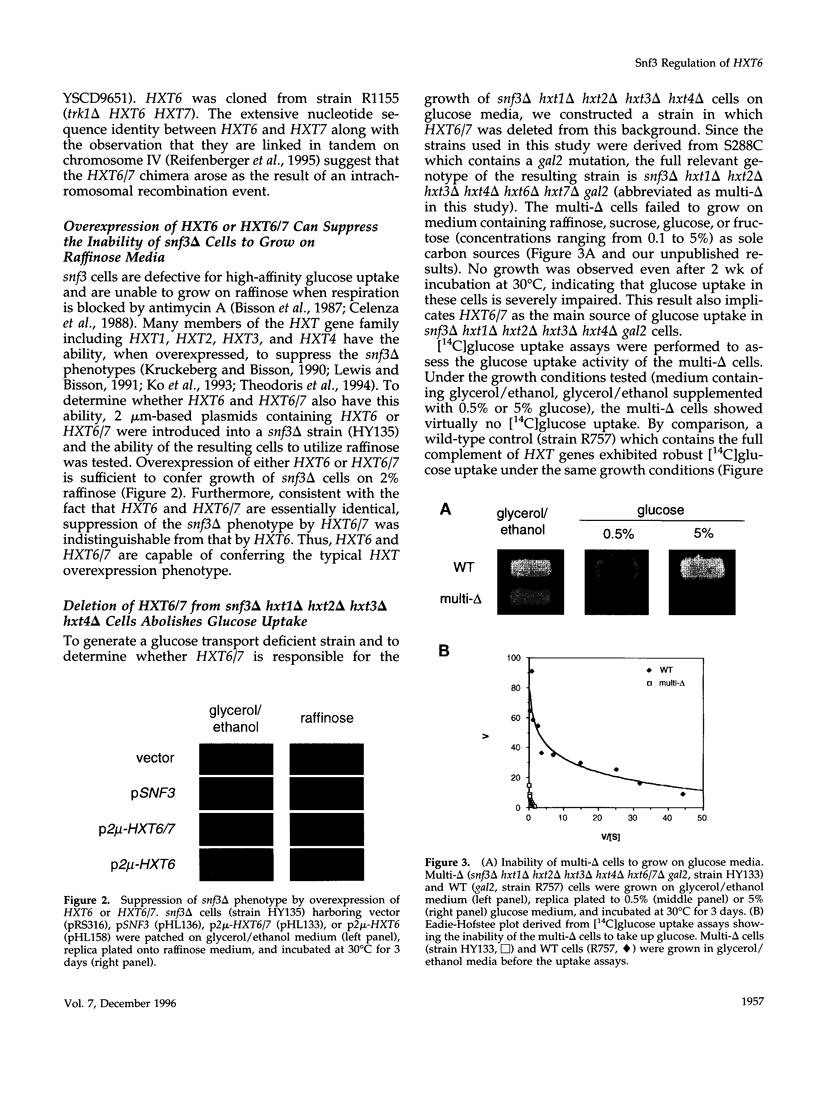
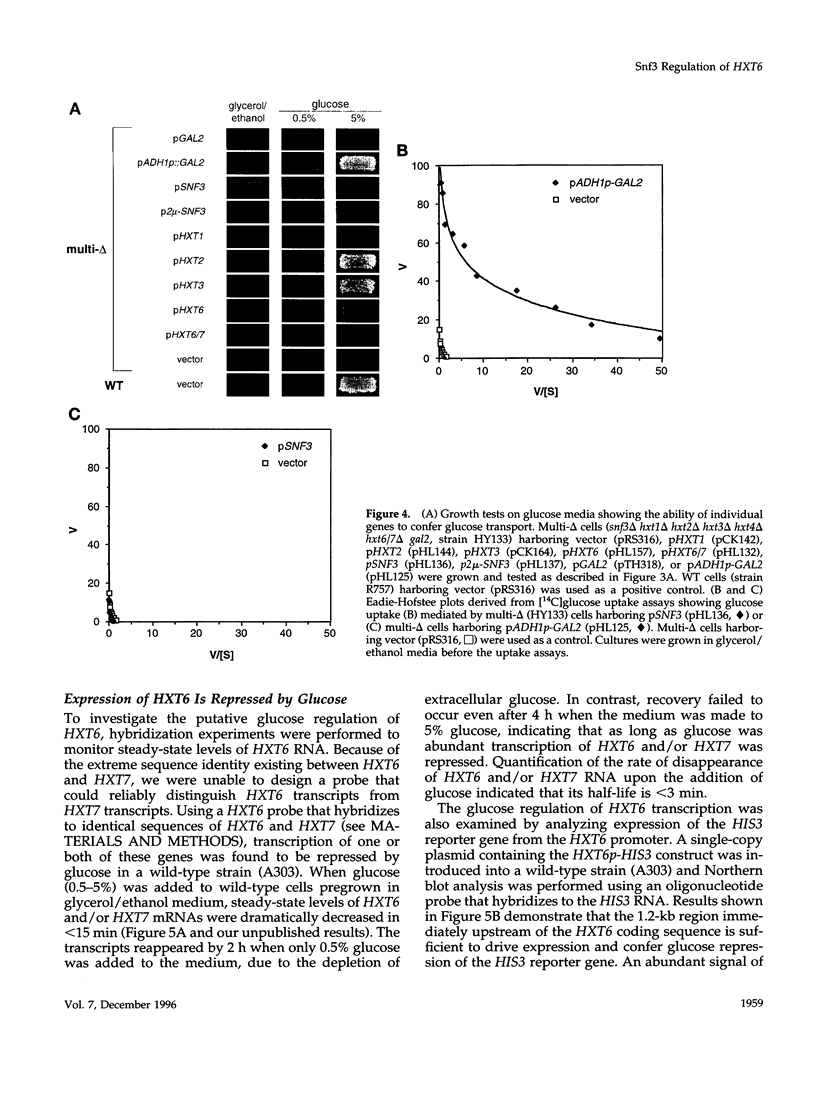
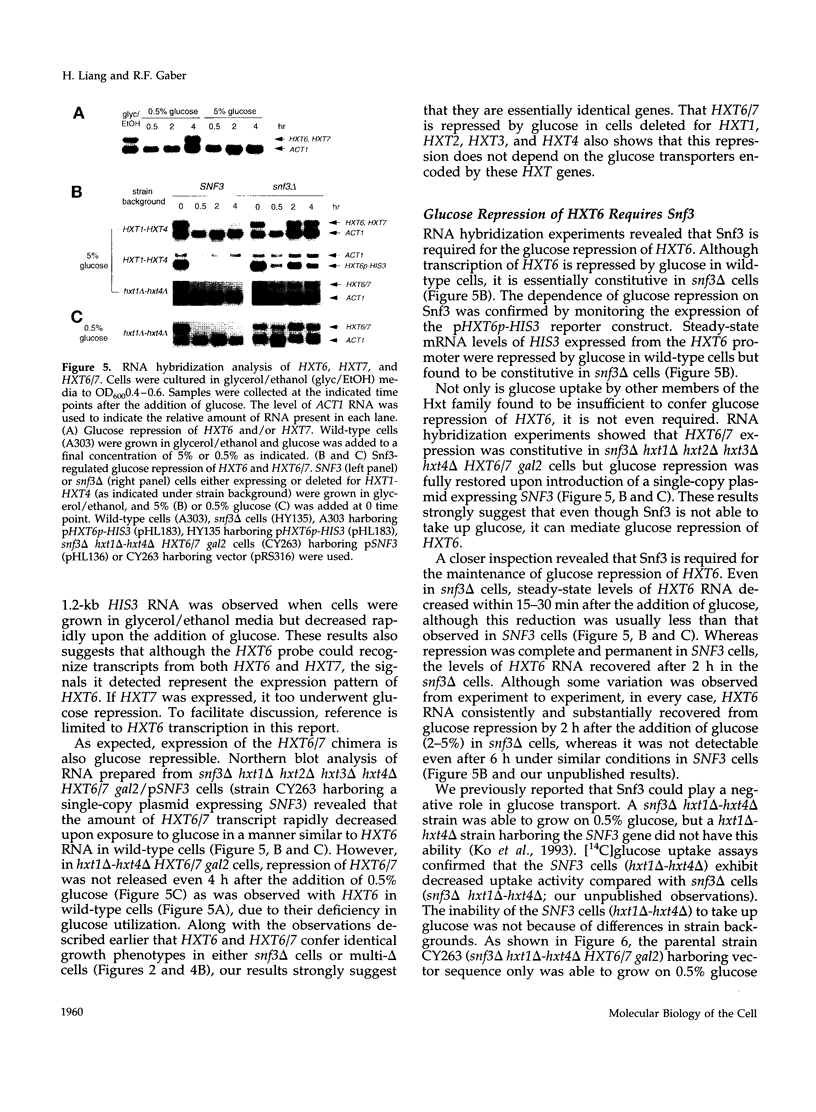
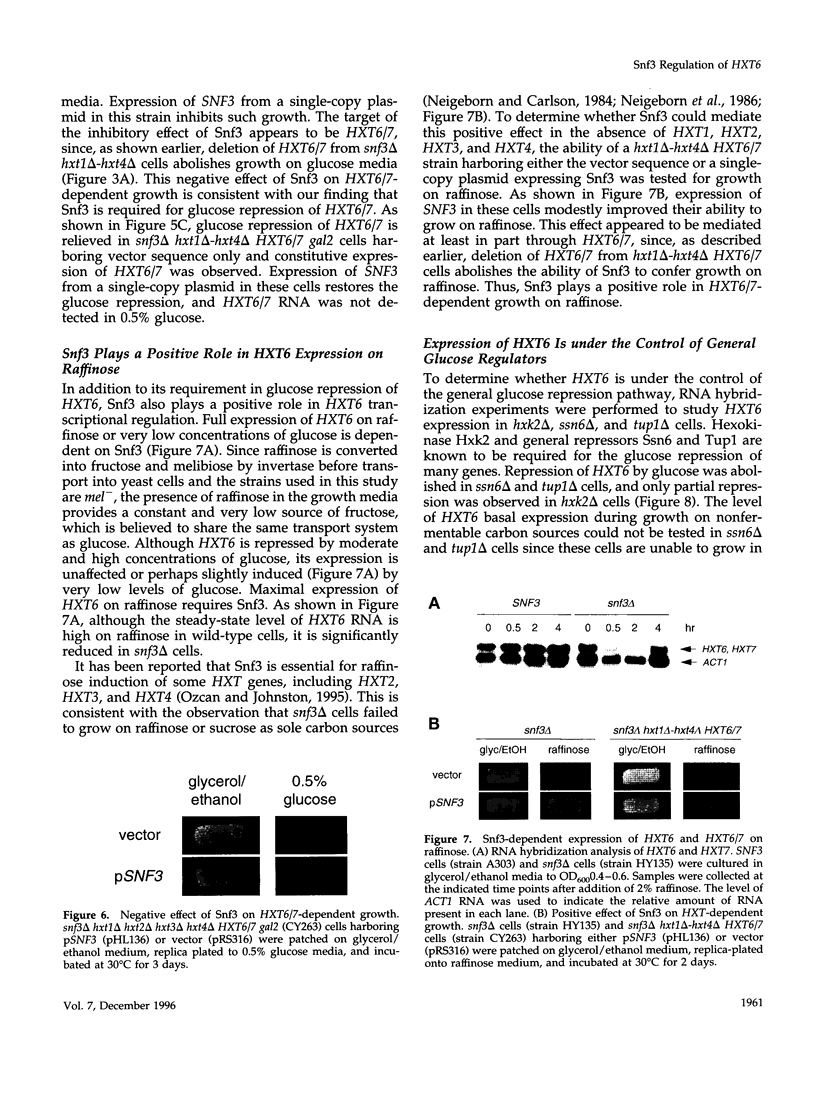
Abstract
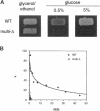
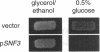
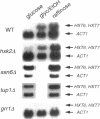
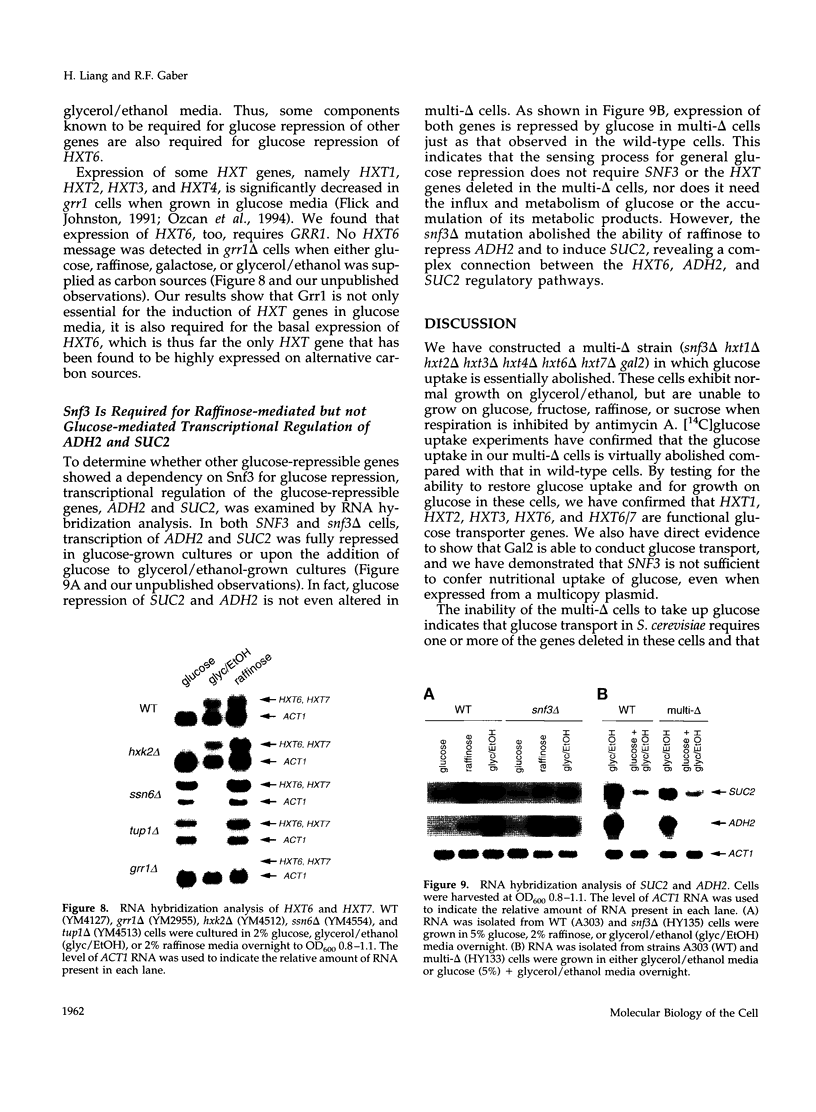
We show that cells deleted for SNF3, HXT1, HXT2, HXT3, HXT4, HXT6, and HXT7 do not take up glucose and cannot grow on media containing glucose as a sole carbon source. The expression of Hxt1, Hxt2, Hxt3, Hxt6, or Gal2 in these cells resulted in glucose transport and allowed growth on glucose media. In contrast, the expression of Snf3 failed to confer glucose uptake or growth on glucose. HXT6 is highly expressed on raffinose, low glucose, or nonfermentable carbon sources but is repressed in the presence of high concentrations of glucose. The maintenance of HXT6 glucose repression is strictly dependent on Snf3 and not on intracellular glucose. In snf3 delta cells expression of HXT6 is constitutive even when the entire repertoire of HXT genes is present and glucose uptake is abundant. In addition, glucose repression of HXT6 does not require glucose uptake by HXT1, HXT2, HXT3 or HXT4. We show that a signal transduction pathway defined by the Snf3-dependent hexose regulation of HXT6 is distinct from but also overlaps with general glucose regulation pathways in Saccharomyces cerevisiae. Finally, glucose repression of ADH2 and SUC2 is intact in snf3 delta hxt1 delta hxt2 delta hxt3 delta hxt4 delta hxt6 delta hxt7 delta gal2 cells, suggesting that the sensing and signaling mechanism for general glucose repression is independent from glucose uptake.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudin A., Ozier-Kalogeropoulos O., Denouel A., Lacroute F., Cullin C. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 1993 Jul 11;21(14):3329–3330. doi: 10.1093/nar/21.14.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D. M., Guarente L. High-efficiency transformation of yeast by electroporation. Methods Enzymol. 1991;194:182–187. doi: 10.1016/0076-6879(91)94015-5. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Coons D. M., Kruckeberg A. L., Lewis D. A. Yeast sugar transporters. Crit Rev Biochem Mol Biol. 1993;28(4):259–308. doi: 10.3109/10409239309078437. [DOI] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Involvement of kinases in glucose and fructose uptake by Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1730–1734. doi: 10.1073/pnas.80.6.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Fraenkel D. G. Transport of 6-deoxyglucose in Saccharomyces cerevisiae. J Bacteriol. 1983 Sep;155(3):995–1000. doi: 10.1128/jb.155.3.995-1000.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F. High-affinity glucose transport in Saccharomyces cerevisiae is under general glucose repression control. J Bacteriol. 1988 Oct;170(10):4838–4845. doi: 10.1128/jb.170.10.4838-4845.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson L. F., Neigeborn L., Carlson M., Fraenkel D. G. The SNF3 gene is required for high-affinity glucose transport in Saccharomyces cerevisiae. J Bacteriol. 1987 Apr;169(4):1656–1662. doi: 10.1128/jb.169.4.1656-1662.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza J. L., Marshall-Carlson L., Carlson M. The yeast SNF3 gene encodes a glucose transporter homologous to the mammalian protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2130–2134. doi: 10.1073/pnas.85.7.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson T. W., Sikorski R. S., Dante M., Shero J. H., Hieter P. Multifunctional yeast high-copy-number shuttle vectors. Gene. 1992 Jan 2;110(1):119–122. doi: 10.1016/0378-1119(92)90454-w. [DOI] [PubMed] [Google Scholar]
- Coons D. M., Boulton R. B., Bisson L. F. Computer-assisted nonlinear regression analysis of the multicomponent glucose uptake kinetics of Saccharomyces cerevisiae. J Bacteriol. 1995 Jun;177(11):3251–3258. doi: 10.1128/jb.177.11.3251-3258.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entian K. D., Kopetzki E., Fröhlich K. U., Mecke D. Cloning of hexokinase isoenzyme PI from Saccharomyces cerevisiae: PI transformants confirm the unique role of hexokinase isoenzyme PII for glucose repression in yeasts. Mol Gen Genet. 1984;198(2):50–54. doi: 10.1007/BF00328699. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Flick J. S., Johnston M. GRR1 of Saccharomyces cerevisiae is required for glucose repression and encodes a protein with leucine-rich repeats. Mol Cell Biol. 1991 Oct;11(10):5101–5112. doi: 10.1128/mcb.11.10.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrmann G. F., Völker B. Misuse of graphical analysis in nonlinear sugar transport kinetics by Eadie-Hofstee plots. Biochim Biophys Acta. 1993 Jan 18;1145(1):180–182. doi: 10.1016/0005-2736(93)90396-h. [DOI] [PubMed] [Google Scholar]
- Gaber R. F., Styles C. A., Fink G. R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo F. J., Moreno E., Lagunas R. The low-affinity component of the glucose transport system in Saccharomyces cerevisiae is not due to passive diffusion. Yeast. 1995 Nov;11(14):1393–1398. doi: 10.1002/yea.320111407. [DOI] [PubMed] [Google Scholar]
- Heredia C. F., Sols A., DelaFuente G. Specificity of the constitutive hexose transport in yeast. Eur J Biochem. 1968 Aug;5(3):321–329. doi: 10.1111/j.1432-1033.1968.tb00373.x. [DOI] [PubMed] [Google Scholar]
- Ko C. H., Liang H., Gaber R. F. Roles of multiple glucose transporters in Saccharomyces cerevisiae. Mol Cell Biol. 1993 Jan;13(1):638–648. doi: 10.1128/mcb.13.1.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komachi K., Redd M. J., Johnson A. D. The WD repeats of Tup1 interact with the homeo domain protein alpha 2. Genes Dev. 1994 Dec 1;8(23):2857–2867. doi: 10.1101/gad.8.23.2857. [DOI] [PubMed] [Google Scholar]
- Kruckeberg A. L., Bisson L. F. The HXT2 gene of Saccharomyces cerevisiae is required for high-affinity glucose transport. Mol Cell Biol. 1990 Nov;10(11):5903–5913. doi: 10.1128/mcb.10.11.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis D. A., Bisson L. F. The HXT1 gene product of Saccharomyces cerevisiae is a new member of the family of hexose transporters. Mol Cell Biol. 1991 Jul;11(7):3804–3813. doi: 10.1128/mcb.11.7.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Carlson L., Celenza J. L., Laurent B. C., Carlson M. Mutational analysis of the SNF3 glucose transporter of Saccharomyces cerevisiae. Mol Cell Biol. 1990 Mar;10(3):1105–1115. doi: 10.1128/mcb.10.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall-Carlson L., Neigeborn L., Coons D., Bisson L., Carlson M. Dominant and recessive suppressors that restore glucose transport in a yeast snf3 mutant. Genetics. 1991 Jul;128(3):505–512. doi: 10.1093/genetics/128.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nehlin J. O., Carlberg M., Ronne H. Yeast galactose permease is related to yeast and mammalian glucose transporters. Gene. 1989 Dec 28;85(2):313–319. doi: 10.1016/0378-1119(89)90423-x. [DOI] [PubMed] [Google Scholar]
- Neigeborn L., Carlson M. Genes affecting the regulation of SUC2 gene expression by glucose repression in Saccharomyces cerevisiae. Genetics. 1984 Dec;108(4):845–858. doi: 10.1093/genetics/108.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigeborn L., Schwartzberg P., Reid R., Carlson M. Null mutations in the SNF3 gene of Saccharomyces cerevisiae cause a different phenotype than do previously isolated missense mutations. Mol Cell Biol. 1986 Nov;6(11):3569–3574. doi: 10.1128/mcb.6.11.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelissen B., Mordant P., Jonniaux J. L., De Wachter R., Goffeau A. Phylogenetic classification of the major superfamily of membrane transport facilitators, as deduced from yeast genome sequencing. FEBS Lett. 1995 Dec 18;377(2):232–236. doi: 10.1016/0014-5793(95)01380-6. [DOI] [PubMed] [Google Scholar]
- Nishizawa K., Shimoda E., Kasahara M. Substrate recognition domain of the Gal2 galactose transporter in yeast Saccharomyces cerevisiae as revealed by chimeric galactose-glucose transporters. J Biol Chem. 1995 Feb 10;270(6):2423–2426. doi: 10.1074/jbc.270.6.2423. [DOI] [PubMed] [Google Scholar]
- Ozcan S., Johnston M. Three different regulatory mechanisms enable yeast hexose transporter (HXT) genes to be induced by different levels of glucose. Mol Cell Biol. 1995 Mar;15(3):1564–1572. doi: 10.1128/mcb.15.3.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan S., Schulte F., Freidel K., Weber A., Ciriacy M. Glucose uptake and metabolism in grr1/cat80 mutants of Saccharomyces cerevisiae. Eur J Biochem. 1994 Sep 1;224(2):605–611. doi: 10.1111/j.1432-1033.1994.00605.x. [DOI] [PubMed] [Google Scholar]
- Reifenberger E., Freidel K., Ciriacy M. Identification of novel HXT genes in Saccharomyces cerevisiae reveals the impact of individual hexose transporters on glycolytic flux. Mol Microbiol. 1995 Apr;16(1):157–167. doi: 10.1111/j.1365-2958.1995.tb02400.x. [DOI] [PubMed] [Google Scholar]
- Rose M., Albig W., Entian K. D. Glucose repression in Saccharomyces cerevisiae is directly associated with hexose phosphorylation by hexokinases PI and PII. Eur J Biochem. 1991 Aug 1;199(3):511–518. doi: 10.1111/j.1432-1033.1991.tb16149.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski R. S., Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989 May;122(1):19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoris G., Fong N. M., Coons D. M., Bisson L. F. High-copy suppression of glucose transport defects by HXT4 and regulatory elements in the promoters of the HXT genes in Saccharomyces cerevisiae. Genetics. 1994 Aug;137(4):957–966. doi: 10.1093/genetics/137.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treitel M. A., Carlson M. Repression by SSN6-TUP1 is directed by MIG1, a repressor/activator protein. Proc Natl Acad Sci U S A. 1995 Apr 11;92(8):3132–3136. doi: 10.1073/pnas.92.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J. F., Emr S. D., Field C., Schekman R. GAL2 codes for a membrane-bound subunit of the galactose permease in Saccharomyces cerevisiae. J Bacteriol. 1986 Apr;166(1):313–318. doi: 10.1128/jb.166.1.313-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallier L. G., Coons D., Bisson L. F., Carlson M. Altered regulatory responses to glucose are associated with a glucose transport defect in grr1 mutants of Saccharomyces cerevisiae. Genetics. 1994 Apr;136(4):1279–1285. doi: 10.1093/genetics/136.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernet T., Dignard D., Thomas D. Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52(2-3):225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Wendell D. L., Bisson L. F. Expression of high-affinity glucose transport protein Hxt2p of Saccharomyces cerevisiae is both repressed and induced by glucose and appears to be regulated posttranslationally. J Bacteriol. 1994 Jun;176(12):3730–3737. doi: 10.1128/jb.176.12.3730-3737.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]