Abstract
Oxidative stress is a pervasive factor in aging and has been implicated in noise-induced cochlear pathology. In this study, we measured the activities of two enzymes that catalyze the removal of hydrogen peroxide (H2O2), catalase and glutathione peroxidase (Gpx), in 3 and 24 month old Fisher-344 rats, and reduced and oxidized glutathione in 3, 12, and 24 month old rats. There was an increase in Gpx activity in vascular tissue (spiral ligament and stria vascularis), but no change in modiolar, sensory or vestibular tissue of the cochlea. The elevation in vascular tissue was age-related. We observed a significant elevation of catalase activity in vestibular tissue, a tendency for age-related elevation in the modiolus, but no change in vascular or sensory cochlear tissue. These findings suggest that increased Gpx activity in vascular cochlear tissue may be an age-related compensation for a decrease in glutathione and a decline in the redox state measured by the ratio of reduced to oxidized glutathione.
Keywords: cochlea, aging, antioxidant enzymes, catalase, glutathione peroxidase, oxidative stress
1. Introduction
Oxidative stress is central to current theories of aging [2, 12, 13] and can affect a number of processes associated with aging including shortening of telomeric DNA, genomic instability, DNA mutations, and increased levels of protein cross linking [26]. Stress responses with associated elevation of reactive oxygen species (ROS) are accompanied by upregulation of antioxidant enzymes [18]. Current theories of aging hold that ROS, uncontrolled by antioxidant enzymes, or alternatively, accumulated ROS-induced damage can be primary signals for cell death.
Two major sources of ROS, superoxide anions (O2•−) and nitric oxide (NO•) can act together, in the form of peroxynitrate, or individually, to affect damage to proteins, lipids and DNA. Hydrogen peroxide, H2O2, is formed in the detoxification of O2•− by superoxide dismutases (SODs), enzymes said to form the first line of defense to oxidative stress. SODs have been extensively studied in cochlear pathologies and age-related hearing loss [7, 10, 24, 25, 29, 30]. In contrast, there are fewer reports of systems for removal of H2O2 in the cochlea. Although H2O2 has no unpaired electrons and is not highly reactive, it is classified as an ROS because it is a precursor for highly reactive and damaging downstream free radicals, like the hydroxyl anion. To prevent the formation of damaging H2O2 metabolites, antioxidant enzymes catalase and glutathione peroxidase function to rapidly convert H2O2 to water.
Catalase, found mainly in peroxisomes, converts H2O2 to water and molecular oxygen [20]. Age dependent changes in catalase activity appear to vary with tissue with reports of increased activity in muscle [15] and decreased activity in liver [32]. Aging effects on catalase mRNA in the rodent cochlea appear to depend on strain and species [6, 30]. Effects on cochlear enzymatic activity have not previously been reported.
Glutathione peroxidases (Gpx’s) catalyze the conversion of H2O2 to water and lipid peroxides to ROH using reduced glutathione as a proton and electron source. Gpx activity during aging also varies with tissue and species [18]. Studies of cochlear Gpx mRNA in Fischer 344 rats and C57BL6/J and CBA mice have not revealed significant age-related differences [6, 30]. However, the 8 rodent Gpx genes have not been exhaustively studied.
Glutathione is a thiol containing tripeptide antioxidant used by a variety enzymes in the detoxification of xenobiotics. It may be conjugated to toxic electrophilic substrates to render them less harmful and to facilitate their removal from cells, or, it may be covalently added to proteins in response to stress or in the regulation of normal cellular processes [8]. Alternatively, reduced glutathione (GSH) may be converted to oxidized glutathione (GSSG) by Gpx in the detoxification of peroxides. Like catalase and Gpx, GSH levels may rise or fall with age and age dependent changes appear to vary across tissues [16]. Our understanding of age-related changes in cochlear GSH metabolism is incomplete. Jiang et al. [17] reported increases in cochlear glutathionylated proteins. Lautermann et al. [21] reported age-related decreased GSH in the auditory nerve of Fischer 344 rats. Age-related changes in GSSG have not been reported due to the paucity of tissue from the cochlea and the low abundance of oxidized glutathione.
Aging Fischer 344/NHsd rats are a popular model for studies age-related hearing loss [3–5, 14, 31]. Audiograms of young rats are relatively flat (20 – 30 dB SPL) from 5 to 10 kHz and then slope to 35 dB SPL at 40 kHz. Hearing loss, reported elsewhere [4], develops progressively with age beginning with high frequencies (75 – 90 dB SPL, 20 – 40 kHz). Spiral ganglion cell [19] or OHC loss [4] do not fully account for the pathology. Remaining OHCs appear dysfunctional by measures of impaired otoacoustic emissions [4] and reduced levels of prestin [5]. Here, we present age-related changes in glutathione, catalase and Gpx.
2. Methods
Animals
Male Fisher-344/NHsd rats (NIA Harlan Colony, Indianapolis) aged 3, 12 or 24 months were housed in a quiet facility (<45 dBA) at least 48 h prior to use. A total of 58 albino rats were used, of which 40 were used for enzyme studies and 18 for glutathione studies. Animals that developed tumors or jaundice were rejected from the study. Experiments were approved by the Institutional Animal Care and Use Committee of the State University of New York at Buffalo in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Enzyme assays
Rats were exposed to CO2 just long enough to eliminate the toe pinch response and then killed by decapitation. Whole blood samples, brains and livers were frozen in liquid nitrogen. Either whole cochlea (preliminary experiments) or four separate tissues were dissected from the inner ear in ice cold PBS. The four discrete regions were 1) vestibular (sacculus, utricle, ampullae, and semicircular canals); 2) lateral wall (stria vascularis and spiral ligament); 3) cochlear sensory epithelium (basilar membrane, inner sulcus, and spiral limbus); and 4) the modiolus (bony modiolus, spiral ganglion, spiral modiolar arterial system and eighth nerve fibers).
Frozen tissue from two ears was thawed, pooled, and homogenized in 30 volumes of ice-cold tissue lysis buffer containing 0.1M sodium phosphate buffer (pH 7.8), 0.1 mM EDTA and a broad spectrum of protease inhibitors (Roche, Complete Protease Cocktail Tablets). Homogenates were centrifuged at 1,000 × g for 10 min at 4°C. Pellets containing unlysed cells and cellular debris were discarded. Protein concentration was determined by the bicinchoninic acid assay (Pierce, Rockford, IL).
Catalase activity in whole cochlea samples (preliminary experiments) or separate cochlear regions was measured in triplicate according to the method of Aebi [1] by monitoring the disappearance of H202 at 240 nm. Activity was determined from calibration curves obtained with purified catalase (Sigma) and reported as units per mg total protein, where 1 U activity = 1 μmol H202 converted to H20 per min.
Gpx activity was measured with a coupled enzyme assay [11]. This assay measures the oxidation of glutathione by Gpx indirectly by following its reduction and the concurrent oxidation of NADPH catalyzed by excess glutathione reductase (Sigma). NADPH oxidation was measured over a 10 min period at 340 nm. Gpx activity was determined using the molar extinction coefficient for NADPH at 340 nm and reported as units per mg total protein where 1 U activity = 1 μmol H202 converted to H20 per min.
Glutathione assay
Dissections consisted of only three tissue fractions. Cochlear sensory epithelium and lateral wall tissues were not separated because catalase and Gpx activities behaved with the same age-related tendencies for these two tissues and the time required for dissection was detrimental to the preservation of reduced and oxidized glutathione. Tissues were frozen in liquid nitrogen and stored at −80 °C under nitrogen. Tissue from 6 rats in each age group was pooled and assayed for GSH and GSSG by a modification of previously reported methods [9, 27]. Pooling of tissues in lieu of assays of biological replicates was necessary in order to obtain measurement of oxidized glutathione, providing data for the first report of redox ratio GSH:GSSG for rat cochlea. In order to provide an indication of the progression of age-related changes in glutathione redox potential, we added an intermediate age (12 months) in addition to the 3 and 24 month aged rats used for the enzyme experiments.
GSH and GSSG were determined using standard curves to obtain concentration in nmol per mg total protein. Briefly, tissue was homogenized in 0.5 ml 10% perchloric acid, 0.85% sodium chloride, and 1 mM bathophenanthroline-disulfonic acid. The homogenate was centrifuged at 9,000×g for 20 min at 4°C. The pellet was used for protein determination by a fluorescent dye binding assay (Quant-it, Invitrogen). The supernatant was used for GSH and GSSG determination. Sulfhydryl groups of GSH were derivatized with iodoacetic acid and γ-diglutamate (γ-glu-glu) was added to each sample as an internal standard. GSH, GSSG and γglu-glu were then derivatized on the alpha-amino group by addition of 1% 2, 4-dinitrofluorobenzene (DNFB) in absolute ethanol. Following derivitization, the reaction mixture was extracted with diethyl ether and methylene chloride in an organic cleanup. The resulting aqueous phase was concentrated by Speed Vac to 100 μl. 50 μl of the concentrate was injected on a Supelcosil LC-18 column (Supelco, 4.6 mm × 250 mm, 5 μm) connected with a Pelliguard LC-18 guard column (Supelco, 4.6 mm × 20 mm, 5 μm). Chromatographic separation was performed using a HP1100 series high performance liquid chromatography (HPLC) system (Agilent Technologies) and the eluant was monitored at 350 nm and 365 nm. Peaks were quantified against standard curves obtained using known concentrations of GSH and GSSG and reported in nmol per mg total protein normalized against γ-glu-glu for variability in the chemical derivitization process. Standard curves for GSH covered the range 0.05 – 100 μg/ml (0.16 – 325 nmol/ml). GSSG standards were used in the range 0.025 – 50 μg/ml (0.04 – 81.6 nmol/ml).
Statistical analysis
For catalase and Gpx assays, measurements for individual animals were performed in triplicate. Mean activities from 5 biological replicate animals for each enzyme and age group were compared using a one-tailed t-test, assuming unequal variance (Microsoft Excel). Statistical analysis of GSH and GSSG data was not possible since detection of GSSG required pooling tissue from 6 rats. In these experiments, an intermediate age group was added to facilitate interpretation.
3. Results
Enzymes
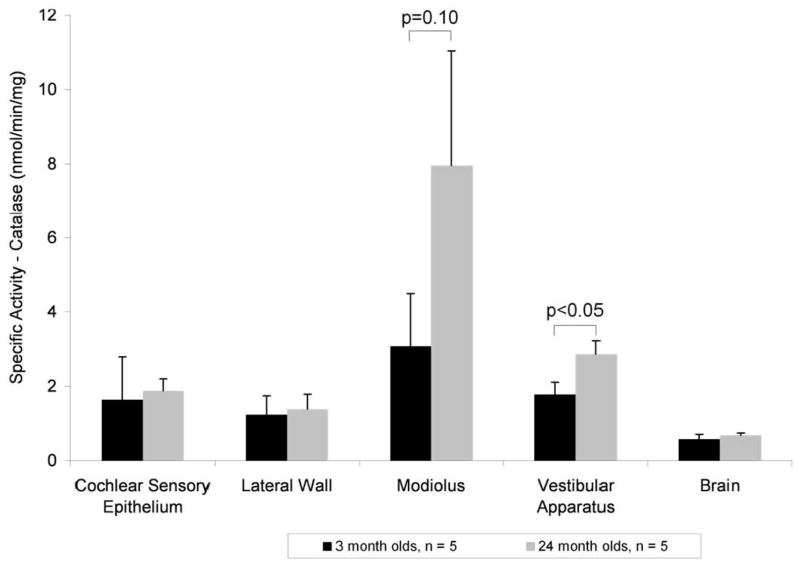
Preliminary enzyme assays, performed on whole cochleae resulted in no significant difference in catalase activities between 3 and 24 months (data not shown). In order to determine if sub-regions of the cochlea undergo age-dependent changes in enzyme activity, we assayed tissue from cochlear sensory epithelium, lateral wall and modiolus separately. Catalase specific activity in young rats ranged from 1 to 2 nmol H202 converted to H20/min/mg protein for the cochlear sensory epithelium, lateral wall and vestibular tissues. Brain catalase was lower, at 0.5 nmol/min/mg, while modiolar activity was higher at 3 nmol/min/mg. There was no significant difference between the activities of 3 and 24 month rats for cochlear sensory epithelium, lateral wall and brain. There was a significant 1.5 fold increase in activity for vestibular tissue from old rats and a tendency for an age-dependent increase in modiolar tissue (Fig. 1).
Figure 1.
Catalase activity in aging rat inner ear. Tissues from rats 3 months (black) and 24 months (gray) assayed for catalase activity with a significant increase in vestibular activity (p < 0.05) and a tendency for an increase in modiolus (p = 0.1). N = 5 rats per age group, means ± standard deviation.
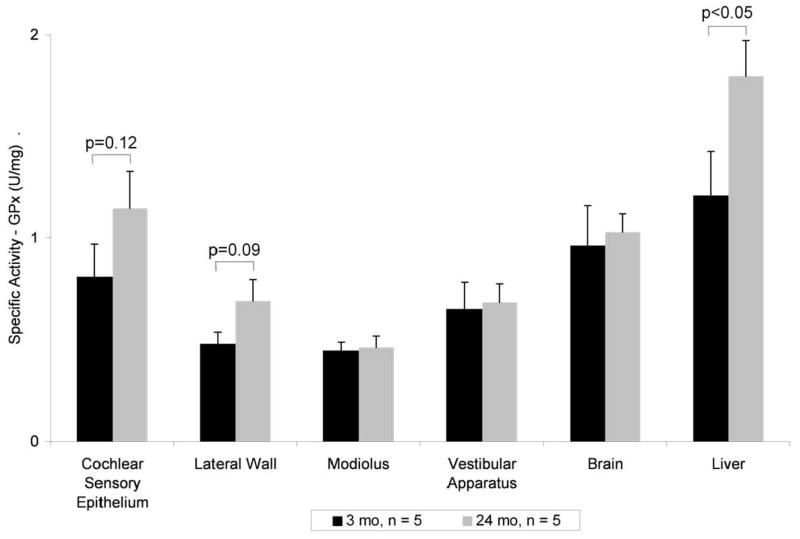
Gpx activity of the cochlear sensory epithelium of young rats (0.8 U/mg protein) was comparable to that of brain and liver and greater than levels measured in lateral wall and modiolus. There was no significant change in activity with age for modiolus, vestibule, or brain. In contrast, there was a significant increase in liver Gpx activity between 3 and 24 months and a tendency for an increase in cochlear sensory epithelium and lateral wall (Fig. 2).
Figure 2.
Glutathione peroxidase (Gpx) activity in aging rat inner ear. Tissues from rats 3 months (black) and 24 months (gray) assayed for Gpx activity with a significant increase in liver (control, p < 0.05) and a tendency for an increase in cochlear sensory epithelium (p = 0.12) and lateral wall (p = 0.09). N = 5 rats per age group, means ± standard deviation.
Glutathione
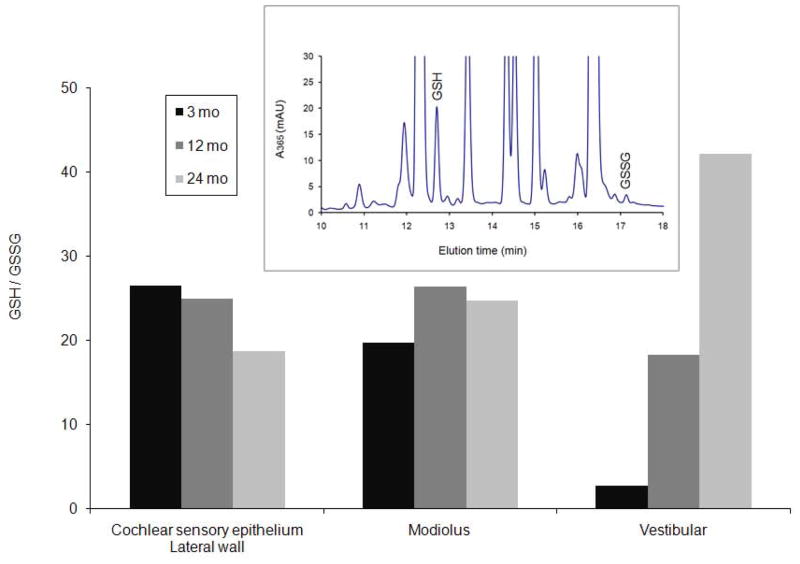
GSH and GSSG were measured by HPLC after derivitization with DNFB (Fig. 3). GSH levels changed little between 3 and 12 months (14% increase for combined cochlear sensory epithelium and lateral wall, 20% increase for modiolus, and 25 % decrease for vestibular). At 24 months, GSH levels dropped 46% of 3 month old levels in combined cochlear sensory epithelium and lateral wall, but returned to within 5% of 3 month levels in both modiolar and vestibular tissue (Table 1). GSSG decreased with age for modiolus and vestibular samples. By 24 months, GSSG for combined cochlear sensory epithelium and lateral wall dropped by 35% from 3 month old levels. (Table 1). GSSG peaks, though small, were within the range of calibration standards used and were clearly discernable from background. The resulting redox state measured by the ratio of GSH:GSSG progressively decreased with age for membranous labyrinth tissue from the cochlea, but increased in modiolar and vestibular tissue (Fig. 3).
Figure 3.
GSH:GSSG in aging rat inner ear. Pooled tissue from rats 3 months (black), 12 months (dark gray), and 24 months (light gray). Inset: Chromatogram from HPLC separation of DNFB-derivatized 3 month cochlear sensory epithelium – lateral wall extracts. Derivatized GSH and GSSG have a detectable adsorption at 365 nm and elute with retention times 12.8 and 17.2 min.
Table 1.
Reduced and Oxidized Glutathione Levels in Cochlear Tissue
| Specific Concentration (nmol/mg rotein)* | ||||
|---|---|---|---|---|
| Metabolite | Tissue | Age: 3 mo | 12 mo | 24 mo |
| GSH | CSE/Lat Wall | 3.45 | 3.94 | 1.587 |
| Modiolus | 5.72 | 6.84 | 5.729 | |
| Vestibular | 2.52 | 1.88 | 2.646 | |
| GSSG | CSE/Lat Wall | 0.130 | 0.158 | 0.085 |
| Modiolus | 0.290 | 0.258 | 0.231 | |
| Vestibular | 0.929 | 0.103 | 0.063 | |
Each value from a single measurement obtained after pooling tissue from 6 rats (12 cochleae).
4. Discussion
The results of the present study suggest that tissues of the inner ear respond to age-related oxidative stress differently. Vestibular and modiolar tissue responded with increased catalase activity with age, while sensory and vascular tissues of the cochlea responded by a tendency to elevate Gpx. Age dependent decrease in GSH:GSSG (Fig. 3) may signal a compensatory upregulation of Gpx activity in sensory epithelium and lateral wall (Fig. 2). In contrast, in tissues where GSH:GSSG increased (modiolus and vestibular), there was no age-related increase in Gpx activity.
A tendency for increased Gpx activity in lateral wall and cochlear sensory epithelium are consistent with a previous report for both selenium-dependent and selenium-independent Gpx activity in aging Fischer 344 rat cochlea [21]. On the other hand, the lack an aging effect on modiolar Gpx activity is inconsistent with the same report which showed a tendency for increased activities of selenium-dependent and –independent activity [21]. Direct comparison of the two reports is complicated by differences in extraction methods, assay methods and the complexity of the rat genome (8 distinct Gpx genes). A careful analysis of the cochlear tissue and cell type specificity and quantitative expression of these genes with age would be useful to sort through the complexity.
To our knowledge, this is the first report of catalase activity in aging cochlea. Unlike Gpx, there is only one catalase gene in rat. In whole cochlea from C57BL/6J mice, catalase mRNA increased progressively over the first 6 months of age [30]. In Fischer 344 rats, catalase mRNA levels did not change between the ages of 8 and 26 months [6]. This agrees with our preliminary data with whole cochlea from similar ages of the same rat (not shown). It was only through dissection of the cochlear tissues that we observed a tendency for increased catalase activity in modiolus (Fig. 1). This tendency in modiolus and the significant age-dependent increase in vestibular catalase activity indicate an increased demand for detoxification of H2O2 in these two compartments of the aging inner ear. The importance of H2O2 removal from inner ear tissues is suggested by higher catalase activity in ear tissues compared to brain.
Age dependent reduction of GSH:GSSG in sensory epithelium and lateral wall is consistent with that in many other tissues [23] and may be related to increased Gpx activity through one of the mechanisms discussed above. Our data are consistent with a previous report of an age-dependent increase of vestibular GSH and a slight reduction of GSH in sensory epithelium from Fischer rat cochleae, but inconsistent with an increase in lateral wall GSH and a significant decrease in modiolar GSH from the same animal [21]. In Fischer 344 rat lung and kidney decline in GSH:GSSG appears to be due to a decrease in expression of the glutathione synthase gene encoding the second enzyme in de novo glutathione synthesis [22], however, in most studies age-dependent depletion of GSH has been assigned to decreased levels of the first enzyme in denovo synthesis, glutamate cysteine ligase (GCL, also known as γ-glutamyl synthase) and nuclear regulatory factor 2, Nrf-2, the transcription factor that signals stress-activated synthesis of GCL [23]. Additional assays of reduced and oxidized glutathione and enzymes related to glutathione metabolism are required to more fully understand the response of this system in aging.
The accumulation of oxidative damage to lipids, proteins and DNA forms the basis of the free radical theory of aging [12]. Though many other factors contributing to aging have been identified, the importance of oxidative damage and the antioxidant system in aging is still undisputed [13]. Studies suggesting the importance of oxidative stress in aging of the inner ear are not surprising [33]. Age-related increases in 4-hydroxynonenal, tyrosine nitration, and glutathionylation of cochlear proteins have been reported [17]. Previous studies from our laboratory and others have indicated increased mitochondrial deletions in mouse spiral ganglion [7, 28], DNA damage in OHCs by terminal deoxynucleotidyl transferase dUTP nick end labeling [14] and decreased levels of OHC motor protein, prestin [5]. Though this loss has not been directly linked to protein oxidation, increased turnover of prestin triggered by oxidative damage is likely related to the mechanisms that result in an accumulation of dysfunctional OHCs in aged Fischer rats [4].
An advantage to measuring enzyme activity as opposed to mRNA, total enzyme level, or even post-translational modification of enzymes is that biological activity is the end result to gene induction and regulation of protein level and modification. However, it is important to note that the enzyme activity results reported here are normalized with respect to total protein and that what is more critical is the aging induced stress response of individual cells and subcellular organelles as exemplified in peroxisomal aging which involves the missorting of catalase [32]. One might expect that studies of the cellular and subcellular distribution of peroxide removal enzymes and metabolites would reveal more striking age-related changes.
Acknowledgments
We acknowledge support from NIH/NIDCD, grant 5R01DC006862-03 awarded to DH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 2.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 3.Bielefeld EC, Coling D, Chen GD, Henderson D. Multiple dosing strategies with acetyl L-carnitine (ALCAR) fail to alter age-related hearing loss in the Fischer 344/NHsd rat. J Negat Results Biomed. 2008;7:4. doi: 10.1186/1477-5751-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bielefeld EC, Coling D, Chen GD, Li M, Tanaka C, Hu BH, Henderson D. Age-related hearing loss in the Fischer 344/NHsd rat substrain. Hear Res. 2008;241:26–33. doi: 10.1016/j.heares.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen GD, Li M, Tanaka C, Bielefeld EC, Hu BH, Kermany MH, Salvi R, Henderson D. Aging outer hair cells (OHCs) in the Fischer 344 rat cochlea: function and morphology. Hear Res. 2009;248:39–47. doi: 10.1016/j.heares.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, Ruan R. Identifying stable reference genes for evaluation of antioxidative enzyme gene expression in auditory cortex and cochlea of young and old Fischer 344 rats. Acta Otolaryngol. 2008:1–9. doi: 10.1080/00016480802311015. [DOI] [PubMed] [Google Scholar]
- 7.Coling DE, Yu KC, Somand D, Satar B, Bai U, Huang TT, Seidman MD, Epstein CJ, Mhatre AN, Lalwani AK. Effect of SOD1 overexpression on age- and noise-related hearing loss. Free Radic Biol Med. 2003;34:873–880. doi: 10.1016/s0891-5849(02)01439-9. [DOI] [PubMed] [Google Scholar]
- 8.Dalle-Donne I, Rossi R, Colombo G, Giustarini D, Milzani A. Protein S-glutathionylation: a regulatory device from bacteria to humans. Trends Biochem Sci. 2009;34:85–96. doi: 10.1016/j.tibs.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Dominick PK, Cassidy PB, Roberts JC. A new and versatile method for determination of thiolamines of biological importance. J Chromatogr B Biomed Sci Appl. 2001;761:1–12. doi: 10.1016/s0378-4347(01)00298-5. [DOI] [PubMed] [Google Scholar]
- 10.Endo T, Nakagawa T, Iguchi F, Kita T, Okano T, Sha SH, Schacht J, Shiga A, Kim TS, Ito J. Elevation of superoxide dismutase increases acoustic trauma from noise exposure. Free Radic Biol Med. 2005;38:492–498. doi: 10.1016/j.freeradbiomed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Flohe L, Gunzler WA. Assays of glutathione peroxidase. Methods Enzymol. 1984;105:114–121. doi: 10.1016/s0076-6879(84)05015-1. [DOI] [PubMed] [Google Scholar]
- 12.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 13.Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann N Y Acad Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 14.Hu BH, Yang WP, Bielefeld EC, Li M, Chen GD, Henderson D. Apoptotic outer hair cell death in the cochleae of aging Fischer 344/NHsd rats. Hear Res. 2008;245:48–57. doi: 10.1016/j.heares.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji LL, Dillon D, Wu E. Alteration of antioxidant enzymes with aging in rat skeletal muscle and liver. Am J Physiol. 1990;258:R918–923. doi: 10.1152/ajpregu.1990.258.4.R918. [DOI] [PubMed] [Google Scholar]
- 16.Ji LL, Hollander J. Antioxidant defence: effects of aging and excercise. Human Kinetics; Champaign: 2000. [Google Scholar]
- 17.Jiang H, Talaska AE, Schacht J, Sha SH. Oxidative imbalance in the aging inner ear. Neurobiol Aging. 2007;28:1605–1612. doi: 10.1016/j.neurobiolaging.2006.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Junqueira VB, Barros SB, Chan SS, Rodrigues L, Giavarotti L, Abud RL, Deucher GP. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hear Res. 1992;59:171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- 20.Kirkman HN, Gaetani GF. Mammalian catalase: a venerable enzyme with new mysteries. Trends Biochem Sci. 2007;32:44–50. doi: 10.1016/j.tibs.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Lautermann J, Crann SA, McLaren J, Schacht J. Glutathione-dependent antioxidant systems in the mammalian inner ear: effects of aging, ototoxic drugs and noise. Hear Res. 1997;114:75–82. doi: 10.1016/s0378-5955(97)00154-8. [DOI] [PubMed] [Google Scholar]
- 22.Liu RM, Dickinson DA. Decreased synthetic capacity underlies the age-associated decline in glutathione content in Fisher 344 rats. Antioxid Redox Signal. 2003;5:529–536. doi: 10.1089/152308603770310176. [DOI] [PubMed] [Google Scholar]
- 23.Maher P. The effects of stress and aging on glutathione metabolism. Ageing Res Rev. 2005;4:288–314. doi: 10.1016/j.arr.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 24.McFadden SL, Ding D, Reaume AG, Flood DG, Salvi RJ. Age-related cochlear hair cell loss is enhanced in mice lacking copper/zinc superoxide dismutase. Neurobiol Aging. 1999;20:1–8. doi: 10.1016/s0197-4580(99)00018-4. [DOI] [PubMed] [Google Scholar]
- 25.McFadden SL, Ding D, Salvi R. Anatomical, metabolic and genetic aspects of age-related hearing loss in mice. Audiology. 2001;40:313–321. [PubMed] [Google Scholar]
- 26.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43:477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Reed DJ, Babson JR, Beatty PW, Brodie AE, Ellis WW, Potter DW. High-performance liquid chromatography analysis of nanomole levels of glutathione, glutathione disulfide, and related thiols and disulfides. Anal Biochem. 1980;106:55–62. doi: 10.1016/0003-2697(80)90118-9. [DOI] [PubMed] [Google Scholar]
- 28.Seidman MD, Bai U, Khan MJ, Quirk WS. Mitochondrial DNA deletions associated with aging and presbyacusis. Arch Otolaryngol Head Neck Surg. 1997;123:1039–1045. doi: 10.1001/archotol.1997.01900100009001. [DOI] [PubMed] [Google Scholar]
- 29.Sha SH, Zajic G, Epstein CJ, Schacht J. Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol. 2001;6:117–123. doi: 10.1159/000046818. [DOI] [PubMed] [Google Scholar]
- 30.Staecker H, Zheng QY, Van De Water TR. Oxidative stress in aging in the C57B16/J mouse cochlea. Acta Otolaryngol. 2001;121:666–672. doi: 10.1080/00016480152583593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka C, Bielefeld EC, Chen GD, Li M, Henderson D. Ameliorative effects of an augmented acoustic environment on age-related hearing loss in middle-aged Fischer 344/NHsd rats. Laryngoscope. 2009 doi: 10.1002/lary.20244. [DOI] [PubMed] [Google Scholar]
- 32.Terlecky SR, Koepke JI, Walton PA. Peroxisomes and aging. Biochim Biophys Acta. 2006;1763:1749–1754. doi: 10.1016/j.bbamcr.2006.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willott JF, Hnath Chisolm T, Lister JJ. Modulation of presbycusis: current status and future directions. Audiol Neurootol. 2001;6:231–249. doi: 10.1159/000046129. [DOI] [PubMed] [Google Scholar]