Abstract
Chikungunya virus (CHIKV) is an alphavirus responsible for a number of large outbreaks. Here we describe the efficient incorporation of CHIKV envelope glycoproteins into lentiviral and rhabdoviral particles. Vectors pseudotyped with CHIKV envelope proteins efficiently transduced many cell types from different species. However, hematopoietic cell types were either partially or completely refractory. A mutation in E1 (A226V) has been linked with expansion of tropism for mosquito species, although differences in in vitro infection of mosquito cell lines have not been noted. However, pseudovirion infectivity assays detected subtle differences in infection of mosquito cells, suggesting an explanation for the changes in mosquito tropism. The presence of C-type lectins increased CHIKV pseudotyped vector infectivity, but not infection of refractory cells, suggesting that they act as attachment factors rather than primary receptors. CHIKV pseudotypes will serve as an important tool for the study of neutralizing antibodies and the analysis of envelope glycoprotein functions.
Keywords: Chikungunya, alphavirus, glycoproteins, lectins, tropism, entry
INTRODUCTION
Chikungunya virus (CHIKV) is an alphavirus belonging to the Togaviridae family and was first isolated in 1952 from a febrile individual in Tanzania (Peters C, 1990; Strauss, 1986). CHIKV is transmitted to humans by several species of mosquitoes, with Aedes aegypti and Ae. albopictus being the two main vectors. Acute infection is characterized by a painful polyarthralgia, high fever, asthenia, headache, vomiting, rash and myalgia (Bodenmann and Genton, 2006; Peters C, 1990; Pialoux et al., 2007). In many patients, a chronic and incapacitating arthralgia persists for months. During the last 50 years, there have been numerous CHIKV outbreaks in East and Southern Africa and in Southeast Asia (Schuffenecker et al., 2006). Though generally not fatal, CHIKV has sickened 1.6 million people in the Indian Ocean region since 2005 (Charrel, de Lamballerie, and Raoult, 2007). Three distinct phylogroups have been described based on E1 sequence of different CHIKV isolates, and microevolution of virus strains has been associated with infection outbreaks (Schuffenecker et al., 2006). In particular, changes in the envelope glycoproteins (gps) of the virus have been described that affect infectivity in different mosquito species. A single change at position 226 of E1 protein has been associated with the adaptation of the virus to more efficiently infect and be transmitted by Ae. albopictus, which is thought to be the major vector of the large scale CHIKV epidemic on La Reunion Island (Tsetsarkin et al., 2007; Vazeille et al., 2007), as well as a recent outbreak in Italy (Bonilauri et al., 2008). This change has also been shown to modulate cholesterol requirement for infection of insect cells by CHIKV (Tsetsarkin et al., 2007), as well as other alphaviruses (Ahn et al., 1999; Lu, Cassese, and Kielian, 1999). Whether there is a correlation between cholesterol requirements and CHIKV fitness increase in Ae. albopictus remains unknown. The adaptation of the virus to the broadly distributed mosquito species Ae. albopictus increases the potential of CHIKV to extend its range further into Europe and America (Tsetsarkin et al., 2007).
Alphaviruses are small enveloped, single stranded, positive polarity RNA viruses (Peters C, 1990). They attach to poorly characterized receptors on many different cell types in various species. Viral entry generally occurs through receptor-mediated endocytosis, and fusion is dependent on endosomal acidification (DeTulleo and Kirchhausen, 1998; Kolokoltsov, Fleming, and Davey, 2006; Marsh and Helenius, 2006; Marsh, Kielian, and Helenius, 1984). CHIKV particles contain three structural proteins: glycosylated El and E2 envelope proteins, embedded in the viral membrane, and a non-glycosylated nucleocapsid protein. Based on similarity to other alphaviruses, E2 mediates receptor attachment, while E1 is a class II viral fusion protein. A third glycoprotein, E3, is not associated with mature virions but released into culture fluids (Simizu et al., 1984), while 6K protein, a membrane-associated peptide created by cleavage of the polyprotein to release E2 and E1, is incorporated into particles at a low level (Gaedigk-Nitschko and Schlesinger, 1990; Lusa, Garoff, and Liljestrom, 1991). There is a critical lack of knowledge of CHIKV biology, contrasting with related model alphaviruses like Sindbis virus (SINV), Semliki Forest virus (SFV), and Ross River virus (RRV). In particular, little is known about the interaction of CHIKV (and of most alphaviruses) with human primary cells. CHIK virus isolates from La Reunion outbreak have been described to infect in vitro primary fibroblasts, and, to a lower extent monocyte-derived macrophages, but not primary periferal blood mononuclear cells, primary lymphocytes and monocytes, nor monocyte-derived dendritic cells (Sourisseau et al., 2007). Studies using live CHIKV have been limited partially because they must be performed at biosafety level 3 (Anon., 2007).
Pseudotyped viruses have important applications as tools for the study of viral glycoprotein function and attachment/entry processes. They can also be used to analyze immune responses and the development of neutralizing antibodies. Furthermore, They can have utility as vaccines, as well as vectors for gene transfer and gene therapy. In particular, due to CHIKV’s apparent preference for muscle satellite cells (Ozden et al., 2007), CHIKV may be useful for therapeutic gene replacement of muscle disease such as muscular dystrophy. Pseudotyped viruses incorporate heterologous viral gps thus acquiring the host range of the virus from which these gps are derived. Early steps in infection, such as receptor binding, membrane fusion, and entry, are determined solely by properties of the nonrelated envelope protein. Also virus envelope gps are the primary targets of neutralizing antibody responses. The epitopes recognized differ sufficiently between virus subtypes and species to distinguish viruses and provide an important basis for disease diagnosis.
Here we describe the successful development and validation of CHIKV pseudovirions. We have assessed CHIKV protein incorporation as well as sensitivity to sera from CHIKV infected individuals. Recapitulation of the cell tropism of the pseudovirions has been performed allowing us to describe several refractory cell lines. We also demonstrate that mutations in E1 glycoprotein mediate cellular tropism differences in mosquito cells. These pseudovirions permit analysis of the host range and function of the Chikungunya virus gps allowing future work that will help to elucidate the pathogenesis and tropism of this as yet poorly characterized virus. They will also improve laboratory safety and security as they can be handled at biosafety level 2.
RESULTS
Chikungunya envelope glycoproteins are incorporated into lentiviral pseudoparticles
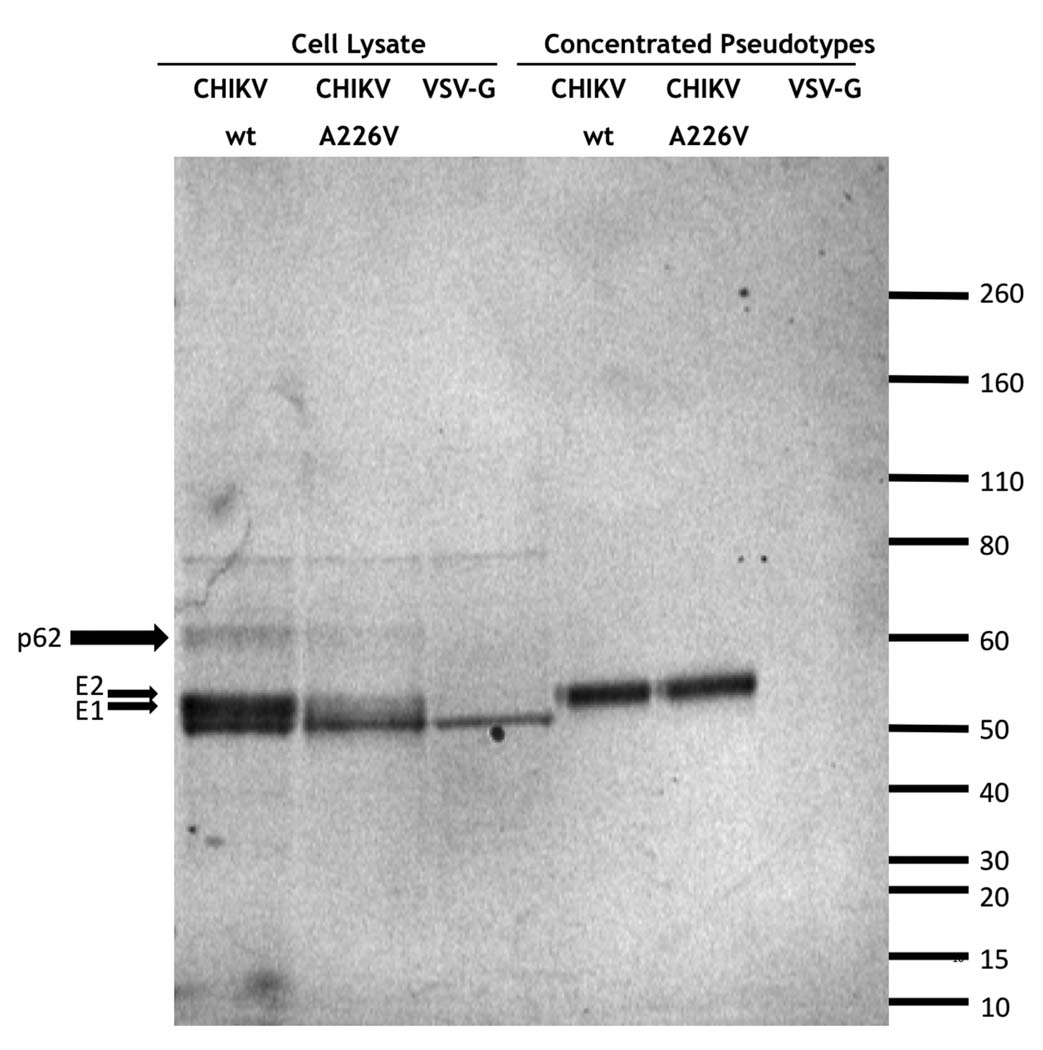
In order to determine if Chikungunya virus (CHIKV) envelope gps were incorporated into HIV pseudoparticles, 293T cells were transfected with pNL-luc, a lentiviral vector encoding luciferase, and a plasmid encoding the CHIKV envelope proteins with and without a point mutation in E1 (A226V) involved in mosquito tropism changes (Tsetsarkin et al., 2007). As controls, 293T cells were also transfected with plasmids encoding vesicular stomatitis virus (VSV) G protein and the pNL-luc vector. After 40 h, the virions obtained were concentrated by ultracentrifugation and the transfected cells were lysed. Both the concentrated virions and the cell lysates were analyzed by western-blot using a mix of 3 different sera from CHIKV infected individuals. CHIKV proteins E1 (52 kDa) and E2 (56 kDa) (that nearly co-migrate in the gel) as well as the p62 (62kDa) precursor protein were detected in both wild type and A226V mutant cell lysates but not in the control VSV-G cell lysate (Fig. 1). Two nonspecific bands of approximately 80 and 50 kDa respectively were also observed in all the cell lysates samples (Fig. 1). Similar amounts of CHIKV envelope gps were also detected in the concentrated wild type and mutant CHIKV pseudoparticles indicating that lentiviral particle production was roughly equivalent in both transfections (Fig. 1A). Thus, CHIKV gps are efficiently produced in 293T cells and are successfully incorporated into the HIV pseudoparticles. Also, the result demonstrates that the CHIKV proteins incorporated into the pseudoparticles are efficiently recognized by antibodies present in the sera of individuals with a prior history of CHIKV infection. Additionally, immunofluorescence studies using anti-CHIKV positive sera on live cells suggested the CHIKV gps were efficiently expressed on the surface of transfected cells (data not shown). Incorporation into murine leukemia virus (MLV)-based retroviral particles was also demonstrated with titers in RD muscle cell line of 9.8 ×106 FFU/ml for CHIKV envelope and 1.3 ×107 FFU/ml for VSV-G.
FIG. 1.
Chikungunya virus envelope protein expression. Western-blot were performed using a mix of human sera from CHIKV infected individuals to detect protein expression in pseudotype producer 293T cell lysates and concentrated HIV-pseudotypes bearing CHIKV wt and E1A226V mutant envelope glycoproteins and VSV G protein. The positions of molecular mass markers (kDa) are shown on the right.
CHIKV pseudovirus infection is inhibited by sera from infected individuals
As expected from previous studies with replication competent CHIKV (Ozden et al., 2007), pseudovirions carrying CHIKV envelope efficiently infected the muscle derived RD cells (1.4 × 107 FFU/ml). To further characterize the specificity of CHIKV pseudotype infection, we tested the ability of sera from individuals previously infected with CHIKV to neutralize wild type and A226V E1 mutant pseudovirion infectivity in RD cells. Similar titers of wild type and mutant CHIKV pseudovirions (15 pg/ml) were incubated with either serum from 9 different infected individuals or a control serum from an uninfected individual at various dilutions and then used to infect RD cells. All sera from CHIKV infected patients were able to inhibit RD cell infection by either wild type or mutant envelope pseudotypes with similar efficiency (Table 1 and Supplementary Fig. 1). Inhibition was dose dependent whereas a negative control serum had little effect, although partial neutralization was seen at the highest concentration (Table 1 and Supplementary Fig. 1). Similar data was obtained with four additional, geographically diverse, negative control sera (data not shown). No neutralization was seen with CHIKV patient serum using control pseudotypes carrying VSV-G (data not shown). These results demonstrate that entry of the pseudotyped viruses is mediated by the CHIKV gps and validates their utility to evaluate CHIKV antibody neutralization capacity.
TABLE 1.
Neutralization of CHIKV pseudovirions
| CHIK WTa | CHIK A226Va | |||
|---|---|---|---|---|
| Sera | ||||
| IC50 | IC90 | IC50 | IC90 | |
| 1 | 3030 | 455 | 4348 | 526 |
| 2 | 3125 | 417 | 3846 | 500 |
| 3 | 3125 | 455 | 3704 | 500 |
| 3317 | 6250 | 833 | 7692 | 1000 |
| 4102 | 4545 | 588 | 6250 | 714 |
| 4743 | 6667 | 833 | 8333 | 1000 |
| 92869 | 909 | 71 | 1000 | 71 |
| 92958 | 2083 | 133 | 1818 | 137 |
| 93137 | 2128 | 161 | 2564 | 133 |
| Control b | 56 | <30 | 38 | <30 |
The values represent the serum dilutions interpolated from the dose-response curves as inhibiting 50 or 90% of luciferase read-out relative to control assays carried out in the absence of serum. < indicates no neutralization at the lowest dilution of serum tested (1 in 30).
Representative uninfected negative control serum
CHIKV pseudovirus infection is inhibited by lysosomotropic agents
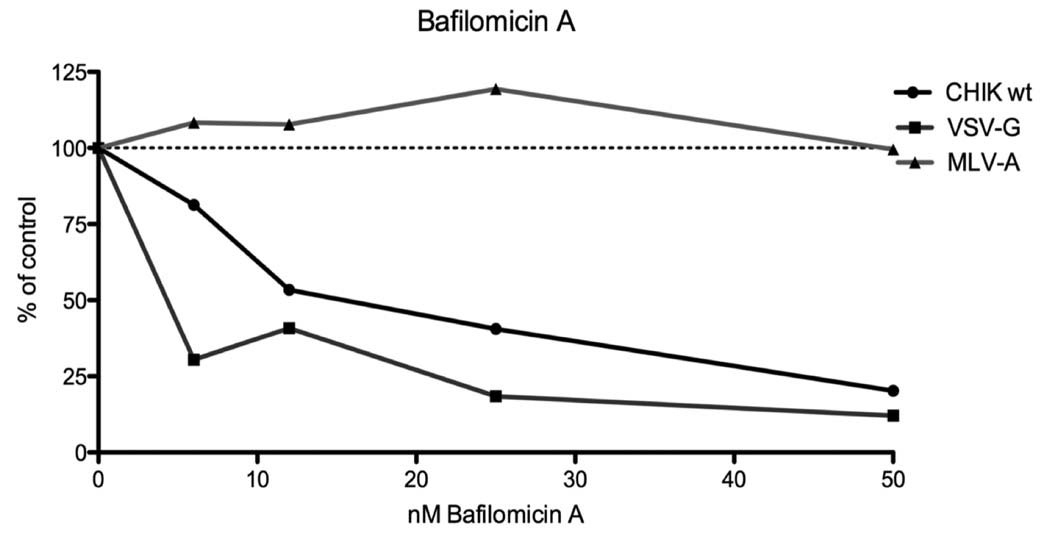
The effects of lysosomotropic agents in pseudovirion infection were studied to further analyze the CHIKV envelope mediated entry process. These agents are known to raise the pH of the endosomal compartment, efficiently blocking infection by viruses dependent on a low-pH step for entry (Marsh and Helenius, 1989; Miller and Lenard, 1980; Ohkuma and Poole, 1978). We chose RD cells as targets for infection because they are susceptible to infection by both VSV-G lentiviral pseudotypes that exhibit pH-dependent entry and amphotropic MLV (MLV-A) envelope lentiviral pseudotypes, whose entry is pH-independent (McClure et al., 1990). Bafilomicin A, an inhibitor of vacuolar H+-ATPases responsible for acidifying endosomes, inhibited transduction of RD cells by both CHIKV and VSV-G pseudotypes (Fig. 2). In contrast, no inhibition was detected in MLV-A pseudotype challenged cells, even with high bafilomicin A concentrations (Fig. 2) indicating that the inhibitory effects of bafilomicin A did not result from post entry inhibition of HIV core replication. Similar results were obtained with the acidotropic weak bases ammonium chloride (NH4Cl) and chloroquine (data not shown). Together, these data indicate that CHIKV envelope mediated entry is dependent on endosomal acidification.
FIG. 2.
Effect of lysosomotropic agents on pseudotype transduction. Bafilomycin A inhibition of RD cell transduction by MLV-A env (triangles), CHIKV gps (squares) and VSV-G (dots) pseudotypes. Results are expressed as percentage of no drug and represent the means of duplicate wells. The data is representative of four experiments.
Analysis of CHIKV pseudovirus tropism in mammalian cells
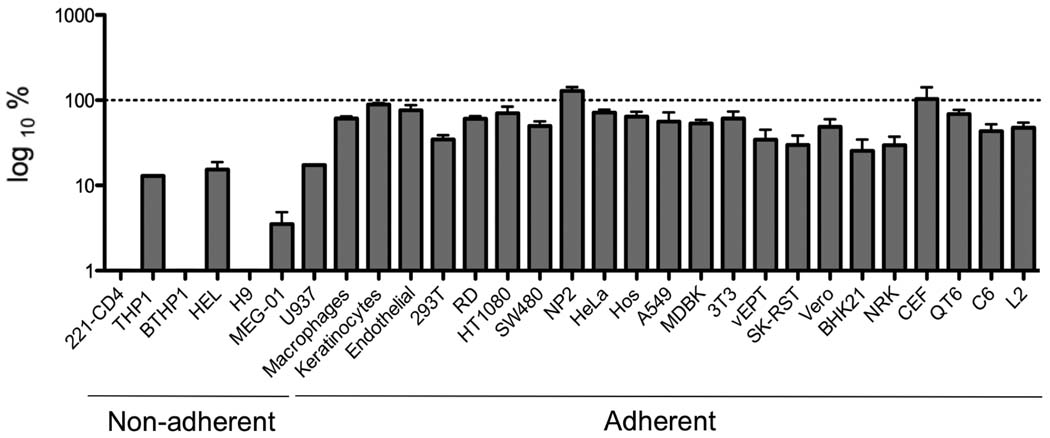
A panel of human adherent and non-adherent cell lines, primary human cells and non-human cell lines was used to characterize the tropism of CHIKV pseudotyped viruses (Table 2). Parallel infections were carried out with HIV particles produced in the absence of a viral envelope protein as a negative control and VSV-G pseudotyped particles, which display a very broad tropism, to distinguish from possible post-entry blockage effects. In this case the HIV vector used was pNL-gfp that carries the Aequorea victoria green fluorescent protein (GFP) gene as a reporter. Virus input of ultracentrifuge pelleted samples was normalized by p24 content prior to infection. The viral titers obtained on the individual cell lines varied quite dramatically (Table 2), but in general this variation was consistent between CHIKV envelope and VSV-G pseudovirions (Fig. 3). GFP expression showed that most cell types listed in Table 2 were permissive for CHIKV pseudotypes demonstrating the wide cellular tropism conferred by CHIKV envelope gps (Fig. 3). However most hematopoietic cell lines were either completely or partially refractory to CHIKV pseudotype infection. Primary, PHA/IL-2 stimulated PBLs were also not infectible by CHIKV pseudovirions. For reasons of better detection, vectors encoding murine CD24 were used in the place of GFP. PBLs were over 99% CD3 positive, and could be easily transduced with pseudovirions bearing VSV-G, resulting in 10.3% of cells staining positive for murine CD24. In contrast, even using 10-fold higher inoculum, CHIKV failed to give any significant transduction above that for no envelope controls (0.25 and 0.15% respectively).
TABLE 2.
HIV pseudovirus titers determined by GFP expression
| Name | Cell type* | Virus titer (FFU/ml) b | |
|---|---|---|---|
| CHIKV ENV | VSV-G | ||
| RD | Muscle | 1.4 × 107 | 2.5 × 107 |
| HT1080 | Fibrosarcoma | 1.4 × 107 | 1.9 × 107 |
| SW480 | Colorectal | 1.2 × 107 | 2.4 × 107 |
| 293T | Kidney epithelial | 7.7 × 106 | 2.6 × 107 |
| NP2 | Brain | 3.1 × 107 | 2.4 × 107 |
| HeLa | Cervical carcinoma epithelial | 5.0 × 106 | 6.7 × 106 |
| Hos | Bone | 2.6 × 107 | 4.1 × 107 |
| A549 | Alveolar epithelial | 1.7 × 107 | 3.0 × 107 |
| Keratinocytes | Primary keratinocytes | 7.4 × 107 | 8.3 × 107 |
| Endothelial | Primary endothelial cells | 2.6 × 107 | 3.4 × 107 |
| Muscle | Primary muscle cells | 3.4 × 107 | ND |
| Name | Cell type* | Virus titer (FIU/ml) c | |
| CHIKV ENV | VSV-G | ||
| THP1 | Monocytes | 4.0 × 104 | 3.1 × 105 |
| BTHP1 | B cell line | – a | 1.1 × 105 |
| HEL | Erytroleukemia | 3.7 × 104 | 2.5 × 105 |
| H9 | CD4 T cell line | – a | 1.3 × 105 |
| MEG-01 | megakaryoblast | 2.1 × 104 | 6.1 × 105 |
| U937 | Promonocytic | 1.6 × 104 | 1.5 × 105 |
| 221-CD4 | Monkey T cell line | – a | 1.7 × 105 |
| Macrophages | Primary monocyte-derived macrophages |
3.9 × 104 | 6.4 × 104 |
| MDBK | Bovine kidney | 4.5 × 104 | 8.7 × 104 |
| 3T3 | Mouse fibroblast | 8.9 × 104 | 1.2 × 105 |
| vEPT | Rabbit kidney | 5.0 × 104 | 1.9 × 105 |
| SK-RST | Pig kidney | 1.2 × 105 | 5.2 × 105 |
| Vero | Monkey kidney | 9.0 × 104 | 2.0 × 105 |
| BHK21 | Hamster kidney | 1.3 × 105 | 3.7 × 105 |
| NRK | Rat kidney | 1.7 × 105 | 5.1 × 105 |
| CEF | Chicken embryo fibroblasts | 1.2 × 105 | 1.3 × 105 |
| QT6 | Quail fibroblast | 8.1 × 104 | 7.5 × 105 |
| C6 | Rat brain | 3.0 × 105 | 7.3 × 105 |
| L2 | Rat lung | 9.1 × 104 | 2.0 × 105 |
Cells are human unless otherwise stated.
FACS data below negative (<1 × 104 FIU/ml)
Titer obtained by counting GFP positive cells under fluorescence microscope
Titer obtained by sample FACS analysis
Results are representative of at least 2 independent experiments
FIG. 3.
Relative cell tropism of CHIKV pseudotypes. GFP expression was used for parallel titration of CHIKV and VSV-G pseudotypes in a panel of cell types. Relative CHIKV pseudotype titer as compared to VSV-G pseudotype titer in each cell type is shown. The dotted line indicates 100 % infectivity for VSV-G pseudotypes. Data represent the average of at least two independent experiments.
The fact that hematopoitic cells were not infectible, suggested that there might be a correlation between cell adhesion and susceptibility. However, no difference in infectivity was found between adherent and suspension cultured 293FR cells (data not shown) indicating that the susceptibility to CHIKV pseudotype infection is most likely independent of cell adhesion. The titers of the VSV-G pseudotypes were consistently higher than that of the CHIKV envelope except in the case of human NP2 glioma cell line and chicken embryo fibroblasts in which CHIKV envelope pseudotypes titers were respectively 26% and 35% higher than those of VSV-G pseudotypes (Fig. 3). This is consistent with a strong tropism for fibroblasts and the CNS observed in in vivo models of CHIKV infection (Couderc et al., 2008). Endothelial human primary cells and human primary keratinocytes were also susceptible to CHIKV pseudotype infection (Fig. 3). In a separate experiment, fetal primary CD146+ myoblasts were also highly susceptible to CHIKV pseudotypes, with a titer of 4.3 × 107 FFU/ml. CD146 is a marker for myoblasts (Cerletti et al., 2006), and thus these results are consistent with previous findings that CHIKV infects muscle precursor cells (Ozden et al., 2007). It has been described that monocytes are insensitive to CHIKV infection while human primary macrophages are productively infected (Sourisseau et al., 2007). Differentiation of THP1 monocytes into macrophages using retinoic acid (Hickstein et al., 1992) resulted in a 4-fold increase in CHIKV pseudotype susceptibility (data not shown) suggesting that CHIKV inability to infect monocytes may be at least partially related to a lack of efficient entry that is overcome by the changes induced after the macrophage differentiation process. HIV does not replicate efficiently in primary monocytes, thus we developed VSV-based pseudovirions (Matsuura et al., 2001) in order to study CHIKV envelope-mediated monocyte infection. experiments have been performed with fresh isolated PBMC, containing primary lymphocytes and monocytes. While good infection was observed in CD14 positive cells (monocytes) using VSV-G envelope as a control with 8.8% of GFP positive cells, little significant infection (1% of GFP positive cells) was seen with the same backbone and CHIKV envelope.
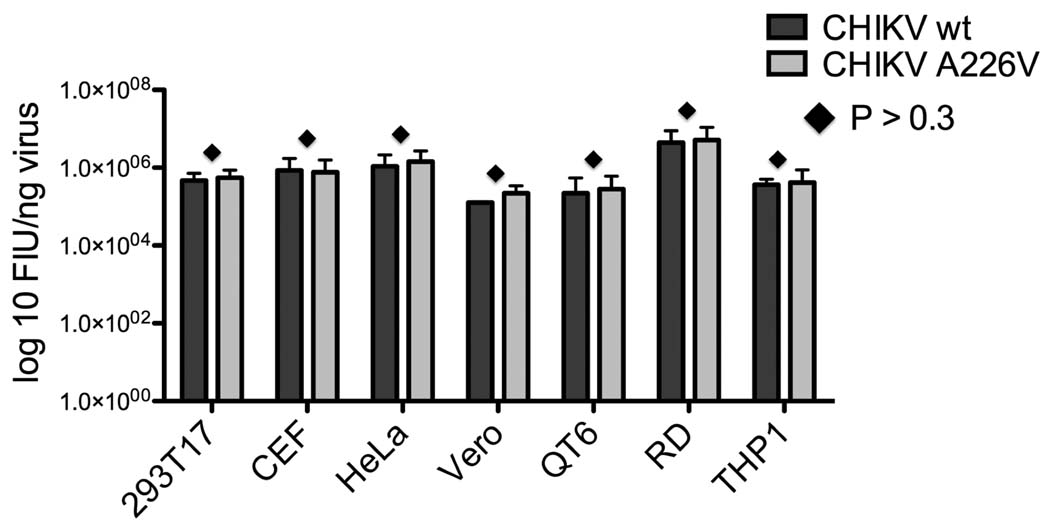
Naturally occurring CHIKV variants carrying an alanine to valine change in E1 (A226V) have been described to alter vector specificity by increasing infectivity for the Ae. albopictus mosquito (Tsetsarkin et al., 2007; Vazeille et al., 2007). Whether this change has any effect in the ability of the virus to infect the mammalian host has not previously been addressed. We therefore compared the infectivity of both wild type and E1 A226V mutant pseudoparticles in different mammalian cell lines. Input was normalized by p24 ELISA analysis prior to infection so that the different cell lines were challenged with equal amounts each pseudotype. Statistical analysis showed no significant difference (P > 0.3) in infectivity between CHIKV envelope variants in any the cell lines tested (Fig. 4). This result indicates that the presence or absence of the change A226V in CHIKV E1 gp does not affect the gross ability of pseudotypes to infect mammalian cells, although we cannot rule out the possibility of changes in the kinetics of infection.
FIG. 4.
Pseudotype infectivity for wt and A226V CHIKV envelopes in mammalian cells. Equal amounts (normalized by p24 ELISA) of pseudotypes bearing CHIKV envelope wt or 226V mutant were titrated in parallel in different cell types. GFP titer was determined by flow cytometry. Each pseudotype was tested in triplicate for each cell type and error bars represent standard deviations (SDs). Differences in viral titers were analyzed by pairwise t-tests. Diamond indicates P > 0.3.
Mutations in E1 glycoprotein mediate cellular tropism differences in mosquito cells
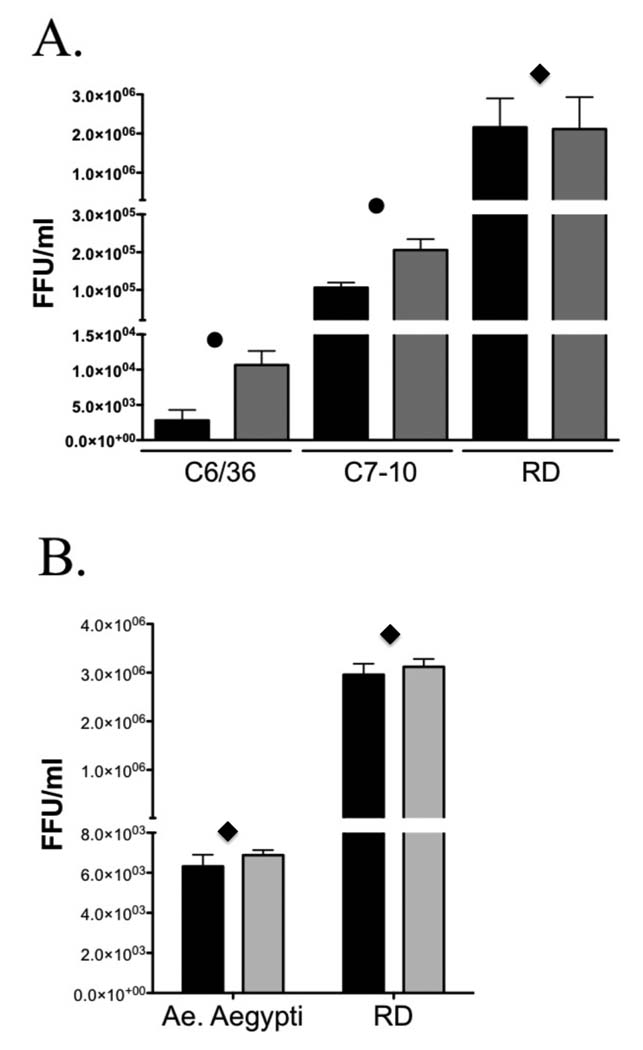
As lentiviral pseudotypes were not sufficiently infectious in mosquito cells we decided to pseudotype VSV with CHIKV envelope to determine the effect of the A226V mutation in mosquito cell entry. Both Ae. albopictus cell lines and Ae. aegypti primary cells were tested for susceptibility to CHIKV pseudotyped VSV. Again, parallel infections were carried out with VSV particles produced in the absence of a viral envelope protein as a negative control and VSV-G pseudotyped particles to distinguish from possible post-entry blockage effects. Using particles first normalized by titering on RD cells, the infectivity of VSV pseudotypes bearing the CHIKV envelope 226V mutation was higher by more than 4 fold in C6/36 and 2 fold in C7-10 Ae. albopictus cell lines when compared to wild type envelope pseudotypes (Fig. 5A). These subtle differences were statistically significant (P<0.05) in multiple independent experiments using different batches of pseudovirions, and demonstrate a role for this E1 mutation in virus binding/entry giving CHIKV 226V a selective advantage in Ae. albopictus cells. However, no such advantage is detected in Ae aegypti cells since no differences in infectivity were observed between CHIKV wild type and 226V mutant in three independent experiments (Fig 5B). The differences in CHIKV infectivity between C6/36 and C7-10 cell lines observed in figure 5A are not specific since similar differences were observed with the control VSV-G enveloped pseudotypes (8.6 × 103 FFU/ml in C6/36 and 1.3 × 105 FFU/ml in C7-10). No pseudotype induced cytopathic effects were observed after 24 h when the analyses were performed.
FIG. 5.
Pseudotype infectivity for CHIKV envelope variants in mosquito cells. VSV pseudotypes bearing CHIKV envelope wt (Black bars) or 226V mutant (Grey bars) were titrated in parallel in (A) Ae. albopictus cell lines C6/36 and C7-10 and RD cells. Four replicates were performed for each pseudotype. (B) Ae. aegypti cells and RD cells. Each pseudotype was tested in triplicate. GFP titer was determined by flow cytometry. Error bars represent standard deviations (SDs). Differences in viral titers were analyzed by pairwise t-tests. Diamond indicates P < 0.3. Dot indicates P< 0.05
CHIKV pseudovirion infectivity is enhanced by the presence of C-type lectins
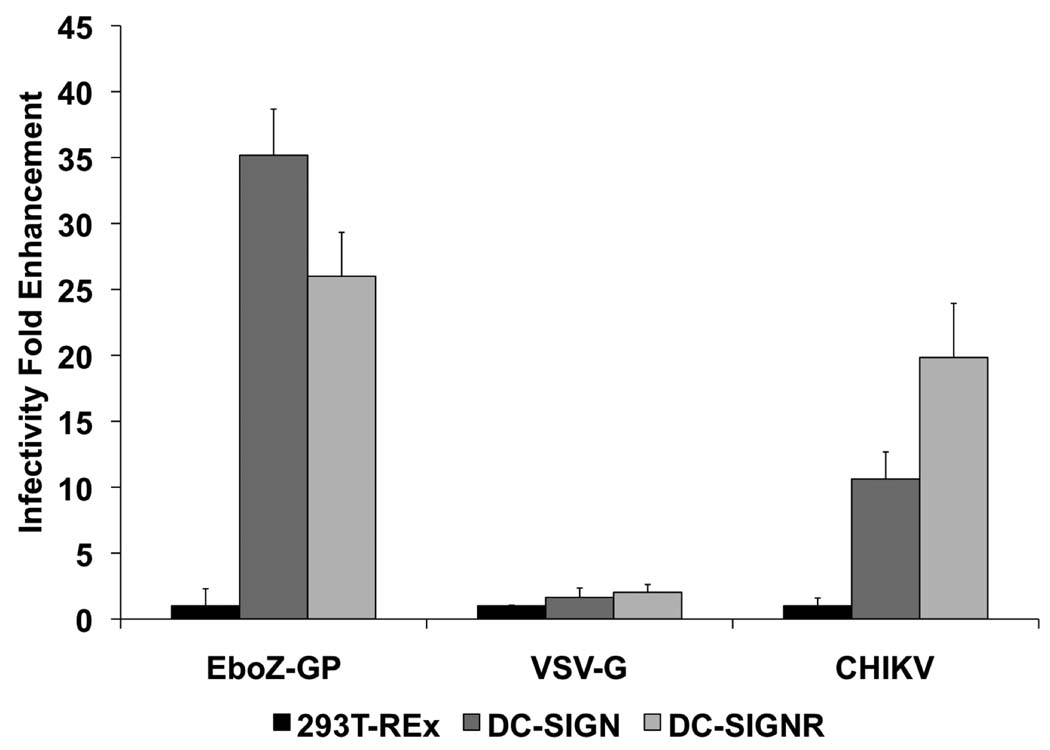
C-type lectins like DC-SIGN and DC-SIGNR have been shown to augment infection of many viruses by interacting with the envelope viral gps (Lozach et al., 2007), playing an important role in virus transmission, tissue tropism, and pathogenesis. To determine the role of lectin expression on the infectivity of CHIKV pseudotypes, we utilized a previously reported 293T-REx cell line system, in which lectin expression was under the control of a tet repressor protein that allows induction of expression with doxycycline (Pohlmann et al., 2001). The induction of lectin expression was verified by fluorescence-activated cell sorter staining (Supplementary Fig. 2) and Western blot (data not shown). We challenged the lectin expressing 293T-REx cells with luciferase reporter pseudotypes bearing CHIKV envelope glycoproteins. Ebola Zaire (EboZ-GP) envelope and VSV-G pseudotypes, whose infectivity is respectively enhanced and unaffected by the presence of lectins (Simmons et al., 2003) were used as positive and negative controls. Infectivity of EboZ-GP envelope pseudotypes was enhanced over 35-fold in DC-SIGN-expressing 293T-REx cells and 26-fold in DC-SIGNR expressing cells, compared with the parental 293T-REx cells (Fig. 6). Similarly, the infectivity of CHIKV envelope pseudotypes was enhanced over 10-fold in DC-SIGN-expressed 293T-REx cells and 19-fold in DC-SIGNR expressed cells (Fig. 6). In contrast, VSV-G infection of either DC-SIGN or DC-SIGNR expressing cells was within 2-fold of parental 293T-REx cell infection. Enhancement of CHIKV pseudotypes infectivity was also observed in susceptible HeLa and RD cell lines expressing DC-SIGN and DC-SIGNR (data not shown). While expression of DC-SIGN on already susceptible cell lines enhanced infection, refractory lines, such as B THP-1, NC-37 and Ramos cell lines, remained resistant to infection, despite stable DC-SIGN expression (data not shown).
FIG. 6.
DC-SIGN and DC-SIGNR enhance infectivity of CHIKV. envelope glycoprotein-bearing pseudotypes in 293T-REx cell lines The data are presented as fold-enhancement of infection in lectin expressing 293T-REx cells compared to parental 293T-REx cells. Error bars indicated standard deviations (SDs) of triplicate values from a representative experiment. Similar results were obtained in three independent experiments.
DISCUSSION
CHIKV characteristics and the widespread distribution of its mosquito vectors make it a potentially highly important emerging pathogen. However, very little is known about the biology of this alphavirus, although its similarity to other alphaviruses suggests general principles that may govern its biological activities. The envelope proteins are the major determinants of alphavirus cell tropism, viral entry and neutralization sensitivity. With the advancement of molecular technologies, several pseudotype reporter viral systems have been developed for different enveloped viruses making the investigation of phenotypic properties of envelope proteins more convenient and efficient (Bartosch et al., 2003; Chan et al., 2006; Fukushi et al., 2005; Ma et al., 1999; Matsuura et al., 2001; Rollman et al., 2007; Simmons et al., 2004; Wool-Lewis and Bates, 1998).
We have successfully exploited the cell surface expression of CHIKV proteins in transfected cells to produce lentiviral particles that are able to transduce cells in a CHIKV envelope-mediated manner. The infectivity of these pseudotyped reporter viruses in CD4 and CXCR4 negative cell lines (Table 2) with a single round of infection, indicates that the CHIKV envelope is directing entry, thus broadening the cell tropism of the resulting HIV particles. CHIKV gps are not only efficiently produced and expressed on the cell surface but are also incorporated in the pseudotyped viruses. Although E1 and E2 proteins migrate closely on gels (Sourisseau et al., 2007) and could not be distinguished by size due to heterogeneous glycosylation, the fact that correct E2 processing requires E1 coexpression makes it likely that both proteins are being expressed. Furthermore, CHIKV pseudovirions present native epitopes on their surfaces that are recognized by neutralizing human sera from individuals previously infected with CHIKV. CHIKV pseudotypes enter cells through a pH dependent pathway, being readily blocked by three inhibitors of endocytic acidification as described for CHIKV (Sourisseau et al., 2007). Thus, CHIKV lentiviral pseudotypes offer the potential to rapidly and easily study native entry pathways of the parental CHIKV in the absence of other cytotoxic effects associated with alphavirus infection.
The host range conferred by CHIKV envelope is very broad, enabling the infection by CHIKV pseudotypes of a variety of cell lines from diverse species and tissues. The infection of many of the cell types used for this work is not unexpected as CHIKV displays a rather wide tropism and has been shown to infect a variety of human and non-human cell types (Glasgow, 1966; Hahon and Zimmerman, 1970; Ozden et al., 2007; Rinaldo, Overall, and Glasgow, 1975; Simizu et al., 1984; Sourisseau et al., 2007). Interestingly, human cell lines A549 and U937, recently described as resistant to CHIKV infection (Sourisseau et al., 2007) are susceptible to infection by CHIKV lentiviral pseudotypes. These results, together with the fact A549 cells are able to bind CHIKV virions (Sourisseau et al., 2007), indicate that the restriction to CHIKV replication in these cells occurs at a post-entry step of viral infection. The exception for CHIKV pseudotypes susceptibility was hematopoietic cell lines, most of which were partially or completely refractory to the infection (Table 2 and Fig. 3). Similar results have been described for replication competent CHIKV, as well as pseudovirions bearing envelopes from the related alphaviruses RRV and SFV (La Linn et al., 2005; Strang et al., 2005). We believe that the failure to transduce those cells is most likely due to the lack or limited expression of the cellular receptor(s), or cellular events involved in internalization, given the efficiency of transduction by VSV-G pseudotypes. These results suggest that CHIKV receptor(s) are present in different host species and thus CHIKV envelope provides a broad specificity to the pseudotyped vectors. Susceptibility of some cell types to Ebola virus has been described to be affected by cell adhesion properties (Dube et al., 2008). Our results show that the differences in CHIKV pseudotypes infectivity between adherent and non-adherent cells are independent of the anchorage-dependency of the cells. The refractory hematopoietic-cell types may be useful for receptor identification. The fact that partially refractory THP1 monocytes increase their susceptibility upon differentiation to macrophages, most likely by induced expression of the virus receptor, makes cells of the monocyte/macrophage lineage potential candidates in aiding the discovery of the CHIKV cell surface receptor(s).
The use of CHIKV pseudotypes also provides a straightforward tool for the functional characterization of envelope gps variants. Single amino acid changes in CHIKV gps such as E1 A226V have been described to affect virus specificity (Tsetsarkin et al., 2007; Vazeille et al., 2007). Moreover, adaptation of CHIKV 226V to Ae. albopictus mosquitoes coincides with CHIKV dependence on cholesterol in the target cell membrane (Tsetsarkin et al., 2007). Similarly, a mutation in the same position of the E1 gp from SFV has been described to affect the cholesterol dependency of the virus (Vashishtha et al., 1998) and, unlike CHIKV (Tsetsarkin et al., 2007), results in the more efficient growth of SFV in Ae. albopictus mosquitoes (Ahn et al., 1999). Whether there is direct correlation between the release from the cholesterol dependence, and the growth advantage in Ae. Albopictus remains unknown. As expected, we have found no effect of E1 A226V mutation in CHIKV pseudotypes infectivity in mammalian cells. However, the presence of the mutation increases CHIKV pseudotypes infectivity in Ae. albopictus cells from 2 to 4 fold but has no effect in Ae. aegypti cells. These results demonstrate a direct effect of the mutation in the CHIKV entry process resulting in differences in cellular tropism. Although both are derived from Singh’s original larva isolates (Singh and Pavri, 1967), several reports suggest differences between C6/36 and C7-10 Ae. albopictus subclones indicating that they may represent different tissue types in the adult insect (Condreay and Brown, 1986; Condreay and Brown, 1988; Miller and Brown, 1992; Miller and Brown, 1993). Thus, it may be that the 226V change confers a selective advantage in multiple cell types within Ae. Albopictus. Despite the demonstrated fitness advantage of E1 226V virus in Ae. albopictus mosquitoes, almost indistinguishable growth kinetics of CHIKV 226A and 226V viruses in Ae. albopictus C6/36 cells have been described (Tsetsarkin et al., 2007). While this appears to be somewhat contradictory to our results that show that the E1 226V CHIKV pseudotype entry in Ae albopictus cells C6/36 and C7-10 is subtly more efficient than the wild type, multiple rounds of replication and post-entry steps of the virus life cycle likely conceal this difference and result in almost equivalent growth kinetics. A putative role for E1 226 position in membrane fusion is consistent with its predicted location in the ij loop, a region believed to interact with the target membrane, in contact with the fusion peptide (Schuffenecker et al., 2006). However, we cannot rule out additional implications of the E1 A226V mutation in other steps of the virus life cycle like virus budding and exit. Whether this more efficient entry of the virus is enough to account for the fitness advantage of the E1 226V mutant in Ae. albopictus mosquitoes and its possible association with cholesterol dependence requires further investigation. CHIKV pseudotypes will facilitate the study of the effect of cholesterol in virus entry independently of the post-entry steps.
Our results suggest that C-type lectins act as CHIKV attachment factors and increase infectivity. DC-SIGN and DC-SIGNR lectins bind virus envelope proteins in a carbohydrate-dependent manner, and are likely recognizing high mannose moieties on the viral gps, which contain at least four predicted N-linked glycosylations (asn-X-ser/thr, where X can be any aa apart from proline; (Hubbard and Ivatt, 1981)). In mouse models of CHIKV infection the liver represents a strong initial target for viral replication, followed by detection of virus in muscle and skin (Couderc et al., 2008). Consistent with this, CHIKV pseudotypes efficiently infect (1.9 × 105 FIU/ml) Huh7 human hepatic cell line. The expression of DC-SIGN and DC-SIGNR, as well as other C-type lectins, in the liver (Lai et al., 2006; Pohlmann et al., 2001) may thus be involved in the early targeting of this organ, while C-type lectins on macrophages and dendritic cells may be involved in disseminating the virus to other tissues.
In this study, we have successfully established retrovirus and rhabdovirus-based pseudotype reporter assays for CHIKV, which to our knowledge, are the first viral reporter systems described for CHIKV. Our results indicate that CHIKV pseudotypes accurately mimic the entry mechanism of the virus and offer a stable and safe source of native antigen thus providing an ideal system for CHIKV immune response analysis, glycoproteins function determination, entry studies and receptor identification. Moreover we have established that mutations in E1 glycoprotein affecting CHIKV entry efficiency mediate alterations in cellular tropism.
MATERIALS AND METHODS
Cells and cell culture
Adherent mammalian cell lines were cultured in DMEM and non-adherent cells were cultured in RPMI, in both cases media was supplemented with 10% FBS and penicillin/streptomycin (15 units/ml). Human endothelial cells (Clonetics) were cultured in Clonetics endothelial cell basal media and human keratinocytes (Clonetics) were cultured in Clonetics keratinocyte cell basal media-2. Human primary blood lymphocytes (PBLs) were isolated as previously described (Simmons et al., 1995) and stimulated in RPMI 1640 containing 20% FCS and 0.5 µg/ml phytohaemagglutinin (PHA, Roche). After 3 days culture, the cells were spun and resuspended in RPMI 1640 containing 20% FCS and 20 units/ml human interleukin-2 (IL-2, Roche). Human primary monocytes were isolated from donors by apheresis and elutriation. Human primary monocyte-derived macrophages were isolated as previously described (Simmons et al., 1995) and cultured in RPMI supplemented with 10% human serum and penicillin/streptomycin (15 units/ml). The C7-10 Ae. albopictus cell line was kindly provided by Victor Stollar (UMDNJ), while C6/36 were obtained from the ATCC. C7-10 and C6/36 were cultured in MEM supplemented with 10% FBS and penicillin/streptomycin (15 units/ml) while Ae aegypti cells (ATCC CCL-125) were cultured in MEM supplemented with 20% FBS and penicillin/streptomycin (15 units/ml). Fetal muscle cells were obtained from elective abortions with the approval of the Committee for Human Research at the University of California, San Francisco and consent of the women undergoing the procedure. The gestational age of the tissues was estimated based on the foot-length of the fetus and ranged from 19 to 24 weeks. Fetal tissues were harvested shortly after termination of the pregnancy and transported to the laboratory on ice in Hank’s balanced salt solution with calcium and magnesium (HBSS) and supplemented with 100 µg/ml gentamicin and 2.5 µg/ml amphotericin B (Invitrogen, Carlsbad, CA). Muscle cells were isolated from tongue or thigh muscles and grown similarly to a previously described method (Ozeki et al., 2006). Briefly, the tissue was washed with HBSS solution and, in the case of thigh muscles, the skin removed and the muscle tissue separated from the bone. The tissues were minced using scissors into pieces ≤ 0.5 cm3. These fragments were digested using a combination of 1% collagenase class D, 2.4 U/ml grade II Dispase and 0.005% DNase (Roche Diagnostic Corporation, Indianapolis, IN) in 20 ml of HBSS for 30–45 min. at 37 °C with gentle agitation. The cell suspension was then passed through a fine wire mesh and any remaining larger fragments disrupted using a glass pestle. The resulting cell suspension was then passed through a 70 m cell strainer (BD Biosciences) to remove any remaining tissue fragments. Cells were washed twice by centrifugation. Muscle cells were cultured on Laminin-1 coated dishes (BD Falcon) in F-10 medium (Invitrogen) supplemented with 20% FBS, 2 µg/ml recombinant human insulin (Roche Diagnostic Corporation), 50 µg/ml gentamicin and 2.5 µg/ml amphotericin B. Staining of cultured muscle cells was performed with CD146-PE (clone 128018, R&D Systems). CD146 is a marker of fetal myoblasts (Cerletti et al., 2006).
For the 293T-REx cell lines, DC-SIGN and DC-SIGNR were expressed under a tet repressor (Invitrogen) and grown in DMEM medium containing 10% FBS, Zeocin (50 µg/ml), and blasticidin (2.5 µg/ml) as described (Pohlmann et al., 2001). The 293T-REx parental cells were maintained in DMEM medium containing 10% FBS and blasticidin (2.5 µg/ml). Lectin expression was induced by addition of 0.1 µg/ml of doxycycline to the medium.
Both true THP-1 cells, a monocyte cell line (ATCC TIB-202), and B-THP-1 cells were used. B-THP-1 have been shown to be a B cell line, probably derived from Raji cells, that were wrongly classified as THP-1 cells (Wu et al., 2004).
Plasmids
Human codon optimized CHIKV E3/E2/E1 was synthesized (Integrated DNA Technologies, Coralville, IA) based on the structural polyprotein sequence for the S27 African prototype strain of CHIKV (Khan et al., 2002). Assuming an amino-acid numeration of 1 for the initiating methionine of capsid protein, an expression cassette for E3/E2/E1 was synthesized consisting of amino acids 262 to 1248, together with an initiating ATG and upstream Kozak sequence. This was subcloned into the mammalian expression plasmid, pCAGGS (Niwa, Yamamura, and Miyazaki, 1991) using SacI and NheI. A naturally occurring A226V mutation in E1 was introduced using Quikchange site directed mutagenesis (Stratagene) in puc57 CHIKV E3/E2/E1. A 750 bp fragment carrying the change was swapped into pCAGGS CHIKV E3/E2/E1 and confirmed by sequencing. Plasmids expressing Ebola Zaire (EboZ), vesicular stomatitis virus (VSV), and amphotropic murine leukemia virus (MLV-A) glycoproteins have been described (Simmons et al., 2002).
Pseudotype production
Lentiviral pseudotypes were produced essentially as described (Simmons et al., 2004) by using 10 µg of luciferase, GFP, or murine CD24 vector (pNL-luc or pNL-gfp, or pNL-HSA based on pNL3-4-R-E-) (Connor et al., 1995) and 30 µg of plasmid-encoding viral envelope. Pseudotyped VSVs were produced essentially as described by Matsuura et al. (Matsuura et al., 2001) by transfecting 293T cells with 16 µg of plasmid-encoding viral envelope and then infecting the cells with recombinant VSV⊗G*-G kindly provided by Dr. Whitt (University of Tennessee) at 0.1 multiplicity. If required, virions were concentrated by ultracentrifuge concentration at 28,000 rpm in a SW28 rotor (Beckman) through a 20% sucrose cushion for 1.5 h at 4 °C. The pellets were resuspended overnight in HBSS at 4 °C.
Pseudovirion infection
After p24 ELISA (Aalto BioReagents, Dublin, Ireland) analysis, lentiviral pseudovirions were normalized to contain an equal number of viral particles. Cells were seeded at 2.5 × 104 in 48-well plates 24 h prior to infection. Appropriate amounts of viral supernatant in 200 µl of medium were added to the plates prior to spin infection (Simmons et al., 2002) for 1.5 h at 2100 × g at room temperature. After a 2 h incubation at 37 °C, 300 µl of fresh medium was added and cells were incubated for 72 h. Virus infection was analyzed by measuring the number of GFP expressing cells either by counting green cells using fluorescence microscopy or by flow cytometric analysis. For VSV pseudotype infection mosquito cells were seeded at 1 × 105 cells in 48-well plates 24 h prior to infection. Appropriate amounts of viral supernatant in 200 µl of medium were added to the plates and incubated for 24 h at 28 °C. Virus infection was analyzed by measuring the number of GFP expressing cells by flow cytometric analysis.
Neutralization of pseudovirions was performed by using sera from CHIKV infected individuals (kindly supplied by Azzedine Assal, EFS Centre-Atlantique, France and Robert S. Lanciotti, Centers for Disease Control & Prevention, Fort Collins). Luc pseudovirions were pretitrated on 293T cells to ensure similar input levels of viruses. Sera was inactivated at 56 °C, serially diluted and mixed with luc reporter pseudovirions at a 1:1 ratio and incubated for 45 min. at room temperature before addition of 50 µl to RD cells seeded at 2 × 104 cells per well in 96-well plates. After spin infection for 1 h at 1600 × g, cells were incubated for 2 h at 37 °C, washed twice and incubated for 40 h. Luciferase activity was assayed from cell lysates as per manufacturer’s instructions (Promega).
Immunological detection of CHIKV envelope glycoproteins
293T cells were transfected as for pseudotype production. The cells were lysed 40 h post-transfection using RIPA buffer, the cell lysate sonicated to shear the DNA and then centrifuged at 2500 × g for 20 min. at 4 °C to pellet cell debris. The supernatant was collected and concentrated as described for pseudotype production. Sample buffer and reducing agent were added to the cell lysates and concentrated pseudovirions and the samples were the analyzed by SDS-PAGE using an 8% Bis-Tris gel (Invitrogen). The separated proteins were transferred to a nitrocellulose membrane and blocked with 2% powdered milk in TBS for 30 min. at room temperature. The membrane was then incubated in TBS-0.05% Tween-20 with a 1:200 dilution of a mix of heat-inactivated sera from CHIKV infected individuals for 1 h at room temperature. After 3 washes, 10 min. each, with TBS-0.05% Tween-20 the membrane was incubated with 1:200 dilution of Alexa 488 goat anti-human antibody (Invitrogen) for 1 h at room temperature. The membrane was then washed with TBS-0.05% Tween-20 three times for 10 min. each, and immunoreactive proteins were visualized using a Molecular Dynamics Storm 860 imaging system in blue fluorescence mode.
Lysosomotropic agents
Cells were incubated with serial dilutions of bafilomicin A, chloroquine, or NH4Cl (Sigma) 1 h before and during spin infection with Luc pseudovirions. The agent was included in the medium for 5 h after infection, before replacement with fresh medium and assaying for transduction 70 h after infection.
Supplementary Material
CHIKV pseudotype neutralization. Neutralization curves for wild type CHIKV envelope and A226V mutant with sera from seven different CHIKV infected individuals. Infectivity of pseudotyped virus is shown as % of no serum control with each point representing the average of one experiment performed in triplicate. Results are representative of two independent experiments.
Enhancement of CHIKV infection by lectins. DC-SIGN and DC-SIGNR expression on 293T-Rex cells was determined 24 hours after induction using an antibody to the AU1 tag. Parental cells are shown as the solid black histogram, while DC-SIGN and DC-SIGNR are dark gray and light gray respectively.
ADNOWLEDGEMENTS
We would like to thank Stefan Pöhlmann (University of Hannover) for T-REx and B-THP-1 cells, Victor Stollar for C7-10 cells and EJ Read and Yelena Dayter for elutriated monocytes. We would also like to thank Azzedine Assal, EFS Centre-Atlantique, France; Michael Whitt, University of Tennessee; and Robert S. Lanciotti, Centers for Disease Control & Prevention, Fort Collins for samples and viruses, and the NIH AIDS Research and Reference Reagent Program for pNL-HSA. We also thank Jacqueline Reeves for critical reading of the manuscript. This work was supported by grant R01AI074986 from the National Institute Of Allergy And Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Anon Biosafety in Microbiological and Biomedical Laboratories. Washigton: US Centers for Disease Control and Prevention, Office of Health and Safety (CDC-OHS); 2007
- Ahn A, Schoepp RJ, Sternberg D, Kielian M. Growth and stability of a cholesterol-independent Semliki Forest virus mutant in mosquitoes. Virology. 1999;262(2):452–456. doi: 10.1006/viro.1999.9932. [DOI] [PubMed] [Google Scholar]
- Bartosch B, Bukh J, Meunier JC, Granier C, Engle RE, Blackwelder WC, Emerson SU, Cosset FL, Purcell RH. In vitro assay for neutralizing antibody to hepatitis C virus: evidence for broadly conserved neutralization epitopes. Proc Natl Acad Sci U S A. 2003;100(24):14199–14204. doi: 10.1073/pnas.2335981100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodenmann P, Genton B. Chikungunya: an epidemic in real time. Lancet. 2006;368(9531):258. doi: 10.1016/S0140-6736(06)69046-6. [DOI] [PubMed] [Google Scholar]
- Bonilauri P, Bellini R, Calzolari M, Angelini R, Venturi L, Fallacara F, Cordioli P, Angelini P, Venturelli C, Merialdi G, Dottori M. Chikungunya virus in Aedes albopictus, Italy. Emerg Infect Dis. 2008;14(5):852–854. doi: 10.3201/eid1405.071144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrel RN, de Lamballerie X, Raoult D. Chikungunya outbreaks-the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- Cerletti M, Molloy MJ, Tomczak KK, Yoon S, Ramoni MF, Kho AT, Beggs AH, Gussoni E. Melanoma cell adhesion molecule is a novel marker for human fetal myogenic cells and affects myoblast fusion. J Cell Sci. 2006;119(Pt 15):3117–3127. doi: 10.1242/jcs.03056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E, Heilek-Snyder G, Cammack N, Sankuratri S, Ji C. Development of a Moloney murine leukemia virus-based pseudotype anti-HIV assay suitable for accurate and rapid evaluation of HIV entry inhibitors. J Biomol Screen. 2006;11(6):652–663. doi: 10.1177/1087057106288881. [DOI] [PubMed] [Google Scholar]
- Condreay LD, Brown DT. Exclusion of superinfecting homologous virus by Sindbis virus-infected Aedes albopictus (mosquito) cells. J Virol. 1986;58(1):81–86. doi: 10.1128/jvi.58.1.81-86.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condreay LD, Brown DT. Suppression of RNA synthesis by a specific antiviral activity in Sindbis virus-infected Aedes albopictus cells. J Virol. 1988;62(1):346–348. doi: 10.1128/jvi.62.1.346-348.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor RI, Chen BK, Choe S, Landau NR. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology. 1995;206(2):935–944. doi: 10.1006/viro.1995.1016. [DOI] [PubMed] [Google Scholar]
- Couderc T, Chretien F, Schilte C, Disson O, Brigitte M, Guivel-Benhassine F, Touret Y, Barau G, Cayet N, Schuffenecker I, Despres P, Arenzana-Seisdedos F, Michault A, Albert ML, Lecuit M. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 2008;4(2):e29. doi: 10.1371/journal.ppat.0040029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeTulleo L, Kirchhausen T. The clathrin endocytic pathway in viral infection. Embo J. 1998;17(16):4585–4593. doi: 10.1093/emboj/17.16.4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube D, Schornberg KL, Stantchev TS, Bonaparte MI, Delos SE, Bouton AH, Broder CC, White JM. Cell adhesion promotes Ebola virus envelope glycoprotein-mediated binding and infection. J Virol. 2008;82(14):7238–7242. doi: 10.1128/JVI.00425-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushi S, Mizutani T, Saijo M, Matsuyama S, Miyajima N, Taguchi F, Itamura S, Kurane I, Morikawa S. Vesicular stomatitis virus pseudotyped with severe acute respiratory syndrome coronavirus spike protein. J Gen Virol. 2005;86(Pt 8):2269–2274. doi: 10.1099/vir.0.80955-0. [DOI] [PubMed] [Google Scholar]
- Gaedigk-Nitschko K, Schlesinger MJ. The Sindbis virus 6K protein can be detected in virions and is acylated with fatty acids. Virology. 1990;175(1):274–281. doi: 10.1016/0042-6822(90)90209-a. [DOI] [PubMed] [Google Scholar]
- Glasgow LA. Leukocytes and interferon in the host response to viral infections. II. Enhanced interferon response of leukocytes from immune animals. J Bacteriol. 1966;91(6):2185–2191. doi: 10.1128/jb.91.6.2185-2191.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahon N, Zimmerman WD. Chikungunya virus infection of cell monolayers by cell-to-cell and extracellular transmission. Appl Microbiol. 1970;19(2):389–391. doi: 10.1128/am.19.2.389-391.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickstein DD, Baker DM, Gollahon KA, Back AL. Identification of the promoter of the myelomonocytic leukocyte integrin CD11b. Proc Natl Acad Sci U S A. 1992;89(6):2105–2109. doi: 10.1073/pnas.89.6.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard SC, Ivatt RJ. Synthesis and processing of asparagine-linked oligosaccharides. Annu Rev Biochem. 1981;50:555–583. doi: 10.1146/annurev.bi.50.070181.003011. [DOI] [PubMed] [Google Scholar]
- Khan AH, Morita K, Parquet Md Mdel C, Hasebe F, Mathenge EG, Igarashi A. Complete nucleotide sequence of chikungunya virus and evidence for an internal polyadenylation site. J Gen Virol. 2002;83(Pt 12):3075–3084. doi: 10.1099/0022-1317-83-12-3075. [DOI] [PubMed] [Google Scholar]
- Kolokoltsov AA, Fleming EH, Davey RA. Venezuelan equine encephalitis virus entry mechanism requires late endosome formation and resists cell membrane cholesterol depletion. Virology. 2006;347(2):333–342. doi: 10.1016/j.virol.2005.11.051. [DOI] [PubMed] [Google Scholar]
- 23.La Linn M, Eble JA, Lubken C, Slade RW, Heino J, Davies J, Suhrbier A. An arthritogenic alphavirus uses the alpha1beta1 integrin collagen receptor. Virology. 2005;336(2):229–239. doi: 10.1016/j.virol.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Lai WK, Sun PJ, Zhang J, Jennings A, Lalor PF, Hubscher S, McKeating JA, Adams DH. Expression of DC-SIGN and DC-SIGNR on human sinusoidal endothelium: a role for capturing hepatitis C virus particles. Am J Pathol. 2006;169(1):200–208. doi: 10.2353/ajpath.2006.051191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozach PY, Burleigh L, Staropoli I, Amara A. The C type lectins DC-SIGN and L-SIGN: receptors for viral glycoproteins. Methods Mol Biol. 2007;379:51–68. doi: 10.1007/978-1-59745-393-6_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YE, Cassese T, Kielian M. The cholesterol requirement for sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73(5):4272–4278. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusa S, Garoff H, Liljestrom P. Fate of the 6K membrane protein of Semliki Forest virus during virus assembly. Virology. 1991;185(2):843–846. doi: 10.1016/0042-6822(91)90556-q. [DOI] [PubMed] [Google Scholar]
- Ma M, Kersten DB, Kamrud KI, Wool-Lewis RJ, Schmaljohn C, Gonzalez-Scarano F. Murine leukemia virus pseudotypes of La Crosse and Hantaan Bunyaviruses: a system for analysis of cell tropism. Virus Res. 1999;64(1):23–32. doi: 10.1016/s0168-1702(99)00070-2. [DOI] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Helenius A. Virus entry: open sesame. Cell. 2006;124(4):729–740. doi: 10.1016/j.cell.2006.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M, Kielian MC, Helenius A. Semliki forest virus entry and the endocytic pathway. Biochem Soc Trans. 1984;12(6):981–983. doi: 10.1042/bst0120981. [DOI] [PubMed] [Google Scholar]
- Matsuura Y, Tani H, Suzuki K, Kimura-Someya T, Suzuki R, Aizaki H, Ishii K, Moriishi K, Robison CS, Whitt MA, Miyamura T. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology. 2001;286(2):263–275. doi: 10.1006/viro.2001.0971. [DOI] [PubMed] [Google Scholar]
- McClure MO, Sommerfelt MA, Marsh M, Weiss RA. The pH independence of mammalian retrovirus infection. J Gen Virol. 1990;71(Pt 4):767–773. doi: 10.1099/0022-1317-71-4-767. [DOI] [PubMed] [Google Scholar]
- Miller DK, Lenard J. Inhibition of vesicular stomatitis virus infection by spike glycoprotein. Evidence for an intracellular, G protein-requiring step. J Cell Biol. 1980;84(2):430–437. doi: 10.1083/jcb.84.2.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Brown DT. Morphogenesis of Sindbis virus in three subclones of Aedes albopictus (mosquito) cells. J Virol. 1992;66(7):4180–4190. doi: 10.1128/jvi.66.7.4180-4190.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, Brown DT. The distribution of Sindbis virus proteins in mosquito cells as determined by immunofluorescence and immunoelectron microscopy. J Gen Virol. 1993;74(Pt 2):293–298. doi: 10.1099/0022-1317-74-2-293. [DOI] [PubMed] [Google Scholar]
- Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108(2):193–199. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- Ohkuma S, Poole B. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc Natl Acad Sci U S A. 1978;75(7):3327–3331. doi: 10.1073/pnas.75.7.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozden S, Huerre M, Riviere JP, Coffey LL, Afonso PV, Mouly V, de Monredon J, Roger JC, El Amrani M, Yvin JL, Jaffar MC, Frenkiel MP, Sourisseau M, Schwartz O, Butler-Browne G, Despres P, Gessain A, Ceccaldi PE. Human muscle satellite cells as targets of Chikungunya virus infection. PLoS ONE. 2007;2(6):e527. doi: 10.1371/journal.pone.0000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozeki N, Lim M, Yao CC, Tolar M, Kramer RH. alpha7 integrin expressing human fetal myogenic progenitors have stem cell-like properties and are capable of osteogenic differentiation. Exp Cell Res. 2006;312(20):4162–4180. doi: 10.1016/j.yexcr.2006.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters CDJ. Alphaviruses. In: Fields BN KD, editor. Fields virology. New York: Raven Press; 1990. pp. 713–761. [Google Scholar]
- Pialoux G, Gauzere BA, Jaureguiberry S, Strobel M. Chikungunya, an epidemic arbovirosis. Lancet Infect Dis. 2007;7(5):319–327. doi: 10.1016/S1473-3099(07)70107-X. [DOI] [PubMed] [Google Scholar]
- Pohlmann S, Baribaud F, Lee B, Leslie GJ, Sanchez MD, Hiebenthal-Millow K, Munch J, Kirchhoff F, Doms RW. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J Virol. 2001;75(10):4664–4672. doi: 10.1128/JVI.75.10.4664-4672.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldo CR, Jr, Overall JC, Jr, Glasgow LA. Viral replication and interferon production in fetal and adult ovine leukocytes and spleen cells. Infect Immun. 1975;12(5):1070–1077. doi: 10.1128/iai.12.5.1070-1077.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman E, Mathy N, Brave A, Boberg A, Kjerrstrom A, van Wely C, Engstrom G, Johansson S, Aperia K, Eriksson LE, Benthin R, Ertl P, Heeney J, Hinkula J, Voss G, Wahren B. Evaluation of immunogenicity and efficacy of combined DNA and adjuvanted protein vaccination in a human immunodeficiency virus type 1/murine leukemia virus pseudotype challenge model. Vaccine. 2007;25(11):2145–2154. doi: 10.1016/j.vaccine.2006.10.057. [DOI] [PubMed] [Google Scholar]
- Schuffenecker I, Iteman I, Michault A, Murri S, Frangeul L, Vaney MC, Lavenir R, Pardigon N, Reynes JM, Pettinelli F, Biscornet L, Diancourt L, Michel S, Duquerroy S, Guigon G, Frenkiel MP, Brehin AC, Cubito N, Despres P, Kunst F, Rey FA, Zeller H, Brisse S. Genome microevolution of chikungunya viruses causing the Indian Ocean outbreak. PLoS Med. 2006;3(7):e263. doi: 10.1371/journal.pmed.0030263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simizu B, Yamamoto K, Hashimoto K, Ogata T. Structural proteins of Chikungunya virus. J Virol. 1984;51(1):254–258. doi: 10.1128/jvi.51.1.254-258.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, McKnight A, Takeuchi Y, Hoshino H, Clapham PR. Cell-to-cell fusion, but not virus entry in macrophages by T-cell line tropic HIV-1 strains: a V3 loop-determined restriction. Virology. 1995;209(2):696–700. doi: 10.1006/viro.1995.1307. [DOI] [PubMed] [Google Scholar]
- Simmons G, Reeves JD, Grogan CC, Vandenberghe LH, Baribaud F, Whitbeck JC, Burke E, Buchmeier MJ, Soilleux EJ, Riley JL, Doms RW, Bates P, Pohlmann S. DC-SIGN and DC-SIGNR bind ebola glycoproteins and enhance infection of macrophages and endothelial cells. Virology. 2003;305(1):115–123. doi: 10.1006/viro.2002.1730. [DOI] [PubMed] [Google Scholar]
- Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci U S A. 2004;101(12):4240–4245. doi: 10.1073/pnas.0306446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons G, Wool-Lewis RJ, Baribaud F, Netter RC, Bates P. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J Virol. 2002;76(5):2518–2528. doi: 10.1128/jvi.76.5.2518-2528.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KR, Pavri KM. Experimental studies with chikungunya virus in Aedes aegypti and Aedes albopictus. Acta Virol. 1967;11(6):517–526. [PubMed] [Google Scholar]
- Sourisseau M, Schilte C, Casartelli N, Trouillet C, Guivel-Benhassine F, Rudnicka D, Sol-Foulon N, Le Roux K, Prevost MC, Fsihi H, Frenkiel MP, Blanchet F, Afonso PV, Ceccaldi PE, Ozden S, Gessain A, Schuffenecker I, Verhasselt B, Zamborlini A, Saib A, Rey FA, Arenzana-Seisdedos F, Despres P, Michault A, Albert ML, Schwartz O. Characterization of reemerging chikungunya virus. PLoS Pathog. 2007;3(6):e89. doi: 10.1371/journal.ppat.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strang BL, Takeuchi Y, Relander T, Richter J, Bailey R, Sanders DA, Collins MK, Ikeda Y. Human immunodeficiency virus type 1 vectors with alphavirus envelope glycoproteins produced from stable packaging cells. J Virol. 2005;79(3):1765–1771. doi: 10.1128/JVI.79.3.1765-1771.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss EGS, J H. Structure and replication of the alphavirus genome. In: Schlesinger SSM, editor. The Togaviruses and Flaviviruses. New York: Plenum Press; 1986. pp. 35–90. [Google Scholar]
- Tsetsarkin KA, Vanlandingham DL, McGee CE, Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3(12):e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashishtha M, Phalen T, Marquardt MT, Ryu JS, Ng AC, Kielian M. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J Cell Biol. 1998;140(1):91–99. doi: 10.1083/jcb.140.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille M, Moutailler S, Coudrier D, Rousseaux C, Khun H, Huerre M, Thiria J, Dehecq JS, Fontenille D, Schuffenecker I, Despres P, Failloux AB. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2(11):e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wool-Lewis RJ, Bates P. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J Virol. 1998;72(4):3155–3160. doi: 10.1128/jvi.72.4.3155-3160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Martin TD, Carrington M, KewalRamani VN. Raji B cells, misidentified as THP-1 cells, stimulate DC-SIGN-mediated HIV transmission. Virology. 2004;318(1):17–23. doi: 10.1016/j.virol.2003.09.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CHIKV pseudotype neutralization. Neutralization curves for wild type CHIKV envelope and A226V mutant with sera from seven different CHIKV infected individuals. Infectivity of pseudotyped virus is shown as % of no serum control with each point representing the average of one experiment performed in triplicate. Results are representative of two independent experiments.
Enhancement of CHIKV infection by lectins. DC-SIGN and DC-SIGNR expression on 293T-Rex cells was determined 24 hours after induction using an antibody to the AU1 tag. Parental cells are shown as the solid black histogram, while DC-SIGN and DC-SIGNR are dark gray and light gray respectively.