Abstract
Electrochemical methods have been widely used to monitor physiologically important molecules in biological systems. This report describes the first application of the scanning electrochemical microscope (SECM) to probe the redox activity of individual living cells. The possibilities of measuring the rate and investigating the pathway of transmembrane charge transfer are demonstrated. By this approach, significant differences are detected in the redox responses given by nonmotile, nontransformed human breast epithelial cells, breast cells with a high level of motility (engendered by overexpression of protein kinase Cα), and highly metastatic breast cancer cells. SECM analysis of the three cell lines reveals reproducible differences with respect to the kinetics of charge transfer by several redox mediators.
Over the past 30 years, electrochemical methods have been developed for probing processes occurring in living cells (1–3). Single cell voltammetry has focused on measurements of concentrations and monitoring the dynamic release of biologically important molecules such as catecholamines, insulin, and anti-cancer drugs (4–9) from living cells. In such experiments, a micrometer-sized ultramicroelectrode (UME) is positioned in close proximity to a cell membrane and used to oxidize (or reduce) the molecules ejected from the cell. The capabilities of electrochemical measurements can be further enhanced by using an UME as a tip in the scanning electrochemical microscope (SECM) (10). The tip is scanned over the surface of a sample, called the substrate, to obtain topographic images and maps of chemical reactivity across the surface. In this way, the kinetics of charge transfer (CT) reactions have been probed at solid/liquid and liquid/liquid interfaces (11). The SECM was also used to image fluxes of oxygen at living cells and to obtain topographic images of biological substrates (12–15).
The objective of this work is to probe reduction–oxidation processes in single cells. Enzymatic redox reactions are essential for many cellular functions [metabolism, protein synthesis (16, 17)]. Characterization of intracellular redox chemistry is complicated by a large number of different redox couples active within the cell. Intracellular redox activity can be probed noninvasively by measuring the rate of transmembrane CT (18). In an SECM experiment, the tip UME is placed in solution containing the oxidized (or reduced) form of a redox mediator. A mediator species is then reduced (or oxidized) at the tip electrode:
 |
1 |
If the tip is positioned near an electrically conductive substrate, the product of reaction [1] diffuses to its surface, where it may be reoxidized (or re-reduced). This process produces an enhancement in the faradaic current at the tip electrode (positive feedback) depending on the tip/substrate separation distance d. Similar increase in the tip current (iT) can be observed if species O is preaccumulated at the substrate. If no regeneration of O occurs, the substrate blocks the diffusion of species O to the tip, so the iT decreases at smaller d (negative feedback). The overall rate of mediator regeneration at the substrate can be evaluated from the tip current-distance (iT − d) curve (10, 11). We used this approach to measure the rate of CT across the membrane of an individual cell.
The mechanism of mediator regeneration by the cell is complicated and may include both transmembrane ion and electron transfers (ET), as shown with model biomimetic systems [phospholipid membranes (19–23)]. For the present work, we will use SECM-based approaches that were developed for liquid/liquid and solid/liquid interfaces (24–26) to investigate the pathway of transmembrane CT and to identify the factors limiting the overall rate. Single cell SECM measurements provide valuable information that is hard to extract from the averaged signal produced by a large number of cells. Measurements at the level of a single cell can be done on a milli- to microsecond time scale, which is more rapid than the minute time scale required to analyze a large cell population (18). The fast response time also allows for detection of short-lived radicals such as reactive oxygen species.
By use of SECM, the present study seeks to compare the intrinsic redox reactivity of cell types that exhibit different motile behaviors. This idea was prompted by observations (27, 28) that oxidants modulate the activity of protein kinase Cα (PKCα), an enzyme that has been linked with motility and metastasis of various cell types (29, 30). Engineered expression of PKCα in nonmotile MCF-10A human breast cells (31) was found to engender a high degree of motility in those cells (11α cells), as previously described (32). Here, we use the SECM to measure and compare the redox reactivity of MCF-10A cells, 11α cells, and overtly metastatic MDA-MB-231 human breast cells (which express high levels of PKCα). With respect to the kinetics of transmembrane charge transfer by a variety of redox mediators, nonmotile and motile cells are observed to exhibit distinct and reproducible differences.
Materials and Methods
Chemicals.
Na4Fe(CN)6, iodine, and potassium iodide were from Fisher Scientific. All aqueous solutions were prepared from deionized water (Milli-Q, Millipore). Menadione (General Biochemicals), 1,2-naphthoquinone (Aldrich), Ru(NH3)6 Cl3 (Strem Chemicals, Newburyport, MA), and other chemicals were reagent grade.
Electrodes.
A two-electrode setup was used with either a 5.5-μm-radius carbon UME tip, a 5-μm Pt tip, or a 1-μm Pt tip; and a 0.25-mm Ag/AgCl reference electrode. The tips were prepared as described previously (10) and polished with 0.05-μm alumina before each experiment.
Cell Culture.
Mid-passage MCF-10A cells, a human breast epithelial cell line, were cultured in DMEM/F12 media (1:1) supplemented with 15% equine serum, insulin (10 μg/ml), epidermal growth factor (20 ng/ml), cholera toxin (100 ng/ml), and hydrocortisone (0.5 μg/ml), and maintained with penicillin (100 units/ml), streptomycin (100 μg/ml), and fungizone (0.5 μg/ml). Cells were passaged at 1:3 to 1:6 every 3 to 4 days. A stable transfectant clone (11α cells) that constitutively expresses bovine PKCα was isolated by neomycin resistance, as previously described (32). Transfectants were maintained in complete growth medium containing 125 μg/ml G418, and cultured up to 20 passages. MDA-MB-231 cells were cultured in Iscove's modified Dulbecco's medium with l-glutamine, 10% FBS, and 1% penicillin/streptomycin. Culture media, serum, and antibiotics (fungizone, penicillin, and streptomycin) were purchased from GIBCO-BRL. All other reagents were obtained from Sigma.
Before each experiment, adherent cells that had been plated at low density in a 60-mm plastic culture dish, were washed with PBS [153 mM Na+/4 mM K+/1 mM Ca2+/1 mM Mg2+/144 mM Cl−/10 mM phosphate (pH 7.4)].
Instrumentation and Procedures.
All measurements were performed at ambient temperature in a plastic culture dish mounted on a horizontal stage of the SECM. The cells were immobilized on the bottom of the dish and were immersed in PBS (pH 7.4) containing the redox mediator at the specified concentration. The SECM apparatus and procedure were described previously (24, 25, 33). Three types of experiments were performed: (i) the iT was recorded as a function of the tip position as the tip was moved laterally in a horizontal (x-y) plane a few micrometers above the cell surface; (ii) similarly, a gray scale image of the cell was obtained by recording variations in tip current while the probe was scanned in the x-y plane above the cell; and (iii) iT vs. d curves were obtained by positioning the tip above the cell and slowly moving it vertically down to the cell surface. The data were acquired by using software generously provided by D. O. Wipf (Mississippi State University). The analysis of iT − d curves was based on an earlier model (24).
Because of negative standard potentials of menadione and naphthoquinone mediators, the deaeration of solutions was necessary in experiments with those mediators. To prevent damage to the cells, oxygen was removed from the medium for a brief period that immediately preceded the actual measurements, i.e., ≈2 min for obtaining an approach curve, and ≈10 min for imaging. After finding an immobilized cell and positioning the tip above it, a flow of nitrogen was quickly passed through a small volume (≈5 ml) of aqueous solution covering the cells. The nearly complete removal of O2 was evident from cyclic voltammetry.
According to previously developed models (24–26), the rate of interfacial charge transfer should appear immeasurably low if the concentration of redox species in solution is much higher than the intracellular concentration of redox centers. In previous SECM measurements, the concentration of the redox mediator in solution was typically in the millimolar range (10). In contrast, significantly lower (μM) concentrations have to be used for experiments with living cells. The possibility of quantitative kinetic measurements with concentrations of redox species in solution as low as 10 μM was confirmed by fitting experimental current-distance curves obtained at both conductive and insulating substrates to the theory.
Results and Discussion
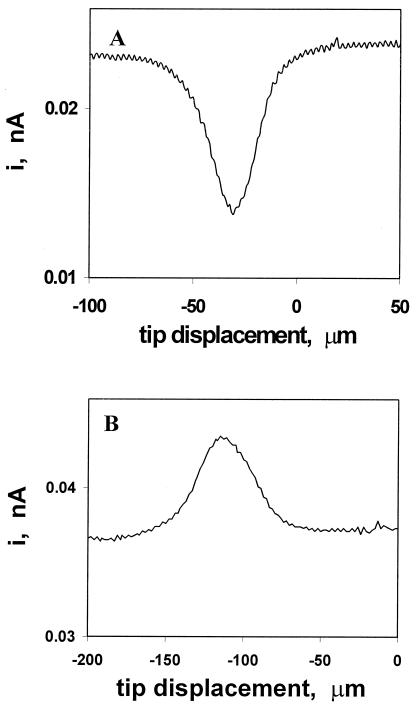
To investigate the pathway of the transmembrane CT reaction, SECM experiments were carried out with different types of redox mediators. When the tip was scanned horizontally above the cells in the presence of a hydrophilic redox mediator (ferrocene carboxylate), the tip current above the cell was significantly lower than that above the plastic surface (Fig. 1A). The cell surface thus impeded diffusion of the mediator to the tip (Fig. 2A). Analogous results obtained with other hydrophilic mediators [e.g., Ru(NH3)63/2+ and Fe(CN)63/4−] show that no unmediated ET occurs across the cell membrane.
Figure 1.
Current vs. tip position dependencies for an SECM tip scanned laterally over a MCF-10A cell in solution containing (A) 50 μM FcCOONa and (B) 40 μM menadione. The tip radius is 5.5 μm.
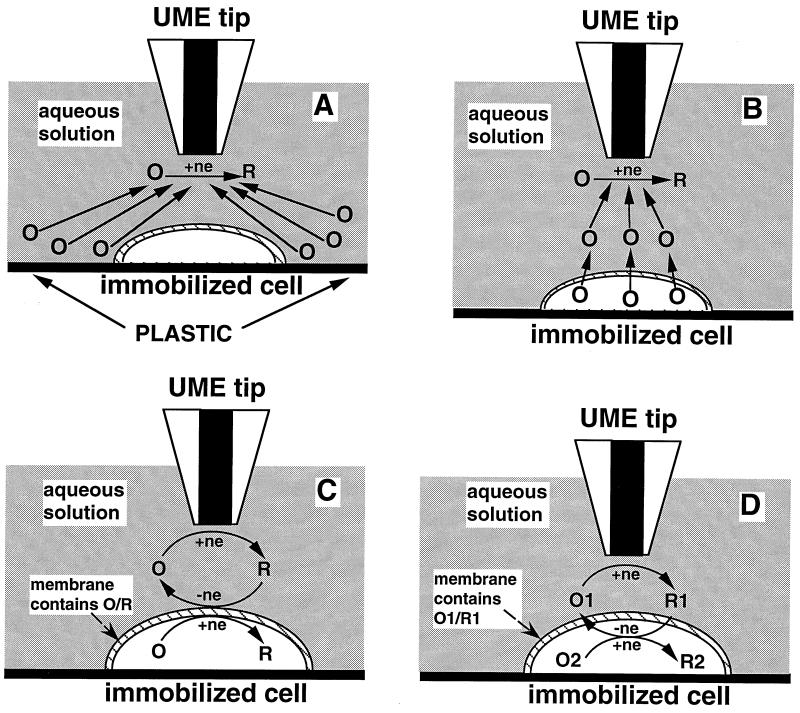
Figure 2.
Schematic diagrams of the SECM experiments with four different types of mediator regeneration. (A) The tip is positioned in the solution close to the cell surface. The lipid cell membrane is impermeable for a hydrophilic redox mediator. Negative feedback is due to the hindered diffusion of redox species to the tip electrode. (B) The UME tip induces the ejection of the redox species, O, from the cell by depleting its concentration near the cell surface via electrolysis. (C) Mediator regeneration proceeds via self-exchange ET reaction. The charge is shuttled across the membrane by the same hydrophobic redox species (O/R). (D) Bimolecular ET between hydrophobic redox mediator (O1/R1) and cell-bound redox moieties (O2/R2).
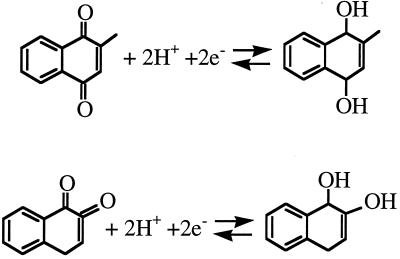
By contrast, with a lipid-soluble mediator (e.g., menadione or 1,2-naphthoquinone), the iT increased when the tip was scanned horizontally above the cell (Fig. 1B). Both oxidized and reduced forms of menadione and 1,2-naphthoquinone are neutral and can cross the cell membrane (Scheme S1). Thus the increase in the IT above the cell in Fig. 1B can be explained either by intracellular oxidation of the reduced mediator species (menadiol) generated by the tip reaction (Fig. 2 C and D), or by diffusion of menadione from a preaccumulated intracellular pool (Fig. 2B). Whatever the mechanism, IT reflects the overall rate of menadione regeneration by the cell.
Scheme 1.
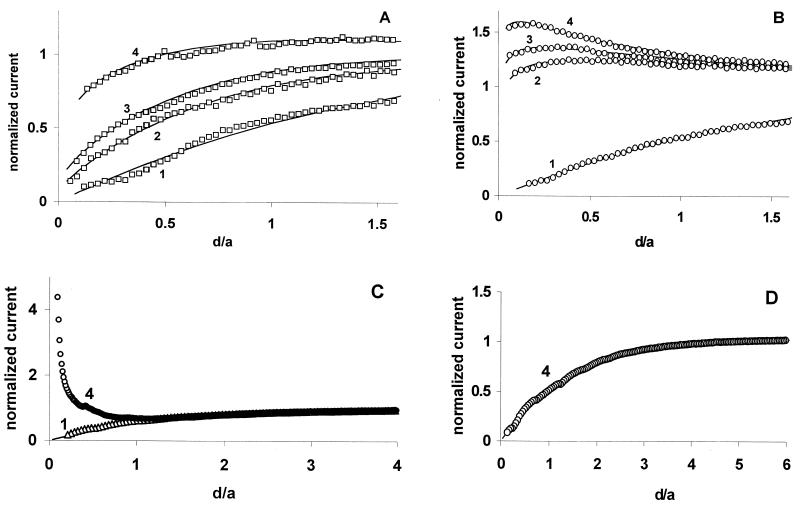
The apparent rate constant of the mediator regeneration process (k) can be extracted from the dependence of the normalized current IT vs. the normalized distance (d/a) between the tip and the cell. The data obtained with a tip approaching the plastic surface of the culture dish (curve 1, Fig. 3A) fit the theory for a diffusion-controlled process with an insulating substrate (solid line). When the same tip approached an MDA-MB-231 cell (metastatic cell) (curve 2), the IT was higher, indicating that the mediator was regenerated by the cell at a measurable rate. The effective heterogeneous rate constant, k = 1.4 × 10−3 cm/s, was obtained by fitting this curve to the SECM theory (solid line) (24). The tip current for an MCF-10A cell (nonmetastatic cell) (curve 4) was significantly higher than the IT obtained for a MDA-MB-231 cell (curve 2), resulting in k = 7 × 10−3 cm/s. Thus, a nonmetastatic cell regenerates menadione at a significantly higher rate than does a metastatic cell. For an 11α cell (MCF-10A cell that overexpresses PKCα), the rate constant (k = 2.4 × 10−3 cm/s from curve 3) was slightly higher than the k value for an MDA-MB-231 cell, but significantly lower than that for a MCF-10A cell.
Figure 3.
An UME tip approaches (1) plastic surface, (2) MDA-MB-231 cell, (3) 11α cell, and (4) MCF-10A cell. Solid lines are the theoretical curves for an insulating substrate (1) and finite heterogeneous kinetics (2, 3, 4). Phosphate buffer (pH 7.4) contained (A) 20 μM menadione, (B) 30 μM 1,2-naphthoquinone, (C) 0.1 mM KI, and (D) 0.1 mM KI + 20 μM I2. (C) Curves for MDA-MB-231 and 11α cells were very similar to curve 4. (D) Curves 1–4 were essentially identical. The tip current is normalized by the iT value measured in the bulk solution. The tip was a 5.5-μm-radius carbon fiber (A and B), and 5-μm-radius Pt (C and D).
The rate of the multistep reaction underlying the observed SECM response can be influenced by a number of factors including the following: (i) the rate of intracellular ET, which is governed either by the intracellular redox potential or concentrations of redox species; (ii) the rate of mass/charge transport across the membrane; and (iii) the kinetics of heterogeneous ET occurring on both sides of the membrane. However, the intracellular redox potential is not the rate-limiting factor for any reaction in Fig. 3. If it were limiting, the effective rate constants for 1,2-naphthoquinone (Fig. 3B) would be much lower than for menadione (Fig. 3A) because the standard potential of menadione is ≈140 mV more negative than that of 1,2-naphthoquinone. However, the k values extracted from curves in Fig. 3B (9 × 10−3 cm/s, 12 × 10−3 cm/s, and 17 × 10−3 cm/s for MDA-MB-231, 11α, and MCF-10A cells, respectively) are somewhat higher than those determined for menadione.
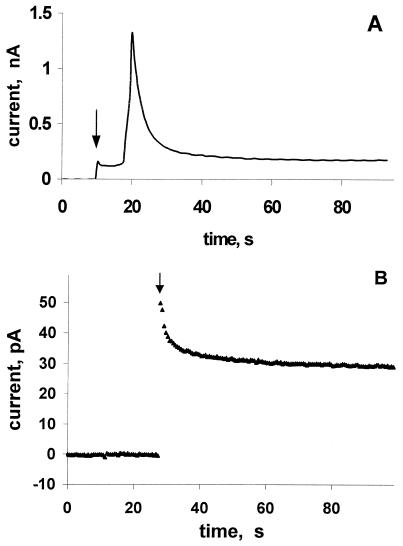
In contrast to quinone mediators, the standard potential of the I3−/I− couple is more positive. In this case, I− is oxidized to I3− by the tip, and I3− enters the cell where it is reduced to I−. Because I− cannot permeate the cell membrane, the CT pathway is easier to identify. The high current in Fig. 3C indicates that the rate of I3− reduction to I− in all three cell types is essentially diffusion controlled and almost identical. In curve 4 of Fig. 3C, iT at the cell surface (d/a << 1, where a is the tip radius) is almost five times higher than in the bulk solution. A tip current this high is possible only when the intracellular concentration of a reducing agent is at least ten times the concentration of I− in solution (24–26). It is not likely therefore that this reducing agent is I−. The addition of a low concentration of I2 to the I− solution quenches the regeneration of I− by the cell, as shown by a drastic decrease in IT (Fig. 3D). In this case, I3− enters the cell and depletes the intracellular concentration of a redox component responsible for I− regeneration. This depletion greatly diminishes the rate of I− regeneration by the cell (Fig. 3D), and would not be observed if either diffusion of preaccumulated I− from the cell (Fig. 2B) or a self-exchange mechanism (Fig. 2C) were responsible for regeneration of I−. Rather, this result is consistent with a bimolecular ET mechanism involving some intracellular reducing agent (Fig. 2D). Additional support for this model comes from iT vs. time dependence (Fig. 4A). Immediately after the application of a positive potential to the tip positioned near the cell surface, iT is low because the membrane is impenetrable to I− and contains no species capable of shuttling the charge. When I3− that is produced by the tip accumulates in the membrane, the iT increases sharply. An increase in conductivity by I3− was previously observed in lipid bilayers (23). A subsequent slow decrease in iT points to the depletion of an intracellular reducing species responsible for regeneration of I−.
Figure 4.
Current vs. time dependencies obtained at an UME tip positioned at a constant distance (about 5 μm) above the cell. Solution contained (A) 0.1 mM KI and (B) 30 μM 1,2-naphthoquinone. Tip potential was (A) 0.64 V and (B) −0.34 V vs Ag/AgCl reference. The arrow indicates when the potential was applied. The tip was (A) 5-μm-radius Pt, and (B) 5.5-μm-radius carbon fiber.
For 1,2-naphthoquinone or menadione, no initial increase in iT with time is observed (Fig. 4B) because both oxidized and reduced forms can enter the cell, and no accumulation of mediator in the membrane is required for charge shuttling. Comparison of Fig. 4 A and B shows that the intracellular concentration of a species capable of oxidizing menadiol is much lower than the concentration of the component reducing I3−. This conclusion is consistent with a much lower current in Fig. 3 A and B as compared with C. The availability of an intracellular oxidant is apparently the rate-limiting factor for menadione and 1,2-naphthoquinone mediators. This idea is further supported by the fact that an increase of menadione concentration in solution from 20 μM to 30 μM resulted in a marked decrease in the apparent rate constant of CT (k = 0.8 × 10−3, 0.9 × 10−3, and 2 × 10−3 cm/s for MDA-MB-231, 11α, and MCF-10A cells, respectively; compare with values in Table 1). According to earlier models (24–26), this observation suggests that a bimolecular ET mechanism (Fig. 2D) is in effect rather than simple diffusion of menadione from the cell (Fig. 2B), or a self-exchange reaction (Fig. 2C).
Table 1.
Variability of heterogeneous rate constants measured for different cell lines with menadione and 1,2-naphthoquinone mediators
| Cell type | Rate constant, ×10−3 cm/s
|
|
|---|---|---|
| Menadione | Naphthoquinone | |
| MCF-10A | 3.8 ± 0.4 (12) | 14 ± 1.1 (15) |
| 11α | 2.0 ± 0.3 (11) | 11 ± 1.0 (13) |
| MDA-MB-231 | 1.5 ± 0.15 (9) | 8 ± 1.2 (11) |
Measurements were made with either 20 μM menadione or 30 μM 1,2-naphthoquinone. The shown uncertainties are 95% confidence intervals. The number of experiments with different cells of the same type is indicated in parentheses.
The slower mediator regeneration rates observed for 11α and MDA-MB-231 cells may be related to a high level of PKCα expression, a common factor in these cell lines. Compared with MCF-10A cells, both cell lines are highly motile and exhibit slower proliferation rates (ref. 32; X.-g. Sun and S.A.R., unpublished data). For 11α cells, the slower proliferative rate was the direct outcome of PKCα overexpression (32). Slower proliferation would coincide with reduced metabolic activity overall, which could include the generation of cellular redox components. Despite slight quantitative differences in the SECM data obtained with individual cells of the same type, the lower oxidizing activity of 11α and MDA-MB-231 cells relative to MCF-10A cells was highly reproducible with different SECM tips and with different lipid-soluble mediators (Table 1). The responses obtained from cells grown in the same dish were essentially constant for about 2 h, after which the k value decreased. The loss of responsiveness presumably coincided with the loss of cell viability because dead cells gave pure negative feedback. As shown in Table 1, the difference in the redox responses given by nonmetastatic (MCF-10A) and metastatic (MDA-MB-231) cells was somewhat larger with menadione than with 1,2-naphthoquinone. Ongoing efforts are seeking to identify a mediator that further enhances this differential.
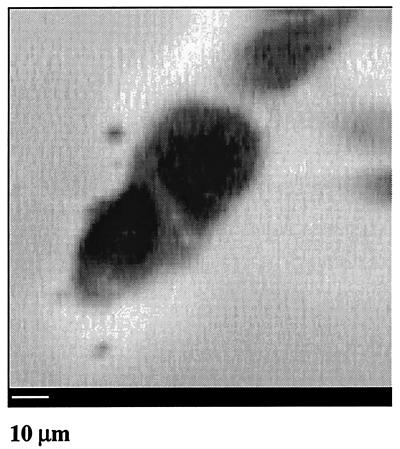
SECM can also be used for electrochemical mapping of redox activity in an individual cell. An image (Fig. 5) was obtained by using 40 μM 1,2-naphthoquinone as mediator for two aggregating MCF-10A cells and a neighboring cell. The bright halo at the cell periphery signifies the region of highest redox activity. A dark, redox inactive area in the center of the cell is a nucleus that is impenetrable to the mediator species.
Figure 5.
Normal human breast (MCF-10A) cells imaged by the SECM with a 1-μm-radius Pt tip and 40 μM 1,2-naphthoquinone as mediator.
This study has shown that the SECM is a suitable tool for probing ET reactions in single biological cells. Effective rate constants have been measured for bimolecular reactions between hydrophobic redox mediators and intracellular redox moieties. Significantly different redox activities of nontransformed breast cells, highly motile breast cells, and metastatic breast cells were observed. Because the scanning UME probe allows one to map transmembrane CT rates with high spatial resolution, a potential application of SECM is to detect aberrant responses in large fields of cells and tumor specimens.
Acknowledgments
Technical assistance with cell culture was provided by Mr. Wei Cheng and Dr. Xiao-guang Sun. This work was supported by the donors of the Petroleum Research Fund administered by the American Chemical Society (M.V.M.), The Gustavus and Louise Pfeiffer Foundation (S.A.R.), and The Professional Staff Congress of The City University of New York.
Abbreviations
- UME
ultramicroelectrode
- SECM
scanning electrochemical microscope
- CT
charge transfer
- ET
electron transfer
- PKCα
protein kinase Cα
References
- 1.Adams R N. Anal Chem. 1976;48:1128A–1134A. doi: 10.1021/ac50008a001. [DOI] [PubMed] [Google Scholar]
- 2.Wightman R M, Kennedy R T, Wiedemann D J, Kawagoe K T, Zimmerman J B, Leszczyszyn D J. In: Microelectrodes: Theory and Applications. Montenegro M I, Queir-s M A, Daschbach J L, editors. Dordrecht: Kluwer; 1991. pp. 453–462. [Google Scholar]
- 3.O'Neill R D. Analyst. 1994;119:767–780. doi: 10.1039/an9941900767. [DOI] [PubMed] [Google Scholar]
- 4.Wightman R M, Jankowski J A, Kennedy R T, Kawagoe K T, Schroeder T J, Leszczyszyn D J, Near J A, Diliberto E M, Jr, Viveros O H. Proc Natl Acad Sci USA. 1991;88:10754–10758. doi: 10.1073/pnas.88.23.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ewing A G, Strein T S, Lau Y Y. Acc Chem Res. 1992;25:440–447. [Google Scholar]
- 6.Kennedy R T, Huang L, Atkinson M A, Dush P. Anal Chem. 1993;65:1882–1887. doi: 10.1021/ac00062a012. [DOI] [PubMed] [Google Scholar]
- 7.Kuhr W G, Pantano P. Electroanalysis. 1995;7:405–416. [Google Scholar]
- 8.Lu H, Gratzl M. Anal Chem. 1999;71:2821–2830. doi: 10.1021/ac9811773. [DOI] [PubMed] [Google Scholar]
- 9.Yi C, Gratzl M. Biophys J. 1998;75:2255–2261. doi: 10.1016/S0006-3495(98)77670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bard A J, Fan F-R F, Mirkin M V. In: Electroanalytical Chemistry. Bard A J, editor. Vol. 18. New York: Dekker; 1994. pp. 243–373. [Google Scholar]
- 11.Bard, A. J. & Mirkin, M. V., eds. Scanning Electrochemical Microscopy (Dekker, New York), in press.
- 12.Lee C M, Kwak J Y, Bard A J. Proc Natl Acad Sci USA. 1990;87:1740–1743. doi: 10.1073/pnas.87.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsionsky M, Cardon Z G, Bard A J, Jackson R B. Plant Physiol. 1997;113:895–901. doi: 10.1104/pp.113.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasukawa T, Kondo Y, Uchida I, Matsue T. Chem Lett. 1998;8:767–768. [Google Scholar]
- 15.Yasukawa T, Kaya T, Matsue T. Anal Chem. 1999;71:4637–4641. [Google Scholar]
- 16.Stryer L. Biochemistry. New York: Freeman; 1995. [Google Scholar]
- 17.Mayfield S P, Danon A. Science. 1994;266:1717–1720. doi: 10.1126/science.7992056. [DOI] [PubMed] [Google Scholar]
- 18.Rabinowitz J D, Vacchino J F, Beeson C, McConnell H M. J Am Chem Soc. 1998;120:2464–2473. [Google Scholar]
- 19.Ottova-Leitmannova A, Tien H T. Progr Surf Sci. 1993;41:337–412. [Google Scholar]
- 20.Girault H H, Schiffrin D J. In: Charge and Field Effects in Biosystems. Allen M J, Usherwood P N R, editors. Turnbridge Wells: Abacus Press; 1984. pp. 171–186. [Google Scholar]
- 21.Moncelli M R, Becucci L, Guidelli R. Biophys J. 1996;70:2716–2722. doi: 10.1016/S0006-3495(96)79841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsionsky M, Bard A J, Mirkin M V. J Am Chem Soc. 1997;119:10785–10792. [Google Scholar]
- 23.Tsionsky M, Zhou J, Amemiya S, Fan F-R F, Bard A J, Dryfe R A W. Anal Chem. 1999;71:4300–4305. doi: 10.1021/ac9903858. [DOI] [PubMed] [Google Scholar]
- 24.Wei C, Bard A J, Mirkin M V. J Phys Chem. 1995;99:16033–16042. [Google Scholar]
- 25.Tsionsky M, Bard A J, Mirkin M V. J Phys Chem. 1996;100:17881–17888. [Google Scholar]
- 26.Barker A L, Macpherson J V, Slevin C J, Unwin P R. J Phys Chem B. 1998;102:1586–1598. [Google Scholar]
- 27.Gopalakrishna R, Anderson W B. Proc Natl Acad Sci USA. 1989;86:6758–6762. doi: 10.1073/pnas.86.17.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rotenberg S A. In: Cellular and Molecular Biology of Nitric Oxide. Laskin J D, Laskin D L, editors. New York: Dekker; 1999. pp. 171–198. [Google Scholar]
- 29.Batlle E, Verdu J, Dominguez D, del Mont Llosas M, Diaz V, Loukili N, Paciucci R, Alameda F, Garcia de Herreros A. J Biol Chem. 1998;273:15091–15098. doi: 10.1074/jbc.273.24.15091. [DOI] [PubMed] [Google Scholar]
- 30.La Porta C A M, Comolli R. Clin Exp Metastasis. 1997;15:568–579. doi: 10.1023/a:1018447531813. [DOI] [PubMed] [Google Scholar]
- 31.Soule H D, Maloney T M, Wolman S R, Peterson W D, Jr, Brenz R, McGrath C M, Russo J, Pauley R J, Jones R F, Brooks S C. Cancer Res. 1990;50:6075–6086. [PubMed] [Google Scholar]
- 32.Sun X-g, Rotenberg S A. Cell Growth Differ. 1999;10:343–352. [PubMed] [Google Scholar]
- 33.Liu B, Mirkin M V. J Am Chem Soc. 1999;121:8352–8355. [Google Scholar]