Abstract
In order to identify novel inhibitors of the ATP-binding cassette transporter ABCG2, a high throughput assay measuring accumulation of the ABCG2 substrate pheophorbide a in ABCG2 overexpressing NCI-H460 MX20 cells was used to screen libraries of compounds. Out of a library of 7325 natural products and synthetic compounds from the National Cancer Institute/Developmental Therapeutics Program (DTP) collection, 18 were found to inhibit ABCG2 at 10 μM. After eliminating flavonoids and compounds of limited availability from the 18 original compounds, 10 of the 11 remaining compounds reversed mitoxantrone resistance in NCI-H460/MX20 cells and prevented ABCG2-mediated BODIPY-prazosin transport in ABCG2-transfected HEK293 cells, confirming an interaction with ABCG2. Based on activity profiles and availability of materials, 5 inhibitors were examined for their ability to compete [125I]-iodoarylazidoprazosin (IAAP) labeling of ABCG2, increase binding of the anti-ABCG2 antibody 5D3, and prevent P-glycoprotein (Pgp)- or multidrug resistance associated protein 1 (MRP1)-mediated transport. At a concentration of 20 μM, all of the compounds reduced IAAP labeling by 50-80% compared to control. All 5 compounds also increased 5D3 labeling of ABCG2, indicating that these compounds are inhibitors but not substrates of ABCG2. None of the compounds affected Pgp-mediated rhodamine 123 transport and only one slightly affected MRP-1 mediated calcein transport at 10 μM, suggesting that the compounds are specific for ABCG2. These five novel inhibitors of ABCG2 activity may provide a basis for further investigation of ABCG2 function and its relevance in multidrug resistance.
Keywords: ABCG2 inhibitors, high throughput screening
Introduction
Acquisition of multidrug resistance has long been recognized as a major obstacle to successful cancer chemotherapy. The multidrug resistance transporter ABCG2 (or Breast Cancer Resistance Protein 1, BCRP1), a member of the ABC (ATP-binding cassette) family of membrane transport proteins, is believed to form a part of the maternal-fetal barrier, the blood-brain barrier, and is known to limit oral absorption of some drugs (1). The normal physiologic function(s) of ABCG2 may be related to transport of a variety of natural substances to prevent intracellular accumulation of toxic compounds (2). ABCG2 is also an important mediator of resistance to a variety of anti-cancer drugs including mitoxantrone, topotecan, irinotecan, flavopiridol, and methotrexate (see references 2-4 for recent reviews). Thus, inhibitors of ABCG2 activity could have important oncologic and pharmacologic applications. Unfortunately, in contrast to related ABC transporters such as P-glycoprotein, few if any clinically useful inhibitors of ABCG2 activity have been developed. In addition to obvious potential therapeutic relevance, development of new specific modulators of ABCG2 will have considerable utility in advancing the understanding of ABCG2 function.
ABCG2 expression has been reported in a wide variety of untreated human solid tumors and its expression implicated in drug resistance in acute myeloid leukemia (AML). Diestra and colleagues were among the first to study a large number of solid tumor samples, finding relatively high expression in tumors of the lung, endometrium, and digestive tract as well as melanoma (5, 6). ABCG2 expression in non-small cell lung cancer tumors was reported to be predictive of a lower response rate in patients receiving platinum-based therapy, despite the fact that platinum compounds are not substrates of ABCG2 (7). Several studies have reported that ABCG2 expression impacts response to chemotherapy or affects progression-free survival (8-10). Gene expression profiling of pretreatment samples from 170 patients with AML revealed a cluster group characterized by high ABC transporter expression and highly resistant disease (11). ABCG2 is also highly expressed in normal and in putative cancer stem cells (2, 12). Inhibitors may therefore increase initial response to chemotherapy or may be useful to gain increased accumulation of molecularly targeted agents to various cancer populations.
Inhibition of ABCG2 is also being pursued to increase oral bioavailability and brain penetration of ABCG2 substrates. Previous studies have shown that coadministration of the ABCG2 inhibitor elacridar with topotecan enhanced oral bioavailability in mice (13) while coadminstration of gefitinib and irinotecan enhanced oral bioavailability and antitumor activity in mice (14). Additionally, inhibition of transporters expressed at the blood-brain barrier, such as Pgp and ABCG2, has been shown to increase brain penetration of gefitinib (15) and topotecan (O. van Tellingen, personal communication), highlighting the need for potent inhibitors of ABCG2.
A high-throughput inhibitor screening assay based on accumulation of a fluorescent ABCG2 substrate, pheophorbide a (PhA), has recently been developed (16). Libraries of synthetic and natural products comprising 7325 compounds were obtained from the National Cancer Institute, Developmental Therapeutics Program (DTP) and screened using this assay. The DTP repository of compounds has proven to be a rich source of both synthetic compounds and natural products as molecularly targeted reagents (17, 18). When applied in the ABCG2 screen, several compounds were identified as novel inhibitors of ABCG2 activity. Activities were confirmed by multiple additional ABCG2 assays. These included sensitization of ABCG2-overexpressing cells to killing by mitoxantrone and activity against ABCG2 in transfected cells. Five of the active compounds were further characterized. Because ABCG2 has overlapping substrate specificity with MRP1 and Pgp and because Pgp, MRP1, and ABCG2 are the major contributors to multidrug resistance in most cancer cells in culture (3), the selected compounds were tested for their ability to affect MRP1 and Pgp activities.
Materials and Methods
Materials
Pheophorbide a (PhA) was obtained from Frontier Scientific (Logan, UT). Fumetrimorgin C (FTC), libraries of pure natural products and synthetic molecules (structural diversity and mechanistic diversity sets) and individual compounds were obtained from the Drug Synthesis and Chemistry Branch, Developmental Therapeutics Program (DTP), Division of Cancer Treatment and Diagnostics, National Cancer Institute (Bethesda, MD). Cell culture media were from Invitrogen (Carlsbad, CA) fetal bovine serum (FBS) from Hyclone (Kansas City, MO), and phosphate-buffered saline (PBS) from Quality Biological (Gaithersburg, MD).
Cell culture
NCI-H460 human lung non-small-cell carcinoma cells (National Cancer Institute, Frederick, MD) were selected for overexpression of ABCG2 by maintenance in RPMI1640/10% FBS supplemented with 20 nM mitoxantrone (19). After removal of mitoxantrone, cells were further grown in the same medium without mitoxantrone for 5-30 days. These cells were designated NCI-H460/MX20. Parental cells (low ABCG2 expression) (19) were maintained in the same medium without mitoxantrone. ABCG2-transfected or MDR1-transfected (i.e. Pgp-expressing) HEK293 cells are maintained in 2 mg/ml G418 as previously described (20). MRP1-transfected HEK293 cells are maintained in 5 μM etoposide.
Screening assay for ABCG2 inhibitors
Accumulation of pheophorbide a, a fluorescent ABCG2 substrate (21), formed the basis of the assay for inhibitors of ABCG2 activity (16). Briefly, NCI-H460/MX20 cells were transferred to black wall, clear bottom 384-well polylysine-coated assay plates (Corning, Corning, NY) and allowed to attach for several hours. Pheophorbide a (1 μM final concentration) was added immediately followed by compounds or vehicle (DMSO/PBS) control and incubated an additional 18 h. After removal of medium and washing with PBS containing Ca2+ and Mg2+, fluorescence intensity was read on a Tecan Safire fluorescence plate reader in bottom read mode, 395 nm excitation, 670 nm emission. Each plate had control wells containing 10 μM (final concentration) FTC. Data were normalized to FTC and reported as % of FTC fluorescence.
Mitoxantrone sensitization
The ability of compounds to sensitize NCI-H460/MX20 cells to killing by mitoxantrone was assessed as described (16). ABCG2-overexpressing cells or parental cells were treated with mitoxantrone in the presence or absence of 10 μM compound (or 1 μM FTC) and cell numbers assessed after 2 d by an XTT assay (22).
Flow cytometry
Compounds identified in the screen were confirmed for their ability to inhibit ABCG2-mediated transport using BODIPY-prazosin as a substrate (20). Five of these were additionally tested for their ability to inhibit Pgp-mediated rhodamine 123 efflux and MRP1-mediated calcein efflux as previously described (20, 23). Briefly, transfected HEK293 cells expressing ABCG2, Pgp or MRP1 were trypsinized and incubated in complete medium (phenol red-free Richter’s medium with 10% FCS and penicillin/streptomycin) containing 200 nM BODIPY-prazosin, 0.5 μg/ml rhodamine 123 or 200 nM calcein AM, respectively, in the presence or absence of the desired concentration of inhibitor for 30 min at 37°C. The positive controls for inhibition of ABC transporters were 10 μM FTC for ABCG2, 3 μg/ml valspodar for Pgp and 25 μM MK-571 for MRP1. Cells were then washed and incubated in substrate-free medium continuing with or without inhibitor for 1 h.
The 5D3 shift assay was performed as described by Ozvegy-Laczka and colleagues with minor modifications (24). ABCG2-transfected HEK293 cells were trypsinized and incubated with 5D3 antibody (1:3500, eBioscience, San Diego, CA) for 2 h in the presence or absence of 20 μM of each of the compounds or 20 μM FTC as a positive control. Cells were subsequently washed and then incubated with APC-labeled goat anti-mouse secondary antibody (1:35) for 30 min after which the cells were washed and analyzed.
Intracellular fluorescence of BODIPY-prazosin, rhodamine 123 or calcein fluorescence was detected with a FACSort flow cytometer equipped with a 488 nm argon laser and 530 nm bandpass filter. APC fluorescence was measured with a 635 nm read diode laser and 561 nm longpass filter. At least 10000 events were collected. Dead cells were eliminated based on propidium iodide exclusion.
Photoaffinity labeling of ABCG2 with [125I]-IAAP
ABCG2 expressed in MCF-7 FLV1000 cells, was photo-labeled with [125I]-IAAP as described previously (25). Briefly, crude membranes (1 mg protein/ml) of MCF-7 FLV1000 cells were incubated with 20 μM of the indicated compound for 10 min at room temperature in 50 mM Tris-HCl, pH 7.5. 3-6 nM [125I]-IAAP (2200 Ci/mmole) (PerkinElmer Life Sciences, Wellesley, MA) was added and the samples were incubated for an additional 5 minutes under subdued light. The samples werethen exposed to ultraviolet (UV, 365 nm) light for 10 min and the labeled ABCG2 was immunoprecipitated using BXP-21 antibody. The radioactivity incorporatedinto the ABCG2 band was quantified using the STORM 860 PhosphorImagersystem (Molecular Dynamics, Sunnyvale, CA) and ImageQuaNT software (Molecular Dynamics).
Data analysis
Apparent IC50 values were calculated from dose-response data using SigmaPlot (SPSS, Inc., Chicago) 4-parameter logistic nonlinear regression analysis. Unless otherwise noted, all data are presented as average ± sem.
Results
Screening
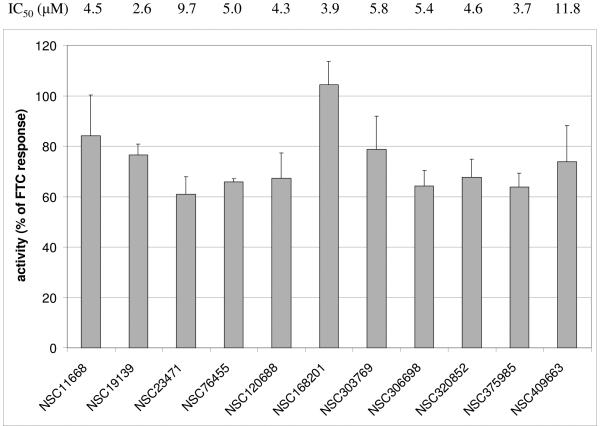
A total of 7325 pure natural products and synthetic compounds comprised the NCI-DTP compound libraries screened using the pheophorbide a assay. Out of these libraries, 18 compounds were identified and confirmed as “hits.” Compounds identified as “hits” in screening (i.e. ≥ 50% of FTC response) were reassayed in quadruplicate and results analyzed by calculation of confidence intervals. A “confirmed hit” was defined as a compound for which reassay showed ≥ 50% of FTC response at a 95% confidence interval (16). After elimination of flavonoids (a well-characterized class of ABCG2 inhibitors) and compounds unavailable for resupply, 11 compounds were further characterized. Figure 1 summarizes the activities of these compounds in the pheophorbide a accumulation assay. The compounds had activities ranging from 60-105% of the activity of FTC.
Figure 1. Activity of compounds in pheophorbide a screening assay.
The compounds listed were assayed for their ability to cause ABCG2-overexpressing cells (NCI-H460/MX20 cells) to accumulate PhA. Each compound was tested at 10 μM (final concentration - bars) and, after resupply, in a dose-response format. Activity for each compound was normalized to the response of 10 μM FTC control wells on the same plate. Error bars represent se (n = 7-9). IC50 values are averages of duplicate determinations for each dose.
Mitoxantrone sensitization (ABCG2-overexpressing cells)
In order to confirm functional relevance of the measured pheophorbide activities, each compound was tested for its ability to restore mitoxantrone sensitivity to cells overexpressing ABCG2 (Figure 2). Unselected NCI-H460 cells were sensitive to killing by mitoxantrone. After 2 d in the presence of 30 μM mitoxantrone, cell numbers were 21.6 ± 1.6% (sd) of control (vehicle). None of the compounds tested were significantly cytotoxic against parental cells (data not shown). Cells selected for ABCG2 overexpression (NCI-H460/MX20) were significantly more resistant to mitoxantrone (see “PBS” column in Figure 2). After mitoxantrone treatment, NCI-H460/MX20 cell number was 56.1 ± 2.1% (sd) of control. In the presence of 1 μM FTC, this was further reduced to 24.5 ± 1.5% (sd). Similar effects were seen with all of the tested compounds (at 10 μM) except for NSC23471 which did not significantly sensitize the cells to mitoxantrone. None of the compounds alone caused significant cell killing in the NCI-H460/MX20 subline (Figure 2).
Figure 2. Sensitization of ABCG2-overexpressing cells to mitoxantrone.
The compounds listed were tested for their ability to sensitize NCI-H460/MX20 cells to killing by mitoxantrone as described in the methods section. Cells were treated for 2 d in the presence or absence of 10 μM compound and 30 μM mitoxantrone and cell number assessed by the XTT assay (22). Cell survival was normalized to buffer control (no mitoxantrone, no compound = 100%). Error bars represent range of duplicate determinations.
Flow cytometry-based prazosin efflux assay
The ability of the 11 compounds to inhibit ABCG2-mediated transport at 10 μM was also confirmed by flow cytometry using a different ABCG2 substrate, BODIPY-prazosin. Ten of the 11 compounds tested were active in inhibiting BODIPY-prazosin efflux (data not shown). Based on relative activities and availability of sufficient quantity of materials for additional assays, five of the compounds, NSC11668, NSC19139, NSC120688, NSC168201, and NSC375985 were selected for further testing. Column 1 of Figure 3 shows the results of a dose-response assay with BODIPY-prazosin with 0.1, 1, or 10 μM of each of the 5 compounds. NSC11668 and NSC168201 were the most potent inhibitors as 0.1 μM of these compounds caused the highest increase in intracellular prazosin fluorescence.
Figure 3. Functional assays of the effects of selected compounds on ABCG2, Pgp, and MRP1.
Column 1: ABCG2 transfected cells were incubated in BODIPY-prazosin in the absence (shaded histogram) or presence of 0.1 (heavy solid line), 1 (solid line), or 10 μM (dashed line) of NSC11668, NSC19139, NSC120688, NSC168201 or NSC375985 as detailed in the methods section. FTC (10 μM, bottom histogram) is shown as a positive control for ABCG2 inhibition. Column 2: ABCG2 transfected cells were incubated with unlabeled 5D3 antibody (1:3500) in the absence (solid line) or presence (dashed line) of 20 μM of the desired compound after which cells were incubated in APC-labeled secondary antibody. FTC (bottom histogram) at a concentration of 20 μM is shown as a positive control. Column 3: MDR1-transfected cells were incubated in 0.5 μg/ml rhodamine 123 in the absence (solid line) or presence (dashed line) of 10 μM of the desired inhibitor as detailed in the methods. Valspodar at 3 μg/ml is included as a positive control for Pgp inhibition (bottom histogram). Column 4: MRP1-transfected cells were incubated in 200 nM calcein AM in the absence (solid line) or presence (dashed line) of 10 μm of the compounds. MK-571 (25 μM) is shown (bottom histogram) as a positive control for inhibition of MRP1 transport.
5D3 antibody binding assay
To confirm their roles as ABCG2 inhibitors (rather than substrates), these 5 compounds were next examined for their ability to increase surface staining of the 5D3 antibody (24). Ozvegy-Laczka, et al. have previously demonstrated that, at high dilution, the 5D3 antibody binds more readily to ABCG2 when ABCG2-transfected cells are incubated with the antibody in the presence of an ABCG2 inhibitor (24). This was believed to be due to the fact that, at low antibody concentrations, 5D3 has a higher affinity for a certain conformation induced by inhibitors of ABCG2, allowing study by flow cytometry. ABCG2-transfected cells were incubated with a high dilution of the 5D3 antibody (1:3500) in the presence or absence of 20 μM of the putative inhibitor. Cells were subsequently incubated with APC-labeled secondary antibody. Figure 3, column 2 shows that, at 20 μM, all of the compounds tested increased 5D3 binding and were comparable to 20 μM FTC shown as a positive control. The change in APC fluorescence was quantitated for each sample and the values are given in Table 1.
Table 1.
Summary of effects of compounds in multiple assays
| Pheophorbide a | MX sensitizationb |
Flow - ABCG2 | IAAP bindinge |
Cross-reactivity (flow) |
||||
|---|---|---|---|---|---|---|---|---|
| totala | IC50 (μM) | 5D3c | BODIPY-prazosind | Pgpf | MRP1g | |||
| NSC11668 | 84.1 | 4.5 | 21.9 | 4.0 | 5.9 | 24.6 | 0.73 | 1.2 |
| NSC19139 | 76.6 | 2.6 | 30.0 | 3.6 | 3.5 | 33.5 | 0.77 | 1.3 |
| NSC120688 | 67.2 | 4.3 | 20.9 | 3.7 | 2.0 | 19.1 | 0.84 | 0.81 |
| NSC168201 | 104.5 | 3.9 | 21.2 | 3.7 | 2.6 | 34.2 | 0.64 | 0.81 |
| NSC375985 | 63.8 | 3.7 | 27.6 | 3.2 | 3.6 | 48.7 | 0.84 | 1.8 |
| FTC | 100 | 0.8 | 24.5 | 3.7 | 3.5 | 32.7 | ||
| MK571 (25 μM) | 3.3 | |||||||
| valspodar (3 μg/mL) |
14.9 | |||||||
| DMSO/PBS | 0 | 56.1 | 1.0 | 1.0 | 100 | |||
% of FTC response
% NCI-H460/MX20 cell survival in the presence of compound and mitoxantrone
5D3 staining: treated/control ratio at 10 μM compound
BODIPY-prazosin efflux: treated/control ratio at 10 μM compound
blocking of 125I-IAAP binding: % of control binding in the presence of 20 μM compound
Pgp inhibition, rhodamine efflux: treated/control ratio at 10 μM compound
MRP1 inhibition, calcein efflux: treated/control ratio at 10 μM compound
Inhibition of Pgp and MRP1
Each of the 5 selected compounds was also tested for its ability to inhibit Pgp and MRP1, other ABC transporters known to confer drug resistance. None of these compounds significantly inhibited Pgp-mediated rhodamine transport (Figure 3, column 3). Complete inhibition of Pgp was observed with valspodar (Figure 3, column 3, bottom histogram). One compound, NSC375985, slightly inhibited MRP1-mediated calcein transport (Figure 3, column 4). This can be contrasted to complete MRP1 inhibition with 25 μM MK571 (Figure 3, column 4, bottom histogram).
Inhibition of IAAP incorporation into ABCG2
To further explore the interaction between the 5 compounds and ABCG2, their ability to inhibit [125I]IAAP incorporation into ABCG2 in membranes isolated from ABCG2-overexpressing MCF-7 FLV1000 cells was studied. Previously IAAP has been shown to be transported by ABG2 and can also be used as a photoaffinity label for this transporter (25). As seen in Figure 4, all 5 compounds (at 20 μM) significantly reduced [125I]-IAAP incorporation into ABCG2. With the exception of NSC375985, all had inhibitory activity comparable to or better than that of FTC. These results suggest that the 5 compounds act at the binding site of IAAP, similar to FTC.
Figure 4. Effect of selected compounds on photoaffinity labeling of ABCG2 with IAAP.
[125I]-IAAP incorporated into the ABCG2 band was quantified as described in ‘materials and methods’. The graph represents the amount of [125I]-IAAP incorporated (average of three independent experiments) into ABCG2 (Y-axis) in the absence (control) or presence of 20 μM of the indicated compound (X-axis).
Discussion
The National Cancer Institute Developmental Therapeutics Program’s libraries of synthetic compounds and purified natural products has been an excellent source of reagents for the study of molecular targets in cancer (17, 18). Application of a high throughput assay for inhibitors of ABCG2 activity (16) has identified a variety of active compounds. As expected, and previously reported, several flavonoids were among the inhibitors identified which served as a validation for the assay (16). However, as a class, flavonoids are well characterized as ABCG2 inhibitors (26, 27) and the screening did not identify any novel flavonoids. Therefore, these were not additionally characterized. Based on further analysis, one of the other compounds identified, NSC23471, appeared to be a false positive result in that it was inactive in alternative assays of ABCG2 activity, mitoxantrone sensitization (Figure 2) and in the BODIPY-prazosin efflux assay (data not shown). Ten new putative inhibitors of ABCG2 have thus been identified. These compounds did not share significant structural features (data not shown) nor did they appear to be similar to previously identified ABCG2 modulators described in the literature.
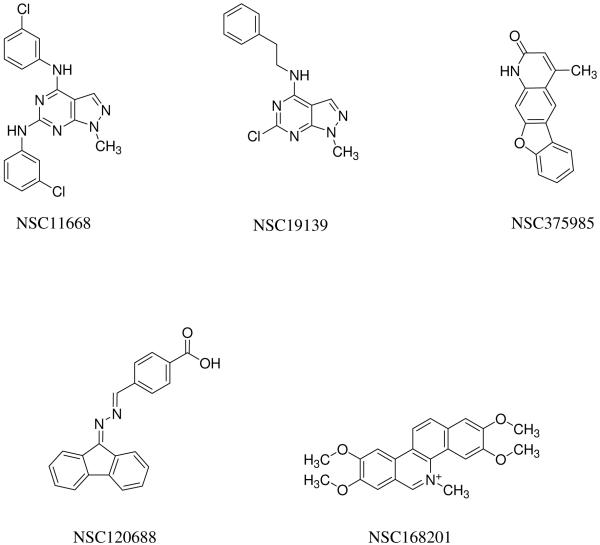
Based on a combination of relative activities in these assays and availability of sufficient material for further study, 5 compounds (NSC11688, NSC19139, NSC120688, NSC168201, and NSC375985) were selected for additional characterization. Figure 5 shows the structures of these compounds as reported by DTP. After resupply of individual compounds from DTP, their identity and purity were confirmed by LC/MS and NMR analysis (data not shown). Although NSC11668 and NSC19139 share some structural features, the members of this set of compounds are not otherwise related. Table 1 summarizes the data obtained with these 5 compounds. All 5 had IC50 values of < 5 μM in the PhA accumulation assay (Figure 1) and they were approximately equally potent in sensitization of cells to mitoxantrone (Figure 2). Flow cytometry data with an alternative substrate (BODIPY-prazosin) and cell line (ABCG2-transfected HEK293 cells) confirmed the activity of the compounds and gave a preliminary ranking of their relative activities (Figure 3, column 1). Inhibition of photoaffinity labeling of ABCG2 with [125I]-IAAP (Figure 4) confirms the interaction of the compounds with the transporter itself and suggests that these compounds act at the drug-substrate binding site(s). Since IAAP is a photoaffinity analog of prazosin, it is thought to label the drug binding site. While reduced IAAP binding could be due to conformational changes induced by the inhibitor, the more likely explanation is that the decreased binding is due to competition of the inhibitor for the drug binding site. Kinetic studies can be performed to determine whether this inhibition of labeling is competitive or non-competitive; the micromolar concentrations required for inhibition suggest that such assays would demonstrate competitive inhibition of drug binding. Finally, the ability of all 5 compounds to increase surface staining of the 5D3 antibody, confirmed their identification as inhibitors (24).
Figure 5.
Structures of selected ABCG2 inhibitors
Comparison of the activities of the five compounds on ABCG2 as compared to two other MDR proteins, Pgp and MRP1, was undertaken. Only one of the compounds (NSC375985) cross reacted weakly with MRP1 and none affected Pgp activity. This result suggests that the compounds are primarily specific for inhibition of ABCG2.
As with other MDR transporters, one of the significant obstacles to development of clinically useful ABCG2 inhibitors is the problem of cytotoxicity (3, 4). The 5 compounds shown in Figure 5 all had low toxicity against parental NCI-H460 cells (data not shown). Similarly, a review of NCI 60-cell data in the DTP website database revealed average GI50 (50% growth inhibition) of 10-70 μM for all 60 cell lines with very little selectivity. These compounds themselves may therefore have relatively low toxicity.
Although the relevance of ABCG2 to clinical drug resistance remains unconfirmed, ABCG2 has been implicated in drug resistance in leukemia (6). Availability of new inhibitors may be able to contribute to increased clinical response in ABCG2-overexpressing tumors. Since ABCG2 has also been reported to be expressed at high levels in the digestive tract and at the blood-brain barrier (28), ABCG2 inhibitors may be able to enhance bioavailability and brain penetration of ABCG2 substrate drugs. Proof of this principle has been demonstrated in a clinical study by Kruitzer et al., who showed that coadministration of GF210918 with topotecan increased oral absorption of topotecan (29).
In conclusion, five novel compounds have been identified from the NCI DTP repository. These compounds are relatively specific inhibitors of the ABCG2 multidrug resistance protein. The compounds identified by this work will provide a selection of novel reagents for studying ABCG2 function and potential clinical relevance as well as potential scaffolds for building newer inhibitors.
Acknowledgements
This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
This Research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
Abbreviations
- ABC
ATP-binding cassette
- AML
acute myeloid leukemia
- BCRP
breast cancer resistance protein
- DTP
Developmental Therapeutics Program (National Cancer Institute)
- FTC
fumetrimorgin C
- IAAP
iodoarylazidoprazosin
- MDR
multidrug resistance
- MRP
multidrug resistance protein
- Pgp
P-glycoprotein
- PhA
pheophorbide a
References
- 1.Robey RW, Polgar O, Deeken J, To KW, Bates SE. ABCG2: determining its relevance in clinical drug resistance. Cancer Metastasis Rev. 2007;26:39–57. doi: 10.1007/s10555-007-9042-6. [DOI] [PubMed] [Google Scholar]
- 2.Krishnamurthy P, Schuetz JD. Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol. 2006;46:381–410. doi: 10.1146/annurev.pharmtox.46.120604.141238. [DOI] [PubMed] [Google Scholar]
- 3.Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5:219–34. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 4.Xu J, Peng H, Zhang J-T. Human multidrug transporter ABCG2, a target for sensitizing drug resistance in cancer chemotherapy. Curr Med Chem. 2007;14:689–701. doi: 10.2174/092986707780059580. [DOI] [PubMed] [Google Scholar]
- 5.Diestra JE, Scheffer GL, Catala I, et al. Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol. 2002;198:213–9. doi: 10.1002/path.1203. [DOI] [PubMed] [Google Scholar]
- 6.Plasschaert SLA, van der Klolk D, de Bont EVSJM, Vellenga E, Kamps WA, de Vries GE. Breast cancer resistance protein (BCRP) in acute leukemia. Leukemia and Lymphoma. 2004;45:649–54. doi: 10.1080/10428190310001597928. [DOI] [PubMed] [Google Scholar]
- 7.Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res. 2004;10:1691–7. doi: 10.1158/1078-0432.ccr-0937-3. [DOI] [PubMed] [Google Scholar]
- 8.Uggla B, Stahl E, Wagsater D, et al. BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res. 2005;29:141–6. doi: 10.1016/j.leukres.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Baderra Z, Faussat AM, Sayada L, et al. MRP3, BCRP, and P-glycoprotein activities are prognostic factors in adult acute myeloid leukemia. Clin Cancer Res. 2005;11:7764–72. doi: 10.1158/1078-0432.CCR-04-1895. [DOI] [PubMed] [Google Scholar]
- 10.Damiani D, Tiribelli M, Calistri E, et al. The prognostic value of P-glycoprotein (ABCB) and breast cancer resistance protein (ABCG2) in adults with de novo acute myeloid leukemia with normal karyotypes. Haematologica. 2006;91:825–8. [PubMed] [Google Scholar]
- 11.Wilson CS, Davidson GS, Martin SB, et al. Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood. 2006;108:685–96. doi: 10.1182/blood-2004-12-4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–84. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 13.Jonker JW, Smit JW, Binkhuis RF, et al. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topotecan. J Natl Cancer Inst. 2000;92:1651–6. doi: 10.1093/jnci/92.20.1651. [DOI] [PubMed] [Google Scholar]
- 14.Stewart CF, Leggas M, Schuetz JD, et al. Gefitinib enhances the antitumor activity and bioavailability of irinotecan in mice. Cancer Res. 2004;64:7491–9. doi: 10.1158/0008-5472.CAN-04-0096. [DOI] [PubMed] [Google Scholar]
- 15.Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–82. doi: 10.1158/0008-5472.CAN-04-2416. [DOI] [PubMed] [Google Scholar]
- 16.Henrich CJ, Bokesch HR, Dean M, et al. A high-throughput cell-based assay for inhibitors of ABCG2 activity. J Biomol Screen. 2006;11:176–83. doi: 10.1177/1087057105284576. [DOI] [PubMed] [Google Scholar]
- 17.Holbeck SL. Update on NCI in vitro drug screen utilities. Eur J Cancer. 2004;40:785–93. doi: 10.1016/j.ejca.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Shoemaker RH. The NCI60 human tumour cell line anticancer drug screen. Nat Rev Cancer. 2006;6:813–23. doi: 10.1038/nrc1951. [DOI] [PubMed] [Google Scholar]
- 19.Robey RW, Honjo Y, van de Laar A, et al. A functional assay for detection of the mitoxantrone resistance protein MXR (ABCG2) Biochim Biophys Acta. 2001;1512:171–82. doi: 10.1016/s0005-2736(01)00308-x. [DOI] [PubMed] [Google Scholar]
- 20.Robey RW, Honjo Y, Morisaki K, et al. Mutations at amino-acid 482 in the ABCG2 gene affect substrate and antagonist specificity. Br J Cancer. 2003;89:1971–8. doi: 10.1038/sj.bjc.6601370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robey RW, Steadman K, Polgar O, et al. Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res. 2004;64:1242–6. doi: 10.1158/0008-5472.can-03-3298. [DOI] [PubMed] [Google Scholar]
- 22.Scudiero DA, Shoemaker RH, Paull KD, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:4827–33. [PubMed] [Google Scholar]
- 23.Alvarez M, Robey R, Sandor V, et al. Using the National Cancer Institute anticancer drug screen to assess the effect of MRP expression on drug sensitivity profiles. Mol Pharmacol. 1998;54:802–14. doi: 10.1124/mol.54.5.802. [DOI] [PubMed] [Google Scholar]
- 24.Ozvegy-Laczka C, Varady G, Koblos G, et al. Function-dependent conformational changes of the ABCG2 multidrug transporter modify its interaction with a monoclonal antibody on the cell surface. J Biol Chem. 2005;280:4219–27. doi: 10.1074/jbc.M411338200. [DOI] [PubMed] [Google Scholar]
- 25.Shukla S, Robey RW, Bates SE, Ambudkar SV. The calcium channel blockers, 1,4-dihydropyridines, are substrates of the multidrug resistance-linked ABC drug transporter, ABCG2. Biochemistry. 2006;45:8940–51. doi: 10.1021/bi060552f. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed-Belkacem A, Pozza A, Munoz-Martinez F, et al. Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. Cancer Res. 65:4852–60. doi: 10.1158/0008-5472.CAN-04-1817. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Yang X, Morris ME. Flavonoids are inhibitors of breast cancer resistance protein (ABCG2)-mediated transport. Mol Pharmacol. 65:1208–1216. doi: 10.1124/mol.65.5.1208. [DOI] [PubMed] [Google Scholar]
- 28.Takano M, Yumoto R, Murakami T. Expression and function of efflux drug transporters in the intestine. Pharmacol Ther. 2006;109:137–61. doi: 10.1016/j.pharmthera.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Kruijtzer CM, Beijnen JH, Rosing H, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol. 2002;20:2943–50. doi: 10.1200/JCO.2002.12.116. [DOI] [PubMed] [Google Scholar]