Abstract
The 12.6-kDa FK506-binding protein (FKBP12.6) is considered to be a key regulator of the cardiac ryanodine receptor (RyR2), but its precise role in RyR2 function is complex and controversial. In the present study we investigated the impact of FKBP12.6 removal on the properties of the RyR2 channel and the propensity for spontaneous Ca2+ release and the occurrence of ventricular arrhythmias. Single channel recordings in lipid bilayers showed that FK506 treatment of recombinant RyR2 co-expressed with or without FKBP12.6 or native canine RyR2 did not induce long-lived subconductance states. [3H]Ryanodine binding studies revealed that coexpression with or without FKBP12.6 or treatment with or without FK506 did not alter the sensitivity of RyR2 to activation by Ca2+ or caffeine. Furthermore, single cell Ca2+ imaging analyses demonstrated that HEK293 cells co-expressing RyR2 and FKBP12.6 or expressing RyR2 alone displayed the same propensity for spontaneous Ca2+ release or store overload-induced Ca2+release (SOICR). FK506 increased the amplitude and decreased the frequency of SOICR in HEK293 cells expressing RyR2 with or without FKBP12.6, indicating that the action of FK506 on SOICR is independent of FKBP12.6. As with recombinant RyR2, the conductance and ligand-gating properties of single RyR2 channels from FKBP12.6-null mice were indistinguishable from those of single wild type channels. Moreover, FKBP12.6-null mice did not exhibit enhanced susceptibility to stress-induced ventricular arrhythmias, in contrast to previous reports. Collectively, our results demonstrate that the loss of FKBP12.6 has no significant effect on the conduction and activation of RyR2 or the propensity for spontaneous Ca2+ release and stress-induced ventricular arrhythmias.
The cardiac Ca2+ release channel (ryanodine receptor, RyR2) governs the release of Ca2+ from the sarcoplasmic reticulum (SR)4 and is essential for excitation-contraction (EC) coupling in cardiac muscle (1). Naturally occurring mutations in RyR2 have been linked to several forms of cardiac arrhythmias, including catecholaminergic polymorphic or bidirectional ventricular tachycardia, catecholaminergic idiopathic ventricular fibrillation, and arrhythmogenic right ventricular dysplasia type 2 (2–4). In addition to its involvement in inherited cardiac arrhythmias, defective RyR2 function has also been implicated in other cardiac abnormalities, including atrial fibrillation and heart failure (HF) (5–7). However, how the RyR2 channel is altered under these conditions is unclear and controversial.
An impaired interaction between RyR2 and the 12.6-kDa FK506 binding protein (FKBP12.6), which is tightly associated with the channel (8), has been proposed to be a major mechanism underlying cardiac dysfunction in HF (9). Marks and coworkers (9) have shown that RyR2 is hyperphosphorylated in HF by the cAMP-dependent protein kinase A at a single residue, Ser-2809, which was originally identified as a unique phosphorylation site for the Ca2+ and calmodulin-dependent protein kinase II (10). They also found that protein kinase A-dependent phosphorylation of RyR2 at Ser-2809 dissociated FKBP12.6 from the channel, which increased the sensitivity of single RyR2 channels to activation by Ca2+ and induced openings to subconductance levels (9, 11). In the heart these FKBP12.6 dissociation-induced alterations in RyR2 function are believed to cause diastolic SR Ca2+ leak, which can result in delayed after-depolarizations and triggered arrhythmias. This diastolic SR Ca2+ leak is also thought to contribute to the reduced SR Ca2+ content often observed in failing cardiac cells (12, 13). In line with this view, K201(JTV519), an experimental cardioprotective and anti-arrhythmic drug (14, 15), was found to stabilize the interaction between FKBP12.6 and RyR2 (16). These observations have led to the proposal that stabilizing the RyR2-FKBP12.6 interaction is a promising strategy for the treatment of HF and cardiac arrhythmias (16).
Many aspects of the theory on the modulation of RyR2 by FKBP12.6 have been called into question by contradictory findings. For example, K201(JTV519) has been shown to abolish spontaneous Ca2+ leak from SR vesicles containing little or no FKBP12.6 (17, 18). We further explored the action of K201 and found that K201 suppressed spontaneous Ca2+ release and [3H]ryanodine binding to RyR2 irrespective of FKBP12.6 association (19). All these observations indicate that the inhibitory action of K201 on RyR2 and spontaneous Ca2+ leak is not mediated by FKBP12.6. The phosphorylation status of RyR2 in HF and its effect on RyR2-FKBP12.6 interaction are also controversial. Jiang et al. (20) showed that the phosphorylation level of RyR2 from failing hearts was indistinguishable from that of normal hearts. Similarly, we have shown that RyR2 is not hyperphosphorylated by protein kinase A in HF (21). We have further demonstrated that RyR2 is phosphorylated by protein kinase A at two major sites, Ser-2030 and Ser-2808 (21), and that stoichiometric phosphorylation of either recombinant or native RyR2 by protein kinase A does not dissociate FKBP12.6 from RyR2 (22). These findings indicate that there is no correlation between protein kinase A-dependent phosphorylation of RyR2 and the dissociation of FKBP12.6 (23). The impact of FKBP12.6 dissociation on the function of the RyR2 channel is also unclear. If the dissociation of FKBP12.6 can markedly alter the gating, conductance, and sensitivity of the RyR2 channel, one would expect that a complete ablation of FKBP12.6 in mice would have a detrimental impact on SR Ca2+ release and cardiac function. Surprisingly, FKBP12.6-null mice display no structural or functional abnormalities at rest in one case (11) and a mild, sex-dependent hypertrophy in the other (24). These observations are inconsistent with the dramatic impairment of single channel function of RyR2 after the dissociation of FKBP12.6 (9, 11) and raise an important question as to whether FKBP12.6 plays an essential role in RyR2 function and cardiac arrhythmias as suggested.
To address this question, in the present study we systematically investigated the impact of FKBP12.6-removal on the function of RyR2 at the molecular, cellular, and intact animal levels. We found that the absence or removal of FKBP12.6 does not induce long-lived subconductance states or alter the sensitivity of the RyR2 channel to activation by Ca2+ or caffeine. We also demonstrated that the presence or absence of FKBP12.6 does not affect the propensity for spontaneous Ca2+ release in HEK293 cells. Interestingly, treatment with FK506 slightly increased the amplitude and decreased the frequency of spontaneous Ca2+ release in HEK293 cells independent of FKBP12.6. Importantly, no stress-induced ventricular arrhythmias were detected in FKBP12.6-null mice. These results challenge the notion that FKBP12.6 is essential for the permeation and activation of the RyR2 channel.
EXPERIMENTAL PROCEDURES
Materials
FK506 was a gift from Astellas Pharma Inc. Soybean phosphatidylcholine was obtained from Avanti Polar Lipids, Inc (Alabaster, AL). [3H]Ryanodine was from PerkinElmer Life Sciences. Anti-FKBP12.0/12.6 and anti-ryanodine receptor (34C) antibodies were purchased from Affinity BioReagents (Golden, CO). CHAPS and other reagents were purchased from Sigma.
Generation of Stable, Inducible HEK293 Cell Lines
Stable, inducible HEK293 cell lines expressing RyR2 or RyR2 and FKBP12.6 were generated using the Flp-In T-REx Core kit from Invitrogen as described previously (19). The double gene construct (RyR2/FKBP12.6) expresses both RyR2 and FKBP12.6, each under the control of a tetracycline operator and the cytomegalovirus and SV40 promoters, respectively (19).
DNA Transfection and Preparation of Cell Lysate from HEK293 Cells
HEK293 cells grown on 100-mm tissue culture dishes were transfected with RyR2 or co-transfected with RyR2 and FKBP12.6 cDNAs using Ca2+ phosphate precipitation as described previously (19, 22). After transfection for 24–26 h, the cells were washed 3 times with PBS (137 mM NaCl, 8 mM Na2HPO4, 1.5 mM KH2PO4, 2.7 mM KCl) plus 2.5 mM EDTA and harvested in the same solution by centrifugation for 8 min at 700 × g in an IEC Centra-CL2 centrifuge. The cells were then washed with PBS without EDTA and centrifuged again at 700 × g for 8 min. The PBS-washed cells were solubilized in a lysis buffer containing 25 mM Tris, 50 mM Hepes (pH 7.4), 137 mM NaCl, 1% CHAPS, 0.5% soybean phosphatidylcholine, 2.5 mM dithiothreitol, and a protease inhibitor mix (1 mM benzamidine, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 2 μg/ml aprotinin, and 0.5 mM phenylmethylsulfonyl fluoride). The mixture was incubated on ice for 1 h. Cell lysate was obtained by centrifugation at 16,000 × g for 30 min twice in a microcentrifuge at 4 °C to remove the unsolubilized materials.
[3H]Ryanodine Binding
Equilibrium [3H]ryanodine binding to cell lysate was performed as described previously (25). Briefly, a binding mixture (300 μl) containing 30 μl of cell lysate (3–5 mg/ml), 25 mM Tris/50 mM Hepes (pH 7.4), 5 nM [3H]ryanodine, a protease inhibitor mix, and various concentrations of CaCl2 as indicated was incubated at 37 °C for 2.5–3.5 h. The binding mixture was diluted with 5 ml of ice-cold washing buffer containing 25 mM Tris (pH 8.0) and 250 mM KCl and immediately filtered through Whatman GF/B filters presoaked with 1% polyethyleneimine. The filters were washed 4 times with 5 ml of ice-cold washing buffer, and the radioactivity associated with the filters was determined by liquid scintillation counting. Nonspecific binding was determined by measuring [3H]ryanodine binding in the presence of 50 μM unlabeled ryanodine. All binding assays were done in duplicate. Data shown are mean ± S.E. for n experiments. The curves for Ca2+ and caffeine-dependent activation of [3H]ryanodine binding were obtained by fitting the data using the MacCurveFit program (Victoria, Australia). Statistical significance was evaluated using the Student’s t test.
Single Cell Ca2+ Imaging of HEK293 Cells
Intracellular Ca2+ transients in stable, inducible HEK293 cells expressing RyR2 or RyR2/FKBP12.6 were measured using single-cell Ca2+ imaging and the fluorescent Ca2+ indicator dye fura-2 acetoxymethyl ester (fura-2 AM) as described previously (19). Cells grown on glass coverslips for 24 or 30 h after induction by 1 μg/ml tetracycline (Sigma) were loaded with 5 μM fura-2 AM in Krebs-Ringer-Hepes (KRH) buffer (125 mM NaCl, 5 mM KCl, 1.2 mM KH2PO4, 6 mM glucose, 1.2 mM, MgCl2, 25 mM Hepes (pH 7.4), plus 0.02% pluronic F-127 (Molecular Probes) and 0.1 mg/ml bovine serum albumin for 20 min at room temperature (22 °C). The coverslips were then mounted in a perfusion chamber (Warner Instruments, Hamden, CT) on an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor 20×/0.75 objective. The cells were continuously perfused with KRH buffer containing 2 mM CaCl2 in the presence or absence of 5 μM FK506 at room temperature (22 °C). 10 mM caffeine was applied at the end of each experiment to confirm the expression of functional RyR2 channels. Time-lapse images (0.33 frames s−1) were captured and analyzed with the Compix Inc. Simple PCI 6 software. Fluorescent intensities were measured from regions of interest centered on individual cells. Only those cells that responded to caffeine were used in analyses.
Isolation of Adult Rat Ventricular Myocytes
All studies with rats were approved by the Animal Care Committee of the University of Calgary and complied with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (Publication no. 85-23, revised 1996). Single rat ventricular myocytes were isolated as described previously (26). Isolated cells were stored at room temperature in a solution containing 20 mM taurine, 5 mg/ml albumin, and 0.5 mM CaCl2 until used for single cell Ca2+ imaging studies.
Single Cell Ca2+ Imaging of Rat Ventricular Myocytes
Freshly isolated rat ventricular myocytes were placed on glass coverslips coated with 0.02% (w/v) gelatin and 10 μg/ml fibronectin and loaded with 5 μM fluo-4 AM Ca2+ (Molecular Probes) plus 0.02% pluronic F-127 in KRH buffer with 1 mM Ca2+ for 20 min at room temperature (22 °C) (19). The cover-slips were mounted in a perfusion chamber on an inverted microscope (Nikon TE2000-S) equipped with an S-Fluor 20×/0.75 objective. The [Ca2+] was then stepped to 3 mM for 5 min before a further increase to 6 mM. The cells were then continuously perfused with KRH buffer containing 6 mM CaCl2 in the presence or absence of 5 μM FK506 at room temperature (22 °C). Time-lapse images were captured every 0.4 s and analyzed with the Compix Inc. Simple PCI 6 software. Fluorescent intensities were measured from regions of interest.
Single Channel Recordings in Planar Lipid Bilayers
Recombinant RyR2 proteins were partially purified from cell lysate by sucrose density gradient centrifugation (27). Heart phosphatidylethanolamine and brain phosphatidylserine (Avanti Polar Lipid) dissolved in chloroform were combined in a 1:1 ratio (w/w), dried under nitrogen gas, and suspended in 30 μl of n-decane at a concentration of 12 mg lipid/ml. Bilayers were formed across a 250-μm hole in a Delrin partition separating two chambers. The cytosolic (cis) chamber (800 μl) was connected to the head stage input of an Axopatch 200A amplifier (Axon Instruments, Inc.). The trans chamber (1.2 ml) was held at virtual ground. A symmetrical solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) was used for all recordings unless indicated otherwise. A 4-μl aliquot (~1 μg of protein) of the sucrose density gradient-purified recombinant RyR2 protein was added to the trans chamber. Spontaneous channel activity was always tested for sensitivity to EGTA and Ca2+. The chamber to which the addition of EGTA inhibited the activity of the incorporated channel was presumed to correspond to the cytoplasmic side of the channel. The direction of single channel currents was always measured from the luminal to the cytoplasmic side of the channel unless mentioned otherwise. Recordings were filtered at 2500 Hz. Free Ca2+ concentrations were calculated using the computer program of Fabiato and Fabiato (28). Data analyses were carried out using the pClamp 8.1 software (Axon Instruments). For single channel recordings of native mouse RyR2 channels, ventricular sarcoplasmic reticulum microsomes from FKBP12.6+/+ and FKBP12.6−/− adult male mice were prepared. RyR2 channels from microsomes were reconstituted into bilayers as described previously (29, 30). In all experiments the luminal (trans) solution contained 50 mM Ca2+, 250 mM HEPES. The cis solution contained 118 mM Tris, 250 mM HEPES (cis and trans pH = 7.4). [Ca2+] in the cis solution was buffered with DiBromo BAPTA. Unless indicated otherwise, all experiments with native mouse RyR2 channels were carried out at a holding potential of 0 mV (transmembrane voltage). Bars at the side of the recordings indicate base-line levels. Open probabilities were obtained from the analysis of channel recordings of 4–8 min in length as described previously (29, 30).
RyR2/FKBP12.6 Pulldown Assays and Immunoblotting
Cell lysates (RyR2 or RyR2/FKBP12.6) or solubilized cardiac microsomes were incubated with protein G-Sepharose (20 μl) that was pre-bound with 1 μl of anti-RyR antibody (34C) at 4 °C for 17–19 h in the presence or absence of 5 μM FK506. The protein G/34C precipitates were washed with ice-cold lysis buffer containing a protease inhibitor mix 3 times, each time for 10 min. The proteins bound to the Sepharose beads or 20 μl of cell lysates were then solubilized by the addition of 20 μl of 2× Laemmli sample buffer (31) plus 5% β-mercaptoethanol and boiled for 5 min. The solubilized samples were then separated by 6.25% SDS-PAGE (31). For the immunoprecipitation experiments, the sample volumes were adjusted to load a similar amount of RyR2 into each lane. The SDS-PAGE-resolved proteins were transferred to nitrocellulose membranes at 45 V for 18–20 h at 4 °C in the presence of 0.01% SDS according to the method of Towbin et al. (32). The nitrocellulose membranes containing the transferred proteins were blocked for 30 min with PBS containing 0.5% Tween 20 and 5% skim milk. The blocked membranes were then incubated with anti-RyR(34C) or anti-FKBP antibodies (both 1:1000) for 1–2 h and washed 3 times for 5 min each in PBS containing 0.5% Tween 20. The membrane was then incubated with the appropriate horseradish peroxidase-conjugated secondary antibody (1:20,000) for 30 min. After washing 3 times for 5 min each in PBS containing 0.5% Tween 20, the RyR2 or FKBP12.6 proteins were detected by enhanced chemiluminescence (Pierce).
Telemetric Electrocardiogram (ECG) Recordings and Stress Tests
All studies with mice were approved by the Animal Care Committee of the University of Calgary and complied with Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (Publication no. 85-23, revised 1996). ECG telemetric transmitters (Data Sciences International, St. Paul, MN) were implanted in the abdominal cavity under general inhalant anesthesia. Body temperature was maintained at 37 °C by use of a heating pad. After 5 days of recovery from surgery, the mice (WT, n = 9, FKBP12.6−/−, n = 9) were injected with epinephrine (2 mg/kg intraperitoneally) and caffeine (120 mg/kg intraperitoneally). ECG was continuously monitored for 10 min before and 60–90 min after injection.
RESULTS
The Removal of FKBP12.6 Does Not Induce Subconductance States in Recombinant or Native Canine RyR2 Channels
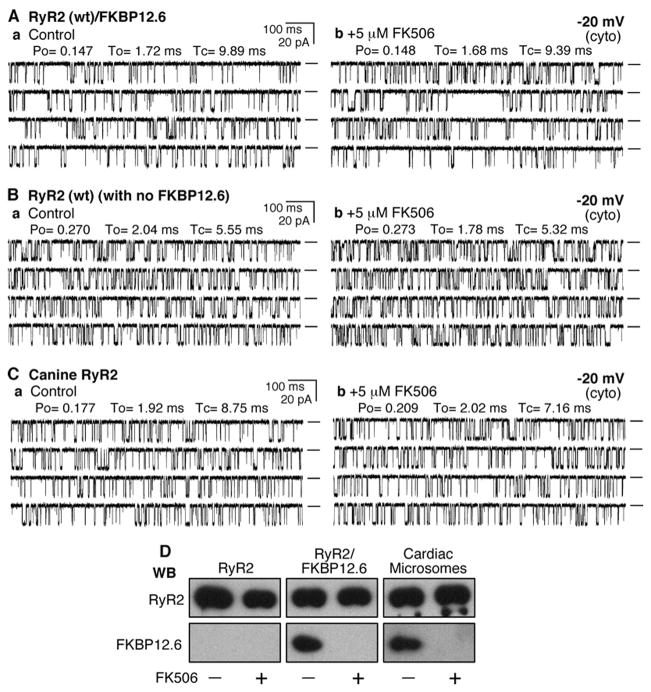
To investigate the role of FKBP12.6 in the conduction of the RyR2 channel, we expressed RyR2 WT alone or with FKBP12.6 in HEK293 cells. The expressed RyR2 channels associated with or without FKBP12.6 were then purified by sucrose-density gradient centrifugation and used for single channel analyses in planar lipid bilayers. We have previously shown that FKBP12.6 is co-immunoprecipitated with RyR2 from HEK293 cells co-transfected with FKBP12.6 (21, 22). Fig. 1A shows the channel activity of a single RyR2 channel from HEK293 cells co-transfected with RyR2 and FKBP12.6 before (Fig. 1Aa) and after (Fig. 1Ab) the addition of 5 μM FK506, an immunosuppressant known to dissociate FKBP12.6 from RyR2. As can be seen, the conductance and gating of the channel remained largely unchanged after the FK506 treatment. The average open probability (Po) after the FK506 treatment was 100 ± 8.4% (n = 7) that before the treatment. These data indicate that dissociation of FKBP12.6 from RyR2 does not induce long-lasting subconductance states.
FIGURE 1. FKBP12.6-removal does not affect the conductance of single RyR2 channels.
Single channel activities of the recombinant RyR2 WT co-expressed with FKBP12.6 (A), recombinant RyR2 WT expressed alone (B), and the native canine RyR2 (C) were recorded in a symmetrical recording solution containing 250 mM KCl and 25 mM Hepes (pH 7.4) at a holding potential of −20 mV. EGTA was added to either the cis or trans chamber to determine the orientation of the incorporated channel. The side of the channel to which the addition of EGTA inhibited the activity of the incorporated channel presumably corresponds to the cytosolic face of the channel. Panel a shows control single channel activities in the presence of ~100 –200 nM cytosolic Ca2+and 45 nM luminal Ca2+. Single channel activities ~10 min after the addition of 5 μM FK506 are shown in panel b. A total of 7 RyR2/FKBP12.6, 10 RyR2 without FKBP12.6, and 8 canine RyR2 channels were tested, and none showed FK506-induced long-lived subconductance states. Openings are downward. Open probability (Po), arithmetic mean open time (To), and arithmetic mean closed time (Tc) are indicated at the top of the traces. A short line to the right of each current trace indicates the base line. D, Western blots (WB)of RyR2 and FKBP12.6 immunoprecipitated with an anti-RyR antibody (34c) from either HEK293 cell lysate expressing RyR2 or co-expressing RyR2 and FKBP12.6 or solubilized canine cardiac microsomes treated with or without 5 μM FK506.
Fig. 1B shows single channel current traces of an RyR2 channel from HEK293 cells transfected with RyR2 alone without FKBP12.6. We have previously shown that RyR2 expressed alone in HEK293 cells is devoid of FKBP12.6 (21, 22). As seen in Fig. 1B, single RyR2 channels devoid of FKBP12.6 displayed a single channel conductance identical to that of RyR2 co-expressed with FKBP12.6 (Fig. 1A) and native canine RyR2 (Fig. 1C). Importantly, single RyR2 channels without FKBP12.6 exhibited no long-lived subconductance states. As with the FKBP12.6/RyR2 channels, FK506 (5 μM) had no significant effect on the conductance or gating of the FKBP12.6-devoid single RyR2 channels (Fig. 1Bb). The average Po after FK506 treatment was 112 ± 5.2% (n = 10, p < 0.236) that before the treatment. These observations demonstrate that the loss of FKBP12.6 does not affect the conductance of the RyR2 channel.
It is clear that the removal of FKBP12.6 does not affect the conductance or gating of the recombinant RyR2 channels. To determine whether the dissociation of FKBP12.6 alters the properties of native RyR2 channels, we tested the effect of FK506 on native canine RyR2 channels incorporated into lipid bilayers. As shown in Fig. 1C, treating single native canine RyR2 channels with 5 μM FK506 did not produce subconductance states or change the gating of the channel. The average Po after the FK506 treatment was 100 ± 4.6% (n = 8) that before the treatment. We have previously shown that FKBP12.6 was co- immunoprecipitated with the recombinant or canine RyR2 and dissociated from RyR2 by rapamycin (19, 21, 22), an analogue of FK506. Similarly, we found that FKBP12.6 was dissociated from the recombinant or native canine RyR2 channels by 5 μM FK506 (Fig. 1D). Hence, taken together, our data indicate that the dissociation of FKBP12.6 does not induce subconductance states in either recombinant or native RyR2 channels.
The Removal of FKBP12.6 Does Not Alter the Ca2+ Dependence or Caffeine Response of [3H]Ryanodine Binding to RyR2
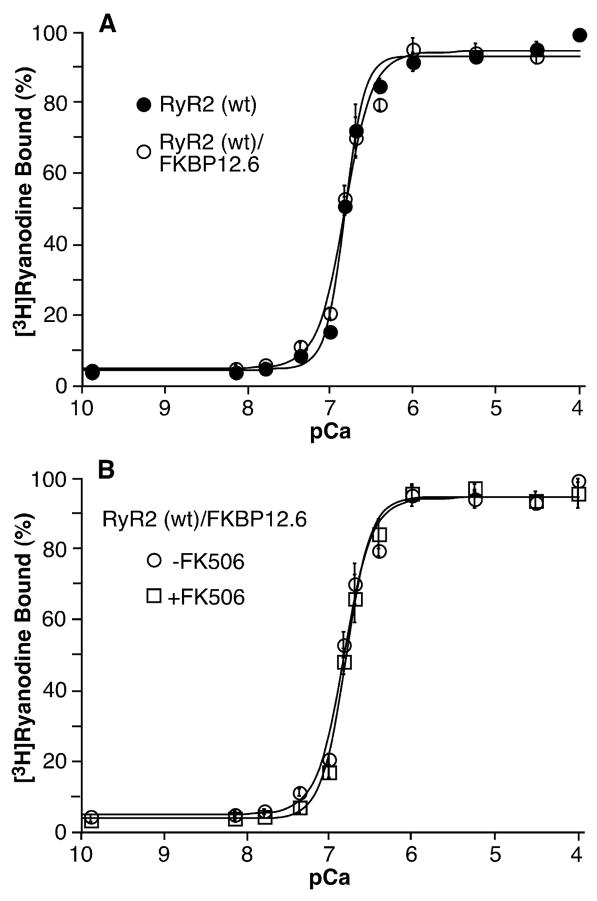
To determine whether the dissociation of FKBP12.6 affects the sensitivity of RyR2 to activation by stimuli, we assessed the effect of FK506 on [3H]ryanodine binding to cell lysates from HEK293 cells transfected with RyR2 alone or co-transfected with RyR2 and FKBP12.6 at various concentrations of Ca2+ or caffeine. As shown in Fig. 2A, the Ca2+ dependence of [3H]ryanodine binding to RyR2 expressed alone (EC50 = 0.16 ± 0.01 μM, n = 5) and RyR2 co-expressed with FKBP12.6 (EC50 = 0.16 ± 0.01 μM, n = 5) is identical. Treatment with 5 μM FK506 did not change the Ca2+dependence of [3H]ryanodine binding to RyR2 co-expressed with FKBP12.6 (Fig. 2B). In the presence of FK506, the EC50 value for [3H]ryanodine binding to RyR2/FKBP12.6 was 0.17 ± 0.02 μM (n = 3), which is similar to that of [3H]ryanodine binding to RyR2/FKBP12.6 in the absence of FK506 (0.16 ± 0.01 μM, n = 5).
FIGURE 2. FKBP12.6-removal does not alter the Ca2+ dependence of [3H]ryanodine binding.
[3H]Ryanodine binding to cell lysate prepared from HEK293 cells expressing RyR2 or expressing both RyR2 and FKBP12.6 was carried out at various Ca2+ concentrations (~0.2 nM to 0.1 mM), 5 nM [3H]ryanodine, and 500 mM KCl. Panel A shows [3H]ryanodine to RyR2 (filled circles) and RyR2/FKBP12.6 (open circles). Panel B shows [3H]ryanodine binding to RyR2/FKBP12.6 in the presence (open squares) or absence (open circles) of 5 μM FK506. Data points shown are the mean ± S.E. from 3–5 separate experiments.
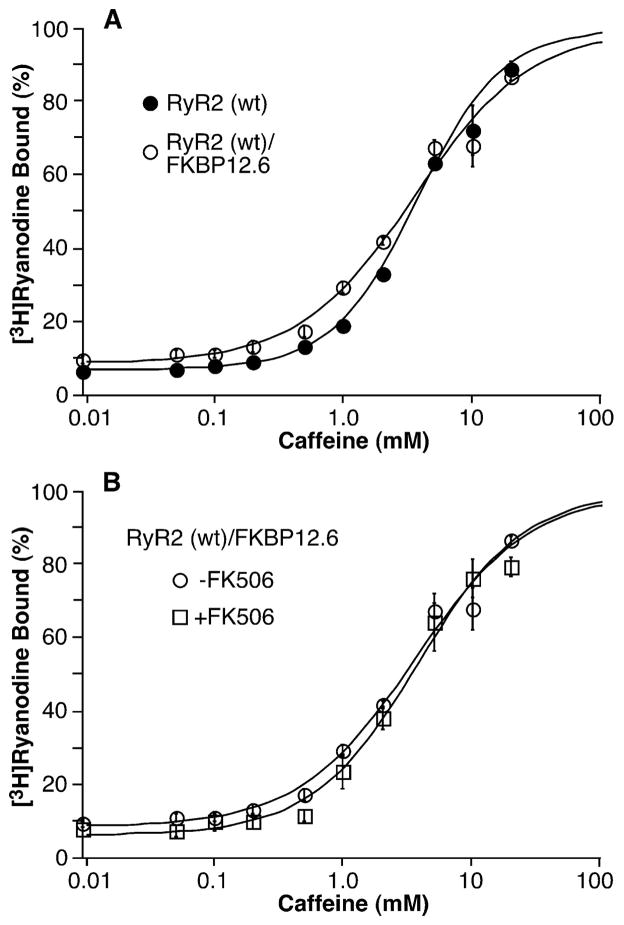
Fig. 3 shows the caffeine response of [3H]ryanodine binding to RyR2 expressed alone or co-expressed with FKBP12.6 in the absence or presence of 5 μM FK506. The caffeine responses of [3H]ryanodine binding to RyR2 co-expressed with FKBP12.6 (EC50 = 3.65 ± 0.22 μM, n = 4) or without FKBP12.6 (EC50 = 4.25 ± 0.46 μM, n = 4) were similar (Fig. 3A). FK506 treatment did not alter the sensitivity of RyR2 to activation by caffeine. The EC50 values for caffeine activation of [3H]ryanodine binding to RyR2 co-expressed with FKBP12.6 in the presence and absence of FK506 were 3.93 ± 0.69 mM (n = 3) and 3.65 ± 0.22 mM (n = 4), respectively. Collectively, these observations indicate that the removal of FKBP12.6 does not affect the sensitivity of RyR2 to activation by Ca2+ or caffeine.
FIGURE 3. FKBP12.6-removal does not alter the sensitivity of RyR2 to activation by caffeine.
[3H]Ryanodine binding to cell lysate prepared from HEK293 cells expressing RyR2 or expressing both RyR2 and FKBP12.6 was carried out at various caffeine concentrations (0.01–20 mM), 5 nM [3H]ryanodine, and 500 mM KCl. Panel A shows [3H]ryanodine to RyR2 (filled circles) and RyR2/FKBP12.6 (open circles). Panel B shows [3H]ryanodine binding to RyR2/FKBP12.6 in the presence (open squares) or absence (open circles) of 5 μM FK506. Data points shown are the mean ± S.E. from four separate experiments.
Effect of FK506 on Store Overload-induced Ca2+ Release (SOICR) in HEK293 Cells Expressing RyR2 and in Rat Cardiac Myocytes
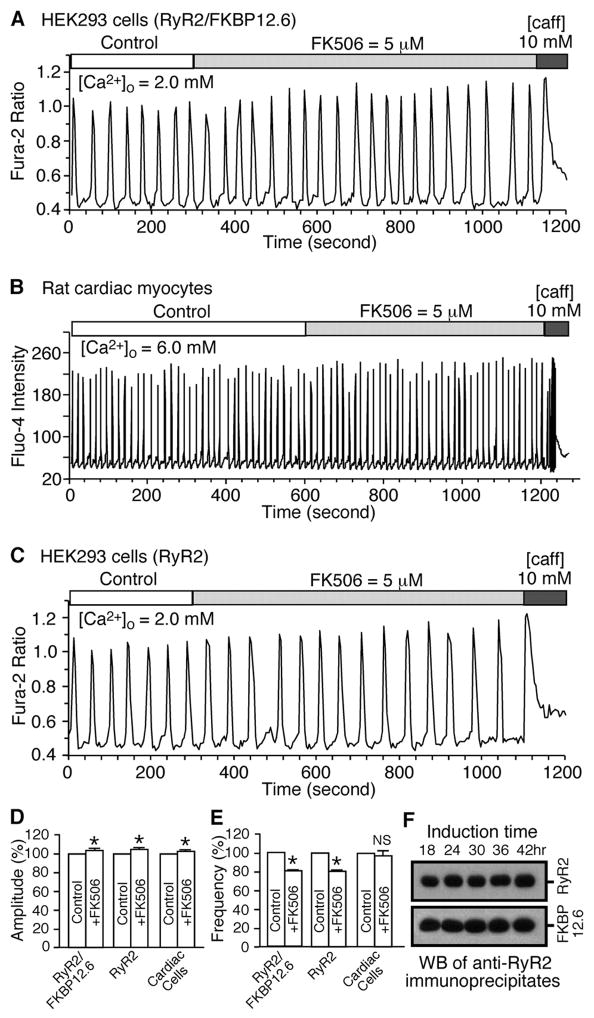
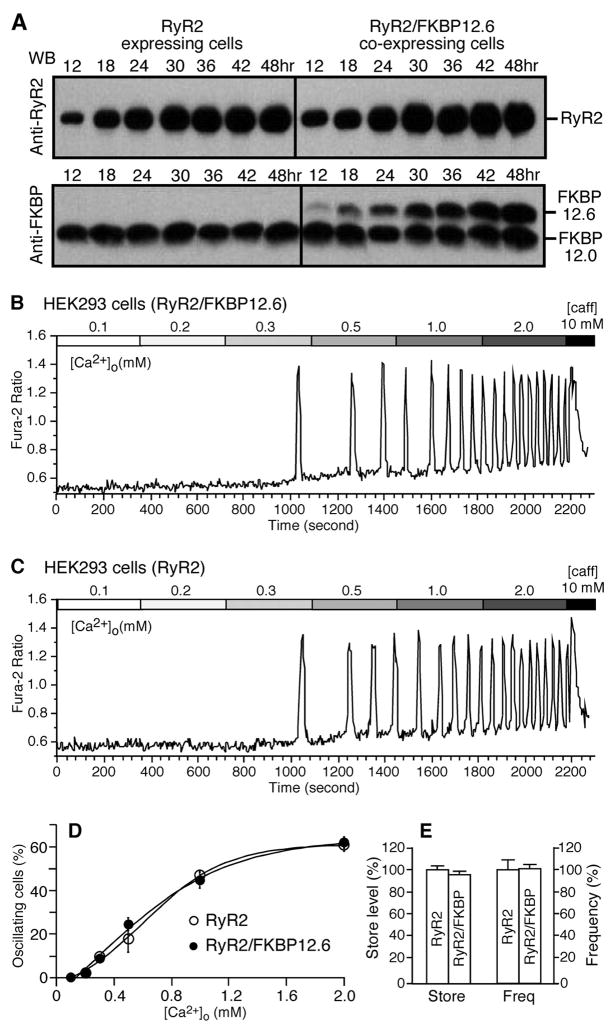
Given the importance of spontaneous Ca2+ release or SOICR in arrhythmogenesis and the potential role of FKBP12.6 in cardiac arrhythmias, we examined whether the removal of FKBP12.6 by FK506 alters the SOICR properties of HEK293 cells expressing RyR2 and cardiac myocytes. To this end, we generated a stable, inducible HEK293 cell line expressing both RyR2 and FKBP12.6. To confirm that RyR2 forms a complex with FKBP12.6 in these cells, we performed immunoprecipitation and immunoblotting experiments. As shown in Fig. 4F, FKBP12.6 was co-immunoprecipitated with RyR2 by an anti-RyR antibody (34C). The levels of co-immunoprecipitated FKBP12.6 were comparable after 24–42 h of induction. Accordingly, the RyR2/FKBP12.6-expressing cells were induced for 24 h. The cells were then loaded with the Ca2+ fluorescent indicator fura-2 AM and perfused with 2 mM external Ca2+ to produce SOICR. After a stable SOICR was achieved, FK506 (5 μM) was added to the perfusate. The addition of FK506 did not increase SOICR activity as would be expected given the common belief that the dissociation of FKBP12.6 enhances the activity of the RyR2 channel. Interestingly and to the contrary, we found that FK506 slightly increased the amplitude to 104.4 ± 0.4% (p < 0.0001) and reduced the frequency of SOICR to 80.5 ± 2.0% (p < 0.002) (n = 172) and (Fig. 4, A, D, and E).
FIGURE 4. Effect of FK506 on SOICR in HEK293 cells and in rat cardiac cells.
HEK293 cells expressing both RyR2 and FKBP12.6 (panel A) or expressing RyR2 alone (panel C) were grown on glass coverslips. The cells were loaded with 5 μM fura-2 AM in KRH buffer for 20 min at room temperature (22 °C). Fura-2 ratios of representative cells in the presence of 2 mM external Ca2+ before and after the addition of 5 μM FK506 are shown. Panel B, freshly isolated rat ventricular myocytes were loaded with fluo-4-AM in KRH buffer for 20 min at room temperature (22 °C). Fluo-4 intensities of a representative cell in the presence of 6 mM external Ca2+ before and after the addition of FK506 (5 μM) are shown. Panels D and E show the impact of FK506 on the amplitude and frequency of Ca2+ oscillations in HEK293 cells expressing both RyR2 and FKBP12.6 or RyR2 alone or in rat cardiac myocytes. The amplitude and frequency were determined during the last 5 min of FK506 treatment and normalized to those during the control recordings before FK506 treatment (100%). A number of RyR2/FKBP12.6 (n = 172), RyR2 (n = 180), and rat cardiac (n = 74) cells from 4 –5 separate experiments were analyzed. Values shown are the mean ± S.E. (*, p < 0.02– 0.0001). Panel F, Western blot (WB) showing the interaction between RyR2 and FKBP12.6 at various induction times with tetracycline. The FKBP12.6-RyR2 complex was immunoprecipitated using the anti-RyR antibody followed by immunoblotting with the anti-RyR (top panel) and anti-FKBP12/12.6 (bottom panel) antibodies. Loadings to the SDS-PAGE gel were adjusted in an attempt to achieve similar loadings of RyR2 in each lane for easy comparison of the levels of associated FKBP12.6 between different induction times. Data shown are representative of three separate experiments. caff, caffeine.
The effect of FK506 on SOICR was also assessed in rat cardiac myocytes. Freshly isolated rat cardiac myocytes were perfused with 6 mM external Ca2+ to induce SOICR. The cells were then switched to perfusate containing 6 mM Ca2+ and 5 μM FK506. As seen in Fig. 4B, perfusion with FK506 did not considerably affect the SOICR activity. A small increase in the amplitude of SOICR was observed (103.0 ± 0.8%, p < 0.0001), but no significant change in the frequency was detected (96.9 ± 6.4%, p < 0.323) (n = 74) (Fig. 4, B, D, and E).
To further investigate whether the modest impact of FK506 on SOICR is mediated by FKBP12.6, we examined the effect of FK506 on SOICR in HEK293 cells expressing RyR2 alone without FKBP12.6. As shown in Fig. 4C, the addition of FK506 (5 μM) was still able to increase the amplitude (104.9 ± 1.6%, p < 0.02) and reduce the frequency (80.5 ± 1.1%, p < 0.0002) (n = 180) of SOICR in these FKBP12.6-deficient cells (Fig. 4, D and E). Taken together, these observations demonstrate that the removal of FKBP12.6 does not enhance spontaneous Ca2+ release in HEK293 cells or in cardiac cells. They also indicate that the effect of FK506 on SOICR is FKBP12.6-independent.
Effect of FKBP12.6 on SOICR in HEK293 Cells Expressing RyR2
To examine the role of FKBP12.6 in SOICR without the interference of FK506, we compared the SOICR activity of HEK293 cells expressing RyR2 alone and HEK293 cells expressing both RyR2 and FKBP12.6. To ensure a comparable level of expression of RyR2 in these cell lines, we examined the time course of expression of RyR2. As shown in Fig. 5A, the expression level of RyR2 and FKBP12.6 increased in both cell lines with increasing induction times. The expression level of RyR2 in RyR2-expressing HEK293 cells after 30 h of induction was found to be comparable with that in RyR2/FKBP12.6-expressing HEK293 cells after 24 h of induction. Hence, SOICR was compared between cells expressing RyR2 after 30 h induction and cells expressing both RyR2 and FKBP12.6 after 24 h induction. It should be noted that FKBP12.6 is absent in HEK293 cells expressing only RyR2 and that the expression levels of the endogenous FKBP12.0 were not changed under these conditions. Analyzing a number of oscillating cells revealed that HEK293 cells expressing RyR2 with (Fig. 5B, n = 508 cells) or without (Fig. 5C, n = 403 cells) FKBP12.6 exhibited the same propensity for SOICR. No significant differences in the level of intracellular Ca2+ store or the frequency of SOICR were detected (Fig. 5E). These data indicate that the loss of FKBP12.6 does not alter the propensity for SOICR.
FIGURE 5. The lack of FKBP12.6 does not alter the SOICR properties.
A, HEK293 cells expressing RyR2 alone (left) or both RyR2 and FKBP12.6 (right) were induced by tetracycline for different lengths of time (12– 48 h). RyR2 and FKBP12.6 proteins from the same amount of cell lysate were immunoblotted (WB) with the anti-RyR antibody (top) or anti-FKBP12.0/12.6 antibody (bottom). Note that FKBP12.6 is in the absence in HEK293 cells expressing RyR2 alone. Fura-2 ratios of single RyR2/FKBP12.6 cells induced for 24 h (B) and of single RyR2 cells induced for 30 h (C) at elevated [Ca2+]o (0.1–2.0 mM) are shown. D, the fraction (%, mean ± S.E.) of RyR2 cells (open circles) and RyR2/FKBP12.6 cells (filled circles) that displayed Ca2+ oscillations at various [Ca2+]o. The total numbers of cells analyzed for Ca2+ oscillations were 403 for RyR2 and 508 for RyR2/FKBP12.6. E, store Ca2+ contents in HEK293 cells expressing RyR2 alone or expressing both RyR2 and FKBP12.6 were determined by measuring the amplitude of caffeine (caff, 10 mM)-induced Ca2+ release, whereas the frequency of Ca2+ oscillations was estimated from the number of peaks in the presence of 2 mM [Ca2+]o. Data shown are the mean ± S.E. from four separate experiments.
Single Channel Properties of RyR2 from FKBP12.6-null Mice
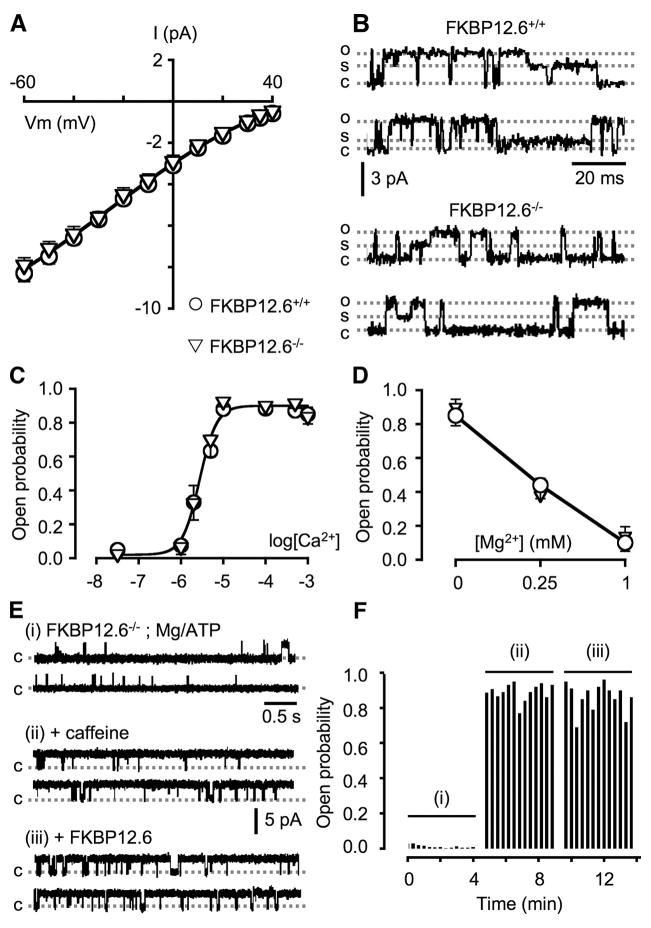
The role of FKBP12.6 in RyR2 function was further investigated using FKBP12.6-null mice. Cardiac microsomes from WT and FKBP12.6-null mice were isolated and reconstituted into planar lipid bilayers. As shown in Fig. 6, A–D, single RyR2 channels from FKBP12.6-null mice displayed properties indistinguishable from those of the WT mice. Their current voltage relationships overlapped (Fig. 6A). Channels openings to various subconductance states, such as those shown in Fig. 6B, were rare. The probability of the subconductance states (the time of the channel in sub-states/the total time of the channel in full-open state) was 0.0005 ± 0.0002 (n = 4) for the WT channels and 0.0004 ± 0.0002 (n = 4) for the FKBP12.6-defficient channels. The Ca2+-dependent activation (Fig. 6C) and Mg2+-dependent inhibition (Fig. 6D) of RyR2 from WT and FKBP12.6-null mice were identical. The FKBP12.6-deficient RyR2 channels also responded to ATP, caffeine (Fig. 6E), ruthenium red, and ryanodine in a manner similar to RyR2 WT (not shown). In addition, the gating properties of RyR2 from FKBP12.6-null mice were not affected by the addition of the exogenous FKBP12.6 protein (Figs. 6, E and F). These observations indicate that the loss of FKBP12.6 does not alter the single channel properties of native mouse RyR2 channels. These findings are consistent with our previous observations that FKBP12.6 removal/association does not have any noticeable effect on the activity of RyR2 (29, 30, 33).
FIGURE 6. Single channel properties of RyR2 from FKBP12.6+/+ and FKBP12.6−/− mice.
A, the I–V relationship of single RyR2 channels activated by 100 μM Ca2+ from FKBP12.6+/+ (open circles) and FKBP12.6−/− mice (open triangles) is shown. The slope conductances of FKBP12.6+/+ and FKBP12.6−/− RyR2 channels were similar (~90 picosiemens at 0 mV). Panel B shows examples of subconductance states, which were rarely observed in either WT or knock-out channels (<0.05% of channels openings). Openings to full conductance state (o) and to subconductance states (s) and the closed state (c) are indicated. Panel C shows the sensitivity of RyR2 from FKBP12.6+/+ (open circles) and FKBP12.6−/− mice (open triangles) to activation by Ca2+. Data points shown are mean ± S.E. (n = 4, each). The Mg2+-dependent inhibition of single RyR2 channels is shown in panel D. Single FKBP12.6+/+ and FKBP12.6−/− RyR2 channels were activated by 5 μM cytosolic Ca2+ and were inhibited by various Mg2+ concentrations in a similar manner. Values are the mean ± S.E. (n = 4, each). E, single FKBP12.6−/−RyR2 channel exhibited low Po at cytosolic [Ca2+] of 2 μM in the presence of 2 mM Mg2+ and 1 mM ATP (free [Mg2+] ~1 mM). The addition of 5 mM caffeine activated the channel (Po ~0.9). Subsequent addition of 1 μM FKBP12.6 did not significantly change the Po of the channel (Po ~0.86) (n = 4). Panel F shows the diary plot of single channel activities shown in panel E.
Impact of FKBP12.6 Deficiency on Stress-induced Ventricular Arrhythmias
It has been shown that one model of FKBP12.6-null mice is highly susceptible to stress-induced ventricular tachycardia (VT) and sudden death (11). As shown above, we found no apparent differences between RyR2 from another FKBP12.6-null mouse strain and WT. Moreover, all our studies suggest that FKBP12.6 removal does not affect single RyR2 channel properties, the sensitivity of RyR2 to activation, or SOICR, a well known trigger of cardiac arrhythmias. Our FKBP12.6-null mice only display some mild cardiac abnormalities (24). Still, the susceptibility of these animals to stress-induced VT was never tested. Accordingly, we decided to address whether VT and sudden death could be readily induced by stress in our FKBP12.6-null mice.
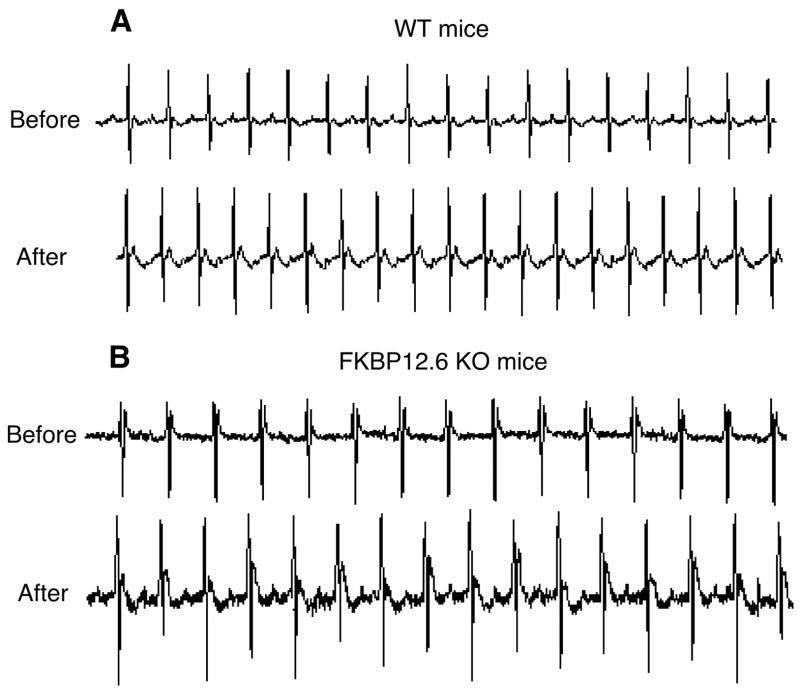
Telemetric ECG devices were implanted into the FKBP12.6-null and WT mice, and their ECGs were monitored before and after the injection of a mixture of epinephrine and caffeine, a combination that has been shown to induce ventricular arrhythmias in mice harboring a RyR2 mutation linked to VT and sudden death (34, 35). Using this approach, we observed that the injection of a combination of epinephrine (2 mg/kg) and caffeine (120 mg/kg) induced no ventricular arrhythmias in either WT (n = 9) or FKBP12.6-null mice (n = 9) (Fig. 7). These observations indicate that FKBP12.6 deficiency does not lead to stress-induced ventricular arrhythmias and sudden death in our FKBP12.6-null mice.
FIGURE 7. FKBP12.6-null mice display no stress-induced ventricular arrhythmias.
WT and FKBP12.6-null mice implanted with ECG telemetric transmitters were injected with epinephrine (2 mg/kg) and caffeine (120 mg/kg). ECG was continuously monitored for 10 min before and 60–90 min after injection. Representative ECG traces from a WT (A) and a FKBP12.6-null mouse (B) before and after the injection are shown. The number of mice tested was nine WT and nine FKBP12.6 knock-out (KO).
DISCUSSION
FKBP12.6, a small RyR2-associated protein, has recently been proposed as a promising therapeutic target for the treatment of HF and cardiac arrhythmias (16). This proposal is largely based on the observations that 1) the level of FKBP12.6 bound to RyR2 was reduced in HF as a result of protein kinase A-dependent hyperphosphorylation of RyR2 (9), 2) the dissociation of FKBP12.6 from RyR2 induced subconductance states and increased the sensitivity of the channel to Ca2+ activation, consequently leading to SR Ca2+ leak (9), 3) an experimental drug, K201(JTV519), which is known to have cardioprotective and anti-arrhythmic properties, inhibited SR Ca2+ leak and cardiac arrhythmias in an FKBP12.6-dependent manner (16), and 4) FKBP12.6-null mice were susceptible to stress-induced ventricular arrhythmias (11). Accordingly, restoring or stabilizing the binding of FKBP12.6 to RyR2 is thought to be an effective strategy for suppressing SR Ca2+ leak and cardiac arrhythmias (16). However, the phosphorylation status of RyR2 by protein kinase A in HF, the impact of protein kinase A-dependent phosphorylation on the FKBP12.6-RyR2 interaction, and the role of FKBP12.6 in the inhibitory action of K201(JTV519) have recently been questioned (19–24, 30, 35). In the present study we have focused on the questions of whether the dissociation of FKBP12.6 alters the conductance and activation of the RyR2 channel and whether the removal of FKBP12.6 enhances the propensity for spontaneous Ca2+ release (SOICR) and the susceptibility to stress-induced ventricular arrhythmias. We found that the removal of FKBP12.6 has little effect on RyR2 function and spontaneous Ca2+ release. Importantly, the ablation of FKBP12.6 does not render mice susceptible to stress-induced ventricular arrhythmias. Taken together, the results of the present study and those of previous studies do not support the notion that impaired RyR2-FKBP12.6 interaction is a major mechanism underlying cardiac dysfunction in HF.
The Role of FKBP12.6 in the Conduction and Activation of the RyR2 Channel
FK506 and rapamycin are two immunosuppressants capable of binding to and dissociating FKBP12.6 from the RyR2 channel. Because of their unique properties, they have been widely used as probes for assessing the role of FKBP12.6 in RyR2 function. It has been shown that treating single RyR2 channels incorporated into planar lipid bilayers with FK506 or rapamycin induced the appearance of various subconductance states and increased the Po of the channel (36, 37). These FK506- or rapamycin-induced changes in single channel behavior were thought to result from the dissociation of FKBP12.6. However, SR membrane vesicles pretreated with FK590, an analogue of FK506, to remove FKBP12.6 before their fusion into planar lipid bilayers displayed single RyR2 channel activities without subconductance states (30). Consistent with this observation, we have demonstrated that single recombinant RyR2 channels expressed in HEK293 cells without FKBP12.6 and single native mouse RyR2 channels from FKBP12.6-null mice exhibited no subconductance states (Figs. 1 and 6). These observations indicate that the removal of FKBP12.6 in and of itself does not induce subconductance states. The exact mechanism by which FK506 or rapamycin induces subconductance states is not clear. The occurrence of subconductance states may be influenced by the concentrations, types, and sources of the experimental drugs used (FK590, FK506, or rapamycin), the preparation, forms (SR membrane vesicles or purified channels), and species of the RyR2 channels, and the recording conditions (bilayer compositions or charge carriers). In any event, it is clear that the drug-induced subconductance states are not due to a lack of FKBP12.6 but may result from a direct effect of the drug or the drug-FKBP12.6 complex on RyR2.
The dissociation of FKBP12.6 is generally believed to increase the sensitivity of RyR2 to Ca2+ activation. However, there is no direct evidence to support this belief. Despite an increase in the Po of the channel as a result of the appearance of long-lived subconductance states, rapamycin did not affect the overall Ca2+dependence of activation of the RyR2 channel (36). The Ca2+ dependence of [3H]ryanodine binding to SR membrane vesicles was also found to remain unchanged after the removal of FKBP12.6 (30). In this study we found that the EC50 values for Ca2+ and caffeine activation of [3H]ryanodine binding to RyR2 co-expressed with or without FKBP12.6 or treated with or without FK506 were indistinguishable (Figs. 2 and 3). We also found that the Ca2+ response of single RyR2 channels from FKBP12.6 null mice is virtually identical to that of single WT channels. Therefore, the removal of FKBP12.6 does not alter the sensitivity of the RyR2 channel to activation by Ca2+or caffeine.
The Significance of FKBP12.6 in SR Ca2+ Release
The significance of FKBP12.6 in SR Ca2+ release has been extensively studied, but its exact role remains largely undefined and controversial. Fleischer and co-workers (38, 39) found that FKBP12.0-stripped or FK590-treated canine skeletal muscle SR vesicles displayed a decreased rate of SR Ca2+ loading, suggesting an increased SR Ca2+ release. Surprisingly, the removal of FKBP12.6 or FK590 treatment had no effect on the rate of SR Ca2+ loading in canine cardiac microsomes (29, 30). Similarly, Prestle et al. (40) showed that rapamycin has no significant effect on the rate of SR Ca2+ uptake in rabbit cardiac myocytes. In contrast, Yano et al. (12) showed that the addition of FK506 to canine cardiac microsomes led to an increase in SR Ca2+ release. Controversies also exist with respect to the impact of FK506 on Ca2+ sparks. It has been shown that FK506 increased, decreased, or had no effect on the amplitude, frequency, or duration of Ca2+ sparks (37, 41–43). The reasons for these different results are unclear, but they could be caused by factors other than FKBP12.6. In this regard, Guo et al. (44) recently showed that rapamycin rapidly dissociated a fluorescent FKBP12 from RyR2 but did not alter the frequency of Ca2+ sparks in permeabilized rat ventricular myocytes, suggesting that the dissociation of FKBP12.6 from RyR2 is unlikely to affect the activity of RyR2. The effect of FK506 on stimulated Ca2+ transients is also inconsistent and may be species-dependent. FK506 increased the amplitude of stimulated Ca2+ transients in rat cardiac myocytes but decreased the amplitude of Ca2+ transients in rabbit cardiac myocytes (45). This difference is thought to be attributable to the different Na+/Ca2+ exchange activities between rat and rabbit cardiac cells (45). On the other hand, Dubell et al. (46) showed no change in Ca2+ transients after FK506 treatment in voltage-clamped rat cardiac cells. Instead, FK506 inhibited outward K+ currents and prolonged the action potential duration (46). Furthermore, Milting et al. (47) found that FK506 has no effect on the contractility of human and rabbit myocardium.
It should be noted that the FK506-induced impact on stimulated Ca2+ transients in both rat and rabbit cardiac cells is sustained, suggesting that FK506 is unlikely to alter only the activity of RyR2. This is because modest modulation of RyR2, for example, by caffeine or tetracaine, has only a transient effect on stimulated Ca2+ transients due to the autoregulation of Ca2+ release by the SR Ca2+ content (48, 49). In line with this view, FK506 has been shown to act on a number of targets in cardiac cells. It inhibits the Na+/Ca2+ exchange and the outward K+ currents (41, 46), the sarcolemmal Na+/K+ pump (50), and the SR Ca2+ pump (51) in addition to inhibiting the phosphatase calcineurin (52). Inhibition of the Na+/Ca2+ exchange current by FK506 is thought to partially account for the enhanced stimulated Ca2+ transients in rat cardiac myocytes by reducing Ca2+ extrusion and, thus, increasing the SR Ca2+ content (41). It is not clear whether this effect is mediated by FKBP12.6 or a direct effect of the drug FK506. Hence, FK506 (or rapamycin) affects multiple targets in cells.
The significance of FKBP12.6 in SR Ca2+ release has also been assessed by knocking out the FKBP12.6 gene in mice or overexpressing it in rabbit cardiac myocytes. The ablation of FKBP12.6 slightly increased the amplitude and prolonged the duration of Ca2+ sparks and enhanced the Ca2+ transients and Ca2+-indcued Ca2+ release gain in mouse cardiac myocytes (24). Hence, the lack of FKBP12.6 is unlikely to be associated with HF, which exhibits a decreased Ca2+-induced Ca2+ release gain (53). On the other hand, the overexpression of FKBP12.6 in rabbit cardiac myocytes decreased the amplitude, frequency, and duration of Ca2+ sparks but augmented stimulated Ca2+ transients and the magnitude of spontaneous Ca2+ waves (40, 42, 54). It is unclear why FKBP12.6 ablation and FKBP12.6 overexpression can both enhance stimulated Ca2+ transients. It should be also noted that FKBP12.6 KO and overexpression both resulted in a maintained impact on Ca2+ transients.
Although modulation of RyR2 has only a transient impact on stimulated Ca2+ transients, it has a maintained effect on spontaneous Ca2+ release (SOICR). Accordingly, in the present study we determined the impact of FK506 or FKBP12.6 on SOICR. We found that coexpression with or without FKBP12.6 did not affect the propensity for SOICR in HEK293 cells. We further demonstrated that FK506 treatment did not reduce the amplitude of SOICR either in HEK293 cells coexpressing RyR2 and FKBP12.6 or in rat cardiac myocytes, as would be expected if FK506 enhances the activity of RyR2. On the contrary, we found that FK506 slightly increased the amplitude and decreased the frequency of SOICR in HEK293 cells and in cardiac myocytes. Interestingly, this effect of FK506 on SOICR is similar to that of tetracaine (55), indicating that FK506 inhibits rather than enhances the activity of RyR2. Importantly, this effect of FK506 was also observed in HEK293 cells expressing RyR2 alone without FKBP12.6, indicating that the effect of FK506 on SOICR is independent of FKBP12.6. These data and those from the FKBP12.6 KO mouse studies indicate that the removal of FKBP12.6 does not increase the propensity for spontaneous Ca2+ release or SOICR, which is consistent with the observation that the dissociation of FKBP12.6 does not alter the conductance and activation of the RyR2 channels.
The Role of FKBP12.6 in Ventricular Arrhythmias
Because defective RyR2 function as a result of naturally occurring mutations is linked to ventricular arrhythmias, altered RyR2 regulation is potentially arrhythmogenic. Based on the observation that the removal of FKBP12.6 from RyR2 dramatically increases the sensitivity of the channel to Ca2+ activation and enhances SR Ca2+ leakage (9), which can lead to delayed after depolarizations (DADs) and triggered arrhythmias, the dissociation of FKBP12.6 itself is believed to trigger or to render the heart susceptible to cardiac arrhythmias (9). However, based on our observation that the removal of FKBP12.6 does not alter RyR2 function or the propensity for SOICR, which is a common defect of disease-linked RyR2 mutations, we expected that, unlike RyR2 mutations, the loss of FKBP12.6 would not result in ventricular arrhythmias. Consistent with this view, we found that mice lacking FKBP12.6 responded to epinephrine and caffeine challenge in a manner indistinguishable from the WT mice. Neither FKBP12.6-null nor WT mice developed ventricular arrhythmias after the injection of caffeine and epinephrine, although the same doses of epinephrine and caffeine induced ventricular tachycardia in mice harboring a Catecholaminergic Polymorphic Ventricular Tachycardia (CPVT) RyR2 mutation, R4496C. Marks and colleagues also generated an FKBP12.6-null mouse line and found no structural and ECG abnormalities or arrhythmias in these mice at rest, but they exhibited exercise-induced ventricular arrhythmias (11). On the other hand, our FKBP12.6-null mice showed mild, sex-dependent cardiac hypertrophy but exhibited no stress-induced VT (24). The reasons for this discrepancy are unknown. Differences in the genetic background may, in part, account for the different phenotypes. Further investigations, particularly a detailed functional characterization of FKBP12.6-null mice, will be required to understand the exact physiological role of FKBP12.6 in RyR2 and cardiac function.
Acknowledgments
We thank Astellas Pharma Inc. for providing the FK506 compound. We also thank Tina Vo for excellent technical assistance.
Footnotes
The abbreviations used are: SR, sarcoplasmic reticulum; EC, excitation-contraction; HF, heart failure; FKBP12.6, 12.6-kDa FK506-binding protein; PBS, phosphate-buffered saline; KRH, Krebs-Ringer-Hepes; WT, wild type; CHAPS, 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate; ECG, electrocardiogram; VT, ventricular tachycardia; SOICR, store overload-induced Ca2+ release.
This work was supported in part by National Institutes of Health Research Grants RO1HL75210 (to S. R. W. C.) and a grant from the Canadian Institutes of Health Research (to S. R. W. C., H. J. D., and A. M. G.).
References
- 1.Bers DM. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Ter Keurs HE, Boyden PA. Physiol Rev. 2007;87:457–506. doi: 10.1152/physrev.00011.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.George CH, Jundi H, Thomas NL, Fry DL, Lai FA. J Mol Cell Cardiol. 2007;42:34–50. doi: 10.1016/j.yjmcc.2006.08.115. [DOI] [PubMed] [Google Scholar]
- 4.Mohamed U, Napolitano C, Priori SG. J Cardiovasc Electrophysiol. 2007;18:791–797. doi: 10.1111/j.1540-8167.2007.00766.x. [DOI] [PubMed] [Google Scholar]
- 5.Hove-Madsen L, Llach A, Bayes-Genis A, Roura S, Rodriguez Font E, Aris A, Cinca J. Circulation. 2004;110:1358–1363. doi: 10.1161/01.CIR.0000141296.59876.87. [DOI] [PubMed] [Google Scholar]
- 6.Vest JA, Wehrens XHT, Reiken SR, Lehnart SE, Dobrev D, Chandra P, Danilo P, Ravens U, Rosen MR, Marks AR. Circulation. 2005;111:2025–2032. doi: 10.1161/01.CIR.0000162461.67140.4C. [DOI] [PubMed] [Google Scholar]
- 7.Yano M, Yamamoto T, Ikeda Y, Matsuzaki M. Nat Clin Pract Cardiovasc Med. 2006;3:43–52. doi: 10.1038/ncpcardio0419. [DOI] [PubMed] [Google Scholar]
- 8.Lam E, Martin MM, Timerman AP, Sabers C, Fleischer S, Lukas T, Abraham RT, O’Keefe SJ, O’Neill EA, Wiederrecht GJ. J Biol Chem. 1995;270:26511–26522. doi: 10.1074/jbc.270.44.26511. [DOI] [PubMed] [Google Scholar]
- 9.Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 10.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 11.Wehrens X, Lehnart S, Huang F, Vest J, Reiken S, Mohler P, Sun J, Guatimosim S, Song L, Rosemblit N, D’Armierto JM, Napolitan C, Memmi M, Priori SG, Lederer WJ, Marks AR. Cell. 2003;113:829–840. doi: 10.1016/s0092-8674(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 12.Yano M, Ono K, Ohkusa T, Suetsugu M, Kohno M, Hisaoka T, Kobayashi S, Hisamatsu Y, Yamamoto T, Noguchi N, Takasawa S, Okamoto H, Matsuzaki M. Circulation. 2000;102:2131–2136. doi: 10.1161/01.cir.102.17.2131. [DOI] [PubMed] [Google Scholar]
- 13.Lehnart SE, Terrenoire C, Reiken S, Wehrens XHT, Song LS, Tillman EJ, Mancarella S, Coromilas J, Lederer WJ, Kass RS, Marks AR. 2006;103:7906–7910. doi: 10.1073/pnas.0602133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaneko N. Drug Dev Res. 1994;33:429–438. [Google Scholar]
- 15.Kumagai K, Nakashima H, Gondo N, Saku K. J Cardiovasc Electrophysiol. 2003;14:880–884. doi: 10.1046/j.1540-8167.2003.03050.x. [DOI] [PubMed] [Google Scholar]
- 16.Wehrens XHT, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR. Science. 2004;304:292–296. doi: 10.1126/science.1094301. [DOI] [PubMed] [Google Scholar]
- 17.Ono K, Yano M, Ohkusa T, Kohno M, Hisaoka T, Tanigawa T, Kobayashi S, Matsuzaki M. Cardiovasc Res. 2000;48:323–331. doi: 10.1016/s0008-6363(00)00191-7. [DOI] [PubMed] [Google Scholar]
- 18.Yano M, Kobayashi S, Kohno M, Doi M, Tokuhisa T, Okuda S, Suetsugu M, Hisaoka T, Obayashi M, Ohkusa T, Kohno M, Matsuzaki M. Circulation. 2003;107:477–484. doi: 10.1161/01.cir.0000044917.74408.be. [DOI] [PubMed] [Google Scholar]
- 19.Hunt DJ, Jones PP, Wang R, Chen W, Bolstad J, Chen K, Shimoni Y, Chen SR. Biochem J. 2007;404:431–438. doi: 10.1042/BJ20070135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- 21.Xiao B, Jiang MT, Zhao M, Yang D, Sutherland C, Lai FA, Walsh MP, Warltier DC, Cheng H, Chen SRW. Circ Res. 2005;96:847–855. doi: 10.1161/01.RES.0000163276.26083.e8. [DOI] [PubMed] [Google Scholar]
- 22.Xiao B, Sutherland C, Walsh MP, Chen SRW. Circ Res. 2004;94:487–495. doi: 10.1161/01.RES.0000115945.89741.22. [DOI] [PubMed] [Google Scholar]
- 23.Stange M, Xu L, Balshaw D, Yamaguchi N, Meissner G. J Biol Chem. 2003;278:51693–51702. doi: 10.1074/jbc.M310406200. [DOI] [PubMed] [Google Scholar]
- 24.Xin HB, Senbonmatsu T, Cheng DS, Wang YX, Copello JA, Ji GJ, Collier ML, Deng KY, Jeyakumar LH, Magnuson MA, Inagami T, Kotlikoff MI, Fleischer S. Nature. 2002;416:334–338. doi: 10.1038/416334a. [DOI] [PubMed] [Google Scholar]
- 25.Li P, Chen SR. J Gen Physiol. 2001;118:33–44. doi: 10.1085/jgp.118.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimoni Y, Chuang M, Abel ED, Severson DL. 2004;555:345–354. doi: 10.1113/jphysiol.2003.055590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang D, Wang R, Xiao B, Kong H, Hunt DJ, Choi P, Zhang L, Chen SRW. Circ Res. 2005;97:1173–1181. doi: 10.1161/01.RES.0000192146.85173.4b. [DOI] [PubMed] [Google Scholar]
- 28.Fabiato A, Fabiato F. J Physiol Paris. 1979;75:463–505. [PubMed] [Google Scholar]
- 29.Barg S, Copello JA, Fleischer S. Am J Physiol. 1997;272:C1726–C1733. doi: 10.1152/ajpcell.1997.272.5.C1726. [DOI] [PubMed] [Google Scholar]
- 30.Timerman AP, Onoue H, Xin HB, Barg S, Copello J, Wiederrecht G, Fleischer S. J Biol Chem. 1996;271:20385–20391. doi: 10.1074/jbc.271.34.20385. [DOI] [PubMed] [Google Scholar]
- 31.Laemmli UK. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 32.Towbin H, Staehelin T, Gordon J. Proc Natl Acad Sci U S A. 1979;76:4350–4434. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Copello JA, Qi Y, Jeyakumar LH, Ogunbunmi E, Fleischer S. Cell Calcium. 2001;30:269–284. doi: 10.1054/ceca.2001.0235. [DOI] [PubMed] [Google Scholar]
- 34.Cerrone M, Colombi B, Santoro M, di Barletta MR, Scelsi M, Villani L, Napolitano C, Priori SG. Circ Res. 2005;96:e77–e82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 35.Liu N, Colombi B, Memmi M, Zissimopoulos S, Rizzi N, Negri S, Imbriani M, Napolitano C, Lai FA, Priori SG. Circ Res. 2006;99:292–298. doi: 10.1161/01.RES.0000235869.50747.e1. [DOI] [PubMed] [Google Scholar]
- 36.Kaftan E, Marks AR, Ehrlich BE. Circ Res. 1996;78:990–997. doi: 10.1161/01.res.78.6.990. [DOI] [PubMed] [Google Scholar]
- 37.Xiao RP, Valdivia HH, Bogdanov K, Valdivia C, Lakatta EG, Cheng H. 1997;500:343–354. doi: 10.1113/jphysiol.1997.sp022025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mayrleitner M, Timerman AP, Wiederrecht G, Fleischer S. Cell Calcium. 1994;15:99–108. doi: 10.1016/0143-4160(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 39.Timerman AP, Ogunbumni E, Freund E, Wiederrecht G, Marks AR, Fleischer S. J Biol Chem. 1993;268:22992–22999. [PubMed] [Google Scholar]
- 40.Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, Smith GL, Hasenfuss G. Circ Res. 2001;88:188–194. doi: 10.1161/01.res.88.2.188. [DOI] [PubMed] [Google Scholar]
- 41.McCall E, Li L, Satoh H, Shannon TR, Blatter LA, Bers DM. Circ Res. 1996;79:1110–1121. doi: 10.1161/01.res.79.6.1110. [DOI] [PubMed] [Google Scholar]
- 42.Gomez AM, Schuster I, Fauconnier J, Prestle J, Hasenfuss G, Richard S. Am J Physiol Heart Circ Physiol. 2004;287:1987–1993. doi: 10.1152/ajpheart.00409.2004. [DOI] [PubMed] [Google Scholar]
- 43.Lukyanenko V, Wiesner TF, Gyorke S. J Physiol (Lond) 1998;507:667–677. doi: 10.1111/j.1469-7793.1998.667bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo T, Huke S, Cornea RL, Fruen BR, Bers DM. Biophys J. 2007;92:2825. (abstr.) [Google Scholar]
- 45.Su Z, Sugishita K, Li F, Ritter M, Barry WH. J Pharmacol Exp Ther. 2003;304:334–341. doi: 10.1124/jpet.102.041210. [DOI] [PubMed] [Google Scholar]
- 46.duBell WH, Wright PA, Lederer WJ, Rogers TB. 1997;501:509–516. doi: 10.1111/j.1469-7793.1997.509bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milting H, Janssen PM, Wangemann T, Kögler H, Domeier E, Seidler T, Hakim K, Grapow M, Zeitz O, Prestle J, Zerkowski HR. Eur J Pharmacol. 2001;430:299–304. doi: 10.1016/s0014-2999(01)01387-5. [DOI] [PubMed] [Google Scholar]
- 48.Eisner DA, Trafford AW, Diaz ME, Overend CL, O’Neill SC. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 49.Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Gyorke S. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lea JP, Sands JM, McMahon SJ, Tumlin JA. Kidney Int. 1994;46:647–652. doi: 10.1038/ki.1994.317. [DOI] [PubMed] [Google Scholar]
- 51.Bultynck G, De Smet P, Weidema AF, Ver Heyen M, Maes K, Callewaert G, Missiaen L, Parys JB, De Smedt H. J Physiol (Lond) 2000;525:681–693. doi: 10.1111/j.1469-7793.2000.t01-1-00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chelu MG, Danila CI, Gilman CP, Hamilton SL. Trends Cardiovasc Med. 2004;14:227–234. doi: 10.1016/j.tcm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 53.Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, McCune SA, Altschuld RA, Lederer WJ. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- 54.Loughrey CM, Seidler T, Miller SL, Prestle J, MacEachern KE, Reynolds DF, Hasenfuss G, Smith GL. J Physiol (Lond) 2004;556:919–934. doi: 10.1113/jphysiol.2003.057166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Overend CL, O’Neill SC, Eisner DA. J Physiol (Lond) 1998;507:759–769. doi: 10.1111/j.1469-7793.1998.759bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]