Abstract
Background
Blood lead concentration has been associated with mortality from different causes in several studies. Many effects of lead exposure that might increase risk of death are likely to result from cumulative exposure, for which bone lead is a better biomarker than blood lead. The association between bone lead levels and mortality has not been explored.
Methods and Results
We prospectively assessed the association between both blood lead and bone lead—analyzed using K-x-ray fluorescence—and mortality among 868 men in the Normative Aging Study. We identified 241 deaths over an average of 8.9 (sd=3.9) years of follow-up. We calculated adjusted hazard ratios (HR) and 95% confidence intervals (CI) using Cox proportional hazards. Compared to the lowest tertile of patella bone lead, the fully adjusted HR in the highest tertile for all cause and cardiovascular mortality (n=137 deaths) were 2.52 (95% CI: 1.17–5.41) and 5.63 (95% CI: 1.73–18.3), respectively. The age, smoking, and race-adjusted HR for ischemic heart disease mortality (n=62 deaths) in the highest tertile was 8.37 (95% CI: 1.29–54.4). Results were similar for tibia lead. Bone lead was not associated with cancer, and blood lead was not associated with any mortality category.
Conclusions
We found bone lead to be associated with all-cause and cardiovascular mortality in an environmentally-exposed population with low blood lead levels. This study suggests that cumulative lead exposure from prior decades of high environmental exposures continues to significantly impact risk of death despite recent declines in environmental lead exposure.
Keywords: cardiovascular diseases, epidemiology, mortality, population, bone lead
The possibility that exposure to lead may contribute to cardiovascular disease is as old as Hippocrates and Vitruvius, who wrote that lead fumes “destroy the vigour of the blood.” 1 Despite such ancient roots the contribution of lead to cardiovascular disease is incompletely understood, although lead and other environmental contaminants are potentially modifiable risk factors for cardiovascular outcomes. Nonetheless, evidence that lead exposure is associated with a variety of adverse cardiovascular outcomes is growing, with the most extensive data being epidemiologic studies relating it to increases in blood pressure and risk of hypertension.2, 3 Controlled animal studies have confirmed these associations.4, 5
A few studies have reported an increased risk of cardiovascular mortality with increasing blood lead levels in data from both the second and third National Health and Nutrition Examination Surveys (NHANES).6–8 Lead in blood has a half-life of approximately 30 days.9 In contrast, lead in bone—a major deposition site for lead in circulation and where the vast majority of lead in the body resides—has a half-life of many years to decades and thus is an indicator of cumulative exposure.9 Many of the known biological effects of lead on the cardiovascular system could be expected to exert their adverse effects chronically, and thus an indicator of cumulative exposure to lead would be expected to be a better biological marker of chronic toxicity than blood lead. The association between bone lead concentration and mortality from cardiovascular or any other causes, however, has not been examined.
To address this issue, we followed participants in the Department of Veterans Affairs (VA) Normative Aging Study (NAS), a longitudinal study of aging among a cohort of community-dwelling elderly men from the greater Boston, Massachusetts, area, who had previously had bone lead measurements taken using K-shell X-Ray Fluorescence (KXRF). In a previous study of ours we found an increased risk of mostly non-fatal (70 of 83 cases) ischemic heart disease with both increasing blood and bone lead levels.10 The current study, however, has longer follow-up enabling us to look specifically at mortality.
METHODS
Study population
This research was conducted on a subgroup of the VA NAS, a multidisciplinary longitudinal study of aging in men established in 1963 when 2,280 men from the Greater Boston area between the ages of 21 and 80 were enrolled.11 Men with a history of treatment for hypertension, systolic blood pressure >140 mmHg, diastolic blood pressure >90 mmHg, or other chronic conditions, including heart disease, diabetes, and cancer, were not admitted into the study. Study cohort members reported for medical examinations every 3–5 years, at which time extensive clinical and other health data were collected. The attrition rate for all causes has been <1% annually. From 1991 through 1999, 868 (68%) of 1,283 active participants gave informed consent for a KXRF measurement of lead in bone. Of the participants with patella and tibia bone lead measurements, 8 and 5, respectively, were excluded because of high uncertainty values (see Bone Lead Measurements section below) leaving a final sample size of 860 for analyses of patella lead and 863 for tibia lead. Measurement of blood lead using atomic absorption on fresh blood samples began in 1992. Of the active participants, 1,235 (96%) provided blood for lead analysis. The research herein was approved by the Human Subjects Committees of the Boston VA Healthcare System, the Brigham and Women’s Hospital, and the Harvard School of Public Health.
Bone Lead Measurements
Bone lead measurements were taken at two anatomical sites, the mid-tibial shaft (midpoint between the tibial plateau and the medial malleolus) and the patella, with an ABIOMED KXRF instrument (ABIOMED, Danvers, MA) as described previously.12 Thirty-minute measurements were taken at each site, after each region had been washed with a 50% solution of isopropyl alcohol. The KXRF beam collimator was sited perpendicular to the flat bony surface of the tibia and at 30° in the lateral direction for the patella. We have previously found the correlation coefficient between KXRF and inductively coupled plasma mass spectrometry (ICP-MS) measurement of lead in cadaver bones to be 0.94 and 0.99 for tibia and patella bone, respectively.13 The average uncertainty around our typical KXRF measurements—equivalent to a standard deviation around the measurement—is approximately 3 μg/g. Tibia (n=5) and patella (n=8) bone lead measurements with estimated uncertainties greater than 10 and 15μg/g bone, respectively, were excluded as these measurements usually reflect excessive participant movement during the measurement.
Blood Lead Measurements
Fresh blood for lead measurement was taken in a special lead-free tube containing EDTA and was sent to ESA Laboratories (Chelmsford, MA). Blood samples were analyzed by Zeeman background-corrected flameless atomic absorption (graphite furnace). The instrument was calibrated before use with National Bureau of Standards Blood Lead Standard Materials. Ten percent of the samples were run in duplicate, 10% were controls and 10% were blanks, analysis of which produced no evidence of external contamination or significant problems with reliability. In tests on reference samples from the Centers for Disease Control and Prevention, the coefficient of variations were 1–8%.
Case ascertainment
Most deaths occurring in this cohort are notified through next of kin or postal authorities. Birthday cards and supplemental questionnaires mailed to participants provided additional opportunities to ascertain the vital records as well as the records of the VA and the Social Security Administration Death Master File to pick up possible unreported deaths. Thus, we have nearly 100% mortality follow-up through March of 2007. For participants who have died, death certificates are obtained from the appropriate state health department. These are reviewed by a board-certified cardiologist (P.V.) to assign cause of death codes according to the 9th revision of the International Classifiction of Disease (ICD-9). Cause specific mortality was classified as cardiovascular disease (ICD-9 codes: 390 to 459), ischemic heart disease (ICD-9 codes 410 to 414 and 429.2), other cardiovascular (no ICD-9 code of 410–414 or 429.2), and cancer (ICD-9 codes 140 to 239) based on any underlying cause listed on the death certificate.
Covariates
Data on all covariates were obtained from the participants’ regularly collected NAS data at the time of the baseline lead measurement. We considered cigarette smoking both as status—never, former, current—and pack-years. Education was categorized into less than high school, high school graduate, vocational/trade school, some college, and completed college/graduate school. Race was coded as white or non-white. Alcohol intake was assessed using a food frequency questionnaire. Participants were asked how many servings per day they drank of beer, wine, and hard liquor, and these were converted to grams of alcohol per day. Responses were categorized into non-drinkers and then successive tertiles of grams per day among those who did drink alcohol. Physical activity was assessed using a modified Paffenbarger scale.14 NAS participants reported how many hours they walked weekly, how many flights of stairs they climbed daily, and the type, frequency, and duration of their participationin sports or recreational activities in hours per week. From these data a physical-activity index was computed in kilocalories per week, which we categorized into quartiles. Weight, height, and blood pressure were assessed by trained staff. The height and weight were used to calculate body mass index (BMI; weight in kilograms divided by the square of height in meters). A participant was considered to have hypertension if their systolic blood pressure was greater than 140 mmHg, their diastolic blood pressure was greater than 90 mmHg, or they reported having been diagnosed with hypertension by a physician. Blood samples were analyzed for total cholesterol and serum high-density lipids (HDL). Participants were asked if they had been diagnosed with diabetes.
Statistical analyses
Participants contributed follow-up time from the date of their first blood or bone lead measurement for the blood lead and bone lead analyses, respectively, to the date of death, or the date of their last contact with the NAS. Direct standardization by age was done for the descriptive statistics to minimize the influence of age—with which bone lead is strongly associated—on the distribution of covariates. The standardization was done by calculating a weighted average of the age-specific averages (continuous variables) or percentages (categorical variables) where the weights were the age-specific proportions (in 5-year groups) of our study population.15 We used Cox proportional hazards regression to estimate hazard ratios (HR) and 95 percent confidence intervals (CI). Multivariable Cox models were stratified on age in months and adjusted for smoking (both status and pack-years), and education. Additonal covariates considered in other models were alcohol intake, physical activity, BMI, total cholesterol, serum high-density lipids (HDL), diabetes, race, and hypertension. To ensure proper temporality and avoid the possibility that disease might alter exposure levels, we ran additional models excluding anyone with cardiovascular conditions or cancer, depending on the model, at baseline. Tests for linear trend across tertiles were computed by including tertile of lead biomarker as a continuous variable in the models. SAS version 9.1 was used for these analyses. We additionally tested for non-linearity of the lead terms using Cox proportional hazards models in the R software package with penalized spline terms for the lead biomarkers.16
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
RESULTS
Compared to NAS participants with measurement of lead in blood but not bone, those with bone lead measurements more often had at least some college education (42% vs 36%) and were ever smokers (78% vs. 70%). Otherwise they were similar, including on blood lead concentration (mean 0.28 vs 0.26 μmol/L [5.7 vs 5.3 μg/dL]). Among the 860 NAS participants with valid patella bone lead measurements, we identified 241 deaths during 7,673 person-years of follow-up, an average of 8.9 years per participant (sd=3.9). The men averaged 67.3 (sd=7.3) years of age at the baseline bone lead measurement. The average patella and tibia bone lead concentrations were 31.2 (sd=19.4) and 21.8 (sd=13.6) μg/g bone mineral, respectively. The average blood lead concentration measured at baseline (1994 ± 3 years) was 0.27 (sd=0.16) μmol/L (5.6 [sd=3.4] μg/dL). The geometric mean of blood lead was 0.23 (inter-quartile range [iqr]: 0.14–0.34) μmol/L (4.8 [iqr: 3–7] μg/dL). The Pearson correlation coefficient between patella bone lead and blood lead measured at the same time was 0.38 and between patella and tibia bone lead was 0.77. Participants with higher patella bone lead concentration at baseline were more likely to be non-white, smokers, and not have more than a high school education (Table 1). Those with higher patella lead concentrations also tended to be slightly less active, slightly more likely to have hypertension, and slightly less likely to have had a stroke. Those with higher patella lead concentrations also tended to be older (Table 1), although this trend reverses among the oldest participants. Among NAS participants less than 80 years of age at the time of bone lead measurement (n=825) the Spearman correlation between age and patella bone lead concentration was 0.27, while among those participants 80 years or older at bone lead measurement (n=35), the Spearman correlation was −0.12.
Table 1.
Baseline characteristics* by patella lead tertile.
| Tertile of patella Pb |
|||
|---|---|---|---|
| 1st | 2nd | 3rd | |
| <22 μg/g | 22–35 μg/g | >35 μg/g | |
| N | 298 | 283 | 279 |
| Deaths, n (%) | 55 (22.8) | 75 (31.1) | 111 (46.1) |
| Age at baseline, mean years (sd) | 65.2 (7.1) | 66.5 (6.5) | 70.2 (7.2) |
| Years of follow-up, mean (sd) | 9.3 (3.7) | 8.9 (4.0) | 8.6 (4.0) |
| White race, % | 97.0 | 97.8 | 93.2 |
| Education, % | |||
| <high school | 5.6 | 11.2 | 14.5 |
| High school grad | 26.3 | 32.4 | 40.7 |
| Technical school | 11.6 | 10.8 | 8.6 |
| Some College | 13.7 | 13.7 | 12.5 |
| College grad | 23.8 | 15.3 | 9.6 |
| Grad school | 16.6 | 12.5 | 6.1 |
| Smoking (at baseline) | |||
| Never smoker, % | 37.3 | 27.5 | 21.4 |
| Former smoker, % | 58.4 | 63.2 | 64.6 |
| Current smoker, % | 4.3 | 9.3 | 10.8 |
| Packyears, mean | 15.1 | 22.1 | 28.9 |
| Total cholesterol (mg/dL), mean | 227 | 225 | 222 |
| Total HDL (mg/dL), mean | 47.2 | 48.5 | 47.0 |
| Body mass index (kg/m2), mean | 27.9 | 27.6 | 27.0 |
| Alcohol (g/day), mean† | 12.4 | 14.1 | 13.9 |
| Physical activity (kcal/wk), mean | 2,644 | 2,560 | 2,392 |
| Diabetes, % | 7.1 | 6.8 | 7.6 |
| Stroke, % | 1.8 | 2.1 | 0.3 |
| Ischemic heart disease, % | 14.8 | 20.3 | 17.8 |
| Cancer, % | 14.4 | 15.8 | 14.8 |
| Hypertension‡, % | 50.4 | 51.8 | 51.8 |
| Patella lead (μg/g), mean | 14.5 | 28.0 | 49.6 |
| Tibia lead (μg/g), mean | 13.9 | 19.5 | 30.6 |
| Blood lead (μmol/L [μg/dL]), mean | 0.22 [4.6] | 0.28 [5.8] | 0.35 [7.2] |
All variables except deaths, age at baseline and years of follow-up are age-adjusted by direct standardization to the entire cohort.
Among the 83%, 82%, and 76% in the 1st, 2nd, and 3rd tertiles, respectively, who reported drinking alcohol
Systolic blood pressure greater then 140 mm Hg, diastolic blood pressure greater than 90 mm Hg, or physician diagnosed hypertension.
The crude HR for all mortality endpoints increased with increasing patella bone lead tertiles (Table 2). In age, smoking status, packyears, and education-adjusted models, however, only all-cause, cardiovascular, and ischemic heart disease deaths showed significant associations with patella lead. For all-cause and cardiovascular mortality, the associations were stronger in the multivariable model that excluded any participants who had ischemic heart disease or history of stroke at baseline. In this multivariable-adjusted model for all-cause mortality, the HR for those in the highest tertile of patella lead compared to the lowest was 2.52 (95% CI: 1.17–5.41; p-for-trend=0.02). In the same multivariable-adjusted model for cardiovascular disease mortality the HR in the highest compared to lowest tertile of patella lead was 5.63 (95% CI: 1.73–18.3; p-for-trend=0.003). Among categories of cardiovascular deaths, the multivariable-adjusted HR for mortality from ischemic heart disease was significantly elevated in the highest patella lead tertile. There were too few ischemic heart disease deaths among participants without heart disease or stroke at baseline to allow for stable multivariable-adjusted models of this outcome in this subset of participants. The multivariable HR for other cardiovascular deaths did not appear to increase with increasing bone lead. Additionally adjusting for hypertension or race in models of patella bone lead did not meaningfully change the results, nor did including alcohol, physical activity, BMI, HDL, cholesterol, and diabetes. In contrast to cardiovascular mortality, cancer mortality did not exhibit an association with patella bone lead in multivariable models.
Table 2.
Hazard ratios (HR; 95% CI) for all-cause, cardiovascular disease, ischemic heart disease, other cardiovascular, and cancer mortality by tertile of patella lead at baseline.
| Tertile of patella Pb |
||||
|---|---|---|---|---|
| 1st | 2nd | 3rd | p-trend | |
| <22 μg/g | 22–35 μg/g | >35 μg/g | ||
| N | 298 | 283 | 279 | |
| Follow-up, years | 2,763 | 2,523 | 2,387 | |
| Allcause | ||||
| Deaths | 55 | 75 | 111 | |
| Crude | Ref | 1.47 (1.04–2.08) | 2.45 (1.77–3.39) | <0.0001 |
| Multivariable 1* | Ref | 1.44 (0.79–3.26) | 1.76 (0.95–3.26) | 0.07 |
| Multivariable 2† | Ref | 1.75 (0.82–3.75) | 2.52 (1.17–5.41) | 0.02 |
| All cardiovascular | ||||
| Deaths | 33 | 41 | 63 | |
| Crude | Ref | 1.36 (0.86–2.15) | 2.33 (1.53–3.55) | <0.0001 |
| Multivariable 1* | Ref | 1.39 (0.61–3.19) | 2.45 (1.07–5.60) | 0.03 |
| Multivariable 2† | Ref | 1.63 (0.51–5.18) | 5.63 (1.73–18.3) | 0.003 |
| Ischemic heart disease | ||||
| Deaths | 14 | 18 | 30 | |
| Crude | Ref | 1.41 (0.70–2.85) | 2.69 (1.42–5.08) | 0.002 |
| Multivariable 1* | Ref | 2.99 (0.40–22.6) | 8.37 (1.29–54.4) | 0.01 |
| Other cardiovascular | ||||
| Deaths | 19 | 23 | 33 | |
| Crude | Ref | 1.31 (0.72–2.41) | 2.07 (1.18–3.64) | 0.01 |
| Multivariable 1* | Ref | 1.01 (0.38–2.70) | 1.16 (0.40–3.39) | 0.79 |
| Multivariable 2† | Ref | 0.64 (0.15–2.80) | 1.35 (0.30–6.09) | 0.73 |
| Cancer | ||||
| Deaths | 21 | 28 | 42 | |
| Crude | Ref | 1.44 (0.82–2.54) | 2.40 (1.42–4.05) | 0.0008 |
| Multivariable 1* | Ref | 1.08 (0.43–2.68) | 0.59 (0.21–1.67) | 0.32 |
| Multivariable 2‡ | Ref | 0.82 (0.26–2.59) | 0.32 (0.08–1.35) | 0.14 |
Adjusted for age, smoking (never/former/current & packyears), and education.
Same model, but excluding the 154 participants (53 deaths) who had heart disease (146) or stroke (11) at baseline.
Multivariable models of cancer excluding 133 participants (34 deaths) with cancer at baseline.
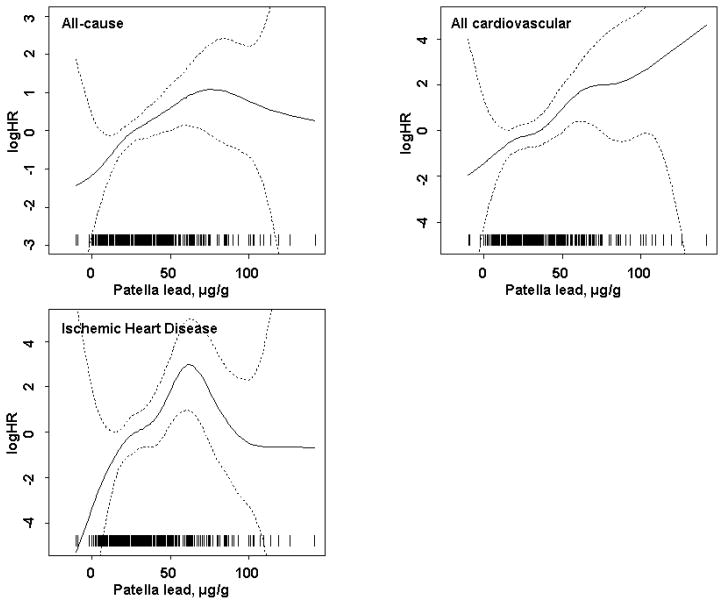
In spline regression models excluding participants with heart disease or stroke at baseline the non-linear component did not reach statistical significance for all-cause (p=0.42) nor all cardiovascular (p=0.80) deaths. Neither did the non-linear component for ischemic heart disease (p=0.10) in the full multivariable model. There was, however, some suggestion that the associations with all-cause mortality and ischemic heart disease leveled off at higher bone lead concentrations although in these ranges the data were sparse and the confidence intervals were wide (Figure 1).
FIGURE 1.
Nonlinear association between patella bone lead concentration and the log of Hazard Ratio (logHR) for all-cause, cardiovascular, and ischemic heart disease adjusted for age, education, smoking status, and packyears of smoking among participants without ischemic heart disease or stroke at baseline. The reference logHR=0 at the mean of patella lead concentration. The estimates are indicated by the solid line and the 95% pointwise confidence intervals by the dashed lines. The p-values for significance of the non-linear component for all-cause, cardiovascular, and ischemic heart disease mortality were 0.42, 0.80, and 0.10, respectively. Patella lead concentrations of all individual participants are indicated by short vertical lines on the abscissa.
The multivariable model results for tibia lead and mortality were much weaker than those for patella lead. In the age, smoking, and education-adjusted models excluding those with existent heart conditions, participants in the highest tertile of tibia lead compared to the lowest had an HR of 2.04 (95% CI: 1.00–4.15) for all-cause mortality, 1.71 (95% CI: 0.67–4.39) for cardiovascular disease mortality, and 1.42 (95% CI: 0.36–5.54) for ischemic heart disease. For non-ischemic heart disease there was more of a suggestion of an effect as the HR in the highest quintile compared to the lowest was 3.08 (95% CI: 0.61–15.6; p for trend=0.19).
While crude associations between blood lead and mortality generally tended towards increasing mortality with increasing blood lead tertile, we did not see associations with blood lead in multivariable-adjusted models (Table 3). These analyses included 1,235 NAS participants with blood lead measures, among whom we identified 326 deaths over 9,770 person-years of follow-up. Furthermore, in analyses restricted to the subset of NAS participants for whom we had bone lead data in which both blood lead (at the time of the bone lead measurement) and bone lead tertiles were included in the model, there were still no associations with blood lead and the associations with bone lead were effectively unchanged.
Table 3.
Hazard ratios (HR; 95% CI) for all-cause, cardiovascular disease, ischemic heart disease, other cardiovascular, and cancer mortality by tertile of blood lead at baseline.
| Tertile of Blood Pb |
||||
|---|---|---|---|---|
| 1st | 2nd | 3rd | p-trend | |
| <0.19 μmol/L (<4 μg/dL) | 0.19–0.29 μmol/L (4–6 μg/dL) | >0.29 μmol/L (>6 μg/dL) | ||
| N | 320 | 561 | 354 | |
| Follow-up, years | 2,472 | 4,431 | 2,867 | |
| Allcause | ||||
| Deaths | 72 | 144 | 110 | |
| Crude | Ref | 1.11 (0.84–1.47) | 1.27 (0.94–1.71) | 0.11 |
| Multivariable 1* | Ref | 0.81 (0.53–1.25) | 0.93 (0.59–1.45) | 0.84 |
| Multivariable 2† | Ref | 0.69 (0.41–1.19) | 0.84 (0.50–1.42) | 0.67 |
| All cardiovascular | ||||
| Deaths | 38 | 84 | 63 | |
| Crude | Ref | 1.23 (0.84–1.80) | 1.40 (0.94–2.09) | 0.10 |
| Multivariable 1* | Ref | 0.86 (0.49–1.51) | 0.99 (0.55–1.78) | 0.96 |
| Multivariable 2† | Ref | 0.63 (0.29–1.38) | 0.69 (0.33–1.47) | 0.44 |
| Ischemic heart disease | ||||
| Deaths | 17 | 36 | 29 | |
| Crude | Ref | 1.19 (0.67–2.11) | 1.44 (0.79–2.61) | 0.23 |
| Multivariable 1* | Ref | 1.13 (0.49–2.62) | 1.30 (0.54–3.17) | 0.55 |
| Multivariable 2† | Ref | 1.02 (0.32–3.21) | 1.04 (0.33–3.22) | 0.95 |
| Other cardiovascular | ||||
| Deaths | 21 | 48 | 34 | |
| Crude | Ref | 1.26 (0.76–2.11) | 1.37 (0.80–2.36) | 0.27 |
| Multivariable 1* | Ref | 0.64 (0.28–1.46) | 0.77 (0.34–1.74) | 0.65 |
| Multivariable 2 | Ref | 0.30 (0.09–1.03) | 0.39 (0.12–1.23) | 0.23 |
| Cancer | ||||
| Deaths | 28 | 57 | 40 | |
| Crude | Ref | 1.12 (0.71–1.76) | 1.17 (0.72–1.91) | 0.52 |
| Multivariable 1* | Ref | 0.84 (0.44–1.62) | 0.67 (0.33–1.37) | 0.26 |
| Multivariable 2‡ | Ref | 1.03 (0.42–2.55) | 0.53 (0.20–1.39) | 0.15 |
Adjusted for age, smoking (never/former/current & packyears), and education.
Same model, but excluding the 212 participants (72 deaths) who had ischemic heart disease (201) or stroke (12) at baseline.
Multivariable models of cancer excluding 197 participants (40 deaths) with cancer at baseline.
DISCUSSION
In this prospective study we found that patella bone lead was associated with a significantly increased rate of all-cause mortality over an average follow-up of almost 10-years. This association appeared to be driven largely by cardiovascular causes of death as patella bone lead was even more strongly associated with these outcomes, in particular ischemic heart disease. These findings were among a population of men with blood lead levels only slightly higher than US averages for men of a similar age,17 and likely well within the range that would be seen around the world, particularly in countries that banned leaded gasoline more recently than the US or have not banned leaded gasoline at all.
While there was little association between patella bone lead and cancer or non-ischemic heart disease, we found that, compared to participants in the lowest tertile of patella bone lead, those in the highest tertile were at a 2 and one half-fold greater risk of all-cause mortality, almost 6-fold greater risk of cardiovascular mortality, and over 8-fold greater risk of ischemic heart disease, although in this last category the confidence interval was very wide and so the specific point estimate should be interpreted with caution. The increase in hazard ratio with increasing tertile of bone lead exposure was relatively monotonic for all three of these endpoints, suggesting linear dose-response relations, and this was also supported by the significant p-values for linear trend. This was also borne out by smoothing analyses in which the generally monotonic trend was apparent (Figure 1), and in none of which did the non-linear components reach statistical significance. However, for all-cause and ischemic heart disease mortality, there was the suggestion that the increasing risk with increasing bone lead lessens at higher lead levels, although the confidence intervals were very wide in this range of lead exposures. For all-cause and all cardiovascular mortality the associations with patella bone lead were stronger in analyses excluding participants with heart disease or stroke at baseline than in analyses including such participants. This suggests that having those conditions changes the lead exposure profile, either by alterations in behavior or physiology. Major strengths of our study include the prospectively followed community-dwelling cohort, and having both blood and bone lead data. Bone lead levels provide a better indicator of cumulative exposure to lead than do blood lead levels because of the much longer half-life of lead in bone.9
There are several limitations to this study that should be recognized. First, our study is restricted to men, the majority of whom are Caucasian. Thus, whether the results generalize to women or minorities remains a question. Additionally, bone lead measurements are not perfectly precise and are made with some error. This measurement error, however, is most likely unrelated to overall or cause specific mortality and thus would be likely to bias results towards the null rather than induce a spurious association. We also adjusted for several covariates that might confound the association between lead and cardiovascular mortality, but, as with any observational study, the possibility of residual confounding by these variables or confounding by other unmeasured variables cannot be completely ruled out. Although 32% of active NAS participants did not participate in bone lead measurements, this group was generally similar to those who did, in particular in blood lead level, which suggests that little bias would be introduced from the non-participation. Finally, in comparison to recent studies examining blood lead data in NHANES,7, 8 the present study was substantially smaller. While the increase in strength of the association between patella lead and ischemic heart disease mortality after adjustment is driven by the inverse association between patella lead and age in the oldest NAS participants, this negative confounding is likely exacerbated by the small number of ischemic heart disease deaths. Overall, though, the fact that we still found significant associations between bone lead and mortality in a sample with over 6 times fewer deaths, however, suggests that bone lead is a particularly good biomarker for these outcomes.
As in this study, bone lead has been found in other studies to be a stronger predictor than blood lead of several other health endpoints (e.g. hypertension,12, 18, 19 electrocardiographic disturbances,20 pulse pressure,21 renal function,22 cognition,23, 24 cataracts25), and suggest that this biomarker should be strongly consider for monitoring environmental exposures relevant for health. Currently blood lead is the only lead biomarker assessed in NHANES and current Occupational Safety and Health Administration (OSHA) standards regarding exposure to lead relate only to blood lead.26 Bone lead, however, may be a more relevant biomarker and a more important indicator of subsequent health events, at least when the exposure assessment is done only once.
No previous studies have examined the association between bone lead and mortality, although several have reported associations with blood lead in data from NHANES.6–8, 27 In the most recent studies from the third (1988–1994) NHANES, blood lead levels were associated with an increased rate of all-cause, cardiovascular, and, in the study not restricted to those with lower blood lead levels, cancer mortality.7, 8 Among adults 20 years of age and older with blood lead concentrations below 0.48 μmol/L (10 μg/dL), the hazard ratio for those with blood lead concentrations in the highest tertile (≥ 0.175 μmol/L [3.63 μg/dL]) was 25% higher than those with blood lead in the lowest tertile (<0.093 μmol/L [1.93 μg/dL]) for all-cause mortality and 55% higher for cardiovascular mortality.7 Among adults 40 years of age and older, the hazard ratio for those with blood lead concentrations of 0.48 μmol/L (10 μg/dL) or more was 59% higher than those with blood lead below 0.24 μmol/L (5 μg/dL) for all-cause mortality and 55% higher for cardiovascular mortality.8 The average age at baseline in one of these studies7 was more than 20 years younger than in the current study, which, given the difference from our findings for blood lead, could suggest that blood lead at earlier ages is more predictive than blood lead at older ages. The other recent paper on blood lead,8 however, found associations even among older participants, although the findings appeared slightly less robust at older ages. In a study of mostly non-fatal ischemic heart disease (70 of 83 cases) in the NAS, however, both blood lead and patella bone lead were significantly associated with myocardial infarction or angina pectoris, although patella lead appeared to be the stronger predictor.10 Three prior studies with only blood lead data, however, did not see an association with that biomarker.28–30
An alternative explanation for the differences in findings for blood lead in our cohort and the NHANES studies is that if exposures to lead in the Greater Boston area were more varied at the time of blood lead assessment than they were for the more national NHANES cohorts (as is possibly suggested by the slightly higher blood lead levels in our cohort), this could result in more fluctuation in blood lead levels. If this were the case, then any single blood lead measure would be less correlated with overall lead exposure in our cohort and show a reduced effect estimate for mortality if it is truly cumulative exposure that is important for mortality outcomes.
There are several mechanisms by which exposure to lead may result in cardiovascular mortality in particular (for review see 2). Lead can have direct effects on the excitability and contractility of the heart, and increase vascular tone and peripheral resistance via effects such as stimulating the renin-angiotensin system, reductions in nitric oxide availability and guanylate cyclase, or increased oxidative stress.31–33 Lead has been found to induce proliferation of vascular smooth cells and fibroblasts and induce atherosclerosis in animal models.34, 35 In addition, neurotoxic effects of lead can affect autonomic control of the heart.36 The most extensive data in humans relates to the association between lead exposure and higher blood pressure or hypertension, but lead exposure has also been associated with many other cardiovascular endpoints,2, 3 including pulse-pressure (an indicator of arterial stiffening),21 electrocardiographic disturbances,20 and ischemic heart disease10 in this same cohort. The association with heart disease deaths persisted after control for blood pressure suggesting that other mechanisms likely mediate the effect of lead on heart disease. Given the mechanisms of these effects of lead on the cardiovascular system, it is not surprising that a cumulative biomarker of lead—exposure such as bone lead—would be a better predictor of cardiovascular mortality than blood, which is a more acute exposure biomarker.
Cardiovascular disease is the leading cause of death in the US and one of the most significant contributors to mortality worldwide.37, 38 Projections of future trends in cardiovascular mortality have important health care planning implications. Cardiovascular mortality has generally shown a steady decrease over the past several decades in developed countries, although the opposite is seen in some developing countries.37, 39, 40 These trends tend to be paralleled by trends in traditional cardiovascular risk factors such as blood pressure, cholesterol levels, and smoking.41, 42 These trends, however, do not explain all the change in cardiovascular mortality, and, at least in some cases, cardiovascular mortality continues to decline despite a leveling off of changes in traditional risk factors.42 Lead exposure has not been considered in this equation, but in the US and many other developed countries environmental exposures have been declining since the mid-1970s.43 These declines may well have contributed to declining cardiovascular mortality rates. Because of the long residence time of lead in bone—a storage site from which it can later re-enter circulation9 —and the aging of the population, the full effects of reducing environmental levels of lead may continue for some time.
In summary, we found that in a population of community-dwelling elderly men with biomarkers of both blood and bone lead, bone lead, but not blood lead, was associated with an increased mortality rate that was particularly evident for cardiovascular disease, and specifically between patella lead and ischemic heart disease.
Acknowledgments
Funding sources
This study was funded primarily by NIEHS R01-ES05257, P42-ES05947, and NIEHS Center Grant P30-ES00002. Dr. Weisskopf was supported by NIEHS K01-#ES012653. Participants were evaluated for measurement of bone lead levels in the outpatient Clinical Research Center of the Brigham and Women’s Hospital with support from NIH grant no. NCRR GCRC M01RR02635. The KXRF instrument used in this work was developed by ABIOMED, Inc., of Danvers, Massachusetts, with support from NIH grant no. SBIR 2R44 ES03918-02. The Normative Aging Study is supported by the Research Services and the Cooperative Studies Program/ERIC of the US Department of Veterans Affairs, and is a research component of the Massachusetts Veterans Epidemiology Research and Information Center (MAVERIC). The views expressed in this paper are those of the authors and do not necessarily represent the views of the US Department of Veterans Affairs.
Footnotes
Weisskopf: Bone lead and mortality
Disclosures
None.
Clinical summary
Genetics is well known to play only a limited role in the etiology of cardiovascular disease. It is now known that in addition to diet, exercise, and other lifestyle and behavioral factors, certain environmental risk factors play a significant role in the general population. Much attention has recently been paid to particulate air pollution and second hand cigarette smoke as such risk factors, for example. This study builds upon another body of research recently indicating that cumulative environmental lead exposure (from decades of exposures to lead in combusted gasoline, paint, water, food cans, etc.) is a risk factor for hypertension and myocardial infarction and goes even farther by linking such exposure directly to increased prospective cardiovascular mortality. Like a number of other recent studies, this investigation also shows that the risk to individuals posed by lead exposure cannot be adequately captured by measuring blood lead levels, which mostly signifies recent, rather than cumulative, exposure. From a clinical perspective, this research highlights the importance of incorporating at least a brief environmental/occupational assessment in the conduct of preventive cardiology and medicine and of advocating for the elimination or minimization of activities that are associated with lead exposure. It also underscores the anticipation surrounding an on going NIH-funded multicenter trial testing the potential value of chelation in reducing cardiovascular risks, the results of which remain pending.
References
- 1.Rowland ID, Howe TN, editors. Vitruvius: Ten Books on Architecture. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- 2.Navas-Acien A, Guallar E, Silbergeld EK, Rothenberg SJ. Lead exposure and cardiovascular disease--a systematic review. Environ Health Perspect. 2007;115:472–482. doi: 10.1289/ehp.9785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navas-Acien A, Schwartz BS, Rothenberg SJ, Hu H, Silbergeld EK, Guallar E. Bone Lead Levels and Blood Pressure Endpoints: A Meta-Analysis. Epidemiology. 2008;19:496–504. doi: 10.1097/EDE.0b013e31816a2400. [DOI] [PubMed] [Google Scholar]
- 4.Boscolo P, Carmignani M. Neurohumoral blood pressure regulation in lead exposure. Environ Health Perspect. 1988;78:101–106. doi: 10.1289/ehp.8878101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmignani M, Boscolo P, Poma A, Volpe AR. Kininergic system and arterial hypertension following chronic exposure to inorganic lead. Immunopharmacology. 1999;44:105–110. doi: 10.1016/s0162-3109(99)00115-0. [DOI] [PubMed] [Google Scholar]
- 6.Lustberg M, Silbergeld E. Blood lead levels and mortality. Arch Intern Med. 2002;162:2443–2449. doi: 10.1001/archinte.162.21.2443. [DOI] [PubMed] [Google Scholar]
- 7.Menke A, Muntner P, Batuman V, Silbergeld EK, Guallar E. Blood lead below 0.48 micromol/L (10 microg/dL) and mortality among US adults. Circulation. 2006;114:1388–1394. doi: 10.1161/CIRCULATIONAHA.106.628321. [DOI] [PubMed] [Google Scholar]
- 8.Schober SE, Mirel LB, Graubard BI, Brody DJ, Flegal KM. Blood lead levels and death from all causes, cardiovascular disease, and cancer: results from the NHANES III mortality study. Environ Health Perspect. 2006;114:1538–1541. doi: 10.1289/ehp.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu H, Rabinowitz M, Smith D. Bone lead as a biological marker in epidemiologic studies of chronic toxicity: conceptual paradigms. Environ Health Perspect. 1998;106:1–8. doi: 10.1289/ehp.981061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain NB, Potula V, Schwartz J, Vokonas PS, Sparrow D, Wright RO, Nie H, Hu H. Lead levels and ischemic heart disease in a prospective study of middle-aged and elderly men: the VA Normative Aging Study. Environ Health Perspect. 2007;115:871–875. doi: 10.1289/ehp.9629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell B, Rose CL, Damon A. The Veterans Administration longitudinal study of healthy aging. Gerontologist. 1966;6:179–184. doi: 10.1093/geront/6.4.179. [DOI] [PubMed] [Google Scholar]
- 12.Hu H, Aro A, Payton M, Korrick S, Sparrow D, Weiss ST, Rotnitzky A. The relationship of bone and blood lead to hypertension. The Normative Aging Study. JAMA. 1996;275:1171–1176. [PubMed] [Google Scholar]
- 13.Aro A, Amarasiriwardena C, Lee ML, Kim R, Hu H. Validation of K x-ray fluorescence bone lead measurements by inductively coupled plasma mass spectrometry in cadaver legs. Med Phys. 2000;27:119–123. doi: 10.1118/1.598863. [DOI] [PubMed] [Google Scholar]
- 14.Paffenbarger RS, Jr, Wing AL, Hyde RT. Physical activity as an index of heart attack risk in college alumni. Am J Epidemiol. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S. Measures of Disease Frequency. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. Philadelphia, PA: Lippincott-Raven Publishers; 1998. pp. 29–46. [Google Scholar]
- 16.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2004. [Google Scholar]
- 17.Pirkle JL, Kaufmann RB, Brody DJ, Hickman T, Gunter EW, Paschal DC. Exposure of the U.S. population to lead, 1991–1994. Environ Health Perspect. 1998;106:745–750. doi: 10.1289/ehp.98106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korrick SA, Hunter DJ, Rotnitzky A, Hu H, Speizer FE. Lead and hypertension in a sample of middle-aged women. Am J Public Health. 1999;89:330–335. doi: 10.2105/ajph.89.3.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BK, Lee GS, Stewart WF, Ahn KD, Simon D, Kelsey KT, Todd AC, Schwartz BS. Associations of blood pressure and hypertension with lead dose measures and polymorphisms in the vitamin D receptor and delta-aminolevulinic acid dehydratase genes. Environ Health Perspect. 2001;109:383–389. doi: 10.1289/ehp.01109383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng Y, Schwartz J, Vokonas PS, Weiss ST, Aro A, Hu H. Electrocardiographic conduction disturbances in association with low- level lead exposure (the Normative Aging Study) Am J Cardiol. 1998;82:594–599. doi: 10.1016/s0002-9149(98)00402-0. [DOI] [PubMed] [Google Scholar]
- 21.Perlstein T, Weuve J, Schwartz J, Sparrow D, Wright R, Litonjua A, Nie H, Hu H. Cumulative community-level lead exposure and pulse pressure: the normative aging study. Environ Health Perspect. 2007;115:1696–1700. doi: 10.1289/ehp.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu MT, Kelsey K, Schwartz J, Sparrow D, Weiss S, Hu H. A delta-aminolevulinic acid dehydratase (ALAD) polymorphism may modify the relationship of low-level lead exposure to uricemia and renal function: the normative aging study. Environ Health Perspect. 2003;111:335–341. doi: 10.1289/ehp.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih RA, Hu H, Weisskopf MG, Schwartz BS. Cumulative lead dose and cognitive function in adults: a review of studies that measured both blood lead and bone lead. Environ Health Perspect. 2007;115:483–492. doi: 10.1289/ehp.9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weisskopf MG, Myers G. Cumulative effect of lead on cognition: is bone more revealing than blood? Neurology. 2006;67:1536–1537. doi: 10.1212/01.wnl.0000246111.21148.ee. [DOI] [PubMed] [Google Scholar]
- 25.Schaumberg DA, Mendes F, Balaram M, Dana MR, Sparrow D, Hu H. Accumulated lead exposure and risk of age-related cataract in men. JAMA. 2004;292:2750–2754. doi: 10.1001/jama.292.22.2750. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz BS, Hu H. Adult lead exposure: time for change. Environ Health Perspect. 2007;115:451–454. doi: 10.1289/ehp.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jemal A, Graubard BI, Devesa SS, Flegal KM. The association of blood lead level and cancer mortality among whites in the United States. Environ Health Perspect. 2002;110:325–329. doi: 10.1289/ehp.02110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kromhout D. Blood lead and coronary heart disease risk among elderly men in Zutphen, The Netherlands. Environ Health Perspect. 1988;78:43–46. doi: 10.1289/ehp.887843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moller L, Kristensen TS. Blood lead as a cardiovascular risk factor. Am J Epidemiol. 1992;136:1091–1100. doi: 10.1093/oxfordjournals.aje.a116574. [DOI] [PubMed] [Google Scholar]
- 30.Pocock SJ, Shaper AG, Ashby D, Delves HT, Clayton BE. The relationship between blood lead, blood pressure, stroke, and heart attacks in middle-aged British men. Environ Health Perspect. 1988;78:23–30. doi: 10.1289/ehp.887823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Iturbe B, Sindhu RK, Quiroz Y, Vaziri ND. Chronic exposure to low doses of lead results in renal infiltration of immune cells, NF-kappaB activation, and overexpression of tubulo interstitial angiotensin II. Antioxid Redox Signal. 2005;7:1269–1274. doi: 10.1089/ars.2005.7.1269. [DOI] [PubMed] [Google Scholar]
- 32.Sharifi AM, Darabi R, Akbarloo N, Larijani B, Khoshbaten A. Investigation of circulatory and tissue ACE activity during development of lead-induced hypertension. Toxicol Lett. 2004;153:233–238. doi: 10.1016/j.toxlet.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 33.Vaziri ND, Khan M. Interplay of reactive oxygen species and nitric oxide in the pathogenesis of experimental lead-induced hypertension. Clin Exp Pharmacol Physiol. 2007;34:920–925. doi: 10.1111/j.1440-1681.2007.04644.x. [DOI] [PubMed] [Google Scholar]
- 34.Fujiwara Y, Kaji T, Yamamoto C, Sakamoto M, Kozuka H. Stimulatory effect of lead on the proliferation of cultured vascular smooth-muscle cells. Toxicology. 1995;98:105–110. doi: 10.1016/0300-483x(94)02984-3. [DOI] [PubMed] [Google Scholar]
- 35.Tarugi P, Calandra S, Borella P, Vivoli GF. Heavy metals and experimental atherosclerosis. Effect of lead intoxication on rabbit plasma lipoproteins. Atherosclerosis. 1982;45:221–234. doi: 10.1016/0021-9150(82)90140-x. [DOI] [PubMed] [Google Scholar]
- 36.Chang HR, Tsao DA, Yu HS, Ho CK. The change of beta-adrenergic system after cessation of lead exposure. Toxicology. 2005;207:73–80. doi: 10.1016/j.tox.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 38.Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, Mass: Harvard University Press; 1996. [Google Scholar]
- 39.Kesteloot H, Sans S, Kromhout D. Dynamics of cardiovascular and all-cause mortality in Western and Eastern Europe between 1970 and 2000. Eur Heart J. 2006;27:107–113. doi: 10.1093/eurheartj/ehi511. [DOI] [PubMed] [Google Scholar]
- 40.Singh RB, Suh IL, Singh VP, Chaithiraphan S, Laothavorn P, Sy RG, Babilonia NA, Rahman AR, Sheikh S, Tomlinson B, Sarraf-Zadigan N. Hypertension and stroke in Asia: prevalence, control and strategies in developing countries for prevention. J Hum Hypertens. 2000;14:749–763. doi: 10.1038/sj.jhh.1001057. [DOI] [PubMed] [Google Scholar]
- 41.Gregg EW, Cheng YJ, Cadwell BL, Imperatore G, Williams DE, Flegal KM, Narayan KM, Williamson DF. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868–1874. doi: 10.1001/jama.293.15.1868. [DOI] [PubMed] [Google Scholar]
- 42.Harald K, Koskinen S, Jousilahti P, Torppa J, Vartiainen E, Salomaa V. Changes in traditional risk factors no longer explain time trends in cardiovascular mortality and its socioeconomic differences. J Epidemiol Community Health. 2008;62:251–257. doi: 10.1136/jech.2007.060707. [DOI] [PubMed] [Google Scholar]
- 43.Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165:2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]