Abstract
Smith-Magenis syndrome (SMS) is a disorder characterized by multiple congenital anomalies and behavior problems, including abnormal sleep patterns. It is most commonly due to a 3.5 Mb interstitial deletion of chromosome 17 band p11.2. Secretion of melatonin, a hormone produced by the pineal gland, is the body’s signal for nighttime darkness. Published reports of 24-hour melatonin secretion patterns in two independent SMS cohorts (US & France) document an inverted endogenous melatonin pattern in virtually all cases (96%), suggesting that this finding is pathognomic for the syndrome. We report on a woman with SMS due to an atypical large proximal deletion (∼6Mb; cen<->TNFRSFproteinB) of chromosome band (17)(p11.1p11.2) who presents with typical sleep disturbances but a normal pattern of melatonin secretion. We further describe a melatonin light suppression test in this patient. This is the second reported patient with a normal endogenous melatonin rhythm in SMS associated with an atypical large deletion. These two patients are significant because they suggest that the sleep disturbances in SMS cannot be solely attributed to the abnormal diurnal melatonin secretion versus the normal nocturnal pattern.
Keywords: Melatonin, Smith-Magenis syndrome, sleep, interstitial deletion 17p11.2, array CGH
INTRODUCTION
Smith-Magenis syndrome (SMS) is a multiple congenital anomaly mental retardation (MCA-MR) syndrome characterized by subtle minor craniofacial anomalies, infantile hypotonia, skeletal findings (brachydactyly, short stature and scoliosis), developmental and expressive language delays, mental retardation, maladaptive and self-injurious behaviors, and sleep disturbances. First delineated in 1986 [Smith et al., 1986], the syndrome is usually due to a common 3.5 Mb interstitial deletion of chromosome 17 band p11.2; however, atypical deletions (larger or smaller) and heterozygous point mutations of the RAI1 gene are also associated with the phenotype [Bi et al., 2006; Girirajan et al., 2005; Gropman et al., 2007; Natacci et al., 2000; Slager et al., 2003]. Several studies have evaluated sleep patterns in SMS patients using subjective and objective measures [Cornelissen et al., 2003; De Leersnyder et al., 2001a; De Leersnyder et al., 2001b; Greenberg et al., 1996; 2000; Smith et al., 1998; Smith and Duncan, 2005]. These problems include difficulties getting to sleep, frequent nocturnal awakenings, early sleep offset and daytime sleepiness with a need for daytime naps [De Leersnyder et al., 2001a; Smith et al., 1998].
Melatonin, a hormone secreted by the pineal gland in the brain, serves as the body’s signal for darkness. Typically, the duration of melatonin secretion is 12 hours, starting 2 hours before habitual sleep onset and returning to daytime low baseline levels 12 hours after onset [Lewy, 1983]. Twenty-four hour melatonin profiles have been studied in SMS in the USA (n=19) [Potocki et al., 2000] and France (n=8) [De Leersnyder et al., 2001a]. Potocki et al. [1997] initially reported abnormal diurnal rhythms of a urinary melatonin metabolite 6-sulphatoxymelatonin (aMT6) in a pilot study of 6 SMS patients, later extended to 19 SMS patients, ranging in age from 8 months to 31 years [Potocki et al., 2000]. Eighteen of 19 SMS patients were documented to have an inverted endogenous melatonin rhythm based on urinary assay of aMT6. Normal urinary aMT6 excretion was described in a single patient (5y old girl, #1153) whose SMS was due to a large uncommon deletion. DeLeersnyder et al., [2001a] investigated the circadian variations in serum melatonin, cortisol and growth hormone among 8 (5M/3F) French children with SMS between ages 4–17 years. Compared to pediatric controls (n=15), SMS children demonstrated an abnormal diurnal melatonin profile with mean melatonin onset (MO) at 6:00AM ± 2.0 (control 9:00 PM±2:00) and peak melatonin at 12:00PM±1:00 (control at 3:30AM±1:30). The total duration of melatonin secretion was also protracted in SMS (15.5hrs±3.5) compared to controls (8hr±1.0). In contrast to diurnal melatonin profile, both serum cortisol and growth hormone followed a normal circadian secretion pattern. Among the 27 SMS patients whose melatonin secretion has been studied, all but one show the novel diurnal melatonin rhythm, with peak values occurring in the morning soon after rising and trough levels occurring at night before bedtime. The inverted melatonin rhythm, felt to be pathognomic of SMS [Gropman et al., 2006], was postulated to be a cause of the disrupted nighttime sleep and daytime sleepiness in these patients [Potocki et al., 2000].
We report a second patient with a cytogenetically confirmed diagnosis of SMS and long-standing sleep difficulties who was documented to have a normal pattern of melatonin secretion and light-suppression of melatonin. Repeat molecular cytogenetic studies, pursued to characterize the deletion breakpoints using a variety of current molecular genetic techniques, identified a large complex ∼6Mb proximal deletion from TNFRSFproteinB to the centromere of chromosome 17. Comparison of our patient to the single other published patient with normal melatonin secretion [Potocki et al., 2000] offers new insights about the sleep phenotype in SMS.
CLINICAL REPORT
The patient, a 20 year old woman with cytogenetically confirmed SMS, was referred to the Oregon Health & Science University (OHSU) at 18 years for formal sleep evaluation to further characterize her clinical sleep patterns and melatonin secretion. The studies, described in greater detail in the Methods section, included plasma dim light melatonin onset (DLMO) profiles, which entails having the patient wear dark glasses to block light and then obtaining plasma melatonin levels; melatonin light suppression tests; plasma cortisol levels; actigraphy; and overnight polysomnography.
Pertinent/Past Medical History: Retrospective chart review confirmed developmental, behavioral, and physical aspects consistent with SMS. The patient was the 3402 gram term product born to a G3P2 29-year-old mother. Prenatal history was significant for maternal neck injury sustained in an automobile accident at 1 month of gestation with two weeks of prescribed pain medication and phenytoin. Fetal movements were notably diminished throughout pregnancy. Other than neonatal temperature instability, the newborn period was uncomplicated. Family history is non-contributory.
Early motor-milestones were delayed with sitting, crawling, and walking at 8 months, 12 months and 18 months, respectively. Speech was significantly delayed, with single words at 2.5 years, two-three word phrases at 5 years with growling and guttural noises, and full sentences at 8 years; her voice was notably hoarse. Early childhood history was significant for numerous middle ear infections and secondary conductive hearing loss; a moderate bilateral sensorineural hearing loss confirmed at 8 years led to bilateral hearing aid amplification.
At 4 years she had a shortened attention span with hyperactivity with significant shift from generalized flat affect noted in early infancy to emotional lability/volatility, and destructive behavior towards objects as well as self-injurious behaviors, specifically head-banging, self-hitting and nail pulling (fingernails and toenails). By 8 years, her care was complicated by disrupted sleep patterns that included poor sleep consolidation with maximal sleep periods of four to five hours, as well as frequent nighttime awakenings during which she would get up and wander around the house. As a pre-teen she preferred adult attention to peers, was affectionate but socially disinhibited (inappropriate social behavior) and was easily frustrated in school, demonstrating anger and impulse control problems, rapid mood shifts, and aggressive outbursts. Behavior was better in school than in the home setting, with destructive and aggressive behaviors directed towards objects, siblings and her mother. Reliable toileting was not present until 10 years and urinary incontinence remains an issue. The only medication tried for behavior was Dimetapp for hyperactivity. She was medication-free during her admissions.
The patient was first evaluated at OHSU as an infant for high myopia and right esotropia requiring surgery at 1 year. Comprehensive developmental pediatric evaluation between 3.5 years and 11.5 years by the Child Development Program at OHSU confirmed global delays in speech/language, mental processing and cognition, fine motor skills and general development (Table I) leading to the diagnoses of autism and global developmental delay/mental retardation (MR). A comparison of testing between age 3 years and11 years show her relatively more delayed at age 11. While her scores at 11 years of age are consistent with published data in SMS patients [Greenberg et al., 1996; Madduri et al., 2006; Martin et al., 2006], the role her maladaptive behaviors played in the measured decline in cognitive function is difficult to assess.
Table I.
Summary of patient‘s developmental and adaptive function by chronologic age
| Chronologic Age | Assessment Tool/measure | Development/Functional Age |
|---|---|---|
| 3y 6mo | Kaufman Assessment Battery for Children (KABC) | 2 year level;2 SD below age mean Sequential Processing 67 (standard scores) |
| Simultaneous Processing 74 | ||
| Mental Processing Composite 68 | ||
| Achievement 72 | ||
| 6y 7mo | Peabody Picture Vocabulary Test (PPVT) | Standard score <40 (2y8m) |
| 11y 3mo | Wechsler Intelligence Scale for Children (WISC-III) | Verbal IQ 46 |
| Performance IQ 65 | ||
| Full scale IQ 53 | ||
| Mild-Moderate MR range | ||
| 11y 3mo | Vineland Adaptive Behavior Scales (VABS) | Composite score 42 ± 8 (age 4y3mo) |
| Communication 40 ± 13 | ||
| Daily living skills 52 ± 11 | ||
| Socialization 46 ± 11 | ||
| Low range of adaptive function | ||
Initial cytogenetic G-banded high resolution chromosome analysis (850 band) pursued at 11 years showed a visible interstitial deletion of 17p11.2 (46, XX, del (17)(p11.2p11.2) consistent with a diagnosis of SMS. Craniofacial findings consistent with the SMS diagnosis (Fig. 1) included broad forehead, low frontal hairline, square-shaped face, deep-set eyes with upslanting palpebral fissures, short upturned nose, short philtrum, open mouth, and maxillary hypoplasia with relative prognathism. Additional manifestations include “self hugging behavior”, hoarse voice, small hands and feet with short tapered fingers, mild digital 2–3 syndactyly of fingers and toes, dry skin especially the hands/feet, evidence of decreased sensation to pain, extreme myopia, bilateral sensory neural hearing loss (aided), grade II/VI systolic ejection murmur, early adrenarche (pubic hair at 11 years) with onset of menses at 13 years, truncal obesity and short stature. At 12 years, height was at the 10th centile, weight at the 50th centile, and OFC at the 95th centile; significant weight gain to 55.5 kg (75th centile) was documented at 13 years coinciding with puberty. Echocardiogram was normal. Routine EEG obtained for suspected seizure disorder was also normal. Clinical follow-up did not show continuing concern for a seizure disorder and no further evaluation or treatment was undertaken.
Figure 1.
Photograph of patient showing the typical facial appearance (a. full face and b. profile) seen in SMS including broad forehead, low frontal hairline, square-shaped face, deep-set eyes with upslanting palpebral fissures, short upturned nose, short philtrum, open mouth posture, and maxillary hypoplasia with relative prognathism.
METHODS
Clinical Sleep Studies
The patient and her family agreed to participate in a pilot study investigating sleep and melatonin secretion in patients with SMS under an IRB-approved protocol at OHSU. The study involved six 24-hour admissions to the General Clinical Research Center at OHSU. The first three admissions were at 18 years; a second round of testing occurred during three admissions two years later. During the first two admissions, plasma melatonin levels were obtained every 30 minutes. These were studied in dim light by having her wear light-suppressing goggles throughout the admission and minimizing exposure to bright light sources such as television or computer screens. On the third admission, a melatonin suppression test was performed by administering a pulse of 2000 lux of light for 1.5 hours at the predicted peak of melatonin secretion. During the second round of testing, hourly plasma cortisol levels were also measured during the fifth admission, concurrent with dim light melatonin levels. Actigraphy data were collected for the six-week periods encompassing admissions at both ages to measure her activity/rest patterns in the home setting.
Melatonin and Cortisol Analysis
Since patients with the common SMS deletion have been shown to have inverted melatonin rhythms [De Leersnyder et al., 2001a; Potocki et al., 2000], we first measured the melatonin profile of the patient. Additionally, we investigated whether her melatonin secretion was suppressed by exposure to light in the normal fashion [Lewy et al., 1980; Lewy et al., 1981]. Blood was drawn from the patient through an intravenous saline lock and centrifuged; the plasma was then decanted into clean tubes and stored at −20º C according to the protocol described by Lewy et al. [2005]. The plasma melatonin concentrations were measured by radioimmunoassay using an antibody raised in the laboratory of Kennaway [Earl et al., 1985; Voultsios et al., 1997] and reagents supplied by ALPCO Diagnostics (Windham, NH). This assay has a lower sensitivity limit of 0.5 pg/ml, and it was validated by GCMS [Lewy and Markey, 1978; Lewy and Sack, 1997]. An automated Immulite chemiluminescent assay system (Diagnostic Products Corporation, Los Angeles) was used to measure plasma cortisol levels (analytical sensitivity was 0.2 µg/dL, average intra-assay coefficient of variation was 7.3%, and inter-assay coefficient of variation was 8.5%).
Polysomnography and Actigraphy
In order to determine the patient’s sleep patterns and to rule out the presence of another primary sleep disorder, actigraphy and overnight polysomnography were performed. A single night of outpatient, overnight polysomnography was performed at the OHSU Sleep Laboratory during the second round of admissions at 20 years. This recording was performed using Tyco Sandman Elite Sleep Diagnosis Software version 6.0 with MMC amplifiers (Nellcor Puritan Bennett (Melville) Ltd. Kanata, Ontario Canada) and an infrared video camera. Four channels of electroencephalography (EEG) were recorded (C3-A2, O1-A2, C4-A1, O2-A1). In addition to the EEG, polysomnography included two channels of electro-oculogram (EOG); 3 channels of electromyogram (EMG) covering cheek, submental, and leg musculature; one channel of electrocardiogram; one channel of airflow using a standard thermister; and two channels of chest and abdominal excursions; and pulse oximetry. The polysomnography data were analyzed for sleep efficiency, latency to sleep onset, REM latency, and percentage of time spent in each sleep stage [Rechtschaffen and Kales, 1968]. The subject wore an activity monitor (Actiwatch®) for 6-weeks in the home setting encompassing admissions at both ages. The Actiwatch® is a wristwatch-sized instrument that contains an accelerometer and provides an objective measure of motor activity, which allows indirect assessment of sleep/wake patterns. Measurements such as these are called actigraphy and are useful for the evaluation of patients who are suspected of having a circadian rhythm abnormality [Morgenthaler et al., 2007]. Sleep onsets, sleep offsets, and sleep efficiency were calculated automatically using the Actiware® software (Mini Mitter/Respironics, Bend, OR) following manual entry of estimated onset and offsets. The data were expressed as activity counts per 1-minute epoch and were stored in on-board memory. Downloaded data were expressed graphically as actigrams. An event marker was used by the patient (with assistance from the caregiver) to record in-bed and out-of-bed times to improve computation of sleep onset and sleep offset times. Activity recorded during the sleep period was used for sleep efficiency calculations. In the case of an occasional missing or anomalous event marker, the rater referred to sleep diaries, which were kept by her care caregiver, for the onset/offset estimate.
Molecular Genetic Studies
Peripheral blood, collected from the patient and her parents, was employed for extraction of genomic DNA and for Epstein Barr Virus (EBV) immortalization of B-lymphocytes, using standard protocols. G-band cytogenetic analysis (750 band level) included fluorescent in situ hybridization (FISH) of the proband’s chromosome preparations. DNA clones for FISH analysis included probes specific for the RAI1 locus (RP1–253P7), a distal SMS-REP (RP11–416I2) and a proximal SMS-REP (RP5–836L9) 17p probe. Additional Bacterial artificial chromosome (BAC) probes for FISH were also used to validate comparative genomic hybridization (CGH) findings, including: RP11–219A15 and RP11–218E15. Chromosome analysis with FISH for the RAI1 locus was also completed on both parents.
Microsatellite marker analysis (STRP) using standard protocols was performed on genomic DNA from the patient and her parents to determine the parental origin of the deletion. STRP markers were located using UCSC Genome Bioinformatics: http://www.genome.ucsc.edu/. Additional tandem repeats in the region were located using DNA sequence from UCSC Genome Bioinformatics (hg18) and Tandem Repeats Finder: http://tandem.bu.edu/trf/trf.html [Benson, 1999]. Primer sequences for these repeat regions were identified using Primer3: http://primer3.sourceforge.net/. The PCR products were separated and analyzed using the Genetic Analyzer 3100 (Applied Biosystems).
Comparative whole genome hybridization array (CGH) was performed using the Agilent® 244K, Human Genome CGH kit allowing an average probe spatial resolution of 9 Kb. Patient DNA was labeled and cohybridized against DNA from a normal female control. Array slides were scanned using an Agilent® microarray scanner, data were extracted from images with Feature Extraction 9.5 software (Agilent®), and CGH profiles were generated using CGH-analytics 3.2 software (Agilent®). Gains or losses were considered if present on at least three tailing probes to avoid false positives.
RESULTS
Melatonin and Sleep
Melatonin Profile: Fig. 2 shows the patient’s melatonin profile at the third 24-hour admission. All three profiles obtained from the first round of admissions show a reproducible rise in dim light melatonin onset around 20:00 hours and a fall about 08:00 hours. The patient’s melatonin profiles were not significantly different from published melatonin profiles and melatonin suppression tests in normal volunteers [Czeisler et al., 1995; Danel and Touitou, 2006; Lewy et al., 1981]. Melatonin suppression was observed after 2000 lux of light was administered between 23:00 hours and 00:30 hours on the third admission. Dim light melatonin onset at 20:00 hours and offset at 08:00 hours, and melatonin suppression to light at age 20 years were identical to those values at age 18 years (results not shown). The 24-hour cortisol profile was normal with a peak at 08:00 hours and nadir at 02:00 hours (data not shown). Actigraphy showed that she had generally decreased levels of activity between 00:00 hours and 06:00 hours but these results were notable in that the period of relative quiet was also punctuated with brief bursts of activity. Standard overnight polysomnography was notable for poor sleep efficiency (69.7%), delayed sleep onset (67.2 min), frequent arousals, and a prolonged wake period from 01:45 to 02:45 (Fig. 3). No significant sleep disordered breathing was present (apnea-hypopnea index = 3.2), with most events being during REM sleep. Sleep staging was notable for increased non-slow wave sleep and very little slow wave sleep with normal amounts of REM (Stage 1:14.5%, Stage 2 = 63.6%, Stage 3 and 4 = 1.1%, REM = 20.8%). There were no significant leg movements. There was no evidence that another primary sleep disorder was significantly contributing to her sleep problems.
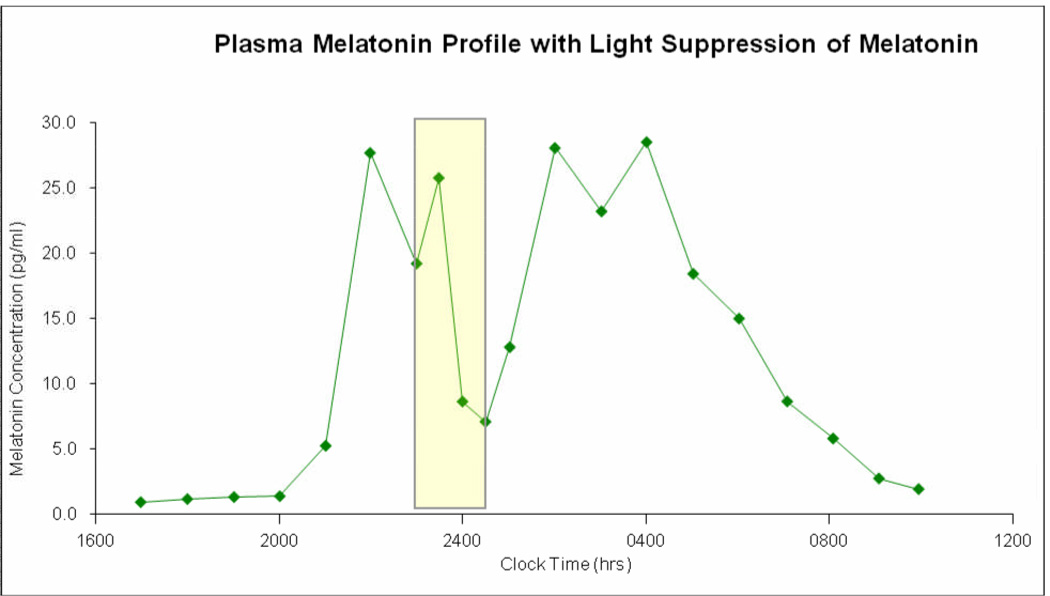
Figure 2.
Plot of clock time (x-axis) versus plasma melatonin concentration (y-axis) showing the normal pattern of melatonin rise in the evening and nadir in the middle of the day for our patient at age 18 years old. This profile was obtained on the third admission and is nearly identical to those obtained on the first two admissions. It illustrates the peak onset of melatonin occurs at about 2200 hours. It also shows that giving a pulse of bright light (indicated by the rectangles) temporarily inhibits melatonin secretion as shown by the drop in melatonin concentration, which rebounds after the light is stopped. This pattern of inhibition of melatonin secretion by light is typical and further illustrates that melatonin secretion and response to light is not abnormal in our patient.
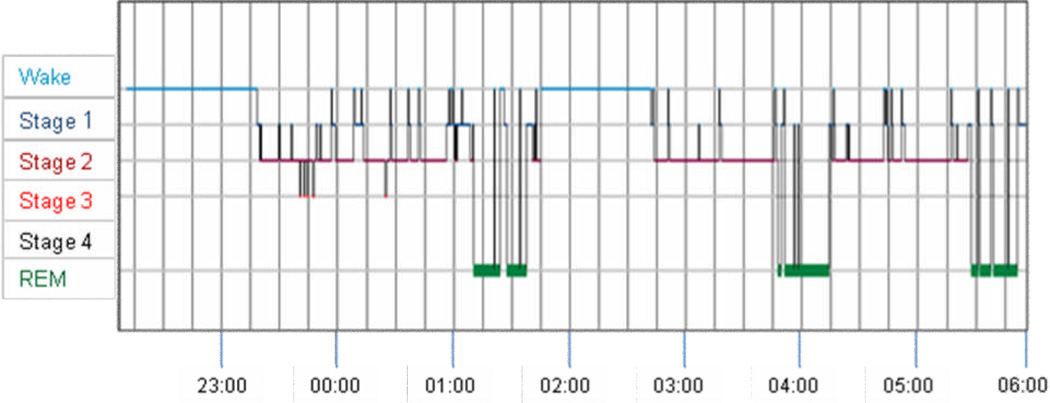
Figure 3.
Hypnogram showing clock time plotted on the x-axis versus sleep stage on the y-axis taken from standard overnight polysomnography on our patient. This figure demonstrates that compared with normative data [Lavie, 2001; Wilson and Nutt, 2008] the patient had increased sleep fragmentation and an extended period of wakefulness between 01:45 and 02:45 AM.
Molecular Genetic Analyses
Repeat molecular cytogenetic studies confirmed a de novo deletion in the patient (46,XX,del(17)(p11.2p11.2). Normal karyotypes without evidence of deletion by FISH (RAI1) were identified in both parents. FISH analysis of metaphase cell preparations on the patient showed deletions for all three FISH probes covering the SMS common deletion: RAI1 region (clone RP1–253P7), distal SMS-REP (RP11–416I2) and proximal SMS-REP (RP5–836L9). The more proximal FISH probe (RP11–218E15) and more distal probe (RP11–219A15) were not deleted (Fig. 4). Genotyping using microsatellite markers (STRP) demonstrated the deletion to be paternally derived (Fig. 4B) and that the deletion extended beyond the proximal SMS -REP region indicating a larger than a typical deletion.
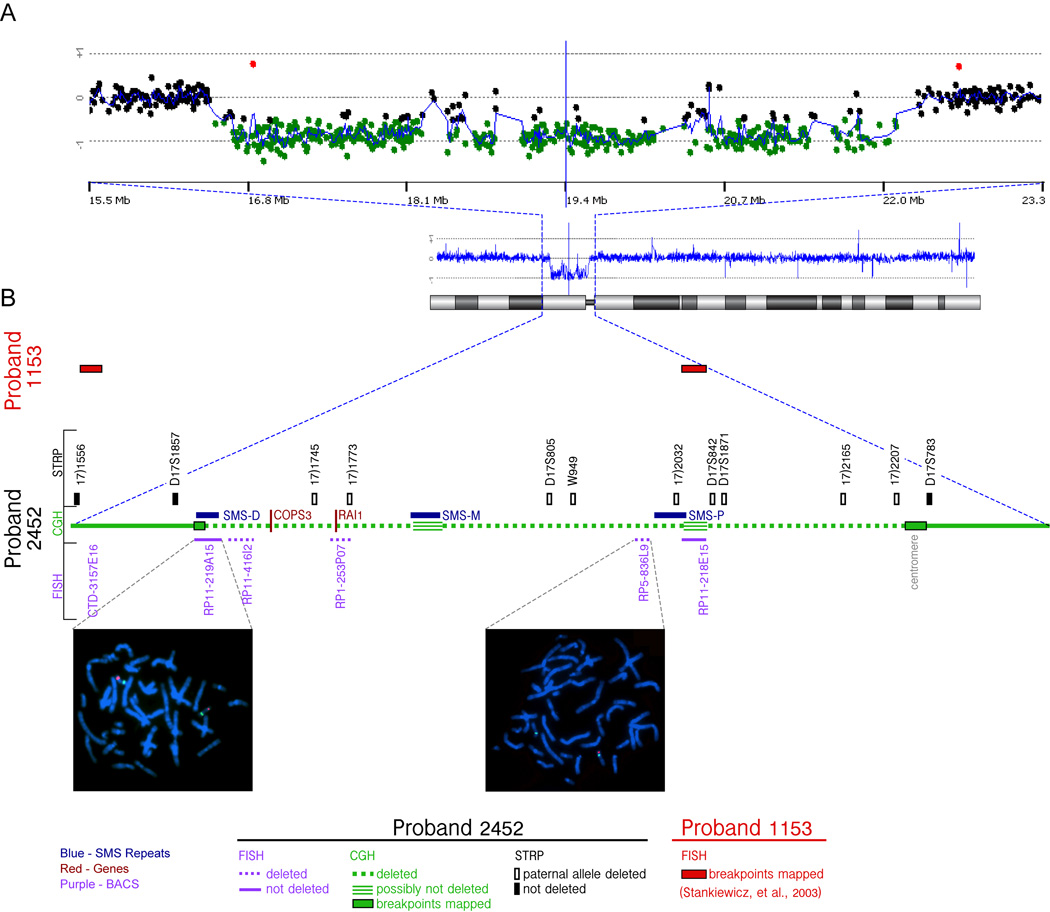
Figure 4.
Molecular cytogenetic characterization of the deletion in the patient (2452).
A) CGH analysis of chromosome 17 for the proband 2452. Ideogram of the chromosome (below), and the CGH profile scaled to the whole chromosome(middle) is shown along with expansion (top) of the deleted area. The zoomed area (15.5 Mb to 23.3 Mb) includes black spots corresponding to the array CGH probes with “balanced” ratio between control and patient DNA, and green spots showing lower ratio corresponding to the “deleted” region. A CGH profile of the whole chromosome (blue line) gives the mean value of the CGH ratios for 5 consecutive spots.
B) The STRP analysis and FISH mapping of the region for the patient 2452 are shown adjacent to the green line representing CGH data. The loss of the paternal alleles (open rectangles) or the presence of both alleles (filled rectangles) for the informative STRP markers are shown. The FISH signals (red) for the BAC RP11-219A15 shows the retention of two copies whereas that for the BAC RP5-836L9 shows deletion of a copy: Green signals indicate a reference probe on 17q or at centromere, respectively.
The two BAC clones, CTD-3157E16 and RP11-218E15, in which the distal and proximal breakpoints for the patient 1153 are reported to reside, are indicated (red rectangles) [Stankiewicz et al., 2003].
The sequence coordinates of all the elements shown in this figure, along with the sequence and allele sizes of the STRP markers in the proband 2452 is shown in Supplementary Table I.
High resolution CGH (Fig. 4A) confirmed the large deletion on the centromeric part of 17p, spanning from the centromere to the distal SMS-REP; a minimum deletion of 5.56 Mb based on the coordinates of the first deleted probes flanking the deletion breakpoints: 16,578,627 to 22,142,572 bp. The first gene affected on the telomeric side was TNFRSF13B and last deleted gene on the centromeric side was FAM27L. However, the large deletion did not appear to be completely hemizygous from TNFRSF3B to FAM27L. At two localizations the CGH ratio between control and patient DNA were normal, indicating that these two short intervals were potentially non-deleted on the patient’s chromosome. The first interval was between 18,265,564 and 18,470,630 (350 Kb) and the second between 20,414,682 to 20,582,325 (167 Kb) (Fig. 4). Non-deletion of the second interval was confirmed by a FISH probe of BAC RP11–218E15 (Fig. 4B).
DISCUSSION
Smith-Magenis syndrome is a complex MCA-MR syndrome that includes significant sleep disorder characterized by decreased total nighttime sleep for age, frequent and prolonged nocturnal awakenings, early morning sleep offset (05:00–06:00) and excessive daytime sleepiness (increased napping). Based on thirty-six published patients undergoing polysomnography (PSG) studies (see Table II) [De Leersnyder et al., 2001a; Potocki et al., 2000], sleep cycle abnormalities were objectively documented in most cases, including specific sleep stage abnormalities with respect to rapid eye movement and slow wave sleep identified in over 50%. Reduced total sleep time (<7hr) was documented in 55.6% (20/36); disrupted REM sleep in 72% (26/36); and frequent spontaneous nighttime awakenings in 86% (31/36). The parentally reported daytime sleepiness (napping) that occurs secondary to chronic sleep disruption was objectively evaluated by MSLT with a reduced latency (<10 minutes) to fall asleep during the daytime identified in half (13/26) of the studied cases [Potocki et al., 2000].
Table II.
Comparison of objective sleep measures and melatonin assays in Smith-Magenis syndrome for our patient (#2452) to published (n=28) cases.
| Case Report | Potocki et al., 2000 | De Leersnyder et al., 2001 | Total Reported | ||
|---|---|---|---|---|---|
| 2452 | n=28 | n=8 | n=37 | ||
| Gender | F | 12F/16M | 3F/5M | 16F/21M | |
| Mean Age (range) | 20y | 9y4m (Range 2y8m-31y) | 9.5y (Range 4–17y) | ||
| Deletion Type/Size | Atypical 5.6 Mb | Common deletion | Atypical deletion | All FISH confirmed deletion size unspecified | |
| n=23 | n=5 | ||||
| (no junction fragment) | |||||
| Complex del: #1221 | |||||
| Small del: #1190, #1456 | |||||
| Large del: #1153*, #1354 | |||||
| SLEEP STUDIES | n=1 | n=28 | n=8 | n=37 | |
| Methodology Used | PSG | PSG (nighttime) | 24-hr PSG | ||
| Actigraphy | MSLT (daytime) | Actigraphy | |||
| Total sleep time | 7.8 hrs (efficiency = 69.7%) | 8/23 (35%) < 7hrs | 4/5 (80%) < 7 hrs | 7/8 (35%) | 20/37 (54%) |
| Mean = 7.5h (5–9hr) | |||||
| All sleep states present* | |||||
| REM sleep abnormalities | REM 20.8% | 13/23 (57%) | 5/5 (100%) | Disrupted in 8/8 (100%) | 27/37 (73%) |
| 8 Decreased | 3 Decreased | ||||
| 5 Increased | 2 Increased | ||||
| Spontaneous awakenings | 29 | ≥ 10 | ≥ 10 | >15 min | 32/37 (86%) |
| 20/23 (87%) | 5/5 (100%) | 6/8 (75%) | |||
| MSLT latency < 10min. | Not done | 10/21 (48%) | 3/5 (60%) | Not done | 13/26 (50%) |
| MELATONIN ASSAY | Serum | Urinary aMT6 (n=19) | Serum (n=8) | n=28 | |
| Melatonin Pattern | Not inverted | Inverted 16/16 | 2/3 inverted | Inverted 8/8 | 26/28 (93%) Inverted |
| 1/3 not inverted | 2/28 (7%) Not inverted | ||||
One patient (#1153) with large deletion did not have inverted melatonin profile; tow atypical deletion cases (#1221 and 1 small (patient number not reported) demonstrated inverted melatonin rhythm (Potocki et al., 2000).
Wrist activity derived sleep estimates using the ActiwatchTM documents disrupted sleep patterns that begin in infancy and continue into adulthood [Gropman et al., 2006]. Infants as young as 1 year show decreased 24-h sleep for age, a pattern that continues into early childhood and school age years; on average, children with SMS generally sleep 1–2 h less per 24 h compared to healthy age-matched controls [Gropman et al., 2006]. While children under 10 years demonstrate increased nocturnal arousals during the second half of the night, children over 10 years appear to have increased evening arousals and more difficulty initiating sleep (“settling”), a pattern often seen in healthy teenagers [Gropman et al., 2007].
The inverted melatonin secretion that occurs in SMS distinguishes the sleep disturbance in SMS from that seen in other children with developmental disabilities. Two independent studies have documented an inverted circadian melatonin rhythm characterized by daytime highs/nighttime lows that appears to be pathognomic of SMS (Table II). Potocki et al., [2000] assayed 6-sulphatoxymelatonin (aMT6s) in 24-hour urine samples in 19 patients with SMS, 16 of whom had common deletions and three with atypical deletions. Eighteen of the 19 demonstrated the novel inverted melatonin rhythm. The single published case with a normal melatonin rhythm was a 5-year-old girl (#1153) found to have an uncommonly large deletion of 17p11.2 [Potocki et al., 2000]. Although this patient’s deletion was not fully characterized in this early report, multiple FISH probes (ZNF179, COPS3, FLI, MFAP) were deleted and the SMS junction fragment associated with common deletion cases was not present on pulsed field gel electrophoresis [Potocki et al., 2000]. Published molecular data pertaining to this same case appears in subsequent reports confirming a large distal 5Mb deletion of paternal origin that spans from CTD-3157E16 to RP11–281E15 [#1153 in [Stankiewicz et al., 2003].
De Leersnyder et al., [2001a] studied 24-hour serum melatonin, cortisol and growth hormone in 8 (5M/3F) children with FISH confirmed SMS (ages 4–17 years) compared to healthy age matched controls (n=15). The SMS group (n=8) all showed a phase shift in melatonin secretion with melatonin onset at 06:00 +/− 2h (control group 20:00 +/− 2) and peak at 12:00 +/−1h (control group: 03:30 +/−1:30h). Compared to controls, all eight SMS patients showed an increased total duration of melatonin secretion (SMS 15.5hr vs. controls 8h) and higher melatonin peak values (SMS 94pg/mL vs. 76 pg/mL Controls). In contrast, 24-hour profiles of serum cortisol and growth hormone were consistent with expected normal circadian phase of secretion.
The degree of variability of melatonin secretion patterns in SMS is not known, since only a limited number of cases have been studied biochemically. However, among the 27 SMS patients in whom 24-hour melatonin secretion was analyzed, 26 (96%) exhibit a diurnal melatonin rhythm shifted by about 12 hours from the normal pattern of secretion [De Leersnyder et al., 2001a; Potocki et al., 2000]. This inverted pattern was also dissociated from normal cortisol and growth hormone secretion patterns [De Leersnyder et al., 2001a]. The fact that melatonin was measurable in daytime serum samples collected under hospital room light conditions [De Leersnyder et al., 2001a] suggests that the expected suppression of melatonin secretion by light is abnormal in SMS.
The present study represents the second case report of a patient with both an uncommonly large 5.56 Mb proximal SMS deletion and a normal melatonin rhythm. She demonstrates a normal expected light suppression of melatonin and a normal cortisol rhythm consistent with the cortisol profiles of SMS patients who have inverted melatonin cycles [De Leersnyder et al., 2001a]. Moreover, she shows the typical pattern of poor nighttime sleep consolidation seen in SMS patients [Greenberg et al., 1996; Potocki et al., 2000; Smith et al., 1998]. Because only one night of polysomnography was performed, adjustment to the unfamiliar environment in the sleep laboratory, a phenomenon called “first night effect”, was a potential confounder. However, the data from the sleep study was consistent with her actigraphy data, suggesting that a first night effect was not a major issue. Overall, the poor nighttime sleep consolidation seen in our patient is significant because it suggests that the disrupted sleep patterns in persons with SMS are not directly linked to disruption of their melatonin rhythm.
Both the patient and patient #1153 reported by Potocki et al. [2000] demonstrate normal melatonin rhythm in combination with disrupted sleep cycles in association with large paternally derived deletions. Comparisons of molecular deletion data for our case (#2452) are summarized in Fig. 4 illustrating FISH, STRP genotyping and CGH array results. Though the patient #1153 was reported by Potocki et al. [2000], the breakpoints were published by Stankiewicz et al. [2003] indicating a large distal deletion. As summarized in Table II, 26/28 (93%) SMS cases demonstrate inverted melatonin; only two published cases, both associated with large atypical deletions (2/28; 7%), exhibit normal melatonin rhythm. Among the twenty SMS cases whose deletion size has been specified (excludes [De Leersnyder et al., 2001a]), the association between deletion type (common vs. atypical) and melatonin pattern (inverted vs. normal) was statistically significant (p=.0315; two-tailed Fisher’s Exact probability test).
These two cases suggest that simple disruptions in melatonin rhythm cannot solely account for the sleep problems seen in SMS patients. One hypothesis, put forth by De Leersnyder et al., [2001a], suggests that the sleep and melatonin abnormalities may be caused by intrinsic dysfunction of the circadian clock. However, the findings of a normal 24-hour rhythm for both cortisol and growth hormone, and recent reports that 24-hour body temperature rhythm is not inverted, argues against the hypothesis of an inverted central clock [Duncan et al., 2004; Gropman et al., 2006]. It is possible that the genetic mechanism that underlies the inverted melatonin remains intact in these two patients. Published clinical reports of RAI1 mutation cases offer subjective evidence of sleep disturbance; however, few have been studied using objective measures to quantify the sleep disturbance and/or melatonin pattern. A recent abstract/preliminary report of urinary aMT6s studies in two SMS RAI1 mutation cases identified inverted melatonin rhythms in both; however, sleep cycle abnormalities using objective polysomnography were only documented in the youngest of the two (ages 11 years and 27 years) [Dang et al., 2007].
Regulation of melatonin secretion may stem from disruption and/or functional regulation of a gene(s) within the common deletion interval or at/near the breakpoints. Within the SMS critical region on chromosome 17, the gene COPS3 (17090864–17125316) encodes subunit 3 of the COP9 signal transduction complex [Potocki et al., 1999]. Wei et al. [1998] reported that the entire COP9 complex is related to the 26S proteasome regulatory complex. The latter has been associated with control of the rate-limiting step in melatonin metabolism by N-acetyltransferase. Thus, there is indirect evidence linking subunit 3 of the COP9 complex with melatonin metabolism.
Since COPS3 is located within the common deletion interval, most individuals with SMS, including our patient, are haploinsufficient for COPS3. It may be that COPS3 haploinsufficiency is more tightly linked to the clinical phenotype of disrupted sleep in SMS patients. It is not known whether COPS3 haploinsufficiency represents a marker for sleep disruption in SMS or whether it is the primary biochemical defect. However, Elsea et al. [1999] evaluated the impact of COPS3 haploinsufficiency in lymphoblast cell lines developed from SMS patients. They found that COPS3 was present at normal levels and COP9 signalsome complex assembly appeared unaffected, but could not rule out an effect of COPS3 haploinsufficiency during development.
The fact that both cases have paternally derived deletions is consistent with Shaw et al. [Shaw et al., 2004] who analyzed uncommon recurrent deletions of 17p11.2 and documented a bias toward paternally derived deletions in contrast to common deletions, which do not exhibit a parent of origin effect [Greenberg et al., 1991; Shaw et al., 2004]. However, the deletion is more distal in #1153 compared to the large proximal deletion in our case (2425). Objective investigation of melatonin patterns and careful characterization of sleep cycle abnormalities in persons with SMS with well-characterized deletions are required to further validate these findings. Elucidation of the gene(s) and underlying mechanism that lead to the unusual inverted circadian melatonin rhythm that occurs in this rare syndrome will be a major contribution to understanding human circadian biology.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to sincerely acknowledge the assistance of Cameron Brick in helping to coordinate this study and in preparing Figure 2. The NHGRI SMS Research Team at NIH gratefully acknowledges Angelica Garcia, BS, Thierry Vilboux, PhD and Carla Ciccone for their able assistance and critical laboratory support to complete the molecular studies on this family. This work was supported in part by the Intramural Research Programs of the National Human Genome Research Institute, National Institutes of Health and by grant number R01 HD42125 from the National Institute of Child Health and Human Development, National Institutes of Health (Dr. Lewy).
REFERENCES
- Benson G. Tandem repeats finder: a program to analyze DNA sequences. Nucleic Acids Res. 1999;27(2):573–580. doi: 10.1093/nar/27.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Saifi GM, Girirajan S, Shi X, Szomju B, Firth H, Magenis RE, Potocki L, Elsea SH, Lupski JR. RAI1 point mutations, CAG repeat variation, and SNP analysis in non-deletion Smith-Magenis syndrome. Am J Med Genet A. 2006;140(22):2454–2463. doi: 10.1002/ajmg.a.31510. [DOI] [PubMed] [Google Scholar]
- Cornelissen G, Halberg F, Tarquini R, Perfetto F, Salti R, Laffi G, Otsuka K. Point and interval estimations of circadian melatonin ecphasia in Smith-Magenis syndrome. Biomed Pharmacother. 2003;57 Suppl 1:31s–34s. doi: 10.1016/j.biopha.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF. Suppression of Melatonin Secretion in Some Blind Patients by Exposure to Bright Light. N Engl J Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Danel T, Touitou Y. Alcohol Consumption Does Not Affect Melatonin Circadian Synchronization in Healthy Men. Alcohol Alcohol. 2006;41(4):386–390. doi: 10.1093/alcalc/agl036. [DOI] [PubMed] [Google Scholar]
- Dang DX, Glaze D, Reiter RJ, Tan DX, Lupski JR, Potocki L. San Diego, CA: 2007. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome patients with RAI1 point mutation. 10/26/07. [Google Scholar]
- De Leersnyder H, De Blois MC, Claustrat B, Romana S, Albrecht U, Von Kleist-Retzow JC, Delobel B, Viot G, Lyonnet S, Vekemans M. Inversion of the circadian rhythm of melatonin in the Smith-Magenis syndrome. J Pediatr. 2001a;139(1):111–116. doi: 10.1067/mpd.2001.115018. [DOI] [PubMed] [Google Scholar]
- De Leersnyder H, de Blois MC, Vekemans M, Sidi D, Villain E, Kindermans C, Munnich A. Beta(1)-adrenergic antagonists improve sleep and behavioural disturbances in a circadian disorder, Smith-Magenis syndrome. J Med Genet. 2001b;38(9):586–590. doi: 10.1136/jmg.38.9.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WE, Morse RS, Krasnewich D, Smith ACM. Genetics, Toronto, Canada: American Society for Human; 2004. Body temperature and sleep disturbance in Smith-Magenis syndrome; p. A814. [Google Scholar]
- Earl CR, D'Occhio MJ, Kennaway DJ, Seamark RF. Serum melatonin profiles and endocrine responses of ewes exposed to a pulse of light late in the dark phase. Endocrinology. 1985;117(1):226–230. doi: 10.1210/endo-117-1-226. [DOI] [PubMed] [Google Scholar]
- Elsea SH, Mykytyn K, Ferrell K, Coulter KL, Das P, Dubiel W, Patel PI, Metherall JE. Hemizygosity for the COP9 signalosome subunit gene, SGN3, in the Smith-Magenis syndrome. Am J Med Genet. 1999;87(4):342–348. [PubMed] [Google Scholar]
- Girirajan S, Elsas LJ, 2nd, Devriendt K, Elsea SH. RAI1 variations in Smith-Magenis syndrome patients without 17p11.2 deletions. J Med Genet. 2005;42(11):820–828. doi: 10.1136/jmg.2005.031211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg F, Guzzetta V, Montes de Oca-Luna R, Magenis RE, Smith AC, Richter SF, Kondo I, Dobyns WB, Patel PI, Lupski JR. Molecular analysis of the Smith-Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2) Am J Hum Genet. 1991;49(6):1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Greenberg F, Lewis RA, Potocki L, Glaze D, Parke J, Killian J, Murphy MA, Williamson D, Brown F, Dutton R, et al. Multi-disciplinary clinical study of Smith-Magenis syndrome (deletion 17p11.2) Am J Med Genet. 1996;62(3):247–254. doi: 10.1002/(SICI)1096-8628(19960329)62:3<247::AID-AJMG9>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Duncan WC, Smith AC. Neurologic and developmental features of the Smith-Magenis syndrome (del 17p11.2) Pediatr Neurol. 2006;34(5):337–350. doi: 10.1016/j.pediatrneurol.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Gropman AL, Elsea S, Duncan WC, Jr, Smith AC. New developments in Smith-Magenis syndrome (del 17p11.2) Curr Opin Neurol. 2007;20(2):125–134. doi: 10.1097/WCO.0b013e3280895dba. [DOI] [PubMed] [Google Scholar]
- Lavie P. Sleep Disturbances in the Wake of Traumatic Events. N Engl J Med. 2001;345(25):1825–1832. doi: 10.1056/NEJMra012893. [DOI] [PubMed] [Google Scholar]
- Lewy AJ. Biochemistry and regulation of mammalian melatonin production. In: Relkin RM, editor. The Pineal Gland New York: Elsevier North-Holland; 1983. pp. 77–128. [Google Scholar]
- Lewy AJ, Emens JS, Lefler BJ, Yuhas K, Jackman AR. Melatonin entrains free-running blind people according to a physiological dose-response curve. Chronobiol Int. 2005;22(6):1093–1106. doi: 10.1080/07420520500398064. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Markey SP. Analysis of melatonin in human plasma by gas chromatography negative chemical ionization mass spectrometry. Science. 1978;201(4357):741–743. doi: 10.1126/science.675255. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Sack RL. Exogenous melatonin's phase-shifting effects on the endogenous melatonin profile in sighted humans: a brief review and critique of the literature. J Biol Rhythms. 1997;12(6):588–594. doi: 10.1177/074873049701200614. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Markey SP. Light suppresses melatonin secretion in humans. Science. 1980;210(4475):1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Wehr TA, Goodwin FK, Newsome DA, Rosenthal NE. Manic-depressive patients may be supersensitive to light. Lancet. 1981;1(8216):383–384. doi: 10.1016/s0140-6736(81)91697-4. [DOI] [PubMed] [Google Scholar]
- Madduri N, Peters SU, Voigt RG, Llorente AM, Lupski JR, Potocki L. Cognitive and adaptive behavior profiles in Smith-Magenis syndrome. J Dev Behav Pediatr. 2006;27(3):188–192. doi: 10.1097/00004703-200606000-00002. [DOI] [PubMed] [Google Scholar]
- Martin SC, Wolters PL, Smith AC. Adaptive and maladaptive behavior in children with Smith-Magenis Syndrome. J Autism Dev Disord. 2006;36(4):541–552. doi: 10.1007/s10803-006-0093-2. [DOI] [PubMed] [Google Scholar]
- Morgenthaler TI, Lee-Chiong T, Alessi C, Friedman L, Aurora RN, Boehlecke B, Brown T, Chesson AL, Jr, Kapur V, Maganti R, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders. An American Academy of Sleep Medicine report. Sleep. 2007;30(11):1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natacci F, Corrado L, Pierri M, Rossetti M, Zuccarini C, Riva P, Miozzo M, Larizza L. Patient with large 17p11.2 deletion presenting with Smith-Magenis syndrome and Joubert syndrome phenotype. Am J Med Genet. 2000;95(5):467–472. doi: 10.1002/1096-8628(20001218)95:5<467::aid-ajmg11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Potocki L, Chen KS, Lupski JR. Subunit 3 of the COP9 signal transduction complex is conserved from plants to humans and maps within the smith-magenis syndrome critical region in 17p11.2. Genomics. 1999;57(1):180–182. doi: 10.1006/geno.1998.5748. [DOI] [PubMed] [Google Scholar]
- Potocki L, Glaze D, Tan DX, Park SS, Kashork CD, Shaffer LG, Reiter RJ, Lupski JR. Circadian rhythm abnormalities of melatonin in Smith-Magenis syndrome. J Med Genet. 2000;37(6):428–433. doi: 10.1136/jmg.37.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Reitter RJ, Glaze D, Lupski JR. Twenty-four hour urinary excretion of 6-sulphatoxymelatonin in Smith-Magenis Syndrome. 1997:A31. [Google Scholar]
- Rechtschaffen A, Kales A. Washington, DC: US Government Printing Office, Public Health Service; 1968. A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. [Google Scholar]
- Shaw CJ, Withers MA, Lupski JR. Uncommon deletions of the Smith-Magenis syndrome region can be recurrent when alternate low-copy repeats act as homologous recombination substrates. Am J Hum Genet. 2004;75(1):75–81. doi: 10.1086/422016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slager RE, Newton TL, Vlangos CN, Finucane B, Elsea SH. Mutations in RAI1 associated with Smith-Magenis syndrome. Nat Genet. 2003;33(4):466–468. doi: 10.1038/ng1126. [DOI] [PubMed] [Google Scholar]
- Smith AC, Dykens E, Greenberg F. Sleep disturbance in Smith-Magenis syndrome (del 17 p11.2) Am J Med Genet. 1998;81(2):186–191. [PubMed] [Google Scholar]
- Smith AC, McGavran L, Robinson J, Waldstein G, Macfarlane J, Zonona J, Reiss J, Lahr M, Allen L, Magenis E. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986;24(3):393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Smith ACM, Duncan WC. Smith-Magenis Syndrome - A developmental disorder with circadian dysfunction. In: Butler MG, Meaney FJ, editors. Genetics of Developmental Disabilities Pediatric Habilitation) Boca Raton, FL: Taylor & Francis Group, LLC; 2005. pp. 419–476. [Google Scholar]
- Stankiewicz P, Shaw CJ, Dapper JD, Wakui K, Shaffer LG, Withers M, Elizondo L, Park SS, Lupski JR. Genome architecture catalyzes nonrecurrent chromosomal rearrangements. Am J Hum Genet. 2003;72(5):1101–1116. doi: 10.1086/374385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voultsios A, Kennaway DJ, Dawson D. Salivary melatonin as a circadian phase marker: validation and comparison to plasma melatonin. J Biol Rhythms. 1997;12(5):457–466. doi: 10.1177/074873049701200507. [DOI] [PubMed] [Google Scholar]
- Wei N, Tsuge T, Serino G, Dohmae N, Takio K, Matsui M, Deng XW. The COP9 complex is conserved between plants and mammals and is related to the 26S proteasome regulatory complex. Curr Biol. 1998;8(16):919–922. doi: 10.1016/s0960-9822(07)00372-7. [DOI] [PubMed] [Google Scholar]
- Wilson S, Nutt D. Insomnia: guide to diagnosis and choice of treatment. Prescriber. 2008;19(8):14–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.