Abstract
Monoamine oxidase A (MAO-A) expression is associated with high-grade prostate cancer. Immunohistochemistry showed that MAO-A is also expressed in the basal epithelial cells of normal prostate glands. Using cultured primary prostatic epithelial cells as a model, we showed that MAO-A prevents basal epithelial cells from differentiating into secretory cells. Under differentiation-promoting conditions, clorgyline, an irreversible MAO-A inhibitor, induced secretory cell-like morphology and repressed expression of cytokeratin 14, a basal cell marker. More importantly, clorgyline induced mRNA and protein expression of androgen receptor (AR), a hallmark of secretory epithelial cells. In clorgyline-treated cells, androgen induced luciferase activity controlled by the promoter of prostate-specific antigen, an AR target gene, in a dose-dependent manner. This activity was blocked by the AR antagonist Casodex, showing that AR is functional. In turn, androgen decreased MAO-A expression in clorgyline-treated, secretory-like cells. Our results demonstrated that cultured basal epithelial cells have the potential to differentiate into secretory cells, and that inhibition of MAO-A is a key factor in promoting this process. Increased expression of MAO-A in high-grade prostate cancer may be an important contributor to its de-differentiated phenotype, raising the possibility that MAO-A inhibition may restore differentiation and reverse the aggressive behavior of high-grade cancer.
Keywords: monoamine oxidase A, secretory differentiation, clorgyline, prostatic epithelial cells, androgen receptor
Introduction
Monoamine oxidase A (MAO-A) is a mitochondrial enzyme that degrades monoamine neurotransmitters including 5-hydroxytryptamine (5-HT, or serotonin) and norepinephrine (NE) (Shih et al., 1999a). Its functions in the nervous system have been extensively studied (Shih et al., 1999b). MAO-A inhibitors have been proven to have therapeutic value in several neurological diseases such as depression (Youdim et al., 2006). In a recent gene expression profiling study using laser captured prostate cancer (PCa) cells, True et al. (2006) showed that MAO-A is one of the most highly over-expressed genes in high-grade PCa (2.4-fold higher in Gleason grade 4/5 compared with grade 3). Immunohistochemical evaluation of tissue arrays with >800 prostate samples confirmed that MAO-A was also over-expressed at the protein level in grade 4/5 cancers (True et al., 2006). Because the progression of PCa from grade 3 to 4/5 marks a critical change from curable to lethal (Stamey et al., 1999; Humphrey, 2004), increased expression of MAO-A in grade 4/5 cancer raises the possibility that activity of this enzyme is a key factor in the increased lethality of high-grade PCa.
The pattern of expression and the function of MAO-A in the human prostate is not known. In the study reported here, we demonstrated basal cell-specific expression of MAO-A in normal prostatic epithelia and identified an in vitro model system to investigate the biological function of MAO-A in basal cells. We considered two hypotheses as a framework for our studies. The first was the hypothesis that the function of MAO-A is to protect prostatic epithelial cells from mitogenic activity of neurotransmitters or catecholamines. There is some limited evidence that NE and 5-HT, likely released by neuroendocrine cells present in the prostate and adrenergic enervation of the prostate (Lepor et al., 1990; Abrahamsson and di Sant’Agnese, 1993), might stimulate prostate epithelial cell growth. For example, it was shown that NE and 5-HT stimulate the proliferation of PCa cell lines (Dizeyi et al., 2004; Palm et al., 2006; Siddiqui et al., 2006). In addition, subcutaneous injections of the α-adrenergic agonist, phenylephrine, induced atypical prostatic hyperplasia in rats (Golomb et al., 1998). In contrast, in vitro studies with primary cultures of prostatic cells showed that stromal cells, but not epithelial cells, responded to NE (Kanagawa et al., 2003). The ability of a cell to respond to NE or 5-HT could be regulated by expression of MAO-A, which would inactivate these factors by oxidative degradation.
Our second hypothesis regarding the role of MAO-A in prostate cells was derived from a recent publication that showed that inhibition of MAO-A induced the differentiation of neural stem cells (NSCs) into serotoninergic neurons (Chiou et al., 2006), suggesting that MAO-A may be an anti-differentiation factor. In the human prostate, two predominant cell populations constitute the normal prostate epithelium: basal cells form an outer layer that adheres to the basement membrane while columnar luminal secretory cells constitute an inner layer (Schalken and van Leenders, 2003). The cell linage relationship between these two epithelial types is not clear. One widely accepted theory is that cells residing in the basal cell layer differentiate into secretory cells to maintain tissue homeostasis (Wang et al., 2001; Long et al., 2005; Tokar et al., 2005), although in mouse prostate it appears that secretory cells can develop independent of basal cells (Kurita et al., 2004). During differentiation, basal cells lose expression of cytokeratins 5 and 14 and acquire expression of cytokeratins 8 and 18 (van Leenders and Schalken, 2003). Most importantly, secretory cells gain expression of androgen receptor (AR) and androgen is a key regulator of the differentiated phenotype (Arnold and Isaacs, 2002). Given the reported role of MAO-A in preventing neuronal differentiation, we generated the hypothesis that the function of MAO-A in prostatic basal epithelial cells is to prevent differentiation into secretory cells. Our results supported the second hypothesis, with implications for the role of MAO-A in high-grade cancer and possibilities for new therapeutic strategies for aggressive cancer.
Material and methods
Cell culture and reagents
Primary cultures of normal human prostatic epithelial (E-PZ) and stromal (F-PZ) cells were established and characterized as previously described (Peehl, 1992; Peehl and Sellers, 2000). 1,25-dihydroxyvitamin D3 (Biomol International, Plymouth Meeting, PA) was prepared at 10mM in DMSO. Transforming growth factor (TGF)-β1 (Peprotech, Inc., Rocky Hill, NJ) was prepared in l0mM citric acid (pH 3.0) at 100µg/ml. All-trans retinoic acid (Sigma-Aldrich, St. Louis, MO) was prepared in DMSO at 1 mM. Clorgyline (Sigma) and pargyline (Sigma) were prepared at l00mM in water. The synthetic androgen R1881 (Perkin Elmer, Waltham, MA) was prepared in ethanol at 10 µM. NE and 5-HT (Sigma) were prepared in water at l00mM. Casodex (Zeneca Pharmaceuticals, Macclesfield, UK) was prepared in ethanol at l0mM.
Immunochemistry
E-PZ cells cultured on eight-well chamber slides were fixed in 2% paraformaldehyde and permeabilized in 95% ice-cold ethanol. Horse serum (10% v/v) was used to block non-specific binding of antibodies. The slide was then incubated at room temperature (RT) for 30 min in the primary antibodies. A rabbit polyclonal antibody against human MAO-A (Santa Cruz Biotechnology Inc., Santa Cruz, CA) (1:1,000), two monoclonal antibodies against human cytokeratins 14 and 18 (BioGenex, San Ramon, CA) (1:1,000), a monoclonal antibody against human p63 (NeoMarkers, Fremont, CA) (1:1,000), and a rabbit polyclonal antibody against human AR sc-816 (Santa Cruz Biotechnology Inc.) (1:3,000) were used. The slides were then washed and incubated with a biotinylated secondary antibody at RT for 30 min, washed and incubated again at RT for another 30 min in peroxidase-conjugated streptavidin. Color was developed with 3,3 diaminobenzidine (DakoCytomation California Inc., Carpinteria, CA). Counterstaining was performed with hematoxylin.
For double immunofluorescent staining of tissue sections, a similar procedure was used except that tissues were first deparaffinized in xylene, and hydrated in a graded series of alcohol. Slides were then incubated in 0.3% hydrogen peroxide in methanol for 15 min and 10% horse serum for 20 min at RT before incubation in the primary antibodies at 4°C overnight. The slides were then washed and incubated with an Alexa Fluro® 488 goat anti-mouse immunoglobulin G (IgG) (Invitrogen, Carlsbad, CA), and an Alexa Fluro® 555 goat anti-rabbit IgG (Invitrogen) at RT for 30 min. Fluorescence signal was visualized using a Nikon Eclipse E800 microscope (Nikon Instruments Inc., Melville, NY).
Cell proliferation assay
Cells were seeded into 96-well flat-bottom microplates at a density of 500 cells/well in complete MCDB 105 (Peehl, 1992) or complete MCDB 105 with factors deleted. After overnight incubation, cells were treated with NE or 5-HT, with or without clorgyline, for 6 days at 37°C and 5% CO2. Proliferation was measured in quadruplicate for each treatment by the sulforhodamine B assay (Skehan et al., 1990).
Quantitative real-time polymerase chain reaction (qRT-PCR) and semi-quantitative RT-PCR
Total RNA from E-PZ and F-PZ cells was isolated using Trizol (Invitrogen) and reverse transcribed using SuperScript™ III Reverse Transcriptase (Invitrogen) according to the manufacturer’s instructions. cDNA product was then mixed with SYBR® GreenER™ qPCR super mix (Invitrogen) and MAO-A forward and reverse primers in the subsequent PCR using a M×3005P® QPCR System (Strategene, La Jolla, CA). Transcript level in each sample was determined in triplicate to minimize the experimental variation (standard deviation was calculated for each reaction). Transcript level of TATA box binding protein (TBP) was assayed simultaneously as an internal control to normalize MAO-A transcript level. Semi-quantitative RT-PCR was performed using GoTaq® DNA polymerase (Promega, Madison, WI) according to the manufacture’s instructions. The primer sequences for MAO-A are 5′-TAAATGGTCTCGGGAAGGTG-3′ (forward) and 5′-CCCAGG GCAGTTACTGATGT-3′ (reverse), for AR are 5′-AGTCCCACT TGTGTCAAAAGC-3′ (forward) and 5′-ACTTCTGTTTCCCTTC AGCG-3′ (reverse), and for TBP are 5′-TGCTGAGAAGAG TGTGCTGGAG-3′ (forward) and 5′-TCTGAATAGGCTGTG GGGTC-3′ (reverse).
Western blotting
Cells were lysed with lysis buffer (pH 7.5, 50 mM HEPES, 0.5% NP-40, 0.25% Na-deoxycholate, 0.1 mM sodium vanadate, 50 mM NaCl, 1mM EDTA, 1mM phenylmethylsulfonyl fluoride). NEPER Nuclear and Cytoplasmic Extraction Reagents from Pierce (Rockford, IL) were used to obtain nuclear proteins for AR detection. Protein concentration was determined using the Bradford assay (Bio-Rad, Hercules, CA), and 20 µg of protein were used for each sample. MAO-A, cytokeratins 14 and 18, and AR were detected using antibodies described above. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was detected with a monoclonal mouse anti-rabbit antibody, MoAb 6C5, which reacts with human GAPDH (Research Diagnostics, Flanders, NJ). A horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG (DaKoCytomation) and an HRP-conjugated goat anti-rabbit IgG were used as secondary antibodies. Signals were visualized with an ECL Plus kit (Amersham Biosciences, Piscataway, NJ).
MAO-A enzymatic activity assay
A bioluminescent enzyme assay of MAO-A was performed using MAO-GloTM Assay kit (Promega) according to manufacture’s instructions with modification (Valley et al., 2006). Briefly, 3 million cultured cells were harvested and lysed in 0.3 ml 1 × Reporter Lysis Buffer (Promega). Twenty-five microliters of cell lysate were incubated with an equal volume of 2 × MAO Substrate Solution at RT for 3 hr. Fifty microliters of Luciferin Detection Reagent were then added and incubated for 20 min at RT. MAO-A activity in each sample was normalized against the protein concentration of the same sample determined using the Bradford assay (Bio-Rad).
Flow cytometry
Cells were detached from plates and incubated with a fluorescein-conjugated monoclonal mouse anti-human CD 57 or isotype IgG (Abcam, Cambridge, UK). The relative amount of cell-bound CD 57 was determined using a BD FACSAria™ flow cytometer (Franklin Lakes, NJ), and analyzed with Flow Jo software. Dead cells were excluded by propidium iodide (Sigma-Aldrich) staining. Ten thousand cells were analyzed for each sample and triplicates were analyzed for each sample to minimize experimental variation.
Luciferase reporter assay
A pGL3 luciferase construct containing a 600-bp super-PSA promoter (sPSA-Luc) as described previously (Yeung et al., 2000) was co-transfected into E-PZ cells with a pRL-null-renilla construct (Promega) using NeoFX reagent (Ambion Inc., Austin, TX). pGL3-sPSA-Luc was a generous gift from Dr. Leland Chung at Emory University (Atlanta, GA). Eight hours after transfection, cells were treated with 1,25-dihydroxyvitamin D3, TGF-β1, all-trans retinoic acid, clorgyline, and R1881. Luciferase activity was measured 48 hr after treatment using the Dual-Luciferase Assay Kit (Promega). The ratio of firefly- to renilla-luciferase was determined to correct for transfection efficiency. Triplicates were analyzed to minimize experimental variation.
Results
MAO-A is expressed in basal epithelial cells of normal prostatic tissue
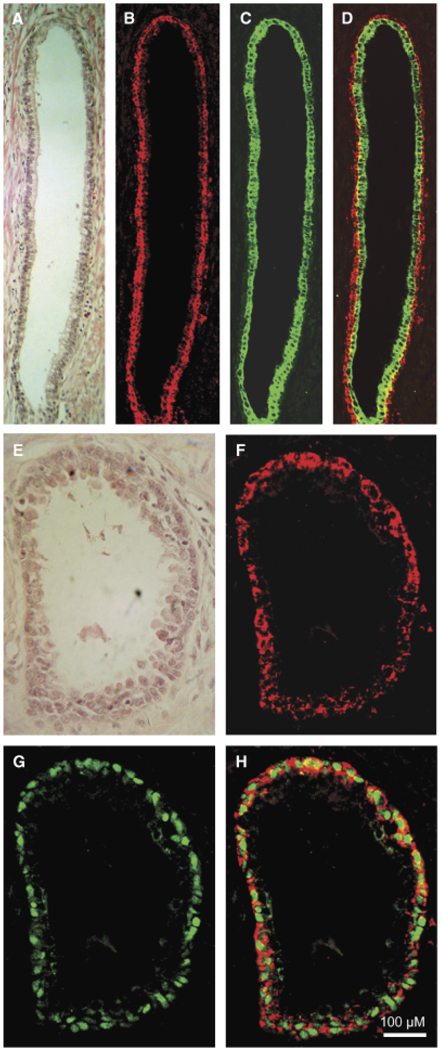
The expression of MAO-A in normal prostatic tissues was evaluated by immunohistochemistry. Double immunofluorescence staining with antibodies against MAO-A and cytokeratin 18, a secretory epithelial cell marker, showed that MAO-A is not expressed in secretory epithelial cells, but exclusively in the basal epithelial cells (Figs. 1A–1D). A similar double-labeling experiment using antibodies against MAO-A and p63, a basal epithelial cell marker present in the nucleus (Signoretti et al., 2000), confirmed the localization of MAO-A to basal epithelial cells (Figs. 1E–1H). MAO-A antibody decorated areas surrounding the nuclei of basal epithelial cells, indicating a cytoplasmic localization. The punctate pattern of staining was consistent with mitochondrial localization of MAO-A. No MAO-A expression was detected in stromal cells of the prostate. Our results demonstrated that MAO-A is specifically expressed by the basal epithelial cells of normal prostatic glands.
Fig. 1.
Monoamine oxidase A (MAO-A) expression in normal prostate tissues. (A) and (E) Prostate glands stained with hematoxylin and eosin display basal (outer layer) and secretory (inner layer) epithelial cells. (B) and (F) Intense staining of MAO-A is observed in basal cells. (C) Strong cytokeratin 18 staining is present in the secretory cells. (D) Merged image of (B) and (C). (G) Robust staining of p63 decorates the nuclei of basal cells. (H) Merged image of (F) and (G). MAO-A and p63 signals do not overlap in the basal cells, showing cytoplasmic localization of MAO-A. The magnification for (A–H) is × 200. For all images, the size bar is 100 µM.
Primary epithelial cell cultures are a suitable model to study MAO-A function
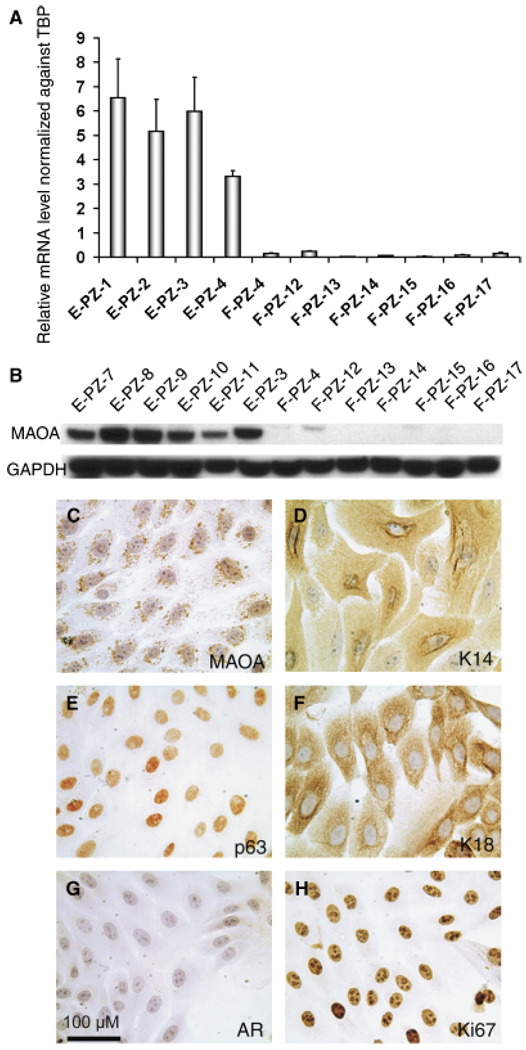
To determine whether cultured normal primary human prostatic epithelial cells (E-PZ) express MAO-A, we examined the transcript level of MAO-A in E-PZ cells as well as in primary cultures of normal prostatic stromal cells (F-PZ) by qRT-PCR. The average level of MAO-A mRNA in E-PZ cells was 50-fold or more higher than that in F-PZ cells (Fig. 2A). In addition, immunoblot analysis showed high levels of MAO-A protein in the E-PZ cells, whereas little or none was detected in F-PZ cells (Fig. 2B). Immunocytochemistry of E-PZ cells illustrated the presence of MAO-A in the cytoplasm of the cells with a punctuate pattern, consistent with mitochondrial localization (Fig. 2C). E-PZ cells also express basal epithelial cell markers including cytokeratin 14 and p63 (Figs. 2D,2E), but not secretory epithelial cell markers such as AR (Fig. 2G). As is typical of primary epithelial cell cultures, E-PZ cells are proliferative as shown by strong Ki67 expression (Fig. 2H), and a subset of cells express cytokeratin 18 (Fig. 2F) which may represent transit amplifying cells (Schalken and van Leenders, 2003). These cells are not fully differentiated into secretory cells because they do not express AR and PSA. Our results demonstrated that E-PZ cells which represent a mixture of basal and transit amplifying cells provide a suitable in vitro model to study MAO-A function.
Fig. 2.
Monoamine oxidase A (MAO-A) expression in normal human prostatic epithelial (E-PZ) and stromal (F-PZ) cells. (A) MAO-A transcript levels in E-PZ cells are much higher than those in F-PZ cells, as determined by quantitative real-time polymerase chain reaction. (B) MAO-A protein is readily detectable in E-PZ cells by Western blotting analysis, whereas little or no MAO-A protein is measurable in F-PZ cells. (C–H) Expression pattern of proteins of interest in E-PZ cells by immunocytochemistry: (C) MAO-A shows a punctuate staining pattern in the cytoplasm, (D) K14, a basal cell marker, is expressed in the cytoplasm, (E) p63, a basal cell marker, is expressed in the nuclei, and (F) K18, a marker of transit amplifying cells and secretory cells, is expressed in the cytoplasm. E-PZ cells are negative for secretory cell markers including AR (G). The proliferative nature of E-PZ cells is shown by labeling with Ki67 (H). The magnification for (C–H) is × 200. For all images, the size bar is 100 µM.
Inhibition of MAO-A does not elicit mitogenic effects of monoamines on E-PZ cells
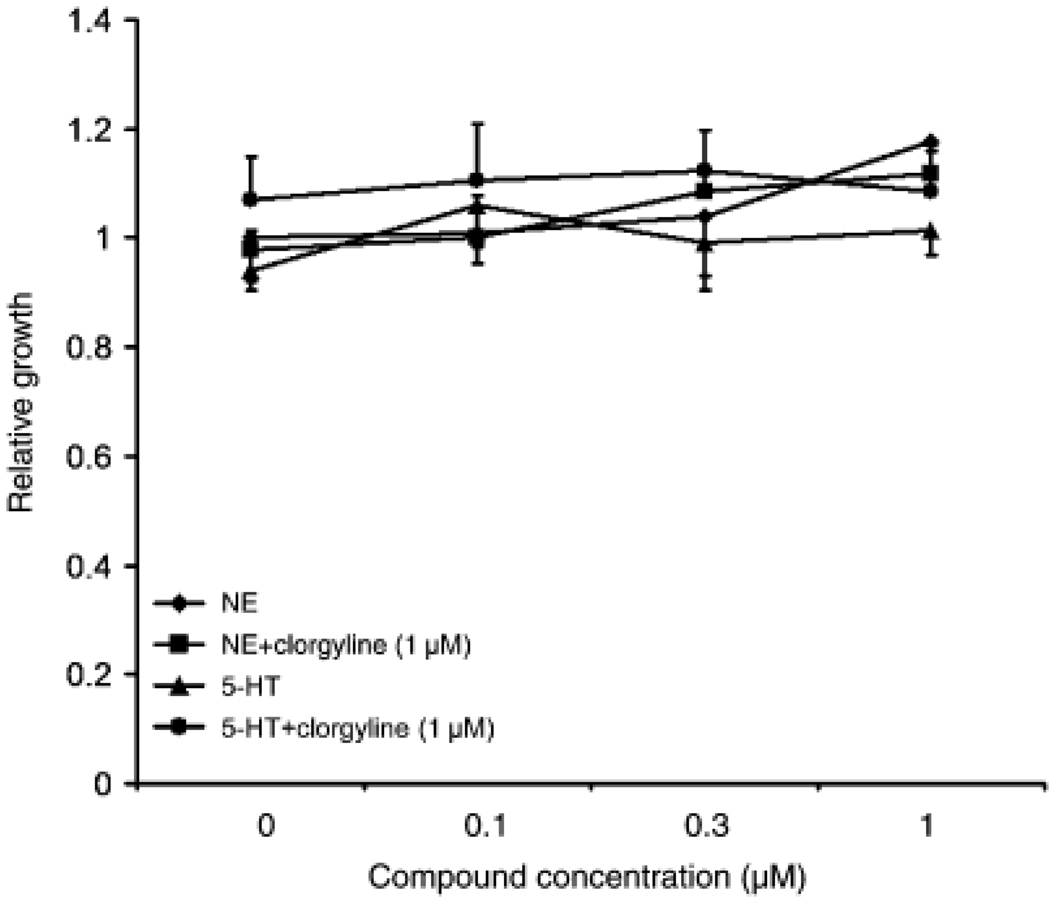
To test the hypothesis that MAO-A may protect basal epithelial cells from mitogenic effects of monoamines such as NE and 5-HT, we evaluated the effects of clorgyline, an irreversible MAO-A inhibitor, on the growth of NE- or 5-HT-treated E-PZ cells. In standard culture medium, clorgyline up to 1 µM had no effect on the growth of E-PZ cells (data not shown). At concentrations of 0.01–1 µM, NE or 5-HT also had no effects on the growth of E-PZ cells (Fig. 3). In the presence of 1 µM clorgyline, NE and 5-HT again failed to exert any growth-promoting effects on E-PZ cells (Fig. 3). We then omitted hydrocortisone (HC) and bovine pituitary extract (BPE) from the culture medium because HC upregulates MAO-A (Ou et al., 2006) which counters the inhibition from clorgyline and BPE may contain monoamines that mask the effects of NE and 5-HT. In this modified medium, still no effects of NE and 5-HT on the growth of E-PZ cells were observed either alone or in combination with clorgyline (data not shown). Taken together, our results indicate that inhibition of MAO-A does not affect the growth of basal epithelial cells or their response to NE or 5-HT; therefore, the function of MAO-A is not to protect basal epithelial cells from mitogenic activity of monoamines in the prostate.
Fig. 3.
Inhibition of monoamine oxidase A (MAO-A) does not elicit mitogenic effects of monoamines on E-PZ cells. Proliferation is measured in quadruplicate for each treatment by the sulforhodamine B assay. Standard deviation is calculated for each sample. Cells treated with 5-hydroxytryptamine (5-HT, or serotonin) and norepinephrine (NE) alone show similar growth rates as control cells. In the presence of clorgyline, no changes are observed in the growth rates of NE- or 5-HT- treated cells compared with control cells.
Inhibition of MAO-A induced AR expression in E-PZ cells
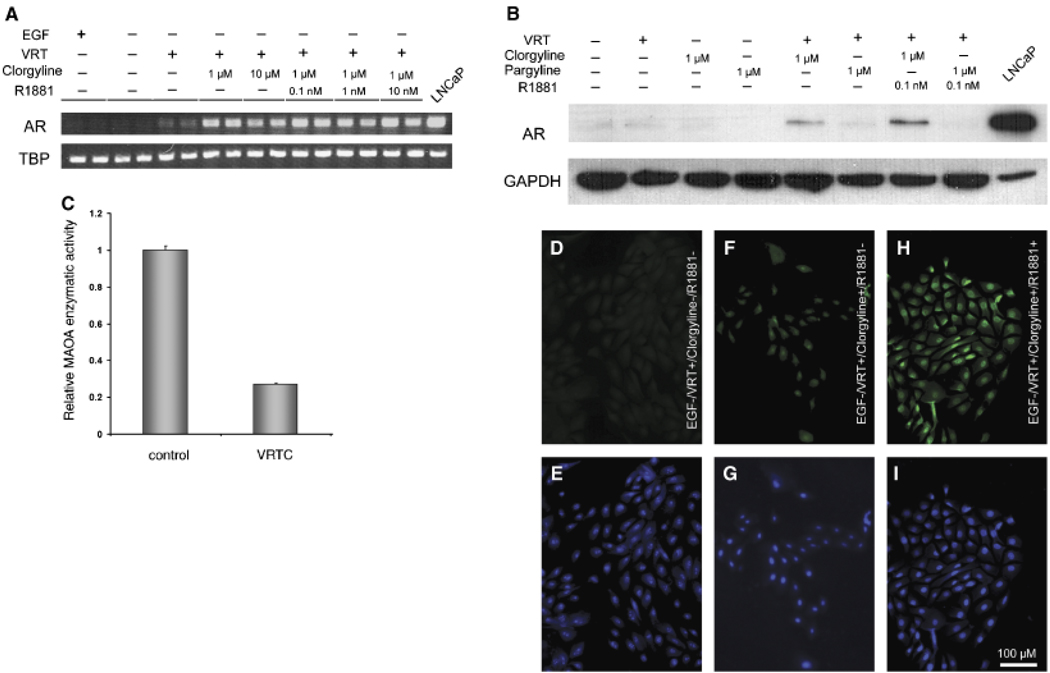
Our alternative hypothesis was that MAO-A blocks basal epithelial cells from differentiation into secretory cells, and that inhibition of its activity would promote differentiation. We examined the effects of MAO-A inhibition on the expression of AR, a hallmark of secretory cells. In standard culture medium, no AR transcript was detected by RT-PCR with or without clorgyline (Fig. 4A). We then considered that other conditions might be required for differentiation in addition to inhibition of MAO-A. First, epidermal growth factor (EGF) was deleted from the culture medium. EGF is a potent mitogen, and proliferation is often counter-inductive for differentiation. Previous reports showed that EGF decreased AR expression in LNCaP cells, a prostate cancer cell line with a secretory cell phenotype (Henttu and Vihko, 1993; Langeler et al., 1993). However, AR transcripts were still undetectable in the absence of EGF with or without clorgyline (Fig. 4A), suggesting that removing EGF was not sufficient to promote differentiation. We then supplemented EGF-deficient culture medium with a cocktail of factors that have shown differentiating activity in prostate and other types of cells (Peehl et al., 1993; Danielpour, 1999; Zhao et al., 1999; Goossens et al., 2002). The individual factors in the cocktail, designated as “VRT,” were 1,25-dihydroxyvitamin D3 (10 nM), all-trans retinoic acid (1 µM) and TGF-βl (1 ng/ml). Indeed, in medium without EGF and with the three putative differentiating factors, AR transcripts became detectable in E-PZ cells after 36 PCR cycles (Fig. 4A). When cells were further treated with 1 µM clorgyline, the level of AR transcript was dramatically increased (Fig. 4A). Androgen (10 nM R1881) did not further increase AR transcript expression in MAO-A-inhibited cells (Fig. 4A). In the absence of VRT, the effect of clorgyline on AR transcription was minimal. AR transcript was only detectable after 50 PCR cycles (data not shown).
Fig. 4.
Inhibition of monoamine oxidase A (MAO-A) induces AR expression in E-PZ cells. (A) AR transcript levels determined by semi-quantitative RT-PCR are increased in cells treated with clorgyline, an irreversible MAO-A inhibitor, and androgen compared with control cells. The treatment conditions are shown on top of each lane (VRT = 1,25-dihydroxyvitamin D3 [10 nM], all-trans retinoic acid [1 µM] and TGF-β1 [1 ng/ml]). Duplicate samples are analyzed for each condition. TBP transcript level in each sample is examined as an internal control for experimental variations. (B) AR protein in nuclear extracts is detectable by Western blotting analysis in clorgyline-treated but not control or pargyline (a preferential MAO-B inhibitor)-treated cells, and AR is further increased in the presence of androgen. GAPDH levels are determined as an internal control for experimental variation. (C) MAO-A enzymatic activity is inhibited by clorgyline in E-PZ cells. Control cells are grown in standard medium. Experimental cells are treated with VRT and clorgyline (1 µM) for 16hr. (D), (F), (H) are immunofluorescent staining of AR protein showing no detectable AR in control cells (D), faint AR signal in clorgyline-treated cells (F), and strong signal in clorgyline- and androgen-treated cells (H). (E), (G), (I) are DAPI staining showing the nuclei of the same cells in (D), (F), (H), respectively. The magnification for (C-H) is × 200. For all images, the size bar is 100µM. TGF-β1, transforming growth factor; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; AR, androgen receptor; TBP, TATA box binding protein.
We further examined the effects of clorgyline on AR protein expression using Western blotting analysis. Nuclear proteins were isolated for this assay given that most of AR is localized in the nucleus. No AR protein was detected in cells grown in control medium (Fig. 4B). AR protein was also undetectable in the presence of VRT alone (Fig. 4B), despite the induction of AR mRNA by these factors (Fig. 4A). However, when cells were treated with 1 µM clorgyline in the presence of VRT, AR protein was clearly visible (Fig. 4B). In addition, androgen significantly increased the level of AR protein in the nuclear extract of clorgyline-treated E-PZ cells although it was much lower than that in LNCaP cells (Fig. 4B), consistent with its role in stabilizing and translocating AR into the nucleus (Georget et al., 2002). Moreover, pargyline, a preferential MAO-B inhibitor (Karoum, 1987), did not induce AR protein expression either alone or in combination with VRT (Fig. 4B), suggesting the induction of AR protein expression in E-PZ cells is clorgyline-specific. Finally, in clorgyline-treated E-PZ cells, MAO-A activity was reduced by 73% compared with cells grown in the control medium (Fig. 4C).
Finally, we examined AR protein expression in clorgyline-treated cells by immunofluorescence. No AR protein was detected in cells treated with VRT alone (Fig. 4D). When cells were treated with 1 µM clorgyline, faint AR labeling was visible in the nuclei of the cells (Fig. 4E). In the presence of 1 nM R1881, much stronger nuclear staining was observed (Fig. 4H). Taken together, our results demonstrated that inhibition of MAO-A induced AR expression at both the mRNA and protein levels, suggesting that inhibition of MAO-A may induce a differentiated, secretory cell phenotype in basal epithelial cells.
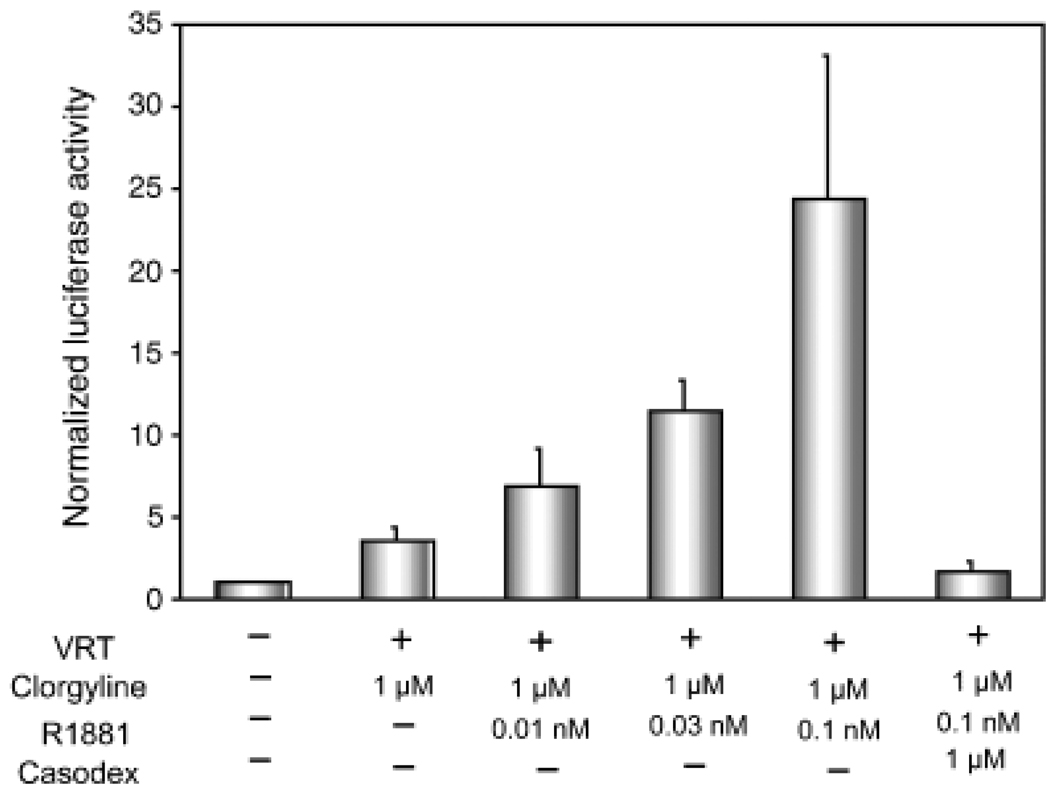
AR is functional in clorgyline-treated E-PZ cells
To further test whether AR is functional in clorgyline-treated cells, we performed reporter assays using a pGL3 luciferase construct containing a 600-bp super-PSA promoter (sPSA-Luc), which exhibits two- to threefold higher activity than the wild type PSA promoter (Yeung et al., 2000). In the presence of VRT, we observed a threefold increase in luciferase activity upon clorgyline treatment (Fig. 5). Androgen further increased luciferase activity in a dose-dependent manner in clorgyline-treated cells (Fig. 5). Furthermore, this induction was AR-dependent because blocking AR function with Casodex reversed the luciferase activity to the basal level (Fig. 5). These results demonstrated that AR is active in clorgyline-treated cells.
Fig. 5.
AR is functional in clorgyline-treated cells. A firefly luciferase reporter gene driven by the super-PSA promoter is transfected into clorgyline-treated cells and control cells. A renilla luciferase reporter construct is co-transfected and its activity is used to normalize the sPSA-controlled firefly luciferase activity. PSA promoter activity is up-regulated by androgen in a dose dependent manner in clorgyline-treated cells. The induction of PSA promoter activity is blocked by Casodex, an AR antagonist. The treatment conditions are shown at the bottom of the graph. Triplicate samples are analyzed and standard deviation is calculated for each treatment. AR, androgen receptor; PSA, prostate-specific antigen.
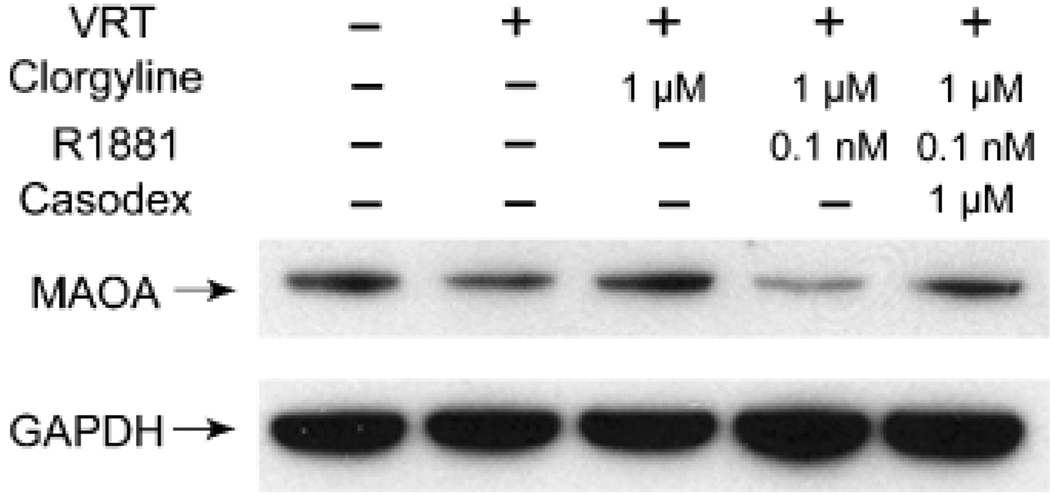
Androgen down-regulates MAO-A expression in clorgyline-treated E-PZ cells
The presence of an androgen response element in the promoter of MAO-A prompted us to examine MAO-A expression itself in clorgyline-treated E-PZ cells by Western blotting analysis. Clorgyline by itself did not alter MAO-A expression, showing that inhibition of enzyme activity had no effect on protein levels, as anticipated. The addition of androgen to clorgyline-treated cells, however, dramatically decreased MAO-A protein, suggesting that androgen negatively regulates MAO-A expression (Fig. 6). The repression of MAO-A expression by androgen is through AR because Casodex restored MAO-A levels to that in control cells (Fig. 6). These results suggest that androgen down-regulates MAO-A expression in clorgyline-treated cells, consistent with the absence of MAO-A in secretory epithelia of prostatic tissues, and that was mediated by an active AR.
Fig. 6.
MAO-A is down-regulated by androgen. Western blotting analysis shows MAO-A expression is significantly decreased in cells treated with clorgyline and androgen compared with control, and Casodex restores the MAO-A level to that of the control. GAPDH levels are determined as an internal control for experimental variations. The treatment conditions are shown on top of each lane. MAO-A, monoamine oxidase A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
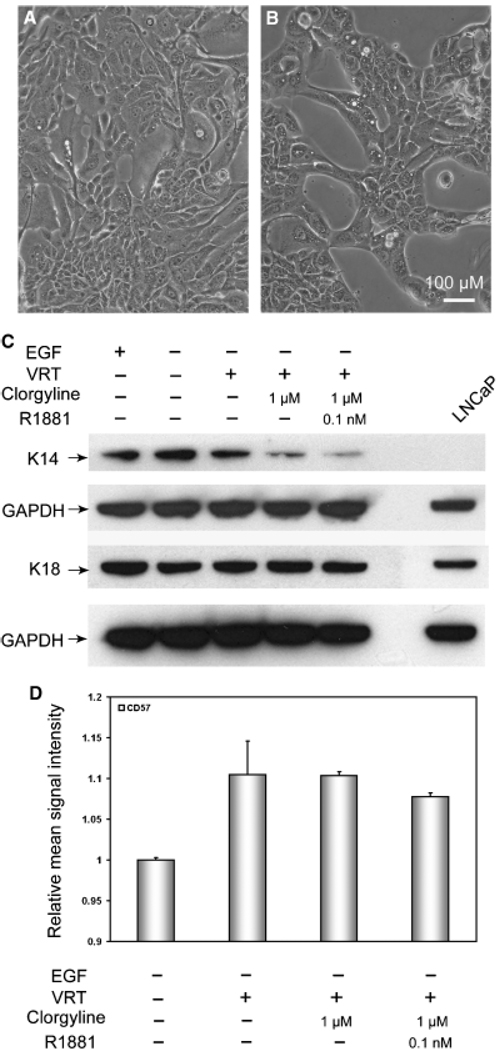
Inhibition of MAO-A induces secretory morphology and represses the basal cell phenotype of E-PZ cells
To determine the extent of secretory differentiation in clorgyline-treated E-PZ cells, we examined the expression of other secretory and basal epithelial cell markers. First, we observed distinctive morphological changes in clorgyline-treated cells. In medium without EGF and with VRT, clorgyline-treated cells were organized in a manner resembling acinar structures of the prostate (Fig. 7B), whereas the control cells were randomly distributed (Fig. 7A). Clorgyline-treated cells, unlike the control cells, were in close contact with the neighboring cells and showed dark organelles in their cytoplasm. This morphological change was not androgen-dependent, since morphology was similar with or without R1881 (data not shown).
Fig. 7.
Inhibition of MAO-A induces secretory morphology and represses the basal phenotype in E-PZ cells. (A) Shows cells grown in control medium are randomly distributed on the plate. (B) Depicts cells treated with clorgyline demonstrating the formation of acinar-like structures. The magnification for (A) and (B) is × 200. For all images, the size bar is 100 µM. (C) Western blotting analysis shows that basal cell K14 expression is greatly decreased in clorgyline-treated cells compared with control, whereas the expression of the transit amplifying/secretory cell K18 is not affected. GAPDH levels were determined as an internal control for experimental variation. The treatment conditions are shown on top of each lane. (D) Flow cytometry analysis shows that CD57 expression is significantly induced by VRT treatment, but not further enhanced by clorgyline or androgen. MAO-A, monoamine oxidase A; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Using Western blotting analysis, we observed a dramatic decrease in the expression of the basal cell-associated cytokeratin 14 in clorgyline-treated cells compared with the control cells (Fig. 7C). Androgen did not further decrease the expression of cytokeratin 14 in these cells (Fig. 7C). In contrast, no increase in the expression of the secretory cell-associated cytokeratin 18 was observed in clorgyline-treated cells compared with the control cells. In addition, we found that the expression of CD 57, a cell surface antigen expressed by secretory epithelial cells (Liu and Peehl, 2001), was increased by VRT treatment (Fig. 7D). However, clorgyline did not further increase the level of CD 57 in E-PZ cells. These results suggest that secretory differentiation takes place in several stages, some induced by VRT alone, others induced by inhibition of MAO-A activity, and yet others induced by androgen in AR-expressing cells generated by inhibition of MAO-A.
Discussion
We have investigated the expression and function of MAO-A in normal basal epithelial cells of the human prostate. Basal epithelial cells of normal glands of the human prostate are K5/K14+, p63+, K8/18 −, AR −, and PSA −. Secretory cells, on the other hand, are K8/K18+, AR+, PSA+, K5/K14 −, and p63 −. Transit amplifying cells simultaneously express basal cell cytokeratins K5/K14 and secretory cell cytokeratins K8 and/or K18 (Bonkhoff et al., 1994; van Leenders et al., 2000; Garraway et al., 2003; Uzgare et al., 2004). These cells are also proliferative, therefore proposed to be cells that are in the process of generating differentiated cell populations but have not yet completely committed to one particular lineage. Our primary cultures appear to represent a mixture of basal and transit amplifying cells. Some cells are K5/14+ and K8/18 −, while others are K5/14+ and K8/18+. They are not fully differentiated into secretory cells, however, since in our standard culture conditions, the cells do not express AR or PSA.
Our results led us to reject our initial hypothesis that the function of MAO-A is to protect basal epithelial cells from mitogenic effects of monoamines present in the prostate. Instead, we found that MAO-A inhibits basal cells from differentiating into secretory cells. First, MAO-A is selectively expressed by basal but not secretory epithelial cells in normal prostatic tissues, signifying a cell-specific function. Second, inhibition of MAO-A activity induced AR expression at both mRNA and protein levels. Third, AR was active in MAO-A-inhibited cells because it mediated PSA promoter driven-lu-ciferase expression. Finally, inhibition of MAO-A repressed the expression of the basal epithelial cell marker, cytokeratin 14, and induced morphological changes resembling secretory differentiation. Our results demonstrated that basal epithelial cells in culture have the potential to differentiate into secretory cells, and inhibition of MAO-A is a key factor in promoting this process. Our results do not contradict the possibility that secretory cells may develop independent of basal cells supported by the work of Kurita et al. (2004) showing that mouse prostatic tissue that developed in urogenital sinus isolated from p63(− / −) embryos contained secretory cells in the absence of basal cells.
Ample evidence has shown that clorgyline is a potent and specific MAO-A inhibitor. Although MAO-A and its isoform MAO-B have 70% amino acid identity, MAO-A has a 500-fold lower IC50 for clorgyline than MAO-B (Geha et al., 2001). In addition, both site-directed mutagenesis and comparison of the crystal structures of MAO-A and MAO-B complexed with their inhibitors showed that a single amino acid in these two isoforms determines their substrate and inhibitor specificities (Geha et al., 2001; Ma et al., 2004). However, clorgyline also has other activities unrelated to inhibition of MAO-A. For instance, clorgyline can change the sensitized response to quinpirole from locomotion to stationary “mouthing” activity in rats by a mechanism apparently not linked to its actions on MAO-A (Richards et al., 2007). Therefore, it cannot be ruled out that the differentiation-promoting effects of clorgyline in E-PZ cells are a result of off target actions.
The role of MAO-A as a regulator of cell differentiation has just recently been discovered. Chiou et al. (2006) demonstrated that inhibition of MAO-A induced the differentiation of NSCs into serotoninergic neurons. Specifically, inhibition of MAO-A significantly increased the percentage of serotonin- and microtubule-associated protein 2 (a marker of neuronal differentiation)-positive cells as well as the production of 5-HT in cultured NSCs. Furthermore, inhibition of MAO-A induced morphological changes in NSCs, supporting a role for inhibition of MAO-A in facilitating dendritic development and neurite expansion. Although elevated Bcl-2 expression through activating extracellular-regulated kinase (ERK) phosphorylation was found in MAO-A-inhibited NSCs, whether the differentiation-promoting effect of MAO-A inhibitor in these cells was mediated by Bcl-2 and ERK is still unknown. Basal prostatic epithelial cells in vitro have been shown to possess certain stem/progenitor cell properties in that they have relatively high proliferation potential and are capable of differentiation into other cell types present in the prostate such as secretory and neuroendocrine cells (Schalken and van Leenders, 2003). Whether MAO-A is a general differentiation-inhibiting mechanism of stem/progenitor cells awaits further investigation.
A hallmark of differentiated prostatic secretory cells is the expression of AR and PSA. Most investigators report that normal primary prostatic epithelial cell cultures express low or no AR or PSA and do not respond to androgen (Peehl, 2005). Attempts to achieve a fully differentiated secretory phenotype of prostate cells in vitro have not been successful. For example, all-trans retinoic acid increases levels of secretory cell cytokeratins 8 and 18 in primary cultures, but the cells continue to express basal cell cytokeratins, and other differentiation markers such as PSA remain low (Peehl et al., 1993). Differentiation of primary prostatic epithelial cells has been more complete in three-dimensional culture models using Matrigel. Lang et al. (2006) demonstrated that immortalized primary epithelial cells formed acinar-like spheroids when cultured in Matrigel, and co-culture of the spheroids with fibroblasts advanced differentiation by inducing AR expression and epithelial polarization. Inhibition of MAO-A, on the other hand, not only induced endogenous AR expression, but also PSA promoter-driven reporter activity mediated by AR, demonstrating a key role of MAO-A in regulating basal cell differentiation. The MAO-A-inhibited cells seem to be more sensitive to androgen than prostate cancer cell lines in that they respond to 10-fold lower doses of androgen. One explanation is that the serum-containing culture medium for cancer cell lines has hormone-binding proteins, which may sequester some of the supplemented androgen. Our culture medium is serum-free; therefore, the amount of androgen added to the medium probably reflects the actual amount of androgen acting on the cells.
Molecular mechanisms and pathways involved in secretory differentiation are poorly understood. Most of the studies on this topic have understandably focused on the role of androgen in this process due to the vital role of androgen in the growth and development of the prostate. Some information has been derived from the exogenous expression of AR in immortalized prostatic epithelial cell lines. Although the characteristics of these cells may differ from cells in vivo to a larger extent than primary cell cultures, nonetheless, these studies shed light on the differentiation-related events mediated by AR. For example, Ling et al. (2001) showed that androgen treatment resulted in up-regulation of secretory cell markers including cytokeratins 8 and 18, and PSA, and down-regulation of c-myc, Bcl-2, and telomerase activity. In addition, Berger et al. (2004) demonstrated that androgen stimulation led to down-regulation of p63. However, AR expression in the presence of androgen is not sufficient to repress basal cell keratins, suggesting that differentiation of prostate epithelial cells involves the interplay of a concert of gene expression, which is regulated by different mechanisms.
The mechanisms of differentiation triggered in E-PZ cells by inhibiting MAO-A remain to be determined. Our results suggest that differentiation occurs in stages, with different inductive factors required for each stage. Although our experiments were not designed to distinguish and characterize these different stages, several conditions seemed to be required to achieve an AR-expressing secretory-like cell in vitro. First, growth-stimulatory factors in the medium are probably counter-inductive for differentiation. We did not observe any cellular changes upon the inhibition of MAO-A until the potent mitogen, EGF, was deleted from the culture medium. In addition to depletion of mitogens, differentiation-promoting factors are also probably required. In EGF-deficient medium, the addition of VRT induced transcription of the AR gene, but AR protein remained undetectable. The secretory cell marker CD57 was also induced in medium without EGF and with VRT. Inhibiting MAO-A in the EGF-deficient medium supplemented with the three differentiation-promoting factors, however, led to morphological changes resembling acinar structures. Cytokeratin 14, a basal cell-specific protein, was also significantly decreased under these conditions. Down-regulation of cytokeratin 14 therefore may be an early step of secretory differentiation. Levels of cytokeratin 18, however, remained constant. Because cytokeratin 18 is present in both transit amplifying or “intermediate” cells and secretory cells, it is possible that cytokeratin 18 levels were already maximal in the cultured cells. Notably, inhibition of MAO-A in EGF-deficient medium with the three differentiation factors induced AR mRNA and protein. The addition of androgen to these cells then promoted additional androgen-regulated features of differentiation, such as PSA expression.
The promotion of differentiation by inhibition of MAO-A has clinical implications relevant to PCa. Our results together with the finding that MAO-A is one of the most highly over-expressed genes in Gleason grade 4/5 compared with grade 3 PCa (True et al., 2006) may explain the poorly differentiated phenotype of high-grade cancer. If inhibition of MAO-A promotes differentiation of grade 4/5 cancer cells as it does normal basal epithelial cells, then antidepressant drugs that target MAO-A may provide a new approach to differentiation therapy for aggressive PCa.
Acknowledgments
We thank Dr. Leland Chung at Emory University (Atlanta, Georgia) for his generous gift of pGL3-sPSA-Luc. Dr. Zhao is supported by National Institutes of Health Grant CA123532 and Dr. Peehl by CA121460.
References
- Abrahamsson PA, di Sant’Agnese PA. Neuroendocrine cells in the human prostate gland. J Androl. 1993;14:307–309. [PubMed] [Google Scholar]
- Arnold JT, Isaacs JT. Mechanisms involved in the progression of androgen-independent prostate cancers: it is not only the cancer cell’s fault. Endocr Relat Cancer. 2002;9:61–73. doi: 10.1677/erc.0.0090061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger R, Febbo PG, Majumder PK, Zhao JJ, Mukherjee S, Signoretti S, Campbell KT, Sellers WR, Roberts TM, Loda M, Golub TR, Hahn WC. Androgen-in-duced differentiation and tumorigenicity of human prostate epithelial cells. Cancer Res. 2004;64:8867–8875. doi: 10.1158/0008-5472.CAN-04-2938. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Stein U, Remberger K. Multidirectional differentiation in the normal, hyperplastic, and neoplastic human prostate: simultaneous demonstration of cell-specific epithelial markers. Hum Pathol. 1994;25:42–46. doi: 10.1016/0046-8177(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Chiou SH, Ku HH, Tsai TH, Lin HL, Chen LH, Chien CS, Ho LL, Lee CH, Chang YL. Moclobemide upregulated Bcl-2 expression and induced neural stem cell differentiation into serotoninergic neuron via extracellular-regulated kinase pathway. Br J Pharmacol. 2006;148:587–598. doi: 10.1038/sj.bjp.0706766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielpour D. Transdifferentiation of NRP-152 rat prostatic basal epithelial cells toward a luminal phenotype: regulation by glucocorticoid, insulin-like growth factor-I and transforming growth factor-beta. J Cell Sci. 1999;112(Pt 2):169–179. doi: 10.1242/jcs.112.2.169. [DOI] [PubMed] [Google Scholar]
- Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate. 2004;59:328–336. doi: 10.1002/pros.10374. [DOI] [PubMed] [Google Scholar]
- Garraway LA, Lin D, Signoretti S, Waltregny D, Dilks J, Bhattacharya N, Loda M. Intermediate basal cells of the prostate: in vitro and in vivo characterization. Prostate. 2003;55:206–218. doi: 10.1002/pros.10244. [DOI] [PubMed] [Google Scholar]
- Geha RM, Rebrin I, Chen K, Shih JC. Substrate and inhibitor specificities for human monoamine oxidase A and B are influenced by a single amino acid. J Biol Chem. 2001;276:9877–9882. doi: 10.1074/jbc.M006972200. [DOI] [PubMed] [Google Scholar]
- Georget V, Terouanne B, Nicolas JC, Sultan C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry. 2002;41:11824–11831. doi: 10.1021/bi0259150. [DOI] [PubMed] [Google Scholar]
- Golomb E, Kruglikova A, Dvir D, Parnes N, Abramovici A. Induction of atypical prostatic hyperplasia in rats by sympathomimetic stimulation. Prostate. 1998;34:214–221. doi: 10.1002/(sici)1097-0045(19980215)34:3<214::aid-pros9>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Goossens K, Deboel L, Swinnen JV, Roskams T, Manin M, Rombauts W, Verhoeven G. Both retinoids and androgens are required to maintain or promote functional differentiation in reaggregation cultures of human prostate epithelial cells. Prostate. 2002;53:34–49. doi: 10.1002/pros.10125. [DOI] [PubMed] [Google Scholar]
- Henttu P, Vihko P. Growth factor regulation of gene expression in the human prostatic carcinoma cell line LNCaP. Cancer Res. 1993;53:1051–1058. [PubMed] [Google Scholar]
- Humphrey PA. Gleason grading and prognostic factors in carcinoma of the prostate. Mod Pathol. 2004;17:292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- Kanagawa K, Sugimura K, Kuratsukuri K, Ikemoto S, Kishimoto T, Nakatani T. Norepinephrine activates P44 and P42 MAPK in human prostate stromal and smooth muscle cells but not in epithelial cells. Prostate. 2003;56:313–318. doi: 10.1002/pros.10267. [DOI] [PubMed] [Google Scholar]
- Karoum F. N-propargylbenzylamine, a major metabolite of pargyline, is a potent inhibitor of monoamine oxidase type B in rats in vivo: a comparison with deprenyl. Br J Pharmacol. 1987;90:335–345. doi: 10.1111/j.1476-5381.1987.tb08963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Medina RT, Mills AA, Cunha GR. Role of p63 and basal cells in the prostate. Development. 2004;131:4955–4964. doi: 10.1242/dev.01384. [DOI] [PubMed] [Google Scholar]
- Lang SH, Smith J, Hyde C, Macintosh C, Stower M, Maitland NJ. Differentiation of prostate epithelial cell cultures by matrigel/stromal cell glandular reconstruction. In Vitro Cell Dev Biol Anim. 2006;42:273–280. doi: 10.1290/0511080.1. [DOI] [PubMed] [Google Scholar]
- Langeler EG, van Uffelen CJ, Blankenstein MA, van Steenbrugge GJ, Mulder E. Effect of culture conditions on androgen sensitivity of the human prostatic cancer cell line LNCaP. Prostate. 1993;23:213–223. doi: 10.1002/pros.2990230304. [DOI] [PubMed] [Google Scholar]
- Lepor H, Shapiro E, Bowsher RR, Henry DP. Determination of norepinephrine levels in the adult human prostate. J Urol. 1990;144:1263–1266. doi: 10.1016/s0022-5347(17)39716-1. [DOI] [PubMed] [Google Scholar]
- Ling MT, Chan KW, Choo CK. Androgen induces differentiation of a human papillomavirus 16 E6/E7 immortalized prostate epithelial cell line. J Endocrinol. 2001;170:287–296. doi: 10.1677/joe.0.1700287. [DOI] [PubMed] [Google Scholar]
- Liu AY, Peehl DM. Characterization of cultured human prostatic epithelial cells by cluster designation antigen expression. Cell Tissue Res. 2001;305:389–397. doi: 10.1007/s004410100419. [DOI] [PubMed] [Google Scholar]
- Long RM, Morrissey C, Fitzpatrick JM, Watson RW. Prostate epithelial cell differentiation and its relevance to the understanding of prostate cancer therapies. Clin Sci (Lond) 2005;108:1–11. doi: 10.1042/CS20040241. [DOI] [PubMed] [Google Scholar]
- Ma J, Kubota F, Yoshimura M, Yamashita E, Nakagawa A, Ito A, Tsukihara T. Crystallization and preliminary crystallographic analysis of rat monoamine oxidase A complexed with clorgyline. Acta Crystallogr D Biol Crystallogr. 2004;60:317–319. doi: 10.1107/S0907444903025770. [DOI] [PubMed] [Google Scholar]
- Ou XM, Chen K, Shih JC. Glucocorticoid and androgen activation of monoamine oxidase A is regulated differently by Rl and Spl. J Biol Chem. 2006;281:21512–21525. doi: 10.1074/jbc.M600250200. [DOI] [PubMed] [Google Scholar]
- Palm D, Lang K, Niggemann B, Drell TLT, Masur K, Zaenker KS, Entschladen F. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer. 2006;118:2744–2749. doi: 10.1002/ijc.21723. [DOI] [PubMed] [Google Scholar]
- Peehl D. Culture of human prostatic epithelial cells. In: Freshney R, editor. Culture of epithelial cells. New York: Wiley-Liss; 1992. [Google Scholar]
- Peehl DM. Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer. 2005;12:19–47. doi: 10.1677/erc.1.00795. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Sellers RG. Cultured stromal cells: an in vitro model of prostatic mesenchymal biology. Prostate. 2000;45:115–123. doi: 10.1002/1097-0045(20001001)45:2<115::aid-pros5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Peehl DM, Wong ST, Stamey TA. Vitamin A regulates proliferation and differentiation of human prostatic epithelial cells. Prostate. 1993;23:69–78. doi: 10.1002/pros.2990230107. [DOI] [PubMed] [Google Scholar]
- Richards TL, Pazdernik TL, Levant B. Clorgyline-induced modification of behavioral sensitization to quinpirole: effects on local cerebral glucose utilization. Brain Res. 2007;1160:124–133. doi: 10.1016/j.brainres.2007.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schalken JA, van Leenders G. Cellular and molecular biology of the prostate: stem cell biology. Urology. 2003;62:11–20. doi: 10.1016/s0090-4295(03)00758-1. [DOI] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Monoamine oxidase: from genes to behavior. Annu Rev Neurosci. 1999a;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih JC, Chen K, Ridd MJ. Role of MAO A and B in neurotransmitter metabolism and behavior. Pol J Pharmacol. 1999b;51:25–29. [PubMed] [Google Scholar]
- Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptaminelA and 1B) receptors in prostate cancer cell proliferation. J Urol. 2006;176:1648–1653. doi: 10.1016/j.juro.2006.06.087. [DOI] [PubMed] [Google Scholar]
- Signoretti S, Waltregny D, Dilks J, Isaac B, Lin D, Garraway L, Yang A, Montironi R, McKeon F, Loda M. p63 is a prostate basal cell marker and is required for prostate development. Am J Pathol. 2000;157:1769–1775. doi: 10.1016/S0002-9440(10)64814-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Stamey TA, McNeal JE, Yemoto CM, Sigal BM, Johnstone IM. Biological determinants of cancer progression in men with prostate cancer. JAMA. 1999;281:1395–1400. doi: 10.1001/jama.281.15.1395. [DOI] [PubMed] [Google Scholar]
- Tokar EJ, Ancrile BB, Cunha GR, Webber MM. Stem/progenitor and intermediate cell types and the origin of human prostate cancer. Differentiation. 2005;73:463–473. doi: 10.1111/j.1432-0436.2005.00047.x. [DOI] [PubMed] [Google Scholar]
- True L, Coleman I, Hawley S, Huang CY, Gifford D, Coleman R, Beer TM, Gelmann E, Datta M, Mostaghel E, Knudsen B, Lange P, Vessella R, Lin D, Hood L, Nelson PS. A molecular correlate to the Gleason grading system for prostate adenocarcinoma. Proc Natl Acad Sci USA. 2006;103:10991–10996. doi: 10.1073/pnas.0603678103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzgare AR, Xu Y, Isaacs JT. In vitro culturing and characteristics of transit amplifying epithelial cells from human prostate tissue. J Cell Biochem. 2004;91:196–205. doi: 10.1002/jcb.10764. [DOI] [PubMed] [Google Scholar]
- Valley MP, Zhou W, Hawkins EM, Shultz J, Cali JJ, Worzella T, Bernad L, Good T, Good D, Riss TL, Klaubert DH, Wood KV. A bioluminescent assay for monoamine oxidase activity. Anal Biochem. 2006;359:238–246. doi: 10.1016/j.ab.2006.09.035. [DOI] [PubMed] [Google Scholar]
- van Leenders G, Dijkman H, Hulsbergen-van de Kaa C, Ruiter D, Schalken J. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab Invest. 2000;80:1251–1258. doi: 10.1038/labinvest.3780133. [DOI] [PubMed] [Google Scholar]
- van Leenders GJ, Schalken JA. Epithelial cell differentiation in the human prostate epithelium: implications for the pathogenesis and therapy of prostate cancer. Crit Rev Oncol Hematol. 2003;46(Suppl):S3–S10. doi: 10.1016/s1040-8428(03)00059-3. [DOI] [PubMed] [Google Scholar]
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–279. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Yeung F, Li X, Ellett J, Trapman J, Kao C, Chung LW. Regions of prostate-specific antigen (PSA) promoter confer androgen-independent expression of PSA in prostate cancer cells. J Biol Chem. 2000;275:40846–40855. doi: 10.1074/jbc.M002755200. [DOI] [PubMed] [Google Scholar]
- Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- Zhao XY, Ly LH, Peehl DM, Feldman D. Induction of androgen receptor by 1alpha,25-dihydroxyvitamin D3 and 9-cis retinoic acid in LNCaP human prostate cancer cells. Endocrinology. 1999;140:1205–1212. doi: 10.1210/endo.140.3.6561. [DOI] [PubMed] [Google Scholar]