Abstract
Purpose of review
Virus-induced wheezing in infancy is a risk factor for asthma, and recent studies have highlighted the role of rhinoviruses in causing acute illnesses and as a possible contributing factor to chronic asthma.
Recent findings
Rhinoviruses (HRV) have long been known as the most common cause of the common cold in infants and children. Recent developments in molecular diagnostics have led to the discovery of new viruses, and have also provided data to implicate HRV as important causes of lower respiratory infections and acute virus-induced wheezing in preschool children. In addition, HRV-induced wheezing episodes appear to identify children who are at increased risk for the subsequent development of childhood asthma.
Summary
Collectively, these findings raise the possibility that LRI with pathogens such as HRV and RSV could participate in the causation of asthma, especially in children with suboptimal antiviral defenses. A variety of experimental models and clinical studies have been used to identify possible mechanisms related to the infection and the ensuing host response that could disturb normal lung and immunologic development to promote asthma. Defining these relationships could lead to new therapeutic and preventive approaches to common forms of childhood asthma.
Keywords: Rhinovirus, respiratory syncytial virus, children, asthma
Introduction
This question of whether respiratory infections with viruses can cause asthma is controversial, and has been argued for decades. Recently, the advent of molecular viral diagnostics has expanded our understanding of the epidemiology of respiratory illnesses in infancy by improved detection rates for known virus, and surprisingly, has led to an onslaught of discovery of previously unrecognized respiratory viruses [1*,2*,3*]. These new diagnostic techniques have been particularly helpful in understanding the role of human rhinoviruses (HRV), which are particularly difficult to grow in tissue culture, in acute illnesses, and in exacerbations of chronic disease such as asthma, chronic obstructive lung disease, and cystic fibrosis. In addition, recent clinical studies have highlighted the role of HRV as an important lower airway pathogen in infancy, and further suggest that children who wheeze with HRV may be at particularly high risk for the subsequent development of asthma. This review will focus on the role of HRV infections in early childhood respiratory illnesses, and discuss clinical evidence and mechanistic studies evaluating a potential role for HRV infections in the initiation of asthma.
Epidemiology of viral infections and wheezing in infancy
Viral respiratory infections are universal in the first few years of life, and cause wheezing illnesses in about 50% of affected infants. Bronchiolitis is the most common wheezing illness in infancy, and can be caused by a number of different viral infections (Table I). Interestingly, despite very different viral replication cycles, the clinical manifestations of infections with these diverse viruses are quite similar. RSV, HRV, and mixed viral infections are the most common causes of these illnesses: RSV infections are responsible for the majority of cases from December through April (offset by 6 months in the southern hemisphere), while HRV infections account for most cases during the rest of the year [4]. HRV, once considered to be limited to the upper airway, is now recognized as an important cause of lower respiratory infections [4,5**,6,7].
Table I.
Viruses* associated with bronchiolitis in infancy
| Viruses | Family | Genome† |
|---|---|---|
| RSV | Paramyxovirus | ssRNA (−) |
| PIV | Paramyxovirus | ssRNA (−) |
| Influenza | Orthomyxovirus | ssRNA (−) |
| MPV | Pneumovirus | ssRNA (−) |
| HRV A, HRV B, HRV C | Picornavirus | ssRNA (+) |
| Enteroviruses | Picornavirus | ssRNA (+) |
| OC43, NL63, HKU1, SARS | Coronavirus | ssRNA (+) |
| Bocavirus | Parvovirus | ssDNA (−) |
| WU, KIP | Polyomavirus | dsDNA |
| Adenoviruses | Arenovirus | dsDNA |
Bold print indicates viruses that were recently discovered using molecular diagnostics.
Abbreviations: ss, single stranded; ds, double stranded; (+) and (−) refer to the polarity of the genome.
HRV are enterovirus species in the Picornaviradae family, and 100–101 prototypical strains have been divided into groups A and B based on patterns of inhibition with certain antiviral compounds, along with molecular analysis of partial genetic sequencing. HRV serotypes were originally identified by growth in tissue culture followed by inhibition with specific antisera; this work was largely completed in the 1980s, although nontypable HRV were occasionally reported. More recently, partial and complete genetic sequencing of viruses detected using molecular techniques have revealed that the number of HRV strains has been severely underestimated, and in fact evidence is growing for a third group of HRV (“HRV C”), that appears to be as different from HRV groups A and B as it is from other enteroviruses [6,8*,9*,10*,11*]. These newly discovered viruses are quite common, and comprise up to 50% of HRV detected in some clinical studies [8*]. Clinical manifestations appear similar to other HRV, and they have been found to contribute to wheezing illnesses in infants, and in children with asthma. The frequency of detection appears to be consistently high in studies conducted in North America, Europe, Asia, and Australia [12].
HRV, either alone or in combination with other viruses [13], are an important cause of wheezing illnesses, and yet are also the viruses most often detected in asymptomatic infants and children [4,14*,15]. The observed diversity in the severity of HRV clinical illness could be related to either host factors or the strain of infecting virus. An outstanding question is whether there are HRV strains that are inherently more virulent, and thus more likely to cause wheezing illnesses. This is certainly the case for other respiratory viruses (e.g. influenza): the large number of strains suggests that this is also true for HRV. Identifying more virulent strains, and the molecular mechanisms that determine this characteristic, will be important in developing new therapeutic strategies aimed at lessening the severity of HRV-related illness.
Rhinovirus and distribution in the lower airway
HRV replicate best at relatively cool temperatures (33–35°C), and so it was long assumed that infections were limited to the upper airway. Although lung parenchyma is at core temperature (37°C), airways are considerably cooler, and temperatures in large and medium sized airways are ideal for HRV replication [16*]. In fact, HRV has been demonstrated in lower airway fluids and cells and after experimental infection of the upper airway [17–19]. Furthermore, there is considerable clinical evidence linking HRV infections to lower respiratory infections in children, including those who are hospitalized for pneumonia [5–7,20]. A recent year-long population-based study of children < 5 year of age found that HRV was detected in 26% of children hospitalized for respiratory symptoms or fever [5]. HRV-related hospitalization rates were especially high for infants, and children with asthma (Table II). These findings suggest the possibility that infections with HRV, like RSV and other respiratory viruses, can directly injure airway tissues during the acute infection. One unique feature of HRV infections is the large number of serotypes and strains – well over 100. Thus, HRV infections occur frequently in young children, and account for significant morbidity related to both upper and lower respiratory illnesses.
Table II.
Age-specific rates for HRV-related hospitalization in children*
| Age (months) | Hospitalization rates† |
|---|---|
| 0–5 | 17.6 (14.9 – 20.6 |
| 6–23 | 6.0 (5.0 – 7.0) |
| 24–59 | 2.0 (1.6 – 2.4) |
| Asthma | |
| Yes | 25.3 (21.6 – 29.5) |
| No | 3.1 (2.7 – 3.5) |
From reference [5]
Rates per 1000 children (95% confidence interval)
HRV infections in infancy and subsequent asthma
The coughing, wheezing, and tachypnea associated with viral respiratory illnesses closely resemble exacerbations of asthma in older children, and 30–50% of children with recurrent virus-induced wheezing in infancy go on to develop asthma. This progression suggests that viral respiratory infections might damage the airways and initiate asthma (Figure 1). It is also possible that the relationship is not causal, and that virus-induced wheezing episodes instead reveal a preexisting tendency for asthma secondary to impaired lung physiology or antiviral responses. A third possibility, which combines elements of the first two, is a “two hit hypothesis” in which viral infections promote asthma mainly in predisposed children [5,21]. Understanding the host-pathogen interactions that determine the severity of respiratory illnesses and long-term sequelae would be of great help in identifying at-risk individuals, and in designing new and more effective treatments and preventive strategies.
Figure 1.
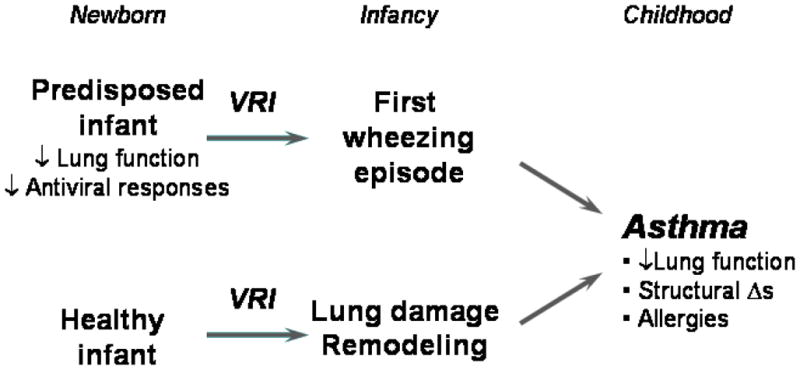
Relationship of viral respiratory infections in early life to the development of asthma.
Risk factors for virus-induced wheezing
Risk factors for bronchiolitis and viral LRI include young age, especially the first 6 months of life, small lung size, and exposure to tobacco smoke [22**]. Lung-specific factors such as preexisting airway hyperresponsiveness [23] and/or limitation to airflow [22] also increase the risk of viral LRI. In addition, several genetic factors modify the risk of RSV-induced wheezing, including polymorphisms in genes encoding surfactant proteins, cytokines, and chemokines [24]. Although data are more limited for HRV infections, polymorphisms in IL-10 may influence the severity of illnesses with this virus [25].
Risk factors for asthma following viral infection
Long-term studies have demonstrated that infants hospitalized with RSV bronchiolitis have a 2–3-fold increase in the risk of developing asthma later in childhood. This risk is further increased by a strong family history of atopy, or the development of atopic features, particularly if this occurs during early childhood.
The type of virus-induced wheezing episode also appears to influence the risk of subsequent asthma. Wheezing illnesses caused by RSV, parainfluenza viruses, or influenza A appear to have similar long-term prognosis. In contrast, a case control study conducted in Finland demonstrated that infants hospitalized with HRV-induced wheezing were found to have a particularly high risk for subsequent asthma, and this relationship persisted at least through the late teen years [26,27**].
This finding is corroborated by the results of two birth cohort studies. The Childhood Origins of Asthma (COAST) study is a high-risk birth cohort study in which families with at least one parent with allergies or asthma were enrolled prenatally, and both immune development and respiratory illnesses were prospectively evaluated [21]. Through the use of PCR technology, viral etiologies were identified in 90% of wheezing illnesses. Notably, moderate to severe HRV infections (with and without wheezing) during infancy were a significant risk factor (OR = 10) for persistent wheezing at age 3 years [4]. Moreover, RV wheezing illnesses in the first three years of life were significantly associated with the development of asthma at age 6 years [28**]. The combination of allergic sensitization and HRV-induced by age 3 was associated with the highest risk of developing asthma. Similarly, in high-risk infants who were followed prospectively in Australia, Kusel and colleagues prospectively evaluated 198 Australian children and compared respiratory illnesses in the first year of life to respiratory outcomes at age 5 years [29**]. Wheezing illnesses with either HRV or RSV were associated with asthma at age 5 years. Interestingly, these associations were only significant in the children with early onset (by age 2 years) allergic sensitization. Both of these studies highlight the role of virus-induced wheezing in infancy, and HRV in particular, in determining the risk for subsequent asthma. Children who develop allergic sensitization at an early age and also wheeze with HRV are at high risk for developing asthma.
How do viral infections affect long-term airway physiology?
Infancy is a period of profound growth and development of the lungs, and these changes are occurring at the time of maximum susceptibility to viral LRI. This coincidence raises a number of questions related to long-term effects of viral infections on the structure and function of the developing lung.
Pre- and postnatal lung development
Lung development begins at about 4 weeks gestation, and continues even after birth.[30] The basic lung architecture, including differentiation of the respiratory airways and differentiation of future respiratory gas exchange (acinar) units, is largely completed by 40 weeks gestation. Postnatally, alveoli multiply (alveolarization), and this process continues for 2–3 years. Lung growth is maximal at this time, and involves continuous “remodeling” throughout childhood. Murine models of gene deletion and overexpression have identified key regulatory factors for lung growth and alveolarization, such as epidermal growth factor [EGF], vascular endothelial growth factor [VEGF], transforming growth factor-β (TGF-β), and platelet-derived growth factor [PDGF].
What happens to lung development when this carefully orchestrated process is disrupted by a viral respiratory infection? First, viral infections damage airway structures via replication, and also by inducing inflammatory immune responses. In vitro models demonstrate that HRV replication is enhanced in epithelium that is damaged, indicating that the barrier function of the epithelial layer is an important component of antiviral defenses [31,32]. Fibroblasts are also quite susceptible to HRV infection, and by extension, loss of the epithelial layer would provide viral particles with access to additional susceptible tissues. More severe infections that cause considerable damage to lung tissues, with directly or by inducing a harmful inflammatory response, could have adverse effects on lung development that lead to chronic lung disease.
Virus-induced antiviral and inflammatory responses
It is likely that the balance between an effective antiviral response and damaging inflammation influence both short and long-term effects on the developing lung. For viruses such as RV, which infect relatively few cells in the airway,[33,34] virus-induced inflammatory responses may be the driving force for airway symptoms and lower airway dysfunction. During the acute infection, epithelial edema and shedding together with mucus production promote airway obstruction and wheezing. Viral RNA activates innate immune responses by binding to molecules such as Toll-line receptor-3 (TLR-3), TLR-7, the dsRNA dependent protein kinase (PKR), RIG-I, and MDA-5.[35–37] These mechanisms activate a host of antiviral effector mechanisms, and also secretion of chemokines to recruit additional inflammatory cells into the airway [38]. Once replication is underway, mononuclear cells strengthen the antiviral response through the secretion of interferons, proinflammatory cytokines and chemokines.[39,40]
Interestingly, there are experimental and clinical data indicate that interferon responses in early life are inversely associated with the severity of viral respiratory illnesses. For example, airway epithelial cells cultured from subjects with asthma were reported to produce reduced amounts of IFN-β, IFN-γ and IFN-λ in response to HRV, and support enhanced viral replication [41*,42**,43**]. Furthermore, there is clinical evidence that babies with low ex vivo interferon responses in early life are more likely to have frequent viral respiratory illnesses, including those associated with wheezing [44**,45*,46*]. These experimental findings suggest that an impaired interferon response could increase the risk of more severe viral respiratory infections in infancy, and perhaps promote long-term damage to airway structures. Interestingly, reduced IFN-γ responses in infancy are also observed in children with atopic features, which could help to explain why atopy is a risk factor for virus-induced wheezing and the progression to asthma (Figure 2) [28**,29**].
Figure 2.
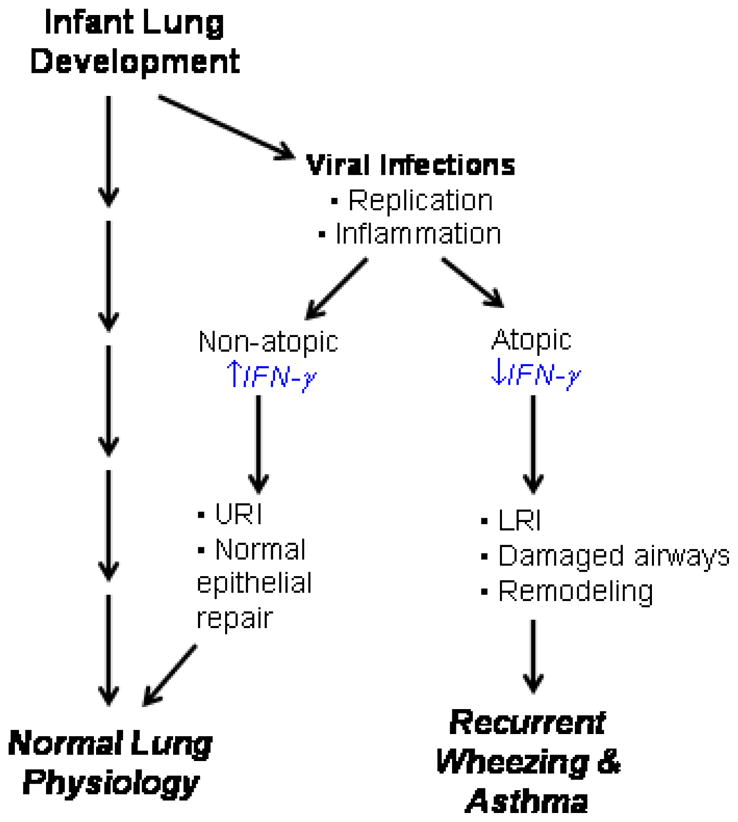
Atopy, wheezing, and asthma. Viral respiratory infections are common during the first 3 years of life, a period of rapid lung development. Children with atopic features tend to have reduced IFN–γ responses, which is also associated with increased susceptibility to viral LRI. Viral replication and the induction of inflammation in the lower airway during this period of rapid development could lead to long term changes in airway structure (fibrosis), abnormal physiology (airway hyperresponsiveness), and clinical asthma.
The coordinate expression of adhesion molecules and secretion of chemokines by airway cells provides a potent stimulus for the recruitment of neutrophils, however, to date there is no convincing evidence that this is either beneficial or harmful to the host. Products of neutrophil activation can damage the airways, and the release of the potent secretagogue elastase can upregulate goblet cell secretion of mucus [47]. However, the rapid recruitment of these cells to the airways suggests that this response is important for host defense. The role of these responses in long-term outcomes has not been determined. HRV infections can also indirectly activate and cause degranulation of eosinophils through a lymphocyte-dependent mechanism [48]. This effect could contribute to the enhanced risk of virus-induced wheeze in children with allergic sensitization [49].
Effects of viral infections on growth factors
Acute infections with HRV and other viral infections can induce the synthesis of several factors that regulate airway remodeling and alveolar development, including VEGF [50,51*], NO [52], TGF-β [53,54], amphiregulin [51*], activin A [51*], and FGF [55]. Furthermore, viral infections can upregulate neurotropins that have the potential to cause remodeling of the airway neural network, and possibly promote nonspecific airway responsiveness [56]. How single or repeated bouts of virus-induced overexpression of these regulators of lung development and remodeling affects the ultimate lung structure and function is not known, but is of interest regarding the long-term effects on lung function and asthma. These questions may be best addressed in animal models, and recently two different models for HRV infections in the mouse have been published [57**,58*], as well as methods for serial passage of mouse epithelial cells for propagation of HRV [59].
Summary and conclusions
The pathologic features and physiologic abnormalities of asthma appear in the first few years of life, during a period of rapid growth and development. These observations raise the possibility that contracting recurrent viral LRI in infancy could be one of several pathways that lead to the development of asthma, especially in children with atopic features. Plausible mechanisms have been proposed, though not yet proven, to relate viral infections in early life to epithelial damage, airway remodeling, and intermittent airway obstruction leading to asthma. New experimental models are needed to answer questions about causality, and the relative importance of hereditary versus environmental or lifestyle-related factors in the progression from virus-induced wheezing to multifactorial childhood asthma.
Results of a recent nonrandomized clinical trial of palivizumab suggest that preventing or moderating the severity of RSV infections in infancy also reduces subsequent asthma [60*]. In addition, there is some evidence that treatment of infants with systemic corticosteroid during an acute HRV wheezing illness can reduce the subsequent risk of recurrent risk [61*]. These studies provide optimism that prevention or early treatment of viral LRI in early childhood could reduce long-term morbidity related to asthma. Further progress in this area would be advanced by the development of effective and practical antiviral strategies for HRV and other respiratory viruses.
Acknowledgments
Support: NIH NIAID U19 AI070503-01, RFP/contract NIH-NIAID-DAIT-02-11, and NIH-NHLBIP01 HL070831
Reference List
- 1*.Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Schnurr D, Ganem D, DeRisi JL, Boushey HA. Pan-viral screening of respiratory tract infections in adults with and without asthma reveals unexpected human coronavirus and human rhinovirus diversity. J Infect Dis. 2007;196:817–825. doi: 10.1086/520816. This paper describes a chip-based microarray that was used to explore the virology associated with acute exacerbations of asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2*.Lee WM, Grindle KA, Pappas TE, Marshall D, Moser M, Beaty E, Shult PA, Prudent J, Gern JE. A high-throughput, sensitive and accurate multiplex PCR-microsphere flow cytometry system for large-scale comprehensive detection of respiratory viruses. Journal of Clinical Microbiology. 2007;45:2626–2634. doi: 10.1128/JCM.02501-06. This is an example of a high-throughput molecular test for all common respiratory viruses that can be used in large scale observational studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3*.Lamson D, Renwick N, Kapoor V, Liu Z, Palacios G, Ju J, Dean A, St GK, Briese T, Lipkin WI. MassTag polymerase-chain-reaction detection of respiratory pathogens, including a new rhinovirus genotype, that caused influenza-like illness in New York State during 2004–2005. J Infect Dis. 2006;194:1398–1402. doi: 10.1086/508551. The Mass Tag technique has been used to identify a number of new pathogens in a variety of clinical and biological situations. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Jackson DJ, Gangnon RE, Evans ME, Li Z, Shult P, Kirk CJ, Reisdorf E, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, Pappas TE, DaSilva DF, Tisler CJ, Gern JE. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116:571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5**.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, Iwane MK, Edwards KM. Rhinovirus-associated hospitalizations in young children. J Infect Dis. 2007;195:773–781. doi: 10.1086/511821. This is an excellent population based study that used molecular and traditional techniques to identify respiratory pathogens in hospitalized children. There were high rates of viral detection, and rhinoviruses were shown to be associated with high rates of illness in infants, and in children with asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kusel MM, de Klerk NH, Holt PG, Kebadze T, Johnston SL, Sly PD. Role of respiratory viruses in acute upper and lower respiratory tract illness in the first year of life: a birth cohort study. Pediatr Infect Dis J. 2006;25:680–686. doi: 10.1097/01.inf.0000226912.88900.a3. [DOI] [PubMed] [Google Scholar]
- 7.Regamey N, Kaiser L. Rhinovirus infections in infants: is respiratory syncytial virus ready for the challenge? . Eur Respir J. 2008;32:249–251. doi: 10.1183/09031936.00076508. [DOI] [PubMed] [Google Scholar]
- 8*.Lee WM, Kiesner C, Pappas T, Lee I, Grindle K, Jartti T, Jakiela B, Lemanske RF, Jr, Shult PA, Gern JE. A diverse group of previously unrecognized human rhinoviruses are common causes of respiratory illnesses in infants. PLoS ONE. 2007;2:e966. doi: 10.1371/journal.pone.0000966. This paper summarizes the identification of over 20 previously unknown strains of HRV, including a number of putative HRV C group viruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9*.Kistler AL, Webster DR, Rouskin S, Magrini V, Credle JJ, Schnurr DP, Boushey HA, Mardis ER, Li H, DeRisi JL. Genome-wide diversity and selective pressure in the human rhinovirus. Virol J. 2007;4:40. doi: 10.1186/1743-422X-4-40. Microarray technology was used to evaluate overall genetic structure of a large number of HRV strains, and to identify areas that are conserved vs. hypervariable in the genomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10*.McErlean P, Shackelton LA, Andrews E, Webster DR, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Distinguishing molecular features and clinical characteristics of a putative new rhinovirus species, human rhinovirus C (HRV C) PLoS ONE. 2008;3:e1847. doi: 10.1371/journal.pone.0001847. Genomic and structural differences among some of the newly described HRV are explored in this article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11*.Lau SK, Yip CC, Tsoi HW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. Clinical features and complete genome characterization of a distinct human rhinovirus (HRV) genetic cluster, probably representing a previously undetected HRV species, HRV-C, associated with acute respiratory illness in children. J Clin Microbiol. 2007;45:3655–3664. doi: 10.1128/JCM.01254-07. This is another large series in which molecular techniques were used to detect new strains of HRV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Briese T, Renwick N, Venter M, Jarman RG, Ghosh D, Kondgen S, Shrestha SK, Hoegh AM, Casas I, Adjogoua EV, koua-Koffi C, Myint KS, Williams DT, Chidlow G, van den BR, Calvo C, Koch O, Palacios G, Kapoor V, Villari J, Dominguez SR, Holmes KV, Harnett G, Smith D, Mackenzie JS, Ellerbrok H, Schweiger B, Schonning K, Chadha MS, Leendertz FH, Mishra AC, Gibbons RV, Holmes EC, Lipkin WI. Global distribution of novel rhinovirus genotype. Emerg Infect Dis. 2008;14:944–947. doi: 10.3201/eid1406.080271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF, Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008;32:314–320. doi: 10.1183/09031936.00161907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14*.Peltola V, Waris M, Osterback R, Susi P, Ruuskanen O, Hyypia T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197:382–389. doi: 10.1086/525542. This is an excellent study that defines HRV detection in healthy and sick individuals within families, and identifies patterns of viral transmission. [DOI] [PubMed] [Google Scholar]
- 15.Alper CM, Doyle WJ, Winther B, Owen HJ. Upper respiratory virus detection without parent-reported illness in children is virus-specific. J Clin Virol. 2008;43:120–122. doi: 10.1016/j.jcv.2008.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16*.McFadden ER, Jr, Pichurko BM, Bowman HF, Ingenito E, Burns S, Dowling N, Solway J. Thermal mapping of the airways in humans. J Appl Physiol. 1985;58:564–570. doi: 10.1152/jappl.1985.58.2.564. This study used a bronchoscope equipped with a thermister to demonstrate that airway temperatures are significantly lower than core temperature. [DOI] [PubMed] [Google Scholar]
- 17.Mosser AG, Vrtis R, Burchell L, Lee WM, Dick CR, Weisshaar E, Bock D, Swenson CA, Cornwell RD, Meyer KC, Jarjour NN, Busse WW, Gern JE. Quantitative and qualitative analysis of rhinovirus infection in bronchial tissues. Am J Respir Crit Care Med. 2005;171:645–651. doi: 10.1164/rccm.200407-970OC. [DOI] [PubMed] [Google Scholar]
- 18.Gern JE, Galagan DM, Jarjour NN, Dick EC, Busse WW. Detection of rhinovirus RNA in lower airway cells during experimentally-induced infection. Am J Respir Crit Care Med. 1997;155:1159–1161. doi: 10.1164/ajrccm.155.3.9117003. [DOI] [PubMed] [Google Scholar]
- 19.Papadopoulos NG, Bates PJ, Bardin PG, Papi A, Leir SH, Fraenkel DJ, Meyer J, Lackie PM, Sanderson G, Holgate ST, Johnston SL. Rhinoviruses infect the lower airways. J Infect Dis. 2000;181:1875–1884. doi: 10.1086/315513. [DOI] [PubMed] [Google Scholar]
- 20.Tsolia MN, Psarras S, Bossios A, Audi H, Paldanius M, Gourgiotis D, Kallergi K, Kafetzis DA, Constantopoulos A, Papadopoulos NG. Etiology of community-acquired pneumonia in hospitalized school-age children: evidence for high prevalence of viral infections. Clin Infect Dis. 2004;39:681–686. doi: 10.1086/422996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lemanske RF., Jr The childhood origins of asthma (COAST) study. Pediatr Allergy Immunol. 2002;13 (Suppl 15):38–43. doi: 10.1034/j.1399-3038.13.s.15.8.x. [DOI] [PubMed] [Google Scholar]
- 22**.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ Group Health Medical Associates. Asthma and wheezing in the first six years of life. N Engl J Med. 1995;332:133–138. doi: 10.1056/NEJM199501193320301. This is a classic study that identified risk factors for wheezing and asthma in a population based birth cohort study. It also provided firm data related to specific phenotypes of wheezing illnesses in children, along with specific risk factors. [DOI] [PubMed] [Google Scholar]
- 23.Palmer LJ, Rye PJ, Gibson NA, Burton PR, Landau LI, LeSouef PN. Airway responsiveness in early infancy predicts asthma, lung function, and respiratory symptoms by school age. Am J Respir Crit Care Med. 2001;163:37–42. doi: 10.1164/ajrccm.163.1.2005013. [DOI] [PubMed] [Google Scholar]
- 24.Singh AM, Moore PE, Gern JE, Lemanske RF, Jr, Hartert TV. Bronchiolitis to asthma: a review and call for studies of gene-virus interactions in asthma causation. Am J Respir Crit Care Med. 2007;175:108–119. doi: 10.1164/rccm.200603-435PP. [DOI] [PubMed] [Google Scholar]
- 25.Helminen M, Nuolivirta K, Virta M, Halkosalo A, Korppi M, Vesikari T, Hurme M. IL-10 gene polymorphism at −1082 A/G is associated with severe rhinovirus bronchiolitis in infants. Pediatr Pulmonol. 2008;43:391–395. doi: 10.1002/ppul.20793. [DOI] [PubMed] [Google Scholar]
- 26.Reijonen TM, Kotaniemi-Syrjanen A, Korhonen K, Korppi M. Predictors of asthma three years after hospital admission for wheezing in infancy. Pediatr. 2000;106:1406–1412. doi: 10.1542/peds.106.6.1406. [DOI] [PubMed] [Google Scholar]
- 27**.Kotaniemi-Syrjanen A, Vainionpaa R, Reijonen TM, Waris M, Korhonen K, Korppi M. Rhinovirus-induced wheezing in infancy--the first sign of childhood asthma? . J Allergy Clin Immunol. 2003;111:66–71. doi: 10.1067/mai.2003.33. In a long-term case-control study, HRV-induced wheezing episodes in infancy were highly predictive of subsequent asthma. The group now has follow-up into the teen years that show consistent results. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28**.Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, Printz MC, Lee WM, Shult PA, Reisdorf E, Carlson-Dakes KT, Salazar LP, DaSilva DF, Tisler CJ, Gern JE, Lemanske RF., Jr Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. In a birth cohort including families with a parental history of allergies or asthma, HRV infections in infancy were the strongest predictor of asthma at age 6 years. The effects of allergic sensitization and HRV-induced wheezing were additive in terms of asthma risk. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29**.Kusel MM, de Klerk NH, Kebadze T, Vohma V, Holt PG, Johnston SL, Sly PD. Early-life respiratory viral infections, atopic sensitization, and risk of subsequent development of persistent asthma. J Allergy Clin Immunol. 2007;119:1105–1110. doi: 10.1016/j.jaci.2006.12.669. In this high-risk birth cohort, early wheezing infections with HRV and RSV were associated with increased risk of subsequent asthma. This effect was driven by children with the onset of allergic sensitization before the age of 2 years. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Thoracic Society ad hoc Statement Committee. Mechanisms and limits of induced postnatal lung growth. Am J Respir Crit Care Med. 2004;170:319–343. doi: 10.1164/rccm.200209-1062ST. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Souza N, Dolganov G, Dubin R, Sachs LA, Sassina L, Sporer H, Yagi S, Schnurr D, Boushey HA, Widdicombe JH. Resistance of differentiated human airway epithelium to infection by rhinovirus. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2004;286:L373–L381. doi: 10.1152/ajplung.00300.2003. [DOI] [PubMed] [Google Scholar]
- 32.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol. 2008;38:517–523. doi: 10.1165/rcmb.2007-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arruda E, Boyle TR, Winther B, Pevear DC, Gwaltney JM, Hayden FG. Localization of human rhinovirus replication in the upper respiratory tract by in situ hybridization. J Infect Dis. 1995;171:1329–1333. doi: 10.1093/infdis/171.5.1329. [DOI] [PubMed] [Google Scholar]
- 34.Mosser AG, Brockman-Schneider RA, Amineva SP, Burchell L, Sedgwick JB, Busse WW, Gern JE. Similar frequency of rhinovirus-infectable cells in upper and lower airway epithelium. J Infect Dis. 2002;185:734–743. doi: 10.1086/339339. [DOI] [PubMed] [Google Scholar]
- 35.Edwards MR, Slater L, Johnston SL. Signalling pathways mediating type I interferon gene expression. Microbes Infect. 2007;9:1245–1251. doi: 10.1016/j.micinf.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Edwards MR, Hewson CA, Laza-Stanca V, Lau HT, Mukaida N, Hershenson MB, Johnston SL. Protein kinase R, IkappaB kinase-beta and NF-kappaB are required for human rhinovirus induced pro-inflammatory cytokine production in bronchial epithelial cells. Mol Immunol. 2007;44:1587–1597. doi: 10.1016/j.molimm.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll- like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- 38.Gern JE, French DA, Grindle KA, Brockman-Schneider RA, Konno S, Busse WW. Double-stranded RNA induces the synthesis of specific chemokines by bronchial epithelial cells. Am J Respir Cell Mol Biol. 2003;28:731–737. doi: 10.1165/rcmb.2002-0055OC. [DOI] [PubMed] [Google Scholar]
- 39.Hall DJ, Bates ME, Guar L, Cronan M, Korpi N, Bertics PJ. The role of p38 MAPK in rhinovirus-induced monocyte chemoattractant protein-1 production by monocytic-lineage cells. J Immunol. 2005;174:8056–8063. doi: 10.4049/jimmunol.174.12.8056. [DOI] [PubMed] [Google Scholar]
- 40.Korpi-Steiner NL, Bates ME, Lee WM, Hall DJ, Bertics PJ. Human rhinovirus induces robust IP-10 release by monocytic cells, which is independent of viral replication but linked to type I interferon receptor ligation and STAT1 activation. J Leukoc Biol. 2006;80:1364–1374. doi: 10.1189/jlb.0606412. [DOI] [PubMed] [Google Scholar]
- 41*.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax. 2002;57:328–332. doi: 10.1136/thorax.57.4.328. Peripheral blood cells that were stimulated ex vivo to induce IFN-γ responses, which were significantly lower in patients with asthma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42**.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med. 2006;12:1023–1026. doi: 10.1038/nm1462. These experiments demonstrated that airway epithelial cells and macrophages from patients with asthma had a significant impairment in HRV-induced IFN-λ responses. The ex vivo responses were inversely correlated with viral load after the volunteers were experimentally inoculated with HRV. [DOI] [PubMed] [Google Scholar]
- 43**.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med. 2005;201:937–947. doi: 10.1084/jem.20041901. IFN-β responses were also found to be deficient in airway epithelial cells cultured in the presence of HRV. The reduced IFN-β responses were associated with impaired apoptosis, which may be an important pathway for the clearance of virus-infected cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44**.Copenhaver CC, Gern JE, Li Z, Shult PA, Rosenthal LA, Mikus LD, Kirk CJ, Roberg KA, Anderson EL, Tisler CJ, DaSilva DF, Heimke HJ, Gentile K, Gangnon RE, Lemanske RF., Jr Cytokine response patterns, exposure to viruses, and respiratory infections in the first year of life. Am J Respir Crit Care Med. 2004;170:175–180. doi: 10.1164/rccm.200312-1647OC. There was a weak inverse correlation between cord blood mononuclear cells IFN-γ responses at the time and the number of viral respiratory illnesses in the first year of life. These relationships were stronger in children with high exposure to other children, suggesting an interaction between low interferon responses and environmental exposure to respiratory viruses. [DOI] [PubMed] [Google Scholar]
- 45*.Gern JE, Brooks GD, Meyer P, Chang AM, Shen K-L, Evans MD, Tisler C, DaSilva D, Roberg KA, Mikus LD, Rosenthal LA, Kirk CJ, Shult PA, Bhattacharya A, Li Z, Gangnon R, Lemanske RF., Jr Bidirectional interactions between viral respiratory illnesses and cytokine responses in the first year of life. J Allergy Clin Immunol. 2006;117:72–78. doi: 10.1016/j.jaci.2005.10.002. This paper evaluated blood mononuclear cell IFN-γ responses to respiratory viruses at birth, and found inverse correlations with the risk of wheezing illnesses in the first year of life. [DOI] [PubMed] [Google Scholar]
- 46.Stern DA, Guerra S, Halonen M, Wright AL, Martinez FD. Low IFN-gamma production in the first year of life as a predictor of wheeze during childhood. J Allergy Clin Immunol. 2007;120:835–841. doi: 10.1016/j.jaci.2007.05.050. This study evaluated interferon responses at 9 months of age, and compared the immunologic results to an exceptionally long period of follow-up, and multiple wheezing phenotypes. [DOI] [PubMed] [Google Scholar]
- 47.Cardell LO, Agusti C, Takeyama K, Stjarne P, Nadel JA. LTB(4)-induced nasal gland serous cell secretion mediated by neutrophil elastase. Am J Respir Crit Care Med. 1999;160:411–414. doi: 10.1164/ajrccm.160.2.9808117. [DOI] [PubMed] [Google Scholar]
- 48.Davoine F, Cao M, Wu Y, Ajamian F, Ilarraza R, Kokaji AI, Moqbel R, Adamko DJ. Virus-induced eosinophil mediator release requires antigen-presenting and CD4+ T cells. J Allergy Clin Immunol. 2008;122:69–77. 77. doi: 10.1016/j.jaci.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med. 1999;159:785–790. doi: 10.1164/ajrccm.159.3.9801052. [DOI] [PubMed] [Google Scholar]
- 50.Lee CG, Yoon HJ, Zhu Z, Link H, Wang Z, Gwaltney JM, Landry M, Elias JA. Respiratory syncytial virus stimulation of vascular endothelial cell growth Factor/Vascular permeability factor. American Journal of Respiratory Cell & Molecular Biology. 2000;23:662–669. doi: 10.1165/ajrcmb.23.5.4188. [DOI] [PubMed] [Google Scholar]
- 51*.Leigh R, Oyelusi W, Wiehler S, Koetzler R, Zaheer RS, Newton R, Proud D. Human rhinovirus infection enhances airway epithelial cell production of growth factors involved in airway remodeling. J Allergy Clin Immunol. 2008;121:1238–1245. doi: 10.1016/j.jaci.2008.01.067. HRV stimulated epithelial cells to produce VEGF, amphiregulin, and activin A, which have all been linked to airway remodeling in asthma. [DOI] [PubMed] [Google Scholar]
- 52.Sanders SP. Asthma, viruses, and nitric oxide. Proc Soc Exp Biol Med. 1999;220:123–132. doi: 10.1046/j.1525-1373.1999.d01-19.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Uhl EW, Castleman WL, Sorkness RL, Lemanske RF, McAllister PK. Parainfluenza virus-induced persistence of airway inflammation, fibrosis, and dysfunction associated with TGF-β1 expression in Brown Norway rats. Am J Respir Crit Care Med. 1996;154:1834–1842. doi: 10.1164/ajrccm.154.6.8970378. [DOI] [PubMed] [Google Scholar]
- 54.Dosanjh A. Transforming growth factor-beta expression induced by rhinovirus infection in respiratory epithelial cells. Acta Biochim Biophys Sin(Shanghai) 2006;38:911–914. doi: 10.1111/j.1745-7270.2006.00234.x. [DOI] [PubMed] [Google Scholar]
- 55.Dosanjh A, Rednam S, Martin M. Respiratory syncytial virus augments production of fibroblast growth factor basic in vitro: implications for a possible mechanism of prolonged wheezing after infection. Pediatric Allergy & Immunology. 2003;14:437–440. doi: 10.1046/j.0905-6157.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 56.Tortorolo L, Langer A, Polidori G, Vento G, Stampachiacchere B, Aloe L, Piedimonte G. Neurotrophin overexpression in lower airways of infants with respiratory syncytial virus infection. Am J Respir Crit Care Med. 2005;172:233–237. doi: 10.1164/rccm.200412-1693OC. [DOI] [PubMed] [Google Scholar]
- 57**.Bartlett NW, Walton RP, Edwards MR, Aniscenko J, Caramori G, Zhu J, Glanville N, Choy KJ, Jourdan P, Burnet J, Tuthill TJ, Pedrick MS, Hurle MJ, Plumpton C, Sharp NA, Bussell JN, Swallow DM, Schwarze J, Guy B, Almond JW, Jeffery PK, Lloyd CM, Papi A, Killington RA, Rowlands DJ, Blair ED, Clarke NJ, Johnston SL. Mouse models of rhinovirus-induced disease and exacerbation of allergic airway inflammation. Nat Med. 2008;14:199–204. doi: 10.1038/nm1713. This group has developed a mouse model of HRV infection. Although viral replication is limited in the mouse, many of the inflammatory responses closely resemble those associated with the common cold, and interactions with allergic inflammatory responses were demonstrated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58*.Newcomb DC, Sajjan US, Nagarkar DR, Wang Q, Nanua S, Zhou Y, McHenry CL, Hennrick KT, Tsai WC, Bentley JK, Lukacs NW, Johnston SL, Hershenson MB. Human rhinovirus 1B exposure induces phosphatidylinositol 3-kinase-dependent airway inflammation in mice. Am J Respir Crit Care Med. 2008;177:1111–1121. doi: 10.1164/rccm.200708-1243OC. This is another demonstration of a potentially useful mouse model of HRV infection, with a nice demonstration of inflammatory responses that are similar to those in humans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brockman-Schneider RA, Amineva SP, Bulat MV, Gern JE. Serial culture of murine primary airway epithelial cells and ex vivo replication of human rhinoviruses. J Immunol Methods. 2008 doi: 10.1016/j.jim.2008.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60*.Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr. 2007;151:34–42. 42. doi: 10.1016/j.jpeds.2007.02.032. Although this study was not randomized, palivizumab treatment reduced the incidence of recurrent wheezing by half. [DOI] [PubMed] [Google Scholar]
- 61*.Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119:570–575. doi: 10.1016/j.jaci.2006.11.003. Children hospitalized with wheezing caused by HRV, but not RSV, had reduced risk of recurrent wheezing if they were treated with oral prednisolone. [DOI] [PMC free article] [PubMed] [Google Scholar]


