Abstract
3-Iodoindoles have been synthesized by the iodocyclization of N,N-dialkyl-o-(1-alkynyl)anilines, obtained by the Pd/Cu catalyzed coupling of terminal acetylenes with N,N-dialkyl-o-iodoanilines. These 3-iodoindoles undergo palladium-catalyzed Sonogashira and Suzuki coupling reactions to yield 1,2,3-trisubstituted indoles. These reactions have been applied to parallel library synthesis utilizing commercially available terminal acetylenes and boronic acids. The aforementioned chemistry has also been carried out on a chlorinated Wang resin as a solid support, affording 1,2,3,5-tetrasubstituted indoles after cleavage from the support. A diverse 42-member library of highly substituted indoles has been synthesized.
Introduction
Indoles are very important in medicinal chemistry and the indole moiety is prevalent in numerous naturally-occurring and synthetic biologically active compounds.1 It is one of the most important nitrogen-containing pharmacophores,2 and is present in various drugs.1b,1g Due to the importance of the indole nucleus, many synthetic approaches to this ring system have been developed in our research group and others and reported in the literature for the synthesis of substituted indoles.3 Biologically active natural products are a good indicator of lead structures that might possess biological activity. Due to the biological importance of compounds containing the indole nuclei, it is quite likely that libraries of low molecular weight indoles will display similar activity and thus serve as valuable tools for drug development. Several methods are known for the synthesis of indoles in solution phase4 and on a solid support5 by combinatorial methods, but 3-iodoindoles have not previously been examined as key intermediates for indole library synthesis.
Yamanaka et al. have reported the coupling of 3-iodoindoles with terminal acetylenes, but satisfactory results were obtained only when the N atom of the indole was protected with an electron-withdrawing 1-methanesulfonyl group.6 With an electron-donating group on the N atom of the 3-iodoindole, the C-I bond is electron-rich and this appears to limit further functionalization at the 3 position of the indole by palladium-catalyzed coupling reactions.
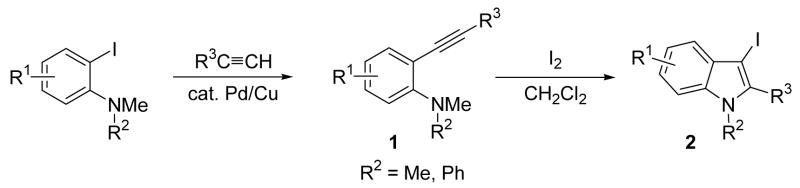
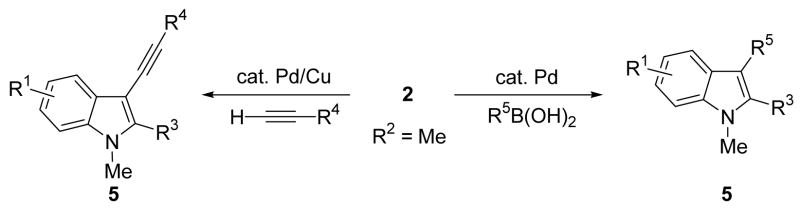
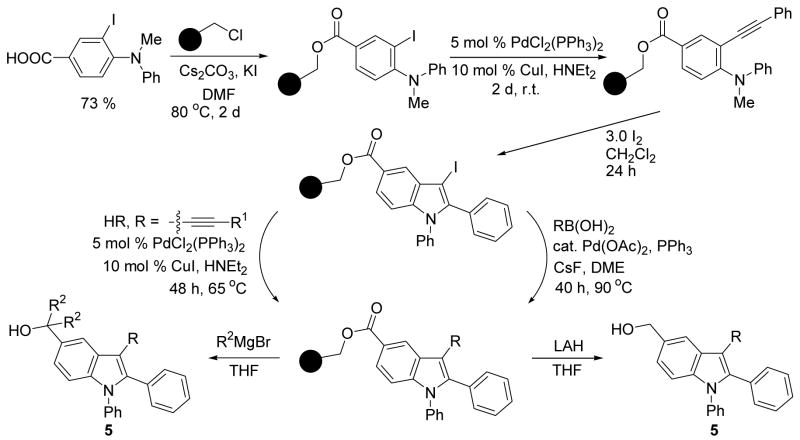
Previously, in our laboratory, we have synthesized N,N-dialkyl-o-(1-alkynyl)anilines (1) by coupling terminal acetylenes with N,N-dialkyl-o-iodoanilines in the presence of a Pd/Cu catalyst, which on iodocyclization yield 3-iodoindoles (2) in excellent yields (Scheme 1).7 We have previously reported individual examples of Sonogashira8 and Suzuki-Miyaura9 cross-coupling reactions, which provide the corresponding 1,2,3-trisubstituted indoles in good yields (Scheme 2).6b We further optimized each of these processes in order to adapt them for library generation. We have previously also reported individual examples of these two coupling reactions on a solid support, followed by cleavage by base.10 The development of a solid phase version of this chemistry allows the multistep synthesis of highly substituted indoles and eliminates cumbersome purification steps. We herein report the successful synthesis of 1,2,3,5-tetrasubstituted indoles on a solid support by slight modifications of our earlier procedure and alternative cleavage reactions (Scheme 3).
Scheme 1.
Scheme 2.
Scheme 3.
Results and Discussion
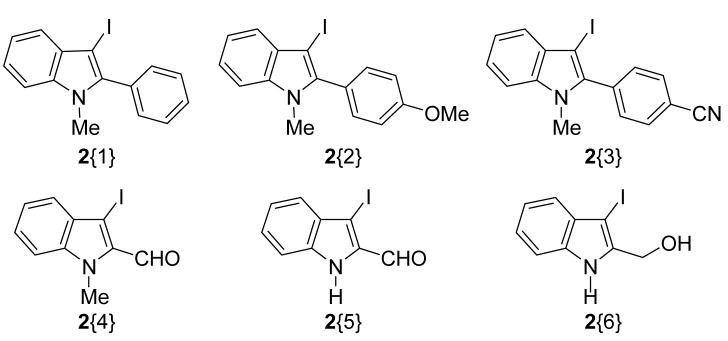
Our previous work on 3-iodoindole synthesis reported good yields of single cyclization products from the corresponding N,N-dimethyl- or N-methyl, N-phenyl-o-(1-alkynyl)anilines (1). After the iodocyclization step in the former case, the N-atom of the 3-iodoindole is protected by a methyl group, and, in the latter case, by a phenyl group. Our desire for a low molecular weight indole library led us to choose methyl as the preferred N-protecting group. Therefore, our choice of R2 was a methyl group in our solution phase library synthesis. 3-Iodoindole 2{1} was synthesized as our basic scaffold by using our previous cyclization method.7 The 3-iodoindoles 2{2} and 2{3} were similarly synthesized from the corresponding N, N-dimethyl-o-(1-alkynyl)anilines 1. Due to certain limitations in the types of R1 and R2 groups that can be employed in our iodocyclization methodology, we synthesized the 3-iodoindoles 2{4}11 and 2{5}12 by literature methods, while the 3-iodoindole 2{6} was obtained by treatment of 2{5} with NaBH4. Thus, we choose a subset of 3-iodoindoles on the basis of the ease of synthesis from readily available starting materials and with different electron-donating and electron-withdrawing functionalities at the 2-position of the indoles (Figure 1).
Figure 1.
3-Iodoindole sublibrary.
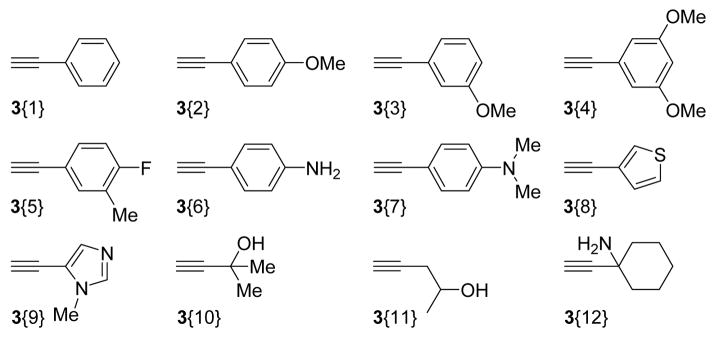
The terminal alkyne sublibrary was chosen on the basis of commercially available acetylenes. Attempts were made to include heteroatoms in the acetylenes that could impart drug-like, hydrogen bond donor and/or acceptor properties to the indoles after Sonogashira coupling (Figure 2). For similar reasons, acetylenes 3{5} and 3{8} were chosen due to the increasing popularity of fluorine13 and sulfur14 atoms in drug molecules.
Figure 2.
Terminal acetylene sublibrary.
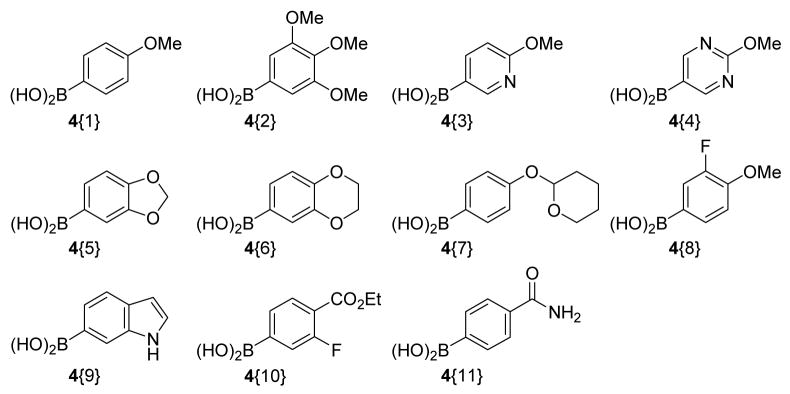
The boronic acids for the Suzuki-Miyaura reactions were also chosen on the basis of their commercial availability and their ability to provide the requisite diversity and drug-like properties to the indole scaffold after subsequent cross-coupling reactions (Figure 3). For instance, the methoxy-containing boronic acids 4{1} and 4{2} were chosen with a view towards increasing the polarity of the substituted indole. The N- heterocyclic boronic acids 4{3}, 4{4}, the O- heterocyclic boronic acids 4{5}, 4{6}, 4{7}, the indolylboronic acid 4{9}, and the benzamido boronic acid 4{11} were chosen to include heteroatoms and increase the drug-like nature of the corresponding indoles. The fluorine-containing acids 4{8} and 4{10} were desirable due to the importance of fluorine in medicinal chemistry.
Figure 3.
Boronic acid sublibrary.
Having chosen these sublibraries, we proceeded to prepare a diverse library of 1,2,3-trisubstituted indoles via solution phase chemistry as outlined in Scheme 2 and 1,2,3,5-tetrasubstituted indoles using a chlorinated Wang resin as the solid support as depicted in Scheme 3. The crude products have been analyzed by LC/MS, followed by purification by preparative HPLC or flash chromatography.
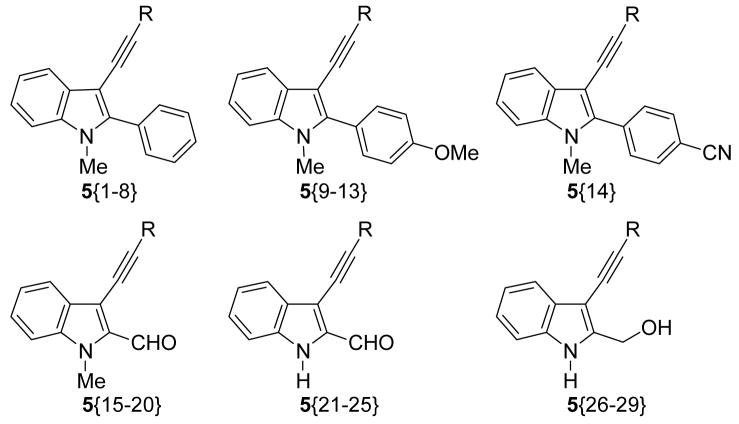
A summary of the results of the library synthesis is provided in Tables 1–3. Most of the crude products were subjected to preparative HPLC. Purities in the range of 70–100% have been achieved after purification. Most of the Sonogashira coupling reactions proceeded well, except for those run with the terminal alkynes 3{11} and 3{12}. Suzuki-Miyaura reactions with the boronic acids 4{10} and 4{11} with electron-withdrawing groups failed to give the desired coupling products. The boronic acids 4{3} and 4{8} gave decent yields of the coupling products 5{32} and 5{33} and excellent purities when reacted with 3-iodoindole 2{2}, but failed to give the corresponding trisubstituted indoles when coupled with 3-iodoindole 2{3}. On the solid support, the cleavage by MeMgBr was successful, but EtMgBr failed to give the anticipated products. Out of a total of 51 palladium-catalyzed processes attempted, around 80% were successful.
Table 1.
Library Data for Compounds 5{1–29}
 | |||
|---|---|---|---|
| compound | R | yielda (%) | purityb (%) |
| 5{1} | 4-MeOC6H4 | 12 | 97 |
| 5{2} | 3,5-dimethoxyphenyl | 38 | 94 |
| 5{3} | 4-fluoro-3-methylphenyl | 47 | 99 |
| 5{4} | 4-H2NC6H4 | 27 | 95 |
| 5{5} | 4-Me2NC6H4 | 30 | 79 |
| 5{6} | 1-amino-1-cyclohexyl | - | - |
| 5{7} | 2-hydroxypropyl | - | - |
| 5{8} | (1-hydroxy-1-methyl)ethyl | 13 | 70 |
| 5{9} | C6H5 | 43 | 99 |
| 5{10} | 3,5-dimethoxyphenyl | 34 | 98 |
| 5{11} | 4-Me2NC6H4 | 59 | 97 |
| 5{12} | 3-thiophenyl | 20 | 93 |
| 5{13} | (1-hydroxy-1-methyl)ethyl | 33 | 90 |
| 5{14} | C6H5 | 45 | 98 |
| 5{15} | C6H5 | 89 | 100 |
| 5{16} | 4-MeOC6H4 | 90 | 97 |
| 5{17} | 3-MeOC6H4 | 79 | 95 |
| 5{18} | 3,5-dimethoxyphenyl | 94 | 91 |
| 5{19} | 1-methyl-1H-imidazol-5-yl | 82 | 90 |
| 5{20} | (1-hydroxy-1-methyl)ethyl | 77 | 95 |
| 5{21} | C6H5 | 52 | 100 |
| 5{22} | 3,5-dimethoxyphenyl | 52 | 90 |
| 5{23} | 1-methyl-1H-imidazol-5-yl | 36 | 98 |
| 5{24} | 3-thiophenyl | 76 | 93 |
| 5{25} | (1-hydroxy-1-methyl)ethyl | 43 | 96 |
| 5{26} | 4-MeOC6H4 | 21 | 86 |
| 5{27} | 3-MeOC6H4 | 36 | 92 |
| 5{28} | 3,5-dimethoxyphenyl | 3 | 100 |
| 5{29} | (1-hydroxy-1-methyl)ethyl | 13 | 95 |
Isolated yield after preparative HPLC.
UV purities determined at 214 nm after preparative HPLC.
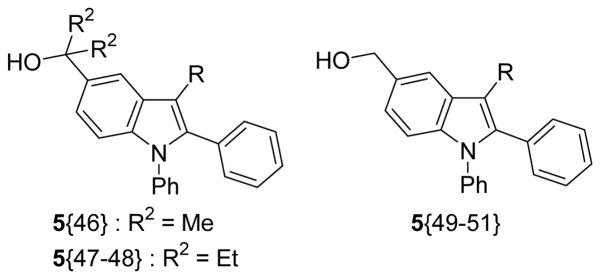
Table 3.
Library Data for Compounds 5{46–51} Synthesized on a Solid Support
 | |||
|---|---|---|---|
| compound | R | yielda (%) | purityb (%) |
| 5{46} | C6H5 | 69c | <90d |
| 5{47} | 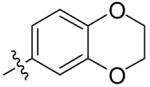 |
- | |
| 5{48} |
|
- | |
| 5{49} | C6H5 | 60c | <90d |
| 5{50} | 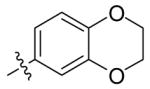 |
54 | 91 |
| 5{51} |
|
64 | 95 |
Isolated yield after preparative HPLC.
UV purities determined at 214 nm after preparative HPLC.
Isolated yield after flash chromatography.
Purities determined by H1 NMR spectroscopy after flash chromatography.
Our goal in synthesizing these low molecular weight heterocycles is for use in high-throughput screening projects. Therefore, we carried out an in silico evaluation of these library members to determine their agreement with Lipinski’s15 “rule of five” and Veber’s rules.16 The SYBYL17 program was used for the calculation of molecular weight, clog P, the number of hydrogen bond donors and acceptors, and the number of rotatable bonds for each library member (Table 4). According to these rules a potential drug molecule is more drug-like and more bioavailable if the clog P value is not more than 5, the molecular weight is less than 500, the hydrogen bond acceptors are not more than 10, the hydrogen bond donors are not more than 5, and the rotatable bonds in the molecule are not more than 12. One Lipinski violation is allowed for potential drug design. All of the indole library members are Lipinski compliant and no molecule has more than one Lipinski violation. The only violation that a molecule in the library had was clog P, which points towards potential solubility and delivery issues.
Table 4.
In silico parameters for gauging oral availability/drug-likeness
| Mean | St. Dev. | Range | |
|---|---|---|---|
| Clog P | 5.1 | 1.9 | 0.6 – 8.0 |
| Mol. Weight | 317 | 53 | 227–433 |
| H-Bond Acceptors | 2.0 | 0.9 | 0 – 4 |
| H-Bond Donors | 0.8 | 0.8 | 0 – 3 |
| Rotatable Bonds | 4.1 | 1.1 | 2 – 6 |
Conclusions
In conclusion, the synthesis of 4-iodoindoles and subsequent palladium-catalyzed Sonogashira and Suzuki-Miyaura cross-coupling reactions with various commercially available terminal alkynes and boronic acids have allowed the construction of a 42-member library of highly substituted indoles. The chemistry has been successfully transferred to a solid support and diversity has been achieved at the 5-position by different cleavage reactions. The average yield of the library was 46% and the average purity after purification was 94%.
Supplementary Material
Table 2.
Library Data for Compounds 5{30–45}
 | |||
|---|---|---|---|
| compound | Ar | yielda (%) | purityb (%) |
| 5{30} | 4-MeOC6H4 | 23 | 100 |
| 5{31} | 3,4,5-trimethoxyphenyl | 79 | 83 |
| 5{32} | 3-fluoro-4-methoxyphenyl | 42 | 100 |
| 5{33} | 2-methoxy-5-pyridinyl | 50 | 98 |
| 5{34} | benzo[1,3]dioxol-5-yl | 39 | 99 |
| 5{35} | 2-methoxy-5-pyrimidinyl | 59 | 99 |
| 5{36} | 4-H2NC(O)C6H4 | - | - |
| 5{37} | 4-EtO2C-3-FC6H3 | - | - |
| 5{38} | 3-fluoro-4-methoxyphenyl | - | - |
| 5{39} | 2-methoxy-5-pyridinyl | - | - |
| 5{40} | 2,3-dihydrobenzo[1,4]dioxin-6-yl | 84 | 89 |
| 5{41} | 2-methoxy-5-pyrimidinyl | 2 | 100 |
| 5{42} | 6-indolyl | 25 | 94 |
| 5{43} | benzo[1,3]dioxol-5-yl | 9 | 100 |
| 5{44} | 6-indolyl | - | - |
| 5{45} | 4-(tetrahydropyran-2-yloxy)phenyl | 9 | 91 |
Isolated yield after preparative HPLC.
UV purities determined at 214 nm after preparative HPLC.
Acknowledgments
We would like to thank Frank Schoenen for useful discussions and David Hill at the University of Kansas NIH Center of Excellence in Chemical Methodologies and Library Development for obtaining the NMR spectra of representative library compounds. We thank the National Institute of General Medical Sciences (R01 GM070620 and R01 GM079593) and the National Institutes of Health Kansas University Chemical Methodologies and Library Development Center of Excellence (P50 GM069663) for support of this research; Johnson Matthey, Inc. and Kawaken Fine Chemicals Co., Ltd. for donations of palladium catalysts; and Frontier Scientific and Synthonix for donations of boronic acids.
Footnotes
Supporting Information Available. Experimental details and full characterization of previously unknown sublibrary members and a representative 20 library members, including 1H and 13C NMR spectra and HRMS data. This information is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For reviews, see: Sundberg RJ. Prog Heterocycl Chem. 1989;1:111.Sundberg RJ. Indoles. Academic Press; New York: 1996. Saxton JE. Nat Prod Rep. 1986;3:357. doi: 10.1039/np9860300353.1987;4:591.1989;6:1.Ezquerra J, Pedregal C, Lamas C. J Org Chem. 1996;61:5804.Toyota M, Ihara M. Nat Prod Rep. 1998;15:327.Gribble GW. J Chem Soc, Perkin Trans. 2000;1:1045.Mori H, Yoshimi N. Mutat Res. 2001;480:201. doi: 10.1016/s0027-5107(01)00200-7.Tokuyama H, Fukuyama T. Kagaku Kogyo. 2001;52:416.Xiong WN, Yang CG, Jiang B. Bioorg Med Chem. 2001;9:1773. doi: 10.1016/s0968-0896(01)00070-0.Lobo AM, Prabhakar S. J Heterocycl Chem. 2002;39:429.
- 2.(a) Balkenhohl F, von dem Bussche-Hünnefeld C, Lansky A, Zechel C. Angew Chem, Int Ed Engl. 1996;35:2289. [Google Scholar]; (b) Nefzi A, Ostresh JM, Houghten RA. Chem Rev. 1997;97:449. doi: 10.1021/cr960010b. [DOI] [PubMed] [Google Scholar]; (c) Loughlin WA. Aust J Chem. 1998;51:875. [Google Scholar]
- 3.(a) Hegedus LS. Angew Chem. 1988;100:1147. [Google Scholar]; (b) Larock RC, Yum EK. J Am Chem Soc. 1991;113:6689. [Google Scholar]; (c) Arcadi A, Cacchi S, Carnicelli V, Marinelli F. Tetrahedron. 1994;50:437. [Google Scholar]; (d) Larock RC, Yum EK, Refvik MD. J Org Chem. 1998;63:7652. [Google Scholar]; (e) Cacchi S, Fabrizi G, Pace P, Marinelli F. Synlett. 1999:620. [Google Scholar]; (f) Arcadi A, Cacchi S, Fabrizi G, Moro L. Eur J Org Chem. 1999;64:1137. [Google Scholar]; (g) Larock RC. J Organomet Chem. 1999;576:111. [Google Scholar]; (h) Rodriguez AL, Koradin C, Dohle W, Knochel P. Angew Chem, Int Ed Engl. 2000;39:2488. doi: 10.1002/1521-3773(20000717)39:14<2488::aid-anie2488>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]; (i) Takeda A, Kamijo S, Yamamoto Y. J Am Chem Soc. 2000;122:5662. [Google Scholar]; (j) Pete B, Toke L. Tetrahedron Lett. 2001;42:3373. [Google Scholar]; (k) Kamijo S, Yamamoto Y. Angew Chem, Int Ed. 2002;41:3230. doi: 10.1002/1521-3773(20020902)41:17<3230::AID-ANIE3230>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]; (l) Kamijo S, Yamamoto Y. J Am Soc Chem. 2002;124:11940. doi: 10.1021/ja0272742. [DOI] [PubMed] [Google Scholar]; (m) Yasuhara A, Takeda Y, Suzuki N, Sakamoto T. Chem Pharm Bull. 2002;50:235. doi: 10.1248/cpb.50.235. [DOI] [PubMed] [Google Scholar]; (n) Rossiter S. Tetrahedron Lett. 2002;43:4671. [Google Scholar]; (o) Zhou T, Chen ZC. Synth Commun. 2002;32:903. [Google Scholar]; (p) Li JJ, Gribble GW. Palladium in Heterocyclic Chemistry. Pergamon; Oxford, UK: 2000. pp. 73–187. [Google Scholar]
- 4.(a) Gorohovsky S, Meir S, Shkoulev V, Byk G, Gellerman G. Synlett. 2003:1411. doi: 10.1023/b:modi.0000047511.72472.ea. [DOI] [PubMed] [Google Scholar]; (b) Jennings LD, Foreman KW, Rush TS, III, Tsao DHH, Mosyak L, Kincaid SL, Sukhdeo MN, Sutherland AG, Ding W, Kenny CH, Sabus CL, Liu H, Dushin EG, Moghazeh SL, Labthavikul P, Petersen PJ, Tuckman M, Ruzin AV. Bioorg Med Chem. 2004;12:5115. doi: 10.1016/j.bmc.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 5.(a) Fagnola MC, Candiani I, Visentin G, Cabri W, Zarini F, Mongelli N, Bedeschi A. Tetrahedron Lett. 1997;38:2307. [Google Scholar]; (b) Smith AL, Stevenson GI, Swain CJ, Castro JL. Tetrahedron Lett. 1998;39:8317. [Google Scholar]; (c) Macleod C, Hartley RC, Hamprecht DW. Org Lett. 2002;4:75. doi: 10.1021/ol016924o. [DOI] [PubMed] [Google Scholar]; (d) Yamazaki K, Kondo Y. J Comb Chem. 2002;4:191. doi: 10.1021/cc010079j. [DOI] [PubMed] [Google Scholar]; (e) Wacker DA, Kasireddy P. Tetrahedron Lett. 2002;43:5189. [Google Scholar]; (f) Yamazaki K, Nakamura Y, Kondo Y. J Chem Soc, Perkin Trans. 2002;119:2137. [Google Scholar]; (g) Yamazaki K, Nakamura Y, Kondo Y. J Org Chem. 2003;68:6011. doi: 10.1021/jo0340307. [DOI] [PubMed] [Google Scholar]; (h) Knepper K, Braese S. Org Lett. 2003;5:2829. doi: 10.1021/ol034851y. [DOI] [PubMed] [Google Scholar]; (i) Tois J, Franzen R, Koskinen A. Tetrahedron. 2003;59:5395. [Google Scholar]; (j) Nicolaou KC, Roecker AJ, Hughes R, van Summeren R, Pfefferkorn JA, Winssinger N. Bioorg Med Chem. 2003;11:465. doi: 10.1016/s0968-0896(02)00386-3. [DOI] [PubMed] [Google Scholar]; (k) Lee SH, Clapham B, Koch G, Zimmermann J, Janda KD. J Comb Chem. 2003;5:188. doi: 10.1021/cc020079z. [DOI] [PubMed] [Google Scholar]; (l) Tanaka H, Ohno H, Kawamura K, Ohtake A, Nagase H, Takahashi T. Org Lett. 2003;5:1159. doi: 10.1021/ol020230d. [DOI] [PubMed] [Google Scholar]; (m) Rosenbaum C, Katzka C, Marzinzik A, Waldmann H. Chem Commun. 2003;15:1822. doi: 10.1039/b305497g. [DOI] [PubMed] [Google Scholar]; (n) Dai WM, Guo DS, Sun LP, Huang XH. Org Lett. 2003;5:2919. doi: 10.1021/ol0350543. [DOI] [PubMed] [Google Scholar]; (o) Wu Z, Ede NJ. Org Lett. 2003;5:2935. doi: 10.1021/ol035153g. [DOI] [PubMed] [Google Scholar]; (p) Rosenbaum C, Baumhof P, Mazitschek R, Mueller O, Giannis A, Waldmann H. Angew Chem, Int Ed. 2004;43:224. doi: 10.1002/anie.200352582. [DOI] [PubMed] [Google Scholar]; (q) Ohno H, Tanaka H, Takahashi T. Synlett. 2004;3:508. [Google Scholar]; (r) Ohno H, Tanaka H, Takahashi T. Synlett. 2005;7:1191. [Google Scholar]; (s) Mun HS, Ham WH, Jeong JH. J Comb Chem. 2005;7:130. doi: 10.1021/cc049922e. [DOI] [PubMed] [Google Scholar]; (t) Sun LP, Dai WM. Angew Chem, Int Ed. 2006;45:7255. doi: 10.1002/anie.200602523. [DOI] [PubMed] [Google Scholar]; (u) Main CA, Petersson HM, Rahman SS, Hartley RC. Tetrahedron. 2008;64:901. [Google Scholar]
- 6.Sakamoto T, Nagano T, Kondo Y, Yamanaka H. Chem Pharm Bull. 1988;36:2248. [Google Scholar]
- 7.(a) Yue D, Larock RC. Org Lett. 2004;6:1037. doi: 10.1021/ol0498996. [DOI] [PubMed] [Google Scholar]; (b) Yue D, Yao T, Larock RC. J Org Chem. 2006;71:62. doi: 10.1021/jo051549p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Sonogashira K. In: In Metal-Catalyzed Cross-Coupling Reactions. Diederich F, Stang PJ, editors. Chapter 5. Wiley-VCH; Weinheim, Germany: 1998. p. 203. [Google Scholar]; (b) Sonogashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975:4467. [Google Scholar]
- 9.(a) Miyaura N, Suzuki A. Chem Rev. 1995;95:2457. [Google Scholar]; (b) Suzuki A. J Organomet Chem. 1999;576:147. [Google Scholar]
- 10.Yao T, Yue D, Larock RC. J Comb Chem. 2005;7:809. doi: 10.1021/cc050062r. [DOI] [PubMed] [Google Scholar]
- 11.Zhang H, Larock RC. Org Lett. 2001;3:3083. doi: 10.1021/ol010124w. [DOI] [PubMed] [Google Scholar]
- 12.Choshi T, Sada T, Fujimoto H, Nagayama C, Sugino E, Hibino S. J Org Chem. 1997;62:2535. doi: 10.1021/jo962038t. [DOI] [PubMed] [Google Scholar]
- 13.(a) Hudlicky M, Pavlath AE, editors. A Critical Review. American Chemical Society; Washington, D. C: 1995. Chemistry of Organic Fluorine Compounds II. ACS Monograph 187. [Google Scholar]; (b) Hiyama T, editor. Chemistry and Applications. Springer-Verlag; New York: 2000. Organofluorine Compounds. [Google Scholar]
- 14.(a) Rajbhoj AS, Gaikwad ST, Chondhekar TK. J Ind Chem Soc. 2007;84:987. [Google Scholar]; (b) Walash MI, El-Brashy AM, Metwally MS, Abdelal AA. Bull Kor Chem Soc. 2004;25:517. [Google Scholar]; (c) Roopnarinesingh ES, Steventon GB, Harris RM, Waring RH, Mitchell SC. Drug Metabolism and Drug Interactions. 2005;21:75. doi: 10.1515/dmdi.2005.21.2.75. [DOI] [PubMed] [Google Scholar]; (d) Hiller KO, Hodd PL, Willson RL. Chem Biol Interact. 1983;47:293. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- 15.(a) Lipinski CA, Lombardo F, Dominay BW, Feeney PJ. Adv Drug Del Rev. 1997;23:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]; (b) Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Adv Drug Del Rev. 2001;46:3. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 16.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, Kopple KD. J Med Chem. 2002;45:2615. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]
- 17.SYBYL, version 8.0. The Tripos Associate; St. Louis, MO: 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.