Abstract
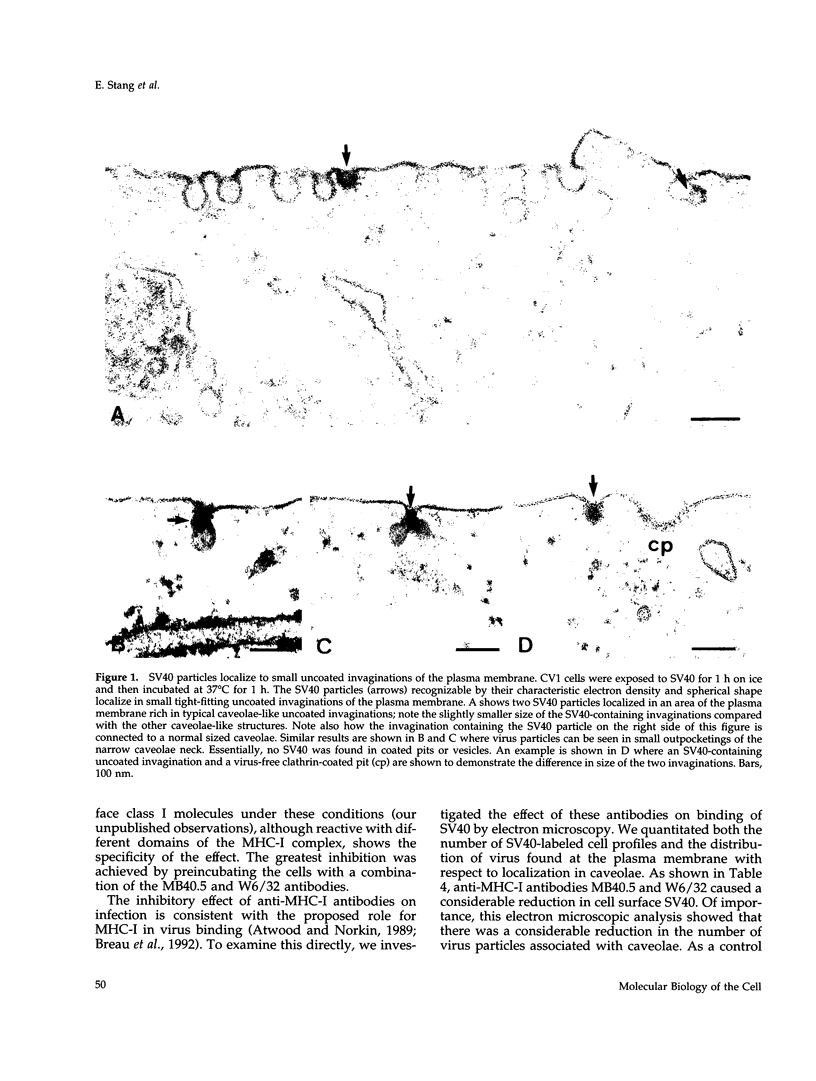
Simian virus 40 (SV40) has been shown to enter mammalian cells via uncoated plasma membrane invaginations. Viral particles subsequently appear within the endoplasmic reticulum. In the present study, we have examined the surface binding and internalization of SV40 by immunoelectron microscopy. We show that SV40 associates with surface pits which have the characteristics of caveolae and are labeled with antibodies to the caveolar marker protein, caveolin-1. SV40 is believed to use major histocompatibility complex (MHC) class I molecules as cell surface receptors. Using a number of MHC class I-specific monoclonal antibodies, we found that both viral infection and association of virus with caveolae were strongly reduced by preincubation with anti-MHC class I antibodies. Because binding of SV40 to MHC class I molecules may induce clustering, we investigated whether antibody cross-linked class I molecules also redistributed to caveolae. Clusters of MHC class I molecules were indeed shown to be specifically associated with caveolin-labeled surface pits. Taken together, the results suggest that SV40 may make use of MHC class I molecule clustering and the caveolae pathway to enter mammalian cells.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Potocytosis of small molecules and ions by caveolae. Trends Cell Biol. 1993 Mar;3(3):69–72. doi: 10.1016/0962-8924(93)90065-9. [DOI] [PubMed] [Google Scholar]
- Atwood W. J., Norkin L. C. Class I major histocompatibility proteins as cell surface receptors for simian virus 40. J Virol. 1989 Oct;63(10):4474–4477. doi: 10.1128/jvi.63.10.4474-4477.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breau W. C., Atwood W. J., Norkin L. C. Class I major histocompatibility proteins are an essential component of the simian virus 40 receptor. J Virol. 1992 Apr;66(4):2037–2045. doi: 10.1128/jvi.66.4.2037-2045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad P. A., Smart E. J., Ying Y. S., Anderson R. G., Bloom G. S. Caveolin cycles between plasma membrane caveolae and the Golgi complex by microtubule-dependent and microtubule-independent steps. J Cell Biol. 1995 Dec;131(6 Pt 1):1421–1433. doi: 10.1083/jcb.131.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasgupta J. D., Watkins S., Slayter H., Yunis E. J. Receptor-like nature of class I HLA: endocytosis via coated pits. J Immunol. 1988 Oct 15;141(8):2577–2580. [PubMed] [Google Scholar]
- Dupree P., Parton R. G., Raposo G., Kurzchalia T. V., Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO J. 1993 Apr;12(4):1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fra A. M., Williamson E., Simons K., Parton R. G. De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8655–8659. doi: 10.1073/pnas.92.19.8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T. Calcium pump of the plasma membrane is localized in caveolae. J Cell Biol. 1993 Mar;120(5):1147–1157. doi: 10.1083/jcb.120.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghitescu L., Fixman A., Simionescu M., Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. J Cell Biol. 1986 Apr;102(4):1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenney J. R., Jr The sequence of human caveolin reveals identity with VIP21, a component of transport vesicles. FEBS Lett. 1992 Dec 7;314(1):45–48. doi: 10.1016/0014-5793(92)81458-x. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L. Novel tyrosine kinase substrates from Rous sarcoma virus-transformed cells are present in the membrane skeleton. J Cell Biol. 1989 Jun;108(6):2401–2408. doi: 10.1083/jcb.108.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet C., Ash J. F., Singer S. J. The antibody-induced clustering and endocytosis of HLA antigens on cultured human fibroblasts. Cell. 1980 Sep;21(2):429–438. doi: 10.1016/0092-8674(80)90479-1. [DOI] [PubMed] [Google Scholar]
- Kartenbeck J., Stukenbrok H., Helenius A. Endocytosis of simian virus 40 into the endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2721–2729. doi: 10.1083/jcb.109.6.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzchalia T. V., Dupree P., Monier S. VIP21-Caveolin, a protein of the trans-Golgi network and caveolae. FEBS Lett. 1994 Jun 6;346(1):88–91. doi: 10.1016/0014-5793(94)00466-8. [DOI] [PubMed] [Google Scholar]
- Li S., Okamoto T., Chun M., Sargiacomo M., Casanova J. E., Hansen S. H., Nishimoto I., Lisanti M. P. Evidence for a regulated interaction between heterotrimeric G proteins and caveolin. J Biol Chem. 1995 Jun 30;270(26):15693–15701. doi: 10.1074/jbc.270.26.15693. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Scherer P. E., Tang Z., Sargiacomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signalling hypothesis. Trends Cell Biol. 1994 Jul;4(7):231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Machy P., Truneh A., Gennaro D., Hoffstein S. Endocytosis and de novo expression of major histocompatibility complex encoded class I molecules: kinetic and ultrastructural studies. Eur J Cell Biol. 1987 Dec;45(1):126–136. [PubMed] [Google Scholar]
- Machy P., Truneh A., Gennaro D., Hoffstein S. Major histocompatibility complex class I molecules internalized via coated pits in T lymphocytes. Nature. 1987 Aug 20;328(6132):724–726. doi: 10.1038/328724a0. [DOI] [PubMed] [Google Scholar]
- Monier S., Parton R. G., Vogel F., Behlke J., Henske A., Kurzchalia T. V. VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell. 1995 Jul;6(7):911–927. doi: 10.1091/mbc.6.7.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M., Peränen J., Schreiner R., Wieland F., Kurzchalia T. V., Simons K. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A. 1995 Oct 24;92(22):10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes J. J., Stollorz V., Peters P. J., Geuze H. J., Ploegh H. L. The biosynthetic pathway of MHC class II but not class I molecules intersects the endocytic route. Cell. 1990 Apr 6;61(1):171–183. doi: 10.1016/0092-8674(90)90224-3. [DOI] [PubMed] [Google Scholar]
- Parton R. G. Caveolae and caveolins. Curr Opin Cell Biol. 1996 Aug;8(4):542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Parton R. G., Joggerst B., Simons K. Regulated internalization of caveolae. J Cell Biol. 1994 Dec;127(5):1199–1215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parton R. G., Simons K. Digging into caveolae. Science. 1995 Sep 8;269(5229):1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Rothberg K. G., Heuser J. E., Donzell W. C., Ying Y. S., Glenney J. R., Anderson R. G. Caveolin, a protein component of caveolae membrane coats. Cell. 1992 Feb 21;68(4):673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Scherer P. E., Okamoto T., Chun M., Nishimoto I., Lodish H. F., Lisanti M. P. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc Natl Acad Sci U S A. 1996 Jan 9;93(1):131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer J. E., Oh P., Pinney E., Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994 Dec;127(5):1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severs N. J. Caveolae: static inpocketings of the plasma membrane, dynamic vesicles or plain artifact? J Cell Sci. 1988 Jul;90(Pt 3):341–348. doi: 10.1242/jcs.90.3.341. [DOI] [PubMed] [Google Scholar]
- Smart E. J., Ying Y. S., Conrad P. A., Anderson R. G. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994 Dec;127(5):1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z., Scherer P. E., Okamoto T., Song K., Chu C., Kohtz D. S., Nishimoto I., Lodish H. F., Lisanti M. P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J Biol Chem. 1996 Jan 26;271(4):2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- Tran D., Carpentier J. L., Sawano F., Gorden P., Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Parton R. G. M-caveolin, a muscle-specific caveolin-related protein. FEBS Lett. 1995 Nov 27;376(1-2):108–112. doi: 10.1016/0014-5793(95)01256-7. [DOI] [PubMed] [Google Scholar]
- van Deurs B., Holm P. K., Sandvig K., Hansen S. H. Are caveolae involved in clathrin-independent endocytosis? Trends Cell Biol. 1993 Aug;3(8):249–251. doi: 10.1016/0962-8924(93)90045-3. [DOI] [PubMed] [Google Scholar]