Abstract
Small (SK) and large conductance (BK) Ca2+-activated K+ channels contribute to action potential repolarization, shape dendritic Ca2+spikes and postsynaptic responses, modulate the release of hormones and neurotransmitters, and contribute to hippocampal-dependent synaptic plasticity. Over the last decade, SK and BK channels have emerged as important targets for the development of acute ethanol tolerance and for altering neuronal excitability following chronic ethanol consumption. In this mini-review, we discuss new evidence implicating SK and BK channels in ethanol tolerance and ethanol-associated homeostatic plasticity. Findings from recent reports demonstrate that chronic ethanol produces a reduction in the function of SK channels in VTA dopaminergic and CA1 pyramidal neurons. It is hypothesized that the reduction in SK channel function increases the propensity for burst firing in VTA neurons and increases the likelihood for aberrant hyperexcitability during ethanol withdrawal in hippocampus. There is also increasing evidence supporting the idea that ethanol sensitivity of native BK channel results from differences in BK subunit composition, the proteolipid microenvironment, and molecular determinants of the channel-forming subunit itself. Moreover, these molecular entities play a substantial role in controlling the temporal component of ethanol-associated neuroadaptations in BK channels. Taken together, these studies suggest that SK and BK channels contribute to ethanol tolerance and adaptive plasticity.
Keywords: Ca2+-Activated K+ Channels, Ethanol Tolerance and Dependence, Adaptive Plasticity, Withdrawal Hyperexcitability
Alcohol (ethanol) addiction is a complex pathology characterized by the development of tolerance and physical dependence and compulsive ethanol-seeking behavior that often manifests as a chronic relapsing syndrome. The development of tolerance, which can be observed in humans as recovery of motor function during a single drinking session, in spite of maintained ethanol levels is believed to predict the likelihood of individuals to develop addiction to ethanol (Heath et al., 1999; Schuckit, 1985). The behavioral manifestations of chronic ethanol consumption likely reflect neuroadaptive changes that have a molecular, cellular, and structural basis. Thus, understanding the neuroadaptations that sustain ethanol tolerance and dependence is of fundamental significance.
Evidence suggests that addiction to ethanol and other drugs involves changes in systems implicated in reward-related associative learning processes. Moreover, studies have demonstrated that plasticity of glutamatergic neurotransmission contributes to the development and maintenance of drug addiction (Tzschentke and Schmidt, 2003), and these aberrant neuroadaptations within the glutamatergic systems play an important role in ethanol addiction (Chandler, 2003; Mulholland and Chandler, 2007). For more than two decades, a number of ligand-gated ion channels and metabotropic receptors have been extensively examined for their putative role in mediating the effects of acute and chronic ethanol on tolerance and adaptive plasticity (Vengeliene et al., 2008). In contrast, few Ca2+-activated and voltage-gated ion channels have received similar attention.
CA2+-ACTIVATED K+ CHANNELS AND NEURONAL EXCITABILITY
In CNS neurons, Ca2+-activated K+ channels modulate neuronal activity by controlling intrinsic excitability, tonic firing frequency, spike frequency adaptations, and action potential repolarization. Ca2+-activated K+ channels are activated by elevations in intracellular Ca2+that occurs during single or trains of action potentials, and their activation is thought to contribute to the prolonged after hyperpolarization (AHP) that follows an action potential. Three types of Ca2+-activated K+ currents [small (SK), intermediate (IK), and large conductance (BK or MaxiK)] have been identified that demonstrate heterogeneous pharmacological and kinetic properties. In mammalian brain, three genes (KCNN1, KCNN2, KCNN3) encode SK channel subunits (SK1, SK2, SK3 and their multiple splice variants—20 for SK1, and three each for SK2 and SK3) which show different patterns of regional expression. SK1 and SK2 channels are highly expressed in hippocampus and cortex, whereas SK3 channels are predominantly detected in thalamus, hypothalamus, ventral tegmental area (VTA), and NAc (Kohler et al., 1996; Sailer et al., 2002; Stocker and Pedarzani, 2000; Stocker et al., 1999). SK channels are solely activated by transient elevations of intracellular Ca2+and have single channel conductances of ~10 pS (Bond et al., 2005; Faber and Sah, 2007). These channels do not possess an obvious Ca2+-binding domain, but instead form functional heteromeric complexes with calmodulin (Allen et al., 2007; Lee et al., 2003; Maylie et al., 2004). Calmodulin constitutively binds to the C-terminal of each of the four SK channel α-subunits and acts as a high-affinity Ca2+sensor with submicromolar affinity. SK channels underlie the medium phase of the AHP and are sensitive to block by the bee venom toxin, apamin (Bond et al., 2005; Faber and Sah, 2007; Stocker, 2004). SK channels are important for shaping postsynaptic responses and controlling intrinsic excitability, dendritic integration, and pacemaker firing (Bond et al., 2005; Fakler and Adelman, 2008).
Large conductance channels are a product of the KCNMA1 (Slo1) gene and its orthologs (e.g., dSlo in Drosophila), and are ubiquitously expressed in the CNS. These channels can be activated by increases in intracellular Ca2+or transmembrane voltage shifts in the positive direction, and demonstrate single channel conductances of ~100 pS under physiological conditions (Latorre and Brauchi, 2006). Evidence suggests that BK channels possess at least three distinct Ca2+binding sites with micromolar affinity that function independently of each other (Latorre and Brauchi, 2006). In addition to contributing to the fast phase of the AHP, BK channels serve to shape dendritic Ca2+spikes and modulate the release of hormones and neurotransmitters (Storm, 1990). Interestingly, recent studies have demonstrated a role for both BK and SK channels in contributing to hippocampal-dependent synaptic plasticity (Lin et al., 2008; Matthews et al., 2008). These findings are relevant as Ca2+-activated K+ channels have been identified as relevant targets in the site of action of ethanol (for review, see Brodie et al., 2007). Thus, altered function of Ca2+-activated K+ channels represents a developing area that is of particular importance in understanding ethanol-associated molecular and behavioral tolerance and adaptive plasticity.
SK CHANNELS, CHRONIC ETHANOL, AND BURST FIRING IN DOPAMINERGIC NEURONS
A recent study (Hopf et al., 2007) addressed the possibility that repeated ethanol exposure and withdrawal leads to altered firing properties of VTA dopamine (DA) neurons through changes in SK channel function. Midbrain DA neurons from the VTA interact with other limbic brain structures that are targets for VTA DA release such as the NAc, amygdala, hippocampus, and prefrontal cortex. Many studies have shown that mesolimbic regions play an important role in motivated and goal-directed behaviors, including addiction to ethanol and other drugs of abuse. Acute ethanol increases VTA neuron firing rate (Brodie et al., 1990; Mereu et al., 1984), and ethanol self-administration enhances NAc DA release (Gonzales and Weiss, 1998). Ethanol self-administration may be reduced by DA receptor antagonists in the NAc (Hodge et al., 1997; Rassnick et al., 1992, 1993) or by inhibiting VTA DA cell activity (Hodge et al., 1993). Several lines of evidence also implicate the mesolimbic system in ethanol seeking and relapse (Gonzales et al., 2004; Katner and Weiss, 1999; McBride et al., 2002). In contrast, previous in vivo and in vitro observations during withdrawal following chronic ethanol generally found reduced firing rates in adult midbrain DA neurons after 1 to 3 days (Bailey et al., 1998; Diana et al., 1993, 1996; Shen, 2003; but see Brodie 1999) or 6 days of withdrawal (Bailey et al., 2001), with no changes in firing rates of active DA neurons three or more weeks after repeated ethanol exposure (Bailey et al., 2001; Shen et al., 2007), although the number of neurons firing was reduced (Shen et al., 2007). There are also mixed results regarding whether decreased VTA activity during early withdrawal reflects reduced excitability (Bailey et al., 1998, 2001; Diana et al., 1993, 1996) or hyperactivity and depolarization block (Shen, 2003; Shen et al., 2007).
Hopf and colleagues (2007) used ex vivo brain slice electrophysiology to characterize whether pacemaker firing and NMDA-induced burst firing in VTA neurons were altered after 5 days of intermittent ethanol exposure and 7 days of withdrawal, and what channels might be altered functionally to explain any observed firing differences. Firing activity of midbrain DA neurons is interesting in that these neurons present two activity patterns. Regular, pacemaker firing may be altered under a number of conditions, including drug withdrawal and schizophrenia (Grace, 2000; Weiss et al., 1992), while phasic, burst firing is associated with strong DA release in VTA terminal regions (reviewed in Overton and Clark, 1997). Burst firing is particularly interesting in that it is thought to signal the salience of a reward (Robinson and Berridge, 2001), and may also represent an error signal that facilitates learning (Schultz, 2002). Although pacemaker and burst firing in VTA neurons is regulated by a number of channels, the results led Hopf and colleagues to focus on SK and the inwardly rectifying hyperpolarization-activated cation channels (HCN, responsible for generating the Ih current) (Johnson and Seutin, 1997; Neuhoff et al., 2002; Seutin et al., 1993). A number of lines of evidence indicate that SK is a primary channel altered in VTA DA neurons by repeated ethanol and withdrawal. The magnitude of the AHP was large in control VTA DA neurons and significantly reduced in ethanol animals, and the SK-selective antagonist apamin produced a greater reduction in the AHP in control relative to ethanol animals (Hopf et al., 2007). Direct measurements of SK with voltage-clamp found reduced total SK currents in ethanol animals, with no changes in input resistance, cell capacitance, SK reversal potential, or the SK time course of decay. Thus, repeated ethanol and withdrawal leads to a significant reduction in VTA SK currents (Fig. 1B). These data are consistent with previous evidence demonstrating a reduced Ca2+-dependent AHP in the hippocampus after 3 weeks abstinence from long-term ethanol intake (Durand and Carlen, 1984).
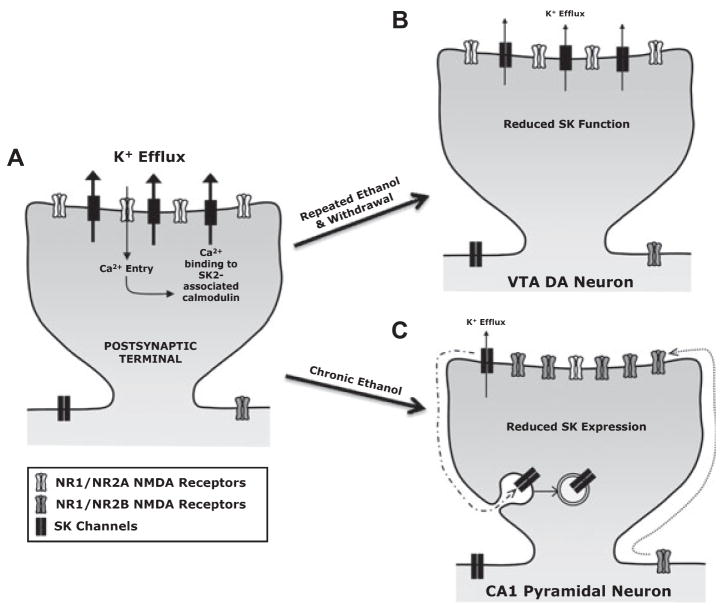
Fig. 1.
Disrupted function of SK2 channel–NMDA receptor feedback loop following chronic ethanol and withdrawal. (A) Studies have demonstrated that SK2 channels localize in the PSD of glutamatergic synapses and form a Ca2+-dependent negative feedback loop with synaptic NMDA receptors (Faber et al., 2005; Lin et al., 2008; Ngo-Anh et al., 2005). Ca2+entry through synaptic NMDA receptors is thought to bind to SK2-associated calmodulin and cause a confirmation change in calmodulin and SK2 channels that leads to K+ efflux. The increased K+ efflux hyperpolarizes the membrane potential and reduces excitability by promoting Mg2+re-block of NMDA receptors. (B) Chronic ethanol and withdrawal reduces SK channel function via an unknown mechanism in VTA DA neurons. The reduction in SK facilitates NMDA receptor-induced burst firing that may affect DA-mediated responses to ethanol or ethanol-related cues. (C) Proposed model for ethanol-induced homeostatic functional uncoupling of the synaptic SK2 channel-NMDA receptor feedback loop within hippocampal postsynaptic terminals. Chronic ethanol produces a homeostatic synaptic targeting of NR2B-containing NMDA receptors, and preliminary evidence suggests that chronic ethanol reduces surface SK2 channels. The opposing effects on SK2 channels and NMDA receptors represent a common homeostatic adaptive response to chronic ethanol exposure, and disruption of this feedback loop by ethanol likely contributes to the development of ethanol tolerance and to aberrant neuronal hyperexcitability during acute withdrawal.
A number of studies have observed that inhibition of SK facilitates the ability of NMDA receptor activation to induce midbrain DA neuron burst firing (e.g., Johnson and Seutin, 1997; Seutin et al., 1993). In agreement, depolarization of control VTA DA neurons with a range of concentrations of NMDA increased pacemaker firing rate but rarely led to burst firing, while coadministration of NMDA with the SK blocker apamin routinely produced burst firing in control neurons (Hopf et al., 2007). In strong contrast, NMDA application produced burst firing in many VTA DA neurons after repeated ethanol and withdrawal, and the level of burst firing was significantly correlated with the level of SK function in each neuron. Furthermore, NMDA-induced depolarization measured under voltage-clamp was not different in ethanol and control neurons, suggesting no changes in NMDA receptor function. Taken together, these results suggest that a major consequence of reduced SK function in animals is to facilitate NMDA-induced burst firing, while strong SK currents in control neurons prevent burst firing. It should be noted that ethanol acutely inhibits NMDA receptor function, which could also alter firing in VTA neurons. However, the animals in the study of Hopf and colleagues (2007) were examined after 7 days withdrawal from ethanol, a time when the ethanol is fully metabolized. Also, no differences in NMDA receptor currents after 7 days withdrawal were observed in this study.
Interestingly, repeated ethanol and withdrawal facilitates burst firing but does not alter VTA pacemaker firing (Hopf et al., 2007). While VTA pacemaker firing is controlled by many channels including Ca2+ and K+, studies of SK and Ih regulation of pacemaker activity have yielded mixed results (see Hopf et al., 2007, for references). One possibility is that Ca2+and some other currents responsible for the strong oscillatory drive in VTA neurons could override the contribution of Ih and SK during pacemaker firing. In this regard, preliminary action potential waveform analysis indicated (although did not definitively demonstrate) there was no changes in sodium channels or fast repolarizing K+ channels after ethanol and withdrawal. Further, voltage-clamp experiments found no differences in the early current response to a hyperpolarizing step, suggesting no differences in IRK+ leak channels. Thus, a number of basic physiological parameters were not altered in the VTA by repeated ethanol and 7 days of abstinence.
A small reduction in Ih current was also observed after repeated ethanol and abstinence, as has been reported after 1 day of withdrawal from repeated ethanol (Okamoto et al., 2006). Reduced Ih retarded the ability of ethanol neurons to recover from hyperpolarization. However, inhibition of Ih in control VTA DA neurons did not facilitate NMDA-induced bursting. These results support the suggestion that reduced SK currents rather than Ih in ethanol animals primarily contributed to the NMDA-induced bursting in ethanol neurons.
Importantly, this exposure paradigm (i.e., 2 injections of 2 g/kg/day for 5 days and withdrawal for 7 days) led to a cross-sensitization to the locomotor-activating effects of cocaine (Hopf et al., 2007), and variants of this paradigm have been observed to produce locomotor sensitization to ethanol in mice (Itzhak and Martin, 1999) and rats (Hoshaw and Lewis, 2001). It has been suggested that locomotor sensitization (also called “behavioral sensitization”) represents an increased positive value attributed to a drug or drug-associated stimulus or environment. Further, cross-sensitization is thought to indicate a common mechanism of expression for sensitization to different drugs and to stress (Robinson and Berridge, 2001). For this reason, and because locomotor sensitization can enhance subsequent drug self-administration (Vezina, 2004), sensitization has been considered a model of altered drug-related motivation (Robinson and Berridge, 2001). However, a recent study showed that ethanol-related sensitization in mice can be apparent without increased DA release, perhaps dissociating the dopaminergic system from enhanced locomotor activation by ethanol (Zapata et al., 2006). Thus, the molecular basis of the cross-sensitization in rats after repeated ethanol exposure and withdrawal remains unclear (Hopf et al., 2007).
It has been proposed that SK channels serve different roles in VTA DA and CA1 pyramidal neurons (Bond et al., 2005). SK channels clearly regulate firing patterns in DA neurons in response to synaptic input, and reduced SK channel function by chronic ethanol greatly facilitates NMDA receptor-induced burst firing, which could increase DA release in VTA terminal regions in response to ethanol, ethanol-related cues, or nonethanol situations such as exposure to cocaine or stress. In hippocampus, SK channels play a role in synaptic plasticity and are involved in a calcium-dependent negative feedback loop with synaptic NMDA receptors (Faber et al., 2005; Hammond et al., 2006; Ngo-Anh et al., 2005), suggesting that alteration of this regulatory feedback loop by chronic ethanol may have profound effects on function of glutamatergic synapses and hippocampal-dependent synaptic plasticity.
DISRUPTED SK2 CHANNEL–NMDA RECEPTOR COUPLING IN HIPPOCAMPAL SYNAPSES
Recent studies have demonstrated that chronic exposure of hippocampal neurons to ethanol results in a compensatory increase in the expression of synaptic NMDA receptors without altering extrasynaptic NMDA receptors (Carpenter-Hyland et al., 2004; Hendricson et al., 2007; Qiang et al., 2007). This homeostatic increase in NMDA receptors is associated with and dependent upon a corresponding increase in the localization of the scaffolding protein PSD-95 at the post-synaptic density (PSD), and with an actin-dependent increase in the size of dendritic spines (Carpenter-Hyland and Chandler, 2006). These observations led to the hypothesis that ethanol-induced plasticity at excitatory synapses involves increases in NR2B-containing NMDA receptors and PSD-95 at the PSD that provides an expanded scaffolding platform for the recruitment and activation of signaling molecules that regulate synaptic plasticity (Carpenter-Hyland and Chandler, 2006; Mulholland and Chandler, 2007). In addition, the compensatory increase in synaptic NMDA receptors following chronic consumption contributes to ethanol withdrawal hyperexcitability and is thought to play a role in tolerance, craving, and relapse. Thus, further characterization of ethanol-associated homeostatic plasticity within glutamatergic synapses may help to identify novel targets for medications development for the treatment of addiction to ethanol and other drugs.
Two independent studies have linked SK channel activation with Ca2+influx through synaptic NMDA receptors (Faber et al., 2005; Ngo-Anh et al., 2005). These studies demonstrate that SK channels localized within glutamatergic synapses in hippocampus and lateral amygdala function to shape postsynaptic potentials and promote Mg2+re-block of NMDA receptors. This led to the suggestion that SK channels and NMDA receptors form a regulatory Ca2+-mediated negative feedback loop within dendritic spines (Faber et al., 2005; Ngo-Anh et al., 2005) (Fig. 1), and this was supported by a recent study that used double label, postembedding immunoglod electron microscopy showing that SK2 channels and NMDA receptors exist in a nanodomain within the same PSD (Lin et al., 2008). Emerging evidence suggests that SK2 channels play a role in hippocampal synaptic plasticity, and that activity-dependent processes regulate the synaptic targeting of SK2 channels in hippocampal dendritic spines (Hammond et al., 2006; Lin et al., 2008). However, it is unknown if these channels are sensitive to the adaptive processes that underlie ethanol tolerance and homeostatic plasticity.
Using well-established in vitro models of chronic ethanol exposure and withdrawal (Carpenter-Hyland et al., 2004; Mulholland et al., 2008; Prendergast et al., 2004), Mulholland and colleagues examined the function and expression of SK channels following ethanol treatment. Chronic treatment of slices with 75 mM ethanol produced a significant reduction in SK2 channel expression in the plasma membrane, and consistent with the work by Hopf and colleagues (2007), the apamin-sensitive SK tail current recorded in CA1 neurons was markedly reduced in slices treated with ethanol (P.J. Mulholland and L.J. Chandler, unpublished observation). These preliminary data suggest that decreases in SK2 channels and opposing increases in synaptic NMDA receptors represent a common homeostatic adaptive response to prolonged reductions in NMDA receptor activity during ethanol exposure (Fig. 1). Moreover, opposing regulation of SK2 channels and NMDA receptors by chronic ethanol leads to a functional uncoupling of the SK2 channel-NMDA receptor Ca2+-dependent negative feedback loop in synapses that may contribute to the development of tolerance and to hyperexcitability during withdrawal.
Currently, medications for treatment of ethanol dependency have limited efficacy and small effect sizes, emphasizing the need to continue to examine mechanisms of ethanol-associated homeostatic plasticity that may identify novel treatment targets and strategies. Recent evidence suggests that positive modulators of SK channels possess potent anticonvulsant properties and reduce seizure activity across a range of in vivo seizure models (Anderson et al., 2006). In addition, overexpression of hippocampal SK2 channels results in enhanced mAHP current, altered LTP, and neuroprotection against glutamatergic insult (Hammond et al., 2006; Lee et al., 2003; Stackman et al., 2002), suggesting that SK channel positive modulators could reduce ethanol withdrawal seizure activity and neurotoxicity. Indeed, preliminary findings demonstrated that focal application of 400 μM 1-EBIO, a SK channel positive modulator, dramatically reduced ethanol withdrawal hyperexcitability in primary hippocampal neurons (P.J. Mulholland and L.J. Chandler, unpublished observation). Moreover, 1-EBIO (10 mg/kg, s.c.) administered at 4 and 8 hours into ethanol withdrawal significantly reduced handling-induced convulsion in mice undergoing withdrawal from chronic (64 hours) ethanol inhalation in vapor chambers. These observations support the suggestion that SK channel positive modulators effectively reduce ethanol withdrawal hyperexcitability both in vitro and in vivo, and may have identified a potential novel therapeutic target. Overall, the results from these studies implicate a role for SK channels in ethanol-associated plasticity in CA1 pyramidal and VTA DA neurons.
BK CHANNELS: MOLECULAR, CELLULAR, AND BEHAVIORAL MECHANISMS UNDERLYING TOLERANCE TO ETHANOL
Although the findings on SK channels are of substantial significance to understanding neuroadaptations to chronic ethanol, a number of studies have also implicated an important role for BK channels in ethanol tolerance and adaptive plasticity. The possibility that Ca2+-activated K+ conductances are relevant targets of ethanol and sedative/hypnotic drugs was first raised by Krjnevic (1972), and advanced by results documenting that clinically relevant concentrations of ethanol (20 mM) increase a Ca2+-dependent AHP in CA1 cells from rat hippocampus, this ethanol action being accompanied by increase in membrane macroscopic conductance (Carlen et al., 1982). In the mid-1990s, it was documented that acute exposure to ethanol (10 to 100 mM for a couple of minutes), indeed, can increase the steady-state activity of BK (or MaxiK) channels of BK, an action that may contribute to ethanol inhibition of neuropeptide (vasopressin and oxytocin) and growth hormone release (Dopico et al., 1996; Jakab et al., 1997). Over the past 10 years or so, BK channels have emerged as one of the most promising ethanol targets (Brodie et al., 2007; Dopico et al., 1996, 1999; Martin et al., 2004). Ethanol activation of BK channels has been reported in a wide variety of neuronal and non-neuronal preparations, and further linked to several pathophysiological processes known to be affected by ethanol exposure, such as neurotransmitter release, nociception, action potential firing in NAc neurons, motor incoordination in Caenorhabditis elegans, and endothelial proliferation (reviewed in Brodie et al., 2007).
Unlike other members of the voltage-gated, TM6 family of K+ channels, BK channels are very sensitive to low (10 to 50 mM) ethanol concentrations in a number of structures (dorsal root ganglion, terminals of magnocellular neurons from the supraoptic nucleus, and NAc somata) (Brodie et al., 2007; Dopico et al., 1996, 1999; Gruss et al., 2001; Martin et al., 2004). The ethanol sensitivity of recombinant BK channels has been mapped to channel regions that are absent in voltage-gated TM6 K+ channels other than BK (Liu et al., 2003; Liu et al., 2006, 2008) (Fig. 2A). The ethanol sensitivity of both native and recombinant BK channels is usually reflected by a concentration-dependent increase in channel open probability without noticeable modifications in ion permeation (Dopico et al., 1996, 1998; Jakab et al., 1997; Martin et al., 2004). Ethanol modulation of BK channel activity is influenced by the channel subunit composition (Dopico et al., 1999; Feinberg-Zadek and Treistman, 2007; Martin et al., 2004). Whereas ethanol increases α and αβ4 BK channel open probability, it does not alter αβ1 BK channel activity (Martin et al., 2004). Interestingly, recent studies indicate that BK channels play an important role in ethanol tolerance. Thus, in C. elegans and in Drosophila, α BK channels are critical for the expression of ethanol acute tolerance at the behavioral level (Cowmeadow et al., 2005, 2006; Davies et al., 2003). However, to date, there is no evidence of their role in higher organisms (e.g., rats and mice).
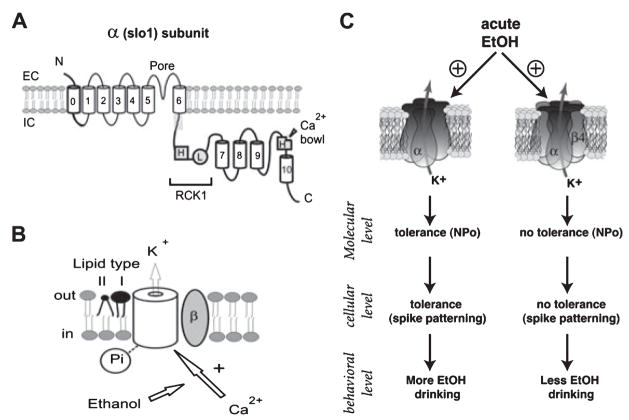
Fig. 2.
Molecular determinants of BK channel responses and adaptation to acute ethanol exposure. (A) Ethanol action on BK channels results from drug modulation of Ca2+-driven gating. Cartoon of the BK channel-forming α subunit, where bold traces highlight regions that are absent in voltage-gated TM6 K+ channels other than BK and control the ethanol sensitivity of this channel type. The main recognition sensors of divalents are labeled with H (high affinity) or L (low affinity); predominantly helical segments are shown as numbered cylinders; EC: extracellular, IC: intracellular. (B) Ethanol modulates Ca2+action after this divalent is recognized by sites shown above (see main text). Upon the fundamental Ca2+-slo1 subunit-ethanol interaction, final drug action is regulated by posttranslational modification of slo (phosphorylation), channel accessory β subunits, and the lipid microenvironment around the channel, with type-I and type-II lipids respectively facilitating and inhibiting ethanol-induced potentiation of channel activity. (C) Role of BK channel β4 subunit on acute ethanol tolerance. At the molecular level, acute ethanol increases α and αβ4 BK channel open probability in HEK-293 cells and MSNs. However, the activity of α BK channels (left column) returns to control levels shortly (7 to 8 minutes) after the beginning of drug exposure, indicating the development of tolerance, while that of αβ4 BK channels (right column) remains potentiated. At the cellular level, acute ethanol depresses excitability of striatal MSNs through its action on BK channels. This effect is transient in neurons expressing α, but not αβ4 BK channels. Behaviorally, in the absence of β4, the changes in locomotor activity following ethanol injection show acute tolerance, whereas acute tolerance of this behavioral measure is severely reduced in mice expressing the β4 subunit. Finally, β4 KO mice, exhibited a marked increase in ethanol consumption, compared to WT mice. Thus, the data at multiple levels of analysis establish the importance of BK β4 on acute ethanol tolerance, and support a link between acute tolerance and drinking behavior.
Potentiation of BK channel activity by acute exposure to ethanol is not a universal phenomenon, with native BK channels in rat supraoptic neuron somata (Dopico et al., 1999) and NAc dendrites (Martin et al., 2004) being refractory to ethanol concentrations below 100 mM. Moreover, some BK channels, such as those from rat aorta (Walters et al., 2000) and channels in native cerebrovascular myocytes are significantly inhibited by ethanol exposure, an ethanol action leading to vasoconstriction (Liu et al., 2004). Notably, variant BK channel responses to ethanol were observed under conditions that ruled out the involvement of freely diffusible cytosolic signals and cell metabolism of ethanol. Rather, variability in BK channel response to ethanol has to be ascribed to specifics in the channel complex itself and/or its surrounding proteolipid environment in the cell membrane (see below).
In addition, variability in BK channel responses to acute ethanol exposure includes a temporal component. Repeated or protracted (>2 minutes) ethanol exposure of native neurohypophysial nerve ending BK channels in isolated membrane patches results in blunted ethanol potentiation (Dopico et al., 1996). This time-dependent reduction in channel responsiveness was also observed when ethanol was probed on BK channel-forming subunits cloned from mouse brain (mSlo, mbr5 variant) in cell-free Xenopus oocyte membrane patches (A.M. Dopico and S.N. Treistman, unpublished observation). Moreover, a recent study documents that the time-course of the acute ethanol response is replicated when the ethanol is probed on cloned hSlo (human brain) channels reconstituted into artificial lipid bilayers (Yuan et al., 2008). Collectively, these results indicate that, as interpreted for the immediate drug response of the naïve system, the time-dependent component of the channel response to acute ethanol is mediated by the channel-forming subunit itself or its immediate proteolipid environment.
To begin to understand the molecular source(s) of variability in BK channel responses to acute exposure to ethanol, Dopico and colleagues explored drug action in highly simplified systems, such as heterologous expression of mutated channel subunits in isolated membrane patches and channel reconstitution into artificial bilayers of controlled lipid composition. Results from membrane patches and bilayers both indicate that acute exposure to ethanol (≤100 mM for <2 minutes) can evoke variant responses (activation, refractoriness, or inhibition) even when a given (cloned) channel-forming subunit (mSlo mbr5) is probed in a controlled proteolipid environment. Such variability is determined by the concentration of activating ligand (i.e., internal Ca2+). Thus, ethanol evokes BK channel potentiation at “low” (<10 μM) and inhibition at “high” (>10 μM) internal Ca2+, this dual pattern resulting from ethanol facilitation of specific Ca2+actions on channel gating (Liu et al., 2008).
To begin to understand the structural bases of the ligand-dependence of BK channel responses to ethanol exposure, the Dopico laboratory studied ethanol action on channel subunits where point mutagenesis rendered nonfunctional each of the known channel regions that participate in sensing of physiological concentrations of divalents (Fig. 2B): (1) the “calcium bowl” (a string of five aspartates), which participates of high-affinity Ca2+-sensing; (2) two key residues (D362, D367) in the regulatory of conductance for potassium (RCK) 1 domain that are key part of the other high affinity Ca2+sensor; and (3) two residues in the RCK1 domain (E374, E399) that are key to define a low affinity site for divalents (Fig. 2A). Mutations in the low-affinity site did not alter ethanol action on channel activity, whether activatory or inhibitory. However, ethanol potentiation of BK channels was suppressed when both calcium-bowl and high affinity RCK1 site nonfunctional, but this ethanol action remained when each high-affinity site was mutated. Thus, as far as activating Ca2+can be sensed by at least one of the channel high-affinity Ca2+sensors, ethanol is able to increase BK channel activity. In addition, the RCK1 high-affinity sensor is necessary and sufficient to sustain the ethanol-induced inhibition of channel activity observed at tens to hundreds of micromolar Ca2+. This inhibition results from ethanol facilitating channel dwelling into a low activity mode that is driven by Ca2+itself. Consistently, mutations that rendered the RCK1 high-affinity sensor nonfunctional ablated channel dwelling into its low activity mode. The fact that Ca2+levels determine the ethanol response of cloned Slo channels is a consequence of the absolute and selective requirement for this activating ion in ethanol action, as the drug does not modify intrinsic-, physiological Mg2+ driven- or voltage-gating of the channel (Liu et al., 2008).
In addition to the fundamental Slo subunit-activating Ca2+-ethanol interaction, a few defined molecular processes circumscribed to the channel complex and its proteolipid environment contribute to determine the final ethanol response of the BK channel complex (Fig. 2B). Beginning with the Slo subunit itself, CaMKII-phosphorylation of Thr107 in bSlo (from bovine aorta) progressively switches ethanol potentiation to inhibition (Liu et al., 2006), indicating that this posttranslational modification overrides ethanol amplification of Ca2+actions.
At low micromolar Ca2+, channel accessory subunits of the β1 type increase the apparent Ca2+-sensitivity of the BK channel and thus could fine-tune the ethanol response. Indeed, after cloning channel-forming (cbv1; AY330293) and β1 (FJ154955) subunits from rat cerebrovascular myocytes, Dopico and colleagues found that the presence of β1 subunits shifts the crossover for ethanol-induced channel activation to inhibition toward lower Ca2+i (≤3 μM), with consequent ethanol inhibition of recombinant BK channels at low micromolar Ca2+ (Bukiya et al., 2009), as found with the native cerebrovascular channel (Liu et al., 2004). Consistently, inhibition or reduced potentiation by ethanol was also reported with hSlo + β1 subunits heterologously expressed (Feinberg-Zadek and Treistman, 2007; Martin et al., 2004).
Finally, ethanol action on the steady-state activity of the BK channel complex (whether activation or inhibition) is primarily determined by drug modulation of the channel long-closed states (Dopico et al., 1996, 1998), the latter being highly sensitivity to the effective shape of the lipid species surrounding the channel complex. Thus, ethanol potentiation is favored by “type-I” (e.g., phosphatidylserine) while blunted by type-II lipids (e.g., phosphatidylethanolamine and cholesterol) (Crowley et al., 2003, 2005). In conclusion, there is solid evidence supporting the idea that the final response of a native BK channel to acute ethanol exposure results from the orchestration of a few molecular players in the channel complex itself and its immediate proteolipid environment (Fig. 2B). Moreover, some of these molecular entities, alone or in concerted fashion, play a key role in controlling the temporal component of BK channel responses to ethanol.
The composition of β subunits in dendritic, somatic, and terminal compartments may regulate the differences in acute ethanol tolerance (Dopico et al., 1996; Knott et al., 2002; Martin et al., 2004). It has previously been demonstrated that BK channel β subunits modulate the two components of ethanol tolerance (Feinberg-Zadek et al., 2008). The first component of BK channel tolerance to ethanol is a decreased sensitivity that occurs within 15 minutes, and the prolonged component is a reduced BK current density that occurs between 6 and 24 hours. In that study, Feinberg-Zadek and colleagues showed that hSlo + β1 BK channel subunits expressed in HEK 293 cells did not demonstrate tolerance to ethanol when compared with hSlo and hSlo + β4 BK channel subunits. These data suggest that subunit composition of BK channels is important for determining the extent of acute and prolonged ethanol tolerance.
In an attempt to further characterize the role played by BK channel subunit composition on ethanol tolerance at the molecular, cellular and behavioral levels, Treistman and colleagues compared the effects of ethanol during a short ethanol exposure on single channel activity of α and αβ4 BK channels expressed in HEK 293 cells, using cell-attached patch clamp recording mode. Consistent with previous reports (Pietrzykowski et al., 2008), 50 mM ethanol markedly potentiated α BK channel open probability that peaked after 2 min and returned to control levels after 6 to 7 minutes (Martin et al., 2008). In HEK 293 cells expressing αβ4 BK channels, ethanol similarly produced an increase in BK channel activity in the first few minutes following drug exposure that remained elevated for more than 10 min (Martin et al., 2008). These data suggest that α, but not αβ4 subunits of the BK channel demonstrate acute tolerance to ethanol.
To determine whether BK channels in neurons would behave in response to ethanol similarly to the channels in transfected HEK cells, Treistman and colleagues recorded BK channels from striatal medium spiny neurons (MSNs), in cell-attached mode. A previous study identified the presence of αβ4 BK channels in the somata of NAc MSNs (Martin et al., 2004). Therefore, it stands to reason that the ethanol response of BK channels in dissociated striatal neurons obtained from wild-type (Wt) mice would mimic that of αβ4 BK channels in HEK 293 cells. Indeed, 50 mM ethanol increased BK channel activity from MSNs, and this increase was sustained throughout the recording session (Martin et al., 2008). In contrast, in neurons from β4 KO mice, 50 mM ethanol initially potentiated BK channel activity, but the activity rapidly returned to predrug levels, indicating the development of acute tolerance. These observations suggest that β4 subunit expression prevents BK channels from exhibiting acute tolerance.
A number of studies have shown that BK channels are an important contributor to the shaping of action potentials in a number of brain regions (Faber and Sah, 2003; Gu et al., 2007; Kang et al., 2000; Meredith et al., 2006; Pitts et al., 2006; Shao et al., 1999; Zhang et al., 2003). Therefore, Treistman and colleagues hypothesized that ethanol, through BK channels, would alter the electrical excitability of MSNs. In acute slices from both Wt and β4 KO mice, 50 mM ethanol drastically depressed MSN excitability shortly (about 3 minutes) after the start of drug perfusion (Martin et al., 2008). In slices from Wt mice, the depression of MSN excitability persisted throughout the duration of the recording. In contrast, in β4 KO mice, at 10 minutes after the start of drug exposure, the number of action potentials was nearly identical to that during the predrug exposure period, demonstrating the presence of acute tolerance to ethanol. To verify that the effects of ethanol on MSN excitability were intrinsic to these neurons and not mediated by the release of neurotransmitters, this experiment was repeated on freshly dissociated MSNs. As in the slice preparation, ethanol depressed neuronal excitability in MSNs from both Wt and β4 KO mice, but while tolerance was observed in the latter it was not observed in the former (Martin et al., 2008). To confirm that the effects of ethanol on action potential patterning were indeed mediated by BK channels, slices were exposed to ethanol in the presence of BK channel blockers. In these conditions, the effects of ethanol were completely blocked (Martin et al., 2008).
It is possible that manipulation of β4 subunit expression could lead to tolerance at the behavioral level, similar to what has been observed at the molecular and cellular level. To test this hypothesis, Treistman and colleagues monitored mouse ambulatory activity before and after (5, 10, and 15 minutes) intraperitoneal ethanol injection that was repeated three times with two-day intervals between injections. On day 1, shortly (5 minutes) following ethanol injection, the locomotor activity of both Wt and β4 KO mice dropped markedly (Martin et al., 2008). Interestingly, WT mouse activity remained depressed for up to 15 minutes after injection. In contrast, β4 KO mouse ambulatory activity returned to nearly preinjection levels within the same time frame, indicating a strong adaptation (acute tolerance) to the sedative effects of ethanol. The difference in the effect of ethanol on ambulation between Wt and KO mice was even more pronounced on days 3 and 5. These differences were not attributable to difference in ethanol metabolism between Wt and KO mice. Using a “drinking-in-the dark” protocol (Rhodes et al., 2005, 2007), β4 KO mice drank nearly 50% more ethanol than their Wt counterpart (Martin et al., 2008). This was selective for ethanol, as they did not differ in their water and sucrose intake, or their dislike for quinine. From these studies, evidence suggests that the BK channel is a key ion channel mediating acute ethanol tolerance at the molecular, cellular and behavioral levels, and that the BK β4 subunit can control the influence of BK on acute tolerance (Fig. 2C). The findings from these studies also suggest that the development of acute tolerance in rodents is a predictor of the propensity to drink more ethanol, consistent with what has been reported in humans.
Previous work has demonstrated that BK channels in the supraoptic nucleus and straitum develop tolerance to ethanol due to decreased sensitivity to ethanol and to reduced BK channel density (Martin et al., 2004; Pietrzykowski et al., 2004). Recently, those findings have been extended to demonstrate that ethanol induces an increase in microRNA miR-9 expression in these regions (Pietrzykowski et al., 2008). The ethanol-induced up-regulation of miR-9 destabilizes BK mRNA containing 3′-UTRs with a miR-9 recognition element leading to a change in BK channel isoforms. These data demonstrate a central role for miR-9 in the action of ethanol in the CNS and suggest that miR-9 up-regulation contributes to the development of acute tolerance to ethanol. Moreover, ethanol regulation of miR-9 also affects additional ethanol-related targets that may possibly play a role in neuronal plasticity and ethanol dependence.
FUTURE DIRECTIONS
Using a multitude of molecular, cellular, and behavioral approaches in brain regions important in ethanol abuse and dependency, it is evident that SK and BK channels are key contributors to ethanol tolerance and adaptive plasticity. These results demonstrate that chronic ethanol leads to reduced SK function in VTA DA and CA1 pyramidal neurons. Further experiments will be required to determine whether SK channel function is reduced in these critical brain regions after voluntary self-administration of ethanol. Does impaired function of SK channels affect DA release in VTA terminal regions, responses to ethanol-related cues, neuronal excitability, or synaptic plasticity? This raises the interesting possibility that SK channel positive modulators might reduce burst firing following repeated ethanol exposure in VTA DA neurons and aberrant withdrawal excitability in hippocampus, and in this way reduce ethanol-seeking behaviors or the expression of cross-sensitization to cocaine.
SK channels are part of a multi-protein complex with protein phosphatase 2, casein kinase 2 (CK2), and calmodulin, and phosphorylation of calmodulin by CK2 decreases SK channel sensitivity to Ca2+ (Faber and Sah, 2007). Thus, it is possible that the reduction in SK channel function by ethanol involves increased CK2 phosphorylation of SK-associated calmodulin. Alternatively, reduced function of SK by prolonged ethanol exposure may be due to down-regulation of surface channels through a protein kinase A (PKA)-dependent process. Indeed, phosphorylation of SK channels by PKA regulates their surface trafficking (Lin et al., 2008). Future studies are necessary to determine the molecular mechanisms underlying ethanol-induced reduction in SK channel function or expression.
Additional evidence has identified plasticity of BK channels that is dependent upon subunit composition and molecular mechanisms that regulate posttranslational reorganization of BK splice variants. Moreover, BK channel activity and the potentiation by ethanol are critically modulated by the proteolipid environment. These studies provide important molecular and behavioral evidence for mechanisms contributing to ethanol tolerance and adaptive plasticity and may have implications for understanding relapse and the development of ethanol dependence. Because of the association between alcohol tolerance and the increased risk for dependency, the gene encoding the BK channel β4 subunit should be further studied as a possible candidate gene for developing alcoholism (Martin et al., 2008). In addition, future work is necessary to determine the mechanisms by which ethanol-induced up-regulation of miR-9 leads to a change in BK channel isoforms.
Acknowledgments
The work described in this review was supported by NIH grants R01 AA01098 (LJC) and R01 AA11560 (AMD). In addition, the work described in the review was supported by funds provided by the state of California for medical research on alcohol and substance abuse through the University of California, San Francisco (AB) and by the Department of the Army, grant no. W81XWH-05-1-0213 (AB). The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick, MD 21702-5014, is the awarding and administering acquisition office. The content of the information represented does not necessarily reflect the position or the policy of the government, and no official endorsement should be inferred.
References
- Allen D, Fakler B, Maylie J, Adelman JP. Organization and regulation of small conductance Ca2+-activated K+ channel multiprotein complexes. J Neurosci. 2007;27:2369–2376. doi: 10.1523/JNEUROSCI.3565-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur J Pharmacol. 2006;546:48–53. doi: 10.1016/j.ejphar.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Bailey CP, Manley SJ, Watson WP, Wonnacott S, Molleman A, Little HJ. Chronic ethanol administration alters activity in ventral tegmental area neurons after cessation of withdrawal hyperexcitability. Brain Res. 1998;803:144–152. doi: 10.1016/s0006-8993(98)00654-4. [DOI] [PubMed] [Google Scholar]
- Bailey CP, O’Callaghan MJ, Croft AP, Manley SJ, Little HJ. Alterations in mesolimbic dopamine function during the abstinence period following chronic ethanol consumption. Neuropharmacology. 2001;41:989–999. doi: 10.1016/s0028-3908(01)00146-0. [DOI] [PubMed] [Google Scholar]
- Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–311. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brodie MS, Scholz A, Weiger TM, Dopico AM. Ethanol interactions with calcium-dependent potassium channels. Alcohol Clin Exp Res. 2007;31:1625–1632. doi: 10.1111/j.1530-0277.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. Arterial smooth muscle BK channel beta1 subunits determine ethanol-induced cerebrovascular constriction. Biophysical J. 2009;96:A416. [Google Scholar]
- Carlen PL, Gurevich N, Durand D. Ethanol in low doses augments calcium-mediated mechanisms measured intracellularly in hippocampal neurons. Science. 1982;215:306–309. doi: 10.1126/science.7053581. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- Carpenter-Hyland EP, Woodward JJ, Chandler LJ. Chronic ethanol induces synaptic but not extrasynaptic targeting of NMDA receptors. J Neurosci. 2004;24:7859–7868. doi: 10.1523/JNEUROSCI.1902-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler LJ. Ethanol and brain plasticity: receptors and molecular networks of the postsynaptic density as targets of ethanol. Pharmacol Ther. 2003;99:311–326. doi: 10.1016/s0163-7258(03)00096-2. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Atkinson NS. The slowpoke gene is necessary for rapid ethanol tolerance in Drosophila. Alcohol Clin Exp Res. 2005;29:1777–1786. doi: 10.1097/01.alc.0000183232.56788.62. [DOI] [PubMed] [Google Scholar]
- Cowmeadow RB, Krishnan HR, Ghezzi A, Al’Hasan YM, Wang YZ, Atkinson NS. Ethanol tolerance caused by slowpoke induction in Drosophila. Alcohol Clin Exp Res. 2006;30:745–753. doi: 10.1111/j.1530-0277.2006.00087.x. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, Dopico AM. Cholesterol antagonizes ethanol potentiation of human brain BKCa channels reconstituted into phospholipid bilayers. Mol Pharmacol. 2003;64:365–372. doi: 10.1124/mol.64.2.365. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Treistman SN, Dopico AM. Distinct structural features of phospholipids differentially determine ethanol sensitivity and basal function of BK channels. Mol Pharmacol. 2005;68:4–10. doi: 10.1124/mol.105.012971. [DOI] [PubMed] [Google Scholar]
- Davies AG, Pierce-Shimomura JT, Kim H, VanHoven MK, Thiele TR, Bonci A, Bargmann CI, McIntire SL. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell. 2003;115:655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- Diana M, Pistis M, Carboni S, Gessa GL, Rossetti ZL. Profound decrement of mesolimbic dopaminergic neuronal activity during ethanol withdrawal syndrome in rats: electrophysiological and biochemical evidence. Proc Natl Acad Sci U S A. 1993;90:7966–7969. doi: 10.1073/pnas.90.17.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diana M, Pistis M, Muntoni A, Gessa G. Mesolimbic dopaminergic reduction outlasts ethanol withdrawal syndrome: evidence of protracted abstinence. Neuroscience. 1996;71:411–415. doi: 10.1016/0306-4522(95)00482-3. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Anantharam V, Treistman SN. Ethanol increases the activity of Ca++-dependent K+ (mslo) channels: functional interaction with cytosolic Ca++ J Pharmacol Exp Ther. 1998;284:258–268. [PubMed] [Google Scholar]
- Dopico AM, Lemos JR, Treistman SN. Ethanol increases the activity of large conductance, Ca2+-activated K+ channels in isolated neurohypophysal terminals. Mol Pharmacol. 1996;49:40–48. [PubMed] [Google Scholar]
- Dopico AM, Widmer H, Wang G, Lemos JR, Treistman SN. Rat supraoptic magnocellular neurones show distinct large conductance, Ca2+-activated K+ channel subtypes in cell bodies versus nerve endings. J Physiol. 1999;519:101–114. doi: 10.1111/j.1469-7793.1999.0101o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand D, Carlen PL. Decreased neuronal inhibition in vitro after long-term administration of ethanol. Science. 1984;224:1359–1361. doi: 10.1126/science.6328654. [DOI] [PubMed] [Google Scholar]
- Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–641. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- Faber ES, Sah P. Ca2 +-activated K+ (BK) channel inactivation contributes to spike broadening during repetitive firing in the rat lateral amygdala. J Physiol. 2003;552:483–497. doi: 10.1113/jphysiol.2003.050120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–1083. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL, Martin G, Treistman SN. BK channel subunit composition modulates molecular tolerance to ethanol. Alcohol Clin Exp Res. 2008;32:1207–1216. doi: 10.1111/j.1530-0277.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- Feinberg-Zadek PL, Treistman SN. Beta-subunits are important modulators of the acute response to alcohol in human BK channels. Alcohol Clin Exp Res. 2007;31:737–744. doi: 10.1111/j.1530-0277.2007.00371.x. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–146. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grace AA. The tonic/phasic model of dopamine system regulation and its implications for understanding alcohol and psychostimulant craving. Addiction. 2000;95(Suppl 2):S119–S128. doi: 10.1080/09652140050111690. [DOI] [PubMed] [Google Scholar]
- Gruss M, Henrich M, Konig P, Hempelmann G, Vogel W, Scholz A. Ethanol reduces excitability in a subgroup of primary sensory neurons by activation of BK(Ca) channels. Eur J Neurosci. 2001;14:1246–1256. doi: 10.1046/j.0953-816x.2001.01754.x. [DOI] [PubMed] [Google Scholar]
- Gu N, Vervaeke K, Storm JF. BK potassium channels facilitate high-frequency firing and cause early spike frequency adaptation in rat CA1 hippocampal pyramidal cells. J Physiol. 2007;580:859–882. doi: 10.1113/jphysiol.2006.126367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, Stackman RW. Small-conductance Ca2 +-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–1853. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Madden PA, Bucholz KK, Dinwiddie SH, Slutske WS, Bierut LJ, Rohrbaugh JW, Statham DJ, Dunne MP, Whitfield JB, Martin NG. Genetic differences in alcohol sensitivity and the inheritance of alcoholism risk. Psychol Med. 1999;29:1069–1081. doi: 10.1017/s0033291799008909. [DOI] [PubMed] [Google Scholar]
- Hendricson AW, Maldve RE, Salinas AG, Theile JW, Zhang TA, Diaz LM, Morrisett RA. Aberrant synaptic activation of N-methyl-D-aspartate receptors underlies ethanol withdrawal hyperexcitability. J Pharmacol Exp Ther. 2007;321:60–72. doi: 10.1124/jpet.106.111419. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Haraguchi M, Erickson H, Samson HH. Ventral tegmental microinjections of quinpirole decrease ethanol and sucrose-reinforced responding. Alcohol Clin Exp Res. 1993;17:370–375. doi: 10.1111/j.1530-0277.1993.tb00778.x. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Samson HH, Chappelle AM. Alcohol self-administration: further examination of the role of dopamine receptors in the nucleus accumbens. Alcohol Clin Exp Res. 1997;21:1083–1091. doi: 10.1111/j.1530-0277.1997.tb04257.x. [DOI] [PubMed] [Google Scholar]
- Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- Hoshaw BA, Lewis MJ. Behavioral sensitization to ethanol in rats: evidence from the Sprague-Dawley strain. Pharmacol Biochem Behav. 2001;68:685–690. doi: 10.1016/s0091-3057(01)00489-0. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Effects of cocaine, nicotine, dizocipline and alcohol on mice locomotor activity: cocaine-alcohol cross-sensitization involves upregulation of striatal dopamine transporter binding sites. Brain Res. 1999;818:204–211. doi: 10.1016/s0006-8993(98)01260-8. [DOI] [PubMed] [Google Scholar]
- Jakab M, Weiger TM, Hermann A. Ethanol activates maxi Ca2+-activated K+ channels of clonal pituitary (GH3) cells. J Membr Biol. 1997;157:237–245. doi: 10.1007/pl00005895. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2 +-activated K+ currents. Neurosci Lett. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Kang J, Huguenard JR, Prince DA. Voltage-gated potassium channels activated during action potentials in layer V neocortical pyramidal neurons. J Neurophysiol. 2000;83:70–80. doi: 10.1152/jn.2000.83.1.70. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Ethanol-associated olfactory stimuli reinstate ethanol-seeking behavior after extinction and modify extracellular dopamine levels in the nucleus accumbens. Alcohol Clin Exp Res. 1999;23:1751–1760. [PubMed] [Google Scholar]
- Knott TK, Dopico AM, Dayanithi G, Lemos J, Treistman SN. Integrated channel plasticity contributes to alcohol tolerance in neurohypophysial terminals. Mol Pharmacol. 2002;62:135–142. doi: 10.1124/mol.62.1.135. [DOI] [PubMed] [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Krjnevic K. Excitable membranes and anesthetics. In: Fink BR, editor. Cellular Biology and Toxicity of Anesthetics. Williams & Wilkins; Baltimore, MD: 1972. pp. 3–9. [Google Scholar]
- Latorre R, Brauchi S. Large conductance Ca2 +-activated K+ (BK) channel: activation by Ca2+ and voltage. Biol Res. 2006;39:385–401. doi: 10.4067/s0716-97602006000300003. [DOI] [PubMed] [Google Scholar]
- Lee WS, Ngo-Anh TJ, Bruening-Wright A, Maylie J, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin: cell surface expression and gating. J Biol Chem. 2003;278:25940–25946. doi: 10.1074/jbc.M302091200. [DOI] [PubMed] [Google Scholar]
- Lin MT, Lujan R, Watanabe M, Adelman JP, Maylie J. SK2 channel plasticity contributes to LTP at Schaffer collateral-CA1 synapses. Nat Neurosci. 2008;11:170–177. doi: 10.1038/nn2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Asuncion-Chin MT, Liu P, Dopico AM. CaM kinase II phosphorylation of slo Thr107 regulates activity and ethanol responses of BK channels. Nat Neurosci. 2006;9:41–49. doi: 10.1038/nn1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Vaithianathan T, Manivannan K, Parrill A, Dopico AM. Ethanol modulates BKCa channels by acting as an adjuvant of calcium. Mol Pharmacol. 2008;74:628–640. doi: 10.1124/mol.108.048694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Liu J, Huang W, Li MD, Dopico AM. Distinct regions of the slo subunit determine differential BK Ca channel responses to ethanol. Alcohol Clin Exp Res. 2003;27:1640–1644. doi: 10.1097/01.ALC.0000094756.41638.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci U S A. 2004;101:18217–18222. doi: 10.1073/pnas.0406096102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GE, Hendrickson LM, Penta KL, Friesen RM, Pietrzykowski AZ, Tapper AR, Treistman SN. Identification of a BK channel auxiliary protein controlling molecular and behavioral tolerance to alcohol. Proc Natl Acad Sci U S A. 2008;105:17543–17548. doi: 10.1073/pnas.0801068105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G, Puig S, Pietrzykowski A, Zadek P, Emery P, Treistman SN. Somatic localization of specific large-conductance calcium-activated potassium channel subtype controls compartimentalized ethanol sensitivity in the nucleus accumbens. J Neurosci. 2004;24:6563–6572. doi: 10.1523/JNEUROSCI.0684-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews EA, Weible AP, Shah S, Disterhoft JF. The BK-mediated fAHP is modulated by learning a hippocampus-dependent task. Proc Natl Acad Sci U S A. 2008;105:15154–15159. doi: 10.1073/pnas.0805855105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554:255–261. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Le AD, Noronha A. Central nervous system mechanisms in alcohol relapse. Alcohol Clin Exp Res. 2002;26:280–286. [PubMed] [Google Scholar]
- Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mereu G, Fadda F, Gessa GL. Ethanol stimulates the firing rate of nigral dopaminergic neurons in unanesthetized rats. Brain Res. 1984;292:63–69. doi: 10.1016/0006-8993(84)90890-4. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ, Carpenter-Hyland EP, Hearing MC, Becker HC, Woodward JJ, Chandler LJ. Glutamate transporters regulate extrasynaptic NMDA receptor modulation of Kv2.1 potassium channels. J Neurosci. 2008;28:8801–8809. doi: 10.1523/JNEUROSCI.2405-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ, Chandler LJ. The thorny side of addiction: adaptive plasticity and dendritic spines. Sci World J. 2007;7:9–21. doi: 10.1100/tsw.2007.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Anh TJ, Bloodgood BL, Lin M, Sabatini BL, Maylie J, Adelman JP. SK channels and NMDA receptors form a Ca2+-mediated feedback loop in dendritic spines. Nat Neurosci. 2005;8:642–649. doi: 10.1038/nn1449. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J Neurosci. 2004;24:8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts GR, Ohta H, McMahon DG. Daily rhythmicity of large-conductance Ca2+-activated K+ currents in suprachiasmatic nucleus neurons. Brain Res. 2006;1071:54–62. doi: 10.1016/j.brainres.2005.11.078. [DOI] [PubMed] [Google Scholar]
- Prendergast MA, Harris BR, Mullholland PJ, Blanchard JA, II, Gibson DA, Holley RC, Littleton JM. Hippocampal CA1 region neurodegeneration produced by ethanol withdrawal requires activation of intrinsic polysynaptic hippocampal pathways and function of N-methyl-D-aspartate receptors. Neuroscience. 2004;124:869–877. doi: 10.1016/j.neuroscience.2003.12.013. [DOI] [PubMed] [Google Scholar]
- Qiang M, Denny AD, Ticku MK. Chronic intermittent ethanol treatment selectively alters N-methyl-D-aspartate receptor subunit surface expression in cultured cortical neurons. Mol Pharmacol. 2007;72:95–102. doi: 10.1124/mol.106.033043. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Pulvirenti L, Koob GF. Oral ethanol self-administration in rats is reduced by the administration of dopamine and glutamate receptor antagonists into the nucleus accumbens. Psychopharmacology. 1992;109:92–98. doi: 10.1007/BF02245485. [DOI] [PubMed] [Google Scholar]
- Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rhodes JS, Ford MM, Yu CH, Brown LL, Finn DA, Garland TJ, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiatry. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW, North RA. Apamin increases NMDA-induced burst-firing of rat mesencephalic dopamine neurons. Brain Res. 1993;630:341–344. doi: 10.1016/0006-8993(93)90675-d. [DOI] [PubMed] [Google Scholar]
- Shao LR, Halvorsrud R, Borg-Graham L, Storm JF. The role of BK-type Ca2+-dependent K+ channels in spike broadening during repetitive firing in rat hippocampal pyramidal cells. J Physiol. 1999;1 (521 Pt):135–146. doi: 10.1111/j.1469-7793.1999.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen RY. Ethanol withdrawal reduces the number of spontaneously active ventral tegmental area dopamine neurons in conscious animals. J Pharmacol Exp Ther. 2003;307:566–572. doi: 10.1124/jpet.103.053371. [DOI] [PubMed] [Google Scholar]
- Shen RY, Choong KC, Thompson AC. Long-term reduction in ventral tegmental area dopamine neuron population activity following repeated stimulant or ethanol treatment. Biol Psychiatry. 2007;61:93–100. doi: 10.1016/j.biopsych.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Stackman RW, Hammond RS, Linardatos E, Gerlach A, Maylie J, Adelman JP, Tzounopoulos T. Small conductance Ca2+-activated K+ channels modulate synaptic plasticity and memory encoding. J Neurosci. 2002;22:10163–10171. doi: 10.1523/JNEUROSCI.22-23-10163.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–770. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proc Natl Acad Sci U S A. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Storm JF. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8:373–382. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Bilbao A, Molander A, Spanagel R. Neuropharmacology of alcohol addiction. Br J Pharmacol. 2008;154:299–315. doi: 10.1038/bjp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Walters F, Covarrubias M, Giangiacomo K, Ellingson G. Potent inhibition of the aortic smooth muscle maxi-K channel by clinical doses of ethanol. Am J Physiol Cell Physiol. 2000;279:C1107–C1115. doi: 10.1152/ajpcell.2000.279.4.C1107. [DOI] [PubMed] [Google Scholar]
- Weiss F, Markou A, Lorang MT, Koob GF. Basal extracellular dopamine levels in the nucleus accumbens are decreased during cocaine withdrawal after unlimited-access self-administration. Brain Res. 1992;593:314–318. doi: 10.1016/0006-8993(92)91327-b. [DOI] [PubMed] [Google Scholar]
- Yuan C, O’Connell RJ, Wilson A, Pietrzykowski AZ, Treistman SN. Acute alcohol tolerance is intrinsic to the BKCa protein, but is modulated by the lipid environment. J Biol Chem. 2008;283:5090–5098. doi: 10.1074/jbc.M708214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata A, Gonzales RA, Shippenberg TS. Repeated ethanol intoxication induces behavioral sensitization in the absence of a sensitized accumbens dopamine response in C57BL/6J and DBA/2J mice. Neuropsychopharmcology. 2006;31:396–405. doi: 10.1038/sj.npp.1300833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Gopalakrishnan M, Shieh CC. Modulation of action potential firing by iberiotoxin and NS1619 in rat dorsal root ganglion neurons. Neuroscience. 2003;122:1003–1011. doi: 10.1016/j.neuroscience.2003.08.035. [DOI] [PubMed] [Google Scholar]