), another difficult human pathogen that invades the host via a mucosal route and establishes a chronic infection. As stated in one review published in 2000, “The generation of complete protection from infection may be unobtainable…” and [an HPV vaccine], “…is certainly not a realistic goal for the foreseeable future” (Stern et al., 2000). Remarkably, within the short time frame between 2000 and 2007, two Phase III clinical trials were published, demonstrating between 90% (Paavonen et al., 2007) and 98% (The FUTURE II Study Group, 2007) efficacy against the most virulent strains of HPV; serotype 16 and serotype 18, the leading causes of cervical cancer (Rogers et al., 2008). Prior to publication of these clinical trials, it was believed that neutralizing antibodies might play some role in protective immunity, but protection from chronic infection and disease would require strong cell-mediated immunity (Stern et al., 2000). Based on current dogma, this would require a live attenuated vaccine vector, since these are more capable of inducing cytotoxic CD8+ T cell responses than non-replicating vaccines. Interestingly, both of these highly effective HPV vaccines are based on non-replicating virus-like particles (VLPs) produced by the synthesis and self-assembly of the major virus capsid protein, L1. Although these represent non-replicating vectors that require an initial 3-dose vaccine regimen, durable immunity appears to be maintained for at least 4 to 5 years (Rogers et al., 2008). If neutralizing antibody responses represent the underlying mechanism of protective immunity and VLP antigens have similar immunogenicity to other vaccine antigens such as tetanus and diphtheria (Amanna et al., 2007), then HPV-specific immunity could last for decades.
5. Duration of vaccine-mediated immunity
5.1 Both live and inactivated vaccines require boosters
It is generally accepted that inactivated vaccines require at least one or two booster vaccinations in order to elicit optimal long-term protective immunity. However, one central dogma of vaccinology is that live vaccines are superior to non-replicating vaccines because with live attenuated vaccine viruses, “one shot induces lifelong immunity”. Although this is indeed the case for many natural viral infections, it not the case following immunization with many live attenuated vaccines (Table 1). The notion that live attenuated vaccines would induce long-term protective immunity is likely based on the experience and epidemiological data derived from immunological memory induced by natural wild-type virus infections (Slifka and Ahmed, 1996). For instance, measles, mumps, and rubella were once considered “childhood diseases” because typically an individual was infected once and then was afforded essentially lifelong immunity thereafter. Although the MMR vaccine is comprised of live, replicating versions of measles, mumps, and rubella viruses, these represent highly attenuated viruses and the lack of long-term protective immunity following a single shot has required the US to require two doses of this vaccine for routine childhood vaccination (Table 1). One might argue that the failure of these attenuated viruses to elicit long-term protective immunity is due to the transient nature of acute viral infections that are cleared and that a chronic or latent viral infection might lead to more sustained antiviral immunity. Introduction of the attenuated varicella zoster vaccine, Varivax®, has provided an opportunity to examine this question. Although this vaccine is immunogenic and generally well-tolerated, protective immunity is not long-lived. The rates of breakthrough varicella changes significantly over time from 1.6 cases/1000 person-years at 1 year after vaccination to 9.0 cases/1000 person-years at 5 years, and 58.2 cases/1000 person-years at 9 years post-vaccination (Chaves et al., 2007). For this reason, the US adopted a 2-dose vaccination regimen in order to counter the short-lived immunity induced by a single dose of Varivax® (Table 1). Although the traditional smallpox vaccine represents a live virus that can provide long-term, often lifelong immunity (Amanna et al., 2007; Crotty et al., 2003; Hammarlund et al., 2003; Putz et al., 2005) most artificially attenuated vaccines including measles, mumps, rubella, varicella zoster virus, polio, and hepatitis A, require booster vaccination for optimal immunity. This indicates that the “one shot induces lifelong immunity” dogma regarding live attenuated vaccines is actually the exception and not the rule.
Table 1.
| Live Vaccines | Inactivated/Subunit Vaccines | ||||
|---|---|---|---|---|---|
| Vaccine | Schedulea | No. Doses | Vaccine | Schedule | No. Doses |
| Rotavirus | 6-14 weeks, 4 months | 2 | Hepatitis B | Birth, 1-2 months, ≥24 weeks | 3 |
| Measles, Mumps, Rubella (MMR) | 12-15 months, 4-6 years | 2 | Diphtheria, Tetanus, Pertussis | 2, 4, 6 months, 15-18 months, 4-6 years | 5 |
| Varicella | 12-15 months, 4-6 years | 2 | Haemophilus Influenzae type B | 2, 4 months, 12-15 months | 3 |
| Oral Poliovirus (OPV)b | 8, 12 weeks, 6-8 months, 4-6 years | 4 | Pneumococcal | 2, 4, 6 months, 12-15 months | 4 |
| Oral Poliovirus (OPV)c | Birth, 6, 10, 14 weeks | 4 | Hepatitis A | 12, 23 months | 2 |
| Inactivated Poliovirus (IPV) | 2, 4 months, 6-18 months | 3 | |||
United States Recommended Immunization Schedule, 2009 (CDC, 2009).
Not administered in the United States since 2000, but vaccination schedule is based on 1999 recommendations.
WHO recommended vaccination schedule for developing nations or where polio is endemic [page 665 in (Sutter et al., 2008)].
5.2 Duration of immunity following vaccination with live or inactivated vaccines
There are not many examples in which the duration of immunity following immunization with either a live vaccine or an inactivated counterpart have been developed against the same pathogen. However, this is the case for a handful of viruses such as polio and hepatitis A and this allows for some interesting comparisons of immunological memory to be made. For instance, one of the main concerns with inactivated vaccines is that immunity may only be short-lived. The polio vaccine is a classic example of this debate (Meldrum, 1999). Proponents of Sabin's live oral poliovirus vaccine (OPV) contended that only the reaction to an active infection would elicit lifelong immunity (Meldrum, 1999) and should therefore be the vaccine of choice [page 18 in (Robertson, 1993)]. In Sweden, OPV was never administered, but vaccination with IPV began in 1957 and following vaccination of up to two-thirds of the population (Bottiger et al., 1972) endemic polio was eliminated by 1962 (Bottiger et al., 1998). Analysis of antiviral antibody responses in children and young adults demonstrated that long-term immunity was remarkably stable – declining by just 20% (or 0% in some instances) over a 5-year span of time that was devoid of natural polio outbreaks (Bottiger, 1987). This proof of durable immunity, together with the rapid success in eradicating polio from Sweden following the introduction of IPV (Bottiger et al., 1998), demonstrate the remarkable efficacy of this inactivated vaccine approach.
Similar to IPV, long-term immunity has been observed following administration of an inactivated hepatitis A vaccine in children (Hammitt et al., 2008). In this study, children were given 3 doses of the hepatitis A vaccine, Havrix®, before 12 months of age and antibody titers were followed for up to 10 years. As with IPV (Bottiger, 1990), an initial drop in antibody titers during the first few years is then followed by a much slower decline. Although decay rates were not calculated, the authors estimated that protective levels of antibodies would be maintained for at least 20-30 years. In contrast, follow-up analysis on a live attenuated hepatitis A vaccine employed in China showed that 28% of children that received a single vaccination showed no detectable anti-hepatitis A virus antibodies at 8 years post-immunization (Wang et al., 2007). In a side-by-side comparison, seroconversion rates and the magnitude of antibody titers following vaccination with Zhepu® (live, attenuated vaccine) or Havrix® (inactivated whole virus vaccine) were not significantly different after primary vaccination (Wang et al., 2004). Following booster vaccination (note: this is a requirement for both live and inactivated vaccines), the inactivated vaccine induced geometric mean titers (GMT) that were 2-fold higher than the boosted antibody responses elicited by the live attenuated vaccine. Remarkably, at 2 years post-vaccination, the antibody response induced by the inactivated vaccine remained about 3-fold higher than that observed following live vaccination (GMT: 655 mIU/mL vs. 218 mIU/mL, respectively). In this particular case, the inactivated vaccine induced substantially better long-term immunity than its live, attenuated counterpart. Likewise, a study in 177 children using a different inactivated whole virus hepatitis A vaccine (Avaxim®) showed that antiviral antibody responses initially declined following booster vaccination, but then antibody titers remained stable for the following two years with no further measurable decay (Dagan et al., 2005). Finally, a study involving 451 adults demonstrated that a single vaccination with inactivated hepatitis A vaccine (Vaqta® or Avaxim®) induced antiviral antibody responses of >10 mIU/mL in 97% of subjects within one month after immunization. Moreover, antiviral antibodies were still detected in 93% of the vaccine recipients for up to 2 years, indicating that a single dose of an inactivated vaccine can be effective at inducing prolonged immune responses (Orr et al., 2006).
6. Conclusions
Re-examination of “Experiments of Nature” involving patients with genetic immunodeficiencies has shed new light on the role of antibody and T cells in protecting against viral infections. Although there are cases in which T cell responses are able to compensate for the lack of humoral immunity, many patients with agammaglobulinemia remain highly susceptible to a wide range of viral infections. Clinical outcome has been improved by repeated administration of high-dose gamma globulin and this has reduced the morbidity and mortality of viral infections in these immunocompromised individuals. When feasible, passive immunotherapy is a useful approach to preventing viral infection and in some cases may be used therapeutically to reduce disease progression and in some cases even prevent lethal infection. Based on these studies, it appears clear that effective vaccines must include the induction of appropriate humoral immune responses – as dramatically illustrated by the failed STEP trial of a T cell-mediated HIV vaccine. Live attenuated vaccines are often effective at inducing protective immunity, but similar to inactivated vaccines, they typically require booster vaccination in order to elicit dependable and durable immunity. This suggests a critical re-evaluation of the vaccine dogma stating that lifelong immunity is attained by a single dose of a live vaccine. If live vaccines have serious adverse events, they will eventually be replaced by safer, more highly attenuated vaccines or by inactivated/non-replicating/subunit vaccines. More work is needed to develop better forms of inactivated vaccines since current approaches, such as formaldehyde fixation, may reduce vaccine efficacy or, as in the case of RSV (Delgado et al., 2009), potentially exacerbate disease rather than prevent it. The best approach to future vaccine design will be to develop immunogenic vaccine formulations that induce robust T cell responses in addition to high quality antibody responses since these are likely to provide a synergistic improvement in protective immunity.
Acknowledgments
This work was supported by NIH grants, R43 AI079898 (I.J.A., M.K.S.) R56 AI076506 (M.K.S.), U54 AI081680 (co-investigator, M.K.S.), UO1 AI082196 (M.K.S.) and Oregon National Primate Research Center grant, RR000163 (M.K.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
OHSU, Dr. Slifka, and Dr. Amanna have a financial interest in Najít Technologies, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU and the Integrity Program Oversight Council.
References
- Alexander LN, Seward JF, Santibanez TA, Pallansch MA, Kew OM, Prevots DR, Strebel PM, Cono J, Wharton M, Orenstein WA, Sutter RW. Vaccine policy changes and epidemiology of poliomyelitis in the United States. Jama. 2004;292:1696–701. doi: 10.1001/jama.292.14.1696. [DOI] [PubMed] [Google Scholar]
- Amanna I, Slifka MK. Public fear of vaccination: separating fact from fiction. Viral Immunol. 2005;18:307–15. doi: 10.1089/vim.2005.18.307. [DOI] [PubMed] [Google Scholar]
- Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens. N Engl J Med. 2007;357:1903–15. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- American Academy of Pediatrics. Revised indications for the use of palivizumab and respiratory syncytial virus immune globulin intravenous for the prevention of respiratory syncytial virus infections. Pediatrics. 2003;112:1442–1446. [PubMed] [Google Scholar]
- Annunziato D, Kaplan MH, Hall WW, Ichinose H, Lin JH, Balsam D, Paladino VS. Atypical measles syndrome: pathologic and serologic findings. Pediatrics. 1982;70:203–9. [PubMed] [Google Scholar]
- Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–6. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- Barrett AD, Monath TP, Barban V, Niedrig M, Teuwen DE. 17D yellow fever vaccines: new insights. A report of a workshop held during the World Congress on medicine and health in the tropics, Marseille, France, Monday 12 September 2005. Vaccine. 2007;25:2758–65. doi: 10.1016/j.vaccine.2006.12.015. [DOI] [PubMed] [Google Scholar]
- Belyakov IM, Earl P, Dzutsev A, Kuznetsov VA, Lemon M, Wyatt LS, Snyder JT, Ahlers JD, Franchini G, Moss B, Berzofsky JA. Shared modes of protection against poxvirus infection by attenuated and conventional smallpox vaccine viruses. Proc Natl Acad Sci U S A. 2003;100:9458–63. doi: 10.1073/pnas.1233578100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortin MM, Rimm AA. Severe combined immunodeficiency disease. Characterization of the disease and results of transplantation. Jama. 1977;238:591–600. [PubMed] [Google Scholar]
- Bottiger M. A study of the sero-immunity that has protected the Swedish population against poliomyelitis for 25 years. Scand J Infect Dis. 1987;19:595–601. doi: 10.3109/00365548709117192. [DOI] [PubMed] [Google Scholar]
- Bottiger M. Polio immunity to killed vaccine: an 18-year follow-up. Vaccine. 1990;8:443–5. doi: 10.1016/0264-410x(90)90244-g. [DOI] [PubMed] [Google Scholar]
- Bottiger M, Gustavsson O, Svensson A. Immunity to tetanus, diphtheria and poliomyelitis in the adult population of Sweden in 1991. Int J Epidemiol. 1998;27:916–25. doi: 10.1093/ije/27.5.916. [DOI] [PubMed] [Google Scholar]
- Bottiger M, Zetterberg B, Salenstedt CR. Seroimmunity to poliomyelitis in Sweden after the use of inactivated poliovirus vaccine for 10 years. Bull World Health Organ. 1972;46:141–9. [PMC free article] [PubMed] [Google Scholar]
- Boulter EA, Zwartouw HT, Titmuss DH, Maber HB. The nature of the immune state produced by inactivated vaccinia virus in rabbits. Am J Epidemiol. 1971;94:612–20. doi: 10.1093/oxfordjournals.aje.a121360. [DOI] [PubMed] [Google Scholar]
- Brown F. Review of accidents caused by incomplete inactivation of viruses. Dev Biol Stand. 1993;81:103–7. [PubMed] [Google Scholar]
- Bruton OC. Agammaglobulinemia. Pediatrics. 1952;9:722–8. [PubMed] [Google Scholar]
- Buchbinder SP, Mehrotra DV, Duerr A, Fitzgerald DW, Mogg R, Li D, Gilbert PB, Lama JR, Marmor M, Del Rio C, McElrath MJ, Casimiro DR, Gottesdiener KM, Chodakewitz JA, Corey L, Robertson MN. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley RH, Schiff RI, Schiff SE, Markert ML, Williams LW, Harville TO, Roberts JL, Puck JM. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J Pediatr. 1997;130:378–87. doi: 10.1016/s0022-3476(97)70199-9. [DOI] [PubMed] [Google Scholar]
- Casadevall A. Antibody-based therapies for emerging infectious diseases. Emerg Infect Dis. 1996;2:200–8. doi: 10.3201/eid0203.960306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadevall A. Passive antibody therapies: progress and continuing challenges. Clin Immunol. 1999;93:5–15. doi: 10.1006/clim.1999.4768. [DOI] [PubMed] [Google Scholar]
- Casadevall A, Scharff MD. Return to the past: the case for antibody-based therapies in infectious diseases. Clin Infect Dis. 1995;21:150–61. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Epidemiology and prevention of vaccine-preventable diseases course textbook. National Immunization Program; Atlanta, GA: 2005a. [Google Scholar]
- CDC. Health Information for International Travel 2005-2006. US Department of Health and Human Services, Public Health Service; Atlanta: 2005b. [Google Scholar]
- CDC. Update on vaccine-derived polioviruses--worldwide, January 2006-August 2007. MMWR Morb Mortal Wkly Rep. 2007;56:996–1001. [PubMed] [Google Scholar]
- CDC. MMWR. Vol. 57 2009. Recommended immunization schedules for persons aged 0 through 18–United States, 2009. [Google Scholar]
- Chaves SS, Gargiullo P, Zhang JX, Civen R, Guris D, Mascola L, Seward JF. Loss of vaccine-induced immunity to varicella over time. N Engl J Med. 2007;356:1121–9. doi: 10.1056/NEJMoa064040. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Chakraborty J, Khan MR, Stanton BF, Kay BA, Khan MU, Yunus M, Atkinson W, et al. Field trial of oral cholera vaccines in Bangladesh. Lancet. 1986;2:124–7. doi: 10.1016/s0140-6736(86)91944-6. [DOI] [PubMed] [Google Scholar]
- Clemens JD, Sack DA, Harris JR, Van Loon F, Chakraborty J, Ahmed F, Rao MR, Khan MR, Yunus M, Huda N, et al. Field trial of oral cholera vaccines in Bangladesh: results from three-year follow-up. Lancet. 1990;335:270–3. doi: 10.1016/0140-6736(90)90080-o. [DOI] [PubMed] [Google Scholar]
- Co MD, Terajima M, Cruz J, Ennis FA, Rothman AL. Human cytotoxic T lymphocyte responses to live attenuated 17D yellow fever vaccine: identification of HLA-B35-restricted CTL epitopes on nonstructural proteins NS1, NS2b, NS3, and the structural protein E. Virology. 2002;293:151–63. doi: 10.1006/viro.2001.1255. [DOI] [PubMed] [Google Scholar]
- Couzi G, Kircher JP. Immunotherapie de la Variole. Bulletin de l'Institut d'hygiene du Maroc. 1941;1:59–68. [Google Scholar]
- Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting Edge: Long-Term B Cell Memory in Humans after Smallpox Vaccination. J Immunol. 2003;171:4969–4973. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- Cutts FT. Measles, The Immunological Basis for Immunization Series. Module 7, World Health Organization Global Programme for Vaccines and Immunization; Geneva, Switzerland: 1993. [Google Scholar]
- Cutts FT. Measles, The Immunological Basis for Immunization Series. Module 7, World Health Organization Global programme for Vaccines and Immunization; Geneva, Switzerland: 1996. p. 20. [Google Scholar]
- Dagan R, Greenberg D, Weber F. Immunogenicity of an inactivated hepatitis A pediatric vaccine: three-year post-booster follow-up. Vaccine. 2005;23:5144–8. doi: 10.1016/j.vaccine.2005.06.017. [DOI] [PubMed] [Google Scholar]
- De Swart RL, Kuiken T, Timmerman HH, van Amerongen G, Van Den Hoogen BG, Vos HW, Neijens HJ, Andeweg AC, Osterhaus AD. Immunization of macaques with formalin-inactivated respiratory syncytial virus (RSV) induces interleukin-13-associated hypersensitivity to subsequent RSV infection. J Virol. 2002;76:11561–9. doi: 10.1128/JVI.76.22.11561-11569.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doblas A, Domingo C, Bae HG, Bohorquez CL, de Ory F, Niedrig M, Mora D, Carrasco FJ, Tenorio A. Yellow fever vaccine-associated viscerotropic disease and death in Spain. J Clin Virol. 2006;36:156–8. doi: 10.1016/j.jcv.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Dubois ME, Yoshihara P, Slifka MK. Antibody-Mediated Protection against Respiratory Viral Infection. Semin Respir Crit Care Med. 2005;26:635–42. doi: 10.1055/s-2005-925527. [DOI] [PubMed] [Google Scholar]
- Edghill-Smith Y, Golding H, Manischewitz J, King LR, Scott D, Bray M, Nalca A, Hooper JW, Whitehouse CA, Schmitz JE, Reimann KA, Franchini G. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–7. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- Eibl MM. History of immunoglobulin replacement. Immunol Allergy Clin North Am. 2008;28:737–64. viii. doi: 10.1016/j.iac.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Endo A, Izumi H, Miyashita M, Taniguchi K, Okubo O, Harada K. Current efficacy of postexposure prophylaxis against measles with immunoglobulin. J Pediatr. 2001;138:926–8. doi: 10.1067/mpd.2001.113710. [DOI] [PubMed] [Google Scholar]
- Enria DA, Barrera Oro JG. Junin virus vaccines. Curr Top Microbiol Immunol. 2002;263:239–61. doi: 10.1007/978-3-642-56055-2_12. [DOI] [PubMed] [Google Scholar]
- Enria DA, Briggiler AM, Sanchez Z. Treatment of Argentine hemorrhagic fever. Antiviral Res. 2008;78:132–9. doi: 10.1016/j.antiviral.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. Smallpox and its eradication, The pathogenesis, immunology, and pathology of smallpox and vaccinia. World Health Organization; Geneva: 1988. p. 1469. [Google Scholar]
- Fischer A. Severe combined immunodeficiencies (SCID) Clin Exp Immunol. 2000;122:143–9. doi: 10.1046/j.1365-2249.2000.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerasimon G, Lowry K. Rare case of fatal yellow fever vaccine-associated viscerotropic disease. South Med J. 2005;98:653–6. doi: 10.1097/01.SMJ.0000157537.11806.DC. [DOI] [PubMed] [Google Scholar]
- Giurca A, Topciu VL, Voiculescu D, Moldovan E, Plavosin L. Investigations on allergic and serological reactions following inoculation of inactivated smallpox vaccines by cutaneous scarification. Virologie. 1976;27:173–7. [PubMed] [Google Scholar]
- Good RA, Zak SJ. Disturbances in gamma globulin synthesis as experiments of nature. Pediatrics. 1956;18:109–49. [PubMed] [Google Scholar]
- Gordon JE, Hughes TP. A study of inactivated yellow fever virus as an immunizing agent. J Immunol. 1935;30:221–234. [Google Scholar]
- Haigwood NL, Montefiori DC, Sutton WF, McClure J, Watson AJ, Voss G, Hirsch VM, Richardson BA, Letvin NL, Hu SL, Johnson PR. Passive immunotherapy in simian immunodeficiency virus-infected macaques accelerates the development of neutralizing antibodies. J Virol. 2004;78:5983–95. doi: 10.1128/JVI.78.11.5983-5995.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund E, Lewis MW, Hansen SG, Strelow LI, Nelson JA, Sexton GJ, Hanifin JM, Slifka MK. Duration of antiviral immunity after smallpox vaccination. Nature Medicine. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- Hammitt LL, Bulkow L, Hennessy TW, Zanis C, Snowball M, Williams JL, Bell BP, McMahon BJ. Persistence of antibody to hepatitis A virus 10 years after vaccination among children and adults. J Infect Dis. 2008;198:1776–82. doi: 10.1086/593335. [DOI] [PubMed] [Google Scholar]
- Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, Legasse AW, Axthelm MK, Oswald K, Trubey CM, Piatak M, Jr, Lifson JD, Nelson JA, Jarvis MA, Picker LJ. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison LH, Halsey NA, McKee KT, Jr, Peters CJ, Barrera Oro JG, Briggiler AM, Feuillade MR, Maiztegui JI. Clinical case definitions for Argentine hemorrhagic fever. Clin Infect Dis. 1999;28:1091–4. doi: 10.1086/514749. [DOI] [PubMed] [Google Scholar]
- Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–4. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle E. A Yellow Fever Vaccine. Br Med J. 1928;1:976–977. doi: 10.1136/bmj.1.3518.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh M, Okuno Y, Hotta H. Comparative analysis of titers of antibody against measles virus in sera of vaccinated and naturally infected Japanese individuals of different age groups. J Clin Microbiol. 2002;40:1733–8. doi: 10.1128/JCM.40.5.1733-1738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janeway C. Use of Concentrated Human Serum g-Globulin in the Prevention and Attenuation of Measles. Bull NY Acad Med. 1945;21:202–222. [PMC free article] [PubMed] [Google Scholar]
- Jenner E. An inquiry into the causes and effects of the variolae vaccinae. Sampson Low; London: 1798. [Google Scholar]
- Jenner E. Further observations on the variolae vaccinae. Sampson Low; London: 1799. [Google Scholar]
- Jenner E. A continuation of facts and observations relative to the variolae vaccinae, or cowpox. Sampson Low; London: 1800. [Google Scholar]
- Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Reed Clark K. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med. 2009;15:901–6. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan C. A non-infectious smallpox vaccine. Lancet. 1962;2:1027–8. doi: 10.1016/s0140-6736(62)92708-3. [DOI] [PubMed] [Google Scholar]
- Kaplan C, Mc CD, Vallet L. A note on the immunogenicity of ultra-violet irradiated vaccinia virus in man. J Hyg (Lond) 1962;60:79–83. doi: 10.1017/s0022172400039322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller MA, Stiehm ER. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev. 2000;13:602–14. doi: 10.1128/cmr.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempe CH, Bowles C, Meiklejohn G, Berge TO, St Vincent L, Babu BVS, Govindarajan S, Ratnakannan NR, Downie AW, Murthy VR. The use of vaccinia hyperimmune gammaglobulin in the prophylaxis of smallpox. Bull WHO. 1961;25:41–48. [PMC free article] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–34. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- Kitchener S. Viscerotropic and neurotropic disease following vaccination with the 17D yellow fever vaccine, ARILVAX. Vaccine. 2004;22:2103–5. doi: 10.1016/j.vaccine.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Wallinga J, Teunis P, Xing S, Mikolajczyk R. Frequency of Adverse Events after Vaccination with Different Vaccinia Strains. PLoS Med. 2006;3 doi: 10.1371/journal.pmed.0030272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugman S, Giles JP, Friedman H, Stone S. Studies on Immunity to Measles. J Pediatr. 1965;66:471–88. doi: 10.1016/s0022-3476(65)80112-3. [DOI] [PubMed] [Google Scholar]
- Laassri M, Lottenbach K, Belshe R, Wolff M, Rennels M, Plotkin S, Chumakov K. Effect of different vaccination schedules on excretion of oral poliovirus vaccine strains. J Infect Dis. 2005;192:2092–8. doi: 10.1086/498172. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–539. doi: 10.1038/314537a0. [DOI] [PubMed] [Google Scholar]
- Lederman HM, Winkelstein JA. X-linked agammaglobulinemia: an analysis of 96 patients. Medicine (Baltimore) 1985;64:145–56. [PubMed] [Google Scholar]
- Lindsey NP, Schroeder BA, Miller ER, Braun MM, Hinckley AF, Marano N, Slade BA, Barnett ED, Brunette GW, Horan K, Staples JE, Kozarsky PE, Hayes EB. Adverse event reports following yellow fever vaccination. Vaccine. 2008;26:6077–82. doi: 10.1016/j.vaccine.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Lu S. Human versus HIV: round 2 defeat in AIDS vaccine development. Expert Rev Vaccines. 2008;7:151–3. doi: 10.1586/14760584.7.2.151. [DOI] [PubMed] [Google Scholar]
- Manmohan S. Vaccine adjuvants and delivery systems. Hoboken, N.J: 2007. [Google Scholar]
- Marennikova SS. The use of hyperimmune antivaccinia gamma-globulin for the prevention and treatment of smallpox. Bull World Health Organ. 1962;27:325–30. [PMC free article] [PubMed] [Google Scholar]
- Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–10. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- McElrath MJ, De Rosa SC, Moodie Z, Dubey S, Kierstead L, Janes H, Defawe OD, Carter DK, Hural J, Akondy R, Buchbinder SP, Robertson MN, Mehrotra DV, Self SG, Corey L, Shiver JW, Casimiro DR. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum ML. The historical feud over polio vaccine: how could a killed vaccine contain a natural disease? West J Med. 1999;171:271–3. [PMC free article] [PubMed] [Google Scholar]
- Melnick JL, Benyesh-Melnick M, Pena R, Yow M. Effectiveness of Salk vaccine. Analysis of virologically confirmed cases of paralytic and nonparalytic poliomyelitis. Jama. 1961;175:1159–62. doi: 10.1001/jama.1961.03040130043010. [DOI] [PubMed] [Google Scholar]
- Moghaddam A, Olszewska W, Wang B, Tregoning JS, Helson R, Sattentau QJ, Openshaw PJ. A potential molecular mechanism for hypersensitivity caused by formalin-inactivated vaccines. Nat Med. 2006;12:905–7. doi: 10.1038/nm1456. [DOI] [PubMed] [Google Scholar]
- Monath TP. In: Yellow fever vaccine. Plotkin S, editor. Vaccines, W.B. Saunders; Philadelphia: 2004. pp. 1095–1176. [Google Scholar]
- Monath TP, Cetron MS, Teuwen DE. In: Yellow fever vaccine. Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, Saunders/Elsevier; 2008. p. 1725. [Google Scholar]
- Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, Guirakhoo F. Clinical proof of principle for ChimeriVax: recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–18. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- Moore JP, Klasse PJ, Dolan MJ, Ahuja SK. AIDS/HIV. A STEP into darkness or light? Science. 2008;320:753–5. doi: 10.1126/science.1154258. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26:1595–7. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka RJ, Buckler-White A, Shibata R, Martin MA. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76:2123–30. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr N, Klement E, Gillis D, Sela T, Kayouf R, Derazne E, Grotto I, Balicer R, Huerta M, Aviram L, Ambar R, Epstein Y, Heled Y, Cohen D. Long-term immunity in young adults after a single dose of inactivated Hepatitis A vaccines. Vaccine. 2006;24:4328–32. doi: 10.1016/j.vaccine.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Paavonen J, Jenkins D, Bosch FX, Naud P, Salmeron J, Wheeler CM, Chow SN, Apter DL, Kitchener HC, Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA, Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P, Struyf F, Wieting SL, Lehtinen MO, Dubin G. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369:2161–70. doi: 10.1016/S0140-6736(07)60946-5. [DOI] [PubMed] [Google Scholar]
- Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–7. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit A. Rapport sur la valeur immunisante des vaccins employes contre le fievre jaune et la valeur therapeutique du serum antiamaril. Bull Acad Med Paris. 1931;105:522–526. [Google Scholar]
- Plotkin SA. Vaccines: correlates of vaccine-induced immunity. Clin Infect Dis. 2008;47:401–9. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Priddy FH, Brown D, Kublin J, Monahan K, Wright DP, Lalezari J, Santiago S, Marmor M, Lally M, Novak RM, Brown SJ, Kulkarni P, Dubey SA, Kierstead LS, Casimiro DR, Mogg R, DiNubile MJ, Shiver JW, Leavitt RY, Robertson MN, Mehrotra DV, Quirk E. Safety and immunogenicity of a replication-incompetent adenovirus type 5 HIV-1 clade B gag/pol/nef vaccine in healthy adults. Clin Infect Dis. 2008;46:1769–81. doi: 10.1086/587993. [DOI] [PubMed] [Google Scholar]
- Pugachev KV, Guirakhoo F, Monath TP. New developments in flavivirus vaccines with special attention to yellow fever. Curr Opin Infect Dis. 2005;18:387–94. doi: 10.1097/01.qco.0000178823.28585.ad. [DOI] [PubMed] [Google Scholar]
- Putz MM, Alberini I, Midgley CM, Manini I, Montomoli E, Smith GL. Prevalence of antibodies to Vaccinia virus after smallpox vaccination in Italy. J Gen Virol. 2005;86:2955–60. doi: 10.1099/vir.0.81265-0. [DOI] [PubMed] [Google Scholar]
- Richie EE, Punjabi NH, Sidharta YY, Peetosutan KK, Sukandar MM, Wasserman SS, Lesmana MM, Wangsasaputra FF, Pandam SS, Levine MM, O'Hanley PP, Cryz SJ, Simanjuntak CH. Efficacy trial of single-dose live oral cholera vaccine CVD 103-HgR in North Jakarta, Indonesia, a cholera-endemic area. Vaccine. 2000;18:2399–410. doi: 10.1016/s0264-410x(00)00006-2. [DOI] [PubMed] [Google Scholar]
- Robertson S. Poliomyelitis, The Immunological Basis for Immunization Series. Module 6, World Health Organization Global Programme for Vaccines and Immunization; Geneva, Switzerland: 1993. p. 24. [Google Scholar]
- Rogers LJ, Eva LJ, Luesley DM. Vaccines against cervical cancer. Curr Opin Oncol. 2008;20:570–4. doi: 10.1097/CCO.0b013e328303e2a1. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Burton DR. Role of antibodies in controlling viral disease: lessons from experiments of nature and gene knockouts. J Virol. 2000;74:9813–7. doi: 10.1128/jvi.74.21.9813-9817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer LA. Antibodies for the prevention and treatment of viral diseases. Antiviral Res. 2000;47:57–77. doi: 10.1016/s0166-3542(00)00111-x. [DOI] [PubMed] [Google Scholar]
- Sawyer WA. Persistence of yellow fever immunity. J Prev Med. 1931;5:413–428. [Google Scholar]
- Schlesinger JJ, Brandriss MW, Cropp CB, Monath TP. Protection against yellow fever in monkeys by immunization with yellow fever virus nonstructural protein NS1. Journal of Virology. 1986;60:1153–5. doi: 10.1128/jvi.60.3.1153-1155.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekaly RP. The failed HIV Merck vaccine study: a step back or a launching point for future vaccine development? J Exp Med. 2008;205:7–12. doi: 10.1084/jem.20072681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sencer D, Langmuir A, Kokko U. Fatal viral encephalitis following 17D yellow fever vaccine inoculation. Report of a case in a 3-year-old child. Jama. 1966;198:671–2. doi: 10.1001/jama.1966.03110190153047. [DOI] [PubMed] [Google Scholar]
- Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980-1996. Jama. 1999;282:1440–6. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- Siegrist CA. In: Vaccine Immunology. Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, Saunders/Elsevier; 2008. p. 1725. [Google Scholar]
- Skerrett SJ. Antibody treatment of lower respiratory tract infections. Semin Respir Infect. 2001;16:67–75. doi: 10.1053/srin.2001.22730. [DOI] [PubMed] [Google Scholar]
- Skull S, Kemp A. Treatment of hypogammaglobulinaemia with intravenous immunoglobulin, 1973-93. Arch Dis Child. 1996;74:527–30. doi: 10.1136/adc.74.6.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK. Immunological memory to viral infection. Curr Opin Immunol. 2004;16:443–50. doi: 10.1016/j.coi.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Slifka MK, Ahmed R. Long-term humoral immunity against viruses: revisiting the issue of plasma cell longevity. Trends Microbiol. 1996;4:394–400. doi: 10.1016/0966-842X(96)10059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slifka MK, Hanifin JM. Dermatol Clin. Vol. 22. 2004. Smallpox: The Basics; pp. 263–74.pp. vi [DOI] [PubMed] [Google Scholar]
- Steinbrook R. One step forward, two steps back--will there ever be an AIDS vaccine? N Engl J Med. 2007;357:2653–5. doi: 10.1056/NEJMp0708117. [DOI] [PubMed] [Google Scholar]
- Stephan JL, Vlekova V, Le Deist F, Blanche S, Donadieu J, De Saint-Basile G, Durandy A, Griscelli C, Fischer A. Severe combined immunodeficiency: a retrospective single-center study of clinical presentation and outcome in 117 patients. J Pediatr. 1993;123:564–72. doi: 10.1016/s0022-3476(05)80951-5. [DOI] [PubMed] [Google Scholar]
- Stern PL, Brown M, Stacey SN, Kitchener HC, Hampson I, Abdel-Hady ES, Moore JV. Natural HPV immunity and vaccination strategies. J Clin Virol. 2000;19:57–66. doi: 10.1016/s1386-6532(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Struchiner CJ, Luz PM, Dourado I, Sato HK, Aguiar SG, Ribeiro JG, Soares RC, Codeco CT. Risk of fatal adverse events associated with 17DD yellow fever vaccine. Epidemiol Infect. 2004;132:939–46. doi: 10.1017/s0950268804002602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter RW, Kew OM, Cochi SL. In: Poliovirus vaccine-live. Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines, Saunders/Elsevier; 2008. p. 1725. [Google Scholar]
- The FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- Turner GS, Squires EJ, Murray HG. Inactivated smallpox vaccine. A comparison of inactivation methods. J Hyg (Lond) 1970;68:197–210. doi: 10.1017/s0022172400028679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkonen P, Hovi T, von Bonsdorff CH, Saikku P, Penttinen K. Age-specific prevalence of complement-fixing antibodies to sixteen viral antigens: A computer analysis of 58,500 patients covering a period of eight years. J Med Virol. 1984;13:131–148. doi: 10.1002/jmv.1890130204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos PF, Luna EJ, Galler R, Silva LJ, Coimbra TL, Barros VL, Monath TP, Rodigues SG, Laval C, Costa ZG, Vilela MF, Santos CL, Papaiordanou PM, Alves VA, Andrade LD, Sato HK, Rosa ES, Froguas GB, Lacava E, Almeida LM, Cruz AC, Rocco IM, Santos RT, Oliva OF. Serious adverse events associated with yellow fever 17DD vaccine in Brazil: a report of two cases. Lancet. 2001;358:91–7. doi: 10.1016/s0140-6736(01)05326-0. [DOI] [PubMed] [Google Scholar]
- Wang XY, Xu Z, Yao X, Tian M, Zhou L, He L, Wen Y. Immune responses of anti-HAV in children vaccinated with live attenuated and inactivated hepatitis A vaccines. Vaccine. 2004;22:1941–5. doi: 10.1016/j.vaccine.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Wang XY, Xu ZY, Ma JC, von Seidlein L, Zhang Y, Hao ZY, Han OP, Zhang YL, Tian MY, Ouyang PY, Zhang ZY, Han CQ, Xing ZC, Chen JC. Long-term immunogenicity after single and booster dose of a live attenuated hepatitis A vaccine: results from 8-year follow-up. Vaccine. 2007;25:446–9. doi: 10.1016/j.vaccine.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Waris ME, Tsou C, Erdman DD, Zaki SR, Anderson LJ. Respiratory synctial virus infection in BALB/c mice previously immunized with formalin-inactivated virus induces enhanced pulmonary inflammatory response with a predominant Th2-like cytokine pattern. J Virol. 1996;70:2852–60. doi: 10.1128/jvi.70.5.2852-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–21. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesselhoeft C. Treatment of scarlet fever and diphtheria. Med Clin North Am. 1936;19:1389–1407. [Google Scholar]
- Whitton JL, Slifka MK, Liu F, Nussbaum AK, Whitmire JK. The regulation and maturation of antiviral immune responses. Adv Virus Res. 2004;63:181–238. doi: 10.1016/S0065-3527(04)63003-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin L, Cone RA, Moench TR, Whaley KJ. Preventing infectious disease with passive immunization. Microbes Infect. 2000;2:701–8. doi: 10.1016/s1286-4579(00)00355-5. [DOI] [PubMed] [Google Scholar]