Abstract
Degenerative retinopathies, including age-related macular degeneration, diabetic retinopathy, and hereditary retinal disorders—major causes of world blindness—are potentially treatable by using low-molecular weight neuroprotective, antiapoptotic, or antineovascular drugs. These agents are, however, not in current systemic use owing to, among other factors, their inability to passively diffuse across the microvasculature of the retina because of the presence of the inner blood–retina barrier (iBRB). Moreover, preclinical assessment of the efficacies of new formulations in the treatment of such conditions is similarly compromised. We describe here an experimental process for RNAi-mediated, size-selective, transient, and reversible modulation of the iBRB in mice to molecules up to 800 Da by suppression of transcripts encoding claudin-5, a protein component of the tight junctions of the inner retinal vasculature. MRI produced no evidence indicative of brain or retinal edema, and the process resulted in minimal disturbance of global transcriptional patterns analyzed in neuronal tissue. We show that visual function can be improved in IMPDH1−/− mice, a model of autosomal recessive retinitis pigmentosa, and that the rate of photoreceptor cell death can be reduced in a model of light-induced retinal degeneration by systemic drug delivery after reversible barrier opening. These findings provide a platform for high-throughput drug screening in models of retinal degeneration, and they ultimately could result in the development of a novel “humanized” approach to therapy for conditions with little or no current forms of treatment.
Keywords: blood–retina barrier, retinitis pigmentosa, RNAi, tight junctions, claudin-5
In transducing photons into electrical energy, the retina has the highest oxygen consumption per weight of any tissue in the human body (1). This, combined with a delicacy characteristic of all neuronal tissues, underlines a requirement for a unique and abundant blood supply capable of sustaining the retina's energy demands while at the same time possessing efficient barriers, excluding potentially harmful bloodborne agents such as pathogens, antibodies, immune cells, and anaphylatoxins (2). There are two sources of blood supply to the retina: the central retinal artery, entering the retina at the optic disc, the arterioles of which terminate in both the inner plexiform layer and outer plexiform layer (OPL) and are readily visualized in images of the retinal surface, and the underlying choroidal circulation, providing nutriment primarily to the outermost layer of the retina, the photoreceptors (3). This outer layer of the retina remains avascular. The retina has two highly evolved and efficient barriers that maintain retinal homeostasis: the inner and outer blood–retina barriers (iBRB and oBRB, respectively). The iBRB comprises retinal endothelial cells, which line retinal microvessels, allowing for the maintenance of blood vessel integrity, preserving vessel homeostasis and mediating highly selective transport of molecules to the surrounding neural tissue (4). The oBRB is made up of a single layer of retinal pigment epithelial (RPE) cells situated at the back of the eye that act as a selective filter to restrict the passage of macromolecules to the outer segments of the retina (5). It is now widely accepted that up to 98% of low-molecular weight drugs cannot passively diffuse across these barriers (6), in part because of the highly evolved “tight junctions” (TJs) formed between adjacent endothelial cells or RPE cells.
TJs are formed at the apical periphery of endothelial cells of the iBRB and RPE of the oBRB, and they perform the dual role of creating a primary barrier to the diffusion of solutes through the paracellular pathway while also maintaining cell polarity (7). TJs are complex and dynamic structures, composed of a series of integral and peripheral membrane proteins. The transmembrane proteins of the tight junction include occludin, junctional adhesion molecule, and claudins 1–24, proteins which extend into the paracellular space, creating the seal characteristic of the TJ (8). There are differences, however, in the molecular components of TJs of oBRBs and iBRBs, and in the context of the current study it is of note that claudin-5 is absent from the TJs of the oBRB in adult mice (9). Claudins have been shown previously to interact homotypically with proteins on adjacent endothelial cells (10) and, in particular, claudin-5 knockout mice have altered blood–brain barrier (BBB) integrity (11). Although removal of claudin-5 compromised BBB function by allowing it to become permeable to molecules of up to ≈800 Da, the barrier could still form, remaining intact and impervious to larger molecules, with fully formed TJs. These mice did, however, die within 10 h of birth, most likely because of the importance of claudin-5 in developmental processes. We have reported recently that levels of claudin-5 can be suppressed in adult mice by using an RNAi approach. Transient suppression of claudin-5 in the microvasculature of the brain causes a size-selective increase in passive diffusion of low-molecular weight molecules from the blood to the brain without any outward adverse detriment to such mice. Indeed, potentially pathological molecules far exceed the size-selectivity of barrier opening when claudin-5 is suppressed (12). Moreover, endogenous bloodborne molecules below 800 Da will be excluded from the brain and retina because of the presence of efflux transporters and specific receptors on the luminal surface of endothelial cells lining the microvasculature of the brain and retina (13).
Here, we report a preclinical and experimental platform technology allowing delivery of low-molecular weight therapeutics to the outer layers of the retina while excluding molecules greater than ≈1 kDa. RNAi-mediated suppression of claudin-5 in the retinas of adult mice causes a transient and size-selective increase in paracellular permeability of microvessels without the development of retinal edema or changes in retinal function as assayed by electrophysiology. Moreover, we demonstrate the therapeutic potential of this technique by enhancing the delivery of two distinct low-molecular weight molecules to the neural retina of two rodent models of retinopathy: IMPDH1−/− mice (14), a model of autosomal recessive retinitis pigmentosa (RP) that lacks the key enzyme involved in the de novo synthesis of GTP (15), and a mouse model of light-induced retinal degeneration (16). These results highlight the safety and efficacy of selective iBRB modulation as an experimental tool for the systemic screening of a wide range of potentially therapeutic molecules in rodent models of retinopathies. Ultimately, a humanized approach involving localized inducible barrier opening could offer the potential to treat conditions where no therapeutic interventions are available, representing a unique example of combining gene therapy with a pharmacological approach to disease treatment.
Results
Suppression of Claudin-5 in the Retina.
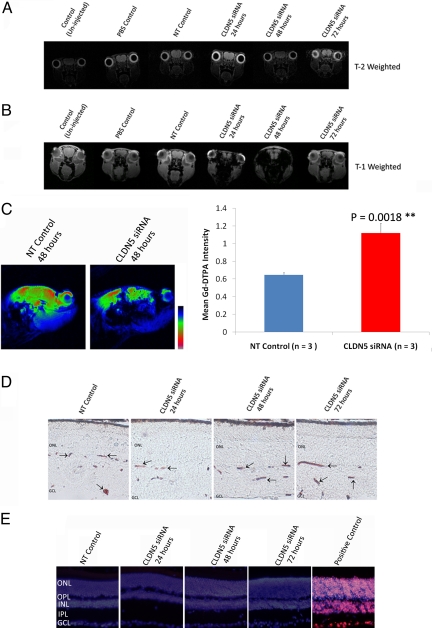
Levels of expression of claudin-5 were significantly decreased 48 h after injection of siRNA targeting claudin-5 compared with the uninjected control, PBS control, and nontargeting (NT) control siRNA-injected mice. Suppression was transient, with levels returning to those observed in the control groups after 72 h (Fig. 1A and Figs. S1–S3). After retinal flatmount analysis of claudin-5 levels after delivery of siRNA, claudin-5 localization in retinal microvessels was manifested as a discontinuous pattern of staining 24 and 48 h after injection of claudin-5 siRNA compared with the control groups (Fig. 1B). These findings were in contrast to the continuous and intense pattern of staining of claudin-5 in the control groups and, indeed, at the 72 h time point after injection of siRNA, levels of claudin-5 in the retina appeared similar to the control groups, highlighting the transient nature of suppression. Although by Western blot analysis, levels of expression of claudin-5 appeared unchanged 24 h after siRNA injection, the pattern of staining in retinal flatmounts was not evident. Therefore, protein expression by Western blot at 24 h after claudin-5 siRNA injection more than likely represents immunoreactive yet non-TJ-associated claudin-5. Immunohistochemical analysis of retinal cryosections showed claudin-5 expression in vessels up to and including the OPL, with levels decreased and mislocalized 24 and 48 h after injection of claudin-5 siRNA (Fig. 1C). Discontinuous and decreased expression of claudin-5 was not evident 72 h after injection. After suppression of claudin-5, electroretinographic (ERG) analysis of retinal function in mice 48 h after siRNA injection showed normal electrophysiological readouts in rod-isolated responses, with average readings of 841 μV in each eye for four mice compared with typical ERG readouts from adult C57/Bl6 mice that had not been exposed to experimental conditions (Fig. 1D).
Fig. 1.
Suppression of claudin-5 in retinal vasculature. (A) Western blot analysis of claudin-5 levels in the neural retinas of mice at 24, 48, and 72 h after injection of siRNA showed a significant decrease in levels 48 h after siRNA injection. **, P = 0.0011. Western blots are representative of three independent experiments. (B) Flatmount analysis of claudin-5 levels in the microvasculature of the neural retina showed decreased levels of expression and aberrant localization of claudin-5. At 24 and 48 h after injection of siRNA, the pattern of claudin-5 staining appeared diffuse and fragmented compared with the control groups. (40× objective.) (C) Claudin-5 expression was isolated to the retinal microvessels, processing as far as the OPL of the retina. At 24 and 48 h after injection of claudin-5 siRNA, levels of claudin-5 appeared mislocalized and fragmented. (40× objective.) (D) ERG analysis of adult C57/Bl6 mice revealed no distinct changes in ERG tracings 48 h after injection of claudin-5 siRNA, with mean ERG readings of 841 μV in each eye from four mice (Upper). This finding was in comparison to standard mean ERG readings of between 800 μV an 1 mV in uninjected mice (Lower).
Transient and Size-Selective iBRB Modulation.
To assess the integrity of the iBRB after suppression of claudin-5, we used two distinct molecules that do not normally passively diffuse across the iBRB. Initially, by using MRI analysis, we observed no distinct changes in T2-weighted images (Fig. 2A), which would readily allow for imaging of ocular edema. T2-weighted imaging revealed no aberrancies in the ocular regions of mice from any experimental group. Moreover, when the contrasting agent gadolinium-diethylene triamine N,N,N′,N′,N′-pentaacetic acid (Gd-DTPA; 742 Da) was injected in mice, we observed large amounts of extravasation from microvessels in the retina. T1-weighted imaging after Gd-DTPA injection showed distinct contrasting in both eyes of mice 24 and 48 h after injection of claudin-5 siRNA (Fig. 2B). Such contrasting was not observed in the control groups of mice, nor was it observed at the 72-h time point after injection of claudin-5 siRNA. Gd-DTPA has a molecular mass of 742 Da and will not freely passively diffuse across microvessels into the retina in homeostatic circumstances. In a sagittal perspective, levels of Gd-DTPA were observed to infiltrate the retina in large quantities at the 48-h time point after siRNA injection compared with NT siRNA control mouse, with densitometric analysis of mice showing significantly increased levels of contrasting agent in the eyes of mice 48 h after injection of claudin-5 siRNA (P = 0.0018; Fig. 2C, double asterisk).
Fig. 2.
Transient and size-selective modulation of iBRB function. (A) MRI with T2-weighted sequence (resolution, 0.141 × 0.141 × 5 μm3; repetition and echo times (TR/TE, 4179.3/36 ms; alpha = 9°), which will allow for edema visualization, showed no signs of ocular edema in any experimental group of mice analyzed (three to five mice per group). All images are in a coronal orientation. (B) After injection of the contrast agent Gd-DTPA (742 Da), however, intense contrasting was observed both 24 and 48 h after injection of claudin-5 siRNA when observed with T1-weighted imaging (resolution, 0.156 × 0.156 × 5 mm3; field of view, 20 × 20 × 17.9 mm3; matrix = 128 × 128 × 30; TR/TE, 500/2.7 ms; flip angle, 30°; number of averages, 3; acquisition time, 2 min 24 sec; repetitions, 10). This contrasting manifested as intense dark contrasting in the ocular region of the mouse. All images are in a coronal orientation. (C) After analysis of mice in a sagittal orientation, it was clear that extravasation of Gd-DTPA was occurring in large quantities. Enhanced signals, pseudocolored in red and green, showed highly significant levels of Gd-DTPA in mice 48 h after injection of claudin-5 siRNA compared with NT control mice. **, P = 0.0018. (D) Postexperimental treatments. Mice were perfused at 24, 48, and 72 h after injection with 5 μL/g body weight of 25 mg/mL microperoxidase. Microperoxidase was subsequently visualized (dark brown/red staining indicated by arrows) in retinal cryosections to be localized within the microvessels of all experimental groups (n = 5 mice per group). GCL indicates ganglion cell layer. (40× objective.) (E) Levels of retinal cell death were assayed by TUNEL staining (red staining in positive control). None of the experimental groups showed signs of retinal cell death at any time point after injection of siRNA. Nuclei were counterstained with DAPI. INL indicates inner nuclear layer; IPL, inner plexiform layer. (40× objective.)
The size-selective nature of transient modulation of the iBRB was readily observable when a molecule of ≈1,900 Da was perfused in mice after clauidn-5 siRNA injection. Microperoxidase (molecular mass, 1,881 Da) was detected in the microvessels of the retinas of all mice after all experimental treatments. In conditions where the iBRB or BBB is damaged, microperoxidase will extravasate in vast quantities, yet this was not the case when claudin-5 was suppressed (Fig. 2D). Size-selective barrier opening observed, as well as being a transient process, did not appear to cause any neuronal cell death in any layer of the retina of mice after analysis of DNA fragmentation in retinal layers after siRNA delivery (Fig. 2E).
Enhanced Delivery of GTP to the Retinas of IMPDH1−/− Mice.
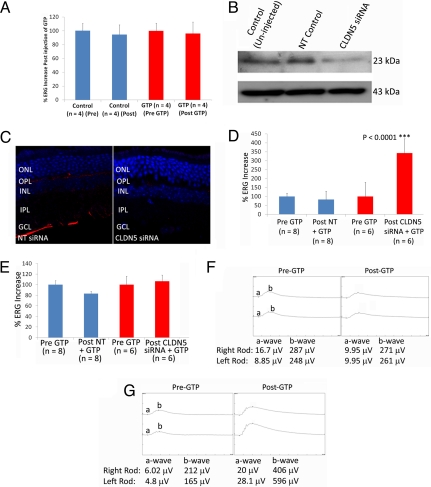
Given our observations of maximum suppression of claudin-5 levels 48 h after injection of siRNA, this was chosen as the optimum time point at which to assess efficacy of therapeutic systemic delivery of compounds to the retina. IMPDH1−/− mice lack the rate-limiting enzyme for the de novo synthesis of GTP, a central mediator of visual phototransduction. With increasing age, these mice develop a mild retinopathy, as evidenced by decreases in electrophysiological readout from the retina in tandem with a thinning of the photoreceptor outer nuclear layer (ONL) in animals older than 10 months (14). GTP has a molecular mass of 523 Da and will not freely passively diffuse across the iBRB if injected systemically. Indeed, we have previously attempted to inject GTP intraocularly to induce increases in ERG in IMPDH1−/− mice (n = 4), but without success (Fig. 3A). At 48 h after injection of siRNA, levels of claudin-5 were significantly decreased in IMPDH1−/− mice (Fig. 3B). Suppression was manifested by a distinct change in the pattern of staining observed in retinal cryosections compared with mice receiving NT siRNA (Fig. 3C). Before experimentation, retinal function of all IMPDH1−/− mice was assessed by ERG, and 48 h before injection of GTP, mice were injected with either an NT or claudin-5-targeting siRNA. Subsequently, after injection of GTP i.p., retinal function was assessed immediately by ERG, and a highly significant increase in rod-isolated b-wave amplitude was observed in those mice that had received claudin-5 siRNA (n = 6) compared with the NT control (n = 8) group (Fig. 3D). Analysis of cone-isolated ERG after enhanced delivery of GTP did not show any significant differences between the experimental groups (Fig. 3E). ERG tracings revealed well-formed a and b waves in mice after enhanced systemic delivery of GTP to the retina (Fig. 3G), in contrast to mice receiving no iBRB modulation (Fig. 3F), where no change in ERG output was observed.
Fig. 3.
Enhanced delivery of GTP to retinas of IMPDH1−/− mice. (A) After analysis of ERG readout in IMPDH1−/− mice, animals were injected with a 3-μL solution containing 0.6 mg of GTP. Mice were again analyzed by ERG, and the percentage change in electrical readout from the retina was plotted. There was no significant difference in ERG readouts in IMPDH1−/− mice after intraocular injection of GTP (n = 4 mice per group). (B) Levels of expression of claudin-5 in the retinas of IMPDH1−/− mice were assessed by Western blotting, with levels decreased 48 h after injection of claudin-5 siRNA. (C) From immunohistochemical analysis of claudin-5 levels (red staining) in retinal cryosections, it was clear that levels were decreased in all retinal layers. Sections were counterstained with DAPI (blue staining). (40× objective.) (D) IMPDH1−/− mice were subjected to ERG analysis of rod function, and 48 h after injection of either an NT or claudin-5 siRNA, mice were administered an injection of GTP, and ERG readout was assessed again. Electrical readout of rod photoreceptors was expressed as percentage changes, and a significant increase (***, P < 0.0001) in rod-isolated ERG was observed in mice receiving claudin-5 siRNA and 3.3 mg of GTP compared with mice receiving NT siRNA and 3.3 mg of GTP. (E) No change in retinal cone function was observed after injection of GTP in either experimental group. (F) Rod-isolated ERG tracings in IMPDH1−/− mice before and after injection of GTP with NT siRNA showed no distinct changes in waveform; however, upon analysis of ERG tracings before and after GTP injection with claudin-5 siRNA, well-formed a and b waves were observed in the retinas of mice.
Enhanced Delivery of ALLM to the Retinas of Light-Exposed BalB/c Mice.
Light-induced retinopathy in albino BalB/c mice has been used previously to induce photoreceptor cell apoptosis. Indeed, many antiapoptotic agents have been screened in vivo by using this technique but by direct intraocular injection of these agents before light ablation (17). Ca2+ activated calpains have been shown to be associated with neuronal cell death in different neuropathological diseases, including retinopathy (18). Calpains are often associated with necrotic cell death; however, they are also found to be activated during apoptosis (19). We chose to assay the effects of enhanced systemic delivery of a known calpain inhibitor, N-acetyl-Leu-Leu-Met-CHO (ALLM). This molecule has a molecular mass of 401 Da and is a potent inhibitor of both calpain I (Ki = 120 nM), calpain II (Ki = 230 nM), cathepsin-L (Ki = 0.6 nM), cathepsin-B (Ki = 100 nM), and the proteasome (20). It does not readily passively diffuse across the iBRB and has been used previously to demonstrate efficacy in preventing photoreceptor apoptosis when intraocularly injected (17). Forty-eight hours before injection of ALLM (20 mg/kg), mice received an injection of either an NT siRNA or a claudin-5 siRNA. Mice were dark-adapted for a further 24 h after injection of ALLM, after which time they were exposed to 6,000-lux white light for 2 h. Mice were returned to the dark room, and 12, 24, and 48 h after light ablation, photoreceptor nuclei were analyzed for TUNEL for identification of end-stage dying cells. With enhanced systemic delivery of ALLM, at 12 h (P = 0.0011; Fig. 4A, **) and 24 h (P = 0.0252; Fig. 4B, *) after light exposure, the retinas of BalB/c mice showed significantly fewer TUNEL-positive cells in the ONL of the retina, directly implying less photoreceptor cell death. ALLM slows the rate of cell death, because 48 h after light exposure (Fig. 4C), the number of TUNEL-positive cells increased in the group of mice receiving ALLM subsequent to claudin-5 siRNA (*, P = 0.0191). Mice analyzed at 4 days and 7 days after light ablation showed no differences in TUNEL-positive cells in the ONL (Fig. S4).
Fig. 4.
Enhanced delivery of a calpain inhibitor to retinas of light-exposed BalB/c mice. Effects of either an NT or claudin-5 siRNA together with a potent calpain inhibitor, ALLM, on light-induced retinal degeneration were assessed in BalB/c mice. Claudin-5 or NT siRNA was administered to BALB/c mice 48 h before injection of 20 mg/kg ALLM. Mice were dark-adapted for 24 h before being exposed to white fluorescent light of 7,900 lux for 2 h. (A) At 12 h after light ablation, TUNEL-positive nuclei present in the ONL of retinal sections of mice injected with claudin-5 siRNA and ALLM were significantly fewer than those injected with NT siRNA. **, P = 0.0011. (B) At 24 h after light ablation, the number of TUNEL-positive cells in mice that received claudin-5 siRNA and ALLM was significantly decreased compared with mice receiving NT siRNA and ALLM. *, P = 0.0252. (C) There were significantly more TUNEL-positive nuclei in retinas of claudin-5 siRNA- and ALLM-injected mice compared with NT siRNA- and ALLM-injected mice at 48 h after light exposure. *, P = 0.0191. (40× objective.)
Discussion
Although degenerative retinopathies, including age-related macular degeneration (AMD), diabetic retinopathy, and RP represent major causes of world blindness, treatment options are currently exceedingly limited (15, 21–24). No form of prevention is available for the nonexudative (dry) form of AMD, apart from dietary intervention. With regard to the exudative (wet) form of AMD, intraocular injection of the monoclonal antibody Lucentis (Genentech Inc), targeting VEGF, is being used in a growing number of cases; however, severe endophthalmitis, resulting in blindness, has been reported to occur in up to 2% of treated patients (25). Proliferative diabetic retinopathy is treatable by scatter and focal laser surgery, but the procedure itself induces significant retinal damage and can involve in excess of 1,000 laser burns to the retinas of diseased patients (26). No form of preventive therapy is yet available for RP, and the immense genetic heterogeneity encountered in this condition is clearly a substantial impediment to the development of gene-based methods of disease prevention (27). On the other hand, many low-molecular weight, potentially therapeutic compounds, including VEGF (28), GPR91 (29), and C3a/C5a anaphylatoxin receptor antagonists (30), as well as many neuroprotective and antiapoptotic drugs, could potentially preserve retinal function or slow photoreceptor cell death in such conditions, yet regular intraocular injection of such compounds is not a feasible strategy for the reasons outlined above. Moreover, systemic administration of such drugs is restricted in view of the very low rates of passive diffusion of these compounds into the retina across the iBRB (31).
We report an experimental, RNAi-mediated approach for transient modulation of iBRB function by targeting the TJ component claudin-5. Claudin-5 plays a role in the formation of paracellular pores or channels that function in mediating selective ion permeability (11, 32). However, the function of claudin-5 in the retina appears to be more dynamic than simply structural. It has recently been reported that mice exposed to hypoxic conditions exhibit down-regulation of claudin-5, which size-selectively disrupts the iBRB, allowing passage of small molecules into the retina, similar to the phenotype seen in claudin-5-deficient mice (33). Moreover, levels of claudin-5 in the brain appear to fluctuate depending on environmental stimuli, such as exposure to alcohol, traumatic brain injury, and stroke (34–36).
To explore the therapeutic potential of transient compromise in barrier integrity, we sought to deliver therapeutic molecules to the retinas of two mouse models of retinopathy. First, we chose a model of autosomal recessive RP, the IMPDH1−/− mouse (14). These animals lack one of the two rate-limiting enzymes of de novo synthesis of GTP, a central mediator of visual phototransduction (15). With increasing age, these mice develop a mild retinopathy, as evidenced by decreases in electrophysiological readout from the retina in tandem with a thinning of the photoreceptor ONL in animals older than 10 months. GTP has a molecular mass of 523 Da, and direct intraocular injection (Fig. 3A) did not have an impact on ERG function in these mice because of a low rate of diffusion of the compound across the iBRB and limited diffusion across intact cell layers of the inner retina. However, when levels of claudin-5 were suppressed, enhanced systemic delivery of GTP to the outer layers of the retina resulted in a significant increase in the rod-isolated ERG response from IMPDH1−/− mice in comparison with those receiving NT siRNA compared with a pre-GTP ERG. ERG readings of up to 600 μV were observed (Fig. 3G). Taking into consideration that a standard ERG readout is between 800 and 1,000 μV, these findings represent significant advances in visual enhancement in these mice. GTP is likely being actively transported into photoreceptors after enhanced systemic delivery; however, it is also feasible to postulate that GTP may passively diffuse across the plasma membranes of photoreceptors, and enhanced delivery will simply allow for increased quantities of GTP to access the neural retina before being rapidly hydrolyzed.
Second, we explored the efficacy of enhanced delivery of an antiapoptotic agent to the retinas of mice before light-induced ablation of photoreceptors. Albino BalB/c mice exposed to varying degrees of intense white light are very susceptible to photoreceptor cell death, due in part to activation of calpains in photoreceptors of the retina (37). To study the effects of increased delivery of ALLM on photoreceptor cell survival, we injected 20 mg/kg of the inhibitor intraperitoneal 24 h before light exposure with and without iBRB modulation. TUNEL staining became apparent 12 h after the initial exposure to light; however, significantly less cell death was observed in the group of mice that received ALLM when the iBRB was modulated with claudin-5 siRNA compared with NT siRNA. ALLM has a molecular mass of 401 Da and will have a low rate of diffusion across the iBRB. Indeed, photoreceptor cell death did not manifest in the claudin-5 siRNA-injected mice until 48 h after light exposure. These findings display enhanced delivery of ALLM to the retina and also highlight the influence increased delivery of this calpain inhibitor has on the rate of photoreceptor cell death.
As a method of enhanced delivery of low-molecular weight therapeutics to the retina, RNAi-mediated suppression of claudin-5 can now be used in animal models of retinopathy to screen a wide range of potentially therapeutic molecules ranging from antiapoptotic agents to antiangiogenic compounds. Not only does suppression of claudin-5 have little impact on neuronal homeostasis, we have also shown minimal disturbance of global transcriptional patterns in neuronal tissue when the BBB is transiently modulated (Tables S1 and S2). This indicates that rather than “breaking” the BBB and iBRB, targeted suppression of claudin-5 selectively modulates barrier function while not causing neuroinflammatory activation, edema, or cell death. Indeed, coupled with commercially available reagents, such as in vivo-jetPEI (Polyplus-transfection), regular barrier opening in experimental rodents could be used for longer-term studies pertaining to drug delivery. This, however, would not be the case in “humanizing” this delivery system, and for the purposes of systemic delivery of drugs targeting degenerative retinal conditions in humans, localized barrier opening could, in principle, be limited to retinal tissues by incorporating barrier-opening machinery into an inducible viral delivery system. In this regard, it is of note that adeno-associated virus (AAV) delivery systems have now been approved for use in a growing number of gene therapy trials, including those recently initiated for the congenital retinopathy Leber's congenital amaurosis (38–40), and recent studies have indicated that AAV9 is capable of targeting vascular endothelial cells, rendering the concept of an inducible AAV9 system for barrier opening potentially feasible (41).
Materials and Methods
Animal Experiments and Experimental Groups.
All studies carried out in the Ocular Genetics Unit at Trinity College Dublin (TCD) adhere to the Association for Research in Vision and Ophthalmology statement for the use of Animals in Ophthalmic and Vision Research. Mice were sourced from Jackson Laboratories and bred on site at the Ocular Genetics Unit in TCD. Extensive Materials and Methods are outlined in SI Text.
In Vivo Delivery of siRNA to Retinal Capillary Endothelial Cells.
Rapid high-pressure, high-volume tail vein injections were carried out essentially as successfully used previously at this laboratory (12, 42, 43).
Antibodies for Western blots were as follows: polyclonal rabbit anti-claudin-5 (1:500; Zymed Laboratories) and polyclonal rabbit anti-β-actin (1:1,000; Abcam). Membranes were washed with Tris buffered saline (0.05 M Tris, 150 mM NaCl, pH 7.5) and incubated with a secondary anti-rabbit (IgG) antibody with HRP conjugates (1:2,500; Sigma–Aldrich), or with anti-mouse (IgG; 1:1,000; Sigma–Aldrich), for 3 h at room temperature. Immune complexes were detected by using ECL. All Western blot analyses were repeated a minimum of three times (n = 3 mice per treatment).
ERG Analysis of C57/Bl6 and IMPDH1−/− Mice.
Extensive details are outlined in SI Text.
Assessment of iBRB Integrity by Perfusion of Tracer Molecules.
After RNAi-mediated ablation of transcripts encoding claudin-5, microperoxidase [5 μL/g body weight of 25 mg/mL microperoxidase (MP-11; 1,862 Da; Sigma–Aldrich] was perfused at 37 °C through the left ventricle of the beating heart of anesthetized mice 24, 48, and 72 h after hydrodynamic delivery of claudin-5 siRNA.
MRI.
After injection of siRNA and by using appropriate controls, iBRB integrity to a molecule of 742 Da (Gd-DTPA) was assessed via MRI using a dedicated small rodent Bruker BioSpec 70/30 magnet system (i.e., 7-T field strength, 30-cm bore diameter) with an actively shielded USR magnet.
TUNEL Analysis.
Data after TUNEL staining were expressed as mean TUNEL-positive cells per section ± SEM, as determined by an individual blinded to the treatment. Results were analyzed by using a two-tailed Student's t test, with P < 0.05 considered significant.
Supplementary Material
Acknowledgments.
We thank Caroline Woods, David Flynn, and Rebecca Robertson for animal husbandry. IMPDH1−/− mice were kindly donated by Dr. Beverly Mitchell. The Ocular Genetics Unit at TCD is supported by Science Foundation Ireland, The Wellcome Trust, European Vision Institute, EVI-Genoret Grant LSHG-CT-2005-512036, Fighting Blindness Ireland, and Enterprise Ireland.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0908561106/DCSupplemental.
References
- 1.Masland RH. The fundamental plan of the retina. Nat Neurosci. 2001;4:877–886. doi: 10.1038/nn0901-877. [DOI] [PubMed] [Google Scholar]
- 2.Gardiner TA, Archer DB, Curtis TM, Stitt AW. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: Implications for pathogenesis. Microcirculation. 2007;14:25–38. doi: 10.1080/10739680601072123. [DOI] [PubMed] [Google Scholar]
- 3.Antonetti DA, Lieth E, Barber AJ, Gardner TW. Molecular mechanisms of vascular permeability in diabetic retinopathy. Semin Ophthalmol. 1999;14:240–248. doi: 10.3109/08820539909069543. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, et al. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: Vascular endothelial growth factor decreases occludin in retinal endothelial cells. Diabetes. 1998;47:1953–1959. doi: 10.2337/diabetes.47.12.1953. [DOI] [PubMed] [Google Scholar]
- 5.Rizzolo LJ, Chen X, Weitzman M, Sun R, Zhang H. Analysis of the RPE transcriptome reveals dynamic changes during the development of the outer blood-retinal barrier. Mol Vis. 2007;23:1259–1273. [PubMed] [Google Scholar]
- 6.Pardridge WM. Molecular biology of the blood-brain barrier. Mol Biotechnol. 2005;30:57–70. doi: 10.1385/MB:30:1:057. [DOI] [PubMed] [Google Scholar]
- 7.Campbell M, et al. Involvement of MAPKs in endostatin-mediated regulation of blood-retinal barrier function. Curr Eye Res. 2006;31:1033–1045. doi: 10.1080/02713680601013025. [DOI] [PubMed] [Google Scholar]
- 8.Fanning AS, Anderson JM. PDZ domains and the formation of protein networks at the plasma membrane. Curr Top Microbiol Immunol. 1998;228:209–233. doi: 10.1007/978-3-642-80481-6_9. [DOI] [PubMed] [Google Scholar]
- 9.Kojima S, Rahner C, Peng S, Rizzolo LJ. Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol. 2002;186:81–88. doi: 10.1007/s00232-001-0137-7. [DOI] [PubMed] [Google Scholar]
- 10.Piontek J, et al. Formation of tight junction: Determinants of homophilic interaction between classic claudins. FASEB J. 2008;22:146–158. doi: 10.1096/fj.07-8319com. [DOI] [PubMed] [Google Scholar]
- 11.Nitta T, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol. 2003;161:653–660. doi: 10.1083/jcb.200302070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campbell M, et al. RNAi-mediated reversible opening of the blood-brain barrier. J Gene Med. 2008;10:930–947. doi: 10.1002/jgm.1211. [DOI] [PubMed] [Google Scholar]
- 13.Pardridge WM. Molecular Trojan horses for blood-brain barrier drug delivery. Curr Opin Pharmacol. 2006;6:494–500. doi: 10.1016/j.coph.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Aherne A, et al. On the molecular pathology of neurodegeneration in IMPDH1-based retinitis pigmentosa. Hum Mol Genet. 2004;13:641–650. doi: 10.1093/hmg/ddh061. [DOI] [PubMed] [Google Scholar]
- 15.Tam LC, et al. Therapeutic benefit derived from RNAi-mediated ablation of IMPDH1 transcripts in a murine model of autosomal dominant retinitis pigmentosa (RP10) Hum Mol Genet. 2008;17:2084–2100. doi: 10.1093/hmg/ddn107. [DOI] [PubMed] [Google Scholar]
- 16.Joly S, Samardzija M, Wenzel A, Thiersch M, Grimm C. Nonessential role of beta3 and beta5 integrin subunits for efficient clearance of cellular debris after light-induced photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2009;50:1423–1432. doi: 10.1167/iovs.08-2432. [DOI] [PubMed] [Google Scholar]
- 17.Sanges D, Comitato A, Tammaro R, Marigo V. Apoptosis in retinal degeneration involves cross-talk between apoptosis-inducing factor (AIF) and caspase-12 and is blocked by calpain inhibitors. Proc Natl Acad Sci USA. 2006;103:17366–17371. doi: 10.1073/pnas.0606276103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perche O, Doly M, Ranchon-Cole I. Caspase-dependent apoptosis in light-induced retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:2753–2759. doi: 10.1167/iovs.06-1258. [DOI] [PubMed] [Google Scholar]
- 19.Paquet-Durand F, Johnson L, Ekström P. Calpain activity in retinal degeneration. J Neurosci Res. 2007;85:693–702. doi: 10.1002/jnr.21151. [DOI] [PubMed] [Google Scholar]
- 20.Sasaki T, et al. Inhibitory effect of di-and tripeptidyl aldehydes on calpains and cathepsins. J Enzyme Inhib. 1990;3:195–201. doi: 10.3109/14756369009035837. [DOI] [PubMed] [Google Scholar]
- 21.Nussenblatt RB, Ferris F., III Age-related macular degeneration and the immune response: Implications for therapy. Am J Ophthalmol. 2007;144:618–626. doi: 10.1016/j.ajo.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhatwadekar AD, et al. Advanced glycation of fibronectin impairs vascular repair by endothelial progenitor cells: Implications for vasodegeneration in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49:1232–1241. doi: 10.1167/iovs.07-1015. [DOI] [PubMed] [Google Scholar]
- 23.Chadderton N, et al. Improved retinal function in a mouse model of dominant retinitis pigmentosa following AAV-delivered gene therapy. Mol Ther. 2009;17:593–599. doi: 10.1038/mt.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Reilly M, et al. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet. 2007;81:127–135. doi: 10.1086/519025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montezuma SR, Sobrin L, Seddon JM. Review of genetics in age related macular degeneration. Semin Ophthalmol. 2007;22:229–240. doi: 10.1080/08820530701745140. [DOI] [PubMed] [Google Scholar]
- 26.Fong DS, Girach A, Boney A. Visual side effects of successful scatter laser photocoagulation surgery for proliferative diabetic retinopathy: A literature review. Retina. 2007;27:816–824. doi: 10.1097/IAE.0b013e318042d32c. [DOI] [PubMed] [Google Scholar]
- 27.Humphries P, Kenna P, Farrar GJ. On the molecular genetics of retinitis pigmentosa. Science. 1992;256:804–808. doi: 10.1126/science.1589761. [DOI] [PubMed] [Google Scholar]
- 28.De Boüard S, et al. Antiangiogenic and anti-invasive effects of sunitinib on experimental human glioblastoma. Neuro Oncol. 2007;9:412–423. doi: 10.1215/15228517-2007-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sapieha P, et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat Med. 2008;14:1067–1076. doi: 10.1038/nm.1873. [DOI] [PubMed] [Google Scholar]
- 30.Edwards AO, Malek G. Molecular genetics of AMD and current animal models. Angiogenesis. 2007;10:119–132. doi: 10.1007/s10456-007-9064-2. [DOI] [PubMed] [Google Scholar]
- 31.Brankin B, Campbell M, Canning P, Gardiner TA, Stitt AW. Endostatin modulates VEGF-mediated barrier dysfunction in the retinal microvascular endothelium. Exp Eye Res. 2005;81:22–31. doi: 10.1016/j.exer.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Matter K, Balda MS. Holey barrier: Claudins and the regulation of brain endothelial permeability. J Cell Biol. 2003;161:459–460. doi: 10.1083/jcb.200304039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koto T, et al. Hypoxia disrupts the barrier function of neural blood vessels through changes in the expression of claudin-5 in endothelial cells. Am J Pathol. 2007;170:1389–1397. doi: 10.2353/ajpath.2007.060693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haorah J, et al. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res. 2005;29:999–1009. doi: 10.1097/01.alc.0000166944.79914.0a. [DOI] [PubMed] [Google Scholar]
- 35.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood-brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 36.McColl BW, Rothwell NJ, Allan SM. Systemic inflammation alters the kinetics of cerebrovascular tight junction disruption after experimental stroke in mice. J Neurosci. 2008;28:9451–9462. doi: 10.1523/JNEUROSCI.2674-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Donovan M, Cotter TG. Caspase-independent photoreceptor apoptosis in vivo and differential expression of apoptotic protease activating factor-1 and caspase-3 during retinal development. Cell Death Differ. 2002;9:1220–1231. doi: 10.1038/sj.cdd.4401105. [DOI] [PubMed] [Google Scholar]
- 38.Maguire AM, et al. Safety and efficacy of gene transfer for Leber's congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bainbridge JW, et al. Effect of gene therapy on visual function in Leber's congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 40.Hauswirth WW, et al. Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: Short-term results of a phase I trial. Hum Gene Ther. 2008;19:979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foust KD, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds A, et al. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- 43.Kiang AS, et al. Toward a gene therapy for dominant disease: Validation of an RNA interference-based mutation-independent approach. Mol Ther. 2005;12:555–561. doi: 10.1016/j.ymthe.2005.03.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.