Abstract
Background
Infection of human immunodeficiency virus (HIV) has been associated with several chronic diseases including pulmonary artery hypertension and atherosclerosis. However, the underlying mechanisms of these vascular complications are largely unknown. The objective of this study was to test a novel hypothesis that HIV Nef, an accessory HIV protein, may directly affect endothelial functions and gene expression in pulmonary arteries.
Methods
Fresh porcine pulmonary artery rings and human pulmonary artery endothelial cells (HPAECs) were treated with HIV Nef for 24 h. With a myograph device, vasomotor function was determined with thromboxane A2 analog, U4661910, for contraction, bradykinin and sodium nitroprusside for relaxation. The expression of endothelial nitric oxide synthase (eNOS) was determined with real time PCR and immunohistochemistry. Nitric oxide (NO) production was determined by Calorimetric Nitric Oxide Assay kit. Superoxide anion levels were detected with lucigenin-enhanced chemiluminescence assay and dihydroethidium (DHE) staining.
Results
The endothelium-dependent vasorelaxation in response to bradykinin was significantly reduced in HIV Nef-treated porcine pulmonary artery rings in a concentration-dependent manner. In response to bradykinin (10-8 mol/L), HIV Nef (10 ng/mL) significantly reduced vasorelaxation by 32% compared with untreated controls (P < 0.05). In addition, HIV Nef significantly decreased eNOS expression in the vessels and HPAECs. HIV Nef at 10 ng/mL significantly decreased NO production in HPAECs by 21% compared with controls (P < 0.05). Furthermore, HIV Nef significantly increased superoxide anion production in porcine pulmonary arteries and HPAECs compared with controls (P < 0.05). Consequently, Mn (III) tetrakis porphyrin, a superoxide dismutase mimic, effectively blocked HIV Nef-induced vasomotor dysfunction and superoxide anion production. The specificity of HIV Nef action was confirmed by anti-Nef antibody blocking and Nef heat-inactivation.
Conclusions
HIV Nef protein significantly decreases endothelium-dependent vasorelaxation in porcine pulmonary arteries. It also reduces eNOS expression and induces oxidative stress in both porcine pulmonary arteries and HPAECs. This study demonstrates a new mechanism of HIV Nef, which causes endothelial dysfunction and may contribute to the human pulmonary artery disease in HIV-infected patients.
Keywords: HIV Nef, pulmonary artery hypertension, endothelial nitric oxide synthase, oxidative stress, pulmonary artery endothelial cells
INTRODUCTION
The World Health Organization and UNAIDS estimate that 33.2 million people were living with HIV at the end of 2007, with 2.5 million new infections and 2.1 million AIDS related deaths in that same year [1]. HIV primarily attacks the immune system, leading to infection and eventual death from opportunistic infections [2]. However, with better understanding and treatment of the disease, the non-infectious complications of HIV infection are becoming apparent. For example, HIV has been associated with pulmonary artery hypertension and atherosclerosis [3-6]. There is a growing body of work which has looked at the vascular endothelium as the silent target of HIV. To date, however, the underlying mechanisms of HIV-associated vascular complications are largely unknown [7].
HIV-related pulmonary arterial hypertension (HIV-PAH) is estimated to affect 0.5% of HIV-infected individuals. The etiology of HIV-PAH is not clear. Because the actual virus has not been identified in the vascular lesions or the endothelium, other potentially causative factors have been the subjects of investigation [8]. These range from inflammatory mediators to viral proteins, genetic predisposition and environmental factors. Among these viral proteins, HIV Nef has been shown to be present in vascular cells of patients with HIV-PAH suggesting a role in the development of severe pulmonary arterial disease [8]. Despite evidence for the role of Nef in HIV-PAH, the pathogenesis is unclear. We hypothesized that HIV Nef, an accessory HIV protein, may directly affect endothelial functions and gene expression in pulmonary arteries.
HIV viral protein Nef (the ‘negative factor’) is a protein that is critical for maintaining high viral loads in HIV infection and accelerating the clinical progression to AIDS [9-12]. It is a small protein without distinct enzymatic activity. Its length is polymorphic with a range of 200-215 amino acids. As an accessory protein, it serves to alter host cell proteins by performing aberrant functions which amplify viral replication [12]. Nef enhances HIV infectivity by downregulating CD4 and major histocompatibility complex I and by activation of p21-activated protein kinase (Pak2) [13]. Nef has been identified in HIV-PAH cells, but not in those with idiopathic PAH or controls [14], suggesting a role in development of PAH in HIV patients.
In this paper, we examine effects of HIV Nef on fresh porcine pulmonary artery rings and human pulmonary artery endothelial cells (HPAECs) including vasomotor function, endothelial nitric oxide synthase (eNOS) expression and superoxide anion production. It is well established that nitric oxide (NO) is a critical molecule in vascular physiology. In pathologic conditions, NO bioavailability is decreased by eNOS inhibition or by interaction with reactive oxygen species (ROS) such as superoxide anion. This study may advance our understanding of HIV-associated pulmonary hypertension and provide new potential targets for therapeutic intervention.
MATERIALS AND METHODS
Chemicals and Reagents
Recombinant HIV-1 Nef protein and HIV-1 Nef antiserum were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Thromboxane A2 analogue U46619, bradykinin, and sodium nitroprusside (SNP) were obtained from Sigma Chemical (St. Louis, Mo). Dihydroethidium (DHE) was purchased from Molecular Probes (Eugene, OR). Dulbecco modified Eagle’s medium (DMEM) was obtained from Life Technologies, Inc (Grand Island, NY). EGM-2 was purchased from Cambrex (San Diego, CA). Lucigenin was obtained from Molecular Probes (Eugene, Ore). Antibody against human eNOS was obtained from BD Transduction Laboratories (Lexington, KY). The biotinylated horse anti-mouse IgG and avidin-biotin complex kit were obtained from Vector Labs (Burlingame, CA). Mn (III) tetrakis (4-benzoic acid) porphyrin (MnTBAP) was purchased from A.G. Scientific (San Diego, CA).
Myograph Model
Fresh porcine lungs were harvested from young adult farm pigs (6-7 months old) at a local slaughterhouse, placed in a container filled with cold PBS solution, and immediately transported to the laboratory. Porcine pulmonary arteries (the third division with 3-4 mm diameter) were isolated and cut into 5-mm rings. The rings were then incubated in DMEM with 1, 10 or 50 ng/mL of Nef at 37°C and 5% CO2 for 24 h. The myograph tension system used in our laboratory has been previously described [15]. Briefly, the rings were suspended between the wires of the organ bath myograph chamber in 6 ml of Kreb’s solution, maintained at 37°C and oxygenated with pure oxygen gas. Rings were slowly subjected stepwise to a predetermined optimal tension of 3 mN, and each ring was precontracted with thromboxane A2 analogue U46619 (3×10-6 mol/L). Following 30-60 min of contraction, a relaxation curve was generated by adding 60 μL of five cumulative additions of the endothelium-dependent vasodilator bradykinin (final concentrations 10-11 to 10-7 mol/L) every 3 min. In addition, SNP (final concentration 10-6 mol/L) was added into the organ bath, and endothelium-independent vasorelaxation was recorded.
Cell Culture
HPAECs were purchased from Cambrex (San Diego, CA). The cells were used at passage 4 to 5. When HPAECs grew to 80%-90% confluence in 6-well plates, they were applied to different treatments with different concentrations (1, 10 or 50 ng/mL) of Nef or co-culturing with MnTBAP (2 μM) for 24 h. Cells cultured in EGM-2 alone were used as negative controls.
Real-Time PCR
Porcine endothelial cells were isolated from cultured porcine pulmonary artery rings by scraping the luminal surface with surgical blades. Total RNA from porcine endothelial cells and HPAECs was isolated using an Ambion RNAqueous-4PCR kit (Austin, TX). Primers of eNOS (human and porcine), human GAPDH and porcine CD31 were designed via the Beacon Designer 2.1 software (Bio-Rad) [16]. Porcine CD31 was used to normalize porcine eNOS mRNA levels in order to exclude potential protein contaminations from porcine smooth muscle cells. For HPAECs, GAPDH was used to normalize the eNOS mRNA levels. The iQ SYBR green Supermix Kit and iCycler iQ Real-time PCR detection system (Bio-Rad) were used in real-time PCR. Controls were performed with no RT (mRNA sample only) or no mRNA (water only) to demonstrate the specificity of the primers and the lack of DNA contamination in samples. Relative mRNA levels of eNOS was presented as 2^[Ct(GAPDH or CD31)-Ct(gene of interest)] as previously described [17].
Immunohistochemistry
Treated porcine pulmonary artery rings were fixed in 10% formalin and embedded in paraffin. Samples were sectioned at a 5 μm thickness and slides were incubated in 0.3% hydrogen peroxide solution to quench endogenous peroxidase activity for 15 min. The monoclonal antibody against human eNOS (1:500) was applied for 30 min at room temperature. After washing with PBS, the sections were incubated with biotinylated secondary antibody for 30 min. An avidin-biotin reaction using peroxidase enzyme was used for protein detection (ABC kit; Vector Laboratories, Burlingham, CA). Immune complexes were detected with diaminobenzidine under the microscope before counterstaining with hematoxylin for 2 min. Images were captured under microscope with an attached SPOT-RT digital camera and software (Diagnostic Instruments, Sterling Heights, Mich).
Nitrite Detection
NO levels released from HPAECs were determined by measuring the accumulation of its stable degradation products, nitrite and nitrate (Griess reaction NO assay kit, Calbiochem). Nitrate is reduced to nitrite by nitrate reductase. Thus, total nitrite levels represent total NO levels. HPAECs were cultured with or without Nef for 24 h. The supernatant was collected and total nitrite levels were measured. Absorbance of the samples was determined at 540 nm wavelength and compared with standard solutions. The amount of nitrite detected was normalized to total proteins of HPAECs (μmol/L/mg).
Superoxide Anion Measurement
Levels of superoxide anion produced by endothelial cells of the porcine arteries were detected using the lucigenin-enhanced chemiluminescence method as previously described in our studies [15]. Six sets of vessel rings in each group were used. Briefly, the rings were cut open longitudinally and trimmed into 5 × 5-mm pieces. An assay tube was filled with 5 μmol/L lucigenin. Time-based reading of the luminometer was recorded. The data, in relative light units per second (RLU/sec) for each sample, were averaged between 5 and 10 min. Values of blank tubes containing the same reagents as the vessel ring samples were subtracted from their corresponding vessel samples. The area of each vessel segment was measured using a caliper and was used to normalize the data for each sample. Final data were represented as RLU/sec/mm2. For detecting superoxide anion in HPAECs, cells were harvested with 0.02% Trypsin/EDTA and adjusted to 1 × 106 cells per FACS tube. DHE (3 μmol/L) was added, and incubated in 37°C for 30 min. ROS in the cells were analyzed by FACS Calibure flow cytometry (Becton Dickinson, San Jose, CA). In each experiment, at least 10,000 events were analyzed.
Statistical Analysis
All data are presented as the mean ± SEM. Inter-group differences were analyzed using one-way ANOVA for comparison of three or more groups. Student’s t-test was used for comparison between two groups. A P value < 0.05 was regarded as significant.
RESULTS
HIV Nef Impairs Vasomotor Functions in Porcine Pulmonary Artery Rings
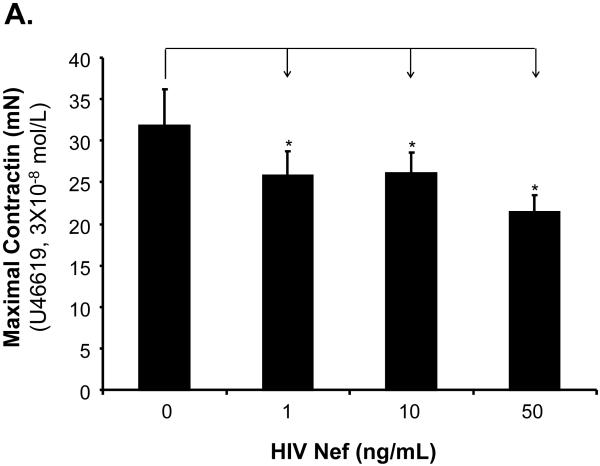
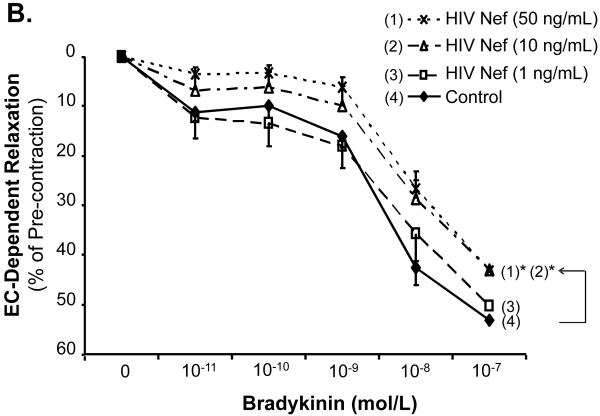
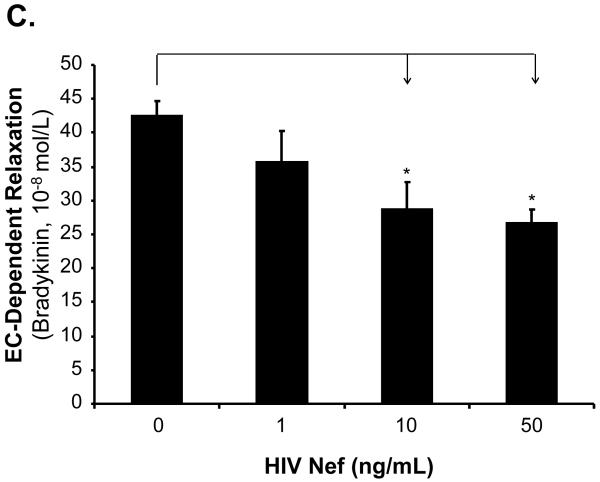
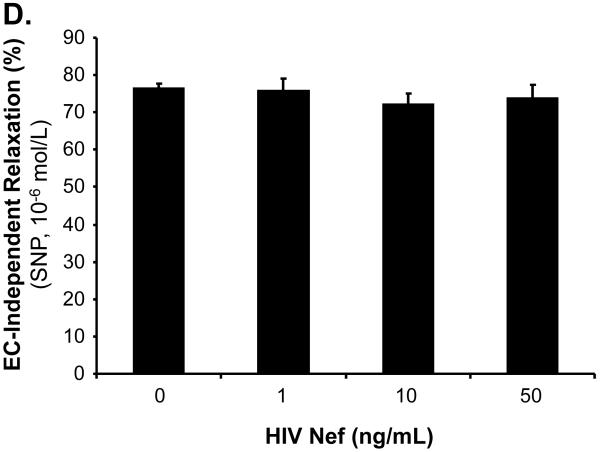
The effects of HIV Nef on vasomotor functions in porcine pulmonary arteries were first tested by a myograph system including vessel contraction (U46619), endothelium-dependent (bradykinin) and endothelium-independent (SNP) relaxation assays. Maximal contraction was reduced in Nef-treated groups in a concentration-dependent manner (Fig. 1A). In response to bradykinin at 10-8mol/L, endothelium-dependent vasorelaxation of the rings was significantly reduced in Nef-treated groups in a concentration-dependent manner (Fig. 1B, C). At 10 or 50 ng/mL of Nef, the endothelium-dependent relaxation was significantly reduced by 32% or 37%, respectively, compared with controls (Fig. 1C, P < 0.05). However, in response to SNP (10-6 mol/L), endothelium-independent vasorelaxation showed no difference between Nef-treated and control groups (Fig. 1D).
FIG 1.
Effects of HIV Nef on vasomotor functions in porcine pulmonary arteries. Porcine pulmonary artery rings were treated with Nef (0, 1, 10 and 50 ng/mL) for 24 h. (A). Maximal contraction of porcine pulmonary artery rings in response to U46619 (3×10-6 mol/L). (B). Precontracted vessels were tested for endothelium-dependent relaxation by adding a series of concentrations of bradykinin (10-11 to 10-7 mol/L). (C). Endothelium-dependent relaxation in response to bradykinin (10-8 mol/L). (D). Endothelium-independent relaxation in response to SNP (10-6 mol/L). *P < 0.05 versus controls (medium only). n = 12.
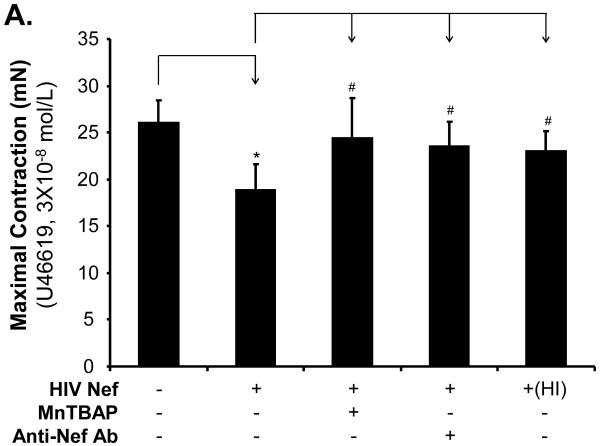
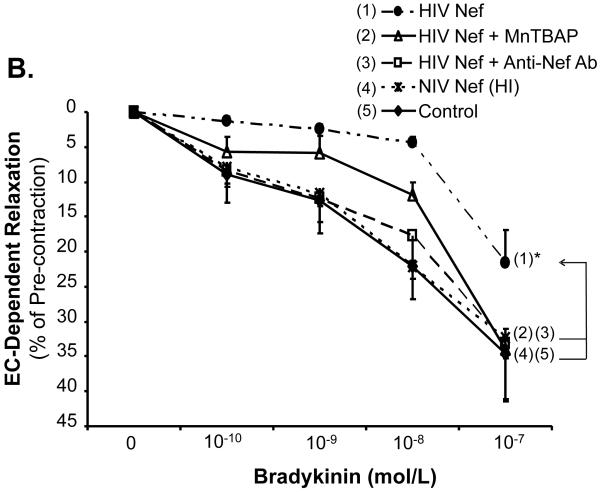
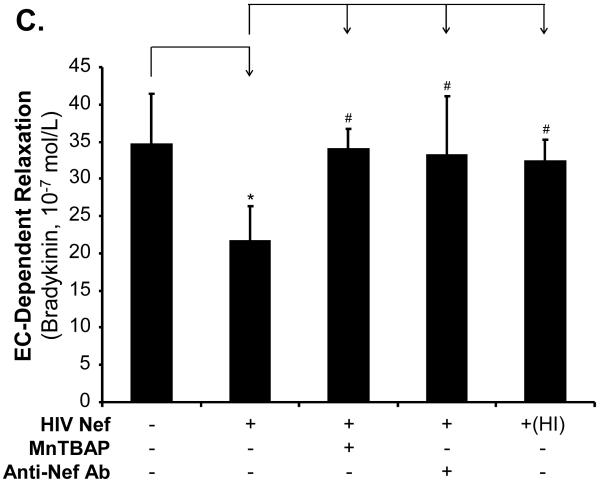
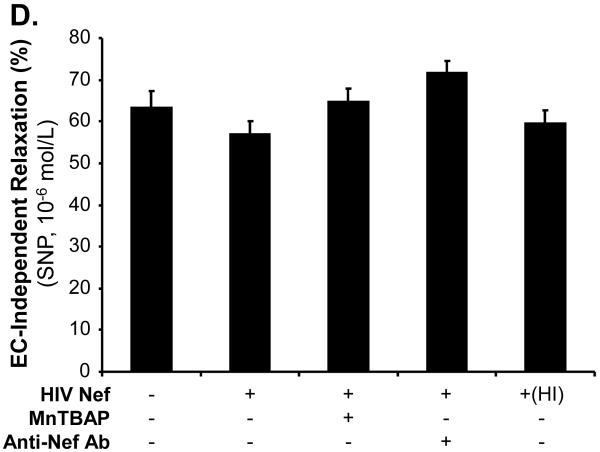
In order to determine the specificity of the Nef action, a neutralizing antibody against Nef was used along with Nef and completely abolished Nef-induced reduction of maximal contraction in response to U46619 (Fig. 2A, P < 0.05), as well as the inhibition of endothelium-dependent vasorelaxation of the rings, compared with the Nef treatment group (Fig. 2B and C, P < 0.05). There was no significant effect of anti-Nef antibody on the endothelium-independent vasorelaxation of the rings (Fig. 2D). Heat-inactivated Nef was also used as a negative control and showed no effect on the maximal contraction, endothelium-dependent and -independent vasorelaxation of the rings (Fig. 2 A-D).
FIG 2.
Effects of MnPBAP, neutralizing antibody to Nef and heat-inactivated Nef on vasomotor dysfunction in porcine pulmonary arteries. Effects of MnPBAP, neutralizing antibody to Nef and heat-inactivated Nef on maximal contraction (A), endothelium-dependent relaxation in response to bradykinin (B, C), and endothelium-independent relaxation in response to SNP (10-6 mol/L) (D). *P < 0.05 versus Nef (10 ng/mL), #P < 0.05 versus controls (medium only), n = 10.
HIV Nef Decreases eNOS Expression in Both Porcine Pulmonary Artery Rings and HPAECs
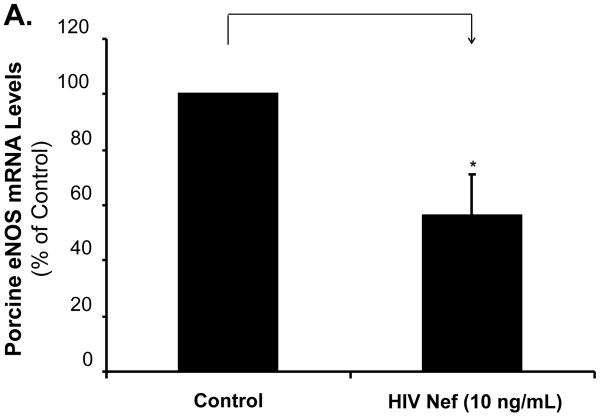
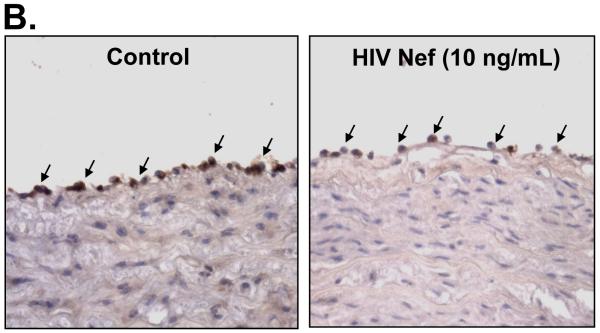
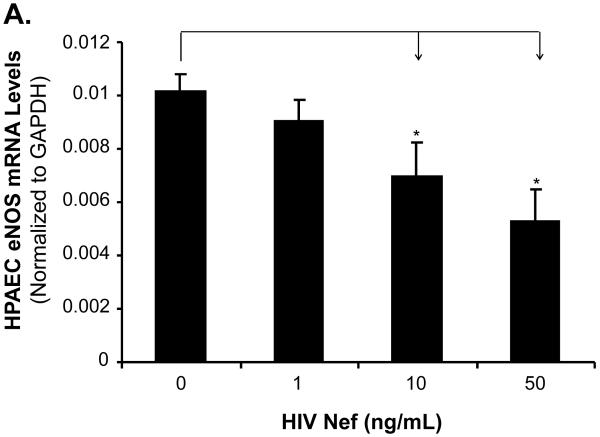
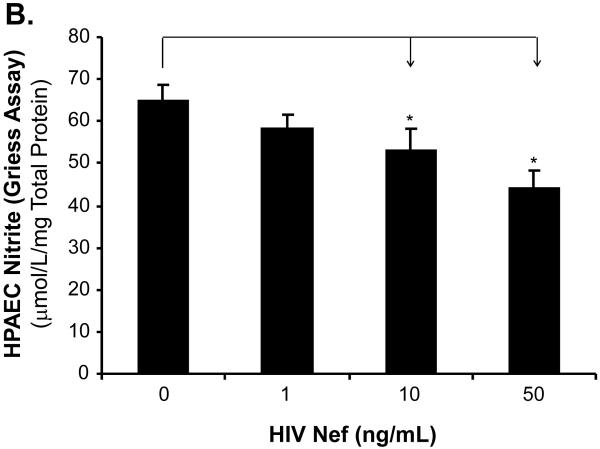
To investigate whether eNOS could be involved in the Nef-induced vasomotor dysfunction in porcine pulmonary arteries, eNOS expression in both treated arterial rings and HPAECs were analyzed by real-time PCR and immunohistochemistry. Significant decreases of eNOS mRNA levels were observed in response to Nef treatment. At 10 ng/mL of Nef, eNOS mRNA levels of arterial rings showed a significant decrease by 43% compared with controls (Fig. 3A, P < 0.05). Immunohistochemistry staining also confirmed the significant decrease in eNOS protein levels in endothelial layers of the porcine arteries (Fig. 3B). Consistent with the effects of Nef on arterial rings, mRNA levels of eNOS in HPAECs were significantly reduced with Nef treatment in a concentration-dependent manner (Fig. 4A, P < 0.05). When cells were treated with Nef (10 or 50 ng/mL) for 24 h, eNOS mRNA levels were decreased by 32% and 48%, respectively, compared with controls (Fig. 4A, P < 0.05). Furthermore, the NO production was evaluated with Calorimetric Nitric Oxide Assay. NO production was substantially reduced in the Nef-treated HPAECs in a concentration-dependent manner. When treated with Nef at 10 or 50 ng/mL, NO production was decreased by 18% and 32% in HPAECs, respectively, compared with controls (Fig. 4B, P < 0.05).
FIG 3.
Effects of HIV Nef on eNOS expression in porcine pulmonary arteries. Porcine pulmonary artery rings were cultured with or without Nef for 24 h, and the total mRNA was purified from endothelial layers of the rings. The eNOS mRNA was measured with real-time PCR. (A). Nef significantly decreased the eNOS mRNA expression compared with controls (n = 4). *P < 0.05 versus controls (medium only). (B). Representative slides showed decreased eNOS immunoreactivity in the endothelium of Nef-treated porcine pulmonary arteries compared with controls. Magnification: ×400.
FIG. 4.
Effects of HIV Nef on eNOS mRNA and NO levels in HPAECs. HPAECs were treated with Nef in a concentration-dependent manner, and eNOS mRNA levels were measured with real-time PCR. (A). The eNOS mRNA levels in HPAECs were significantly decreased in Nef-treated cells in a concentration-dependent manner, compared with controls. n = 4. (B). NO release from HPAECs was determined by measuring the culture medium for the accumulation of its stable degradation products, nitrite and nitrate using Griess assay method. Nef substantially decreased NO release compared with controls (n = 4). *P < 0.05 versus controls (medium only).
HIV Nef Increases ROS Production in Both Porcine Pulmonary Artery Rings and HPAECs
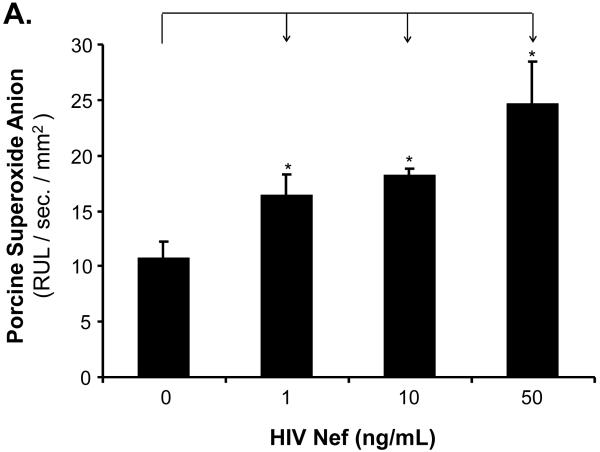
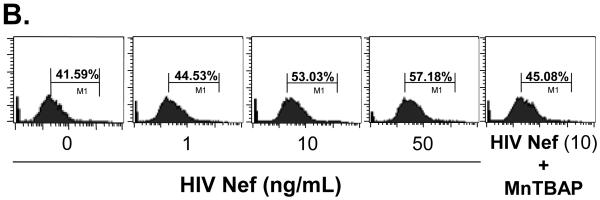
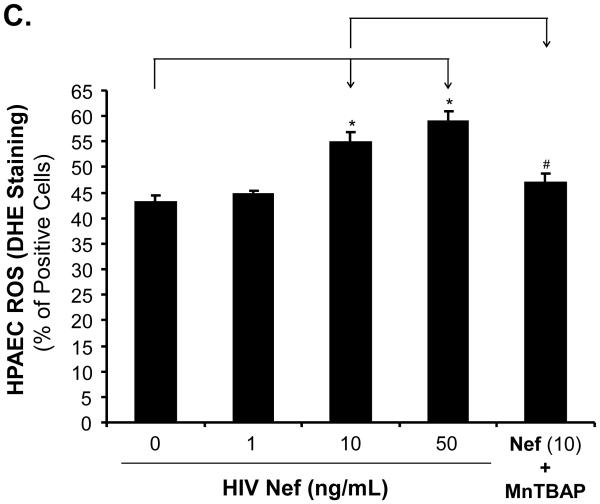
To investigate whether oxidative stress could play a role in the Nef-induced vasomotor dysfunction in porcine pulmonary arteries, superoxide anion production from the arterial rings and HPAECs was analyzed with lucigenin-enhanced chemiluminescence and DHE staining, respectively. Superoxide anion from the arterial rings was significantly increased in Nef-treated vessels in a concentration-dependent manner. At 10 or 50 ng/mL, Nef treatments showed increases of superoxide anion from the rings by 54% or 70%, respectively, compared with controls (Fig. 5A, P < 0.05). Moreover, superoxide anion levels were also investigated with flow cytometry using DHE staining in HPAEC. DHE becomes ethidium bromide upon interaction with superoxide anion and produces red fluorescence when excited. When HPAECs were cultured with 10 or 50 ng/mL of Nef for 24 h, the superoxide anion was substantially increased by 27% or 37%, respectively, compared with controls (Fig. 5B and C, P < 0.05). Moreover, antioxidant MnTBAP, a SOD mimic, effectively abolished Nef-induced increased superoxide anion levels (Fig. 5B and C). Furthermore, in order to determine the role of ROS in Nef-induced decrease in vasocontraction and vasorelaxation of porcine pulmonary arteries, MnPBAP (2 μM) was used to pre-treat porcine pulmonary arteries, followed by Nef (10 ng/mL) treatment for 24 h. As shown in Fig. 2 A-C, MnPBAP markedly blocked the reduction of contraction and relaxation of porcine pulmonary arteries induced by Nef, indicating oxidative stress is involved in Nef-induced endothelial dysfunction. However, MnPBAP did not affect endothelium-independent vasorelaxation of porcine pulmonary arteries (Fig. 2D).
FIG. 5.
Effects of HIV Nef on ROS production in porcine pulmonary arteries and HPAECs. (A) Superoxide anion levels in the endothelial layer of porcine pulmonary arteries were tested with lucigenin-enhanced chemiluminescence assay. Nef significantly increased the superoxide anion levels of the vessel rings in a concentration-dependent manner compared with controls. (B, C) Superoxide anion levels in HPAECs were stained with DHE and analyzed by FACS Calibure flow cytometry. Superoxide anion levels in Nef-treated HPAECs were substantially increased. Co-culture with antioxidant MnPBAP effectively blocked Nef -induced increase of superoxide anion in HPAECs. *P < 0.05 versus controls (medium only), #P < 0.05 versus Nef (10 ng/mL). n = 3.
DISCUSSION
Non-infectious complications of HIV are becoming more prominent as the survival of HIV-infected patients improves due to better anti-retroviral therapy and prophylaxis against opportunistic infections. Well documented among these non-infectious complications are those associated with accelerated cardiovascular disease [2]. Autopsy reports were the first to describe the association between coronary artery disease and HIV infection [1]. HIV-PAH has recently gained attention as a devastating non-infectious complication of HIV. Although a rare disease in itself, its prevalence in HIV infected patients is much higher that that seen in the general population suggesting the virus itself or as a consequence of infection, is linked to the development of the disease [4]. The prevalence of HIV-PAH has been estimated to be 1:1,200 (0.5%) in HIV-infected individuals as compared to 1-2:1,000,000 with idiopathic PAH in the general population [4]. It is postulated that the incidence of HIV-PAH is higher as the asymptomatic patients are not included in the published studies.
The exact mechanism for the pathogenesis of HIV-PAH is yet to be clearly defined. Affected vessels in all forms of PAH including that associated with HIV, show similar pathology [4-6]. The underlying vasculopathy shows severe angioproliferative disorder, in which vessel occlusion results from medial hypertrophy and smooth muscle proliferation, endothelial proliferation and fibrosis [8]. There is currently no evidence to show a direct role of HIV in the pathogenesis of HIV-PAH and the virus itself has not been detected in the pulmonary endothelial cells [8]. This has led to the investigation of HIV associated production mediators such as cytokines, growth factors and vasoconstrictors as well as HIV accessory proteins such as Nef, for their potential role in indirectly causing endothelial dysfunction. Although direct infection of vascular endothelial cells with HIV virus has not been shown, extracellular plasma levels of Nef at 10 ng/mL have been found in HIV-infected patients [14]. Entry of Nef protein via the CXCR4 chemokine receptor into lymphocytes has been demonstrated [14]. This receptor is also expressed on the endothelial cells in the lung vessels, suggesting that Nef may be present in the endothelial cells in the absence of active infection.
Vasomotor dysfunction is an important mechanism of idiopathic PAH. The monolayer vascular endothelium has the vital function of regulating vascular tone, smooth muscle cell proliferation, coagulation and fibrinolysis, permeability, cell adhesion and migration [6]. We selected Nef at concentrations of 1, 10 and 50 ng/mL based on that Nef plasma levels of 10 ng/mL are seen in HIV-infected patients [14]. Using a well-defined myograph tension system, our experiments demonstrated that HIV Nef protein significantly decreases endothelium-dependent vasorelaxation in porcine pulmonary arteries. The endothelium-dependent vasorelaxation in response to bradykinin was significantly reduced in HIV Nef-treated porcine pulmonary artery rings in a concentration-dependent manner (1, 10, and 50 ng/mL). In response to bradykinin (10-8 mol/L), HIV Nef (10 ng/mL) significantly reduced vasorelaxation by 32% compared with untreated controls (P < 0.05). To our knowledge, this is the first report to show an independent effect of Nef protein on endothelium-dependent vasorelaxation. The impaired pulmonary artery endothelium is subject to injury, the preceding event to all causes of PAH. Endothelial dysfunction begins a cascade of events leading to medial wall thickening, muscularization of the nonmuscular pulmonary arteries, and wide spread proliferative changes in pulmonary vessels.
A key player in the endothelial cells is nitric oxide (NO). NO maintains vascular integrity. Impaired availability of bioactive NO results in the loss of vasomotor relaxation in pulmonary arteries leading to vascular thickening and right ventricular hypertrophy in experimental rat models [4]. In clinical trials, the administration of NO has been shown to attenuate PAH clinically and experimentally [4-6]. NO modulates the expression of growth factors, vascular smooth cell proliferation, collagen synthesis, angiogenesis, signal transducer activation, platelet aggregation, and cell adhesion [18,19]. Through all these mechanisms, NO-dependent signaling pathways control the concentration of cellular antioxidants, modulate apoptosis and regulate cellular response to inflammation, thereby providing cytoprotection [19].
NO is a short-lived free radical, generated from L-arginine by catalytic activity of eNOS [20]. Using real time PCR and immunohistochemistry, we measured the expression of eNOS in both porcine pulmonary arteries and HPAECs. We found that HIV Nef significantly decreased eNOS expression in the vessels and HPAECs. Using Calorimetric Nitric Oxide Assay kit, we also measured the NO production in HPAECs. We discovered HIV Nef decreased NO production in HPAECs in a concentration-dependent manner. For example, HIV Nef at 10 ng/mL significantly decreased NO production in HPAECs by 21% compared with controls (P < 0.05). In addition, the bioavailability of NO is also impaired by its reaction with ROS such as superoxide anion.
ROS such as superoxide anion react with NO and lessen its bioavailabilty, effectively decreasing its ability to maintain the integrity of the endothelium [21]. Using lucigenin-enhanced chemiluminescence assay and DHE staining, we measured superoxide anion production in porcine pulmonary arteries and HPAECs. We found that HIV Nef (10 ng/mL) significantly increased superoxide anion production in porcine pulmonary arteries and HPAECs compared with controls (P < 0.05). All of these findings contribute to the decreased NO availability and the consequent endothelial dysfunction which may ultimately lead to PAH.
HIV Nef has previously been shown to be associated with complex pulmonary vascular lesions in SHIV-Nef infected Macaques. Marecki et al. demonstrated that these lesions were found in macaques monkeys infected with the SHIV-nef (a chimeric viral construct containing the nef gene in an SIV backbone) and not in those solely infected with SIV [9,14]. From this unexpected observation, they were able to conclude that HIV-Nef may play a role in the development of complex pulmonary arterial lesions similar to those seen in patients with HIV-PAH. Acheampong and coworkers demonstrated that either exogenous or intracellular Nef induced apoptosis of human brain microvascular endothelial cells [22]. HIV Nef’s role in the development of HIV-PAH is not clearly defined. Nef-induced apoptosis, growth factor release, proliferation and/or differentiation are proposed avenues of Nef-mediated endothelial dysfunction and ultimately vascular lesions leading to PAH.
In summary, we have successfully demonstrated HIV Nef protein significantly decreases endothelium-dependent vasorelaxation in porcine pulmonary arteries. It also reduces eNOS expression and induces oxidative stress in both porcine pulmonary arteries and HPAECs. The specificity of this Nef action was further confirmed by anti-Nef antibody blocking and Nef-heat inactivation. This study demonstrates new mechanisms of HIV Nef causing endothelial dysfunction, which may contribute to the human pulmonary artery disease in HIV-infected patients.
ACKNOWLEDGMENTS
This work is partially supported by a research grant (HL083471 to CC) from the National Institutes of Health (NIH) and by the Michael E. DeBakey Department of Surgery, Baylor College of Medicine and Michael E. DeBakey VA Medical Center, Houston, Texas. Recombinant HIV-1 Nef protein and HIV-1 Nef antiserum were obtained from the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH. Dr. Patrick Duffy has won the 2009 Association of Academic Surgery Research Resident Award competition at the 4th Annual Academic Surgical Congress, February 3-6, 2009 in Sanibel Harbour Resort in Fort Myers, Florida.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented at the 4th Annual Academic Surgical Congress, February 3-6, 2009 in Sanibel Harbour Resort in Fort Myers, Florida.
REFERENCES
- 1.Boccara F. Cardiovascular complications and atherosclerotic manifestations in the HIV-infected population: type, incidence and associated risk factors. AIDS. 2008;22(Suppl 3):S19–26. doi: 10.1097/01.aids.0000327512.76126.6e. [DOI] [PubMed] [Google Scholar]
- 2.Glesby MJ. Coronary heart disease in HIV-infected patients. Curr HIV/AIDS rep. 2005;2:68–73. doi: 10.1007/s11904-005-0021-7. [DOI] [PubMed] [Google Scholar]
- 3.Voelkel N, Carlyne D, Flores S. From viral infection to pulmonary arterial hypertension: a role for vir al proteins? AIDS. 2008;22(Suppl 3):S49–S53. doi: 10.1097/01.aids.0000327516.55041.01. [DOI] [PubMed] [Google Scholar]
- 4.Petitpretz P, Brenot F, Azarian R, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary hypertension. Circulation. 1994;89:2722–7. doi: 10.1161/01.cir.89.6.2722. [DOI] [PubMed] [Google Scholar]
- 5.Mehta NJ, Khan RN, Mehta RN, et al. HIV-related pulmonary hypertension: Analytic revie of 131 cases. Chest. 2008;118:1133–41. doi: 10.1378/chest.118.4.1133. [DOI] [PubMed] [Google Scholar]
- 6.Limsukon A, Saeed AI, Ramasamy V, et al. HIV-related pulmonary hypertension. Mt Sinai J Med. 2006;73:1037–44. [PubMed] [Google Scholar]
- 7.Andrade AC, Cotter BR, et al. Endothelial dysfunction and cardiovascular disease in HIV-infected patient. Braz J Infect Dis. 2006;10:139–45. doi: 10.1590/s1413-86702006000200012. [DOI] [PubMed] [Google Scholar]
- 8.Marecki JC, Cool CD, Parr JE, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected Macaques. Am J Respir Crit Care Med. 2006;174:437–45. doi: 10.1164/rccm.200601-005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lindwasser OW, Chaudhuri R, Bonifacino JS. Mechanisms of CD4 downregulation by the Nef and Vpu proteins of primate immunodeficiency viruses. Curr Mol Med. 2007;7:171–84. doi: 10.2174/156652407780059177. [DOI] [PubMed] [Google Scholar]
- 10.Wildum S, Schindler M, Munch J, et al. Contribution of Vpu and Nef to CD4 down-modulation and resistance of human immunodeficiency virus type 1-infected T cells to superinfection. J Virol. 2006;80:8047–59. doi: 10.1128/JVI.00252-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laguette N, Benichou S, Basmaciogullari S. HIV-1 Nef incorporation into virions does not Increase infectivity. J Virol. 2008 doi: 10.1128/JVI.01633-08. EPUB ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schaeffer E, Geleziunas R, Greene WC. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J Virol. 2001;75:2993–3000. doi: 10.1128/JVI.75.6.2993-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster JL, Garcia JV. HIV-1 Nef: at the crossroads. Retrovirology. 2008;5:84. doi: 10.1186/1742-4690-5-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marecki J, Cool C, Voelkel N, et al. Evidence for vascular remodeling in the lungs of macaques infected with simian immunodeficiency virus/HIV NEF recombinant virus. Chest. 2005;128(Suppl 6):S621–2. doi: 10.1378/chest.128.6_suppl.621S-a. [DOI] [PubMed] [Google Scholar]
- 15.Chai H, Yang H, Yan S, Li M, Lin PH, Lumsden AB, Yao Q, Chen C. Effects of 5 HIV protease inhibitors on vasomotor function and superoxide anion production in porcine coronary arteries. J Acquir Immune Defic Syndr. 2005;40:12–19. doi: 10.1097/01.qai.0000172368.05327.7b. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Chai H, Wang H, Yao Q, Chen C. SerumAmyloid A induces Endothelial Dysfunction in Porcine Coronary Arteries and Human Coronary Artery Endothelial Cells. Am J Physiol Heart Circ Physiol. 2008;295:H2399–408. doi: 10.1152/ajpheart.00238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Mu H, Chai H, Liao D, Yao Q, Chen C. Human immunodeficiency virus protease inhibtor ritonavir inhibits cholesterol efflux from human macrophage-derived foam cells. Am J Pathol. 2007;171:304–314. doi: 10.2353/ajpath.2007.060965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad and ugly. AM J Physiol. 1996;271:C1424–37. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- 19.Ganz P, Vita JA. Testing endothelial vasomotor function: nitric oxide, a multipotent molecule. Circulation. 2003;108:2049–53. doi: 10.1161/01.CIR.0000089507.19675.F9. [DOI] [PubMed] [Google Scholar]
- 20.Vane JR, Anggard EE, Botting RM. Regulatory functions of the vascular endothelium. N Engl J Med. 1990;323:27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- 21.Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195–9. doi: 10.1136/pmj.79.930.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acheampong EA, Parveen Z, Muthga LW, et al. Human immunodeficiency virus type I Nef potently induces apoptosis in primary human brain microvascular endtothelial cells via activation of caspases. J Virology. 2005;79:4257–69. doi: 10.1128/JVI.79.7.4257-4269.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]