Abstract
Molecular mechanisms that regulate in situ activation of ryanodine receptors (RY) in different cells are poorly understood. Here we demonstrate that caffeine (10 mM) released Ca2+ from the endoplasmic reticulum (ER) in the form of small spikes in only 14% of cultured fura-2 loaded beta cells from ob/ob mice. Surprisingly, when forskolin, an activator of adenylyl cyclase was present, caffeine induced larger Ca2+ spikes in as many as 60% of the cells. Forskolin or the phosphodiesterase-resistant PKA activator Sp-cAMPS alone did not release Ca2+ from ER. 4-Chloro-3-ethylphenol (4-CEP), an agent that activates RYs in other cell systems, released Ca2+ from ER, giving rise to a slow and small increase in [Ca2+]i in beta cells. Prior exposure of cells to forskolin or caffeine (5 mM) qualitatively altered Ca2+ release by 4-CEP, giving rise to Ca2+ spikes. In glucose-stimulated beta cells forskolin induced Ca2+ spikes that were enhanced by 3,9-dimethylxanthine, an activator of RYs. Analysis of RNA from islets and insulin-secreting βTC-3-cells by RNase protection assay, using type-specific RY probes, revealed low-level expression of mRNA for the type 2 isoform of the receptor (RY2). We conclude that in situ activation of RY2 in beta cells requires cAMP-dependent phosphorylation, a process that recruits the receptor in a functionally operative form.
Keywords: Islets of Langerhans/caffeine/calcium
Ryanodine receptors (RY) are Ca2+ channels in the endoplasmic reticulum (ER) composed of four ≈550-kDa RY protomers and four molecules of FKBP12 or FKBP12.6. The latter are isoforms of the 12-kDa binding protein for the immunosuppressant drug FK506. cDNAs for three RYs have been cloned. Ryanodine RY1, RY2, and RY3 receptors are products of three genes (1). Truncated and splice variants of RYs have been described, all of which might associate in the form of homo- or heterotetramers to produce large diversity of the channels. FKBP12 and FKBP12.6 associate with RY1 and RY2, respectively, and stabilize the channel (2). Studies in cell-free systems have demonstrated that activity of RYs can be modulated by numerous factors including phosphorylation–dephosphorylation, Ca2+, Mg2+, and cyclic ADP ribose (3, 4). However, it is less clear how activity of RYs is modulated in their native environment in the intact cells.
High expression of RYs typically occurs in a few electrically excitable cells, e.g., striated muscles and some neurons. In most cells, the level of expression of RYs is low, often making their detection by conventional methods difficult (1). The existence and roles of RYs in beta cells have remained controversial. Some investigators claim crucial importance of RYs in Ca2+ signaling in beta cells (5–7), whereas others favor the idea of total absence of them (8). One difficulty in resolving the controversy derives from difficulty in obtaining a large number of pure beta cells, which are nested in the islet with many other cells. For this reason most studies have attempted to obtain indirect information on the presence of RYs, by measuring cytoplasmic-free [Ca2+] ([Ca2+]i) in single beta cells and relying on agents such as caffeine, ryanodine, sulfhydryl agents, and cyclic ADP ribose (9–11). Development of ligands capable of discriminating between the isoforms of RYs so far has been met with limited success (12, 13). Instead, sensitive molecular biological techniques are being used for identification of RY types in different tissues (14).
Caffeine remains the most useful tool for activating RYs. Unfortunately, the effects of caffeine on [Ca2+]i in beta cells are complex. When applied to resting beta cells, high concentrations of caffeine (e.g., 10–50 mM) increase [Ca2+]i by inhibiting K+ATP channels and thereby depolarizing the plasma membrane and allowing Ca2+ entry through voltage-gated Ca2+ channels (10). On the other hand, when applied to beta cells whose [Ca2+]i is first raised by depolarization, caffeine reduces [Ca2+]i by inhibiting the L-type voltage-gated Ca2+ channels. In contrast to these, a consistent Ca2+-releasing effect of caffeine in beta cells has been difficult to demonstrate (10, 11, 15). These observations prompted us to find out the conditions that could ensure more consistent activation of RYs by caffeine. We demonstrate that in intact beta cells, cAMP-dependent phosphorylation is an important requirement for achieving activation of RYs by pharmacological probes as well as by physiological stimuli. Using a sensitive RNase protection assay and type-specific probes, we demonstrate that the RY isoform involved in this process in beta cells is RY2.
MATERIALS AND METHODS
Materials.
4-chloro-3-ethylphenol was from Aldrich. Sp-cAMPS was from Biolog, Bremen, Germany. 3,9-Dimethylxanthine was from Fluka. Fura-2 AM and Indo-1 were from Molecular Probes Europe. Ryanodine (≈98% pure) was from Calbiochem. β-Alanyl ryanodine was a gift from Henry R. Besch, Jr. (Indiana University).
Measurements of [Ca2+]i in Beta Cells.
Islets from ob/ob mice were isolated and cells were cultured on glass coverslips for 24–48 hr in RPMI 1640 medium, supplemented with 10% fetal bovine serum (10). Coverslips were incubated in RPMI 1640 with 0.1% BSA and 0.6 μM fura-2 AM for 35 min at 37°C. Cells were then incubated for a further 10 min in the basal medium containing 125 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 1.28 mM CaCl2, 25 mM Hepes, 3 mM glucose, and 0.1% BSA (pH 7.4). The coverslip was mounted in a chamber on an inverted microscope. The temperature inside the chamber was 37°C. The microscope was connected to a SPEX fluorolog-2 CM1T11I system. The excitation wavelengths were generated by two monochromators, and the emitted light, selected by a 510-nm filter, was monitored by a photomultiplier. Cells were excited at 1 Hz, and the length of time for data collection at each point was 0.33 s. The emissions at the excitation wavelengths of 340 nm (F340) and 380 nm (F380) were used to calculate the fluorescence ratio (R340/380). Single cells or clusters of two to three cells, isolated optically, were studied by a ×40, oil-immersion lens. Rmax and Rmin were determined by using thin films of external standards containing fura-2 and 2 M sucrose (16). The Kd for Ca2+-fura-2 was taken as 224 nM.
Preparation of Low-Ca2+ Medium.
Low-Ca2+ medium was prepared by adding EGTA to the basal medium. The concentration of EGTA required was determined by using a computer program (17). Actual free [Ca2+] was checked by Ca2+ electrode (Orion, Boston) and calibration buffers (World Precision Instruments, Sarasota, FL).
Measurements of [Ca2+]i in Skeletal Muscle Cells.
Fibers dissected from the flexor brevis muscle of the hind limb of NMRI mice were mounted between a force transducer and a holder at a length giving maximum tetanic force. Fibers were superfused by Tyrode solution (18). Indo-1 was injected into single fibers and [Ca2+]i measured as described (18). The excitation light was 360 ± 5 nm, and light emitted at 405 ± 5 and 510 ± 5 nm was measured. The ratio of the signal at 405 nm to that at 510 nm was converted to [Ca2+]i by using an intracellularly established calibration curve (18). The Kd for indo-1 was 373 nM.
Identification of Ryanodine Receptor mRNA.
RNA was isolated from ob/ob mouse islets, βTC-3 cells, mouse skeletal muscle, heart, and brain. Poly(A)+ RNA was selected by using the Poly(A)Quick mRNA Isolation Kit (Stratagene).
cDNAs for mRNA analysis.
Mouse RY1, RY2, and RY3 cDNA subclones were used (14). Subclone MB9-Eco Sty/XhoI contained 840 bp of the 3′ coding region of RY1 cDNA. Subclone MB3B contained 1,500 bp of the 3′ coding region of RY2 cDNA. MTII11 contained 2,000 bp of 3′ coding region of RY3 cDNA. MB9-Eco Sty/XhoI was linearized with AvaII and transcribed with T7 RNA polymerase (Boehringer Mannheim) in the presence of [α-32P]rUTP, to obtain a 350-bp antisense RY1 probe. MB3B was linearized with StyI and transcribed with T7 polymerase to obtain a 300-bp antisense RY2 probe. MTII11 was linearized with BglII and transcribed with T3 RNA polymerase to obtain a 420-bp RY3 probe. The probes were purified on a 4% polyacrylamide, 7 M urea gel.
RNase protection assay.
RNA was analyzed by RNase protection assay using the RPAII kit (Ambion, Austin, TX). Usually, 1–60 μg of the RNA was hybridized to 1 × 105 cpm of the RNA probe. The hybrids were subjected to digestion with 8 units RNase T1 and 0.2 Kunitz units RNase A and separated on a 6% polyacrylamide, 7 M urea sequencing gel. The gel was transferred to filter paper, dried, and exposed to screens for phosphor imaging. The resulting bands were analyzed and quantified by the PhosphorImager SF (Molecular Dynamics) and imagequant software.
RESULTS
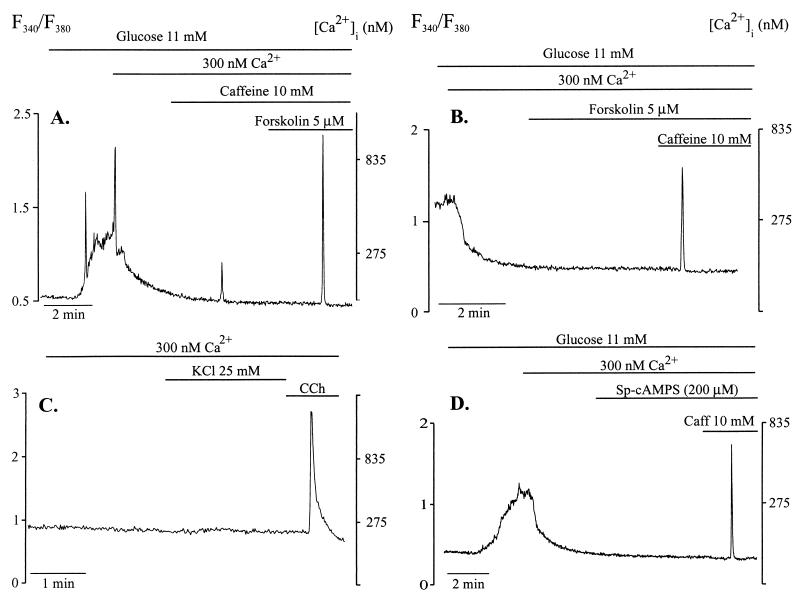
A previous protocol for detecting release of Ca2+ by caffeine from ER in beta cells resulted in a low success rate (10). In the present study we used a different protocol, arbitrarily designed to increase the probability and magnitude of Ca2+ release by the xanthine drug. First, we stimulated the cells with 11 mM glucose, in the presence of basal medium containing 1.28 mM Ca2+. When the glucose-induced increase in [Ca2+]i reached its peak, the medium was changed to a low-Ca2+ medium. The [Ca2+] of this medium, as measured by Ca2+-selective electrodes, was ≈300 nM. When [Ca2+]i returned to basal level, we added 10 mM caffeine, which induced a Ca2+ spike. We then added 5 μM forskolin in the continued presence of caffeine. As illustrated in Fig. 1A, this maneuver resulted in a Ca2+ spike much larger than that obtained when caffeine was used alone. Forskolin alone did not increase [Ca2+]i in any of the 15 cells examined (Fig. 1B). The increase in [Ca2+]i induced by caffeine could not be due to Ca2+ entry from the extracellular space, because depolarization by KCl, in the presence of 300 nM Cao2+, did not increase [Ca2+]i (Fig. 1C). Under similar conditions, Sp-cAMPS (200 μM), a phosphodiesterase-resistant membrane-permeable cAMP analogue that activates PKA, also did not increase [Ca2+]i (Fig. 1D). However, as illustrated in Fig. 1D, Sp-cAMPS also permitted caffeine to produce Ca2+ spikes.
Figure 1.
Caffeine-induced Ca2+ release from ER is enhanced by forskolin. Cells were perifused with basal medium containing 1.28 mM Cao2+ and stimulated with glucose (11 mM). At the time indicated, cells were perifused with a 300-nM Cao2+ medium. (A) Caffeine (10 mM) alone induced a small Ca2+ spike. In the presence of forskolin (5 μM), caffeine induced a much larger Ca2+ spike. The trace is representative of experiments repeated twice with same result. (B) Forskolin alone did not increase [Ca2+]i in any of 15 cells. (C) In the presence of 300 nM Cao2+, depolarization by KCl did not affect [Ca2+]i but carbachol increased [Ca2+]i. (D) Sp-cAMPS (200 μM), a phosphodiesterase-resistant cAMP analogue, did not change [Ca2+]i by itself in any of six cells tested, but permitted a caffeine-induced-Ca2+ spike.
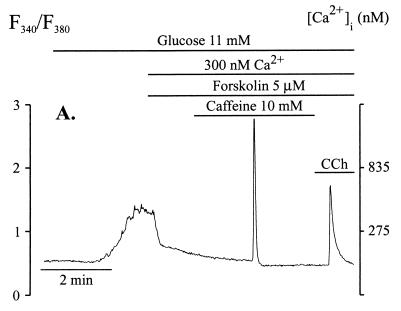
Examination of a large number of cells revealed that when 10 mM caffeine was used alone, a Ca2+ spike was obtained in only 14% of cells examined (n = 21). This value increased to ≈60% (n = 21) when forskolin was added before the addition of caffeine (Fig. 2). The interval between the arrival of caffeine to the chamber and the occurrence of the Ca2+ spike varied between cells and could be as long as 5 min (median 33 s, n = 11). In the presence of forskolin, increase in [Ca2+]i during the caffeine-induced spike was 825 nM ± 135 nM (n = 10). The spikes were consistent in shape with half-height width of 4.3 ± 0.3 s (n = 10). As shown in Fig. 2, after release of Ca2+ by forskolin and caffeine, addition of carbachol induced further Ca2+ release, indicating that caffeine released Ca2+ from only part of the ER Ca2+ pools and not from the Ins(1,4,5)P3-sensitive pool (Fig. 2).
Figure 2.
In forskolin-treated beta cells, caffeine released Ca2+ from an Ins(1,4,5)P3-insensitive pool. Conditions of experiments were as in the legend to Fig. 1. Forskolin (5 μM) was added before the addition of caffeine. Caffeine (10 mM), in the continued presence of forskolin, induced Ca2+ spikes in 13 of 21 cells examined. Carbachol (10 μM) released further Ca2+ from the Ins(1,4,5)P3-sensitive pool.
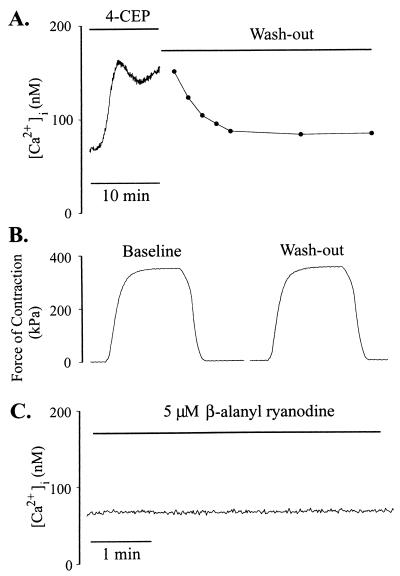
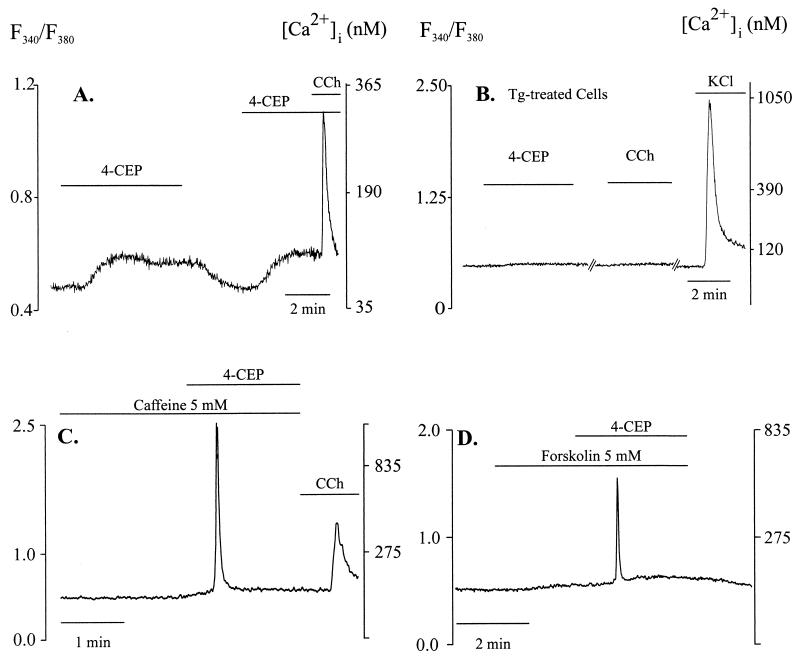
Because the existence of RYs in beta cells has been a matter of controversy and caffeine has pleiotrophic effects (10), we used another activator of RY, namely, 4-chloro-3-ethylphenol (4-CEP) (19). Using skeletal muscle, a cell type in which expression of RYs is well defined, we examined whether 4-CEP can activate RYs in situ and at resting conditions. In muscle cells injected with Indo-1, 4-CEP (200 μM) increased [Ca2+]i, which reached a plateau in ≈3 min (Fig. 3A) and reversed on washout. To test whether this phenolic compound might damage cells, we measured the force of contractions of fibers on electrical stimulation before and after 8 min of exposure to 4-CEP. As shown in Fig. 3B, the forces of contraction under the two conditions were equal. β-Alanyl ryanodine, an activator-selective analog of ryanodine (20), does not increase [Ca2+]i in beta cells (11). We tested whether this ryanodine analog could increase [Ca2+]i in muscle cells. As shown in Fig. 3C, in contrast to marked Ca2+ release by 4-CEP, application of 5 μM β-alanyl ryanodine did not increase [Ca2+]i in muscle cells. In beta cells 4-CEP increased [Ca2+]i dose-dependently, with minimum concentration needed for a rise in [Ca2+]i being ≈100 μM. Clear and consistent increase was obtained with 500 μM 4-CEP, and the substance was used at this concentration in subsequent experiments (Fig. 4A). [Ca2+]i began to rise immediately on arrival of the compound to the chamber and increased from 65 ± 6 nM (n = 15) to 102 ± 5 nM with a t1/2 of 22 ± 4 s. The magnitude of [Ca2+]i increase ranged from 11 to 66 nM (36 ± 3 nM, n = 15). [Ca2+]i remained elevated during continued presence of 4-CEP, but completely reversed on washout (Fig. 4A). Addition of carbachol after 4-CEP-induced increase in [Ca2+]i elicited further Ca2+ release (Fig. 4A), indicating that 4-CEP did not empty the Ins(1,4,5)P3-sensitive pool. Ca2+ release by 4-CEP was observed even when extracellular Ca2+ (Cao2+) was buffered to ≈300 nM (data not shown). In Fig. 4B, ER Ca2+ pools were depleted by treating the cells with 250 nM thapsigargin for 35 min. In these cells 4-CEP or carbachol did not increase [Ca2+]i, whereas membrane depolarization by KCl did.
Figure 3.
Effect of 4-CEP on [Ca2+]i and contraction in skeletal muscle cells. [Ca2+]i was measured as described in Materials and Methods. (A) 4-CEP induced an increase in [Ca2+]i that reversed on washout. (B) Tetanic contraction was produced by giving a train (300 ms) of supramaximal current pulses (0.5 ms) at 100 Hz. The forces of contractions before addition of 4-CEP and after 8 min of exposure to the substance and washout were equal in magnitude. (C) β-Alanyl ryanodine (10 μM) did not increase [Ca2+]i. The traces are representative of three to four experiments.
Figure 4.
4-CEP releases Ca2+ from ER in beta cells. [Ca2+]i was measured in the presence of 1.28 mM Cao2+. (A) 4-CEP (500 μM) induced a gradual increase in [Ca2+]i. Carbachol (CCh) added in the continued presence of 4-CEP released further Ca2+. Similar results were obtained in all of 16 experiments. (B) Intracellular Ca2+ pools were depleted by treating the cells with 250 nM thapsigargin for 35 min. Under these conditions, neither 4-CEP nor carbachol increased [Ca2+]i, but depolarization with KCl did. The trace is representative of three experiments. (C) Caffeine (5 mM) was present in the perifusion medium. 4-CEP (500 μM), added in the presence of caffeine, induced Ca2+ spikes in three of five cells. In the absence of caffeine, 4-CEP did not induce a Ca2+ spike in any of the 16 cells examined (c.f. A). (D) When added in the presence of forskolin, 4-CEP induced Ca2+ spikes in 7 of 13 cells. 4-CEP alone did not induce a Ca2+ spike in any of the 16 cells tested.
Because caffeine sensitizes RYs, we tested whether treatment of cells by a low concentration of caffeine could affect Ca2+ release by 4-CEP. In experiments illustrated in Fig. 4C, 5 mM caffeine was present in the incubation and perfusion media. 4-CEP, applied in the presence of caffeine, induced large Ca2+ spikes in three of five cells examined. In the absence of caffeine, although 4-CEP promoted a minor increase in [Ca2+]i, it did not induce Ca2+ spikes in any of the 16 cells examined. To test whether cAMP-dependent phosphorylation could affect Ca2+ release by 4-CEP, we tested the effect of forskolin on the release of the cation by 4-CEP. In forskolin-treated cells, 4-CEP induced a Ca2+ spike in 7 of 13 cells (Fig. 4D). In the absence of forskolin, 4-CEP did not induce a Ca2+ spike in any of the 16 cells examined. To test whether ryanodine could inhibit Ca2+ release by 4-CEP, we treated cells with 300 μM ryanodine for 45 min. However, such treatment did not cause significant inhibition of Ca2+ release by 4-CEP (Δ[Ca2+]i = 38 ± 3 nM in controls and 28 ± 4 nM in the ryanodine-treated group; n = 11, P = 0.16).
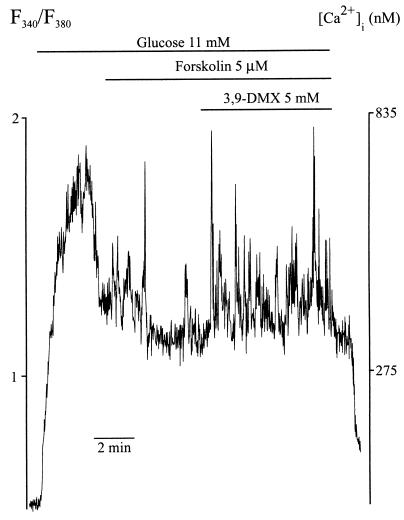
We then tested whether a RY might be involved in a glucose-induced increase in [Ca2+]i. As shown in Fig. 5, addition of forskolin to beta cells whose [Ca2+]i was first elevated by glucose stimulation resulted in the appearance of a few Ca2+ spikes. To test whether a RY might be involved in generation of some of these spikes, we used 3,9-dimethylxanthine (3,9-DMX). This xanthine derivative, like caffeine, is an activator of RY (21) and produces Ca2+ spikes when used in protocols like that of Fig. 1 (data not shown). However, unlike caffeine, 9-substituted methylxanthines do not inhibit cAMP phosphodiesterases (ref. 22 and our unpublished results). Another reason for not using caffeine in these experiments was that it inhibits plasma membrane Ca2+ channels (10). As shown in Fig. 5, forskolin-induced Ca2+ spikes were enhanced by 5 mM 3,9-DMX in all of the cells examined, suggesting involvement of RYs in the generation of such spikes. Under similar conditions but in the absence of forskolin, 3,9-DMX induced diminutive and low-frequency spikes in a smaller proportion of cells.
Figure 5.
Involvement of RYs in the generation of Ca2+ spikes in glucose-stimulated beta cells. [Ca2+]i was measured in fura-2 loaded cells perifused by the basal medium. Addition of forskolin (5 μM) to glucose-stimulated beta cells induced a few Ca2+ spikes. Forskolin-induced Ca2+-spikes were further enhanced by 3,9-DMX in all of the eight cells examined.
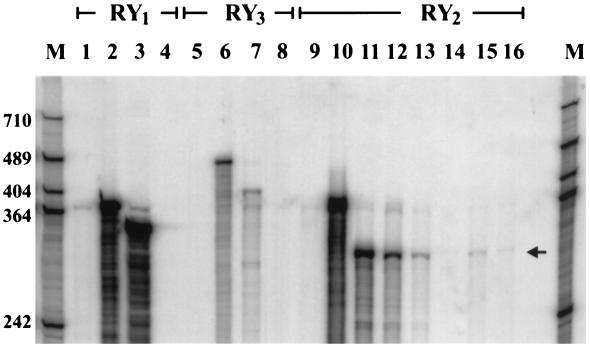
To detect the isoforms of RYs that are expressed in beta cells, we analyzed RNA obtained from ob/ob islets, where ≈95% of the endocrine cells are beta cells. Because expression of RYs in beta cells might be low, we used RNase protection assay instead of Northern blot analysis to obtain greater sensitivity. We used radiolabeled cRNA fragments of mouse RY1, RY2, and RY3 as probes that could identify regions demonstrating a high degree of divergence between the isoforms. The lengths of the protected RY1–3 cRNA fragments were demonstrated by incubating the respective probes with RNA from mouse skeletal muscle (RY1, 350 bp, Fig. 6, lane 3), heart (RY2, 300 bp, lanes 11 and 12), and brain (RY3, 420 bp, lane 7), respectively. The RY2 cRNA probe was protected by mRNA (lane 13) and total RNA (lane 14) from islets. The length of the protected segment was shorter than the undigested probe and equal to the length of the segment protected by RNA from heart (lanes 11 and 12). We considered the possibility that cells other than beta cells in the islet might be the source of RY2 mRNA. To exclude this, we examined RNA from an insulin-secreting cell line, βTC-3. As shown in Fig. 6, lanes 15 and 16, positive signals with appropriate lengths also were obtained with RY2 probe and βTC-3 RNA. We also examined RNA from βTC-3 cells with RY1 and RY3 probes, but no convincing signals were detected. These results suggest the presence of RY2 or RY2-like transcripts in beta cells. The bands, however, were faint and the estimated amount of transcript in islets was at least 1,000-fold less, compared with the amount per microgram of total RNA detected in heart.
Figure 6.
Detection of mRNA of RYs in beta cells by RNase protection assay. Radiolabeled cRNA probes of RY1 (lanes 1–4), RY3 (lanes 5–8), and RY2 (lanes 9–15) were incubated with RNA, digested with RNase, and separated on a 6% polyacrylamide gel containing 7 M urea. RY1: lane 1, digested probe without sample RNA; lane 2, full-length probe without RNase treatment; lane 3, 5 μg total RNA from skeletal muscle; lane 4, 60 μg total RNA from βTC-3 cells. RY3: lane 5, digested probe without sample RNA; lane 6, full-length probe without RNase treatment; lane 7, 30 μg total RNA from brain; lane 8, 60 μg total RNA from βTC-3 cells. RY2: lane 9, digested probe without sample RNA; lane 10, full-length probe without RNase treatment; lanes 11 and 12, 5 and 1 μg total RNA from heart, respectively; lane 13, 15 μg mRNA from islets; lane 14, 60 μg total RNA from islets; lane 15, 15 μg mRNA from βTC-3 cells; lane 16, 60 μg total RNA from βTC-3 cells. Molecular weight (in bases) of protected fragment was determined by pBluescript(SK−) digested with HpaII and labeled by fill-in reaction using Klenow enzyme and [α-32P]dCTP. The figure represents experiments repeated three times.
DISCUSSION
Previous studies, using experimental conditions adequate for detecting depolarization- and Ins(1,4,5)P3-induced increases in [Ca2+]i, failed to obtain Ca2+ release by caffeine in beta cells (10, 11, 23). We suspected that the protocols used in those studies were not adequate to achieve the conditions for in situ activation of RYs. Through preliminary experiments we worked out a new protocol, which when used in this study yielded Ca2+ release by caffeine. The current protocol, which uses prior stimulation of the cells by glucose, is likely to improve cellular energy status and ensure a higher degree of filling of the Ca2+ stores, thus promoting RY-mediated Ca2+ release. However, even under these conditions, Ca2+ release by caffeine was observed in only a small proportion of cells. The most significant finding of the present study was that prior application of the adenylyl cyclase activator forskolin dramatically increased the number of cells responding to caffeine and the magnitude of Ca2+ release induced by the xanthine drug. Such Ca2+ release could not be an effect of cAMP through direct or phosphorylation-mediated gating of ER Ca2+ channels. This is evident because elevation of the cellular cAMP level, as would occur on exposure to forskolin alone, did not release Ca2+, an observation consistent with previous studies (24). Similarly, high concentrations of Sp-cAMPS, a phosphodiesterase-resistant analogue of cAMP that activates PKA, also did not release Ca2+ by itself but mimicked the effect of forskolin by permitting caffeine to produce Ca2+ spikes.
4-Chloro-m-cresol is a new tool for activating RYs (19). We demonstrate that 4-CEP, a lipophilic analogue of 4-chloro-m-cresol, induced small but consistent Ca2+ release from ER in beta cells. 4-CEP-induced Ca2+ release was enhanced by caffeine, indicating that the release was mediated by a RY. However, 4-CEP-induced Ca2+ release was not inhibited significantly by ryanodine, an observation consistent with a previous report (25). This may be because of the difficulty in delivering ryanodine into attached cells and in achieving the conditions for ryanodine binding in intact cells. This view is supported by the lack of effect of β-alanyl ryanodine in our skeletal muscle experiments (Fig. 3C). Conditions that favor ryanodine binding in cell-free systems, e.g., high ionic activity and low [Mg2+], cannot be obtained in living cells (3). It is noteworthy that as with caffeine-induced Ca2+ release, 4-CEP-induced Ca2+ release also was enhanced by forskolin, giving rise to Ca2+ spikes.
Ca2+ release through the RYs and requirement of cAMP-dependent phosphorylation in this process was demonstrated even under conditions of physiological stimulation of beta cells by glucose. Thus once [Ca2+]i was raised by glucose, addition of forskolin alone induced Ca2+ spikes. Apparently forskolin-induced phosphorylation of RYs made them available for activation by Ca2+, the endogenous agonist of RY2. That RYs were involved in this process was further supported by the observation that in glucose-stimulated cells, forskolin-induced Ca2+ spikes were enhanced by 3,9-DMX. This methyl xanthine, like caffeine, is an activator of RYs but, in contrast to the latter 3,9-DMX, does not inhibit Ca2+ entry through the plasma membrane Ca2+ channels, does not depolarize the plasma membrane, and probably does not inhibit cAMP-phosphodiesterases (10, 21, 22). Our RNase protection assay detected low-level expression of a RY2-like transcript in beta cells. As alluded to earlier, in many other cells the level of expression of RY mRNAs also is low, usually requiring reverse transcription–PCR amplification for their detection (26). The physiological significance of RY2-mediated Ca2+ release in beta cells is not fully clear. However, despite their low level these channels may play a role in complex Ca2+ signaling in the beta cells by virtue of their high conductance or strategic intracellular location.
cAMP-dependent phosphorylation has been shown to modulate activity of RY2 in cell-free systems (27). Here we demonstrate that RY2 is not readily activated by caffeine in the live beta cells. But once RY2 or intermediate protein(s) involved in the regulation of the channel is phosphorylated by PKA, Ca2+ release occurs in the form of spikes. Such spikes are likely to represent synchronous activation of multiple RY2. It appears that in the absence of PKA phosphorylation, RY2 is maintained in an inactive state probably via the inhibitory effect of intracellular Mg2+ (27). PKA-mediated phosphorylation apparently enables RY2 to overcome this inhibition, thus effectively recruiting the receptors in a form that can be readily activated. Some of the controversial results on RYs in beta cells reported so far may be accounted for by the differences in the state of phosphorylation of the receptor (or its regulatory proteins) under different experimental conditions. The implications of these findings can be extended to other cells where RY2 is expressed at a low level. Thus, experimental protocols that cannot ensure cAMP-mediated phosphorylation of RY2 are less likely to obtain Ca2+ release by caffeine.
Acknowledgments
Financial support was obtained from the Swedish Medical Research Council (03X-09890, 19X-00034), Juvenile Diabetes Foundation Int., Nordic Insulin Foundation Committee, Swedish Diabetes Association, The Swedish Society of Medicine, National Board of Health and Welfare, Funds of the Karolinska Institute, Telethon, and European Economic Community (to V.S.). F.H.A. was supported by the Baylor College of Medicine–Karolinska Institute research exchange program. M.S.I. has a position at the Swedish Medical Research Council.
ABBREVIATIONS
- [Ca2+]i
cytoplasmic-free [Ca2+]
- Cao2+
extracellular Ca2+
- 4-CEP
4-chloro-3-ethylphenol
- RY1
RY2, and RY3, type 1, 2, and 3 ryanodine receptor
- 3
9-DMX, 3,9-dimethylxanthine
- ER
endoplasmic reticulum
References
- 1.Sorrentino V. Adv Pharmacol. 1995;33:67–90. doi: 10.1016/s1054-3589(08)60666-3. [DOI] [PubMed] [Google Scholar]
- 2.Brillantes A M B, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella M C, Jayaraman T, Landers M, Ehrlich B E, Marks A R. Cell. 1994;77:513–523. doi: 10.1016/0092-8674(94)90214-3. [DOI] [PubMed] [Google Scholar]
- 3.Ogawa Y. Crit Rev Biochem Mol Biol. 1994;29:229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- 4.Lee H C. Physiol Rev. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]
- 5.Islam M S. Ph.D. thesis. Sweden: Karolinska Institute; 1994. [Google Scholar]
- 6.Takasawa S, Nata K, Yonekura H, Okamoto H. Science. 1993;259:370–373. doi: 10.1126/science.8420005. [DOI] [PubMed] [Google Scholar]
- 7.Gromada J, Dissing S, Bokvist K, Renström E, Frøkjær-Jensen J, Wulff B S, Rorsman P. Diabetes. 1995;44:767–774. doi: 10.2337/diab.44.7.767. [DOI] [PubMed] [Google Scholar]
- 8.Rutter G A, Theler J-M, Li G, Wollheim C B. Cell Calcium. 1994;16:71–80. doi: 10.1016/0143-4160(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 9.Islam M S, Larsson O, Berggren P O. Science. 1993;262:584–585. doi: 10.1126/science.8211188. [DOI] [PubMed] [Google Scholar]
- 10.Islam M S, Larsson O, Nilsson T, Berggren P O. Biochem J. 1995;306:679–686. doi: 10.1042/bj3060679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Islam M S, Kindmark H, Larsson O, Berggren P O. Biochem J. 1997;321:347–354. doi: 10.1042/bj3210347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.El-Hayek R, Lokuta A J, Arevalo C, Valdivia H H. J Biol Chem. 1995;270:28696–28704. doi: 10.1074/jbc.270.48.28696. [DOI] [PubMed] [Google Scholar]
- 13.Wong P W, Pessah I N. Mol Pharmacol. 1996;49:740–751. [PubMed] [Google Scholar]
- 14.Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V. J Cell Biol. 1995;128:893–904. doi: 10.1083/jcb.128.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willmott N J, Galione A, Smith P A. Cell Calcium. 1995;18:411–419. doi: 10.1016/0143-4160(95)90056-x. [DOI] [PubMed] [Google Scholar]
- 16.Poenie M. Cell Calcium. 1990;11:85–91. doi: 10.1016/0143-4160(90)90062-y. [DOI] [PubMed] [Google Scholar]
- 17.Föhr K J, Warchol W, Gratzl M. Methods Enzymol. 1993;221:149–157. doi: 10.1016/0076-6879(93)21014-y. [DOI] [PubMed] [Google Scholar]
- 18.Westerblad H, Allen D G. J Physiol. 1993;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- 19.Zorzato F, Scutari E, Tegazzin V, Clementi E, Treves S. Mol Pharmacol. 1993;44:1192–1201. [PubMed] [Google Scholar]
- 20.Humerickhouse R A, Bidasee K R, Gerzon K, Emmick J T, Kwon S, Sutko J L, Ruest L, Besch H R., Jr J Biol Chem. 1994;269:30243–30253. [PubMed] [Google Scholar]
- 21.Rousseau E, Ladine J, Liu Q Y, Meissner G. Arch Biochem Biophys. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 22.Beavo J A, Rogers N L, Crofford O B, Hardman J G, Sutherland E W, Newman E V. Mol Pharmacol. 1970;6:597–603. [PubMed] [Google Scholar]
- 23.Lund P E, Gylfe E. Naunyn Schmiedebergs Arch Pharmacol. 1994;349:503–509. doi: 10.1007/BF00169140. [DOI] [PubMed] [Google Scholar]
- 24.Fournier L, Whitfield J F, Schwartz J L, Begin-Heick N. J Biol Chem. 1994;269:1120–1124. [PubMed] [Google Scholar]
- 25.Li G D, Wollheim C B, Pralong W F. Cell Calcium. 1996;19:535–546. doi: 10.1016/s0143-4160(96)90063-9. [DOI] [PubMed] [Google Scholar]
- 26.Bennett D L, Cheek T R, Berridge M J, De Smedt H, Parys J B, Missiaen L, Bootman M D. J Biol Chem. 1996;271:6356–6362. doi: 10.1074/jbc.271.11.6356. [DOI] [PubMed] [Google Scholar]
- 27.Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]