Abstract
Kisspeptin neurons in the arcuate nucleus play a pivotal role in the regulation of hypothalamic GnRH secretion in higher primates. To examine whether kisspeptin also influences the function of the primate pituitary directly, two experiments were performed in adult male rhesus monkeys. First, the distribution of kisspeptin containing cells in the pituitary was described using fluorescence immunohistochemistry. Second, the secretion of non-gonadotrophin adenohypophysial hormones (GH, prolactin and TSH) and cortisol in response to iv kisspeptin administration was examined. Eight animals were deeply anesthetised and transcardially perfused with 4% paraformaldehyde. Fluorescence immunohistochemistry was performed on 25 μm thick free-floating pituitary sections to localise immunopositive kisspeptin cells and to examine their relationship with immunostaining for LH, FSH, GH, prolactin, α-MSH, ACTH and GnRH. Kisspeptin cells were found in the intermediate lobe of all animals, and in four monkeys this neuropeptide was also observed in cells scattered in the periphery of the anterior lobe. Kisspeptin colocalised with α-MSH immunopositive cells in the intermediate lobe and, in 50% of the monkeys, with ACTH immuunopositive cells in the periphery of the adenohypophysis. There was no evidence for colocalisation of kisspeptin with gonadotrophs, somatotrophs or lactotrophs. Beaded kisspeptin axons were observed in the neural lobe. In addition, assay of plasma samples that had been collected for an earlier study documenting kisspeptin-10 induced LH release in male monkeys revealed that kisspeptin administration failed to influence circulating concentrations of GH, prolactin, TSH and cortisol. Release of all 4 of these non-gonadotrophic hormones, however, was stimulated markedly by NMDA, which is considered to act centrally. While the morphological findings of the present study are consistent with the notion that kisspeptin may act directly at the level of the pituitary, the nature of such an action remains to be defined.
Keywords: Kisspeptin, Monkey, Pituitary, Corticotroph, Melananotroph
Introduction
It is now well established that kisspeptin, which is encoded by the KiSS1 gene and signals at the kisspeptin receptor, KiSS1R (also referred to as G protein coupled receptor 54), provides a direct and robust stimulus to the hypothalamic network of GnRH neurons that govern gonadotrophin secretion from the anterior pituitary (1–4). As such, kisspeptin signalling has been proposed to represent the major central drive to the neuroendocrine axis governing gonadal function (2).
The question of whether kisspeptin signalling directly at the level of the pituitary is physiologically important, however, is less clear. In the rodent pituitary, KiSS1R immunostaining has been noted in a subpopulation of LHβ positive cells (5) and in Percoll fractioned ovine pituitary cells RT-PCR analysis indicated KiSS1R expression in gonadotrophs, lactotrophs and somatotrophs (6). KiSS1R mRNA has also been detected by RT-PCR in extracts of whole rat and human pituitary (5, 7–10). With regard to the ligand, an immunohistochemical study of rat pituitary has revealed kisspeptin staining in LHβ positive gonadotrophs and in unidentified cells (5). KiSS1 expression has been demonstrated by RT-PCR in extracts of whole pituitary from rat, ovine and human (5,7,8,10). It should also be noted that kisspeptin may reach the anterior pituitary from the hypothalamus, as indicated by the findings of kisspeptin in hypophysial-portal blood of sheep (6) and in perfusates of the monkey median eminence region (11).
Direct evidence for an endocrine/paracrine role of kisspeptin in regulating pituitary hormone release is inconsistent. In sheep, in which the hypothalamus and pituitary were surgically disconnected, iv administration of kisspeptin-10 failed to induce LH secretion (6) suggesting that kisspeptin induction of gonadotropin release is mediated exclusively by an indirect action of the peptide on the brain. While in vitro studies employing rat pituitary cells in primary culture indicated that kisspeptin had no effect on gonadotropin secretion (12,13), other studies of rat, ovine, bovine and porcine pituitary gland have described minor stimulatory effects on LH (6,10,14). Regarding non-gonadotrophic pituitary hormones, iv bolus administration of human kisspeptin-10 has been shown to increase circulating levels of GH in prepubertal Holstein heifers (15). A modest stimulatory effect of kisspeptin-10 on GH and prolactin release has also been reported in studies of cultured anterior pituitary cells from rodent and bovine (10,16).
For the foregoing reasons, we conducted the following two experiments. In the first, pituitary glands harvested during recent studies of the structural interactions between kisspeptin and GnRH neurones in the hypothalamus of the male monkey (17) were employed, using immunohistochemistry, to determine whether kisspeptin is expressed in the primate hypophysis and, if so, to attempt to identify the phenotype of the immunopositive kisspeptin cells. In the second, blood samples that had been banked after completing a study in which an iv bolus of kisspeptin-10 was administered to adult male monkeys to provoke an LH discharge (18), were used to determine whether kisspeptin also elicited release of non-gonadotrophic pituitary hormones. The results of these two experiments are described here.
Material and Methods
Animals
A group of eight adult male rhesus monkeys (Macaca mulatta, 5.6–14.7 kg body weight) were used to study the localisation of kisspeptin in the pituitary gland. Seven of the animals ranged in age from 4–13 years; the remaining animal was of unknown age. The monkeys were maintained under controlled photoperiod (lights on between 0700–1900 h) and temperature (approximately 21°C) in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. The experimental procedures were approved by the University of Pittsburgh Institutional Animal Care and Use Committee. Localisation of kisspeptin in the hypothalami of some of these animals has been previously reported (17). In addition, a group of five intact adult male rhesus monkeys (6–11 years old, 8–13 kg body weight), described in a previously published study examining kisspeptin induced LH secretion (18), provided the plasma samples for the endocrine component of the present investigation. These samples had been stored at -20°C.
Experimental protocols
Experiment 1. Immunohistochemical localisation of kisspeptin in the pituitary
Eight animals were used in this experiment. One to five months prior to collection of the pituitary gland, four of the animals were castrated as described previously (19). For this purpose, monkeys were first sedated with ketamine hydrochloride (10–20 mg/kg body weight, im; Ketaject, Phoenix Scientific Inc., St. Joseph, MO), anesthetised with 1.5–2.0 % isoflurane (Abbott Animal House, North Chicago, IL) in oxygen, and bilaterally castrated under sterile surgical conditions. Post-surgically, the animals received a single, daily im injection of penicillin (300,000U, Bicillin L-A, Wyeth Laboratories, Philadelphia, PA) and twice-daily im injections of ketoprofen (2 mg/kg body weight, Ketofen, Fort Dodge Animal House, Fort Dodge, IA) for four days. Before collecting the pituitary, blood samples were taken by venipuncture while the animals were sedated with ketamine hydrochloride. In order to perfuse the brain and pituitary, the animals were first sedated with ketamine hydrochloride and then deeply anesthetised with sodium pentobarbital (approximately 30 mg/kg body weight, iv; Nembutal sodium solution, Abbott Laboratories, North Chicago, IL). The thoracic cavity was exposed, the dorsal aorta clamped and the head perfused transcardially at a flow rate of approximately 40 ml/min: initially with approximately 1 L of physiological saline containing 2% sodium nitrite, and 5,000 U of heparin/L and immediately followed with 2–3 L of 4% paraformaldehyde in 0.1M PBS (pH 7.2). Following perfusion, the brain was removed from the cranium and the pituitary from the sella turcica. The pituitary was then immersed in 4% paraformaldehyde for a further 1–2 h. Tissue was then transferred to a 30% sucrose solution (in 0.1M PBS, pH 7.2) for at least 24 h at 4°C. In most cases, transverse 25 Zm sections of the pituitary gland were cut on a freezing microtome and stored in an antifreeze cryoprotectant solution at −20°C (20).
Experiment 2. Effects of human kisspeptin-10 on anterior pituitary hormone secretion
The effects of iv kisspeptin-10 administration on anterior pituitary hormone secretion were studied by assaying the concentration of GH, prolactin, TSH and cortisol (surrogate for ACTH) in plasma samples that had been collected in a previously published study (18) to document kisspeptin induced LH release in a group of five male monkeys under the control (vehicle infused) condition, which comprised a continuous saline (with 2.8% DMSO) infusion for four days. Three h before termination of the vehicle infusion, a bolus iv injection of kisspeptin-10 (10 or 30 μg) was administered and this was followed one h later with an iv injection of NMDA (10 mg/kg). NMDA is a glutamate receptor agonist that provides the hypothalamus with a non-specific neurochemical stimulus that leads to a generalised discharge of pituitary hormones (21). Note that doses of kisspeptin-10 employed were as much as 3-fold higher than the dose of 2 μg/animal that elicits an LH discharge resembling a spontaneous episode of secretion (22). A series of blood samples was collected immediately before and at 10, 20, 30, and 50 min after the injection of both kisspeptin and NMDA. For the purpose of the present study, these blood samples were assayed for GH, prolactin, TSH and cortisol. LH concentrations are reproduced from Ramaswamy et al. (18).
Antibodies and blocking peptides
The kisspeptin antibody (GQ2) was raised in sheep against synthetic human kisspeptin-54 and was kindly provided by Dr. Stephen Bloom (Imperial College London, Hammersmith Hospital, London, UK). The specificity of GQ2 has been previously documented by Dhillo et al. (23) for use in radioimmunoassay and validated by us for immunocytochemical studies of monkey hypothalamus (18). GQ2 was used at a dilution of 1:120,000 as previously described (17). Alexa Fluor 488 donkey anti-sheep IgG (Invitrogen Corporation, Carlsbad, CA) was used as the fluorescent secondary antibody to detect GQ2, as previously described.
For identification of pituitary gonadotrophs (both LH and FSH expressing), lactotrophs, somatotrophs, melanotrophs and corticotrophs the following antibodies and dilutions were used: polyclonal rabbit anti-recombinant cynomolgus (monkey) LH (1:100,000; AFP342994), polyclonal rabbit anti-recombinant cynomolgus (monkey) FSH (1:100,000; AFP782594), polyclonal rabbit anti-human prolactin (1:100,000; Anti-h-PRL-3, AFPC11580) (all obtained from Dr. A. F. Parlow, NHPP, Harbor-UCLA Medical Center, Torrance, CA), polyclonal rabbit anti-synthetic human α-MSH (1:1600; ImmunoStar, Hudson, WI) and polyclonal rabbit anti-human GH (1:20,000; Abcam Inc. Cambridge, MA). In all of these cases, Cy3-conjugated AffiniPure donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc., West Groove, PA) was used as the fluorescent secondary antibody. The first 13 amino acids of ACTH are identical to the entire sequence of α-MSH (24) and, as stated in the manufacturers instructions, the anti-synthetic human α-MSH cross-reacts with ACTH. This property of the antibody was exploited in the present study to localise corticotrophs in the anterior pituitary. The polyclonal anti-GnRH antibody (LR1, raised against [D-Lys6] GnRH in rabbit) was provided by Dr. Robert Benoit (Montreal General Hospital, Canada) and used at a dilution of 1:100,000 with Cy3-conjugated AffiniPure donkey anti-rabbit IgG as secondary antibody, exactly as previously described for studies of monkey hypothalamus (17).
For preabsorption control experiments, α-MSH (10 μg/ml; Phoenix Pharmaceuticals, Inc., Burlingame, CA), human kisspeptin-54 (5 μg/ml; Phoenix Pharmaceuticals, Inc., Burlingame, CA), recombinant cynomolgus (monkey) LH (5 μg/ml; AFP6936A), recombinant cynomolgus (monkey) FSH (5 μg/ml; AFP6853A), human prolactin (1 μg/ml; NIDDK-hPRL-I-7, AFP9900) (LH, FSH, and prolactin from Dr. A. F. Parlow, NHPP, Harbor-UCLA Medical Center, Torrance, CA), and recombinant human GH (1 μg/ml; 1067-GH, R & D Systems, Inc. Minneapolis, MN) were used.
Fluorescence immunohistochemistry
Dual fluorescence immunohistochemistry was performed on free-floating pituitary sections in the following combinations: kisspeptin and GnRH (with kisspeptin preabsorption control) and kisspeptin and α-MSH or LH or FSH or prolactin or GH (with α-MSH, LH, FSH, prolactin, and GH preabsorption controls, respectively). Sections were rinsed at room temperature in 50 mM KPBS (pH 7.3, 8×15 min), incubated for 30 min in 3% hydrogen peroxide (CVS Pharmacy, Inc., Woonsocket, RI), and rinsed again in 50 mM KPBS (6×5 min). They were then incubated overnight at 4°C on a shaker in KPBS buffer containing 5% normal horse serum (Vector Laboratories, Inc., Burlingame, CA), 0.05% Triton X-100 and 5% BSA (Sigma Chemical Co., St. Louis, MO) to block non-specific binding. Sections were incubated for 48 h at 4°C on a shaker in a cocktail of the primary antibodies prepared in the KPBS-horse serum buffer, and then washed in 50 mM KPBS (4×5 min) at room temperature. To detect dual immunofluorescence, sections were incubated for 1 h in the dark at room temperature in a cocktail of respective secondary antibodies in KPBS-horse serum buffer at a dilution of 1:200. After washing, again in 50 mM KPBS (4×5 min), sections were mounted on slides (Fisherbrand Superfrost Plus, Fisher Scientific, Pittsburgh PA) treated with subbing solution (0.1% gelatin and 0.01% chromium potassium sulphate), allowed to dry in the dark, coverslipped using Fluoromount-G™ (SouthernBiotech, Birmingham, AL) and stored at 4°C until analyses.
The specificity of kisspeptin, α-MSH, LH, FSH, prolactin and GH immunostaining was established by preabsorbing with corresponding antibodies overnight on a shaker at 4°C before incubation with tissue. As noted above, specificity of LR1 had been previously established for monkey hypothalamus (25).
Confocal microscopy
Imaging of fluorescence labelling was performed using an Olympus FV1000 confocal microscope equipped with a four-laser system (Multi AR laser, HeNe G laser, HeNe R laser, and LD405/440 laser diode) with transmitted light, DIC, and complete integrated image analysis software system (Olympus America Inc. Melville, NY). The excitation and emission wavelengths for Alexa Flour 488 (green) and Cy3 (red) were 488/520 nm and 543/56 nm, respectively. Between 25–30 optical sections along the z-axis were collected at 1 Zm intervals. Composite digital images were then converted to TIFF format, and imported into Adobe Photoshop (Adobe Photoshop CS2, version 9.0, Adobe Systems Incorporated, San Jose, CA) and colour balance was generally adjusted.
Hormone assays
Plasma LH levels were measured using a homologous (macaque) RIA employing recombinant monkey LH (NHPP/NICHD-rec.mo.LH-RP-1, AFP6936A) as the standard, as described previously (26). The minimal detectable concentration of the LH assay ranged from 0.12–0.32 ng/ml, and the mean intra- and inter-assay coefficients of variation were 9 and 16 %, respectively.
Plasma prolactin, GH, TSH, and cortisol levels were measured using the following commercially available assay kits: Active prolactin IRMA kit (Diagnostic Systems Labs, Inc. Webster, TX), a double antibody human GH RIA kit, Coat-A-Count TSH IRMA kit, and Coat-A-Count cortisol RIA kit (all from Diagnostic Products Corporation, Los Angeles, CA). The minimal detectable concentration of prolactin, GH, TSH, and cortisol was 0.1 ng/ml, 0.9 ng/ml, 0.03 μIU/ml, and 0.9 ng/ml, respectively. The inter- and intra-assay coefficients of variation of these commercial kits were less than 6 and 12 %, respectively.
Results
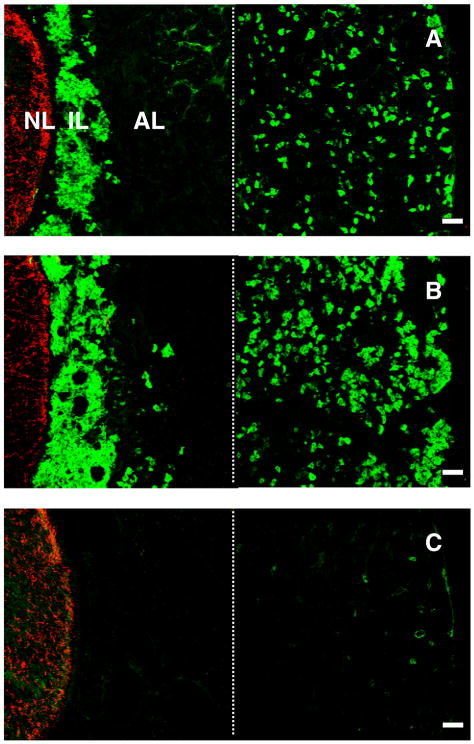
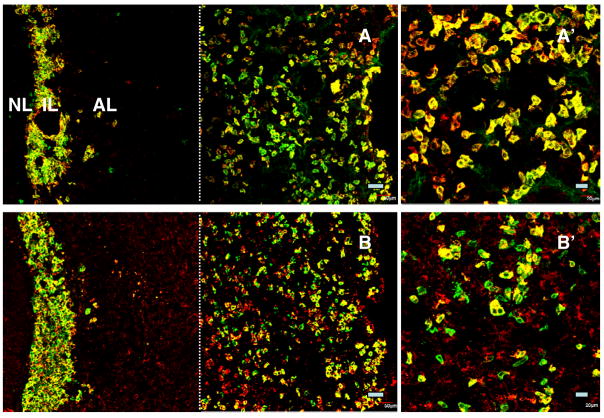
Kisspeptin immunoreactivity in the monkey pituitary was consistently observed in the cells of the intermediate lobe, which, as a result, was clearly delineated adjacent to the neural lobe that was readily recognised by a dense and unambiguous network of GnRH axonal projections running dorsoventrally (Fig. 1). All cells in the intermediate lobe appeared to exhibit intense kisspeptin staining, and this was eliminated by preabsorption of the primary antibody with synthetic peptide (Fig. 1). Kisspeptin immunopositive cells were also found in the anterior lobe of the pituitary in four of the monkeys (two intact and two castrated animals; Fig. 1). These cells, however, were not found throughout the anterior pituitary, but were restricted to the periphery of the anterior lobe (Fig. 1). Kisspeptin positive cells in the anterior lobe were more numerous in castrated animals (Fig. 1). Kisspeptin staining in the anterior lobe, like that in the pars intermedia, was abolished by preabsorption of the kisspeptin primary antibody with the synthetic peptide (Fig. 1).
Figure 1.
Confocal projections (20X; 1 μm optical sections) of dual immunofluorescence images illustrating the distribution of GnRH fibres (red fluorescence, Cy3) in the neural lobe (NL) and kisspeptin cells (green fluorescence, Alexa 488) in the intermediate lobe (IL) and anterior lobe (AL) in the pituitary of an intact (A) and castrated (B) male monkey. Abundant kisspeptin cells were localised throughout the IL, but in the AL their distribution was restricted to peripheral regions. Panel C represents a control experiment wherein the kisspeptin primary antibody was preabsorbed with human kisspeptin-54 in a cocktail including the GnRH primary antibody. The residual fluorescence in the peripheral anterior lobe in this panel is presumably of a non-specific nature. Note the photomicrographs were generated by creating a montage of two images separated by the thin broken white vertical line: the left hand image captured NL, IL and AL immediately adjacent to the IL, while the right hand image shows peripheral AL. A major area of AL has therefore been excluded. Scale bar, 50 μm.
In the neural lobe, kisspeptin beaded axons were found intermingled with those of GnRH, and this was particularly apparent in one animal in which sections through the pituitary were cut in a coronal plane rather than transversely (Fig. 2). There was no evidence of colocalisation of kisspeptin and GnRH.
Figure 2.
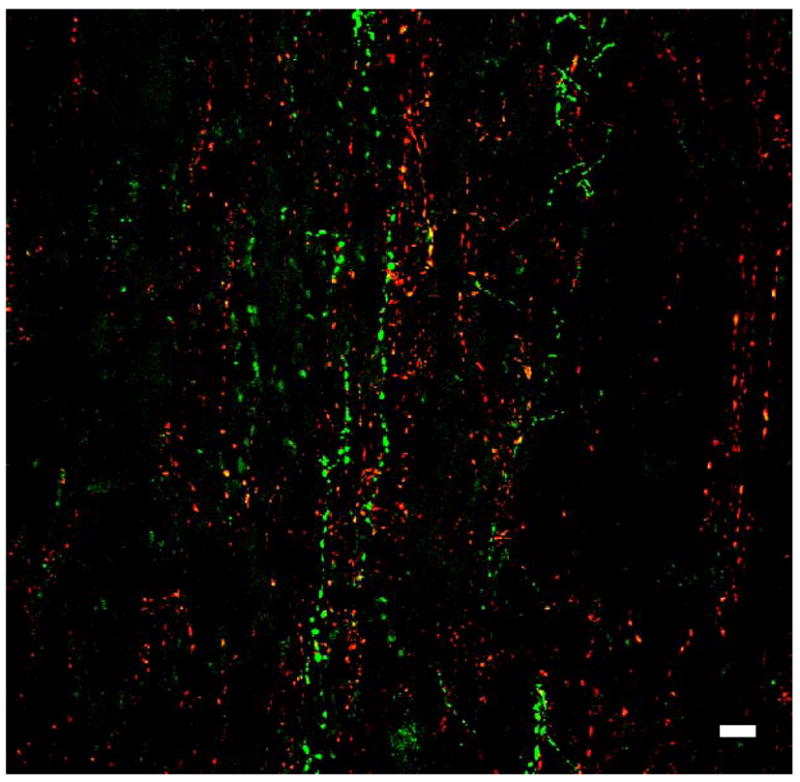
A confocal projection (60X; 1 μm optical sections) of a dual immunofluorescence image showing innervation of the neural lobe by GnRH (red fluorescence, Cy3) and kisspeptin (green fluorescence, Alexa 488) beaded axons from a coronal section of the pituitary from an intact male monkey. Although these axons intermingled, the peptides were not colocalised. Scale bar, 10 Zm.
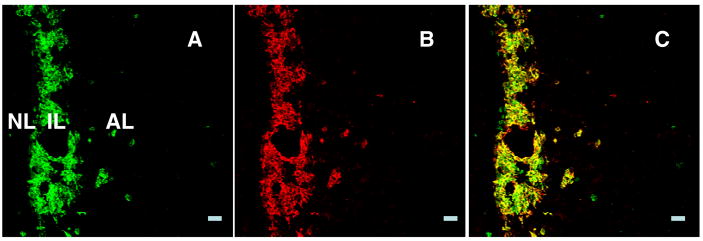
As expected, α-MSH immunopositive cells were found throughout the intermediate lobe and colocalised with kisspeptin (Fig. 3).
Figure 3.
Confocal projections (20X; 1 μm optical sections) of immunofluorescence images of kisspeptin (A) and α-MSH (B) immunopositve cells and their merged profile (C) in the intermediate lobe of the pituitary from an intact male monkey. Almost every cell in this region of the pituitary coexpresses kisspeptin and α-MSH (C). Scale bar, 50 μm.
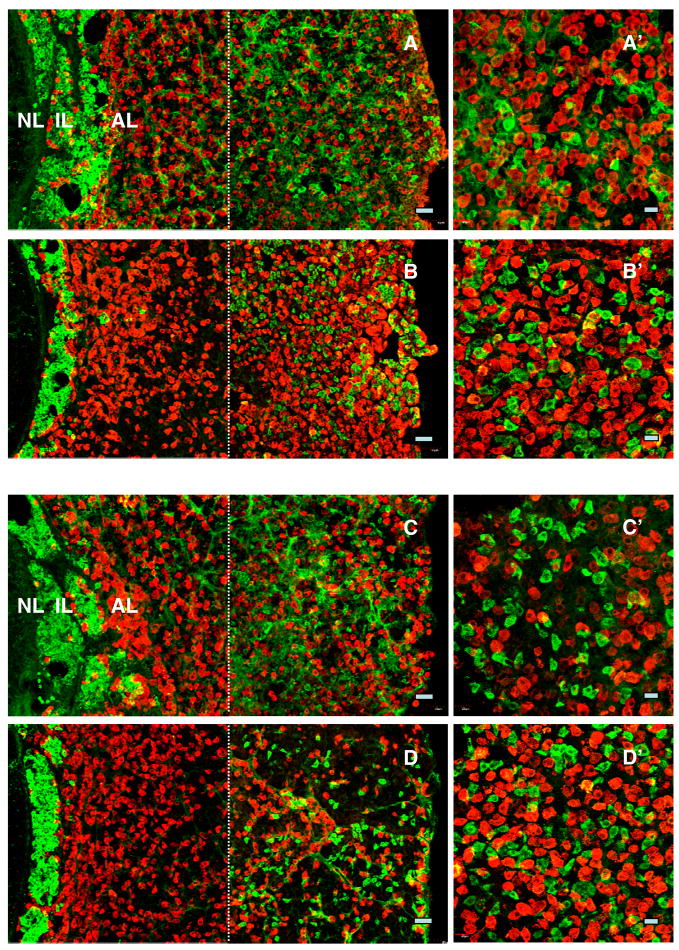
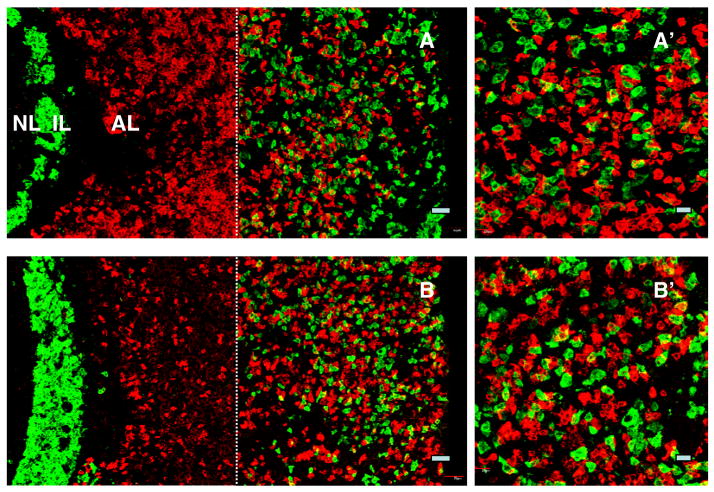
As also expected, LH, FSH, GH and prolactin immunoreactive cells were present in abundance throughout the anterior lobe in all animals. The relationship between kisspeptin positive cells in the periphery of the anterior pituitary and those staining for gonadotropins, GH, prolactin and ACTH was examined in the four animals in which kisspeptin immunoreactivity was found in the anterior lobe. There was no evidence of colocalisation of kisspeptin with either gonadotrophs, somatotrophs or lactotrophs (Figs. 4 and 5). In contrast, the majority of corticotrophs exhibited co-staining with kisspeptin (Fig. 6).
Figure 4.
Confocal projections (20X; 1 μm optical sections) of dual immunofluorescence images of kisspeptin cells (green fluorescence, Alexa 488) in relation to LH (panels A and B) and FSH (red fluorescence, Cy3, panels C and D) immunopositive cells in pituitary from an intact (panels A and C) and a castrated (panels B and D) monkey. Kisspeptin cells were distributed in the intermediate lobe (IL) and towards the periphery of the anterior lobe (AL), whereas gonadotropin immunopositive cells were found throughout the AL. Absence of colocalisation of kisspeptin with either LH (A′ and B′) or FSH (C′ and D′) secreting cells is shown in higher magnification confocal projections (40X; 1 μm optical sections). In cases where orange fluorescence was observed, examination of individual optical 1 μm sections revealed that the merged optical signal was due to the adjacent location of kisspeptin cells and gonadotrophs. Note the left hand photomicrographs were again generated by creating a montage of two images separated by the thin broken white vertical line: the left hand image captured the NL, IL and AL immediately adjacent to IL, while the right hand image shows peripheral AL. Scale bars, 50 μm (A–D) and 20 μm (A′–D′).
Figure 5.
Confocal projections (20X; 1 μm optical sections) of dual immunofluorescence images of kisspeptin cells (green fluorescence, Alexa 488) in relation to GH (top panels) and prolactin (lower panels) immunopositive cells (red fluorescence, Cy3) in the pituitary from a castrated monkey. Kisspeptin cells were distributed in the intermediate lobe (IL) and peripheral aspects of the anterior lobe (AL), whereas somatotrophs (A) and lactotrophs (B) were found throughout the AL. Absence of colocalisation of kisspeptin with either GH (A′) or prolactin (B′) secreting cells is shown at higher magnification (40X; 1 μm optical sections). In cases where orange fluorescence was observed, examination of images of individual optical 1 μm sections revealed that the merged optical signal was due to the adjacent location of kisspeptin cells and somatotrophs or lactotrophs. Note the left hand photomicrographs were again generated by creating a montage of two images separated by the thin broken white vertical line: the left hand image captured the NL, IL and AL immediately adjacent to IL, while the right hand image shows peripheral AL. Scale bars, 50 μm (A,B) and 20 μm (A′, B′).
Figure 6.
Confocal projections (20X; 1 μm optical sections) of dual immunofluorescence images of kisspeptin (green fluorescence, Alexa 488) and α-MSH immunopositive cells (red fluorescence, Cy3) in the intermediate lobe (IL) and in the anterior lobe (AL) of the pituitary from an intact (A) and a castrated (B) monkey. The majority of cells in the IL (melanotrophs) expressed both peptides (A and B). Colocalisation of kisspeptin and α-MSH was also observed in peripheral areas of AL (A and B). This double label staining of presumptive corticotrophs is also shown in higher magnification (40X; 1 μm optical sections) in panels A′ and B′. Note the left hand photomicrographs were again generated by creating a montage of two images as described in previous figures. Scale bars, 50 μm (A and B) and 20 μm (A′, B′).
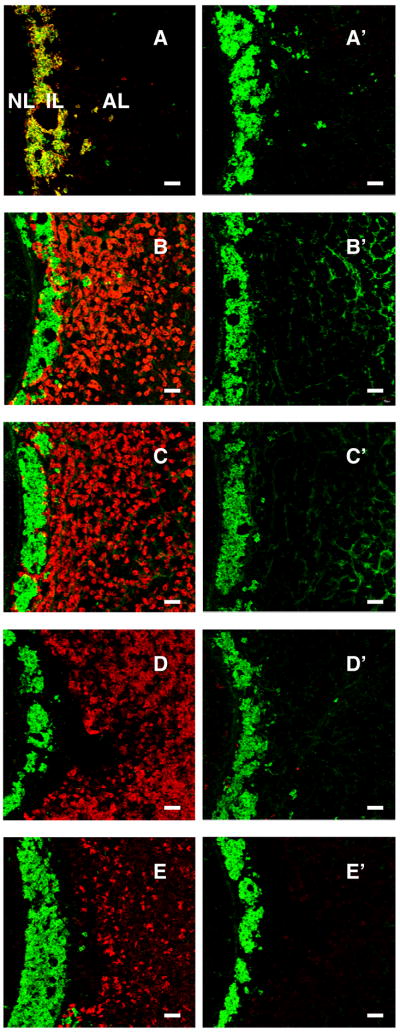
The specificity of staining for α-MSH/ACTH, LH, FSH, GH and prolactin was confirmed in control experiments in which preabsorption of each of the primary antibodies with their respective antigens completely eliminated the immunohistochemical signals in the pituitary gland (Fig. 7).
Figure 7.
Confocal projections (x20; 1 μm optical sections) of dual immunofluorescence images of kisspeptin (green fluorescence, Alexa 488) and either α-MSH (A), LH (B), FSH (C), GH (D) or prolactin (E) immuopositive cells (red fluorescence, Cy3) are shown in the left hand panels and compared in the right hand panels with images obtained after preabsorption of the respective primary antibodies with the appropriate antigen. Preabsorption in each of the foregoing cases was performed in a cocktail including the kisspeptin primary antibody. Scale bar, 50 μm.
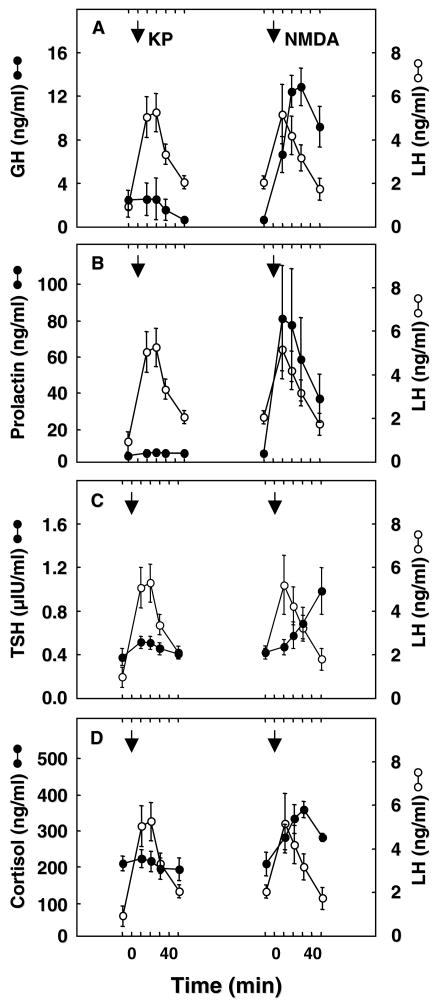
Fig. 8 shows the effect of bolus iv injections of kisspeptin-10 and of NMDA on circulating levels of LH, GH, prolactin, TSH and cortisol. As we have previously described (17), kisspeptin elicited a robust discharge of LH, but concomitant changes in circulating concentrations of GH, prolactin, TSH and cortisol were unremarkable. In contrast, NMDA elicited a generalised discharge of the pituitary hormones and of cortisol (Fig. 8).
Figure 8.
The effect of iv bolus injections of kisspeptin-10 (KP, 10 or 30 μg/monkey) and NMDA (10 mg/kg) on plasma concentrations of GH (Panel A), prolactin (Panel B), TSH (Panel C), and cortisol (Panel D), all shown in closed circles, in intact adult male rhesus monkeys. For comparison, changes in circulating LH concentrations (open circles) during these treatments are also shown. In striking contrast to the robust discharge of LH elicited by kisspeptin administration, kisspeptin was without effect on GH, PRL, TSH and cortisol. Iv injections of NMDA, on the other hand, resulted in a robust discharge of the three pituitary hormones and of cortisol. Data points show mean±SE and arrows indicate time of the iv injections of kisspeptin or NMDA. Note LH profiles are reproduced from Fig. 5 (18) with the permission of the Publisher.
Discussion
To the best of our knowledge, this qualitative immunohistochemical study of the rhesus monkey hypophysis provides the first description of the distribution of kisspeptin immunopositive cells in the pituitary of any primate species, and adds to that previously reported for the rat (5). The specificity of the immunohistochemical procedure as applied to the monkey pituitary was confirmed in control experiments in which kisspeptin staining was abolished by preabsorption of the primary antibody with the full-length peptide. The most striking finding was the abundance of intensely staining kisspeptin cells in the intermediate lobe of the monkey pituitary that, not surprisingly, also stained for α-MSH. Although an occasional kisspeptin cell was found in the intermediate lobe of the rat pituitary (5), all cells in this region of the monkey gland appeared to be positive for this peptide. KiSS1 mRNA has been detected in whole pituitary from several species, but tissue specific expression of this gene in the intermediate lobe has not been studied. The function of the intermediate lobe of the pituitary in higher primates is unclear and therefore it is difficult to even begin to speculate as to the role of kisspeptin in this region of the gland.
In addition to the intermediate lobe, kisspeptin immunoactive cells were also found in the anterior lobe of the pituitary in half of the animals (two intact and two castrated animals). The reason for the inter-animal variability in kisspeptin staining in this lobe of the pituitary is unclear, although, in the case of the castrates, the monkeys in which kisspeptin was detected were younger than the two animals that lacked staining (3 and 7 years versus 9 and 12 years). This relationship, however, was not reproduced in the intact animals. Kisspeptin staining in the agonadal monkeys was unrelated to the duration of castration. Kisspeptin immunopositive cells were confined to more peripheral areas of the anterior lobe of the pituitary and, although not quantitated, the pituitary from the two castrates appeared to have the most cells. The latter observation is consistent with findings in both the male monkey and male rodent that in the castrate situation expression of KiSS1 in the mediobasal hypothalamus is upregulated (27–30). In the four monkeys in which kisspeptin cells were found in the anterior pituitary, extensive colocalisation with ACTH expressing cells was observed. In contrast to the pituitary, where proopiomelanocortin (the prohormone for α-MSH) expressing cells in both the intermediate and anterior lobes also stained for kisspeptin, in hypothalamus of the ewe kisspeptin neurones in the arcuate nucleus, as revealed by immunohistochemistry, are distinct from those expressing proopiomelanocortin (31).
As expected, LH or FSH containing cells were widely distributed throughout the anterior lobe, but there was no evidence for colocalisation of kisspeptin with either gonadotropin. This contrasts with the situation in the rat where all LHβ positive cells were immunostained for kisspeptin, although some kisspeptin immunostaining was also noted in non-LH cells (5).
Similar to gonadotrophs, immunopositive somatotrophs and lactotrophs were also distributed throughout the anterior lobe of the monkey pituitary but no evidence for expression of kisspeptin in either of these populations of non-gonadotrophic hormone-secreting cells was evident.
While as previously described exogenous bolus iv injection of kisspeptin-10 markedly activated the GnRH-LH axis (18), the site of this action of kisspeptin is unlikely to be at the level of the pituitary, because pre-treatment with a GnRH-R antagonist blocks kisspeptin induced LH discharges (22,32). Similarly, in sheep, in which the hypothalamus and pituitary were surgically disconnected, iv administration of kisspeptin failed to induce LH secretion (6), suggesting also that kisspeptin induction of gonadotropin release is mediated exclusively by an indirect action of the peptide on the brain. Several in vitro studies employing rat pituitary cells in primary culture have also reported the absence of an effect of kisspeptin on the release of gonadotropins (12,13). Interestingly, Navarro et al. (13) found that while kisspeptin alone does not stimulate FSH, co-administration with GnRH had a moderate stimulatory effect. On the other hand, additional in vitro studies of rat pituitary cells, and of primary cell cultures derived from ovine, bovine, and porcine pituitaries have described minor stimulatory effects of kisspeptin on LH (6,10,14).
The present study also failed to provide evidence for an action of kisspeptin on the release of the non-gonadotrophic pituitary hormones, namely GH, prolactin, TSH, or ACTH (as reflected by plasma cortisol levels) in the monkey. In the case of GH, this contrasts with the finding in the Holstein heifer where iv kisspeptin administration (5 μg/kg body wt) has been reported to elicit the release of this anterior pituitary hormone (15). Additionally, a modest stimulatory effect of kisspeptin on release of non-gonadotrophic hormones such as GH and prolactin has been reported when high doses of kisspeptin (>1000 nM) were administered to cultures of rodent and bovine pituitary cells (10,16). Although only two doses (10 or 30 μg; approximately 1–3μg/kg body wt) of kisspeptin-10 were used in the present study, it is to be noted that they were similar to or higher than that (2 μg/animal; <1μg/kg body wt) in an earlier study of the juvenile monkey (22), which elicited physiological LH responses. The foregoing considerations, taken together with the absence of non-reproductive phenotypes in transgenic mice, in which KiSS1 or GPR54 was deleted (33–35), suggest that kisspeptin action directly at the level of the pituitary is unlikely to serve a fundamental role in adenohypohysial physiology.
The absence of an effect of kisspeptin on the release of GH, prolactin, TSH or ACTH in the present study may also be interpreted as the lack of a central action of kisspeptin to stimulate discharges of the release/inhibiting factors governing the secretion of these non-gonadotrophic pituitary hormones. The competence of these neuroendocrine axes to respond to a challenge was confirmed by the finding that NMDA, a glutamate receptor agonist which provides a generalised stimulus to the neuroendocrine hypothalamus (21), elicited robust increases in LH, GH, prolactin, TSH and ACTH, the latter being reflected by the cortisol response.
As expected, a GnRH axonal network was readily identified in the neural lobe of the pituitary (36). Interestingly, as previously described for the median eminence (17), kisspeptin beaded axons were also found intermingled with GnRH fibres in this region of the monkey pituitary. In cases where kisspeptin has been measured in the peripheral circulation (i.e., men and non-pregnant women), the concentrations of this neuropeptide were reported to be below the detectable limit of the assay (23). Thus, the function of kisspeptin in the neural lobe, like that in the adenohypophysis, remains to be established. With regard to the former, it is interesting to note that the median eminence and infundibulum share a common capillary bed in the monkey (37), and that iv administration of kisspeptin has been reported to increase plasma oxytocin in female rats (9).
In summary, we report for the first time the distribution of kisspeptin containing cells in the primate pituitary. Using immunohistochemistry, intense kisspeptin positive cells were found throughout the intermediate lobe, and these cells also expressed α-MSH. Kisspeptin beaded axons were found intermingled with those of GnRH in the neural lobe. Kisspeptin positive corticotrophs were also found in 40% of the pituitaries, but kisspeptin was not found in either gonadotrophs, somatotrophs or lactotrophs. Studies of the immunohistochemical colocalisation of kisspeptin and TSH remain to be performed. While the morphological findings are not inconsistent with the notion that kisspeptin may have a physiological role directly at the level of the pituitary, the observation that iv administration of kisspeptin failed to influence the secretion of either GH, TSH, prolactin or ACTH (as reflected by cortisol) and that kisspeptin induced gonadotropin release appears to be mediated at the hypothalamic level suggests that kisspeptin action at the level of the anterior pituitary is unlikely to be of fundamental importance in adenohypophysial physiology.
Acknowledgments
We are grateful to Dr. Stephen R. Bloom (Imperial College London, London, UK) for the generous gift of GQ2, the polyclonal antibody to human kisspeptin-54. We also thank the Primate Core (Mr. Mike Cicco, Ms. Rachel Roslund, and Ms. Lisa Nieman-Vento) and Assay Core (Ms. Carolyn Phalin) Staff of the Pittsburgh Specialized Cooperative Centers Program in Reproduction and Infertility Research for expert technical assistance, and Mr. Karthik Dwarki, a student trainee, for assisting with some of the immunohistochemical experiments. Supported by NIH R01 HD13254 and U54 HD08610.
References
- 1.Seminara SB, Crowley WF., Jr Kisspeptin and GPR54: Discovery of novel pathway in reproduction. J Neuroendocrinol. 2008;20:727–731. doi: 10.1111/j.1365-2826.2008.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roa J, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. New frontiers in kisspeptin/GPR54 physiology as fundamental gatekeepers of reproductive function. Front Neuroendocrinol. 2008;29:48–69. doi: 10.1016/j.yfrne.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Gottsch ML, Clifton DK, Steiner RA. From KiSS1 to kisspeptins: An historical perspective and suggested nomenclature. Peptides. 2009;30:4–9. doi: 10.1016/j.peptides.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plant TM, Ramaswamy S. Kisspeptin and the regulation of the hypothalamic-pituitary-gonadal axis in the rhesus monkey (Macaca mulatta) Peptides. 2009;30:67–75. doi: 10.1016/j.peptides.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Richard N, Galmiche G, Corvaisier S, Caraty AS, Kottler M-L. KiSS-1 and GPR54 genes are co-expressed in rat gonadotrophs and differentially regulated in vivo by oestradiol and gonadotrophin-releasing hormone. J Neuroendocrinol. 2008;20:381–393. doi: 10.1111/j.1365-2826.2008.01653.x. [DOI] [PubMed] [Google Scholar]
- 6.Smith JT, Rao A, Pereira A, Caraty A, Millar RP, Clarke IJ. Kisspeptin is present in ovine hypophysial portal blood but does not increase during the preovulatory luteinizing hormone surge: Evidence that gonadotropes are not direct targets of kisspeptin in vivo. Endocrinology. 2008;149:1951–1959. doi: 10.1210/en.2007-1425. [DOI] [PubMed] [Google Scholar]
- 7.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, Terao Y, Kumano S, Takatsu Y, Masuda Y, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Kitada C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- 8.Muir AI, Chamberlain L, Elshourbagy NA, Michalovich D, Moore DJ, Calamari A, Szekeres PG, Sarau HM, Chambers JK, Murdock P, Steplewski K, Shabon U, Miller JE, Middleton SE, Darker JG, Larminie CGC, Wilson S, Bergsma DJ, Emson P, Faull R, Philpott KL, Harrison DC. AXOR12, a novel human G protein-coupled receptor, activated by the peptide KiSS-1. J Biol Chem. 2001;276:28969–28975. doi: 10.1074/jbc.M102743200. [DOI] [PubMed] [Google Scholar]
- 9.Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden J-M, Le Poul E, Brezillon S, Tyldesley R, Suarez-Herta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez-Pascual E, Martinez-Fuentes AJ, Pinilla L, Tena-Sempere M, Malagon MM, Castano JP. Direct pituitary effects of kisspeptins: Activation of gonadotrophs and somatotrophs and stimulation of luteinising hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- 11.Keen KL, Wegner FH, Bloom SR, Ghatei MA, Teresawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkey in vivo. Endocrinology. 2008;149:4151–4157. doi: 10.1210/en.2008-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsui H, Takatsu Y, Kumano S, Matsumato H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Comm. 2004;320:383–388. doi: 10.1016/j.bbrc.2004.05.185. [DOI] [PubMed] [Google Scholar]
- 13.Navarro VM, Castellano JM, Fernandez-Fernandez R, Tovar S, Roa J, Mayen A, Barreiro ML, Casanueva FF, Agular E, Dieguez C, Pinilla L, Tena-Sempere M. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology. 2005;146:1689–1697. doi: 10.1210/en.2004-1353. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on luteinizing hormone secretion from bovine and porcine anterior pituitary cells. Animal Reprod Sci. 2008;103:360–365. doi: 10.1016/j.anireprosci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Kadokawa H, Matsui M, Hayashi K, Matsunaga N, Kawashima C, Shimizu T, Kida K, Miyamoto A. Peripheral administration of kisspeptin-10 increases plasma concentrations of GH as well as LH in prepubertal Holstein heifers. J Endocrinol. 2008;196:331–334. doi: 10.1677/JOE-07-0504. [DOI] [PubMed] [Google Scholar]
- 16.Kadokawa H, Suzuki S, Hashizume T. Kisspeptin-10 stimulates the secretion of growth hormone and prolactin directly from cultured bovine anterior pituitary cells. Anim Reprod Sci. 2008;105:404–408. doi: 10.1016/j.anireprosci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM. Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology. 2008;149:4387–4395. doi: 10.1210/en.2008-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148:3364–3370. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 19.Simorangkir DR, Ramaswamy S, Marshall GR, Plant TM. In the adult male monkey (Macaca mulatta), unilateral orchidectomy in the face of unchanging gonadotropin stimulation results in partial compensation of testosterone secretion by the remaining testis. Endocrinology. 2004;145:5115–5120. doi: 10.1210/en.2004-0824. [DOI] [PubMed] [Google Scholar]
- 20.Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [DOI] [PubMed] [Google Scholar]
- 21.Gay VL, Plant TM. N-methyl-D,L-aspartate elicits hypothalamic gonadotropin-releasing hormone release in prepubertal male rhesus monkeys (Macaca mulatta) Endocrinology. 1987;120:2289–2296. doi: 10.1210/endo-120-6-2289. [DOI] [PubMed] [Google Scholar]
- 22.Plant TM, Ramaswamy S, DiPietro MJ. Repetitive activation of hypothalamic G protein-coupled receptor 54 with intravenous pulses of kisspeptin in the juvenile monkey (Macaca mulatta) elicits a sustained train of gonadotropin-releasing hormone discharges. Endocrinology. 2006;147:1007–1013. doi: 10.1210/en.2005-1261. [DOI] [PubMed] [Google Scholar]
- 23.Dhillo WS, Chaudhri OB, Patterson M, Thompson EL, Murphy KG, Badman MK, McGowan BM, Amber V, Patel S, Ghatei MA, Bloom SR. Kisspeptin-54 stimulates the hypothalamic-pituitary-gonadal axis in human males. J Clin Endocrinol Metab. 2005;90:6609–6615. doi: 10.1210/jc.2005-1468. [DOI] [PubMed] [Google Scholar]
- 24.Bertolini A, Tacchi R, Vergoni AV. Brain effects of melanocortins. Pharmacol Res. 2009;59:13–47. doi: 10.1016/j.phrs.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Goldsmith PC, Thind KK, Perera AD, Plant TM. Glutamate-immunoreactive neurons and their gonadotropin-releasing hormone-neuronal interactions in the monkey hypothalamus. Endocrinology. 1994;134:858–868. doi: 10.1210/endo.134.2.7905410. [DOI] [PubMed] [Google Scholar]
- 26.El Majdoubi M, Ramaswamy S, Sahu A, Plant TM. Effects of orchidectomy on levels of the mRNAs encoding gonadotropin-releasing hormone and other hypothalamic peptides in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2000;12:167–176. doi: 10.1046/j.1365-2826.2000.00433.x. [DOI] [PubMed] [Google Scholar]
- 27.Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin-releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology. 2004;80:264–272. doi: 10.1159/000083140. [DOI] [PubMed] [Google Scholar]
- 28.Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmentally and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing hormone activity of KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- 29.Simth JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA. Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology. 2005;146:2976–2984. doi: 10.1210/en.2005-0323. [DOI] [PubMed] [Google Scholar]
- 30.Shibata M, Friedman RL, Ramaswamy S, Plant TM. Evidence that down regulation of hypothalamic KiSS-1 expression is involved in the negative feedback action of testosterone to regulate luteinising hormone secretion in the adult male rhesus monkey (Macaca mulatta) J Neuroendocrinol. 2007;19:432–438. doi: 10.1111/j.1365-2826.2007.01549.x. [DOI] [PubMed] [Google Scholar]
- 31.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CVR, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 32.Shahab M, Mastronardi C, Seminara SB, Crowley WF, Jr, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: A potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seminara SB, Messager SM, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof K, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Jr, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 34.Lapatto R, Pallais JC, Zhang D, Chan Y-M, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB. KiSS−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148:4927–4936. doi: 10.1210/en.2007-0078. [DOI] [PubMed] [Google Scholar]
- 35.d’Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman A-J, Livne I, Witkin JW. The gonadtropin-releasing hormone neuronal systems: Immunocytochemistry and in Situ hybridization. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. New York: Raven Press; 1994. pp. 1683–1710. [Google Scholar]
- 37.Page RB, Bergland RM. The neurohypophyseal capillary bed. 1. Anatomy and arterial supply. Am J Anat. 1976;148:345–358. doi: 10.1002/aja.1001480305. [DOI] [PubMed] [Google Scholar]