Abstract
Schistosomes develop successfully in susceptible snails but are encapsulated and killed in resistant ones. Mechanism(s) shaping these outcomes involves the parasites ability to evade the snail’s defenses. RNA analysis from resistant (BS-90), non-susceptible (LAC2) and susceptible (NMRI) juvenile Biomphalaria glabrata to Schistosoma mansoni revealed that stress related genes, heat shock protein 70 (Hsp 70) and reverse transcriptase (RT), were dramatically co-induced early in susceptible snails, but not in resistant/non-susceptible ones. These transcripts were, however, down regulated upon exposure to irradiated parasites although penetration behavior of irradiated vs normal parasites were the same, indicating that Hsp 70 regulation was elicited by infection and not injury. Understanding molecular events involved in stress response transcriptional regulation of Hsp 70 in juvenile snails could pave a way towards the identification of genes involved in schistosome/snail interactions.
Keywords: trematode, Schistosoma mansoni infection, juvenile Biomphalaria glabrata, susceptible, resistant, non-susceptible, stress response, irradiation, miracidia, heat shock protein 70, reverse transcriptase
Introduction
Schistosomiasis remains prevalent in tropical developing countries where an estimated 600 million people are at risk for exposure (Fenwick, 2006). The parasitic schistosome (causative agent of the disease) life cycle involves two hosts; the larval miracidia infect freshwater snails where they develop into cercariae, infective to the human host. In the absence of an effective vaccine, control efforts to reduce disease transmission rely on the use of molluscicides and targeted drug therapy e.g. in school aged children. Although these intervention programs, especially when combined with aggressive surveillance, help to curtail transmission for the short-term, re-infection in both snail and human populations however poses a challenge for the long-term control of the disease. An alternative control method based on the ability to block transmission of the parasite in the snail-host has, therefore, been suggested (Hubendick, 1958).
The outcome of the snail/schistosome interaction is governed by the genetic make-up of both the snail and parasite (Lewis, et al., 2001) with successful miracidial development occurring only in susceptible/compatible but not resistant/incompatible snails. To bring the goal of intramolluscan transmission blocking strategies to fruition, an understanding of mechanism(s) underlying the complex outcomes of snail/schistosome infections is required. To investigate events early after miracidial infection of the snail, we examined genes that are differentially regulated upon early exposure to miracidia in juvenile Biomphalria glabrata snails that are resistant (BS-90), susceptible (NMRI), or non-susceptible (LAC2) to infection. The LAC2-line was derived from NMRI snails that failed to accommodate parasite infection as previously described (Cooper, et al., 1994). In resistant snails, invading miracidia are encapsulated and killed by mechanisms involving hemolymph and hemocyte components of the snail’s innate defense system (Adema and Loker, 1997, Bayne and Yoshino, 1989). Non-susceptible (LAC2) snails inhibit parasite development but resistance may be incomplete. Our initial gene expression studies using a suppression subtractive cDNA cloning approach revealed the specific induction of several novel genes, including the stress response heat shock protein 70 (Hsp 70), in juvenile susceptible, but not in resistant snails (Ittiprasert et al. manuscript submitted). Contrary to previous studies that used adult snails, however, Lockyer et al. (Lockyer, et al., 2004) reported the specific induction of Hsp 70 in resistant but not susceptible snails upon parasite infection. To resolve this uncertainty regarding the differential gene expression of Hsp 70 between resistant and susceptible snails, especially during early time points when subjected to stress from heat shock (abiotic stress) and following parasite (biotic stress) infection, we decided to further examine the differential Hsp 70 induction in parasite resistant vs susceptible juvenile snails before and after either heat shock or infection. In addition, modulation of the reverse transcriptase (RT) encoding domain of the snail non-LTR retrotransposon, nimbus, in relation to stress (heat shock and infection) was also investigated (Raghavan, et al., 2003, Raghavan, et al., 2007). Mobile genetic elements (MGEs), such as non-LTR-retrotransposons have been shown to be activated by pathogens, environmental stress, and wounding (Mhiri, et al., 1997, Wessler, 1996). Although factors that elicit the activation of nimbus RT in the snail are unknown, in preliminary studies we showed the induction of the nimbus RT transcript upon S. mansoni infection of two different susceptible snail-lines (M-line and NMRI). The RT transcript was, however, neither induced in the resistant (BS-90) nor in the non-susceptible snail-line (LAC2) (Knight, et al., in press). Thermal stress induction of the nimbus RT is investigated for the first time in this study, but transcriptional activation of Hsp 70 in response to heat shock has previously been investigated in the B. glabrata embryonic cell-line (Bge) as well as in other mollusks (Cellura, et al., 2006, Laursen, et al., 1997, Piano, et al., 2002). Furthermore, Hsp 70 has been reported as being differentially regulated during the intra-molluscan larval (sporocyst and cercarial) stages of the parasite (Neumann, et al., 1993). We therefore reasoned that a stress-related response operating in both snail host and parasite could be involved in the dynamics of successful/unsuccessful outcomes of the snail host/parasite relationship.
Gamma-irradiated or UV attenuated miracidia fail to develop in susceptible B. glabrata (Lie, et al., 1983, Ruelas, et al., 2007). Therefore, to rule out any possibility that changes measured in expression levels of Hsp 70 and RT between the aforementioned snail strains following infection may be attributable to an injury mediated response (sustained during miracidial penetration of the snail) rather than to the developing parasites, we performed the experiments described herein using either normal or attenuated parasites for snail exposures.
Here, we report that S. mansoni infection of B. glabrata juvenile snails causes the induction (up-regulation) of the stress response gene Hsp 70. This induction was dramatic in the susceptible juvenile snail and occurred early post exposure and coincidentally, within the same time period with elevated expression of the RT of the non-LTR-retrotransposon, nimbus (Raghavan, et al., 2007). In contrast, inductions of both loci were less pronounced and occurred following a longer lag time (post exposure) in resistant and non-susceptible snails exposed to normal miracidia, and was absent in all snail strains following exposure to attenuated miracidia. We propose that further investigation of the differential regulation of stress-related co-transcribed genes in juvenile B. glabrata in relation to S. mansoni infection may provide a novel opportunity towards identifying mechanism(s) leading to arresting parasite development in the snail host.
Materials and Methods
Snail
Snails (4–6 mm in diameter) of the parasite resistant (100%) BS-90 (Paraense and Correa, 1963), susceptible NMRI (Newton, 1955) and non-susceptible LAC2 lines were used in these studies. The LAC2 snail was derived from NMRI snails (F0) after 17 generations of selection (self-fertilization) from susceptible snails that failed to support parasite infection (Cooper, et al., 1994). The LAC2 line exhibits 25% susceptibility compared to 95–100% susceptibility of parent NMRI snails. Prior to either heat shock or parasite exposure, snails were treated overnight with ampicillin (100 μg/ml) and maintained in aerated sterile freshwater. Unstressed snails were maintained at room temperature (between 23–28°C) or subjected to stress by heat shock at 32°C (water bath) for different time periods (0, 5, 1, 2, 3 hours). The heat shock temperature of 32°C was chosen because at this temperature, no significant difference in the death rate was detected between the resistant and susceptible snails. For parasite exposures, snails were exposed individually to either normal or irradiated miracidia (10/snail) for different time points (0, 5, 10, 24 and 48 hours). Irradiation of miracidia was performed immediately after hatching eggs using a Mark 1 cesium-137 irradiator (JL Shepherd, San Fernando, CA) at NIAID, National Institute of Health (Bethesda, Maryland). Irradiated parasites (irradiated at 5–20 Krad, 2,126 rads/min), were used immediately for the snail exposures.
Analysis of miracidium penetration behavior
To determine the influence of radiation on the ability of miracidia to penetrate B. glabrata, BS-90 and NMRI snails (5.5–6.0 mm in diameter) were exposed to either normal miracidia or irradiated miracidia. Using a micropipette and a dissecting microscope, 20 ± 1 miracidia were counted as they were drawn up into the glass pipette then placed into each well of 12-well plates. Approximately 2 ml of aged tap water was added to each well prior to placing the snail into the well. At various time intervals over a one-hour period, the wells were examined for the presence of free-swimming miracidia.
Qualitative RT-PCR
Whole body RNA from either unexposed juvenile (4–6 mm in diameter) or adult snails (8–9 mm in diameter) was extracted using RNA Bee as previously described (Miller, et al., 2001). Trace amounts of contaminating genomic DNA were removed by treating all RNA samples with DNase I prior to using for cDNA synthesis according to manufacturer’s instructions (Promega, WI). Control first strand reactions were also done without reverse transcriptase to eliminate all possibility that second strand amplification products would be non-specific i.e. generated from residual DNA contamination in the RNA samples. Second strand reactions were performed as previously described (Raghavan, et al., 2003) using B. glabrata Hsp 70 [Genbank Acc.no. L44127, (Laursen, et al., 1997)] gene-specific primers F: 5′-AGGCGTCGACATTCAGGTCTA-3′ and R: 5′-TGGTGATGTTGTTGGTTTTACCA-3′or primers for the B. glabrata myoglobin housekeeping gene (Raghavan, et al., 2003). Second strand reactions were also conducted substituting water in place of cDNA template as negative control. PCR reactions (25 μl) containing 1 μl 10 mM dNTPs, 0.125 μl GoTaq DNA polymerase (5U/μl; Promega, WI), 2.5 μl 10× PCR buffer, 1.25 μl of each 10 μM primers and 3 μl of heat inactivated (65°C) 1st strand cDNA and 11.72 μl of sterile water were performed as follows; 94°C denaturation for 5 min, followed by 30 cycles of 94°C for 30 sec, 58°C for 30 sec and 72°C for 30 sec and 7 min at 72°C for the final extension. Amplicons (10 μl) were resolved by TBE agarose gel (1.2%) electrophoresis and visualized by ethidium bromide staining.
Quantitative RT-PCR
RNA samples of 9 individual resistant (BS-90), non-susceptible (LAC2) and susceptible (NMRI) snails exposed at different time periods (0, 5, 10, 24 and 48 hours) to either normal or irradiated miracidia were used. DNA contaminating RNA samples was removed by DNase I treatment (Promega Corporation, WI) as described previously (Miller, et al., 2001). Eighty nanograms of DNase-treated RNA was analyzed by Real Time quantitative RT-PCR using FullVelocity SYBR Green QRT-PCR master mix according to manufacturer’s instructions (Stratagene, CA). At the start of each assay, the validation method (ABI manufacturer’s instructions) and melting curve was optimized by using different input RNA (four different sample dilutions) containing Hsp 70 and myoblogin (house keeping gene) to confirm that the amplification efficiencies of both genes were equal and contained a single peak at the expected temperature to indicate target-specific amplification (data not shown). The 25 μl final reaction volume contained; 200 nM of each gene specific primers for nimbus RT (Raghavan, et al., 2003) and Hsp 70 or 50 nM of the housekeeping gene, myoglobin primers (Raghavan, et al., 2003). Reactions were performed in a one-step format with the first stand cDNA synthesis and Real time PCR amplification done in a single tube in triplicate as described previously (Knight, et al., 2009). Each reaction contained a no template negative control to rule out non-specific amplification from contamination in the buffers and data was normalized using myoglobin as the reference housekeeping gene. The transcript levels of Hsp 70 and nimbus RT during different time points following exposure were normalized relative to myoglobin expression. Fold differences of gene expression were calculated by comparative Ct method with the formula indicated below (Livak and Schmittgen, 2001):
P-values were calculated by comparing the delta-Ct value (N= 9) for each group using a Student’s t-test to determine if the differentially expressed transcripts between unexposed and exposed groups (5–48 hours) were significant.
Results
Hsp 70 and nimbus RT are constitutively expressed in B. glabrata snails
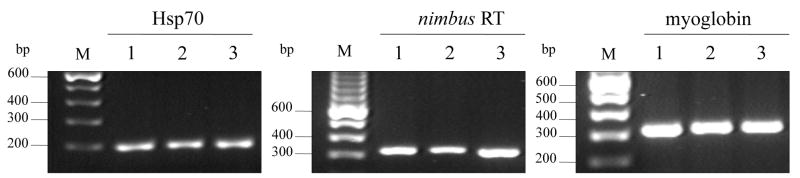
To further investigate expression of Hsp 70 in normal unchallenged snails, qualitative RT-PCR was performed using RNA from pools of resistant, non-susceptible or susceptible snails. cDNA templates prepared with and without reverse transcriptase in the first strand reaction were amplified by PCR using primers corresponding to Hsp 70 B. glabrata sequence of this gene in Genbank [Acc.no. L44127 (Laursen, et al., 1997)], overlapping a recently cloned Hsp 70 EST (Acc.no. GH717124) isolated from a suppressive subtracted cDNA library containing enriched sequences from the exposed susceptible snail (Ittiprasert, et al. manuscript submitted). Hsp 70 gene specific primers (forward and reverse) utilized in the present study encompassed nucleotide regions 1,480 to 1,679 bp of the existing cDNA sequences in Genbank (for an expected PCR product size of 199 bp) and did not overlap nucleotide regions (1,081 to 1,341 bp) amplified by the Hsp 70 primer sequences (forward and reverse) utilized by Lockyer et al. [Acc. No. CK136129 (Lockyer, et al., 2004)]. As shown in Figure 1, amplification of cDNA template prepared from normal resistant (lane 1, BS-90), non-susceptible (lane 2, LAC2) and susceptible (lane 3, NMRI) snails using the Hsp 70 gene specific primers produced amplicons of the expected size (199 bp) and with the same intensity in all snail strains. Parallel amplifications using equal amounts of the same first strand cDNA from the snails with primers corresponding to the nimbus RT, (Raghavan, et al., 2003) and the housekeeping myoglobin gene, resulted in expected size products of 324 bp and 350 bp, respectively indicating similar constitutive expression of these transcripts in all snail strains. Control reactions using cDNA prepared by excluding reverse transcriptase in the first strand reaction showed no PCR products were obtained, an indication that there was no genomic DNA contamination in the RNA used for the first strand cDNA reaction (data not shown). Likewise, in PCR conducted where cDNA templates were omitted in the reaction (negative controls) no amplicons were synthesized indicating there was no contamination in the reaction buffers utilized. Based on these results we can conclude that qualitative end point second strand PCR of cDNA templates prepared from unstressed snails reveals the constitutive expression of Hsp 70, nimbus RT and myoglobin. Relative differences between the strains of the basal expressions of Hsp 70, nimbus RT, and myoglobin were, however, difficult to measure using this assay. The results also confirmed the constitutive expression of nimbus RT in B. glabrata as we previously reported (Raghavan, et al., 2003). Similar qualitative analysis of adult snail RNA (of all three strains) also showed high levels of expression of Hsp 70 and nimbus RT in unstressed older snails (data not shown).
Figure 1.
Constitutive expressions of Hsp 70, nimbus RT and myoglobin transcripts in normal (1) resistant BS-90 (2) non-susceptible LAC2, and (3) susceptible NMRI snails anylysed by qualitative RT-PCR using the same cDNA templates but with primer sets (forward and reverse) corresponding to the specific transcripts. Amplicons of the expected size, 199 bp for Hsp 70, 324 bp for nimbus RT, and 350 bp for myoglobin are shown by ethidium bromide staining after agarose gel electrophoresis. The molecular size (bp) of the 100 bp ladder loaded in parallel is shown on the left.
Thermal stress induces differences in the magnitude and temporal expression of Hsp 70 and nimbus RT transcripts between susceptible and resistant snails
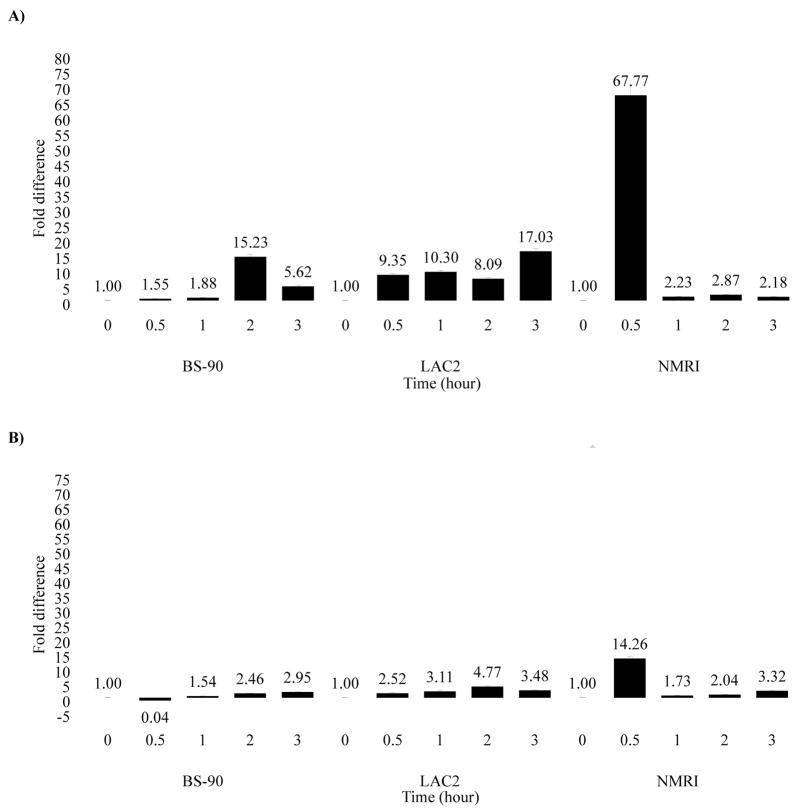
We used quantitative real time PCR to assess differences in the levels of induction of Hsp 70 and nimbus RT in resistant, non-susceptible and susceptible juvenile snails following heat shock. Thus kinetics of expression of transcripts encoding Hsp 70 (Fig. 2A), in response to heat shock, was performed using RNA from individual resistant (BS-90), non-susceptible (LAC2) or susceptible (NMRI) snails maintained for different time periods at 32°C, temperature above the normal laboratory maintenance for B. glabrata (23–28°C). Expression of Hsp 70 was assessed relative to the level in snails incubated at ambient temperature (23°C) and normalized using the housekeeping gene; myoglobin.
Figure 2.
Real-time Q-RT-PCR analysis of the differential gene expression of (A) Hsp 70 and (B) nimbus RT in snails that are resistant (BS-90), non-susceptible (LAC2) and susceptible (NMRI) following incubation at either 23 °C or 32 °C for various time periods (0.5 to 3 hours). Fold difference of gene expression was calculated by comparing expression levels of the transcripts between unstressed and stressed snails by the comparative Ct method using the formula described in Materials and Methods. Significant P-values of < 0.05, < 0.01 and < 0.005 are indicated by *, ** and ***, respectively to show the significance of gene expression by using Student’s t-test.
In susceptible NMRI snails, a significant increase (68 fold) in Hsp 70 expression was detected within 30 minutes after heat shock proceeded by significantly reduced levels (2.2 fold) after 3 hours (Fig. 2A). In contrast, Hsp 70 induction (15 fold) in the BS-90 snails occurred only after 2 hours lag time (post-heat-shock) followed by reduced (5.6 fold) expression after 3 hours. A similar slower and less intense response to heat shock was manifested in the LAC2 snail where expression of Hsp 70 increased gradually throughout the 3 hours incubation period. Likewise, induction (14.3 fold) of the nimbus RT transcript post-heat shock (Fig. 2B) occurred within the same 30 minutes time frame as observed for the elevated expression of Hsp 70 in NMRI snails. In contrast, BS-90 and LAC2 snails showed significantly lower levels of induction of the nimbus RT transcript when challenged by thermal stress. Thus, both Hsp 70 and nimbus RT are induced differentially both in magnitude and temporal expression, between these susceptible, non-susceptible and resistant snails in response to thermal stress causing the strong and early co-induction of these transcripts in the juvenile susceptible snail, but slower and less significant induction in either resistant or non-susceptible snails.
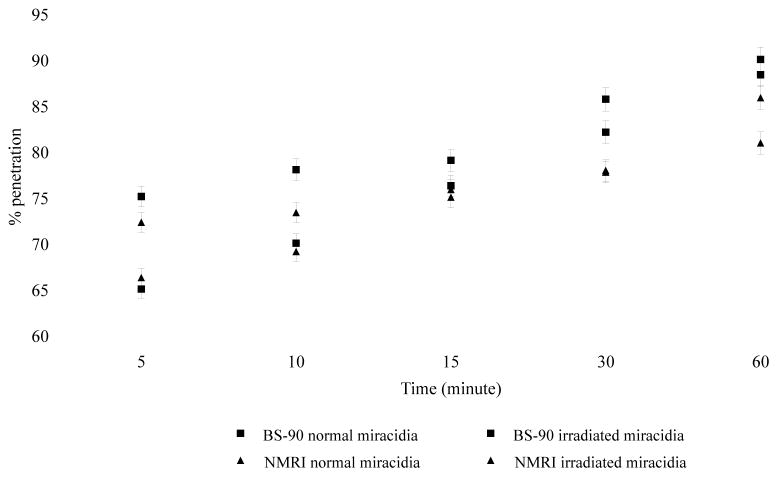
Gamma (γ)-irradiation does not impair penetration of miracidia, but affects their development in the snail
To determine if γ-irradiation would impair snail penetration by miracidia, we compared the ability of normal and 20 Krad irradiated miracidia to penetrate either the resistant or susceptible snail. Under light microscopy, no behavioral differences were observed between irradiated or normal miracidia in their ability to search, probe, attach and penetrate snail tissues. Most miracidia in each group, especially during the early time points, attached either to tentacles or the leading edge of the head-foot of the snail before penetration. In addition, no behavioral differences were observed between miracidia exposed to the different snail stocks. Penetration over the period of exposure (1 hour) revealed a rapid initial penetration (defined as a reduction in number of free-swimming miracidia) within 5 minutes (65–73% penetration) followed by a gradual increase to 85% penetration (Fig. 3). No difference was detected between the ability of irradiated vs non-irradiated miracidia to penetrate snail tissues in the different snail strains. Furthermore, while susceptible snails exposed to normal miracidia shed viable cercariae within 4 weeks, none of the strains infected with irradiated miracidia produced cercariae by 3 months post-exposure.
Figure 3.
Comparision of the penetration ability of normal miracidia and miracidia attenuated by irradiation (20 Krads) in either resistant or susceptible snails. Penetration behavior of normal and attenuated miracidia was observed microscopically over one hour time period and parasites remaining in water at different time intervals (5, 10, 15, 30, 60 minutes) counted to estimate the % penetration. Error bar = standard deviation (n = 12)
We were confident, therefore, that whatever differences might be detected following exposure to normal vs. irradiated miracidia, could not be attributable to the lack of penetration by the irradiated miracidia.
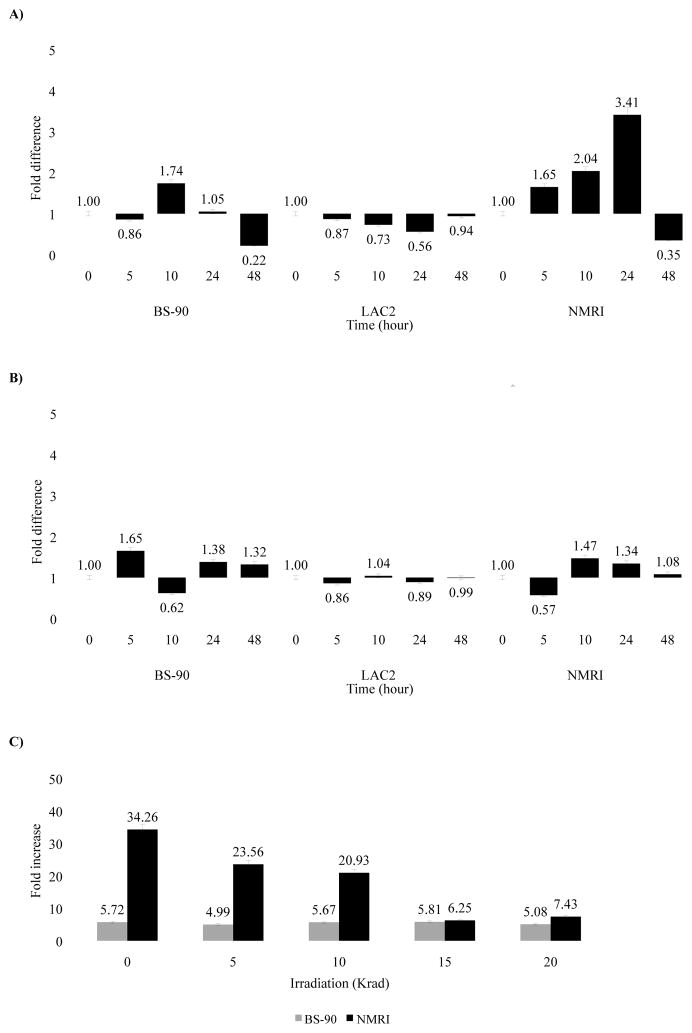
A stress response, manifested by elevated Hsp 70 expression, occurs in juvenile susceptible snails exposed to normal but not attenuated miracidia
To examine induction of Hsp 70 in snails exposed to either normal or attenuated miracidia, RNA samples from all snail strains were analyzed after exposure to normal (Fig. 4A) or attenuated (Fig. 4B) miracidia by real time PCR. As shown in Figure 4A, following different times (0 to 48 hours) of exposure of NMRI snails to normal miracidia, a gradual increase in Hsp 70 expression occurred, reaching a 3.41 fold increase at 24 hours. However, BS-90 snails exposed also to normal miracidia showed no change in lower levels of induction within the same time period. Similarly, no increase in expression was detected in the LAC2 snails. In contrast (Fig. 4B), snails exposed to 20 Krad irradiated-miracidia showed insignificant induction of Hsp 70 in all snails, including NMRI strain at all times after exposure to attenuated parasites.
Figure 4.
Real-time Q-RT-PCR analysis of the differential gene expression of Hsp 70 in resistant (BS-90), non-susceptible (LAC2) and susceptible (NMRI) snails in either unexposed snails (0 hour) or upon exposure for various time periods (5 to 48 hours) to (A) non-irradiated miracidia, (B) 20 Krad irradiated miracidia, or (C) exposed for 5 hours to miracidia attenuated with different doses of irradiation (5, 10, 15, 20 Krad). Fold difference in gene expression was calculated by comparing expression levels of transcripts between unstressed and stressed snails by the comparative Ct method using the formula described in Materials and Methods. Significant P-values of < 0.05 is indicated by * to show the significance of gene expression determined using Student’s t-test.
To assess whether miracidia attenuated with different doses of irradiation would affect the expression of Hsp 70 in juvenile snails in a dose dependent fashion, changes in expression of Hsp 70 was monitored in resistant and susceptible snails at 5 hours post-exposure to either normal miracidia (0 Krad) or to those attenuated at 5, 10, 15 or 20 Krad (Fig. 4C) radiation. Induction of Hsp 70 in susceptible snails was inversely related to the dose of irradiation used to attenuate the parasites. In BS-90 snails, exposure to either normal or similarly-attenuated parasites did not significantly alter the levels of the Hsp 70 expression.
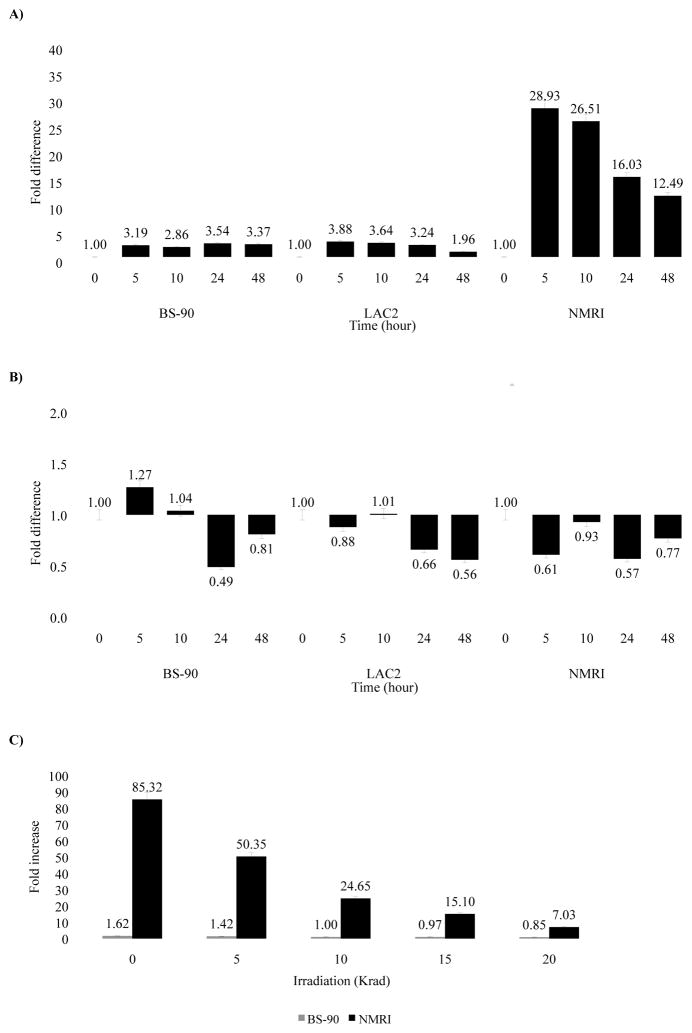
Co-induction of nimbus RT and Hsp 70 transcripts occurs in susceptible, but not resistant snails upon exposure to normal miracidia
To determine whether the kinetics of nimbus RT induction coincides with that of Hsp 70 following exposure to either normal or attenuated miracidia, we examined the expression of the nimbus RT transcript (Raghavan, et al., 2003, Raghavan, et al., 2007) in the RNA samples described above. Susceptible snails exposed 5 hours to normal miracidia showed a significant increase (28.9 fold) in nimbus RT with a steady decrease (26.51 to 12.49 fold) in the levels of expression between 10 to 48 hours post-exposure (Fig. 5A). In contrast, no dramatic induction of nimbus RT was seen in either the BS-90 or LAC2 snails during the same time period post exposure. Similarly, no significant induction of nimbus RT occurred in all strains upon exposure to attenuated miracidia (Fig. 5B). Comparable experiments using adult snails of all strains (resistant, non-susceptible, susceptible) showed that after exposure to normal miracidia, snails at this age showed insignificant changes in the expression of nimbus RT transcript (data not shown).
Figure 5.
Real-time Q-RT-PCR analysis of the differential expression of nimbus RT in resistant (BS-90), non-susceptible (LAC2) and susceptible (NMRI) snails in either non-exposed snails (0 hour) or upon exposure for various time periods (5 to 48 hours) to (A) non-irradiated miracidia, (B) 20 Krad irradiated miracidia, or (C) exposed for 5 hours to miracidia attenuated with different doses of irradiation (5, 10, 15, 20 Krad). Fold difference in gene expression was calculated by comparing expression levels of the transcripts between unstressed and stressed snails by the comparative Ct method using the formula described in Materials and Methods. Significant P-values of < 0.05, < 0.01 and < 0.005 are indicated by *, ** and ***, respectively to show the significance of gene expression determined using Student’s t-test.
We next examined whether varying the dose of irradiation to the miracidia would affect changes observed in the expression of RT in a dose dependent fashion. Snails were exposed to either normal miracidia (0 Krad) or to parasites subjected to increasing doses of irradiation (5, 10, 15 or 20 Krad). At 5 hours post-exposure, RT induction in NMRI snails (Fig. 5C) correlated inversely with the dose of irradiation applied to the miracidia. Comparable to results in Figure 5A, exposing the juvenile susceptible snails to normal parasites produced a similarly strong induction of RT (85.32 fold increase) at 5 hours that declined when these snails were exposed to parasites treated with increasing doses of irradiation. No such modulation occurred in the juvenile resistant snails.
Discussion
This study demonstrates that both heat shock and infection elicit a stress response in juvenile B. glabrata manifested by the differential induction of Hsp 70 and nimbus RT transcripts in susceptible, non-susceptible and resistant snails. Although the degree of upregulation is different when snails are stressed either by heat or infection, the kinetics of induction of these transcripts coincided (induced within the same time period) in the susceptible snail. In other studies, e.g. in the silkworm (Bombyx mori), Kimura et al. (Kimura, et al., 2001) showed that retrotransposon SINE Bm1-RNA induced upon virus infection (baculovirus) occurs without the coincidental expression of Hsp 70 reflecting that, in this case, different RNA polymerases (II and III) direct the expression of these two genes in response to stress. In our study, the coincidental transcription of Hsp 70 and nimbus RT in the juvenile susceptible snail in response to heat or infection may, therefore, indicate that a common molecular pathway governs the transcription of these genes in the snail.
The lower levels of induction of Hsp 70 and nimbus RT in the resistant and non-susceptible (BS-90 and LAC2, respectively) juvenile snails could reflect a major behavioral difference towards stress (from heat or infection) in these snails compared to susceptible juvenile snails. It is possible that these clear differences may be a factor in why these snails respond differently to the parasite. The less dramatic/slower induction of Hsp 70 and nimbus RT in response to stress in these snails may represent a genetic trade-off associated with the cost of resistance/non-susceptibility. The existence of costs to resistance/non-susceptibility of B. glabrata to schistosomes and another digenean trematode, Echinostoma caproni has been previously reported (Langand, et al., 1998). It will therefore, be interesting to investigate whether similar behavioral differences towards stress exist in genetically selected juvenile B. glabrata snails that are either resistant or susceptible to echinostomes (Langand, et al., 1998, Mitta, et al., 2005). Increased expression of transcripts corresponding to retrotransposons in response to stress has been observed in several studies leading to suggestions that MGEs represent another class of stress genes in the cell (Kimura, et al., 2001, Li, et al., 1999, Teneng, et al., 2007, Wessler, 1996). Although the induction of Hsp 70 following bacterial infection in mollusks has been described (Cellura, et al., 2006, Cellura, et al., 2007, Song, et al., 2006), this is the first study to show the elevated co-expression of nimbus RT of retrotransposon RT and Hsp 70 in a snail in response to a metazoan parasite infection. Other open reading frame (ORF) domains within nimbus (Raghavan, et al., 2007) in response to stress have yet to be investigated.
Heat shock proteins are a class of proteins (including Hsp 70 among others) that function as molecular chaperones in protein folding and transport. Although high levels of these proteins are triggered in response to an increase in damaged or abnormal proteins brought on by a variety of stress factors (Lindquist and Craig, 1988, Welch, 1987, Young, 1990), they are also known to function in a housekeeping cell-repair capacity (Gething and Sambrook, 1992). Thus, our observation that Hsp 70 is expressed constitutively in unstressed circumstances in the resistant, non-susceptible and susceptible snails, as has also been previously reported for other mollusks, may not be surprising (Martynova, et al., 2007). These results are, however, different from those previously reported (Lockyer, et al., 2004). Lockyer, et al. used differential display RT-PCR to analyze changes in gene expression profiles following S. mansoni infection of resistant and susceptible snails. From their results Hsp 70 expression was not detected in normal snails (neither resistant nor susceptible snails) unless infected. Also, unlike our observations, the up-regulation of Hsp70 was only detected in resistant but not susceptible snails upon parasite exposure.
Several reasons may account for the discrepancies between these two studies. One possible explanation may be that in the earlier study, adult but not juvenile snails were used for infection. Age-related changes in the constitutive expression of Hsp 70 in mollusks have not been completely resolved. From a recent study, however the mitochondrial chaperone Hsp 60 transcript was shown to decrease substantially with age in two different species of mollusks, while Hsp 70 levels remained unchanged with age (Ivanina, et al., 2008). Similar age related studies to monitor differences in levels of expression of Hsp 70 in adult vs juvenile B. glabrata snails have not yet been conducted. Age variation in B. glabrata susceptibility to S. mansoni infection however has been well documented with snails, sometimes displaying susceptibility as juveniles but not as adults (Richards and Minchella, 1987). It is possible, therefore, that the more pronounced differential expression of both Hsp 70 and nimbus RT in susceptible juvenile snails, compared to resistant snails (in response to infection), could reflect the fact that as juveniles B. glabrata snails are more susceptible to infection than their adult counterparts (Niemann and Lewis, 1990). Although a molecular explanation has not yet been found for the noted variation with age in B. glabrata susceptibility to schistosomes, it may be reasonable to assume that molecular changes made by the parasite in juvenile and adult snails may not necessarily be identical. We already know that the genetics of snail resistance to schistosome infection in adult and juvenile snails are markedly different, with the former displaying a single gene Mendelian trait (with resistance dominant), while juvenile resistance to the parasite is complex (Lewis, et al., 2001). Thus, on the basis of this age-related difference in the genetics of susceptibility and the aforementioned inherent variations associated with age in snail behavior towards the parasite we may be unable to draw direct comparisons between our study and previous reports regarding the differential Hsp 70 regulation in B. glabrata/S. mansoni infections without taking into account age groups of snails utilized for each study.
Another plausible explanation for the noted differences between our data and the earlier (Lockyer, et al., 2004) study could also be due to variations (insertions and deletions) we found in the gene specific forward primer sequence utilized for RT-PCR in the previous study. When aligned to existing B. glabrata Hsp 70 genomic and cDNA sequences in GenBank considerable sequence variation was detected at the 3′-end of their forward primer. In the present study, gene specific Hsp 70 primers were deliberately designed from in silico cDNA sequences showing sequence similarity to a cloned Hsp 70 EST isolated from a susceptible snail suppressive subtractive library (Ittiprasert et al. manuscript submitted). These primers encompassed a region within the full-length sequence that did not overlap the region amplified by primers used by Lockyer et al. (Lockyer, et al., 2004). Because different isoforms of Hsp 70 can be expressed in mollusks in response to stress (Encomio and Chu, 2007) it is possible that the region we amplified may not have been available in templates employed by Lockyer et al. (Lockyer, et al., 2004) due to inconsistencies and variations in methods used to prepare RNA samples from the two labs. Recent amplifications undertaken using primer sequences (both forward and reverse), as reported by Lockyer et al. (Lockyer, et al., 2004) using conditions reported by these workers with our cDNA templates (from both adult and juvenile snails) produced multiple amplicons (size range from 200–700 bp), contrary to the single 263 bp band that was expected.
Since a pronounced defense response is known to operate in resistant B. glabrata snails upon S. mansoni infection, it is possible that the more tolerant behavior towards stress seen in these snails may allow for more immediate turnover of immune related proteins that help prevent parasite development. In this vein we have shown for some recently described transcripts that may play a role in disease resistance in the snail, e.g. proteolytic enzymes, Cathepsin B, (Myers, et al., 2008) and the antioxidant enzyme, peroxiredoxin (Knight, et al., 2009) that enhanced early induction of these genes differentiates resistance and non-susceptibility from snail susceptibility upon parasite exposure.
We do not yet know whether Hsp 70 or retrotransposon transcripts from incoming parasites play a role in circumventing snail host innate defenses. Since several gene profiling studies (Taft, et al., 2009, Verjovski-Almeida, et al., 2003) have shown the existence of these transcripts (Hsp 70 and nimbus RT) among ESTs generated from the intramolluscan stage parasite (sporocysts) it is reasonable to speculate that stress related genes (from both the snail host and parasite) may play some as yet undiscovered role in the snail host/parasite relationship. Thus, it is plausible that the eventual sequencing of Hsp 70 promotor/enchancer regions of the different snails utilized in this study (and of the parasite) may help to explain the dramatic differences in Hsp 70 expression seen here between resistant/susceptible juvenile snails in their response to infection.
Profiles of other known stress related transcripts, such as metallothionine, the heavy metal binding protein that is induced in response to environmental pollutants such as cadmium have not yet been assessed between the snails used in the present study. However, in a previous study (Salice and Roesijadi, 2002) showed that cadmium toxicity was significantly higher in BS-90 snails than in NMRI snails we used for the present study. Taken together, these results suggest that it may be prudent to systematically evaluate interactive effects of various stressors (elevated temperature, parasite infection, environmental pollution) on the survival of resistant and susceptible snails, especially as it is anticipated that resistant snails may one day be used as a form of biological control to help combat schistosomiasis.
Finally, exposure to attenuated and non-attenuated parasites shows differential gene regulation of both Hsp 70 and nimbus RT. This would indicate that some product from the developing parasite and not injury (e.g. during penetration) causes the induction of these genes. We do not yet know the factor(s) from the developing miracidia that might trigger the transcription of these genes.
In conclusion, we report that normal schistosome infection of susceptible but not resistant/non-susceptible juvenile snails causes the coincidental induction of the stress response gene Hsp 70 and the RT domain of the snail non-LTR-retrotransposon, nimbus. We suggest, therefore, that the induction of Hsp 70 and nimbus RT may serve as early biomarkers of parasite infectivity in the juvenile susceptible snail and may help to identify additional genes co-transcribed during early stages of host infection. The induction of both transcripts by normal, but not irradiated attenuated, miracidia indicates that understanding of the regulation of stress-related transcripts could provide a mechanism to disrupt parasite development in the snail.
Acknowledgments
We wish to thank Ms. Patricia Caspar for her assistance with the irradiation experiments. We also thank Drs Allen Cheever, Joanna Bridger and Alan Sher for their support, helpful discussions and critique of the manuscript. This work was funded by NIH-NIAID grant no. R01-AI63480
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adema C, Loker E. Specificity and immunobiology of larval digenean snail associations. In: Fried B, Graczyk TK, editors. Advances in Trematode Biology. CRC Press; Boca Raton, FL: 1997. pp. 229–263. [Google Scholar]
- 2.Bayne CJ, Yoshino TP. Depterminants of compatibility in mollusc-trematode parasitism. American Zoologist. 1989;29:339–409. [Google Scholar]
- 3.Cellura C, Toubiana M, Parrinello N, Roch P. HSP70 gene expression in Mytilus galloprovincialis hemocytes is triggered by moderate heat shock and Vibrio anguillarum, but not by V. splendidus or Micrococcus lysodeikticus. Developmetal & Comparative Immunology. 2006;30:984–997. doi: 10.1016/j.dci.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Cellura C, Toubiana M, Parrinello N, Roch P. Specific expression of antimicrobial peptide and HSP70 genes in response to heat-shock and several bacterial challenges in mussels. Fish and Shellfish Immunology. 2007;22:340–350. doi: 10.1016/j.fsi.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Cooper LA, Richards CS, Lewis FA, Minchella DJ. Schistosoma mansoni: relationship between low fecundity and reduced susceptibility to parasite infection in the snail Biomphalaria glabrata. Experimental Parasitology. 1994;79:21–28. doi: 10.1006/expr.1994.1055. [DOI] [PubMed] [Google Scholar]
- 6.Encomio VG, Chu FL. Heat shock protein (Hsp70) expression and thermal tolerance in sublethally heat-shocked eastern oysters Crassostrea virginica infected with the parasite Perkinsus marinus. Disease of Aquatic Organism. 2007;76:251–260. doi: 10.3354/dao076251. [DOI] [PubMed] [Google Scholar]
- 7.Fenwick A. Waterborne infectious diseases--could they be consigned to history? Science. 2006;313:1077–1081. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 8.Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 9.Hubendick B. A possible method of schistosome-vector control by competition between resistant and susceptible strains. Bulletin of the World Health Organization. 1958;18:113–116. [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanina AV, Sokolova IM, Sukhotin AA. Oxidative stress and expression of chaperones in aging mollusks. Comparative Biochemistry and Physiology. Part B, Biochemistry & Molecular Biology. 2008;150:53–61. doi: 10.1016/j.cbpb.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Kimura RH, Choudary PV, Stone KK, Schmid CW. Stress induction of Bm1 RNA in silkworm larvae: SINEs, an unusual class of stress genes. Cell Stress Chaperones. 2001;6:263–272. doi: 10.1379/1466-1268(2001)006<0263:siobri>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knight M, Bridger J, Ittiprasert W, Odoemelam E, Masabanda J, Miller A, Raghavan N. Endogeneous retrotransposon sequences of the Schistosoma mansoni intermediate snail host, Biomphalaria glabrata. In: Brindley PJ, editor. Mobile Genetic Elements in Metazoan Parasites. In press. [Google Scholar]
- 13.Knight M, Raghavan N, Goodall C, Cousin C, Ittiprasert W, Sayed A, Miller A, Williams DL, Bayne CJ. Biomphalaria glabrata peroxiredoxin: effect of Schistosoma mansoni infection on differential gene regulation. Mol Biochem Parasitol. 2009;167:20–31. doi: 10.1016/j.molbiopara.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langand J, Jourdane J, Coustau C, Delay B, Morand S. Cost of resistance, expressed as a delayed maturity, detected in the host-parasite system Biomphalaria glabrata/Echinostoma caproni. Heredity. 1998;80:320–325. [Google Scholar]
- 15.Laursen JR, di Liu H, Wu XJ, Yoshino TP. Heat-shock response in a molluscan cell line: characterization of the response and cloning of an inducible HSP70 cDNA. Journal of Invertebrate Pathology. 1997;70:226–233. doi: 10.1006/jipa.1997.4686. [DOI] [PubMed] [Google Scholar]
- 16.Lewis FA, Patterson CN, Knight M, Richards CS. The relationship between Schistosoma mansoni and Biomphalaria glabrata: genetic and molecular approaches. Parasitology. 2001;123(Suppl):S169–179. doi: 10.1017/s0031182001007831. [DOI] [PubMed] [Google Scholar]
- 17.Lie KJ, Jeong KH, Heyneman D. Acquired resistance in snails. Induction of resistance to Schistosoma mansoni in Biomphalaria glabrata. Internatioanl Journal for Parasitology. 1983;13:301–304. doi: 10.1016/0020-7519(83)90041-3. [DOI] [PubMed] [Google Scholar]
- 18.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 19.Li T, Spearow J, Rubin CM, Schmid CW. Physiological stresses increase mouse short interspersed element (SINE) RNA expression in vivo. Gene. 1999;239:367–372. doi: 10.1016/s0378-1119(99)00384-4. [DOI] [PubMed] [Google Scholar]
- 20.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 21.Lockyer AE, Noble LR, Rollinson D, Jones CS. Schistosoma mansoni: resistant specific infection-induced gene expression in Biomphalaria glabrata identified by fluorescent-based differential display. Experimental Parasitology. 2004;107:97–104. doi: 10.1016/j.exppara.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 22.Martynova MG, Bystrova OA, Shabelnikov SV, Margulis BA, Prokofjeva DS. Hsp70 in the atrial neuroendocrine units of the snail, Achatina fulica. Cell Biology International. 2007;31:413–419. doi: 10.1016/j.cellbi.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Mhiri C, Morel JB, Vernhettes S, Casacuberta JM, Lucas H, Grandbastien MA. The promoter of the tobacco Tnt1 retrotransposon is induced by wounding and by abiotic stress. Plant Molecular Biology. 1997;33:257–266. doi: 10.1023/a:1005727132202. [DOI] [PubMed] [Google Scholar]
- 24.Miller AN, Raghavan N, FitzGerald PC, Lewis FA, Knight M. Differential gene expression in haemocytes of the snail Biomphalaria glabrata: effects of Schistosoma mansoni infection. Int J Parasitol. 2001;31:687–696. doi: 10.1016/s0020-7519(01)00133-3. [DOI] [PubMed] [Google Scholar]
- 25.Mitta G, Galinier R, Tisseyre P, Allienne JF, Girerd-Chambaz Y, Guillou F, Bouchut A, Coustau C. Gene discovery and expression analysis of immune-relevant genes from Biomphalaria glabrata hemocytes. Developmental & Comparative Immunology. 2005;29:393–407. doi: 10.1016/j.dci.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Myers J, Ittiprasert W, Raghavan N, Miller A, Knight M. Differences in cysteine protease activity in Schistosoma mansoni-resistant and -susceptible Biomphalaria glabrata and characterization of the hepatopancreas cathepsin B Full-length cDNA. Journal of Parasitology. 2008;94:659–668. doi: 10.1645/GE-1410R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann S, Ziv E, Lantner F, Schechter I. Regulation of HSP70 gene expression during the life cycle of the parasitic helmith Schistosoma mansoni. European Journal of Biochemisty. 1993;212:589–596. doi: 10.1111/j.1432-1033.1993.tb17697.x. [DOI] [PubMed] [Google Scholar]
- 28.Newton WL. The establishment of a strain of Australorbis glabratus which combines albinism and high susceptibility to infection with Schistosoma mansoni. Journal of Parasitology. 1955;41:526–528. [PubMed] [Google Scholar]
- 29.Niemann GM, Lewis FA. Schistosoma mansoni: influence of Biomphalaria glabrata size on susceptibility to infection and resultant cercarial production. Experimental Parasitology. 1990;70:286–292. doi: 10.1016/0014-4894(90)90110-x. [DOI] [PubMed] [Google Scholar]
- 30.Paraense W, Correa L. Variations in susceptibility of populations of Australorbis blabratus to a strain of Schistosoma mansoni. Revista do Instituto de Medicina Tropical de São Paulo. 1963;5:15–22. [PubMed] [Google Scholar]
- 31.Piano A, Asirelli C, Caselli F, Fabbri E. Hsp70 expression in thermally stressed Ostrea edulis, a commercially important oyster in Europe. Cell Stress Chaperones. 2002;7:250–257. doi: 10.1379/1466-1268(2002)007<0250:heitso>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raghavan N, Miller AN, Gardner M, FitzGerald PC, Kerlavage AR, Johnston DA, Lewis FA, Knight M. Comparative gene analysis of Biomphalaria glabrata hemocytes pre-and post-exposure to miracidia of Schistosoma mansoni. Molecular and Biochemical Parasitology. 2003;126:181–191. doi: 10.1016/s0166-6851(02)00272-4. [DOI] [PubMed] [Google Scholar]
- 33.Raghavan N, Tettelin H, Miller A, Hostetler J, Tallon L, Knight M. Nimbus (BgI): an active non-LTR retrotransposon of the Schistosoma mansoni snail host Biomphalaria glabrata. International Journal for Parasitology. 2007;37:1307–1318. doi: 10.1016/j.ijpara.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Richards CS, Minchella DJ. Transient non-susceptibility to Schistosoma mansoni associated with atrial amoebocytic accumulations in the snail host Biomphalaria glabrata. Parasitology. 1987;95 (Pt 3):499–505. doi: 10.1017/s0031182000057929. [DOI] [PubMed] [Google Scholar]
- 35.Ruelas DS, Karentz D, Sullivan JT. Sublethal effects of ultraviolet b radiation on miracidia and sporocysts of Schistosoma mansoni: intramolluscan development, infectivity, and photoreactivation. Journal of Parasitology. 2007;93:1303–1310. doi: 10.1645/GE-1227.1. [DOI] [PubMed] [Google Scholar]
- 36.Salice CJ, Roesijadi G. Resistance to cadmium and parasite infection are inversely related in two strains of a freshwater gastropod. Environmetal Toxicology and Chemistry. 2002;21:1398–1403. [PubMed] [Google Scholar]
- 37.Song L, Wu L, Ni D, Chang Y, Xu W, Xing K. The cDNA cloning and mRNA expression of heat shock protein 70 gene in the haemocytes of bay scallop (Argopecten irradians, Lamarck 1819) responding to bacteria challenge and naphthalin stress. Fish Shellfish Immunology. 2006;21:335–345. doi: 10.1016/j.fsi.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 38.Taft AS, Vermeire JJ, Bernier J, Birkeland SR, Cipriano MJ, Papa AR, McArthur AG, Yoshino TP. Transcriptome analysis of Schistosoma mansoni larval development using serial analysis of gene expression (SAGE) Parasitology. 2009:1–17. doi: 10.1017/S0031182009005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teneng I, Stribinskis V, Ramos KS. Context-specific regulation of LINE-1. Genes Cells. 2007;12:1101–1110. doi: 10.1111/j.1365-2443.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 40.Verjovski-Almeida S, DeMarco R, Martins EA, Guimaraes PE, Ojopi EP, Paquola AC, Piazza JP, Nishiyama MY, Jr, Kitajima JP, Adamson RE, Ashton PD, Bonaldo MF, Coulson PS, Dillon GP, Farias LP, Gregorio SP, Ho PL, Leite RA, Malaquias LC, Marques RC, Miyasato PA, Nascimento AL, Ohlweiler FP, Reis EM, Ribeiro MA, Sa RG, Stukart GC, Soares MB, Gargioni C, Kawano T, Rodrigues V, Madeira AM, Wilson RA, Menck CF, Setubal JC, Leite LC, Dias-Neto E. Transcriptome analysis of the acoelomate human parasite Schistosoma mansoni. Nature Genetics. 2003;35:148–157. doi: 10.1038/ng1237. [DOI] [PubMed] [Google Scholar]
- 41.Welch WJ. The mammalian heat shock (or stress) response: a cellular defense mechanism. Adv Exp Med Biol. 1987;225:287–304.5. doi: 10.1007/978-1-4684-5442-0_26. [DOI] [PubMed] [Google Scholar]
- 42.Wessler SR. Turned on by stress. Plant retrotransposons. Current Biology. 1996;6:959–961. doi: 10.1016/s0960-9822(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 43.Young DB. Stress proteins and the immune response. Antonie Van Leeuwenhoek. 1990;58:203–208. doi: 10.1007/BF00548934. [DOI] [PubMed] [Google Scholar]