Abstract
Irradiation damage to salivary glands is a common iatrogenic consequence of treatment for head and neck cancers. The subsequent lack of saliva production leads to many functional and quality-of-life problems for affected patients and there is no effective conventional therapy. To address this problem, we developed an in vivo gene therapy strategy involving viral vector-mediated transfer of the aquaporin-1 cDNA to irradiation-damaged glands and successfully tested it in two pre-clinical models (irradiated rats and miniature pigs), as well as demonstrated its safety in a large toxicology and biodistribution study. Thereafter, a clinical research protocol was developed that has received approval from all required authorities in the United States. Patients are currently being enrolled in this study.
1 Introduction
Each year, worldwide, ∼500,000 individuals are diagnosed with a malignancy in the head and neck region.1 Most of these patients will receive treatment that includes surgery ± chemotherapy and therapeutic irradiation (IR). While IR is quite effective as adjunctive therapy for the cancer, it can also damage adjacent normal tissues. Salivary glands are quite sensitive to IR. Indeed, IR leads to a dramatic loss of the fluid secreting salivary acinar cells, resulting in severe glandular hypofunction (a diminished production of saliva) in most patients (Vissink et al. 2003; Nagler and Baum 2003). The reason for this damage remains enigmatic, as salivary acinar cells are well differentiated and very slowly dividing, the opposite of the classical target cell for IR sensitivity. If patients have sufficient functional acinar tissue post-IR, it is possible to treat their salivary hypofunction with cholinergic drugs (Shiboski et al. 2007). However, most post-IR patients have salivary glands characterized by inadequate acinar cell mass with non-fluid secreting duct cells surviving and predominating (Vissink et al. 2003; Nagler and Baum 2003). There is no effective conventional therapy for these patients, a situation that led us to consider the use of gene therapy, i.e., in vivo gene transfer, as a therapeutic approach to repair damaged glands (Baum et al. 1999). The gene of choice was human aquaporin-1 (hAQP1).
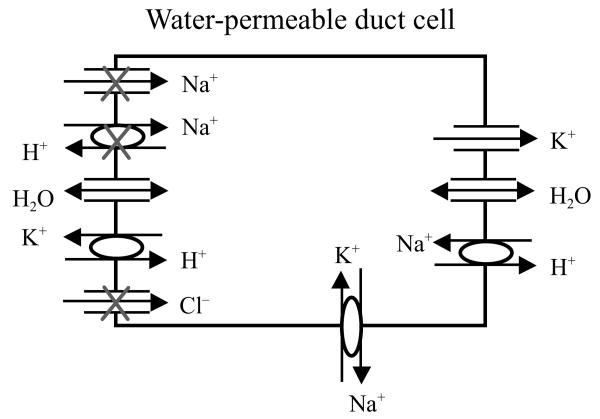
The specific strategy we adopted is depicted in Fig. 1. This strategy was based on the understanding of salivary fluid secretion and salivary duct cell physiology available in 1991 (Baum 1993). In this figure, a surviving duct cell in an IR-damaged salivary gland is illustrated. We hypothesized that it would be possible for this cell to secrete fluid through the transfer of a gene encoding a functional, non-polarized water channel protein. Our hypothesis included a view that the duct cells could generate an osmotic gradient, lumen > interstitium, in the absence of any significant acinar cell secretion and the expression of a water channel in these normally water impermeant cells would in turn permit osmotically driven transepithelial fluid flow into the lumen. The specific hypothesis, in brief, is as follows. In the absence of an isotonic acinar cell secretion, most of the membrane transport proteins then-known to be present in the duct cell luminal membrane would be inactive owing to the low luminal concentrations of their respective substrates; these include the epithelial sodium channel, the cystic fibrosis transmembrane conductance regulator (i.e., a chloride channel) and the sodium/proton exchanger. However, a potassium/proton exchanger in the luminal membrane would be active and able to exchange intracellular potassium for a proton that could be generated in the lumen from the dissolution of CO2 in the small amount of diffused water that normally should be present. The CO2 would dissociate to yield the exchangeable proton and HCO3−, resulting in a KHCO3 osmotic gradient. As shown, the cDNA for hAQP1 has been transferred and it assumes a non-polarized distribution all around the plasma membrane. Water then would be able to flow across the duct cell into the lumen in response to the hypothesized KHCO3 gradient. It is important to recognize that this hypothetical mechanism is still unproven, but the gene transfer strategy, as is presented below, has been successfully utilized in small and large animal pre-clinical models.
Fig. 1.
Schematic diagram of the hypothesized mechanism for fluid secretion following AdhAQP1-mediated gene transfer to duct cells in an irradiated salivary gland. The surviving duct cell is presented in a simplified form, based on our understanding ∼1991. The duct lumen is to the left and the interstitium is to the right. The three apical ion pathways with Xs will be inoperable in the absence of an isotonic primary secretion, which normally is made by acinar cells. We have hypothesized that duct cells could generate a KHCO3 gradient (lumen > interstitium) enabling fluid to flow into the duct lumen following expression of the transgene hAQP1. This figure is modified from Vitolo and Baum (2002), and is based on the experiments presented in Delporte et al. (1997)
2 Gene Transfer Vector: Construction and In Vitro Characterization
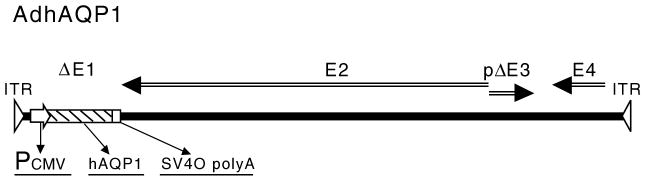
To transfer the hAQP1 cDNA into duct cells we chose to use a first generation, serotype 5 adenoviral (Ad5) vector (Delporte et al. 1997). In earlier studies we showed Ad5 vectors were extremely efficient for in vivo gene transfer to rodent salivary glands (Mastrangeli et al. 1994). The AdhAQP1 vector was constructed (Delporte et al. 1997) and a schematic depiction of this vector is shown in Fig. 2. The vector consists of an Ad5 genome modified by a deletion in the E1 gene region, which is important to prevent replication, and a partial deletion in the E3 region. All other Ad5 genes remain in the vector. The transgene expression cassette was placed into the deleted E1 region via homologous recombination and includes a cytomegalovirus promoter/enhancer to drive expression, the hAQP1 cDNA, and a simian virus-40 polyadenylation signal.
Fig. 2.
Schematic diagram of AdhAQP1. ITR inverted terminal repeat; Pcmv cytomegalovirus promoter/enhancer; hAQP1 human aquaporin-1 cDNA; SV40 polyA simian virus 40 polyadenylation signal; ΔE1 deletion of adenoviral E1 sequences; E2 adenoviral E2 genes; pΔE3 partial deletion/modification of adenoviral E3 sequences; E4 adenoviral E4 genes
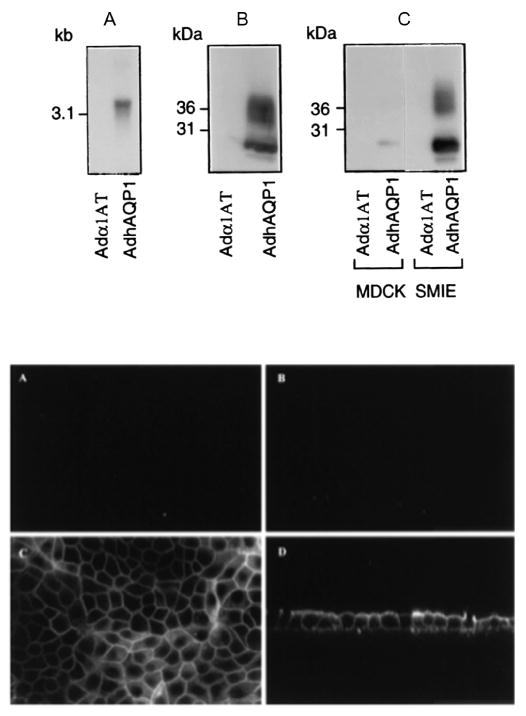
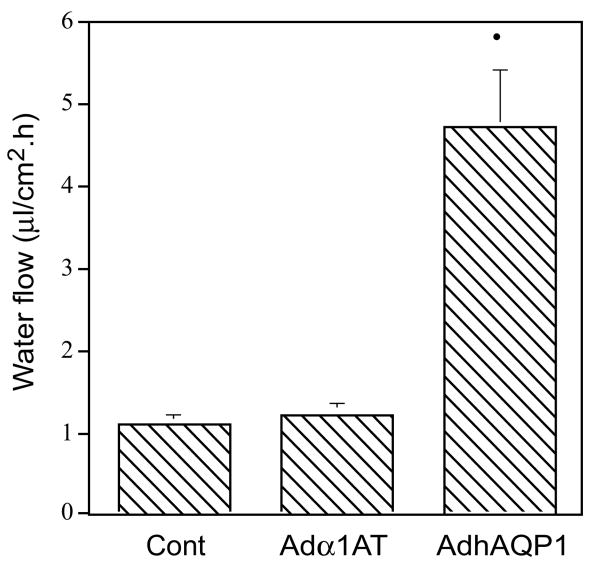
Initial studies of the function and potential utility of this vector were performed in vitro with several epithelial cell types (Delporte et al. 1997). As shown in the upper panel of Fig. 3, when compared to transduction with a control Ad5 vector, 293 (human embryonic kidney), MDCK (canine kidney) and SMIE (rat submandibular gland) cells all begin to express hAQP1 after transduction with AdhAQP1. Furthermore, and essential for our hypothesis, the expressed hAQP1 assumes a non-polarized distribution in MDCK cells (Fig. 3, lower panel). Importantly, as shown in Fig. 4, the transgenic hAQP1 protein is functional and results in the net movement of fluid, from a basal to an apical direction, in response to an imposed osmotic gradient (apical 400 mosm; basal 300 mosm) (Delporte et al. 1997). The treatment of MDCK cells with an irrelevant vector had no effect on fluid movement (same as control cells), while treatment of cells with AdhAQP1 resulted in a ∼fivefold increase in net fluid secretion (Delporte et al. 1997).
Fig. 3.
Human aquaporin-1 expression in epithelial cells in vitro. Upper panel: (a) Northern blot using RNA from 293 cells transduced with either AdhAQP1 or a control vector, Adα1AT. (b) Western blot of crude membranes from 293 cells transduced with AdhAQP1 or the control vector. (c) Western blot of crude membranes from MDCK and SMIE cells transduced with AdhAQP1 or the control vector. Note that in the Western blots the monomeric non-glycosylated hAQP1 protein migrates at ∼28kDa, while multiple glycosylated forms are seen at slightly higher molecular weights. This figure originally was published as Fig. 1 in (Delporte et al. 1997). Lower panel: Localization of transgenic hAQP1 expressed in MDCK cells. Confluent MDCK cells were grown on filters and transduced for 24 h with either Adα1AT (a) and (b) or AdhAQP1 (c) and (d). Cell layers were then examined by confocal microscopy after immunofluorescent staining with an antibody to hAQP1. (a) and (c) are in the x–y plane, while (b) and (d) are in the x–z plane. This figure originally was published as Fig. 2 in (Delporte et al. 1997)
Fig. 4.
Net fluid secretion rate of MDCK cells with and without transduction by AdhAQP1. Cells were either untreated (control, Cont), or transduced with either AdhAQP1 or a control vector, Adα1AT, and water flow measured in response to an apical (400 mosm) >basal (300 mosm) osmotic gradient. The results are expressed as water flow in microliters of fluid per cm2 per hour and are mean values ± SEMs. This figure originally was published as Fig. 3 in (Delporte et al. 1997)
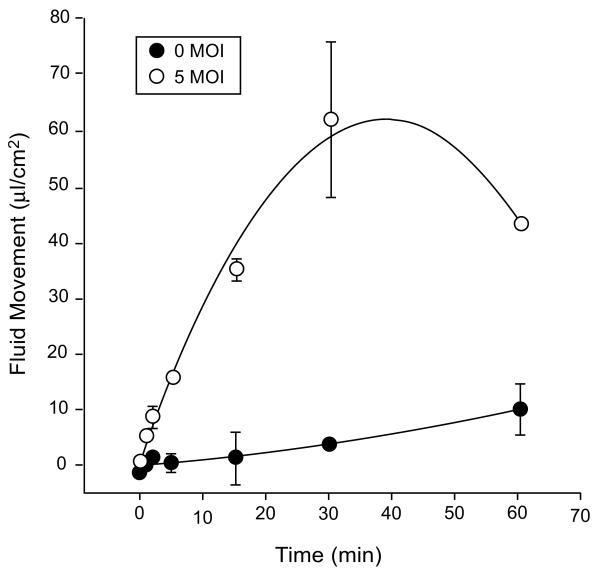
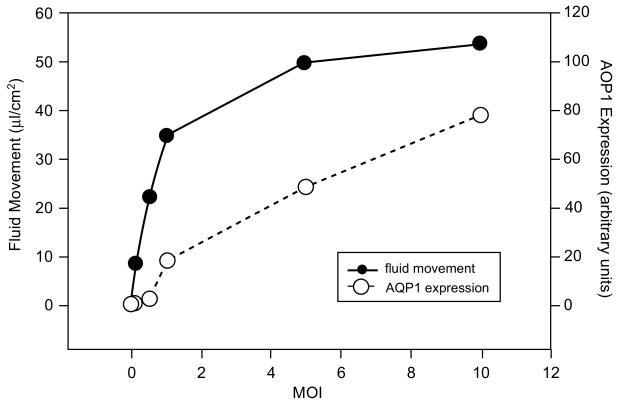
We further characterized the pharmacological properties of the AdhAQP1 vector using the SMIE cell line. As shown in Fig. 5, following transduction of cells at a MOI (multiplicity of infection) of 5, i.e. five infectious units (plaque-forming units, pfu)/cell, fluid movement across SMIE cell monolayers is linear for 15–30 min (Delporte et al. 1998). After 30 min, the transepithelial fluid movement across non-transduced cells was ∼5μl cm−2, while following transduction with AdhAQP1 this value was ∼60μl cm−2. As is shown in Fig. 6, osmotically obliged fluid movement across SMIE cells was AdhAQP1 dose dependent (Delporte et al. 1998). Fluid movement sharply increased at doses from 0.1, 0.5–1.0 MOI and began to plateau thereafter. Interestingly, significant fluid movement occurred at relatively low levels of hAQP1 expression in these monolayers, e.g., the amount of hAQP1 yielding ∼50% of maximal fluid movement was <10% of the maximal hAQP1 protein expression observed.
Fig. 5.
Time course of fluid movement across SMIE cell monolayers. SMIE cells were transduced with AdhAQP1 (MOI = 5) or not, and after 24 h fluid movement was measured in response to an osmotic gradient as shown in Fig. 4. The results are expressed as water flow in microliters of fluid per cm2 per hour and are mean values ± SEMs. This figure originally was published as Fig. 2 in (Delporte et al. 1998)
Fig. 6.
Effect of vector concentration on fluid flow across SMIE cells. After reaching confluence, SMIE cell monolayers were transduced at the indicated MOI and fluid movement then measured for 15 min. Thereafter, crude membranes from each cell monolayer were prepared, electrophoresed and subjected to Western blotting with antibody to hAQP1. The films were scanned with a laser densitometer to quantify the amount of hAQP1 expressed (indicated in arbitrary units). This figure originally was published as Fig. 4 in (Delporte et al. 1998)
3 Pre-Clinical Model Testing
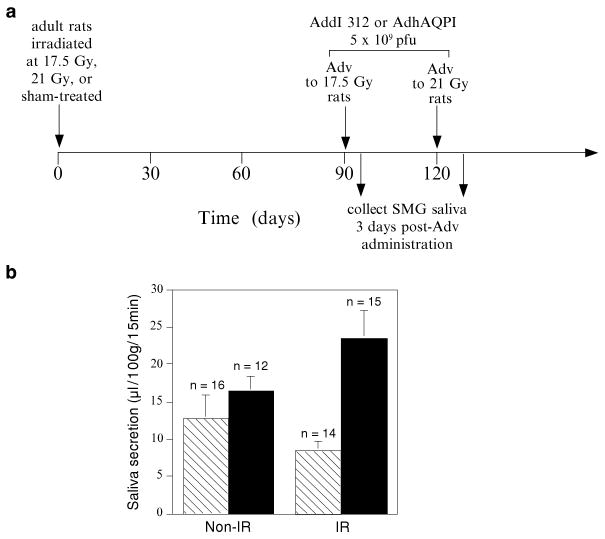
In order to determine if the AdhAQP1 vector was effective in restoring salivary flow to IR-damaged salivary glands, we used a rat IR model with which we previously had considerable experience (Nagler et al. 1998). Additionally, we knew that Ad5 vectors administered to rat salivary glands direct high levels of transgene expression in this tissue (Mastrangeli et al. 1994; Baum et al. 2002). For experimental convenience, we employed single radiation doses (either 17.5 or 21 Gray, Gy), rather than a fractionated scheme that is more typical of the clinical situation. Rodent salivary glands are considered relatively IR-resistant, so the IR doses used were high given the size of the animals (∼300g). A time line depicting the general experimental design of this study is shown in Fig. 7a. Animals were irradiated and then followed for either 90 (the 17.5 Gy group) or 120 (the 21 Gy group) days, providing moderate and severe IR damage models, respectively. At the appropriate time point animals were administered 5 × 109 pfu of the AdhAQP1 vector or a control Ad5 vector (Addl312, without any encoded transgene). The infectious dose used corresponds to ∼5 × 1011 vector genomes and was delivered into both submandibular glands via intra-oral cannulation of the main excretory duct. After 3 days we collected whole saliva from each animal. The results were generally similar with both radiation groups, demonstrating administration of the AdhAQP1 vector increased salivary flow rate in the irradiated rats (Fig. 7b, Table 1). As shown in Fig. 7b, 17.5 Gy leads to a modest, ∼30%, decrease in salivary flow (control rats receiving IR plus the Addl312 vector) and administration of the AdhAQP1 vector to these rats resulted in a dramatic increase in salivary flow. As shown in Table 1, IR with 21 Gy leads to a marked decrease in salivary flow, ∼65%, in the Addl312 vector-treated rats. Conversely, salivary flow in rats receiving the AdhAQP1 vector was similar, independent of whether or not they were irradiated. Immunocytochemical examination of sections prepared from glands transduced with AdhAQP1 showed high levels of hAQP1 immunolabelling in both acinar and duct cells, (in this rat IR model many acinar cells survive IR treatment, but shrink in size (O'Connell et al. 1999a), somewhat unlike the situation in human glands). Nonetheless, these overall results strongly supported our original hypothesis that hAQP1 gene transfer could lead to a correction in salivary flow rates in irradiated animals. Interestingly, the [K+] in saliva was ∼40% greater in samples collected from AdhAQP1-treated rats compared to that from rats treated with Addl312 (Delporte et al. 1997).
Fig. 7.
(a) Time line of initial study of AdhAQP1 efficacy in irradiated rats. Rats were irradiated on day zero with either 17.5 Gy or 21 Gy, or sham-irradiated. After 90 (17.5 Gy) or 120 (21 Gy) days, AdhAQP1 or a control vector (Addl312) was administered to both submandibular glands and 3 days later saliva was collected. (b) Function of transgenic hAQP1 in vivo. Salivary flow rates obtained in animals irradiated (17.5 Gy) or not, and administered either AdhAQP1 or Addl312. Rats administered Addl312 are shown in the hatched bars, while rats administered AdhAQP1 are shown in the black bars. The results are expressed as saliva secretion in microliters per 100 g body weight per 15 min and are mean values ± SEMs. This figure originally was published as Fig. 4b in (Delporte et al. 1997)
Table 1.
Effect of AdhAQP1 transduction on salivary secretion by rat submandibular glands after irradiation with 21 Gy
| Experimental group | Addl312 (n) | AdhAQP1 (n) |
|---|---|---|
| Sham-irradiated | 36.6±6.8 (4) | 28.4±8.0 (6) |
| 21 Gy irradiated | 13.2±3.7 (6) | 30.6±3.5 (9) |
This table is modified from Table 1 of (Delporte et al. 1997). The data shown are salivary flow rates (μl/100g body weight per 15 min; mean ± SEMs) for the number of rats in parentheses. Animals were either sham irradiated or their salivary glands were exposed to 21 Gy. Four months after irradiation, animals received 5 × 109 plaque forming units of either a control Ad5 vector (Addl312) or AdhAQP1 to each submandibular gland. Three days later saliva was collected
A critical step in the development of a gene therapy is demonstration of efficacy, and scaling, to a large animal model. Originally, for this purpose we conducted experiments in rhesus macaques, but the results were equivocal, likely because of the small number of animals available to us (five; including one control, with two animals in each of two dosage groups (O'Connell et al. 1999b)). Subsequently, we developed a more affordable, large animal salivary gland IR damage model using the miniature pig parotid gland (Li et al. 2005). In addition, we demonstrated that it was possible to scale the expression of an Ad5 vector-encoded reporter transgene (luciferase) between 20 g mice and ∼25–30kg miniature pigs (Li et al. 2004). Consequently, we conducted an IR experiment in miniature pigs similar in general design to that used in rats (see Figs. 7a and 8a, and (Shan et al. 2005)).
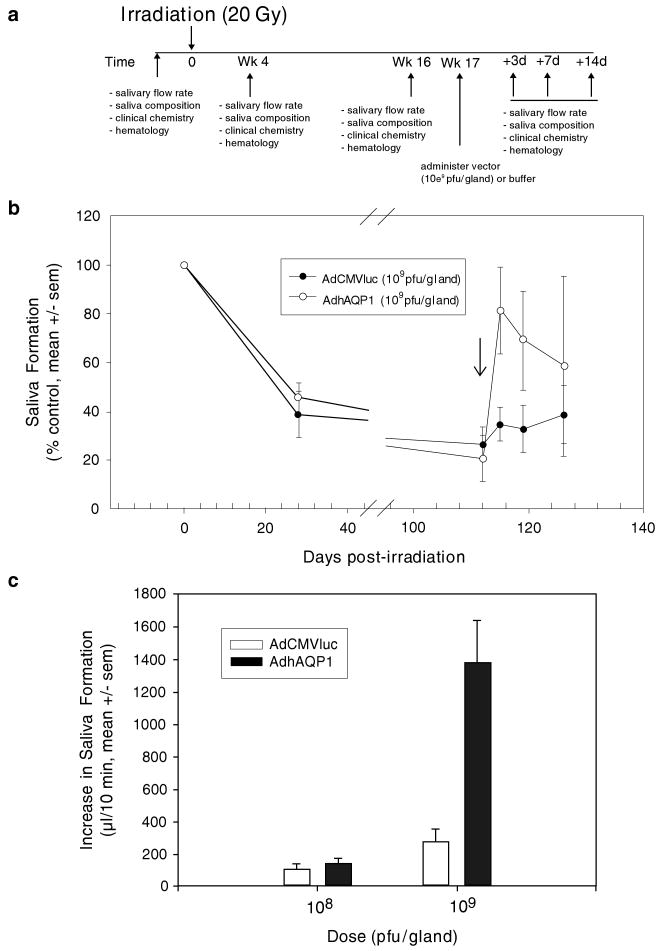
Fig. 8.
The effect of AdhAQP1 vector administration on parotid salivary secretion in irradiated miniature pigs. (a) Time line of study. Animals (three separate cohorts) in these experiments were followed longitudinally. Either AdhAQP1, a control vector (AdCMVluc, encoding luciferase), or buffer was given as indicated. (b) Pattern of parotid salivary flow following irradiation and vector administration. Parotid salivary flow rates (in μl per 10 min; average of two measurements) prior to irradiation were normalized to 100% and data at other time-points are shown as a percentage of that initial value. The arrow indicates the time point when vectors were administered. (c) Effect of AdhAQP1 dose on parotid saliva secretion. All data shown are mean values ± SEMs. This figure originally was published as part of Fig. 1 in (Shan et al. 2005)
We irradiated one miniature pig parotid gland with a single dose of 20 Gy, again for experimental convenience (Fig. 8a). Importantly, the IR dose used was slightly less than that used in rats, which were ∼1% the size of the miniature pigs. Prior to IR we collected saliva twice from the parotid glands of each animal and normalized the average value to 100% secretion levels. We then irradiated the animals and followed them longitudinally for ∼4 months (see Fig. 8a). Within 4 weeks average parotid salivary flow rates in irradiated glands had decreased by ∼60% and by 16 weeks salivary flow was reduced by ∼80% (Fig. 8b). Next, all animals were treated with either the AdhAQP1 vector or a control vector (for this study an Ad5 vector encoding luciferase). The maximum total vector dose administered to each parotid gland was 109 pfu, i.e., only 20% of the total vector dose used for rats. This dose, when normalized to the size of the targeted gland, is the same dose that we previously showed scaled from mice (Li et al. 2004). Three days after vector delivery, the 109 pfu AdhAQP1 dose resulted in a dramatic increase in parotid saliva flow rates, to ∼80% of pre-IR control levels (Fig. 8b). Thereafter, flow rates began to decrease (at days 7 and 14), but on average still remained above those seen for glands receiving the control vector. Additionally, we showed that vector efficacy was dose-dependent, i.e., a 108 pfu/gland dose being without effect (Fig. 8c). When we examined formalin-fixed parotid gland sections by immunocytochemistry with an antibody directed at hAQP1, we saw high levels of hAQP1 immunolabelling only in duct cells. The gland sections also showed an apparent loss of acinar cells with replacement by connective tissue, as seen in humans. Although acinar cells were seen in the sections, none were transduced by vector. Interestingly, the [K+] in parotid saliva from animals transduced with the AdhAQP1 vector was decreased ∼40% from the 16 week post-IR level to an average level approximating that seen prior to IR. This was different from what we expected, based on our hypothesis and the results of the rat study mentioned previously. However, the total amount of K+ secreted by AdhAQP1-transduced glands was greater than that seen in the control glands. Although still unclear, this result may indicate an osmotic driving force operating in the transduced miniature pig duct cells different than we anticipated, or be reflective of a secretion arising only from the transduction of duct cells in these animals, i.e., unlike the mixed acinar and duct cell transduction seen in rats.
4 Toxicology and Biodistribution Studies
Based on the miniature pig experiments, it seemed that the AdhAQP1 vector could prove useful in human studies. Accordingly, it was imperative to conduct a detailed safety evaluation of this vector. We conducted such a study in male and female rats. The study conformed to the U.S. Food and Drug Administration's (FDA's) Good Laboratory Practice (GLP) guidelines (Zheng et al. 2006). Two hundred animals (equal number by gender) were divided into four dosage groups: zero vector (vehicle alone), 2 × 108 vector genomes, 8 × 109 vector genomes, or 2 × 1011 vector genomes. Vector was delivered to a single submandibular gland and thereafter, animals were evaluated on days 3, 15, 29, 57 and 92.
Administration of the AdhAQP1 vector led to no animal mortality or morbidities, and no adverse events were noted clinically (Zheng et al. 2006). Additionally, no neoplasms were detected in any animal studied. In male rats we observed vector-related chronic focal inflammatory lesions in the targeted gland, such as we had reported previously (Adesanya et al. 1996). Interestingly, this was not noted in female rats. Additionally, we observed some other minor gender related effects (in females) that were vector, but not dose, dependent. For example, in male rats we found no vector-associated alterations in multiple clinical chemistry and hematology parameters. Likewise, female rats did not show any changes in clinical chemistry values (Fig. 9); serum levels of alanine aminotransferase, creatine phosphokinase, lactate dehydrogenase and blood urea nitrogen were unaffected by vector treatment in female rats (Zheng et al. 2006). However, female rats displayed a small reduction in body weight (Fig. 10), which also was associated with a similarly small decrease in food consumption ((Zheng et al. 2006), not shown). Additionally, female, but not male, rats showed some evidence of persistent systemic inflammation (small increase in the total and segmented white blood cell number). While each of these gender related changes was quite small, it will be important to be attentive to possible gender differences in responses to AdhAQP1 administration in the clinical study described below.
Fig. 9.
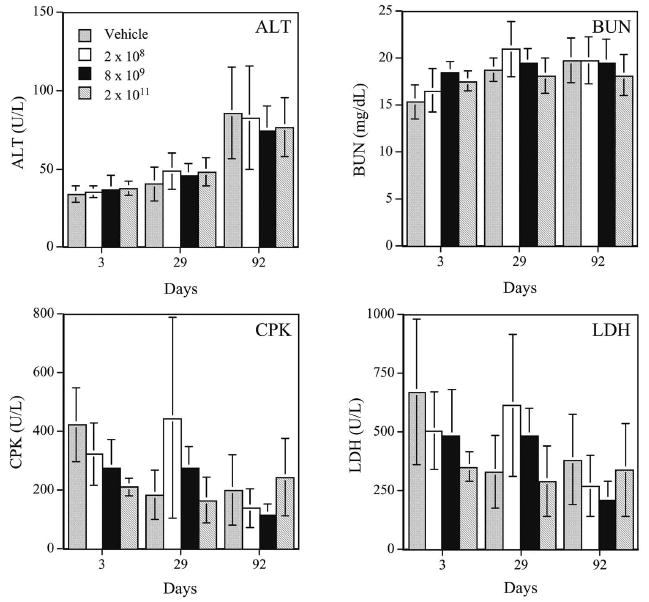
Representative clinical chemistry results seen with female rats treated, or not, with various doses of AdhAQP1. ALT alanine aminotransferase (∼liver damage); CPK creatine phosphokinase (∼heart damage); BUN blood urea nitrogen (∼kidney function); LDH lactate dehydrogenase (indicates general tissue damage). Data shown are mean values ± SD. Dosage groups are indicated (vector genomes of AdhAQP1 administered). This figure was published originally as Fig. 4 in (Zheng et al. 2006)
Fig. 10.
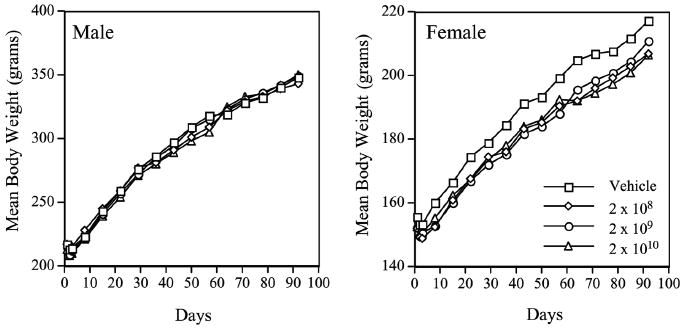
Animal body weights during AdhAQP1 toxicology study. Left panel: Mean body weight in grams is depicted for all four groups of male rats over the time course of this study. Right panel: Mean body weight in grams is depicted for all four groups of female rats over the time course of this study. The animal study groups are as indicated (doses in vector genomes of AdhAQP1). This figure was published originally as Fig. 1 in (Zheng et al. 2006)
We also conducted a detailed QPCR necropsy of all animals in order to determine AdhAQP1 vector biodistribution (Zheng et al. 2006). We sampled the following tissues for this study (Table 2): brain, left and right [the targeted gland] submandibular glands (separately), left and right parotid glands (separately), left and right sublingual glands (separately), buccal mucosa, palatal mucosa, tongue, floor-of-the-mouth mucosa, left and right mandibular lymph nodes (separately), spleen, heart, lung, small intestine, large intestine, kidney, liver, gonads (both testes and ovaries), blood and saliva. Three days after delivery of 2 × 1011 vector genomes of AdhAQP1, vector was detected primarily in the targeted right submandibular gland (Table 2). The median copy number in these samples was 2.24 × 103 copies μg−1 DNA. At later time points vector was found in ∼half of the targeted glands. Thus, following AdhAQP1 vector delivery to a single rat salivary gland, vector can persist in many animals for a long time, even though expression of the transgenic hAQP1 protein was not seen after day 15 (Zheng et al. 2006). Additionally, AdhAQP1 was frequently found in the right sublingual gland (days 3–57 the median value was 1.86 × 102 copies/μg DNA), which is not surprising since sublingual glands can often share a common main excretory duct with submandibular glands. Furthermore, AdhAQP1 was never detected in any blood sample and only detected in a single saliva sample on day 3. Also, only 5 of 90 non-oral samples tested positively in the sensitive QPCR assay used (Zheng et al. 2006). The limited biodistribution of the vector is consistent with the minimal toxicity indicated by clinical chemistry, hematology and pathology analyses. Finally, and quite importantly, QPCR assays showed no evidence for the generation of replication competent adenovirus in blood or saliva samples. Thus, in aggregate this GLP safety study showed that localized delivery of AdhAQP1 to rat salivary glands occurs without significant toxicity.
Table 2.
Tissue distribution of AdhAQP1 in rats administered 2 × 1011 vector genomes in the right submandibular gland
| Tissue | Day 3 | Day 29 | Day 92 |
|---|---|---|---|
| Left parotid | 0/10 | 0/10 | 0/10 |
| Left SMG | 4/10 | 1/10 | 3/10 |
| Left SLG | 0/10 | 0/10 | 1/10 |
| Left mandibular lymph node | 1/10 | 0/10 | 1/10 |
| Right parotid | 1/10 | 0/10 | 0/10 |
| Right SMG | 9/10 | 4/10 | 4/10 |
| Right SLG | 2/10 | 3/10 | 7/10 |
| Right mandibular lymph node | 2/10 | 0/10 | 1/10 |
| Buccal mucosa | 0/10 | 0/10 | 0/10 |
| Floor-of-mouth mucosa | 3/10 | 0/10 | 0/10 |
| Palatal mucosa | 0/10 | 0/10 | 0/10 |
| Tongue | 0/10 | 0/10 | 0/10 |
| Brain | 0/10 | 0/10 | 0/10 |
| Spleen | 2/10 | 0/10 | 1/10 |
| Liver | 1/10 | 0/10 | 0/10 |
| Lung | 2/10 | 0/10 | 0/10 |
| Kidney | 0/10 | 0/10 | 0/10 |
| Gonads | 0/10 | 0/10 | 0/10 |
| Blood | 0/10 | 0/10 | 0/10 |
| Saliva | 1/10 | 0/10 | 0/10 |
This table is modified from Table 2 of (Zheng et al. 2006). The data shown come from PCR-necropsies of experimental animals and represent the number of real time PCR-positive samples per total number of samples assayed. The target gland was the right submandibular gland, and these data are shown in italics. As is clear from the data, when the AdhAQP1 vector was administered to a single gland most of the vector was localized to that gland, and very little was found outside adjacent oral tissues
5 Clinical Protocol
In October of 2005, we submitted a clinical protocol, “Open-label, dose-escalation study evaluating the safety of a single administration of an adenoviral vector encoding human aquaporin-1 to one parotid salivary gland in individuals with irradiation-induced parotid salivary hypofunction”, for initial review by our Institutional Review Board (IRB). The purpose of this clinical protocol is to test the safety of AdhAQP1, with some measures of efficacy, i.e., a Phase 1, dose escalation study, in adult patients with established IR-induced parotid gland hypofunction. The targeted tissue site for the AdhAQP1 vector in this protocol is a single parotid gland and safety is being monitored by measurements of an extensive battery of conventional clinical and immunological parameters. The primary outcome measure for biological efficacy is parotid gland salivary output, with secondary assessment of subjective improvements in symptoms of xerostomia. Five doses of vector are to be administered (Table 3), with three patients in each dosage group. A maximum of 21 patients can be enrolled, depending on the occurrence of protocol-related toxicities. Of note, the fourth dosage level (5.8 × 109 vector genomes) is roughly comparable, on per microliter infused basis, to the dose that was effective in increasing salivary flow in the miniature pig study described above. Additionally, the highest dose to be administered (3.5 × 1010 vector genomes) is a dose that has not been associated with any toxicity in previous clinical studies employing Ad5 vectors for other tissues, e.g., lung, heart, skin, tumors [e.g., (Harvey et al. 2002)]. It is also a dose that is 1,000 times lower than the dose (3.8 × 1013 vector genomes) that led to an Ad5 vector-related death in a clinical trial at the University of Pennsylvania in 1999 (Raper et al. 2003).
Table 3.
Dosage schedule for the AdhAQP1 clinical trial
| Dosage group | Vector (genomes/gland) | Vector (genomes/μl) |
|---|---|---|
| 1 | 4.8 × 107 | 1 × 105 |
| 2 | 2.9 × 108 | 5.8 × 105 |
| 3 | 1.3 × 109 | 2.6 × 106 |
| 4 | 5.8 × 109 | 1.2 × 107 |
| 5 | 3.5 × 1010 | 0.7 × 108 |
This table shows the number of AdhAQP1 vector genomes to be administered to a single parotid gland in subjects (n = 3) in each of the five dosage groups approved for clinical study. The administered doses are presented as both the total dose to be administered (middle column) and the number of vector genomes to be administered per μl infusate, i.e., assuming an infusate volume of 500μl (right column). Note that an additional three subjects can be enrolled if 1 of 3 subjects in a given dosage group experiences a dose-limiting toxicity. Also, if there are no adverse events in the three assigned subjects in the highest dosage group, an additional three subjects can be studied at that dose. Thus, a maximum of 21 patients can be studied under the approved clinical protocol. See website given in footnote 2 for additional information about this protocol
During the IRB review process the protocol was also submitted to the NIH Institutional Biosafety Committee and the NIH Recombinant DNA Advisory Committee. All of these review bodies gave approval to the submitted protocol, with minor modifications. The protocol was then submitted to the FDA as part of an Investigational New Drug (IND) application, which received approval, with some additionally required minor modifications, on August 30, 2006 (IND number 13,102). A general description of the protocol can be found at the publicly accessible clinical trials.gov web site,2 including detailed information on inclusion and exclusion criteria. Following the IND approval, a considerable amount of time was required to develop the operational infrastructure to conduct the trial. This included, in addition to staffing, extensive (and continuing) work with a Contract Research Organization (CRO) that helped us to develop all of the case report forms required, establishing a secure electronic database, developing and implementing a clinical specimen electronic tracking plan (a total of > 4,500 patient samples will be generated for testing) and establishing a study monitoring and protocol compliance plan. In addition, the CRO convened a Data and Safety Monitoring Board (DSMB) specifically to provide oversight of this study. The DSMB will examine all clinical safety data and all adverse event reports and will decide at each step whether escalation to the next higher dosage level is permissible.
Once the infrastructure was essentially established (∼July 1, 2007), we were able to begin recruiting and pre-screening patients for eligibility to enroll in this protocol. As of the date of submission of this chapter (December 2007), we have pre-screened 43 individuals, of whom ∼10% are initially eligible based on our conservative inclusion and exclusion criteria. These patients are then screened much more extensively. Each patient enrolled will be followed for one year and we expect the study will likely be completed in 2010.
Acknowledgments
The authors' research is supported by the intramural research program of the National Institute of Dental and Craniofacial Research.
Footnotes
E. Beitz (ed.), Aquaporins, Handbook of Experimental Pharmacology 190,
References
- Adesanya MR, Redman RS, Baum BJ, et al. Immediate inflammatory responses to adenovirus-mediated gene transfer in rat salivary glands. Hum Gene Ther. 1996;7:1085–1093. doi: 10.1089/hum.1996.7.9-1085. [DOI] [PubMed] [Google Scholar]
- Baum BJ. Principles of saliva secretion. Ann N Y Acad Sci. 1993;694:17–23. doi: 10.1111/j.1749-6632.1993.tb18338.x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Wang S, Cukierman E, et al. Re-engineering the functions of a terminally differentiated epithelial cell in vivo. Ann N Y Acad Sci. 1999;875:294–300. doi: 10.1111/j.1749-6632.1999.tb08512.x. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Wellner RB, Zheng C. Gene transfer to salivary glands. Int Rev Cytol. 2002;213:93–146. doi: 10.1016/s0074-7696(02)13013-0. [DOI] [PubMed] [Google Scholar]
- Delporte C, O'Connell BC, He X, et al. Increased fluid secretion after adenoviral-mediated transfer of the aquaporin-1 cDNA to irradiated rat salivary glands. Proc Natl Acad Sci U S A. 1997;94:3268–3273. doi: 10.1073/pnas.94.7.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delporte C, Hoque AT, Kulakusky JA, et al. Relationship between adenovirus-mediated aquaporin 1 expression and fluid movement across epithelial cells. Biochem Biophys Res Commun. 1998;246:584–588. doi: 10.1006/bbrc.1998.8668. [DOI] [PubMed] [Google Scholar]
- Harvey BG, Maroni J, O'Donoghue KA, et al. Safety of local delivery of low- and intermediate-dose adenovirus gene transfer vectors to individuals with a spectrum of morbid conditions. Hum Gene Ther. 2002;13:15–63. doi: 10.1089/10430340152712638. [DOI] [PubMed] [Google Scholar]
- Li J, Zheng C, Zhang X, et al. Developing a convenient large animal model for gene transfer to salivary glands in vivo. J Gene Med. 2004;6:55–63. doi: 10.1002/jgm.476. [DOI] [PubMed] [Google Scholar]
- Li J, Shan Z, Ou G, et al. Structural and functional characteristics of irradiation damage to parotid glands in the miniature pig. Int J Radiat Oncol Biol Phys. 2005;62:1510–1516. doi: 10.1016/j.ijrobp.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A, O'Connell B, Aladib W, et al. Direct in vivo adenovirus-mediated gene transfer to salivary glands. Am J Physiol. 1994;266:G1146–1155. doi: 10.1152/ajpgi.1994.266.6.G1146. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Baum BJ. Prophylactic treatment reduces the severity of xerostomia following irradiation therapy for oral cavity cancer. Arch Otolaryngol Head Neck Surg. 2003;129:247–250. doi: 10.1001/archotol.129.2.247. [DOI] [PubMed] [Google Scholar]
- Nagler RM, Baum BJ, Miller G, et al. Long-term salivary effects of single-dose head and neck irradiation in the rat. Arch Oral Biol. 1998;43:297–303. doi: 10.1016/s0003-9969(97)00120-9. [DOI] [PubMed] [Google Scholar]
- O'Connell AC, Redman RS, Evans RL, et al. Radiation-induced progressive decrease in fluid secretion in rat submandibular glands is related to decreased acinar volume and not impaired calcium signaling. Radiat Res. 1999a;151:150–158. [PubMed] [Google Scholar]
- O'Connell AC, Baccaglini L, Fox PC, et al. Safety and efficacy of adenovirus-mediated transfer of the human aquaporin-1 cDNA to irradiated parotid glands of non-human primates. Cancer Gene Ther. 1999b;6:505–513. doi: 10.1038/sj.cgt.7700078. [DOI] [PubMed] [Google Scholar]
- Raper SE, Chirmule N, Lee FS, et al. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol Genet Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Shan Z, Li J, Zheng C, et al. Increased fluid secretion after adenoviral-mediated transfer of the human aquaporin-1 cDNA to irradiated miniature pig parotid glands. Mol Ther. 2005;11:444–451. doi: 10.1016/j.ymthe.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Shiboski CH, Hodgson TA, Ship JA, et al. Management of salivary hypofunction during and after radiotherapy. Oral Surg. 2007;103(Suppl):S66.e1–19. doi: 10.1016/j.tripleo.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Vissink A, Jansma J, Spijkervet FK, et al. Oral sequelae of head and neck radiotherapy. Crit Rev Oral Biol Med. 2003;14:199–212. doi: 10.1177/154411130301400305. [DOI] [PubMed] [Google Scholar]
- Vitolo JM, Baum BJ. The use of gene transfer for the protection and repair of salivary glands. Oral Dis. 2002;8:183–191. doi: 10.1034/j.1601-0825.2002.02865.x. [DOI] [PubMed] [Google Scholar]
- Zheng C, Goldsmith CM, Mineshiba F, et al. Toxicity and biodistribution of a first-generation recombinant adenoviral vector encoding aquaporin-1 after retroductal delivery to a single rat submandibular gland. Hum Gene Ther. 2006;17:1122–1133. doi: 10.1089/hum.2006.17.1122. [DOI] [PubMed] [Google Scholar]