Abstract
Objectives
The National Institute of Allergy and Infectious Disease classifies Francisella tularensis as a Category A priority pathogen. Despite the availability of drugs for treating tularaemia, the mortality in naturally acquired cases can still approach 30%. In addition, the usefulness of existing drugs for treatment in response to exposure or for prophylaxis is limited because of toxicity and delivery concerns. The aim of this study was to assess the efficacy of the lead alkyl-substituted diphenyl ether, SBPT04, in the F. tularensis murine model of infection.
Methods
SBPT04 was delivered by intraperitoneal (ip) and oral (po) routes, and mice were monitored for morbidity, mortality and relapse of disease. Pharmacokinetic studies were performed to evaluate bioavailability. Phase I and Phase II metabolism of SBPT04 was assessed in mouse and human microsomes.
Results
SBPT04, a potent inhibitor of the enoyl-ACP reductase enzyme ftuFabI, has efficacy against F. tularensis in the murine model of infection when delivered by both ip and po routes. SBPT04 delivered ip cleared infection by day 4 of treatment, and SBPT04 delivered po resulted in delayed dissemination. Importantly, SBPT04 delivered ip or po demonstrated efficacy with no signs of relapse of disease. Pharmacokinetic studies show increased serum concentrations following ip delivery compared with po delivery, which correlates with the observed survival rate of 100%.
Conclusions
In addition to being a potent lead, this work substantiates substituted diphenyl ethers as a platform for the development of novel broad-spectrum chemotherapeutics to other bacterial agents in addition to F. tularensis.
Keywords: Francisella tularensis, fatty acid, FabI, efficacy, mouse model
Introduction
Francisella tularensis is a facultative intracellular bacterium that can cause severe, acute and often fatal respiratory disease in humans. The ability of this highly infectious and virulent bacterial pathogen to be readily aerosolized along with its history as a potential biological weapon has led to the possibility that it could be used deliberately in an act of bioterrorism.1–4 Consequently, the National Institute of Allergy and Infectious Disease has classified F. tularensis as a Category A priority pathogen and has stated the need for the development of novel chemotherapeutics that can be used rapidly and effectively to treat infections caused by this organism. While some broad-spectrum antibiotics are currently indicated for Category A and B pathogens, concern is growing that the widespread and aggressive use of key antibiotics and potential engineering of specifically weaponized strains will result in the development of drug-resistant organisms. Thus, there is an important need for new broad-spectrum compounds with enhanced therapeutic effects compared with existing frontline treatments.
Current treatment regimens for F. tularensis infections utilize streptomycin and gentamicin, along with the alternative drugs tetracycline, chloramphenicol and fluoroquinolones. However, the efficacy of these regimens is limited because streptomycin and gentamicin have toxic side effects and neither can be orally (po) administered, while tetracycline and chloramphenicol are associated with relapse rates as high as 10%. Fluoroquinolones have also shown promise in a limited number of cases; however, clinical experience is limited and their role in treating severe disease is unknown. In animal studies, gatifloxacin, moxifloxacin and ciprofloxacin prevented disease during the treatment period, but significant failure rates occurred after the cessation of therapy.6–9 Since existing drugs have limited potency and efficacy, and it is unknown whether drug-resistant organisms might be used in an intentional release, it is prudent that novel chemotherapeutics with reduced side effects and that prevent relapse of disease compared with existing frontline drugs be developed.
Historically, clinically relevant drugs target DNA, RNA, protein and glycans of the cell wall. Currently, however, emerging alternative targets for novel antibacterial drug discovery include the enzymes of type II fatty acid biosynthesis (FASII). In particular, FabI, the FASII enoyl-ACP reductase, has been demonstrated as a promising broad-spectrum target for chemotherapeutic intervention.11–14 The utility of diphenyl ethers to target FabI in F. tularensis has been demonstrated by our own drug discovery programme, which is founded on the observation that the diphenyl ether triclosan is a potent inhibitor of the FabI enzyme in other organisms.11,13,15–23 The frontline tuberculosis drug isoniazid is an inhibitor of the FabI enzyme from Mycobacterium tuberculosis,24 and several drug discovery programmes have targeted the FabI enzyme from organisms such as Staphylococcus aureus and Plasmodium falciparum.25–28 Using structure-based drug design strategies, we have developed a series of substituted diphenyl ethers that are potent inhibitors of the M. tuberculosis FabI enzyme and that are active against multidrug-resistant strains of M. tuberculosis.29,30 In addition, selected compounds are also nanomolar inhibitors of FabI from S. aureus and have excellent in vitro activity against methicillin-resistant S. aureus.29
Recently, we identified several alkyl diphenyl ethers that are potent inhibitors of the F. tularensis enoyl-ACP reductase (ftuFabI), some of which have efficacy in an animal model of tularaemia infection when delivered intraperitoneally (ip).14 The high potency of the diphenyl ether-based ftuFabI inhibitors against F. tularensis supports the exploitation of the FASII pathway for the development of novel tularaemia drugs. In particular, we have shown that one of these compounds, SBPT04, has activity in a murine model of tularaemia infection. Accordingly, the work presented here extends our previous studies by assessing bioavailability and drug metabolism of the most potent pre-clinical lead, SBPT04, in addition to efficacy when delivered po. Also, this research further investigates the mode of action of SBPT04 through DNA microarray transcriptional profiling of F. tularensis under drug treatment conditions. These studies provide critical information for lead optimization that will be used to advance substituted diphenyl ether-based compounds into pre-clinical and clinical trials. Here, we demonstrate in vivo efficacy against F. tularensis in the mouse model of infection, with enhanced bioavailability and decreased toxicity compared with that of the parent compound and other analogues.
Methods
Bacterial strains, culture conditions and mice
LVS and Schu4 strains (OH, USA) were cultured in modified Mueller–Hinton (MMH) broth (0.025% ferric pyrophosphate, 2% IsoVitaleX and 0.1% glucose) at 37°C with constant shaking overnight, aliquotted into 1 mL samples, frozen at −80°C and thawed just before use as previously described.31 Frozen stocks were titrated by enumerating viable bacteria from serial dilutions plated on MMH agar (0.025% ferric pyrophosphate, 2% IsoVitaleX, 0.1% glucose and 0.025% fetal bovine serum) as previously described.31 The number of viable bacteria in frozen stock vials varied by <5% over a 10 month period. These stocks were used to start cultures for MIC and MBC analyses.
Six-week-old female ICR mice were purchased from Charles River Laboratories. All mice were housed in sterile microisolater cages in the laboratory animal resources facility or in the Biohazard Research Building BSL-3 facility at Colorado State University (Fort Collins, CO, USA), and were provided with sterile water and food ad libitum. All research involving animals was conducted in accordance with animal care and use guidelines, and animal protocols approved by the Animal Care and Use Committee at Colorado State University.
MIC and MBC determination
MICs were determined for F. tularensis LVS and Schu4 strains. F. tularensis was grown to mid-log phase and diluted to provide an inoculum of ∼105 cells per well when dispensed into a 96-well microtitre drug plate. Compounds in the drug plates were tested at 2-fold serial concentrations from 0.06 to 128.0 mg/L in triplicate. Optical density (OD) readings were obtained after 18–20 h of incubation at 37°C. Microplate Alamar Blue assay (MABA), a colorimetric drug susceptibility testing method that indicates bacterial viability, was also used as described with minor modifications for Francisella.32 MIC values were determined by plotting the percentage inhibition calculated from spectrophotometric plate readings (570 and 600 nm) using GraFit software. Similarly, MBCs were determined by plating onto MMH agar plates 10-fold serial dilutions from wells indicating a percentage inhibition of ≥90% via MABA analysis. The minimal concentration demonstrating a ≥3 log reduction of growth was considered the MBC.
Cytotoxicity testing
African green monkey kidney cells (Vero cells) were grown in RPMI 1640 medium supplemented with 1.5 g/L sodium bicarbonate, 10 mL/L 100 mM sodium pyruvate, 140 mL/L 100× non-essential amino acids, 100 mL/L penicillin–streptomycin solution (10 000 IU/10 000 mg/L) and 10% bovine calf serum at 37°C in a 5% CO2 incubator with 75% humidity. Compounds were evaluated at 2-fold serial diluted concentrations, starting at 100 mg/L. Following compound addition, cells were incubated for 72 h at 37°C in a 5% CO2 incubator. The cells were then washed with PBS, CellTiter 96 AQueous One solution was added to each well and plates were incubated for 4 h at 37°C. Plates were read at 490 nm using a spectrophotometric plate reader and the absorbance readings were used to calculate the 50% lethal concentration (LC50) using GraFit software as previously described.33
Mouse infection, drug dosing and cfu determination
Mice were infected with Schu4 via a whole-body low-dose aerosol as previously described, with minor modifications.34,35 Conscious mice within a stainless steel basket were exposed to the Schu4 strain of F. tularensis by aerosol exposure in a Glascol Inhalation Exposure System (Glas-Col, Inc., Terre Haute, IN, USA). Prior to exposure, the nebulizer was loaded with bacteria diluted in PBS to a concentration of ∼5 × 106 cfu/mL. Mice were exposed to a total of ∼4 × 107 bacteria, aerosolized into a volume of 5 cubic feet over a period of 30 min, followed by a 20 min period of cloud decay in which airflow was maintained without introducing additional bacteria. Mice were monitored for morbidity and mortality twice daily for a period of 14 days, at which time survivors were euthanized. This inoculum routinely results in 100% mortality and a mean time to death of 5–6 days following infection. Mice were treated ip or po with SBPT04 or triclosan at 50, 100 or 200 mg/kg prior to infection and continuing daily for 4 additional days. SBPT04 was formulated in 5% ethanol/water, while triclosan was formulated in 8% Solutol (BASF) and 5% ethanol/water. Control mice were inoculated with saline. Mice were sacrificed at 2 and 4 days post-infection for cfu determination in lungs and spleens. Mouse organs were homogenized in sterile PBS and 100 µL of homogenate was serial diluted (10−1–10−8) and plated on MMH agar plates, which were then incubated at 37°C for 48 h, at which time cfu were enumerated.
Transcriptional profiling under drug treatment
Transcriptional profiling experiments consisting of three independent biological replicates were performed to study the response of genes to drugs over a 2 h period. Bacterial cultures were grown to an OD600 of 0.4, diluted 1:2 and distributed into 150 mL aliquots. The cultures were incubated for 1 h at 37°C prior to the addition of 2× MIC concentrations of SBPT04. Total RNA was isolated as described 2 h post-treatment.33 Competitive hybridizations were performed on F. tularensis whole genome microarrays (Rocky Mountain Regional Center of Excellence Genomics Proteomics Core, U54 AI065357) with fluorescently labelled cDNAs generated using direct labelling from 5 µg of total RNA as described previously.33 Data reduction and analysis were performed using Genepix software (Axon). Transcriptional activity of open reading frames (ORFs) considered statistically significant were those that were differentially regulated by ≥1.5-fold, with a P value of ≤0.05.
Bioavailability, pharmacokinetics/pharmacodynamics and in vivo metabolite studies for SBPT004
A pharmacokinetic study was conducted in male C57/BL6 mice via intravenous (iv), po or ip administration of SBPT04. The dose levels were 20 mg/kg [5% ethanol/D5W (5% dextrose/water)] for iv, 50 and 200 mg/kg (5% ethanol) for po, and 200 mg/kg (5% ethanol/D5W) for ip. Blood samples for each route of administration were collected from retro-orbital puncture at eight timepoints, with three mice being sacrificed at each timepoint. SBPT04 and its major Phase I and Phase II metabolites were monitored in each sample with LC/MS/MS methodology (the lower limit of quantification was 1 ng/mL in plasma).
Results
SBPT04 is a potent inhibitor of F. tularensis and has low cytotoxicity
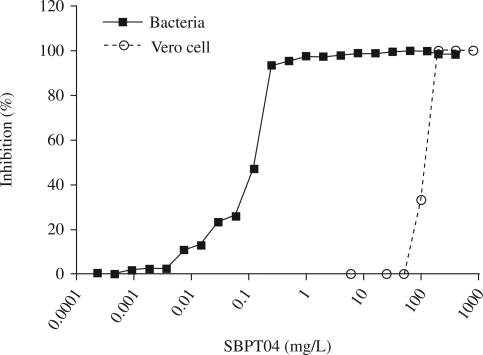
SBPT04 has an MIC of 0.16 ± 0.06 mg/L and an MBC of 0.25 mg/L. Most significantly, SBPT04 is more potent in vitro on a gravimetric basis than current drugs clinically used to treat F. tularensis infections. For example, the MICs determined for various clinical strains of F. tularensis with streptomycin and gentamicin fall into a range of 2–4 and 1–2 mg/L, respectively, as compared with SBPT04 with an MIC range of 0.12–0.25 mg/L.36 To assess the potential safety window of SBPT04, its cytotoxicity was evaluated in Vero cells. The LC50 for SBPT04 was determined to be 101 ± 2 mg/L, which was comparable to or better than current drugs that were also evaluated in our assay, and resulted in a measured 3 log concentration difference between MIC and LC50 values (Figure 1 and Table 1). This represents a significant difference in the selectivity index between tissue toxicity and bactericidal dose.
Figure 1.
MIC and cytotoxicity of SBPT04. Percentage inhibition of growth of F. tularensis Schu4 and Vero cells over different concentrations of SBPT04.
Table 1.
MIC, LC50 and selectivity index of SBPT04 and clinically used drugs to treat F. tularensis
| Drug | MIC (mg/L) | LC50 (mg/L) | SI |
|---|---|---|---|
| SBPT04 | 0.2 | 100 | 500 |
| Streptomycin | 4.0 | >100 | 25 |
| Gentamicin | 2.0 | >100 | 50 |
| Doxycycline | 1.0 | 3 | 3 |
SI, selectivity index.
Transcriptional response to SBPT04
To assess the mode of action and response of F. tularensis to treatment with SBPT04, whole genome transcriptional profiling was performed. Treatment with SBPT04 for 2 h at 2× MIC resulted in the differential regulation of 734 ORFs (P values ≤ 0.05), with 204 ORFs differentially regulated by ≥1.5-fold. The general trend in the transcriptional response caused by SBPT04 treatment included an overall reduction in gene transcripts encoding products involved in cell wall and lipid synthesis, intermediary metabolism and respiration, and RNA and protein synthesis (Table 2). Repressed genes included those associated with fatty acid biosynthesis (acc, fabI, fabH, fabZ), ATP synthesis (atpA, atpB, atpD, atpG, purK), NADH utilization (nuoA, nuoH, nuoI, nuoK, nuoM, nuoN, nadE), menaquinone biosynthesis methyltransferase (ubiE), RNA synthesis (rpoA1, rpoA2, rpoC), ribosomal proteins (rpmA, rpmD, rpsL, rpsI, rpsA, rpsS, rpsD, rpsN, rpsM, rpsK, rpsE, rpsH, rplT, rplQ, rplC, rplX, rplF, rplJ, rplA) that encode the 30S and 50S multisubunit ribosomal complex responsible for protein synthesis, and an ATP-dependent protease (lon). The down-regulation of these functions is consistent with a reduction in replication potential, metabolic function and overall cell viability. In addition, genes associated with stress responses were induced during treatment. These genes included the spoT, gro and sos genes. Of the functional categories, those assigned to insertion sequences and phages (71%), regulation (30%), unknown (21%), and virulence, detoxification and adaptation (28%) were the highest percentages induced. Thus, the transcriptional response of F. tularensis treated with SBPT04 demonstrates that overall metabolic activity was reduced within the treatment period of 2 h, despite the elucidated stress responses.
Table 2.
Differentially regulated genes by functional classification after 2 h of exposure to SBPT04 at 2× MIC
| Functional classification | Total | ≥1.5-Fold | ≤1.5-Fold | Up-regulated (%) | Down-regulated (%) |
|---|---|---|---|---|---|
| Cell wall and cell wall processes | 95 | 8 | 16 | 8 | 17 |
| Conserved hypothetical/hypothetical | 189 | 34 | 9 | 18 | 5 |
| Information pathways | 90 | 4 | 27 | 4 | 30 |
| Insertion sequences and phages | 38 | 27 | 0 | 71 | 0 |
| Intermediate metabolism/respiration | 165 | 12 | 23 | 7 | 14 |
| Lipid metabolism | 18 | 1 | 3 | 6 | 17 |
| Unknown | 100 | 21 | 6 | 21 | 6 |
| Regulatory proteins | 10 | 3 | 1 | 30 | 10 |
| Virulence/detoxification/adaptation | 29 | 8 | 1 | 28 | 3 |
Total genes in analysis = 1820, with 734 genes having P values ≤ 0.05.
SBPT04 has efficacy and controls dissemination in the F. tularensis murine model of infection
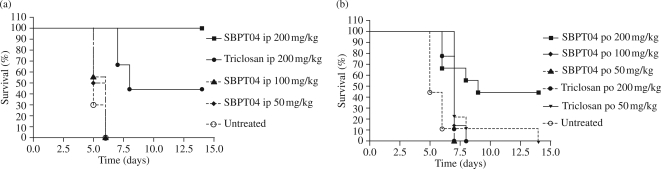
The efficacy of SBPT04 and the parent compound triclosan was determined in the F. tularensis murine model of infection when delivered ip and po beginning on day 1 of infection. For these studies, mice were infected with F. tularensis Schu4 (n = 15/group) via low-dose aerosol. Drugs were administered once a day for 5 days beginning on day 1, and mice were monitored for 30 days for morbidity, mortality and disease relapse. Untreated control mice in the ip study had a 0% survival rate with a median survival of 5 days, whereas treatment with 200 mg/kg triclosan delivered ip resulted in a 44% median survival. Significantly, treatment with 200 mg/kg SBPT04 delivered ip prevented death of all infected animals, resulting in a 100% survival rate (P < 0.001) (Figure 2a). Importantly, mice did not show any signs of relapse or disease for 30 days following 5 days of treatment. This observation is consistent with the lack of cfu in the lungs and spleens following treatment (Figure 3). To assess whether SBPT04 had efficacy when delivered po, mice were again infected with a low-dose aerosol of F. tularensis Schu4 and dosed once a day for 5 days as before. Untreated control animals in the po study had a 0% survival and a median survival of 5 days similar to that observed for ip delivery. In contrast, SBPT04 when administered po at 200 mg/kg significantly (P < 0.01) extended the median survival to 9 days (Figure 2b). Notably, signs of relapse or disease were not observed in the surviving animals for 30 days after 5 days of treatment.
Figure 2.
Efficacy testing of SBPT04 in the F. tularensis mouse model of infection. Survival of mice treated with SBPT04 or the lead pharmacophore triclosan delivered at different doses delivered (a) ip or (b) po. Mice were dosed once a day for 5 days following aerosol infection and observed until day 14.
Figure 3.
Bacterial burdens in the lungs and spleen during treatment with SBPT04. F. tularensis Schu4 cfu recovered from the lungs (a and c) and spleen (b and d) following treatment with different doses of SBPT04 delivered ip (a and b) or po (c and d). Bacterial burden was determined by cfu (log10 cfu/mL) recovered from organs at days 2 and 4 of treatment with SBPT04. Bars indicate means of biological replicates.
To assess the in vivo activity and efficacy of SBPT04 delivered ip or po, bacterial growth in the lungs and spleen was determined at varying doses on days 2 and 4 of infection. Three mice from each treatment group and control group were selected, euthanized, and the bacterial loads in the lungs and spleen were determined by plating and enumeration of cfu. Untreated mice generally displayed 5 log10 and 6 log10 cfu in the lung at days 2 and 4, respectively (Figure 3a and c). Although the bacteria in the spleen at day 2 were minimal, dissemination to the spleen was observed at day 4 with 6 log10 cfu (Figure 3b and d). Doses of ≤100 mg/kg SBPT04 delivered either ip or po did not significantly affect bacterial growth in the lungs or spleens at day 4 of infection when compared with control animals. SBPT04 when delivered ip at 200 mg/kg reduced bacterial growth in the lungs at day 2 and significantly cleared bacteria by day 4 (P < 0.01) (Figure 3a). Similar trends were observed in the spleen with SBPT04 delivered ip at 200 mg/kg, where no bacteria were detected (P < 0.01) (Figure 3b). When delivered po at 200 mg/kg, SBPT04 was able to reduce bacterial growth in the lungs at day 2 (P ≤ 0.05) but was unable to control the extent of burden in the lungs (Figure 3c) or spleen (Figure 3d) at day 4 of infection. This is consistent with the observed differences in efficacy (Figure 2a and b) and demonstrates correlations with median survival, bacterial burden in the lungs, control of dissemination and growth in the spleen during treatment.
Pharmacokinetics and metabolism of SBPT04
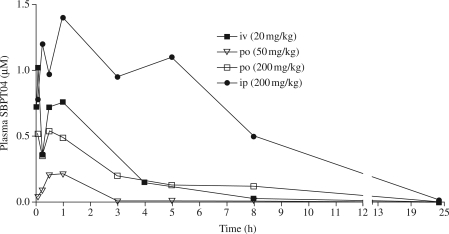
A two-pronged approach consisting of pharmacokinetic analysis and metabolite studies was employed to identify the physiochemical properties of SBPT04 that influence po bioavailability and the specific structural liabilities of SBPT04. The pharmacokinetic parameters that were determined included AUC, Cmax, Tmax, F (bioavailability) and t1/2 for SBPT04 following dosing at 50 mg/kg and 200 mg/kg po, and 200 mg/kg ip in order to estimate the therapeutic dose and pharmacokinetic characteristics associated with efficacy (Figure 4 and Table 3). The AUC of SBPT04, which evaluates drug exposure, was 0.61 or 3.3 mg·h/L when delivered po at 200 mg/kg or ip at 200 mg/kg, respectively. Delivery of SBPT04 at 200 mg/kg po resulted in a Cmax value of 0.54 µmol/L with a Tmax of 0.5 h, whereas a 200 mg/kg dose delivered ip yielded a Cmax value of 1.41 µmol/L with a Tmax of 1.0 h. Comparison of the AUC and Cmax values from 200 mg/kg po or 200 mg/kg ip provides an estimate of the minimal values needed for a compound to demonstrate efficacy. Delivery of SBPT04 ip at 200 mg/kg afforded a therapeutic effect index (AUC/MIC) of 20.6 and a Cmax/MIC of 2. When delivered po at 200 mg/kg or ip at 200 mg/kg, SBPT04 had t1/2 values of 2.08 and 3.04 h, respectively. Together, these values reveal that a compound that exceeds 0.6 mg·h/L, a maximum serum concentration greater than the MIC of the compound and a t1/2 of >3 h demonstrates efficacy in the F. tularensis mouse model of infection. The bioavailability (F) of SBPT04 was assessed to determine the fraction of the dose reaching the systemic circulation unchanged after administration. SBPT04 delivered po at 200 mg/kg had an F value of 8.94 and SBPT04 delivered ip at 200 mg/kg had an F value of 48.56. The observed >5-fold difference in F values for the two routes of administration represents the fraction of therapeutically active drug that reaches the systemic circulation after intestinal absorption or potential first-pass metabolism and is available at the site of action.
Figure 4.
Bioavailability and in vivo pharmacokinetics. Bioavailability was assessed by administering SBPT04 to C57/BL6 mice via iv, po or ip routes and determining the amount of compound in blood over 24 h.
Table 3.
In vivo pharmacokinetic parameters for SBPT04 delivered iv, po or ip in male C57/BL6 mice
| Route and dosage |
||||
|---|---|---|---|---|
| iv 20 mg/kg | po 50 mg/kg | po 200 mg/kg | ip 200 mg/kg | |
| MRT (h) | 1.9 | |||
| Vss (L/kg) | 56.25 | |||
| Vd (L/kg) | 69.06 | |||
| CL (mL/min/kg) | 493.09 | |||
| AUC (μM·h) | 2.5 | 0.17 | 2.24 | 12.14 |
| AUC (mg·h/L) | 0.675 | 0.046 | 0.61 | 3.3 |
| AUC (mg·h/L)/MIC (mg/L) | 4.22 | 0.29 | 3.81 | 20.63 |
| t1/2 (h) | 1.62 | 0.54 | 2.08 | 3.04 |
| t1/2 (h) (O-glucuronide) | 3.38 | 3.24 | 2.57 | 5.48 |
| Tmax (h) | 1 | 0.5 | 0.5 | 1 |
| Cmax (μmol/L) | 0.75 | 0.2 | 0.54 | 1.41 |
| F (bioavailability) (%) | 2.69 | 8.94 | 48.56 | |
MRT, mean residence time.
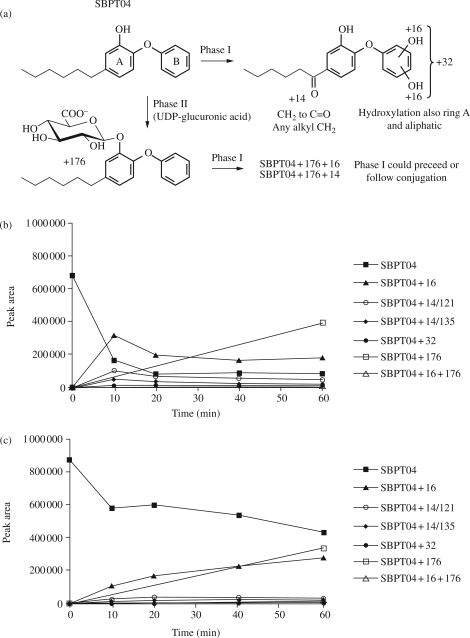
Phase I and Phase II metabolism of SBPT04 was assessed using mouse liver microsomes (MLMs) and human liver microsomes (HLMs) with LC/MS/MS methodology (Figure 5a). Studies conducted with MLMs and HLMs revealed similar oxidative metabolites, with the primary difference being a less extensive profile and an extended half-life in HLMs (Figure 5b and c). In MLMs the t1/2 of SBPT04 was determined to be 4.1 min, while HLMs exhibited a t1/2 of 46 min. Phase I analysis revealed the formation of mono- and bis-hydroxy derivatives of SBPT04 as well as a ketone metabolite (Figure 5). Phase II studies revealed that SBPT04 was also susceptible to conjugation, with the O-glucuronide metabolite being most abundant. Based on the Phase I and Phase II analyses, Phase I metabolism is a minimal contributor to the depletion of SBPT04 and Phase II O-glucuronidation is the primary eliminator of active SBPT04 from circulation in vivo. Together, these results are consistent with the notion that metabolism of SBPT04 is less extensive in human microsomes. It is known that there are differences in drug metabolism between human and rodent species.37,38 Therefore, from this information a significantly longer half-life of SBPT04 is anticipated in humans, which would allow for a reduction in the efficacious dose of SBPT04 and increased duration between dosing in the treatment regimen.
Figure 5.
Phase I and Phase II metabolism of SBPT04. (a) Phase I and Phase II metabolites of SBPT04. (b) Phase I and Phase II metabolism of SBPT04 in MLMs over 60 min. (c) Phase I and Phase II metabolism of SBPT04 in HLMs over 60 min.
Discussion
The development of a novel drug class with activity against F. tularensis that prevents relapse of disease after withdrawal of treatment requires a therapeutically relevant molecular target, and a chemotherapeutic with favourable physiochemical characteristics and rapid efficacy. The microbial fatty acid biosynthesis pathway (FASII) is a validated target for novel antibacterial drug discovery11–13,39 and, using structure-based design, we have developed a series of substituted diphenyl ethers that are potent FASII FabI enzyme inhibitors. Importantly, the activity of the FabI inhibitors against several bacterial pathogens confirms that this enzyme is a sensitive target for the development of broad-spectrum chemotherapeutics.14,29,30,33 In particular, the utility of ftuFabI as a target for drug discovery in F. tularensis has been substantiated by our own antibacterial discovery programme, which has resulted in the discovery of SBPT04, a compound that has promising in vivo activity in an animal model of tularaemia infection.14 Here, we extend our studies on this promising molecule and probe the mechanism of action and in vivo properties of SBPT04.
Monitoring the bacterial response to treatment and general metabolic activity can assess the mode of action and impact a drug has on bacterial viability. Visualization of the transcriptionally active genes revealed that SBPT04 treatment resulted in an overall reduction in intermediary metabolism and respiration, RNA and protein synthesis, and macromolecular synthesis. The down-regulation of these functions is consistent with a reduction in replication potential, metabolic function and overall cell viability due to treatment. Stress, detoxification and adaptation processes were also observed in the transcriptional response to treatment, as revealed by the up-regulation of spoT, groS, recG, visC, sodC and pilQ. However, despite the increase in these processes, SBPT04 is a potent inhibitor of cell growth and viability. The induction of detoxification processes in F. tularensis is similar to the responses in other organisms to diphenyl ethers and, like in those bacteria, the response was not associated with increased resistance to treatment.30,33
SBPT04 demonstrated potency against whole bacteria at concentrations 1000-fold lower than those that demonstrated Vero cell toxicity. When assessed over a range of concentrations, SBPT04 demonstrated a <2-fold difference in drug concentration between MBC and MIC, a key characteristic of potent bactericidal drugs and an important property for a drug to have enduring efficacy. Significantly, there was no observed relapse in disease over 30 days following a 5 day ip or po treatment with SBPT04. This is in contrast to studies with current therapeutics, such as doxycycline and ciprofloxacin, or with the alternative fluoroquinolones moxifloxacin and gatifloxacin, where relapse of disease was noted,8,9,40 although we acknowledge the possibility that alternative routes of administration, dosing regimens or drug properties could account for some of the observed differences in efficacy. Nevertheless, the observed relapse with clinically relevant drugs raises the important concern of relapse in humans following treatment with currently recommended therapies. Of note, SBPT04 is more potent in vitro against LVS and Schu4 than several current clinically used drugs, and demonstrates efficacy in mice without the associated toxicity and relapse for the treatment of pulmonary tularaemia infections.
The factors of absorption, distribution, metabolism and elimination in conjunction with the relative susceptibility of the bacteria to the drug affect the extent of killing in vivo. It has been established that the duration of the drug concentration relative to the MIC for the pathogen (i.e. AUC/MIC) is directly related to bacterial killing in animal models of infection.41 SBPT04 demonstrates a concentration-dependent killing mechanism, as our experiments illustrate a dose-dependent efficacy under a short duration of treatment. For drugs that exhibit concentration-dependent killing, the higher the drug concentration the greater the extent of bacterial killing. Thus, maintenance of the serum drug concentration above the MIC over an appropriate time is critical to establish effective killing and overall therapeutic effect. When delivered at 200 mg/kg po, SBPT04 only achieved an AUC/MIC of 3.8 in comparison with 20.6 when delivered at 200 mg/kg ip. The observed increase in efficacy of SBPT04 when delivered by ip compared with po is attributed to the >5-fold increase in the AUC/MIC.
The mechanism behind the observed bactericidal activity, even after drug concentrations fall below MIC, is related to the time required for an organism to recover from exposure.41,42 Despite the fact that the t1/2 of 3.3 h was modest with regards to the 24 h dosing interval, SBPT04 effectively clears bacteria from the lungs and prevents dissemination, suggesting a post-antibiotic sub-MIC effect. Compounds were cleared from the serum, but may have persisted in tissues at effective levels and offer extended killing beyond the visualized T>MIC or AUC/MIC ratios. Over time, intracellular concentrations of the drug can build if diffusion is efficient and the drug can linger inside the cell to continue to be effective, even though demonstrated serum concentrations have fallen below MIC. Phase I metabolites are minimal contributors to the depletion of SBPT04, and O-glucuronidation is the primary metabolic modification of SBPT04 that affects and limits the levels of SBPT04 in circulation. This information is useful for the design and optimization of next-generation compounds. Remodelling SBPT04 to bypass Phase II metabolism would extend serum levels and increase the t1/2, thereby prolonging antibacterial efficacy at lower doses. Finally, the fact that SBPT04 is a slow-onset inhibitor of ftuFabI with an in vitro residence time of 140 min on the enzyme target may also contribute to the post-antibiotic activity of SBPT04 against intracellular pathogens like F. tularensis.14 Notably, triclosan, which has an MIC of 0.00018 mg/L and is thus significantly more potent in vitro than SBPT04, has lower in vivo activity that can be directly related to the much shorter residence time of triclosan on the target.14
Importantly, in regard to the development of novel chemotherapeutics with broad-spectrum activity, the identification of substituted diphenyl ethers that are slow-onset inhibitors of FabI affords the opportunity to target other bacterial pathogens. Not only have diphenyl ethers demonstrated efficacy against F. tularensis, but with the mechanistic knowledge regarding the enzyme interaction and inhibition, this pharmacophore is suitable for development as a platform for broad-spectrum chemotherapeutics. Notably, animals treated with SBPT04 did not show signs of relapse after withdrawal of treatment. This is particularly significant given the virulence of priority pathogens and the clinical record of currently used drugs for treatment. SBPT04 demonstrated better efficacy when delivered ip compared with po, which reflects the lower bioavailability of the drug resulting from po administration. Modifications to both the structure of the drug as well as formulation are currently being made to increase the bioavailability of SBPT04 at the sites of infection, and reduce Phase I and Phase II metabolism.
In summary, these studies provide a platform for the rational design of ‘next generation’ substituted diphenyl ether compounds with greater efficacy and less metabolic liability, which would allow sufficient efficacy at lower doses against a variety of medically important bacterial pathogens.
Funding
This work was supported by New Opportunities funding from the Rocky Mountain Regional Center of Excellence (AI065357) to R. A. S. and the Northeast Biodefense Center (AI057158) to P. J. T., as well as NIH grants AI44639 and AI70383 to P. J. T.
Transparency declarations
None to declare.
K. E. performed the screening, transcriptional analysis and animal studies. K. E. prepared figures for publication and wrote the manuscript (with R. A. S. and P. J. T.). N. L. M. and R. A. B. supported the animal studies. S. E. K. assisted screening and cytotoxicity testing. C. a. E., H. L. and T. J. S. performed the chemistry. D. L. K. provided analysis support. P. J. T. and R. A. S. contributed to the design of the study, data interpretation and manuscript preparation.
Acknowledgements
Dedicated to the memory of Dr Dennis L. Knudson, a committed and valued colleague whose death occurred during the preparation and writing of this manuscript.
We gratefully recognize the post-genomics resource and instrumentation, and animal models expertise provided by the Genomics Proteomics Core and the Animal Models Core in the Rocky Mountain Regional Center of Excellence (AI065357), respectively.
References
- 1.WHO. Health Aspects of Chemical and Biological Weapons. Geneva: World Health Organization; 1970. [Google Scholar]
- 2.CDC. Biological and chemical terrorism: strategic plan for preparedness and response. Recommendations of the CDC Strategic Planning Workgroup. MMWR Recomm Rep. 2000;49:1–14. [PubMed] [Google Scholar]
- 3.Oysten P, Sjostedt A, Titball R. Tularemia: bioterrorism defence renews interest in Francisella tularensis. Nat Rev Microbiol. 2004;2:967–79. doi: 10.1038/nrmicro1045. [DOI] [PubMed] [Google Scholar]
- 4.Dennis D, Inglesby T, Henderson D, et al. Tularemia as a biological weapon—medical and public health management. JAMA. 2001;285:2763–73. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield R, Bronze M. Prevention and treatment of bacterial diseases caused by bacterial bioterrorism threat agents. Drug Disc Today. 2003;8:881–8. doi: 10.1016/s1359-6446(03)02847-2. [DOI] [PubMed] [Google Scholar]
- 6.Limaye A, Hooper C. Treatment of tularemia with fluoroquinolones: two cases and review. Clin Infect Dis. 1999;29:922–4. doi: 10.1086/520458. [DOI] [PubMed] [Google Scholar]
- 7.Maurin M, Mersali N, Raoult D. Bactericidal activities of antibiotics against intracellular Francisella tularensis. Antimicrob Agents Chemother. 2000;44:3428–31. doi: 10.1128/aac.44.12.3428-3431.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell P, Eley S, Fulop M, et al. Efficacy of ciprofloxacin and doxycycline against experimental tularemia. J Antimicrob Chemother. 1998;41:461–5. doi: 10.1093/jac/41.4.461. [DOI] [PubMed] [Google Scholar]
- 9.Steward J, Piercy T, Lever MS, et al. Treatment of murine pneumonic Francisella tularensis infection with gatifloxacin, moxifloxacin or ciprofloxacin. Int J Antimicrob Agents. 2006;27:439–43. doi: 10.1016/j.ijantimicag.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Tarnvik A, Chu M. New approaches to diagnosis and therapy of tularemia. Ann NY Acad Sci. 2007;1105:378–404. doi: 10.1196/annals.1409.017. [DOI] [PubMed] [Google Scholar]
- 11.Heath RJ. Bacterial fatty-acid biosynthesis: an antibacterial drug target waiting to be exploited. Drug Disc Today. 2001;6:715. doi: 10.1016/s1359-6446(01)01881-5. [DOI] [PubMed] [Google Scholar]
- 12.Campbell JW, Cronan JE., Jr. Bacterial fatty acid biosynthesis: targets for antibacterial drug discovery. Annu Rev Microbiol. 2001;55:305–32. doi: 10.1146/annurev.micro.55.1.305. [DOI] [PubMed] [Google Scholar]
- 13.Heath RJ, White SW, Rock CO. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl Microbiol Biotechnol. 2002;58:695–703. doi: 10.1007/s00253-001-0918-z. [DOI] [PubMed] [Google Scholar]
- 14.Lu H, England K, am Ende C, et al. Slow-onset inhibition of the FabI enoyl reductase from Francisella tularensis: residence time and in vivo activity. ACS Chem Biol. 2009;4:221–31. doi: 10.1021/cb800306y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath RJ, Rock CO. Fatty acid biosynthesis as a target for novel antibacterials. Curr Opin Investig Drugs. 2004;5:146–53. [PMC free article] [PubMed] [Google Scholar]
- 16.Levy CW, Roujeinikova A, Sedelnikova S, et al. Molecular basis of triclosan activity. Nature. 1999;398:383–4. doi: 10.1038/18803. [DOI] [PubMed] [Google Scholar]
- 17.McMurry LM, Oethinger M, Levy SB. Triclosan targets lipid synthesis. Nature. 1998;394:531–2. doi: 10.1038/28970. [DOI] [PubMed] [Google Scholar]
- 18.Parikh SL, Xiao G, Tonge PJ. Inhibition of InhA, the enoyl reductase from Mycobacterium tuberculosis, by triclosan and isoniazid. Biochemistry. 2000;39:7645–50. doi: 10.1021/bi0008940. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X, Janson CA, Court RI, et al. Molecular basis for triclosan activity involves a flipping loop in the active site. Protein Sci. 1999;8:2529–32. doi: 10.1110/ps.8.11.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slayden RA, Barry CE., III The role of KasA and KasB in the biosynthesis of meromycolic acids and isoniazid resistance in Mycobacterium tuberculosis. Tuberculosis (Edinb) 2002;82:149–60. doi: 10.1054/tube.2002.0333. [DOI] [PubMed] [Google Scholar]
- 21.Slayden RA, Lee RE, Barry CE., III Isoniazid affects multiple components of the type II fatty acid synthase system of Mycobacterium tuberculosis. Mol Microbiol. 2000;38:514–25. doi: 10.1046/j.1365-2958.2000.02145.x. [DOI] [PubMed] [Google Scholar]
- 22.Stewart MJ, Parikh S, Xiao G, et al. Structural basis and mechanism of enoyl reductase inhibition by triclosan. J Mol Biol. 1999;290:859–65. doi: 10.1006/jmbi.1999.2907. [DOI] [PubMed] [Google Scholar]
- 23.Ward WH, Holdgate GA, Rowsell S, et al. Kinetic and structural characteristics of the inhibition of enoyl (acyl carrier protein) reductase by triclosan. Biochemistry. 1999;38:12514–25. doi: 10.1021/bi9907779. [DOI] [PubMed] [Google Scholar]
- 24.Rawat R, Whitty A, Tonge PJ. The isoniazid–NAD adduct is a slow, tight-binding inhibitor of InhA, the Mycobacterium tuberculosis enoyl reductase: adduct affinity and drug resistance. Proc Natl Acad Sci USA. 2003;100:13881–6. doi: 10.1073/pnas.2235848100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heerding DA, Chan G, DeWolf WE, et al. 1,4-Disubstituted imidazoles are potential antibacterial agents functioning as inhibitors of enoyl acyl carrier protein reductase (FabI) Bioorg Med Chem Lett. 2001;11:2061–5. doi: 10.1016/s0960-894x(01)00404-8. [DOI] [PubMed] [Google Scholar]
- 26.Kuo MR, Morbidoni HR, Alland D, et al. Targeting tuberculosis and malaria through inhibition of enoyl reductase: compound activity and structural data. J Biol Chem. 2003;278:20851–9. doi: 10.1074/jbc.M211968200. [DOI] [PubMed] [Google Scholar]
- 27.Miller WH, Seefeld MA, Newlander KA, et al. Discovery of aminopyridine-based inhibitors of bacterial enoyl-ACP reductase (FabI) J Med Chem. 2002;45:3246–56. doi: 10.1021/jm020050+. [DOI] [PubMed] [Google Scholar]
- 28.Seefeld MA, Miller WH, Newlander KA, et al. Indole naphthyridinones as inhibitors of bacterial enoyl-ACP reductases FabI and FabK. J Med Chem. 2003;46:1627–35. doi: 10.1021/jm0204035. [DOI] [PubMed] [Google Scholar]
- 29.am Ende CW, Knudson SE, Liu N, et al. Synthesis and in vitro antimycobacterial activity of B-ring modified diaryl ether InhA inhibitors. Bioorg Med Chem Lett. 2008;18:3029–33. doi: 10.1016/j.bmcl.2008.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sullivan TJ, Truglio JJ, Boyne ME, et al. High affinity InhA inhibitors with activity against drug-resistant strains of Mycobacterium tuberculosis. ACS Chem Biol. 2006;1:43–53. doi: 10.1021/cb0500042. [DOI] [PubMed] [Google Scholar]
- 31.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J Immunol. 2005;175:6792–801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 32.Franzblau SG, Witzig RS, McLaughlin JC, et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol. 1998;36:362–6. doi: 10.1128/jcm.36.2.362-366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyne ME, Sullivan TJ, am Ende CW, et al. Targeting fatty acid biosynthesis for the development of novel chemotherapeutics against Mycobacterium tuberculosis: evaluation of A-ring-modified diphenyl ethers as high-affinity InhA inhibitors. Antimicrob Agents Chemother. 2007;51:3562–7. doi: 10.1128/AAC.00383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J Immunol. 2007;178:4538–47. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 35.Orme IM, Collins FM. Prophylactic effect in mice of BCG vaccination against nontuberculous mycobacterial infections. Tubercle. 1985;66:117–20. doi: 10.1016/0041-3879(85)90076-5. [DOI] [PubMed] [Google Scholar]
- 36.Baker CN, Hollis DG, Thornsberry C. Antimicrobial susceptibility testing of Francisella tularensis with a modified Mueller–Hinton broth. J Clin Microbiol. 1985;22:212–5. doi: 10.1128/jcm.22.2.212-215.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caldwell J. The current status of attempts to predict species differences in drug metabolism. Drug Metab Rev. 1981;12:221–37. doi: 10.3109/03602538108994030. [DOI] [PubMed] [Google Scholar]
- 38.Cheung C, Gonzalez FJ. Humanized mouse lines and their application for prediction of human drug metabolism and toxicological risk assessment. J Pharmacol Exp Ther. 2008;327:288–99. doi: 10.1124/jpet.108.141242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu H, Tonge PJ. Inhibitors of FabI, an enzyme drug target in the bacterial fatty acid biosynthesis pathway. Acc Chem Res. 2008;41:11–20. doi: 10.1021/ar700156e. [DOI] [PubMed] [Google Scholar]
- 40.Piercy T, Steward J, Lever MS, et al. In vivo efficacy of fluoroquinolones against systemic tularaemia infection in mice. J Antimicrob Chemother. 2005;56:1069–73. doi: 10.1093/jac/dki359. [DOI] [PubMed] [Google Scholar]
- 41.Jacobs M. Anti-infective pharmacodynamics—maximizing efficacy, minimizing toxicity. Drug Disc Today. 2004;1:505–12. [Google Scholar]
- 42.Craig W. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]