Abstract
Dicer gene dcl2, required for the RNA silencing antiviral defense response in the chestnut blight fungus Cryphonectria parasitica, is inducible upon mycovirus infection and promotes viral RNA recombination. We now report that the antiviral defense response requires only one of the four C. parasitica Argonaute-like protein genes, agl2. The agl2 gene is required for the virus-induced increase in dcl2 transcript accumulation. Agl2 and dcl2 transcripts accumulated to much higher levels in response to hairpin RNA production or infection by a mutant CHV1-EP713 hypovirus lacking the suppressor of RNA silencing p29 than to wild-type CHV1-EP713. Similar results were obtained for an agl2-promoter/EGFP-reporter construct, indicating that p29-mediated repression of agl2 transcript accumulation is promoter-dependent. Significantly, the agl2 deletion mutant exhibited stable maintenance of non-viral sequences in recombinant hypovirus RNA virus vectors and the absence of hypovirus-defective interfering (DI) RNA production. These results establish a key role for an Argonaute gene in the induction of an RNA silencing antiviral defense response and the promotion of viral RNA recombination. They also provide evidence for a mechanism by which a virus-encoded RNA silencing suppressor represses the transcriptional induction of an RNA silencing component.
Keywords: Cryphonectria parasitica, mycovirus, defective interfering RNA, silencing suppressor, RNA virus vector
An RNA-based antiviral defense response, related to RNA interference (1), serves as a key component of the innate immunity repertoire in plants, invertebrates, and fungi (2, 3). Common elements of this response across Kingdoms include the action of conserved ribonucleases: members of the Dicer-like and Argonaute-like protein families (4). Dicer nucleases recognize viral double-stranded (ds) and structured RNAs and use the associated RNase III-type activity to process these RNAs into small RNAs of 21–24 nts in length, termed virus-derived small (vs) RNAs. The vsRNAs are incorporated into an effector complex with the aid of an Argonaute family protein. One strand of the vsRNA is removed and the remaining guide strand then targets the effector complex to the cognate viral RNA, which is cleaved, or sliced, by the Argonaute-associated RNase H-like activity.
Four Dicer proteins drive the RNA silencing pathways in the model plant, Arabidopsis thaliana (5). All four Dicers have been implicated in antiviral RNA silencing (3, 6–8). Studies with Drosophila melanogaster (9, 10) and with mosquitoes (11) have identified a role for Dicer-2, the Dicer involved in siRNA production, in insect antiviral defense. The contribution to antiviral defense of the second Dicer, Dicer-1, remains unclear due to its essential role in microRNA processing and development (12).
Antiviral RNA silencing has been demonstrated in the filamentous Ascomycete fungi Cryphonectria parasitica, the chestnut blight fungus (13), and Aspergillus nidulans (14). Only one of the two C. parasitica Dicer genes, dcl2, was shown to be required for antiviral RNA silencing (13) and the production of vsRNAs (15), while the second Dicer gene, dcl1, was shown to be dispensable. Expression of the C. parasitica dcl2 gene was also shown to be significantly induced in response to mycovirus infection by a mechanism that was repressed (15) by the hypovirus CHV1-EP713-encoded suppressor of RNA silencing p29 (16). A similar induction and repression of Dicer gene expression has not yet been reported for infected plants or animals. A surprising role for C. parasitica DCL2 in viral RNA recombination, the production of internally deleted, defective interfering (DI) RNAs and the instability of foreign gene sequences in recombinant RNA virus vectors, was recently demonstrated (17).
A role for Argonaute proteins in antiviral RNA silencing was described for AGO2 in D. melanogaster (18, 19) and for AGO1 in A. thaliana (20). Subsequent studies have identified contributions to antiviral plant defense by AGO2 and AGO5 (21) as well as AGO7 (8). Members of the PIWI subclass of the Argonaute protein family in Drosophila, AGO3, PIWI (AGO4), and Aubergine (AGO5), have been shown to restrict Drosophila X virus (22) and AGO2, and possibly AGO3, were reported to be critical for inhibition of alphavirus replication in mosquitoes Anopheles gambiae (23) and Aedes aegypti (11).
We now report that only one of the four Argonaute-like protein genes encoded by C. parasitica, agl2, is required for antiviral RNA silencing. We also show that agl2 is required for the induction of dcl-2 expression in response to virus infection and promotes the viral RNA recombination events that accompany the RNA silencing antiviral response. Finally, we present evidence that the hypovirus-encoded RNA silencing suppressor p29 represses agl2 transcriptional induction in response to virus infection.
Results
Cryphonectria parasitica Encodes Four Argonaute-Like Protein Genes.
The number of Argonaute-like protein genes encoded by filamentous fungi range from zero for the corn pathogen Ustilago maydis to as many as eight reported for the mushroom Coprinus cinereus, used as a model system for developmental studies (24). Four Argonaute-like protein (agl) genes, agl1, agl2, agl3, and agl4 (Fig. S1A), were identified through inspection of the C. parasitica draft genome sequence (http://genome.jgi-psf.org/Crypa1/Crypa1.home.html).
Hammond et al. (25) have proposed a paralogous grouping system for the components of fungal RNA silencing pathways based on the two silencing pathways identified for N. crassa. Fungal Dicer and Argonaute proteins related to N. crassa Dicer-2 and Argonaute protein QDE-2 involved in RNA silencing in the vegetative stage (26, 27), called Quelling, are referred to as members of group Q while the corresponding proteins related to Dicer-1 and Argonaute SMS-2 (28, 29), involved in meiotic silencing of unpaired DNA (MSUD), are placed in group M. Phylogenetic analysis of the four C. parasitica agl1–4 gene products showed clustering of AGL4 with the group M Argonaute proteins, while AGL1, AGL2, and AGL3 all clustered with the group Q Argonautes (Fig. S1B). However, the tissue distribution for the C. parasitica agl gene transcripts was not strictly consistent with predicted roles in either Quelling (vegetative tissue) or MSUD (reproductive tissue). Transcripts for all four agl genes were detected in perithecial tissue, while transcripts for agl3 and agl4 were undetectable in vegetative tissue, in which transcripts for agl2 were the most abundant, 25-fold higher than agl1 transcripts at day 7 (Fig. S2).
Disruption of C. parasitica Argonaute-Like Gene agl2, but Not agl1, agl3, or agl4, Dramatically Increased Susceptibility to Hypovirus Infection.
Deletion mutant strains were constructed for each of the agl genes in an effort to identify Argonaute-like proteins that contribute to the antiviral RNA silencing pathway. Gene disruptions were confirmed by Southern analysis (Fig. S3 shows representative Southern analyses of agl2 disruption mutants) and RT-PCR. As indicated in Fig. 1Top, the colony morphology for each of the four agl mutant strains was indistinguishable from the parental strain EP155. Additionally, the mutant strains were found not to be altered in asexual sporulation or virulence on dormant chestnut stems.
Fig. 1.
Effect of hypovirus CHV1-EP713 infection on C. parasitica Argonaute-like gene disruption mutants. Images are shown of the colony morphology for virus-free wild-type strain EP155 and four Argonaute-like gene disruption mutants Δagl1, Δagl2, Δagl3, and Δagl4 (Top) and the corresponding strains infected with hypovirus CHV1-EP713 (Middle). (Bottom) The colony morphology for a complemented Δagl2 strain infected with hypovirus CHV1-EP713 is shown. The Δagl2 deletion mutant was complemented with a genomic DNA clone of the agl2 coding region cloned into plasmid pCPXNBn1 to generate the complementation plasmid pCAGL2, which contains the benomyl resistance cassette and the C. parasitica glyceraldehydes-3-phosphate dehydrogenase promoter (31). Cultures were grown for 7 days on PDA.
The four agl gene disruption mutant strains were independently infected with hypovirus CHV1-EP713, either by transfection of spheroplasts with infectious transcripts (30) or by anastomosis with CHV1-EP713 infected strain EP155 (31). Mutant strains Δagl1, Δagl3, and Δagl4 all responded to CHV1-EP713 infection with phenotypic changes that were indistinguishable from that exhibited by CHV1-EP713-infected wild-type strain EP155. In contrast, a very severe slow growth phenotype was observed for the CHV1-EP713-infected Δagl2 mutant strain (Fig. 1 Middle). Complementation of the Δagl2 mutant with the intact agl2-coding domain restored the wild-type response to hypovirus infection (Fig. 1 Bottom).
AGL2 Is Required for Induction of dcl2 Expression in Response to Virus Infection.
The increased severity of symptoms exhibited by the Δagl2 mutant strain in response to hypovirus infection (Fig. 1) is consistent with the coordinated action of DCL2 to produce vsRNAs (15) and AGL2 to incorporate vsRNAs into the RISC complex, leading to targeted slicing of viral RNA. An alternative, but not mutually exclusive, possibility is that AGL2 is required for dcl2 transcriptional induction upon virus infection. To explore this possibility, we examined agl2 and dcl2 transcript levels in CHV1-EP713-infected wild-type and mutant strains. As shown in Fig. 2, agl2 transcript levels increased by approximately 2-fold compared to the 10–15-fold increase observed for dcl2 transcript accumulation following CHV1-EP713 infection. Significantly, the induction in dcl2 transcript levels observed in wild-type infected strains does not occur in hypovirus infected Δagl2 mutant strains, indicating that AGL2 is required for induction of dcl2 expression in response to virus infection (Fig. 2). In contrast, the modest 2-fold increase in agl2 transcript accumulation observed for the CHV1-EP713-infected wild-type strain (EP155) also occurred in the infected Δdcl2 strain.
Fig. 2.
Accumulation of agl2 and dcl2 transcripts in response to hypovirus infection. The relative levels of agl2 (gray columns) and dcl2 (black columns) were measured by semiquantitative real-time RT-PCR for wild-type strain EP155, strain EP155 infected with full-length hypovirus CHV1-EP713 (EP155/CHV1), virus-free and CHV1-EP713-infected mutant strain Δagl2 (Δagl2 and Δagl2/CHV1, respectively), virus-free and CHV1-EP713-infected mutant strain Δdcl2 (Δdcl2 and Δdcl2/CHV1, respectively), and strain EP155 infected with a CHV1-EP713 mutant that lacks the p29 suppressor of RNA silencing (EP155/Δp29).
We previously reported that the virus-induced increase in dcl2 transcript accumulation was repressed by a hypovirus-encoded suppressor of RNA silencing p29 (15). That is, the accumulation of dcl2 transcripts increased by >30 fold in response to infection by the hypovirus mutant isolate lacking p29 (Δp29) compared to a >10-fold increase in response to wild-type CHV1-EP713 infection. As shown in Fig. 2, agl2 transcript accumulation also increased to a significantly higher level (14-fold) in response to Δp29 infection compared to the 2-fold increase in response to CHV1-EP713 infection.
Differences in Transcript Accumulation Profiles for agl2 and dcl2 in Response to Virus Infection and Hairpin dsRNA Production.
Choudhary et al. (32) reported that the expression of both the N. crassa Dicer gene dcl-2 and the Argonaute gene qde-2, each involved in transgene silencing (27), was significantly induced in response to hairpin dsRNA production. Since the transcript accumulation profiles for C. parasitica dcl2 and agl2 genes in response to hypovirus infection (Fig. 2) differed significantly from the hairpin dsRNA-mediated induction profiles reported for N. crassa orthologous genes dcl-2 and qde-2, we examined the response of C. parasitica dcl2 and agl2 expression to hairpin dsRNA production. The C. parasitica strain EP155 expressing EGFP from a nuclear transgene (16) was transformed with a EGFP hairpin-silencing vector plasmid pGSN2GFP and six independent single-spored transformants that exhibited >70% level of GFP silencing (Fig. 3) were examined by real-time RT-PCR to determine the level of dcl2 and agl2 transcript accumulation. As shown in Fig. 3, agl2 and dcl2 transcript levels increased by approximately 15- and 60-fold, respectively, in the silenced strains (T01, T03, T05, T12, T20, and T21) relative to a pGSN2GFP transformed strain that did not exhibit silencing (T23 in Fig. 3A) or the untransformed cpEGFP strain. Thus the accumulation profiles for agl2 and dcl2 transcripts in response to hairpin dsRNA production were not dissimilar to the N. crassa qde-2 and dcl-2 induction profiles in response to hairpin dsRNA production, but were quite different from the accumulation profiles observed for the response to hypovirus infection (Fig. 2). Moreover, the profiles for agl2 and dcl2 transcript accumulation in response to Δp29 were very similar to the profiles observed in response to hairpin dsRNA production (compare Figs. 2 and 3B). These results indicate that p29 has a very significant negative effect on the transcriptional induction of the RNA silencing response and the relative accumulation of agl2 and dcl2 transcripts in response to hypovirus infection.
Fig. 3.
Accumulation of agl2 and dcl2 transcripts in response to hairpin dsRNA production. (A) Histogram showing fluorescence measurements of EGFP-expressing C. parasitica strain cpEGFP following transformation with the hairpin RNA silencing plasmid pGSN2GFP (see Materials and Methods) that contains inverted copies of the EGFP gene. The level of EGFP fluorescence was silenced by greater than 70% for transformants T01, T03, T05, T12, T20, and T21 relative to the level of fluorescence measured for the parental strain cpEGFP. No silencing of EGFP fluorescence was observed for transformant T23. Four replicate measurements are shown for each independent transformant. (B) Histogram showing relative accumulation of agl2 (gray columns) and dcl2 (black column) transcripts in the corresponding parental cpEGFP strain and hairpin RNA vector pGSN2GFP transformed strains shown in A as measured by real-time RT-PCR. Note the correspondence between increased accumulation of both agl2 and dcl2 transcripts and hairpin dsRNA-mediated EGFP silencing.
Agl2 Expression in Response to Virus Infection Is Promoter-Dependent.
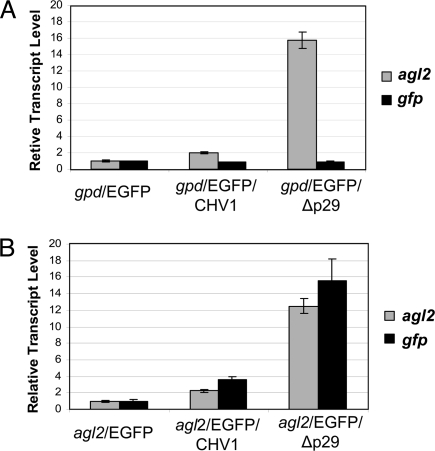
To more closely examine the agl2 transcriptional induction response to hypovirus infection, we tested the response of an agl2 promoter-reporter construct. The 1,566-bp region immediately upstream of the start ATG codon was connected to the EGFP coding sequence and the resulting plasmid construct was used to transform wild-type C. parasitica strain EP155. A promoter/reporter construct consisting of the promoter region of the C. parasitica glyceraldehyde-3-phosphate dehydrogenase (gpd) gene fused to the EGFP coding sequence (see Materials and Methods) served as a control. Resulting transformed strains were infected independently with full-length CHV1-EP713 and the Δp29 deletion mutant virus. As shown in Fig. 4 for representative transformants, the GFP reporter transcript accumulation pattern for the agl2-promoter/EGFP-reporter strain mirrored the native agl2 transcript accumulation pattern with a minimal response to CHV1-EP713 infection and a much larger increase in response to Δp29 infection (Fig. 4B). In contrast, GFP transcript levels were unchanged in the transformants containing the control gpd-promoter/EGFP-reporter construct following infection by either wild-type or Δp29 mutant virus (Fig. 4A). Thus the changes in agl2 expression levels in response to wild-type and the Δp29 mutant hypovirus infections appear to be promoter-dependent.
Fig. 4.
Response of an agl2-promoter/EGFP-reporter to virus infection. C. parasitica strains gpd/EGFP and agl2/EGFP produced by transformation of wild-type strain EP155 with promoter/reporter plasmids pCNE2GP and pA2PG, consisting of the C. parasitica glyceraldehyde-3-phosphate dehydrogenase (gpd) promoter or the agl2 promoter, respectively, fused to the EGFP coding domain (see Materials and Methods), were infected with full-length hypovirus CHV1-EP713 or the mutant hypovirus Δp29 that lacked the p29 suppressor of RNA silencing. The accumulation levels of gfp transcripts and native agl2 transcripts were determined by real-time RT-PCR (Materials and Methods). (A) The level of gfp (black columns) and native agl2 (gray columns) transcript accumulation are shown for a representative gpd-promoter/EGFP-reporter strain following infection by CHV1-EP713 (gpd/EGFP/CHV1) or the Δp29 mutant virus (gpd/EGFP/Δp29) relative to the levels found in the virus-free gpd-promoter/EGFP-reporter control strain set to a value of one. (B) A similar analysis is shown for a representative agl2-promoter/EGFP-reporter strain.
AGL2 Contributes to Viral RNA Recombination.
Hypovirus defective-interfering (DI) RNAs, which are produced at a high frequency in wild-type C. parasitica and Δdcl1 mutant strains, were not produced in detectable levels in Δdcl2 mutant strains (15). The contribution of AGL2 to DI RNA production was examined by transfecting C. parasitica wild-type, Δagl1, and Δagl2 mutant strains with infectious, in vitro synthesized, hypovirus CHV1-EP713 coding strand RNA transcripts and testing for DI RNA production with time following infection. As indicated in Fig. 5, DI RNA production was observed in CHV1-EP713-infected wild-type and Δagl1 mutant strains within several transfers (Fig. 5A, lanes 2 and 3) while DI RNAs were not observed in Δagl2 (lane 4) or Δdcl2 (lane 5) mutant strains even after extensive subculturing.
Fig. 5.
Argonaute AGL2 contributes to hypovirus RNA recombination. (A) Agarose (1%) gel analysis of total RNA isolated from virus-free wild-type strain EP155 (lane 1); hypovirus CHV1-EP713-infected strain EP155 (lane 2); CHV1-EP713-infected Δagl1 mutant strain (lane 3); CHV1-EP713-infected Δagl2 mutant strain (lane 4); and CHV1-EP713-infected Δdcl2 mutant strain (lane 5). The lane marked “M” contains 1-kb DNA size markers. The migration positions of replicative dsRNAs, corresponding to full-length CHV1-EP713 and related Defective Interfering (DI) RNAs, and full-length CHV1-EP713 ssRNA are indicated by arrows at the right. The asterisks indicate the migration positions of C. parasitica ribosomal RNAs. (B) Cartoon representation of recombinant hypvoirus EGFP vector Ctp40[2AEGFP] that contains insertions of the EGFP gene and the Foot and Mouth Virus 2A protease gene at the 3′-end of the p40 coding region of hypovirus CHV1-EP713 (33). (C) Fluorescence micrographs of Argonaute-like gene disruption strains infected by transfection with transcripts derived from recombinant hypovirus EGFP vector ctp40[2AEGFP]. The infected fungal strains were repeatedly transferred to new PDA plates following 7 days of growth. The colonies shown in this figure were photographed after two transfers, by which time fluorescence had been lost in the Ctp40[2AEGFP]-infected wild-type strain EP155 (i) and the infected Δagl1 mutant strain (ii). Fluorescent EGFP expression was retained, however, in Ctp40[2AEGFP]-infected Δagl2 and Δdcl2 mutant strains (iii and iv, respectively) and continued to be expressed for over six subcultures examined in this experiment. Fluorescence micrographs were taken at 5-day-old PDA cultures using Olympus DP70 microscope as described in Materials and Methods. Corresponding light micrographs were shown in v–viii.
The potential contribution of AGL2 to non-viral nucleotide sequence instability was tested by transfecting wild-type and Δagl1 and Δagl2 mutant strains with vector construct Ctp40[2AEGFP], which contains the enhanced green fluorescent protein (EGFP) coding domain preceded by the foot and mouth disease virus (FMDV) 2A protease domain inserted into the p40 coding region just upstream of the ORF A-ORF B junction (Fig. 5B). As reported previously for wild-type C. parasitica strains (33, 17) EGFP expression was lost from Ctp40[2AEGFP] infected wild-type and the Δagl1 mutant strains (Fig. 5C, i and ii, respectively) within two transfers (2 weeks after recovery from regeneration plates), but was retained in the infected Δagl2 and Δdcl2 mutant strains (Fig. 5C, iii and iv, respectively) and continued to be expressed for prolonged periods of subculturing in excess of six transfers tested in this study.
Discussion
We recently demonstrated that RNA silencing serves as an antiviral defense response in fungi by showing that deletion of the C. parasitica Dicer gene dcl2 results in a dramatic increase in susceptibility to mycovirus infections while deletion of the second Dicer gene, dcl1, had no effect on host response to mycovirus infection (13). The similar increase in virus-induced symptoms reported here for one of the four C. parasitica Argonaute-like gene deletion mutant strains, Δagl2, confirms a central role of RNA silencing as a fungal antiviral defense response and indicates that this response is mediated in C. parasitica by a single Dicer gene and a single Argonaute gene. We were also able to show that AGL2 is required for the previously described (15) induction of dcl2 transcription in response to mycovirus infection and that AGL2, like DCL2 (17), promotes viral RNA recombination.
The requirement of a single Dicer gene, dcl2, and a single Argonaute gene, agl2, for RNA silencing antiviral defense in C. parasitica allows an examination of the activation of this response in the absence of potential contributions from multiple Dicers and Argonautes, as occurs in plants, and potentially, in animal cells (3). The picture to emerge from this study indicates that the hypovirus-encoded suppressor of RNA silencing p29 greatly influences both dcl2 and agl2 transcript accumulation in response to virus infection and that AGL2 also plays a regulatory role in the induction of the RNA silencing antiviral defense response. As reported earlier for dcl2 transcripts (15), agl2 transcripts accumulate to a much higher level (14-fold vs. 2-fold increase) in response to infection by a mutant hypovirus that lacks p29 than to wild-type CHV1-EP713 virus (Fig. 2). Moreover, the profiles of dcl2 and agl2 transcript accumulation in response to the Δp29 mutant virus were very similar to the profiles in response to hairpin dsRNA production (compare Figs. 2 and 3). We also note that the response to Δp29 infection was specific for the Dicer and Argonaute genes that are required for antiviral defense. Transcript levels for dcl1 (15) and agl1 (Fig. 2) were increased by less than 2-fold and transcripts for agl3 and agl4 were undetectable in Δp29 virus-infected mycelia. Finally, the similar transcript accumulation profiles observed for the native agl2 gene and the integrated agl2-promoter/EGFP-reporter constructs in response to the wild-type and Δp29 mutant hypoviruses (Fig. 5) indicate that the influence of p29 on agl2 expression is to a significant degree promoter-dependent. The combined results suggest a mechanism in which a viral RNA silencing suppressor represses the transcriptional induction of the RNA silencing pathway.
A regulatory role for the agl2 gene product in the activation of the RNA silencing antiviral defense pathway was exposed by the failure of dcl2 transcripts to accumulate in response to virus infection in the Δagl2 mutant strain (Fig. 2). This observation suggests that increased expression of agl2 precedes the increased expression of dcl2 following virus infection. In this regard, Choudhary et al. (32) reported that the activation of Argonaute gene qde-2 expression preceded the activation of dcl-2 expression following the induction of dsRNA production in N. crassa. It is conceivable that AGL2 may play an early role in sensing or transducing the signal arising from the sensing of viral dsRNA.
The absence of DI RNA production and the stability of hypovirus vectors expressing EGFP observed in the agl2 deletion mutant strains confirms that an intact RNA silencing pathway promotes viral RNA recombination. We previously postulated (17) that the RNA silencing pathway promotes viral RNA recombination by liberating 5′ and 3′ fragments of the viral RNA. Within the context of the classical copy-choice model for viral RNA recombination (described in 34), this would promote disengagement of the viral RNA-dependent-RNA-polymerase and nascent RNA strand from 3′-template fragments and provide 5′-fragments as substrate for template switching and RNA strand completion. Both DCL2 and AGL2 could contribute to fragmentation of viral RNA by dicing of structured RNA regions or vsRNA-guided slicing, respectively. However, a determination of the precise role that AGL2 may play in promoting viral RNA recombination and the relative contributions of DCL2 and AGL2 to this process is complicated by the fact that dcl2 expression is not activated in response to virus infection in the Δagl2 mutant strain (Fig. 2). Experiments are in progress to determine whether viral RNA recombination is reduced in the Δagl2 strain because of the absence of AGL2 or because of the low level of dcl2 expression. A precise understanding of the mechanisms by which RNA silencing promotes viral RNA recombination could lead to the design of more effective viral expression vectors and a clearer view of the emergence of new viruses.
Materials and Methods
Fungal Strains and Culture Conditions.
C. parasitica strains were maintained on potato dextrose agar (PDA; Difco) at 22–24 °C with a 12-h light-dark cycle at 1,300–1,600 l×. Fungal cultures used for nucleic acid and protein preparations were grown for 7 days under similar conditions on cellophane overlaying PDA (PDA-cellophane). Wild-type C. parasitica strain EP155 (ATCC 38755) and the isogenic strain infected with hypovirus CHV1-EP713, strain EP713 (ATCC 52571), have been described by Chen and Nuss (35) while RNA silencing mutants of strain EP155 containing disruption of Dicer genes dcl1 and dcl2 (Δdcl1 and Δdcl2, respectively) were generated in the study described by Segers et al. (13).
Nucleic Acid Preparation and Analysis.
C. parasitica genomic DNA was isolated as described in Sun et al. (31). Total RNA was extracted from virus-free and hypovirus infected C. parasitica strains as described by Parsley et al. (36) with the exception that 5 M LiCl was routinely used for precipitation to collect total host and viral single stranded RNAs as well as viral double stranded RNAs. Real-time RT-PCR analysis of transcript accumulation was performed at least twice, in triplicate for each transcript measured, with at least two independent RNA preparations. Transcript abundance was calculated by using the ΔCt method relative to the amount of internal control transcript with conditions as described previously by Parsley et al. (36).
Transformation and Viral RNA Transfection.
Preparation and transformation of C. parasitica spheroplasts were carried out essentially according to Churchill et al. (37). Hygromycin (40 μg/mL), benomyl (0.4 μg/mL), or G418 (20 μg/mL), depending on the selectable marker used, was included in the growth medium to provide selection of transformants. Hyphal anastomosis, sporulation assays, virulence assays, and statistical analysis were performed as described in Sun et al. (31).
Infection of fungal strains with hypovirus CHV1-EP713 and hypovirus vector Ctp40[2AEGFP] (33) was initiated by transfection of fungal spheroplasts with hypovirus transcripts generated in vitro from a viral cDNA clone using the protocol developed by Chen et al. (30). Transfected fungal strains were transferred to new PDA plates every 7 days.
Molecular Cloning and Gene Disruption.
Argonaute-like genes were identified from the C. paracitica draft genome sequence (http://genome.jgi-psf.org/Crypa1/Crypa1.home.html) produced by the Joint Genome Institute using the consensus sequences of PAZ and PIWI domains, as well as the sequences of the known fungal Argonaute proteins. Sequence data for C. parasitica agl1, agl2, agl3, and agl4 have been submitted to the GenBank with the accession numbers GQ250184, GQ250185, GQ250186, and GQ250187, respectively.
Homology-mediated gene disruption of agl1, agl2, agl3, and agl4 was performed as described by Sun et al. (31) with the exception that the hygromycin resistance gene cassette was used instead of the G418 resistance gene cassette.
Hairpin RNA Silencing and Promoter/Reporter Expression Studies.
Hairpin dsRNA-triggered silencing of enhanced green fluorescent protein (EGFP) expression was performed using the pGSN2GFP silencing vector plasmid to transform a C. parasitica strain expressing EGFP, cpEGFP (33). Plasmid pGSN2GFP is similar to the hairpin structure producing plasmid pGS-2GFP (described in ref. 16), except that it has a neomycin resistance gene cassette in place of the benomyl resistance cassette (31). Neomycin-resistant transformants were selected on G418-containing PDA plates and single spore isolates were obtained for each transformant and grown on PDA (20 mL/plate) for 5 days. Four mycelial plugs (5-mm diameter) were sampled with a cork borer from each actively growing fungal colony and placed, with the mycelium side facing up, in microtiter plate wells (Costar 3915 flat bottom black polystyrene 96-well plates). A BioTek FL 600 Multifunction Fluorescence and Absorbance Microplate Reader (BioTek Instruments, Inc.) was used to measure fluorescence with 485-nm excitation and 530-nm emission filters.
Construction of promoter/reporter transformation plasmids used the base plasmid pCPXHY1 (38), which contains the C. parasitica glyceraldehyde-3-phosphate dehydrogenase gene (gpd) promoter (1.6 kb) and terminator sequences. The hygromycin resistance gene cassette in pCPXHY1 was replaced with neomycin resistance gene cassette (31) and the EGFP coding sequence was cloned between the gpd promoter and terminator sequences resulting in the plasmid pCNE2GP. The gpd promoter sequence in the plasmid pCNE2GP was then replaced with agl2 promoter sequence (1,566 bp) to produce pA2PG. EP155 spheroplasts were transformed with pCNE2GP or pA2PG and transformants were selected on G418-containing PDA plates.
Supplementary Material
Acknowledgments.
This work was supported in part by Public Health Service Grants GM55981 and AI076219.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0907552106/DCSupplemental.
References
- 1.Fire A. RNA-triggered gene silencing. Trends Genet. 1999;15:358–363. doi: 10.1016/s0168-9525(99)01818-1. [DOI] [PubMed] [Google Scholar]
- 2.Aliyari R, Ding SW. RNA-based viral immunity initiated by the Dicer family of host immune receptors. Immunol Rev. 2009;227:176–188. doi: 10.1111/j.1600-065X.2008.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding SW, Voinnet O. Antiviral immunity directed by small RNAs. Cell. 2007;130:413–426. doi: 10.1016/j.cell.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hammond SM. Dicing and slicing: The core machinery of the RNA interference pathway. FEBS Lett. 2005;579:5822–5829. doi: 10.1016/j.febslet.2005.08.079. [DOI] [PubMed] [Google Scholar]
- 5.Xie Z, et al. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004;2:E104. doi: 10.1371/journal.pbio.0020104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blevins TR, et al. Four plant Dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucleic Acids Res. 2006;34:6233–6246. doi: 10.1093/nar/gkl886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moissiard G, Voinnet O. RNA silencing of host transcripts by cauliflower mosaic virus requires coordinated action of the four Arabidopsis Dicer-like proteins. Proc Natl Acad Sci USA. 2006;103:19593–19598. doi: 10.1073/pnas.0604627103. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Qu F, Ye X, Morris TJ. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc Natl Acad Sci USA. 2008;150:14732–14737. doi: 10.1073/pnas.0805760105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galiana-Arnoux D, Dostert C, Schneemann A, Hoffman JA, Imler JL. Essential function in vivo for Dicer-2 in host defense against RNA viruses in Drosophila. Nat Immunol. 2006;7:590–597. doi: 10.1038/ni1335. [DOI] [PubMed] [Google Scholar]
- 10.Wang XH, et al. RNA interference directs innate immunity against viruses in adult Drosophila. Science. 2006;312:452–454. doi: 10.1126/science.1125694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campbell CL, et al. Aedes aegypti uses RNA interference in defense against Sindbis virus infection. BMC Microl. 2008;8:47. doi: 10.1186/1471-2180-8-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YS, et al. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- 13.Segers GC, Zhang X, Deng F, Sun Q, Nuss DL. Evidence that RNA silencing functions as an antiviral defense mechanism in fungi. Proc Natl Acad Sci USA. 2007;104:12902–12906. doi: 10.1073/pnas.0702500104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammond TM, Andrewski MD, Roossinck MJ, Keller NP. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryotic Cell. 2008;7:350–357. doi: 10.1128/EC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang X, Segers GC, Sun Q, Deng F, Nuss DL. Characterization of hypovirus-derived small RNAs generated in the chestnut blight fungus by an inducible DCL-2-dependent pathway. J Virol. 2008;82:2613–2619. doi: 10.1128/JVI.02324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segers GC, van Wezel R, Zhang X, Hong Y, Nuss DL. Hypovirus papain-like protease p29 suppresses RNA silencing in the natural fungal host and in a heterologous plant system. Eukaryotic Cell. 2006;5:896–904. doi: 10.1128/EC.00373-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X, Nuss DL. A host dicer is required for defective viral RNA production and recombinant virus vector RNA instability for a positive sense RNA virus. Proc Natl Acad Sci USA. 2008;105:16749–16754. doi: 10.1073/pnas.0807225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li H, Li WX, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- 19.Van Rij RP, et al. The RNA silencing endonuclease Argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006;20:2985–2995. doi: 10.1101/gad.1482006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morel J-B, et al. Fertile hypomorphic Argonaute (ago1) mutants impaired in post-transcriptional gene silencing and virus resistance. Plant Cell. 2002;14:629–639. doi: 10.1105/tpc.010358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda A, Iwasaki S, Watanabe T, Utsumi M, Watanabe Y. The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol. 2008;49:493–500. doi: 10.1093/pcp/pcn043. [DOI] [PubMed] [Google Scholar]
- 22.Zambon RA, Vakharia VN, Wu LP. RNAi is an antiviral immune response against a dsRNA virus in Drosophila melanogaster. Cell Microbiol. 2006;8:880–889. doi: 10.1111/j.1462-5822.2006.00688.x. [DOI] [PubMed] [Google Scholar]
- 23.Keene KM, Foy BD, Sanchez-Vargas I, Beaty BJ, Blair CD, Olson KE. RNA interference acts as a natural antiviral response to O'nyong-nyong virus (Alphavirus; Togaviridae) infection of Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:17240–17245. doi: 10.1073/pnas.0406983101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakayashiki H, Kadotani N, Mayama S. Evolution and diversification of RNA silencing proteins in fungi. J Mol Evol. 2006;63:127–135. doi: 10.1007/s00239-005-0257-2. [DOI] [PubMed] [Google Scholar]
- 25.Hammond TM, Bok JW, Andrewski MD, Reyes-Dominguez Y, Scazzocchio C, Keller NP. RNA silencing gene truncation in the filamentous fungus Aspergillus nidulans. Eukaryotic Cell. 2008;7:339–349. doi: 10.1128/EC.00355-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Catalanotto C, et al. Redundancy of the two dicer genes in transgene-induced posttranscriptional gene silencing in Neurospora crassa. Mol Cell Biol. 2004;24:2536–2545. doi: 10.1128/MCB.24.6.2536-2545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Catalanotto C, Azzalin G, Macino G, Cogoni C. Gene silencing in worms and fungi. Nature. 2000;404:245. doi: 10.1038/35005169. [DOI] [PubMed] [Google Scholar]
- 28.Alexander WG, et al. DCL-1 co-localizes with other components of the MSUD machinery and is required for silencing. Fungal Genet Biol. 2008;45:719–727. doi: 10.1016/j.fgb.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 29.Lee DW, Pratt RJ, McLaughlin M, Aramayo R. An Argonaute-like protein is required for meiotic silencing. Genetics. 2003;164:821–828. doi: 10.1093/genetics/164.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen B, Choi GH, Nuss DL. Attenuation of fungal virulence by synthetic infectious hypovirus transcripts. Science. 1994;264:1762–1764. doi: 10.1126/science.8209256. [DOI] [PubMed] [Google Scholar]
- 31.Sun Q, Choi GH, Nuss DL. Hypovirus-responsive transcription factor gene pro1 of the Chestnut Blight Fungus Cryphonectria parasitica is required for female fertility, asexual spore development, and stable maintenance of hypovirus infection. Eukaryotic Cell. 2009;8:262–270. doi: 10.1128/EC.00338-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choudhary S, et al. A double-stranded-RNA response program important for RNA interference efficiency. Mol Cell Biol. 2007;27:3995–4005. doi: 10.1128/MCB.00186-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki N, Geletka LM, Nuss DL. Essential and dispensable virus-encoded replication elements revealed by efforts to develop hypoviruses as gene expression vectors. J Virol. 2000;74:7568–7577. doi: 10.1128/jvi.74.16.7568-7577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White KA, Morris TJ. Defective and defective interfering RNAs of monopartite plus-strand plant viruses. Curr Top Microbiol Immunol. 1999;239:1–17. doi: 10.1007/978-3-662-09796-0_1. [DOI] [PubMed] [Google Scholar]
- 35.Chen B, Nuss DL. Infectious cDNA clone of hypovirus CHV1-Euro7: A comparative virology approach to investigate virus-mediated hypovirulence of the chestnut blight fungus Cryphonectria parasitica. J Virol. 1999;73:985–992. doi: 10.1128/jvi.73.2.985-992.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parsley TB, Chen B, Geletka LM, Nuss DL. Differential modulation of cellular signaling pathways by mild and severe hypovirus strains. Eukaryotic Cell. 2002;1:401–413. doi: 10.1128/EC.1.3.401-413.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Churchill ACL, Ciufetti LM, Hansen DR, Van Etten HD, Van Alfen NK. Transformation of the fungal pathogen Cryphonectria parasitica with a variety of heterologous plasmids. Curr Genet. 1990;17:25–31. [Google Scholar]
- 38.Choi GH, Nuss DL. Hypovirulence of chestnut blight fungus conferred by an infectious viral cDNA. Science. 1992;257:800–803. doi: 10.1126/science.1496400. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.