Abstract
Both CCN family 2/connective tissue growth factor (CCN2/CTGF) and bone morphogenetic protein (BMP)-2 play an important role in cartilage metabolism. We evaluated whether or not CCN2 would interact with BMP-2, and examined the combination effect of CCN2 with BMP-2 (CCN2-BMP-2) on the proliferation and differentiation of chondrocytes. Immunoprecipitation-western blotting analysis, solid-phase binding assay and surface plasmon resonance (SPR) spectroscopy showed that CCN2 directly interacted with BMP-2 with a dissociation constant of 0.77 nM as evaluated by SPR. An in vivo study revealed that CCN2 was co-localized with BMP-2 at the pre-hypertrophic region in the E18.5 mouse growth plate. Interestingly, CCN2-BMP-2 did not affect the BMP-2/CCN2-induced phosphorylation of p38 MAPK but caused less phosphorylation of ERK1/2 in cultured chondrocytes. Consistent with these results, cell proliferation assay showed that CCN2-BMP-2 stimulated cell growth to a lesser degree than by either CCN2 or BMP-2 alone, whereas the expression of chondrocyte marker genes and proteoglycan synthesis, representing the mature chondrocytic phenotype, was increased collaboratively by CCN2-BMP-2 treatment in cultured chondrocytes. These findings suggest that CCN2 may regulate the proliferating and differentiation of chondrocytes by forming a complex with BMP-2 as a novel modulator of BMP signalling.
Keywords: chondrocytes, CCN family 2/connective tissue growth factor (CCN2/CTGF), BMP signalling, BMP-2, endochondral ossification
INTRODUCTION
Endochondral ossification is initiated by the condensation of mesenchymal cells and the subsequent differentiation of them into chondrocytes within the condensates (1, 2). Chondrocytes proliferate and produce many kinds of extracellular matrix (ECM) molecules characteristic of cartilage, such as type II collagen, aggrecan, link proteins and hyaluronate (1, 2). Once embedded in ECM, these cells differentiate into pre-hypertrophic and hypertrophic chondrocytes (1, 2). Hypertrophic chondrocytes, in which cell growth is arrested, eventually mineralize the surrounding matrices, allowing the invasion of blood vessels and osteoblasts (1, 2). Finally, the cartilage is replaced by bone. In this differentiation process, a number of growth factors, such as transforming growth factor (TGF)-β (3), insulin-like growth factor (IGF, 4), bone morphogenetic proteins (BMPs, 5) and CCN family 2/connective tissue growth factor (CCN2) have been implicated (6). Among them, CCN2 is highly expressed in the pre-hypertrophic region of growth plate (6). As a member of the CCN family, it consists of four distinct structural modules, i.e. insulin-like growth factor binding protein-like (IGFBP), von Willebrand type C repeat (VWC), thrombospondin type 1 repeat (TSP1) and carboxy-terminal cysteine knot (CT). Also, CCN2 promotes multiple steps of the endochondral ossification, such as proliferation, maturation and hypertrophy of chondrocytes (6, 7). In addition, we reported earlier that CCN2 functions to maintain the integrity of the cartilage tissues in vivo (8). These findings suggest that CCN2 plays a very important role in chondrocyte metabolism. In fact, it has been reported that Ccn2-deficient mice die soon after birth, as a result of, at least in part, severe skeletal abnormalities associated with impaired endochondral ossification (9). These and other findings indicate that CCN2 is an essential growth factor for regulation of the proliferation, maturation and hypertrophy of chondrocytes (7, 9). Recently, it was reported that CCN2 interacted with many growth factors critically involved in cartilage metabolism, such as TGF-β, BMP-4 (10), and vascular endothelial growth factor (VEGF, 11) and that CCN2 modified the activity of each growth factor. Therefore, CCN2 may control the network of growth factors during endochondral ossification; and thus it may be called a ‘signal conductor’ with novel functions.
BMP-2 is also a multifunctional growth factor, and it was originally defined by its ability to induce ectopic bone and cartilage formation in vivo (12). Although it was reported that BMP-2 promoted the proliferation, maturation and hypertrophy of chondrocytes in vitro (5, 13, 14), newborn transgenic mice, in which Bmp-2 had been inactivated in a limb-specific manner, had normal skeletons (15). These findings suggest that other BMPs present in the developing limb can compensate for the loss of BMP-2. Until now, more than 30 BMP family members have already been described, and they have been classified into several subgroups according to their structural similarities (16). In particular, BMP-2 and BMP-4 are highly related molecules, and both molecules have potent bone-forming activity (17). These findings indicate that the functions of BMP-2 and BMP-4 are interchangeable during bone formation in the limb. In fact, it was reported that the loss of both BMP-2 and BMP-4 in a limb-specific manner resulted in a delay in cartilage development and in a severe impairment of osteogenesis (18). Furthermore, the BMP receptor type 1A (Bmpr1a), BMP receptor type 1B (Bmpr1b) double-deficient mice exhibited severe defects in chondrogenesis and osteogenesis (19). Taken together, these results suggest that BMP signalling is essential for endochondral ossification, and that BMP-2 and BMP-4 compensate each other to transduce sufficient BMP signalling to allow cartilage cells to differentiate.
Although it has been already reported that CCN2 interacts with BMP-4 and inhibits the action of BMP-4 in early embryonic patterning (7), investigation of the interaction of CCN2 with BMP-2 as well as BMP-4 may reveal the novel function of CCN2 in BMP signalling required for cartilage development. Therefore, we investigated whether or not CCN2 directly interacts with BMP-2 and examined the combinational effect of CCN2 with BMP-2 on chondrocyte proliferation and differentiation. In this study, we demonstrated that CCN2 directly interacted with BMP-2 and promoted CCN2/BMP-2-induced proteoglycan synthesis, whereas proliferation of chondrocytes was interfered with the combination. These findings suggest that CCN2 has both antagonistic effect and agonistic effect on BMP-2.
MATERIALS AND METHODS
Materials
Dulbecco’s modified Eagle’s medium (DMEM), α-modification of Eagle’s medium (αMEM), and fetal bovine serum (FBS) were purchased from Nissui Pharmaceutical (Tokyo, Japan), ICN Biomedicals (Aurora, OH), and Cancera International (Rexcalale, ON, Canada), respectively. Plastic dishes and multiwell plates were obtained from Greiner Bio-One (Frickenhausen, Germany). Hybond-N membrane and [α-32P]dCTP (specific activity: 110 TBq/mmol) were from GE Healthcare UK (Little Chalfont, United Kingdom), and [35S]sulfate (37 MBq/ml) was from PerkinElmer (Waltham, MA). Hyaluronidase and anti-β-actin were obtained from Sigma (St Louis, MO). Anti-phospho-extracellular signal-regulated kinase (ERK)1/2, and anti-phospho-p38 were from Promega (Madison, WI); and anti-ERK1/2, anti-p38, and anti-phospho-Smad1/5/8, from Cell Signalling Technology (Beverly, MA). Anti-BMP-2 was purchased from R & D Systems (Minneapolis, MN); and anti-HA, from Covance (Princeton, NJ). Anti-CCN2 serum was raised in rabbits, and recombinant CCN2 (rCCN2) was purified as previously reported (20). For binding assays and surface plasmon resonance (SPR) analysis, polyhistidine (His)-tagged rCCN2 and each of the four modules of the CCN2 were purchased from Biovendor (Heidelberg, Germany), or were produced by Escherichia coli harbouring the corresponding expression plasmids. Recombinant BMP-2 (rBMP-2) was kindly provided by Dr K. Sugama of Osteopharma (Osaka, Japan).
Animals
BalbC/129sv hybrid CCN2+/− mice were crossbred to obtain wild-type (WT) and Ccn2-deficient mice, which were used at E18.5 (9). These mice were euthanized to obtain the rib cage for cell culture and the metatarsal bone with surrounding tissues for immunostaining. All mice were genotyped by using PCR. The Animal Committee of Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences approved all of the procedures.
Cell Culture
Cells of the human chondrosarcoma-derived cell line HCS-2/8 (21) were inoculated at a density of 4 ×104 cells/cm2 into 96-well or 24-well multiplates containing DMEM supplemented with 10% FBS and were cultured at 37°C under 5% CO2 in air. Primary cultures of chondrocytes isolated from the ventral half of the rib cages of E18.5 mouse embryos were prepared as described previously (22). The isolated chondrocytes were seeded at a density of 1 ×105 cells/cm2 into 3.5 cm dishes in αMEM containing 10% FBS and were then cultured at 37°C under 5% CO2 in air.
Western Blot Analysis
HCS-2/8 cells were transfected with a CCN2 expression plasmid with an HA-tag by using Fugene6 reagent (Roche, Basel, Switzerland). After 2 days, the cell lysate was collected, and immunoprecipitation was performed with anti-BMP-2 antibody. Then, the HA-tagged CCN2 was detected in the immunoprecipitated sample by western blotting by using an anti-HA antibody. Western blot analysis was performed as described previously (23).
Solid-Phase Binding Assay
A 96-well multiplate was pre-coated with rBMP-2 (0.3–10 μg/ml) at 4°C overnight. Then, rCCN2 with a His-tag was added to each well, and incubation was carried out at 4°C for 6 h. After washing each well, we measured the optical absorbance (450 nm) representing the binding of CCN2 to BMP-2 by conducting a colorimetric assay using the anti-His antibody.
Immunohistochemistry
Mouse metatarsal bones with surrounding tissues were dissected and fixed in 10% formalin overnight at 4°C before being embedded in paraffin. Five micrometre sections were mounted on glass slides, deparaffinized and treated with hyaluronidase (25 mg/ml) for 30 min at room temperature. Immusnohistochemistry was performed with a Histofine kit (Nichirei; Tokyo, Japan) as described previously (23). Colour was developed with diaminobenzidine (DAB), and sections were counterstained with methyl green. The proliferative population of chondrocytes in growth cartilage tissues was determined using a commercial proliferating cell nuclear antigen (PCNA) staining kit (Zymed laboratories, Carlsbad, CA), following the manufacturer’s instructions.
Evaluation of Proteoglycan Synthesis
Mouse costal chondrocytes or HCS-2/8 cells were grown to sub-confluence in 24-well multiplates containing αMEM or DMEM supplemented with 10% FBS, respectively. Thereafter, the medium was replaced with that containing 0.5% FBS and rCCN2, rBMP-2 or both. [35S]sulfate (37 MBq/ml) dissolved in PBS was added to the culture at a final concentration of 370 kBq/ml at 5 h after the addition of these factors, and incubation was continued for another 17 h. After labelling, the cultures were digested with 1 mg/ml actinase E, and the radioactivity of the material precipitated with cetylpyridinium chloride was measured in a scintillation counter.
Northern Blot Analysis
Total RNAs were prepared by using ISOGEN reagent (Nippon Gene, Tokyo, Japan) from mouse costal chondrocytes stimulated with rCCN2, rBMP-2 or their combination for 6 h. Then, 10 μg of total RNA was subjected to electrophoresis on a 1% formaldehyde-agarose gel and subsequently transferred onto Hybond-N filters (GE Healthcare). Northern blot analysis was performed as described previously (23). Specific PCR products of aggrecan cDNA and linearized plasmids containing mouse-type II collagen (22), type X collagen (9) and Runt domain transcription factor (Runx2/Cbfa1, 24) cDNAs were used as probes.
Surface Plasmon Resonance Spectroscopy
Specific interaction between CCN2 and BMP-2 was analysed by using a BIAcore X (GE HealthCare). rCCN2 coupling, blocking and regeneration of a CM5 chip were performed according to the manufacturer’s protocol. rBMP-2 in HBS-EP buffer (10 mM HEPES, 0.15 M NaCl, 3 mM EDTA and 0.005% Tween 20; pH 7.4) at several concentrations was perfused at a flow rate of 10 μl/min at 25°C over the control surface or a surface bearing immobilized CCN2, and the resonance changes were recorded. The response on the control surface was subtracted from that on the CCN2-conjugated surface. The dissociation constants (Kd) were determined by using BIA evaluation software.
Proliferation Assay
For measurement of cell proliferation, HCS-2/8 cells were inoculated into 96-well multiplates at a density of 1 ×104/well, and then the cells were stimulated with rCCN2, rBMP-2 or their combination for 24 h. Thereafter, the proliferation of these cells was determined by conducting a TetraColor ONE assay according to the recommended protocol (Seikagaku Co. Tokyo, Japan). Briefly, 10 μl of TetraColor ONE solution was added to 100 μl of medium of each culture. After the incubation for up to 4 h at 37°C, the absorbance of culture medium was measured at a wavelength of 450 nm.
Statistical Analysis
Unless otherwise specified, all experiments were repeated at least twice, and similar results were obtained. Statistical analysis was performed by using Student’s t-test.
RESULTS
Interaction of CCN2 with BMP-2 Through Its CT Domain
It has been reported that CCN2 directly binds to BMP-4 through its VWC module (10). Because not only BMP-4 but also BMP-2 plays an important role in chondrocyte proliferation and differentiation during endochondral ossification (13, 14), we investigated the involvement of CCN2 in BMP-2 signalling of chondrocytes. As shown in Fig. 1, we found that CCN2 interacted with BMP-2 similarly as with BMP-4. An HA epitope-tagged construct of full-length CCN2 was generated, and the protein was produced in HCS-2/8 cells. After 2 days, cell lysates were collected and immunoprecipitation was performed with anti-BMP-2 antibody in cell lysate in the presence or absence of rBMP-2. Western blot analysis of the immunoprecipitated samples was performed by using anti-HA antibody. As a result, HA-tagged CCN2 was detected only in the presence of rBMP-2 (Fig. 1A). In addition, to test whether CCN2 directly interacted with BMP-2, or not, we performed a solid-phase binding assay. As shown in Fig. 1B, CCN2 directly bound to BMP-2 in a BMP-2-dependent manner. Subsequently, a binding assay using each recombinant module protein of CCN2 was performed to determine which module was responsible for the binding to BMP-2. As shown in Fig. 1C, the CT module of CCN2 distinctly bound to BMP-2, whereas VWC and IGFBP modules slightly bound to it. To further verify if CT or amino-terminal module plays an important role in binding to BMP-2, we generated a N-terminal IGFBP-VWC and C-terminal TSP1-CT fragments and evaluated their binding ability. As shown in Fig. 1D, while both fragments significantly interacted with BMP-2, interaction of TSP1-CT fragment of CCN2 with BMP-2 was stronger than that of IGFBP-VWC fragment. These results indicate that not only the CT module, but also the IGFBP-VWC modules are responsible for the binding to BMP-2. Furthermore, the binding affinity of CCN2 for BMP-2 was determined by SPR analysis (BIAcore systems). Kinetic measurements using different concentrations of CCN2 yielded a Kd of 0.77 nM for BMP-2 (Fig. 1E). These data indicate that CCN2 can directly bind to BMP-2 through its CT domain.
Fig. 1. Complex formation between CCN2 and BMP-2.
(A) Western blot analysis of CCN2 binding to rBMP-2 after immunoprecipitation with anti-BMP-2. HCS-2/8 cells were inoculated at a density of 5 ×105/well into a 6-well plate; and the next day, the cells were transfected with a CCN2 expression plasmid containing an HA tag. After 2 days, the cell lysates were collected and incubated with or without 1 μg rBMP-2 at 4°C overnight. Then, immunoprecipitaion was performed with anti-BMP-2 antibody at 4°C. After 24 h, the immunocomplexes were captured by adding agarose-protein G beads. The beads were washed, and precipitates were subjected to SDS-PAGE and western blotting with anti-HA antibody. Representative results for total cell lysate (input) and treatment without rBMP-2 (−) and with rBMP-2 (+) are shown. The arrow indicates the signal for CCN2-HA. (B) Solid-phase binding assay for binding of full-length CCN2 to rBMP-2. Ninety-six multiplate wells pre-coated with different concentrations of rBMP-2 (0.3, 1, 3 and 10 μg/ml) were incubated with 6 ×His-tagged rCCN2 (500 ng/ml) at 4°C for 6 h. Total binding was determined by measuring immunoreactivity towards the His-tag. Background binding was determined by measuring the immunoreactivity towards the His-tagged CCN2 in the wells pre-coated with BSA at the same concentration of each rBMP-2. Data were calculated by the subtracting background binding from the total binding. Data represents mean ±SD of triplicate samples. (C) Solid-phase binding assay for measuring the binding of each CCN2 module to rBMP-2. CCN2 or its modules (25 μM) were incubated in the wells pre-coated with rBMP-2 at 4°C for 6 h. Bound proteins were determined under the same conditions as in ‘B’. Data are presented as the mean ±SD of triplicate samples. (D) Solid-phase binding assay for the evaluation of the binding of IGFBP-VWC or TSP1-CT fragment of CCN2 to rBMP-2. (Upper panel) Full-length CCN2, IGFBP-VWC or TSP1-CT fragment (1 μg/ml) was incubated in the wells pre-coated with rBMP-2 (3 μg/ml) at 4°C for 6 h. Bound proteins were determined under the same conditions as in ‘B’. Data are presented as the mean ±SD of triplicate samples. (Lower panel) Recombinant full-length CCN2, IGFBP-VWC and TSP1-CT fragment were separated by SDS–PAGE and visualized by coomassie brilliant blue (CBB) staining. (E) Determination of the binding affinity of rBMP-2 for CCN2 by SPR analysis. Four different concentrations of rBMP-2 ranging between 5 nM and 30 nM were passed through the immobilized CCN2 on the CM5 sensor chip (Time 0 s), and kinetic experiments were performed. After the rBMP-2 flow was stopped (arrowhead), dissociation was monitored by a decrease in the resonance units.
Co-Localization of CCN2 with BMP-2 in Pre-Hypertrophic Region of the Growth Plate
Growth plate chondrocytes are horizontally organized into distinct zones. The zones reflect the sequential differentiation stages of chondrocyte proliferation, maturation and hypertrophy (Fig. 2A and B). In wild type mice, proliferative and pre-hypertrophic chondrocytes were stained with anti-PCNA antibody, which has been used as a marker for cell proliferation, but the immunoreactivity of PCNA was not observed in the hypertrophic chondrocytes therein (Fig. 2C). These findings suggest that not only proliferative chondrocytes, but also pre-hypertrophic chondrocytes, which are the major CCN2 producer in growth plate, retain proliferative activity. Because it was shown that CCN2 directly bound to BMP-2 in vitro, we investigated whether or not CCN2 was co-localized with BMP-2 in the growth plate using an immunohistochemical analysis with anti-CCN2 and anti-BMP-2 antibodies in E18.5 WT and Ccn2-deficient metatarsal growth plates. As shown in Fig. 2G and H , both CCN2 and BMP-2 were localized in pre-hypertrophic region of the growth plate, thus suggesting that CCN2 might interact with BMP-2 in vivo. On the other hand, as also shown in Fig. 2D–F, J–L, and consistent with previous studies (9), an enlarged hypertrophic zone and reduced PCNA signal and safranin-O staining were seen in the growth plate in Ccn2-deficient compared with that in WT littermates; and as expected, immunoreactivity for CCN2 was not detected (Fig. 2J). Signals indicating immunoreactivity for BMP-2 were detected in chondrocytes in the proliferative cell layer, but not in the pre-hypertrophic zone (Fig. 2K). This pattern of BMP-2 distribution in the Ccn2-deficient mice was significantly altered from that in the WT mice. These findings suggest that CCN2 regulates the localization of BMP-2 during endochondral ossification.
Fig. 2. Co-localization of CCN2 and BMP-2 in the growth plate of wild-type and Ccn2-deficient metatarsal bones.
Sections of the growth plates of E18.5 metatarsal bone (A, B, D, E, G–L) and femur (C, F) in wild-type (A–C, G–I) and Ccn2-deficient (D–F, J–L) littermates were stained with safranin-O (A, B, D, E), and immunostained with anti-PCNA antibody (C, F), anti-CCN2 antibody (G, J), anti-BMP-2 antibody (H, K) or normal rabbit IgG as a negative control (I, L). The primary antibodies were visualized by immunoperoxidase, and then the sections were counterstained with methyl green (G–L) or hematoxylin (C, F). Panels ‘A’ and ‘D’ are low-power magnification views of the growth plate, and panels ‘B, G–I’ and ‘E, J–L’ show high-power magnification of the area delimited by black square in ‘A’ and ‘D’, respectively. Bidirectional arrows indicate the length of the hypertrophic zones (A, D). In the wild type, immunostaining with PCNA was detected from proliferative to pre-hypertrophic zone (C), and both CCN2 and BMP-2 were mainly localized in the pre-hypertrophic zone of the growth plate (G, H). In the Ccn2-deficient mice, signals for immunoreactivity of PCNA were decreased compared with wild type (F), and CCN2 immunostaining was not detected (J). Signals for immunoreactivity to BMP-2 were located in the proliferative zone (K). Bars in ‘A and D’ and in ‘B, C, E, F and G–L’ represent 100 μm and 50 μm, respectively.
Combination of CCN2 and BMP-2 Modulates the CCN2 or BMP-2 Signalling in Chondrocytes
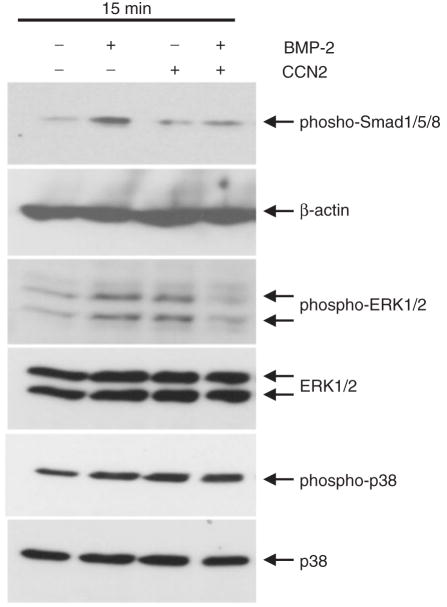
Because CCN2 interacted with BMP-2 in vitro and co-localized with BMP-2 in vivo, we tested whether the combination of CCN2 and BMP-2 modulated CCN2 or BMP-2 signalling in chondrocytes, or not. As shown in Fig. 3, we performed western blot analysis of mouse costal chondrocytes stimulated with rCCN2, rBMP-2 or their combination for 15 or 30 min by using anti-phospho-Smad1/5/8, anti-phospho-ERK1/2 and anti-phospho-p38 antibodies. The levels of Smad1/5/8 and ERK1/2 phosphorylation were increased by 50 ng/ml rBMP-2 and the phosphorylation of ERK1/2 was induced by 100 ng/ml rCCN2. However, the degree of their phosphorylation by the combination of rCCN2 and rBMP-2 was rather decreased compared with that by either factor alone (Fig. 3). Similar results were obtained in HCS-2/8 cells (data not shown). On the other hand, phospho-p38 levels were not affected by the combination of rCCN2 and rBMP-2. After 30 min of stimulation, no effect on Smad1/5/8, ERK1/2 or p38 phosphorylation was detected (data not shown). These findings suggest that the co-presence of CCN2 and BMP-2 modulates BMP-2 or CCN2 signalling in chondrocytes.
Fig. 3. Combinational effect of CCN2 and BMP-2 on the activation of Smad1/5/8 and MAPK pathways in mouse chondrocytes.
After the first passage, mouse chondrocytes were cultured in 3.5 cm dishes until they had become sub-confluent. Then, the cells were stimulated with 50 ng/ml rBMP-2, 100 ng/ml rCCN2 or the combination of CCN2 (100 ng/ml) and rBMP-2 (50 ng/ml) for 15 min. The mixture of CCN2 with BMP-2 was prepared 2 h before stimulation and was kept on ice to allow hetero complex formation. Cell lysates were collected, and western blot analysis was performed with anti-phospho-Smad1/5/8, anti-β-actin, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38 and anti-p38 antibodies. The band of phospho-Smad1/5/8 was increased in cells treated with rBMP-2, and the bands of both phospho-ERK1/2 and phospho-p38 were increased in the cells treated with rCCN2 or rBMP-2 after 15 min of treatment. Note that combination of CCN2 and BMP-2 decreased the activation of phospho-Smad1/5/8 and phospho-ERK1/2 but not that of phospho-p38.
Effect of Combination of CCN2 and BMP-2 on the Proliferation and Differentiation of HCS-2/8 Cells
Because the combination of CCN2 and BMP-2 modulated CCN2 or BMP-2 signalling, we next investigated the biological significance of the combination of CCN2 and BMP-2 in HCS-2/8 cells. HCS-2/8 cells have retained the mature chondrocytic phenotype during a number of passages, and their responsiveness to growth factors and cytokines is similar to that of primary chondrocytes (21). Therefore, we first studied the effect of the combination of CCN2 and BMP-2 on the proliferation of the cells. As shown in Fig. 4A, rCCN2 or rBMP-2 tested alone stimulated the proliferation of the cells, but their combination showed no stimulatory effect. Second, to investigate the effect of the combination of CCN2 and BMP-2 on the differentiation of HCS-2/8 cells, we evaluated proteoglycan synthesis, which is a good marker of differentiated chondrocytes, in the cells stimulated by the combination of rCCN2 and rBMP-2. As shown in Fig. 4B, either rBMP-2 or rCCN2 alone stimulated the proteoglycan synthesis, which was consistent with previous studies (13, 14, 25). Interestingly, when the combination of CCN2 and BMP-2 was added to the culture of HCS-2/8 cells, proteoglycan synthesis was additively increased. These findings suggest that the combination of CCN2 and BMP-2 collaboratively promoted the differentiation of HCS-2/8 cells but that the proliferation was rather interfered with by the combination.
Fig. 4. Combinational effect of CCN2 and BMP-2 on the proliferation and differentiation of HCS-2/8 cells.
(A) Effect of the combination of CCN2 and BMP-2 on the proliferation of HCS-2/8 cells. HCS-2/8 cells in DMEM containing 10% FBS were inoculated at a density of 1 ×104/well into the wells of a 96-well multiplate and cultured for 24 h. Then, the cells were stimulated with final concentrations of 50 ng/ml rBMP-2, 100 ng/ml rCCN2 or the combination of CCN2 (100 ng/ml) and BMP-2 (50 ng/ml) for 24 h. The mixture of CCN2 with BMP-2 was prepared and kept on ice for 2 h before the stimulation. The proliferation assay was performed as described in ‘MATERIALS AND METHODS’ section. Column 1 represents cells cultured with PBS (control); column 2, those with rBMP-2; column 3, those with rCCN2; and column 4, those with the combination of both. Asterisks indicate significant differences (P < 0.05) between values indicated by the brackets. Each column shows the mean value and SD of the results from 8 wells. (B) Effect of the combination of CCN2 and BMP-2 on proteoglycan synthesis in HCS-2/8 cells. Once HCS-2/8 cells had reached sub-confluence, they were treated with PBS (column 1), rBMP-2 (column 2), rCCN2 (column 3) or both (column 4) for 22 h at 37°C. At 5 h after the addition of these factors, [35S]sulfate was added to the cultures; and radioactivity incorporated into proteoglycans was measured 17 h later. Values represent the means ± SD of four independent cultures. Column 1 represents cells cultured with PBS (control), column 2, those with rBMP-2; column 3, those with rCCN2; and column 4, those with both CCN2 and BMP-2. Asterisks indicate significant differences (P < 0.05) between values indicated by the brackets.
Effect of Combination of CCN2 and BMP-2 on the Expression of Chondrocyte Marker Genes and Differentiation of Mouse Primary Chondrocytes
To clarify in detail the differentiation of chondrocytes stimulated with the combination of CCN2 and BMP-2, we performed northern blot analysis of various cartilage differentiation markers, i.e. type II collagen, aggrecan, type X collagen and Runx2/Cbfa1. As shown in Fig. 5A, the expression of type II collagen and aggrecan, which are good markers of mature chondrocytes, was promoted by the combination of rCCN2 and rBMP-2 better than that by each factor alone. In addition, the combination of rCCN2 and rBMP-2 also stimulated the expression of type X collagen and Runx2/Cbfa1, which are markers of hypertrophic chondrocytes, to a greater degree than each factor alone. Furthermore, to confirm whether or not combination of CCN2 and BMP-2 stimulated proteoglycan synthesis in a synergistic or additive manner, we treated mouse costal chondrocyte cultures with the combination of rCCN2 and rBMP-2 (Fig. 5B). As shown in Fig. 5B, the combination of rCCN2 and rBMP-2 increased proteoglycan synthesis additively, consistent with the result in Fig. 4B. These results suggest that the combination of CCN2 and BMP-2 stimulates chondrocyte differentiation better than CCN2 or BMP-2 alone.
Fig. 5. Combinational effect of CCN2 and BMP-2 on expression of the differentiated phenotype of chondrocytes in mouse chondrocytes in culture.
(A) Northern blot analysis of the expression of type II collagen, aggrecan, type X collagen and Runx2/Cbfa1 in mouse chondrocytes. Mouse primary chondrocytes were re-plated into 6 cm dishes, and they were then cultured until they had reached confluence. Thereafter, the medium was replaced with serum-free medium containing rBMP-2 (50 ng/ml), rCCN2 (100 ng/ml) or the combination of CCN2 and BMP-2; and total RNA was prepared 6 h later. Northern blot analysis was performed as described in ‘MATERIALS AND METHODS section’. Hybridization signals of cartilage marker genes in the autoradiogram and methylene blue-stained rRNA on the same membrane are displayed. Representative results of treatment with PBS (lane 1), rBMP-2 (lane 2), rCCN2 (lane 3) and their combination (lane 4) are shown. The amounts of cartilage marker genes were normalized to the amounts of 28S rRNA. (B) Effect of the combination of CCN2 and BMP-2 on proteoglycan synthesis in mouse chondrocytes. After mouse chondrocytes of passage 1 had reached sub-confluence, the medium was replaced with αMEM containing 0.5% FBS and PBS (lane 1), rBMP-2 (50 ng/ml, lane 2), rCCN2 (100 ng/ml, lane 3) or the combination of CCN2 and BMP-2 (lane 4); and incubation was further continued for 22 h at 37°C. After 5 h of stimulation, [35S]sulfate was added to the cultures; and radioactivity incorporated into proteoglycans was then measured. Values represent means ± SD of four cultures. Asterisks indicate significant differences (P < 0.05) between values indicated by the brackets.
DISCUSSION
In this study, we demonstrated that CCN2 directly interacted with BMP-2. It was previously reported that CCN2 directly bound BMP-4 through its VWC domain and that CCN2 inhibited BMP-4 action (10). The same study also showed, by western blot analysis, that the binding of BMP-4 to CCN2 could be competed by an excess of BMP-2, which suggests that CCN2 also binds to BMP-2 through the same domain (10). This VWC domain is 80% homologous to chordin, which is a BMP antagonist in terms of the cysteine residues (26). Therefore, this finding indicates that CCN2 may be a novel BMP antagonist. In this study, we investigated whether CCN2 directly binds to BMP-2 and functions as a BMP-2 antagonist, or not. BMP-2 has various effects on cartilage metabolism (13, 14). It was reported that BMP-2 stimulated the proliferation, maturation and hypertrophy of chondrocytes in vitro (13, 14), but apparently contradictory results were obtained in an in vivo study using genetically modified mice (15–18). These findings suggest that there are many factors regulating BMP signalling in vivo. In the solid-phase binding assay using each recombinant module protein, we showed that CCN2 bound to BMP-2 mainly through its CT module and partially through its IGFBP and VWC modules (Fig. 1C). In addition, we confirmed that binding of TSP1-CT fragment to BMP-2 was more prominent than that of IGFBP-VWC fragment (Fig. 1D). Inkson et al. reported that [CXXXCXC] and [CCXXC] motifs, which are typical chordin cysteine-rich (CR) sequences, have been conserved in the VWC domain of CCN2 binding to BMPs (26). It should be noted that reversed [CXCXXXC] and [CXXCC] motifs are present in the CT module of CCN2. Therefore, BMP-2 may bind to CCN2 probably via these sequences in the CT module as well.
The crystal structure of CCN2 is still unknown. Previously, we raised several monoclonal antibodies against CCN2 by using a recombinant full-length CCN2 protein as an immunogen, and located the epitopes in the module in CCN2. As a result, we found that two of five monoclonal antibodies recognized the VWC module (27). These results indicate the strong antigenicity of the VWC module and suggest that this module is exposed on the molecular surface. In addition, it has been suggested that CCN2 forms a dimer via the CT domain (28), and that CT domain also directly interacts with ECM proteins, such as fibronectin and heparan sulfate (28, 29). Under the condition that CCN2 is overexpressed in the cells, the binding of BMP-2 to the CT domain may not occur, because this domain is engaged in the dimerization of CCN2 or interacts with ECM. Thus, the VWC module may be more accessible than the CT module. If so, the VWC module may be a major interface for CCN2-BMP interaction. Nevertheless, we showed the binding of CT module to BMP-2 and the modest binding of IGFBP and VWC modules in this binding study; hence, binding of VWC module to BMP-2 may require the collaboration of the IGFBP module to confer the full binding activity. In fact, we showed that the amount of total binding of TSP1-CT and IGFBP-VWC fragments to BMP-2 was comparable with the binding of full-length CCN2. These findings suggest that both IGFBP-VWC and CT domains contribute to the interaction of CCN2 with BMP-2.
BMPs transduce signals by binding to heteromeric complexes of type I and type II serine/threonine kinase receptors. The binding of BMPs to the receptor complex results in the phosphorylation of intracellular Smads, which then are translocated to the nucleus, where they regulate the transcription of the target genes (5, 16, 30). On the other hand, it has been also reported that BMPs transduce signals by the activation of mitogen-activated protein kinase (MAPK) pathways (5, 30) and that the activation of MAPK, especially the p38 pathway, plays an important role in chondrocyte differentiation (31). In addition, the expression of the Runx2/Cbfa1 gene, which induces cartilage hypertrophy, is up-regulated by rBMP-2 treatment (30, 32). In this study, we showed that phosphorylation of Smad1/5/8 induced by BMP-2 treatment was inhibited by the co-presence of CCN2. Furthermore, the activation of ERK1/2 and p38 MAPK pathways was induced by BMP-2, but phosphorylation of ERK1/2 was dramatically decreased by the co-presence of CCN2 with BMP-2 in chondrocytes (Fig. 3). These results suggest that CCN2 binds to BMP-2 and regulates the BMP signalling pathways. Typical antagonists, such as noggin, chordin and follistatin, bind to BMPs and prevent them from interacting with their receptors (30, 33). The major difference between CCN2 and other BMP antagonists is that CCN2 itself is a growth factor and activates the signalling to the nucleus. We reported that the phosphorylation of ERK1/2 was involved in chondrocyte proliferation and that the phosphorylation of p38 MAPK was involved in chondrocyte differentiation induced by rCCN2 (34). In fact, we showed presently that the co-presence of CCN2 with BMP-2 inhibited chondrocyte proliferation and promoted proteoglycan synthesis in HCS-2/8 cells (Fig. 4). In addition, this co-presence increased the gene expression of chondrocyte differentiation markers, such as type II collagen, aggrecan and type X collagen, and promoted proteoglycan synthesis in mouse chondrocytes as well as in HCS-2/8 cells (Fig. 5). Also, we reported that PD98059, which is a specific MEK inhibitor, blocked chondrocyte proliferation and stimulated proteoglycan synthesis in HCS-2/8 cells (34). Considering these findings, a decrease in ERK1/2 signalling caused by the complex formation of CCN2 with BMP-2 may lead to the inhibition of the proliferation and promotion of the differentiation of chondrocytes. The expression of type X collagen mRNA, which is a marker of chondrocyte hypertrophy, was also increased by the combination of CCN2 and BMP-2 treatment, in spite of the decreased Smad1/5/8 phosphorylation representing the classical BMP signalling. However, we also showed that the gene expression of Runx2/Cbfa1 was increased by the combination of CCN2 and BMP-2 treatment of chondrocytes (Fig. 5). Therefore, we propose that the level of expression of type X collagen mRNA was increased due to the up-regulation of Runx2/Cbfa1 induced by stimulation from the combination of CCN2 and BMP-2. Taken together, these findings indicate that CCN2 interacted with BMP-2 to inhibit chondrocyte proliferation and to promote differentiation, thus suggesting that CCN2 modulates not only BMP signalling but also its own signalling to regulate the chondrocyte proliferation and differentiation as a ‘signal conductor’. We conclude that CCN2 has both antagonistic effect and agonistic effect of BMP-2 during endochondral ossification.
Acknowledgments
FUNDING
This work was supported in part by the programs Grants-in-Aid for Medical and Dental Postgraduate Education (to A.M.) and Exploratory Research (to M.T.) of the Ministry of Education, Culture, Sports, Science, and Technology, Japan, and Grants-in-Aid for Scientific Research (S) (to M.T.) and (C) (to S.K.) from Japan Society for the Promotion of Sciences.
We thank Drs Takako Hattori and Harumi Kawaki for their helpful suggestions and are also grateful to Yoko Tada for secretarial assistance.
Abbreviations
- E
embryonic day
- ECM
extracellular matrix
- ERK1/2
extracellular signal-regulated kinase 1/2
- HA
influenza virus hemagglutinin
- His
histidine
- MAPK
mitogen-activated protein kinase
- phospho
phosphorylation
- rCCN2
recombinant CCN2 protein
- WT
wild type
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–348. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- 2.Shimizu H, Yokoyama S, Asahara H. Growth and differentiation of the developing limb bud from the perspective of chondrogenesis. Develop Growth Differ. 2007;49:449–454. doi: 10.1111/j.1440-169X.2007.00945.x. [DOI] [PubMed] [Google Scholar]
- 3.Redini F, Galera P, Mauviel A, Loyau G, Pujol JP. Transforming growth factor β stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234:172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- 4.Trippel SB. Role of insulin-like growth factors in the regulation of chondrocytes. In: Adolphe M, editor. Biological Regulation of the Chondrocytes. CRC Press; Boca Raton, FL: 1992. pp. 161–190. [Google Scholar]
- 5.Yoon BS, Lyons KM. Multiple functions of BMPs in chondrogenesis. J Cell Biochem. 2004;93:93–103. doi: 10.1002/jcb.20211. [DOI] [PubMed] [Google Scholar]
- 6.Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, Tezuka K, Takigawa M. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–273. doi: 10.1210/endo.141.1.7267. [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 9.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abreu JG, Ketpura NI, Reversade B, de Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–221. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 12.Wozney JM. Bone morphogenetic proteins. Prog Growth Factor Res. 1989;1:267–280. doi: 10.1016/0955-2235(89)90015-x. [DOI] [PubMed] [Google Scholar]
- 13.Valcourt U, Ronzière MC, Winkler P, Rosen V, Herbage D, Mallein-Gerin F. Different effects of bone morphogenetic proteins 2, 4, 12, and 13 on the expression of cartilage and bone markers in the MC615 chondrocyte cell line. Exp Cell Res. 1999;251:264–274. doi: 10.1006/excr.1999.4584. [DOI] [PubMed] [Google Scholar]
- 14.Shukunami C, Ohta Y, Sakuda M, Hiraki Y. Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp Cell Res. 1998;241:1–11. doi: 10.1006/excr.1998.4045. [DOI] [PubMed] [Google Scholar]
- 15.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, Einhorn T, Tabin CJ, Rosen V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 16.von Bubnoff A, Cho KW. Intracellular BMP signaling regulation in vertebrates: pathway or network? Dev Biol. 2001;239:1–14. doi: 10.1006/dbio.2001.0388. [DOI] [PubMed] [Google Scholar]
- 17.Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am. 2008;90:14–18. doi: 10.2106/JBJS.G.01109. [DOI] [PubMed] [Google Scholar]
- 18.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:2116–2130. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon BS, Ovchinnikov DA, Yoshii I, Mishina Y, Behringer RR, Lyons KM. Bmpr1a and Bmpr1b have overlapping functions and are essential for chondrogenesis in vivo. Proc Natl Acad Sci USA. 2005;102:5062–5067. doi: 10.1073/pnas.0500031102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida T, Nakanishi T, Shimo T, Asano M, Hattori T, Tamatani T, Tezuka K, Takigawa M. Demonstration of receptors specific for connective tissue growth factor on a human chondrocytic cell line (HCS-2/8) Biochem Biophys Res Commun. 1998;247:905–909. doi: 10.1006/bbrc.1998.8895. [DOI] [PubMed] [Google Scholar]
- 21.Takigawa M, Tajima K, Pan HO, Enomoto M, Kinoshita A, Suzuki F, Takano Y, Mori Y. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989;49:3996–4002. [PubMed] [Google Scholar]
- 22.Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (connective tissue growth factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, Takano-Yamamoto T, Takigawa M. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–275. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- 24.Yamaai T, Nakanishi T, Asano M, Nawachi K, Yosimichi G, Ohyama K, Komori T, Sugimoto T, Takigawa M. Gene expression of connective tissue growth factor (CTGF/CCN2) in calcifying tissues of normal and cbfa1-null mutant mice in late stage of embryonic development. J Bone Miner Metab. 2005;23:280–288. doi: 10.1007/s00774-004-0600-5. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, Takigawa M. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol. 2002;192:55–63. doi: 10.1002/jcp.10113. [DOI] [PubMed] [Google Scholar]
- 26.Inkson CA, Ono M, Kuznetsov SA, Fisher LW, Gehron Robey P, Young MF. TGF-β1 and WISP-1/CCN4 can regulate each other’s activity to cooperatively control osteoblast function. J Cell Biochem. 2008;104:1865–1878. doi: 10.1002/jcb.21754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minato M, Kubota S, Kawaki H, Nishida T, Miyauchi A, Hanagata H, Nakanishi T, Takano-Yamamoto T, Takigawa M. Module-specific antibodies against human connective tissue growth factor: utility for structural and functional analysis of the factor as related to chondrocytes. J Biochem. 2004;135:347–354. doi: 10.1093/jb/mvh042. [DOI] [PubMed] [Google Scholar]
- 28.Brigstock DR, Steffen CL, Kim GY, Vegunta RK, Diehl JR, Harding PA. Purification and characterization of novel heparin-binding growth factors in uterine secretory fluids: identification as heparin-regulated Mr 10,000 forms of connective tissue growth factor. J Biol Chem. 1997;272:20275–20282. doi: 10.1074/jbc.272.32.20275. [DOI] [PubMed] [Google Scholar]
- 29.Hoshijima M, Hattori T, Inoue M, Araki D, Hanagata H, Miyauchi A, Takigawa M. CT domain of CCN2/CTGF directly interacts with fibronectin and enhances cell adhesion of chondrocytes through integrin α5β1. FEBS Lett. 2006;580:1376–1382. doi: 10.1016/j.febslet.2006.01.061. [DOI] [PubMed] [Google Scholar]
- 30.Tsumaki N, Yoshikawa H. The role of bone morphogenetic proteins in endochondral bone formation. Cytokine Growth Factor Rev. 2005;16:279–285. doi: 10.1016/j.cytogfr.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Stanton LA, Underhill TM, Beier F. MAP kinases in chondrocyte differentiation. Dev Biol. 2003;263:165–175. doi: 10.1016/s0012-1606(03)00321-x. [DOI] [PubMed] [Google Scholar]
- 32.de Crombrugghe B, Lefebvre V, Nakashima K. Regulatory mechanisms in the pathways of cartilage and bone formation. Curr Opin Cell Biol. 2001;13:721–727. doi: 10.1016/s0955-0674(00)00276-3. [DOI] [PubMed] [Google Scholar]
- 33.Balemans W, Vau Hul W. Extracellular regulation of BMP signaling in vertebrates: a cocktail of modulators. Dev Biol. 2002;250:231–250. [PubMed] [Google Scholar]
- 34.Yosimichi G, Kubota S, Nishida T, Kondo S, Yanagita T, Nakao K, Takano-Yamamoto T, Takigawa M. Roles of PKC, PI3K and JNK in multiple transduction of CCN2/CTGF signals in chondrocytes. Bone. 2006;38:853–863. doi: 10.1016/j.bone.2005.11.016. [DOI] [PubMed] [Google Scholar]