Abstract
Oseltamivir (Tamiflu) is now being stockpiled by several governments as a first line treatment for an anticipated outbreak of avian influenza caused by H5N1. However, abnormal behaviors and death associated with the use of Tamiflu has developed into a major issue in Japan where Tamiflu is often prescribed for seasonal influenza. Thus, it is critical to determine neuropsychiatric effects of oseltamivir and to establish methods for safe administration. Using juvenile rats and rat hippocampal slices, we investigated whether oseltamivir has adverse effects on the central nervous system. Systemic injection of oseltamivir (50mg/kg i.p.) produced no change in behavior within 2 hours. However, prior injection of oseltamivir significantly altered the duration of loss of lightning reflex following ethanol injection (3.3 g/kg, i.p.). Ethanol injection in the presence of oseltamivir also resulted in enhanced hypothermia. In the CA1 region of hippocampal slices, oseltamivir (100 μM) induced paired pulse facilitation in population spikes without changes in excitatory postsynaptic potentials. Similarly, 3 μM oseltamivir carboxylate, the active metabolite of oseltamivir, facilitated neuronal firing, though the facilitation did not involve GABAergic disinhibition. Moreover, oseltamivir carboxylate produced further facilitation following administration of 60 mM ethanol. These findings indicate that oseltamivir has effects on the central nervous system, especially when combined with other agents.
Keywords: Tamiflu, oseltamivir, abnormal behaviors, adverse reaction, drug interaction, avian influenza
An outbreak of avian influenza caused by H5N1, which may kill millions of people worldwide, is expected in the near future. To minimize the risk, governments of several countries are stockpiling oseltamivir (Tamiflu®), an antiviral agent. However, the safety of oseltamivir is questioned because accidental deaths and altered behaviors have been reported after Tamiflu ingestion (www.forbes.com/feeds/ap/2007/04/04/ap3582952.html, www.nature.com/news/2007/070319/full/446358a.html). Tamiflu has been regularly prescribed as a treatment for seasonal influenza in Japan and Japanese consumption accounts for up to 70–80% of all Tamiflu use worldwide. Importantly, 70 deaths and more than 100 cases of abnormal behaviors have been reported associated with Tamiflu use in Japan, with special attention to cases involving children and adolescents. Serious effects include sudden deaths in toddlers and altered behaviors, including suicide, in teenagers. Mechanisms responsible for the altered behaviors are not certain but have prompted the use of animal models to determine potential adverse actions. Oseltamivir is metabolized to oseltamivir carboxylate (OCB) [2] and other metabolites in the body [15]. Although different sensitivities to OCB among races have been suggested [9], there is no literature describing neuronal actions of oseltamivir or OCB. It is possible that OCB has effects on the central nervous system (CNS) because neuraminidase, a key enzyme inhibited by OCB, plays a role in CNS development and impulse conduction [1, 13]. Following inhibition of neuraminidase, certain drugs that alter neuronal function may exhibit augmented CNS effects. Although it is believed that oseltamivir and OCB do not readily pass the blood brain barrier (BBB), high doses damage the brain in experimental animals (http://www.fda.gov/cder/foi/label/2006/021087s033lbl.pdf), suggesting permeability of BBB to OCB. OCB is also likely to reach the CNS if the BBB is immature or impaired. Furthermore, BBB permeability to OCB could be enhanced by the presence of solvents such as alcohol, which is known to increase BBB permeability to other agents, such as horseradish peroxidase [14]. This also raises the possibility that it the putative effects of Tamiflu result from interactions with other CNS-active drugs. Large numbers of Japanese teenagers consume alcohol, though use of other abused drugs is less common [17]. In the present study, we examined whether oseltamivir alters the effects of alcohol when systemically administered to rats. We also determined whether oseltamivir and OCB have neuronal effects in rat hippocampal slices in the presence or absence of ethanol.
All experiments were performed in accordance with the guidelines of the Washington University Animal Study Committee. Every effort was made to minimize the number of animals used and their suffering in all experimental procedures.
To determine acute sensitivity to ethanol, male albino rats (postnatal day 30 ± 2) were administered single injections of ethanol (3.3 g/kg, i.p. as 26% v/v in saline). Two hours before ethanol injection, saline or oseltamivir in saline (2% volume of body weight) was injected (i.p.). After being sedated by ethanol, rats were placed on their backs and the time required for them to right themselves spontaneously was monitored [5, 19]. Because prior saline injection alters the duration of loss of lighting reflex (LORR), we tested various doses of ethanol in the present study and found that 3.3 g/kg is the most suitable at this age. Rectal temperature was measured every hour.
Hippocampal slices were prepared from male albino rats at postnatal day 31 ± 3. Under isoflurane anesthesia, animals were decapitated and hippocampi were quickly dissected into gassed (95 % O2 - 5 % CO2) artificial cerebrospinal fluid (ACSF) containing (in mM): NaCl 124, KCl 5, CaCl2 2, NaHCO3 22, NaH2PO4 1.25, MgSO4 2 and glucose 10 at 4–6°C [20]. Transverse slices (500 μm thick) were cut from the septal half of the hippocampus with a vibrotome (WPI, Sarasota, FL, USA). After at least 1 hour at 30°C in standard ACSF, individual slices were transferred to a recording chamber and perfused continuously (2 ml/min) with fresh solution at 30°C. Evoked synaptic responses were elicited with 0.1–0.2 msec constant current pulses through a bipolar electrode placed in the Schaffer collateral-commissural pathway using a Grass S88 stimulator with SIU5 stimulus isolation unit (Grass, Astro-Med, Inc. West Warwick, RI, USA).
Extracellular recordings were obtained from the pyramidal cell layer and dendritic area of the CA1 region using 5–10 MΩ glass electrodes filled with 2 M NaCl. The electrode for recording population spikes (PSs) was positioned in the pyramidal cell layer such that the latency of the negative peak was the shortest and the peak of the second positive phase after the PS was at its apex. Using this approach, the peak of the positive wave was not masked by the negative PS and gave an accurate measure of the amplitude of the somatic excitatory postsynaptic potential (EPSP). This allowed us to measure somatic EPSPs as the maximal height of the positive wave in the cell body layer compared to baseline. Although the initial slope of the somatic EPSP may better reflect the conducted synaptic input from the dendrites, PSs evoked by stronger stimuli can mask the initial slope making it difficult to measure. Data were discarded when somatic EPSP amplitude was suppressed by PSs with increasing stimulus intensity. PS amplitude was measured as the height from the apex of the first positive peak to the most negative point of the spike (Fig. 1A). Dendritic EPSPs were measured as the maximal slope. In paired-pulse experiments, we compared changes in somatic EPSPs and PSs with changes in dendritic EPSPs by delivering two pulses of fixed intensity at intervals of 21 or 42 msec. Waveforms were monitored by applying two pulses at an interval of 21 msec every minute at an intensity that evoked a 20–40% maximal 2nd PS based on a baseline input-output curve taken before each experiment. In preliminary studies, 30 μM 6-cyano-7-nitroquinoxaline-2, 3-dione (CNQX), a non- N-methyl-D-aspartic acid (NMDA) receptor antagonist, totally eliminated the responses, indicating that the observed responses are not contaminated with responses other than non-NMDA excitatory amino acid receptors.
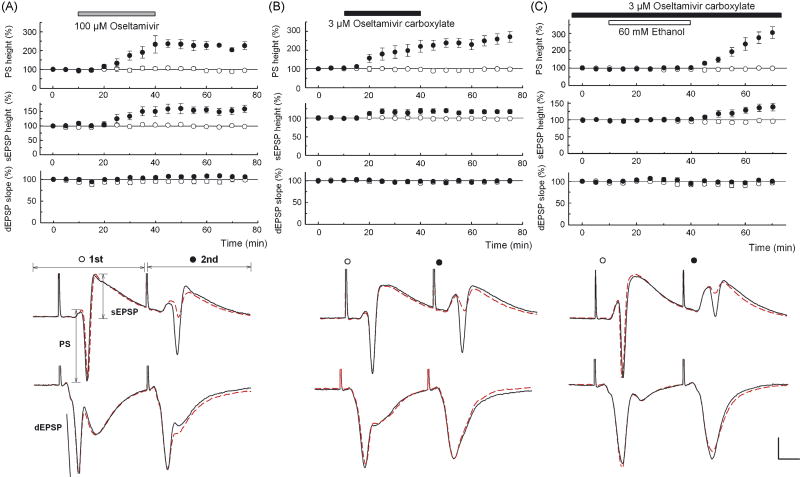
Figure 1.
A, B. Oseltamivir (100 μM, A) and 3 μM OCB (B) facilitated 2nd PS and 2nd somatic EPSPs (sEPSPs) with little change in other parameters. C. In slices preincubated with 3 μM OCB for 2 hours, 60 mM ethanol (open bar) did not change PSs, sEPSPs or dendritic EPSPs (dEPSPs). However, following washout of ethanol, 2nd PS and 2nd sEPSPs were augmented. Traces depict PSs (top) and dEPSPs (bottom in each panel) recorded 10 min before (dotted lines) and 30 min after (solid lines) drug administration (A, B) or 30 min after washout of ethanol (C). Scale: 1 mV, 5 msec.
For studies examining the involvement of gamma-aminobutyric acid (GABA)A receptors, picrotoxin (PTX) was administered to slices cut between CA1 and CA3. PTX was dissolved in ethanol as a 20 mM stock solution and diluted to 1 μM. Although PTX dissolved in DMSO has been used at 50 μM, we found that 1 μM PTX is sufficient to diminish GABAergic inhibition in the CA1 region without eliciting epileptiform discharges if dissolved in ethanol [11]. However, at a higher concentration (3 μM), PTX elicited epileptiform discharges that made it difficult to analyze synaptic waveforms.
The test solution of oseltamivir was prepared by dissolving a Tamiflu tablet (75 mg) in water or in saline. OCB was obtained from Toronto Research Chemicals Inc. (North York, ON. M3J2J8, Canada). Other chemicals were obtained from Sigma-Aldrich (St. Louis, MO 63178, USA).
To determine whether oseltamivir alters animal behavior, we examined systemic injections of the drug in juvenile rats in the absence and presence of ethanol. Intraperitoneal injections of oseltamivir (50 mg/kg) produced no significant change in behavior within 2 hours (N = 14). However, in rats also treated with ethanol, oseltamivir resulted in a dramatic decrease in ethanol-induced sedation. Control rats that received saline 2 hours prior to ethanol (3.3 g/kg, i.p.) exhibited an immediate LORR that lasted 63.1 ± 3.3 min (N = 5). Rats pretreated with oseltamivir exhibited a markedly shortened time to initial awaking (26.6 ± 11.0 min, P < 0.05, N = 8) and no LORR was observed in three rats. Additionally, rectal temperatures measured one hour after ethanol were significantly lower in rats treated with oseltamivir (see Supplement). This suggests that oseltamivir can modulate the actions of CNS drugs such as ethanol, even if oseltamivir alone does not cause behavioral changes.
We subsequently examined whether oseltamivir alters neuronal function in the CA1 region of rat hippocampal slices using a paired pulse stimulation paradigm to determine effects on synaptic transmission and neuronal firing. When Schaffer collateral inputs to CA1 are stimulated at a 21 msec interval, paired-pulse facilitation of dendritic EPSPs is typically observed. This facilitationis accompanied by paired-pulse depression of somatic EPSPs and PSs, reflecting diminished propagation of excitatory inputs to the soma and diminished pyramidal cell firing. Administration of 100 μM oseltamivir for 30 min gradually enhanced 2nd PSs and this facilitation lasted 30 min after washout without affecting 1st PSs (Fig. 1A). Although dendritic EPSPs were not affected, 2nd but not 1st somatic EPSPs were also facilitated, suggesting changes in propagation of inputs from synapses to soma during paired stimulation. Administration of 30 μM oseltamivir partially facilitated 2nd PSs (N = 3), but effects were unclear at 10 μM (N = 3). Administration of 3 μM OCB, the active metabolite, facilitated 2nd PSs (N = 5, Fig. 1B) and 2nd somatic EPSPs without affecting other parameters, suggesting that OCB is responsible for the enhanced excitability. At 1 μM, OCB failed to facilitate 2nd PSs (N = 3). The facilitation of 2nd PSs and 2nd somatic EPSPs by 3 μM OCB was observed even when the interval of stimulation was lengthened to 42 msec.
In follow up to our behavioral studies, we also examined interactions with ethanol in slices pretreated with 3 μM OCB. No changes in EPSPs or PSs were observed during administration of 60 mM ethanol. However, after washout of ethanol, facilitation in 2nd PSs (N = 8, Fig. 1C) accompanied by an increase in 2nd somatic EPSPs was observed without change in other parameters. In naïve slices, all parameters remained stable during and after administration of 60 mM ethanol (N = 5, P < 0.01 vs. OCB-treated).
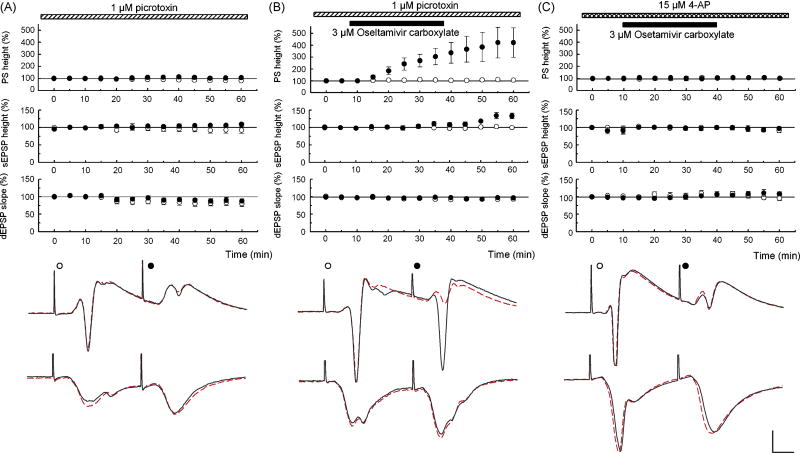
The changes in PSs and somatic EPSPs could reflect effects on GABAergic inhibition and/or conduction of synaptic responses from dendrites to soma [11, 12]. To determine whether effects on GABA-mediated inhibition are relevant, we pretreated slices with 1 μM PTX to block GABAA receptors. In these slices, 3 μM OCB further facilitated 2nd PS (N = 5, Fig. 2B) and 2nd somatic EPSPs. In slices treated with PTX alone, all parameters were stable (N = 5, Fig. 2A). The failure of PTX to mask the facilitating action of OCB indicates that OCB does not produce GABAergic disinhibition. To test the involvement of A-type potassium channels that are highly expressed in dendrites [4, 10], slices were treated with 15 μM 4-aminopyridine (4-AP), an A-type potassium channel blocker. Administration of 4-AP facilitated PSs and somatic EPSPs, consistent with enhanced dendritic propagation. Forty min after introduction of 4-AP, 3 μM OCB was administered, but failed to further facilitate 2nd PSs (N = 5, Fig. 2C) or 2nd EPSPs, suggesting that 4-AP and OCB share mechanisms to facilitate neuronal activity.
Figure 2.
A. PTX (1 μM) was administered starting 40 min before time 0. PSs, somatic EPSPs (sEPSPs) and dendritic EPSPs (dEPSPs) evoked by the first (open circles) and second pulses (filled circles) are stable during PTX. B. In PTX, 3 μM OCB facilitated 2nd PSs and sEPSPs without altering other parameters. C. 4-AP (15 μM) was administered starting 40 min before time 0. Unlike PTX, all parameters were stable with 3 μM OCB in the presence of 4-AP. Traces depict PSs (top) and dEPSPs (bottom) at time 0 (dotted lines) and 60 min (solid lines). Scale: 1 mV, 5 msec.
Although it is believed that oseltamivir and OCB do not readily cross the BBB, they may reach the CNS if combined with other drugs or if the BBB is impaired. It is also possible that other metabolites derived from oseltamivir have actions in the CNS. Our observations in brain slices show that OCB has clear effects on neuronal excitability. Neuraminidase, the enzyme targeted by OCB, can modulate synaptic function [3] and possibly alter propagation of signals based on the fact that many ion channels are glycosylated [6, 16]. Alterations in signal propagation may be responsible for the facilitation of the 2nd PSs because we observed dissociation between enhanced somatic EPSPs and unchanged dendritic EPSPs during administration of OCB, reflecting a form of neuronal hyperexcitability. Like OCB, but to a lesser extent, ethanol may also inactivate neuraminidase [7]. In addition, ethanol reduces gangliosides through depletion of sialic acid, and this may contribute to excitatory effects when sialidase (neuraminidase) is inactivated [8]. The rebound increase in 2nd PSs following ethanol administration with OCB may thus represent a correlate underlying behavioral changes associated with oseltamivir use. It is plausible that medications containing ethanol or CNS stimulants simultaneously taken with antiviral agents contribute to behavioral changes. Ethanol alone can lower body temperature [18]. However, we observed further depression of body temperature with oseltamivir. Even if antiviral agents do not cause psychosis by themselves, it is possible that they modulate the CNS effects of other drugs when taken together. Besides ethanol, caffeine, ephedrine and codeine could be candidates. Because of concerns over an impending pan-epidemic of avian flu and the belief that oseltamivir is relatively safe, there is an urgent need to understand factors contributing to the altered behavior and suicides associated with antiviral drug use.
Supplementary Material
Acknowledgments
This work was supported in part by NIH grants MH077791, NS057105, AA12951 and AG184334.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crain SM, Shen KF. Neuraminidase inhibitor, oseltamivir blocks GM1 ganglioside-regulated excitatory opioid receptor-mediated hyperalgesia, enhances opioid analgesia and attenuates tolerance in mice. Brain Res. 2004;995:260–266. doi: 10.1016/j.brainres.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 2.He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64–0802. Clin Pharmacokinet. 1999;37:471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- 3.Hipp FX, Gielen W, Davies MA, Hinzen DH. Blocking action of intracellularly injected neuraminidase on central synapses in vivo. Pflugers Arch. 1980;385:45–50. doi: 10.1007/BF00583914. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman DA, Magee JC, Colbert CM, Johnston DK. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 5.Izumi Y, Kitabayashi R, Funatsu M, Izumi M, Zorumski CF. A single day of ethanol exposure during development has persistent effects on bi-directional plasticity, N-methyl-D-aspartate receptor function and ethanol sensitivity. Neuroscience. 2005;136:269–279. doi: 10.1016/j.neuroscience.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Johnson D, Montpetit ML, Stocker PJ, Bennett ES. The sialic acid component of the beta1 subunit modulates voltage-gated sodium channel function. J Biol Chem. 2004;279:44303–44310. doi: 10.1074/jbc.M408900200. [DOI] [PubMed] [Google Scholar]
- 7.Kishore GS, Carubelli R. Effect of acute ethanolic intoxication on the neuraminidase activity of rat liver Golgi apparatus. Biochim Biophys Acta. 1977;497:101–111. doi: 10.1016/0304-4165(77)90142-8. [DOI] [PubMed] [Google Scholar]
- 8.Klemm WR, Mathew J, Maring RG. Acute alcohol decreases gangliosides in mouse brain. Alcohol. 1988;5:215–219. doi: 10.1016/0741-8329(88)90055-9. [DOI] [PubMed] [Google Scholar]
- 9.Li CY, Yu Q, Ye ZQ, Sun Y, He Q, Li XM, Zhang W, Luo J, Gu X, Zheng X, Wei L. A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential link with severe adverse reactions to oseltamivir. Cell Res. 2007;17:357–362. doi: 10.1038/cr.2007.27. [DOI] [PubMed] [Google Scholar]
- 10.Magee JC, Carruth M. Dendritic voltage-gated ion channels regulate the action potential firing mode of hippocampal CA1 pyramidal neurons. J Neurophysiol. 1999;82:1895–1901. doi: 10.1152/jn.1999.82.4.1895. [DOI] [PubMed] [Google Scholar]
- 11.Murayama K, Zorumski CF, Izumi Y. Effects of neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one on ethanol-mediated paired-pulse depression of population spikes in the CA1 region of rat hippocampal slices. Neurosci Lett. 2006;394:28–32. doi: 10.1016/j.neulet.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 12.Nagashima K, Zorumski CF, Izumi Y. Nitrous oxide (laughing gas) facilitates excitability in rat hippocampal slices through gamma-aminobutyric acid A receptor-mediated disinhibition. Anesthesiology. 2005;102:230–234. doi: 10.1097/00000542-200501000-00034. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez JA, Piddini E, Hasegawa T, Miyagi T, Dotti CG. Plasma membrane ganglioside sialidase regulates axonal growth and regeneration in hippocampal neurons in culture. J Neurosci. 2001;21:8387–8395. doi: 10.1523/JNEUROSCI.21-21-08387.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart PA, Hayakawa EM, Carlen PL. Ethanol and pentobarbital in combination increase blood-brain barrier permeability to horseradish peroxidase. Brain Res. 1988;443:12–20. doi: 10.1016/0006-8993(88)91593-4. [DOI] [PubMed] [Google Scholar]
- 15.Sweeny DJ, Lynch G, Bidgood AM, Lew W, Wang KY, Cundy KC. Metabolism of the influenza neuraminidase inhibitor prodrug oseltamivir in the rat. Drug Metab Dispos. 2000;28:737–741. [PubMed] [Google Scholar]
- 16.Tyrrell L, Renganathan M, Dib-Hajj SD, Waxman SG. Glycosylation alters steady-state inactivation of sodium channel Nav1.9/NaN in dorsal root ganglion neurons and is developmentally regulated. J Neurosci. 2001;21:9629–9637. doi: 10.1523/JNEUROSCI.21-24-09629.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wada K, Price RK, Fukui S. Reflecting adult drinking culture: prevalence of alcohol use and drinking situations among Japanese junior high school students in Japan. J Stud Alcohol. 1998;59:381–386. doi: 10.15288/jsa.1998.59.381. [DOI] [PubMed] [Google Scholar]
- 18.Wilson E, Waring WS. Severe hypotension and hypothermia caused by acute ethanol toxicity. Emerg Med J. 2007;24:e7. doi: 10.1136/emj.2006.041590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res. 1998;22:914–920. [PubMed] [Google Scholar]
- 20.Zorumski CF, Mennerick S, Izumi Y. Assesment of synaptic effects of nitric oxide in hippocampal neurons. In: Maines, editor. Methods in Neurosciences: Form Nitric oxide synthase: characterization and functional analysis. Vol. 31. Academic Press; San Diego: 1996. pp. 283–299. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.