Abstract
Vascular endothelial growth factor (VEGF) is essential for establishing vascularization and regulating chondrocyte development and survival. We have demonstrated that VEGF regulates the expression of CCN2/connective tissue growth factor (CCN2/CTGF) an essential mediator of cartilage development and angiogenesis, suggesting that CCN2 functions in down-stream of VEGF, and that VEGF function is mediated in part by CCN2. On the other hand, the phenotype of Ccn2 mutant growth plates, which exhibit decreased expression of VEGF in the hypertrophic zone, indicates that Vegf expression is dependent on Ccn2 expression as well. Therefore, we investigated the molecular mechanisms underlying the induction of VEGF by CCN2 using a human chondrocytic cell line, HCS-2/8. Hypoxic stimulation (5% O2) of HCS-2/8 cells increased VEGF mRNA levels by ~8 fold within 6 h as compared with the cells cultured under normoxia. In addition, VEGF expression was further up-regulated under hypoxia in HCS-2/8 cells transfected with a Ccn2 expression plasmid. Hypoxia-inducible factor (HIF)-1α mRNA and protein levels were increased by stimulation with recombinant CCN2 (rCCN2). Furthermore, the activity of a VEGF promoter that contained a HIF-1 binding site was increased in HCS-2/8, when the cells were stimulated by rCCN2. These results suggest that CCN2 regulates the expression of VEGF at a transcriptional level by promoting HIF-1α activity. In fact, HIF-1α was detected in the nuclei of proliferative and pre-hypertrophic chondrocytes of wild-type mice, whereas it was not detected in Ccn2 mutant chondrocytes in vivo. This activation cascade from CCN2 to VEGF may therefore play a critical role in chondrocyte development and survival.
Keywords: CCN family 2/connective tissue growth factor (CCN2/CTGF), Vascular endothelial growth factor (VEGF), Hypoxia-inducible factor (HIF)-1α, Chondrocyte differentiation, Signal conductor
Introduction
During endochondral ossification in a developing bone, chondrocytes become organized into growth plates and undergo maturation, hypertrophic differentiation, calcification, and apoptosis [1]. When the chondrocytes differentiate into hypertrophic cells, they produce angiogenic stimulators and attract surrounding blood vessels [2]. Vascular invasion plays an important initial role in the replacement of mineralized cartilage with bone and marrow [2], and migration of vascular endothelial cells is regulated by several factors, including vascular endothelial growth factor (VEGF) [2] and CCN family 2/connective tissue growth factor (CCN2/CTGF: CCN2) [3–5]. It is well known that VEGF is a strong stimulator of angiogenesis [2]. On the other hand, CCN2 is a cysteine-rich, extracellular matrix (ECM)-associated, heparin-binding protein, encoded by a gene belonging to a group known as the CCN family, which has 6 distinct members [3–5]. Previously, we reported that CCN2 was highly expressed in pre-hypertrophic chondrocytes [6] and that this factor promoted the proliferation and differentiation of chondrocytes and osteoblasts [7,8]. CCN2 also stimulates the proliferation and migration of vascular endothelial cells [9]. On the basis of these in vitro findings, CCN2 is believed to promote all stages of endochondral ossification by enhancing proliferation, maturation and hypertrophy of chondrocytes and calcification of cartilage matrix, and the invasion of endothelial cells into cartilage [10,11]. Indeed, we have also shown that CCN2 promotes regeneration of articular cartilage in vivo without causing undesired calcification [12]. Because of such multifunctionality, CCN2 has been attracting the interest of researchers as a factor with novel functions; which may be called as a “signal conductor”.
Ccn2-deficient mice die soon after birth, as a result in part of severe skeletal abnormalities associated with impaired chondrocyte proliferation and ECM production [13]. The most striking histological feature of theCcn2-deficient growth plate is an enlarged hypertrophic zone, and this phenotype may be due to the defective remodeling of the cartilage ECM by chondroclasts/osteoclasts, impaired invasion of blood vessels, and inability to support the subsequent formation of an osteoid matrix by osteoblasts [13]. Interestingly, VEGF immunostaining per cell was decreased in the expanded hypertrophic zone in newborn Ccn2 mutants [13]. These findings indicate that CCN2 may regulate the expression of Vegf in hypertrophic chondrocytes, and may promote the invasion of endothelial cells into the hypertrophic zone via induction of VEGF in vivo. Recently, Kuiper et al. reported that vascular outgrowth of embryonal mouse metatarsals of wild-type and Ccn2 heterozygous mice was significantly enhanced by VEGF stimulation, but that of Ccn2 mutant metatarsals was not [14]. On the other hand, it was also reported that exogenous administration of CCN2 and VEGF in back or hindlimb ischemic lesions in mice inhibited VEGF-induced angiogenesis as a result of the binding of VEGF by CCN2 [15,16]. Taken together, these findings suggest that CCN2 controls the bioactivity of VEGF by exhibiting either positive or negative functions, depending upon microenvironmental conditions.
It is known that VEGF is necessary for chondrocyte development and survival during endochondral ossification [17]. On the basis of this finding, we hypothesized that CCN2 produced by pre-hypertrophic chondrocytes regulates the expression of VEGF, and that CCN2 controls the function of VEGF during cartilage development [13]. To investigate the mechanistic basis of this hypothesis, we employed a useful immortalized human chondrocyte cell line, HCS-2/8. These cells have retained a mature chondrocytic phenotype during a number of serial passages, which is represented by the fact that they synthesize collagen II, IX, and XI, aggrecan, link protein and integrins produced by chondrocytes [18–20]. Low levels of expression of alkaline phosphatase (ALPase) and type X collagen, which are the markers of hypertrophic chondrocytes, are found under normal growth conditions, but expression of these markers shows similar responsiveness to growth factors, cytokines, and vitamins as observed in primary chondrocytes [18–20]. Therefore, in this study, we investigated the effect of CCN2 on Vegf expression and function in chondrocytes using HCS-2/8 cells and Ccn2-deficient murine chondrocytes.
Materials and methods
Materials
Dulbecco’s modified Eagle’s medium (DMEM), α-modification of Eagle’s medium (αMEM), and fetal bovine serum (FBS) were purchased from Nissui Pharmaceutical Co. Ltd. (Tokyo, Japan), ICN Biomedicals (Aurora, OH) and Cancera International (Rexcalale, ON, Canada), respectively. Plastic dishes and multiwell plates were obtained from Greiner Bio-One (Frickenhausen, Germany). Hybond-N+ membrane and α[32P]dCTP (specific activity: 110 TBq/mmol) were from GE Healthcare UK ltd. (Little Chalfont, England), and α[32P]UTP (specific activity: 111 TBq/mmol) were from PerkinElmer, Inc. (Waltham, MA). Actinomycin D and hyaluronidase were obtained from Sigma (St. Louis, MO). Anti-VEGF antibody came from Santa Cruz Biotechnology (Santa Cruz, CA); and anti-HIF-1α antibody, from Chemicon International, Inc. (Temecula, CA). Recombinant CCN2/CTGF (rCCN2) was purified as previously reported [21].
Cell culture and hypoxia condition
HCS-2/8 cells [18–20] were inoculated at a density of 4×104 cells/cm2 in DMEM supplemented with 10% FBS and were cultured at 37 °C under 5% CO2 in air. Primary cultures of chondrocytes isolated from the ventral half of the rib cage of wild-type or Ccn2-deficient mouse embryos (E18.5) were prepared as described previously [22]. The isolated chondrocytes were seeded at a density of 1×105 cells/cm2 into 35 mm dishes in αMEM containing 10% FBS and were then cultured at 37 °C under 5% CO2. Cells derived from each embryo were plated out independently, and corresponding tail samples were collected for genotyping at the same time. Hypoxia experiments were performed for the indicated time periods in a humidified triple gas model BL-40M incubator (BIO-LABO, Tokyo, Japan) calibrated to deliver 5% CO2, 5% O2 and 90% N2 at 37 °C [23].
Northern blot analysis
Total RNAs were prepared by using ISOGEN reagent (Nippon gene, Tokyo, Japan) from HCS-2/8 cells exposed to normoxic or hypoxic conditions for indicated time periods and from the cells transfected with a Ccn2 or Hif-1α expression plasmid. Then, 10 μg of total RNA was subjected to electrophoresis on a 1% formaldehyde-agarose gel and transferred onto Hybond-N+ filters (GE Healthcare). Northern blot analysis was performed as described previously [24]. Specific PCR product of Vegf obtained by using primers that had been reported previously and linear human Hif-1α plasmid were used as a probe [22].
Quantitative real-time PCR analysis
Total cellular RNA was isolated as described above and was reverse-transcribed to cDNA using the Takara RNA PCR kit (AMV) Version. 3.0 (Takara Shuzo, Tokyo, Japan). Amplification reactions were performed with a SYBR® Green Real-time PCR Master Mix (Toyobo; Tokyo, Japan) by using a LightCycler (Roche; Basel, Switzerland). The primer sequences were as follows: Vegf (NM_001025250.2) forward 5′-CCCATGAAGTGATCAAGTTC-3′ and reverse 5′-ATCCGCAT-GATCTGCATGG-3′; Hif-1α (NM_001530.2) forward 5′-TGCTCAT-CAGTTGCCACTTCC-3′ and reverse 5′-CCAAATCACCAGCATCCAGAAGT-3′; Gapdh (NM_001476707) forward 5′-GCCAAAAGGGTCATCATCTC-3′ and reverse 5′-GTCTTCTGGGTGGCAGTGAT-3′; 18S ribosomal RNA (rRNA) (NR_003286) forward 5′-TCCTGCCAGTAGCATATGCTG-3′ and reverse 5′-AGAGGAGCGAGCGAC-CAAAGG-3′.
Western blot analysis
Proteins separated by SDS-polyacrylamide gel electrophoresis (PAGE) were transferred to a polyvinylidene difluoride (PVDF) membrane (Bio-Rad; Hercules, CA) by using a semi-dry blotting apparatus (AE-6677, ATTO Co. Ltd. Tokyo, Japan). Western blot analysis was carried out essentially as described [25].
Plasmids and luciferase assay
The reporter vectors VEGF promoter (−1910/+379)-LUC, 1HRE/WT-LUC, 2HRE-LUC, and 1HRE/HM-LUC were kindly provided by Dr. G. Semenza [26]. HCS-2/8 cells were inoculated at a density of 4×104 cells/cm2 into 6 multiwell plate in DMEM supplemented with 10% FBS and transfected with 1 μg reporter plasmid in combination with 0.1 μg of pRL-TK (internal control: Promega, Madison, WI) by using Fugene6 reagent (Roche). The Dual Luciferase System (Promega) was used as described previously [23,27]. Quantification of firefly and Renilla luciferase activities and calculation of relative ratios were carried out manually with a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA).
Enzyme-linked immunosorbent assay (ELISA)
HCS-2/8 cells before confluency were replenished with fresh medium, and the cells were exposed to normoxic or hypoxic condition for the indicated time periods. The cell lysate and conditioned medium were harvested at the indicated time points and stored at −80 °C until being assayed. VEGF concentrations were determined using a commercial ELISA kit (R & D systems, Inc. Minneapolis, MN), following the manufacturer’s instructions. Samples from three independent experiments were analyzed in duplicate, and the mean and standard deviation were calculated.
Nuclear run-on analysis
Nuclear run-on assays were carried out essentially as described [23]. Briefly, nuclei were isolated from HCS-2/8 cells stimulated by CCN2 (100 ng/ml) or PBS under hypoxia condition, and these nuclei were labeled in reaction buffer containing a mixture of ATP, CTP, and GTP (2.5 mM each) and 1.56 MBq [α-32P]UTP (PerkinElmer). After digestion with RNase-free DNase I (Promega) and proteinase K (Invitrogen, Carlsbad. CA), the radiolabeled transcripts were extracted using ISOGEN reagent (Nippon gene) and further purified on a spin column. Five μg of linear plasmid DNA containing full-length human HIF-1α and β-actin were blotted into Hybond-N membranes (GE Healthcare), and hybridization was carried out by using ULTRA-hyb (Applied Biosystems, Foster, CA) at 42 °C for 72 h. After washing with 2 × SSC, 0.1% SDS and 0.2 × SSC, 0.1% SDS, the results were quantified by autoradiography.
Immunohistochemistry
Mouse femurs were dissected and fixed in 10% formalin overnight at 4 °C. The tissues were embedded in paraffin. Longitudinal sections were cut at a thickness of 5 μm and mounted on slide glass. After deparaffinization, hyaluronidase (25 mg/ml) treatment was carried out for 30 min at room temperature. Immunohistochemistry was performed as described previously [22]. Color was developed by using 3, 3′-diaminobenzidine tetrachloride (DAB). Finally, the sections were counterstained with methyl green. Control specimens incubated with a diluted non-specific immunoglobulin showed no detectable signals. The experiment was approved by the Animal Committee of the Okayama University Graduate School of Medicine, Dentistry, and Phamaceutical Sciences.
Statistical analysis
Unless otherwise specified, all experiments were repeated at least twice, and similar results were obtained. Statistical analysis was performed by using Student’s t test.
Results
Hypoxia increases Vegf mRNA and protein level in HCS-2/8 cells
Because cartilage is hypoxic prior to the invasion of endothelial cells from surrounding tissues during endochondral ossification [28], we previously examined the expression profiles of a variety of genes induced by hypoxic conditions, and identified 10 genes that ranked high in relative expression levels under hypoxia versus normoxia [23]. The induction ratio of Vegf by hypoxia was very high scores among these genes. Therefore, the time course of Vegf gene expression was analyzed in more detail by Northern blotting with total RNA isolated from HCS-2/8 cells after 1.5, 3, 6, and 12 h of hypoxic exposure. As shown in Fig. 1A, Vegf mRNA expression was maximally up-regulated by 6 h of hypoxic exposure to a level approximately 8 fold higher than that under normoxia. In addition, to investigate Vegf induction by hypoxia at the protein level, we quantified the amount of VEGF proteins by enzyme-linked immunosorbent assay (ELISA). As shown in Fig. 1B, the VEGF levels in the supernatant and cell layer exposed to hypoxia for 12 h were significantly increased compared to those from normoxic cells. These results indicated that hypoxia induced VEGF production in chondrocytic cells in vitro. Next, to investigate the mechanism of up-regulation of Vegf mRNA under hypoxia, we performed luciferase assays with a Vegf promoter (−1910/+379)-luciferase reporter plasmid in HCS-2/8 cells transfected with an expression vector of Hif-1α which is known to mediate hypoxic induction of Vegf. As shown in Fig. 1C, relative luciferase activity of the Vegf promoter in HCS-2/8 cells transfected with the Hif-1α expression vector was significantly increased compared with cells transfected with empty vector under hypoxic conditions, where HIF-1α is stable. These results indicated that the expression of VEGF was up-regulated through HIF-1α under hypoxia in chondrocytes.
Fig. 1.
Hypoxia-induced VEGF165 expression in HCS-2/8 cells. (A) HCS-2/8 cells were exposed to normoxic (N) or hypoxic (H) conditions for indicated time periods. Total RNA was extracted and analyzed by Northern blotting for Vegf mRNA expression. (Upper panel) Hybridization signals for Vegf in the autoradiogram and signals for ribosomal RNA in the methylene blue-stained membrane are displayed. (Lower panel) Quantification of the fold-induction of Vegf mRNA signal at the indicated time periods. Relative fold-induction by hypoxic versus normoxic conditions is shown. Mean values of the results of two experiments are displayed with standard deviations. (B) Hypoxia-increased secretion of VEGF by HCS-2/8 cells. After the cells had reached subconfluence, the medium was replaced with fresh medium, and the cells were placed under the normoxic (open column) or hypoxic (closed column) conditions for indicated time periods. Then, the cell culture supernatant and cell layer fraction were harvested. Quantification of VEGF165 was performed using an ELISA system. Asterisks indicate significant differences from normoxia (*p<0.05, **p<0.01). Results are presented as the mean±standard deviation of duplicates. (C) Effect of HIF-1α on the Vegf promoter activity under hypoxic conditions. HCS-2/8 cells were co-transfected with 1 μg of the Vegf promoter (−1910/+379)-driven firefly luciferase reporter plasmid, 0.1 μg of TK promoter-driven Renilla luciferase reporter plasmid (pRL-TK, internal control), and 1 μg of Hif-1α expression plasmid. After 24 h, the cells were exposed to normoxic or hypoxic conditions for 24 h, and the cells were assayed for luciferase activities. Relative luciferase activities are presented as relative values of the measured luminescence of firefly luciferase versus Renilla luciferase. Mean values of the results of duplicates are presented with standard deviations. Asterisks indicate significant differences from the cells transfected with the empty vector under hypoxia (*p<0.05).
CCN2 regulates the expression of VEGF in chondrocytes
It was previously reported that VEGF expression was reduced in the hypertrophic region of the Ccn2−/− growth plate [13]. To investigate the effect of loss of CCN2 on the expression of Vegf in vitro, we isolated chondrocytes from rib cages of E18.5 wild-type and Ccn2-deficient mice. As shown previously [13] and in Fig. 2A, both mRNA and protein levels of VEGF were decreased in Ccn2-deficient chondrocytes compared with those in wild-type cells. Therefore, we investigated the effect of overexpressed Ccn2 on the expression of Vegf. As shown in Fig. 2B, expression levels of VEGF were increased in HCS-2/8 cells transfected with a Ccn2 expression plasmid, or with a Hif-1α expression plasmid (positive control) under hypoxic conditions where HIF-1α is stable (Fig. 2B). These results suggest that CCN2 regulates the expression of VEGF in chondrocytes under hypoxic conditions.
Fig. 2.
Effect of CCN2 on the expression of VEGF in chondrocytes. (A: left panel) Expression of Vegf164 gene in wild-type and Ccn2-deficient chondrocytes. Wild-type (open column) and Ccn2-deficient chondrocytes (closed column) were cultured until reaching confluent. Total RNA was collected and quantitative real-time RT-PCR analysis was performed using mouse homologue; Vegf164 and Gapdh specific primers. Data are presented as mean and standard deviation of duplicate cultures. (A: right panel) Western blot analysis of VEGF protein in lysates of wild-type and Ccn2-deficient chondrocytes. Levels of VEGF are decreased in Ccn2-deficient chondrocytes. (B) HCS-2/8 cells were transfected with a Ccn2-expression plasmid (Ccn2), a Hif-1α-expression plasmid (Hif-1α), or empty vector (Cont). After 24 h, the cells were exposed to normoxic or hypoxic conditions for 24 h, and total RNA was isolated using ISOGEN reagent. Northern blotting for Vegf165 mRNA expression and methylene blue staining for 28S ribosomal RNA were performed.
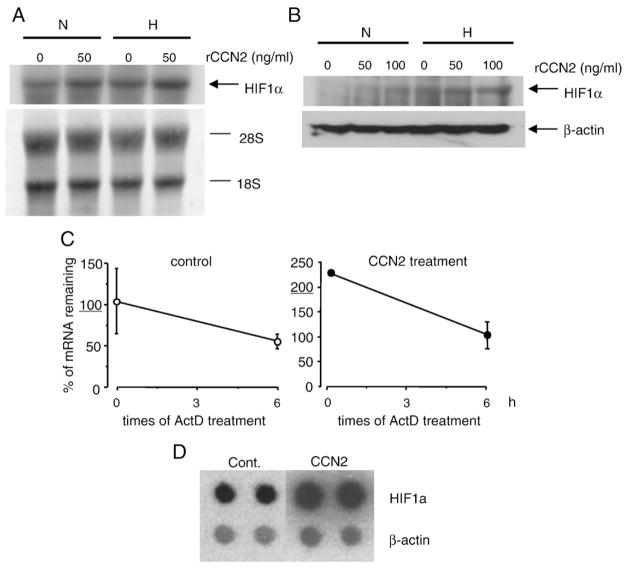
CCN2 induces Hif-1α expression in HCS-2/8 cells
In order to further investigate the mechanism of Vegf regulation in HCS-2/8 cells, we examined whether or not the expression of HIF-1α was induced by CCN2. First, we found that mRNA levels of HIF-1α in HCS-2/8 cells were higher after hypoxia for 24 h than after normoxia (Fig. 3A). Next, we examined whether or not CCN2 regulated the expression of HIF-1α in HCS-2/8 cells. As shown in Fig. 3A, recombinant CCN2 (rCCN2) was able to stimulate HIF-1α mRNA levels both under hypoxia and normoxia conditions. Consistent with Fig. 3A, HIF-1α protein level was also increased by addition of rCCN2 in a dose-dependent manner both under hypoxia and normoxia (Fig. 3B). These results indicate that CCN2 regulates the expression of HIF-1α in HCS-2/8 cells. It is well known that HIF-1α is rapidly degraded via ubiquitin–proteasome pathway under normoxia [29]. To investigate whether or not CCN2 mediates the stabilization of HIF-1α mRNA, we performed quantitative real-time PCR analysis of HIF-1α mRNA in HCS-2/8 cells stimulated by rCCN2, following a time course after the addition of actinomycin D (10 μg/ml). The degradation profile of HIF-1α mRNA in HCS-2/8 cells did not change with rCCN2 treatment (Fig. 3C). Therefore, to investigate whether or not the transcription of Hif-1α was increased by rCCN2 under hypoxia condition, nuclear run-on assay was performed. As shown in Fig. 3D, treatment of CCN2 increased the amount of Hif-1α transcripts under hypoxia condition. These findings indicate that CCN2 up-regulate transcription rate of HIF-1α, but not alter the stability of HIF-1α mRNA.
Fig. 3.
Effect of CCN2 on the expression of Hif-1α in HCS-2/8 cells. (A) HCS-2/8 cells were cultured until reaching sub-confluent in DMEM containing 10% FBS. Then, rCCN2 was added to the experimental cultures at the concentration of 50 ng/ml. PBS of the same volume was added to the control cultures. After 24 h, these cells were exposed to normoxia (N) or hypoxia (H). Total RNA was isolated 24 h later, and Northern blot analysis was performed using specific probe for Hif-1α. Hybridization signals of Hif-1α in the autoradiogram and methylene blue-stained rRNA on the same membrane are displayed. The arrow indicates the signal for Hif-1α. (B) Western blot analysis of HIF-1α protein in cell lysate from HCS-2/8 cells stimulated by rCCN2. HCS-2/8 cells pre-treated with 50 or 100 ng/ml rCCN2 were exposed to normoxic (N) or hypoxic (H) conditions for 24 h, and the cell lysate was corrected. Western blot analysis was performed as described in “Materials and methods”. The arrow indicates the signal for HIF-1α or β-actin. Levels of HIF-1α are increased in cells treated with rCCN2 both normoxic and hypoxic conditions. (C) Degradation of Hif-1α mRNA in HCS-2/8 cells in the presence or absence of 50 ng/ml rCCN2. HCS-2/8 cells were cultured until being sub-confluent in DMEM containing 10% FBS. Then, rCCN2 was added to the experimental cultures at the concentration of 50 ng/ml. After 24 h, the cells were treated with 10 μg/ml actinomycin D (ActD) for 6 h. The relative amount of Hif-1α mRNA remaining after the treatment with ActD was quantified by real-time RT-PCR analysis and shown as a percentage versus the control sample at time 0. Data presented are mean and standard deviation relative to 18S ribosomal RNA level of two separate reactions using mRNA from different cultures. (D) Effect of rCCN2 on rate of Hif-1α transcription in HCS-2/8 cells. Nuclei from PBS- or rCCN2-treated cells were isolated and used for run-on transcription assays. Autoradiograms of labeled transcripts representing Hif-1α and β-actin as an internal control are shown.
The fragment −1006/−954 mediates activation of the human VEGF promoter by rCCN2 in HCS-2/8 cells
It was reported that within the human VEGF promoter, the major hypoxia responsible element (HRE) motif and Smad-binding motif are located within −1006/−954, and that HIF-1α and Smad proteins interacted with these regions [26,30]. Therefore, to examine whether VEGF promoter activity was increased in cis through the −1006/−954 fragment in HCS-2/8 cells by stimulation with rCCN2, we obtained plasmids containing fragments of the VEGF promoter placed upstream of an SV40 promoter-driven firefly luciferase gene [26]. These plasmids, 1HRE/WT-LUC, 2HRE-LUC, and 1HRE/HM-LUC contain a single −1006/−954 fragment, its duplex, and the fragment containing a mutation in the HIF-1 consensus motif at −974 in the backbone of the luciferase reporter vector pGL2-p (Promega), respectively [26,30]. HCS-2/8 cells stimulated by rCCN2 were co-transfected with one of these reporter plasmids, and reporter gene assay was performed after hypoxic exposure. As shown in Fig. 4A, luciferase activity from the 1HRE/WT-LUC reporter plasmid was increased in HCS-2/8 cell lysate stimulated by rCCN2. On the other hand, with 1HRE/HM-LUC reporter plasmid, luciferase activity did not change with rCCN2 (Fig. 4A). As expected, reporter gene expression from 2HRE-LUC was increased by rCCN2 at the dose of 50 and 100 ng/ml under hypoxic condition in HCS-2/8 cells (Fig. 4B). However, it was not increased by rCCN2 stimulation under normoxia. Taken together, these results indicate that rCCN2-mediated VEGF expression is mediated through HIF-1α.
Fig. 4.
Effect of CCN2 on the activation of HRE at −1006/−954 of the human VEGF promoter. (A) HCS-2/8 cells pre-treated with 100 ng/ml rCCN2 (closed column) or the same volume of PBS (open column) were transiently transfected either with the firefly luciferase reporter construct, 1HRE/WT-LUC containing the HRE at −1006/−954 of VEGF promoter, or its mutant, 1HRE/HM-LUC and thymidine kinase promoter-Renilla luciferase reporter plasmid (pRL-TK, internal control). After 24 h, cells were exposed to hypoxia (5% oxygen), and the cells were then assayed for luciferase activities. The ordinate shows the mean and standard error of relative value standardized against control culture of the measured luminescence of firefly versus Renilla luciferase of duplicate cultures. Relative luciferase activity of control culture is represented as 1.0. (B) HCS-2/8 cells pre-treated with 50 or 100 ng/ml rCCN2 (closed column) or same volume of PBS (open column) were transiently transfected with the reporter construct, 2HRE-LUC. After 24 h, the cells were exposed to normoxia or hypoxia (5% oxygen) for 24 h, and the cells were then assayed for luciferase activities. The ordinate shows the mean and standard error of relative value of the measured luminescence of firefly versus Renilla luciferase of duplicate cultures.
Production of VEGF and nuclear translocation of HIF-1α are decreased in Ccn2-deficient growth plate
As shown in Figs. 5A and E, safranin-O staining revealed reduced levels of proteoglycans in cartilage throughout Ccn2−/− growth plates. This finding indicates that cartilage ECM production is reduced in Ccn2−/− growth plates [13]. In wild-type mice, pre-hypertrophic chondrocytes were stained with anti-VEGF antibody; in addition, the immunoreactivity of VEGF was also shown in the proliferative chondrocytes therein (Figs. 5B, C; arrows) [31]. On the other hand, in Ccn2 mutant mice, VEGF signal in the proliferative and pre-hypertrophic regions was slightly detected (Figs. 5F, G). These findings suggest that CCN2 regulates the expression of Vegf in proliferative and pre-hypertrophic chondrocytes. It has been reported that proliferative and pre-hypertrophic zone of growth plate is under hypoxic condition [28]. Therefore, to investigate the role of HIF-1α, which was a key regulator involved in the expression of Vegf under hypoxia, we analyzed the localization of HIF-1α by immunohistochemistry. As shown in Fig. 5D, we detected HIF-1α in the nucleus of wild-type proliferative and pre-hypertrophic chondrocytes (arrows). In contrast, HIF-1α was not detected in the nuclei of the corresponding chondrocytes of Ccn2 mutant mice (Fig. 5H). These results suggest that distribution of HIF-1α is regulated by CCN2 in the growth plate under hypoxic condition.
Fig. 5.
Immunolocalization of VEGF and HIF-1α in sections of the growth plate cartilage of wild-type and Ccn2 mutant mice. Sections of the growth plates of E18.5 femurs in wild-type (A–D) and Ccn2 mutant (E–H) littermates were stained with safranin-O (A, E), and immunostained with anti-VEGF antibody (B, C, F and G), and with anti-HIF-1α antibody (D, H). The primary antibodies were visualized by immunoperoxidase, and then the sections were counterstained with methyl green. Images in C and G are magnifications of the boxed regions in B and F respectively. In the wild type, VEGF was localized in proliferative and pre-hypertrophic zone of the growth plate (C; arrows). In the Ccn2 mutant, it was slightly detected in proliferative and pre-hypertrophic zones (G). On the other hand, HIF-1α was localized in the nuclei of epiphyseal cartilage cells in the wild type (D: arrows), but in Ccn2 mutant, low level of expression was seen in the nuclei of epiphyseal cartilage cells (H). Bars in A, B, E, F and C, D, G, H represent 100 μm, and 50 μm, respectively.
Discussion
In this study, we clarified that CCN2 regulated gene expression of HIF-1α mRNA transcriptionally in HCS-2/8 cells (Fig. 3), and these findings indicate that CCN2 induces VEGF transcription at least in part via the activation of HIF-1α in HCS-2/8 cells under hypoxic conditions. However, under normoxic conditions, the expression of VEGF was not induced by CCN2. In normoxia, HIF-1α is unable to interact with HRE DNA sequence on the VEGF promoter due to decreased stability of HIF-1α, mediated the oxygen-regulated degradation [29]. Therefore, we hypothesized that even if CCN2 induces HIF-1α production, HIF-1α is rapidly degraded by proteasome in normoxia, whereas in hypoxia, increased stability of HIF-1α, permits its binding to HRE on the VEGF promoter. This idea was further supported by the data in Fig. 4A, showing no increase in promoter activity in HCS-2/8 cells treated with rCCN2 and transfected with a VEGF reporter plasmid containing a mutated HRE. Taken together, these findings suggest that up-regulation of VEGF induced by rCCN2 is mediated by HIF-1α activation. We reported that CCN2 was expressed in pre-hypertrophic region previously [32]. VEGF production was observed in a similar region [13,31] (Fig. 5). Therefore, we suspected that CCN2 and VEGF interacted with each other. In fact, it was reported that CCN2 directly bound to VEGF [15], and that CCN2 cleaved by matrix metalloproteinases (MMPs)-2, 3, or 7 regulated VEGF functions in vitro [15,33,34]. It should be also noted that VEGF induced the expression of CCN2 [35,36]. Here, although localization of VEGF was distinctly detected in pre-hypertrophic and hypertrophic regions in the wild type, it was decreased and found scattered in these region of Ccn2 mutant mice ([13], Fig. 5). These observations suggest that CCN2 and VEGF in pre-hypertrophic region participate in a feedback loop to regulate both the induction and activity each protein.
It is well known that VEGF is strong angiogenic factor. More recently it has been shown that one of the VEGF receptors, neuropilin (Npr) 1 is expressed in epiphyseal chondrocytes [17], suggesting that this receptor might be involved in mediating the effects of VEGF in epiphyseal chondrocytes. In addition, the results of cartilage-specific Vegf conditional knockout (CKO) mice showed delayed invasion of blood vessels into primary ossification centers and massive cell death in joint and epiphyseal regions [17]. These findings suggest that VEGF plays a significant role in chondrocyte development and survival as well as cartilage vascularization. Furthermore, these phenotypes of Vegf CKO mice were similar to those of Hif-1α CKO mice, indicating that VEGF expression is closely related to HIF-1α functions [17,28]. However, expression of Vegf was detected in Hif-1α CKO hypertrophic chondrocytes at E14.5 [17], suggesting that HIF-1α function is not a prerequisite for Vegf expression. On the other hand, in Ccn2 mutant mice, hypertrophic zones were expanded, and late stages of cartilage vascularization were impaired; however at E14.5, no differences in the level of Vegf expression could be detected in long bones between Ccn2 mutants and wild-type littermates [13]. Because Vegf expression pattern in Ccn2 mutants at E14.5 is similar to that in Hif-1α CKO mice, mutual regulation of gene expression is suspected between CCN2 and HIF-1α. In fact, there is an HRE DNA sequence in the CCN2 proximal promoter, and it has been shown that HIF-1α regulates the expression of CCN2 under hypoxia [37,38]. Collectively, this previous finding and the results of this study suggest that there is a positive feedback mechanism between CCN2 and HIF-1α in chondrocytes. On the other hand, many previous reports indicate that CCN2 either promoted or inhibited tumor growth and angiogenesis; CCN2 expression was increased in breast cancer [23] and hepatoma cells [39], compared with the corresponding normal tissue cells, whereas it was rather decreased in lung adenocarcinoma [40]. It was reported that CCN2 promoted proteasomal HIF-1α degradation and inhibited VEGF gene expression in human lung adenocarcinoma cell lines [40]. Because HIF activation can lead to changes in tumor behavior [41], these findings suggest that differential effects of CCN2 on tumor phenotype may be due to the conditional induction or degradation of HIF-1α by CCN2 in different tumor microenvironments.
In summary, we have shown that Hif-1α expression is induced by CCN2 in chondrocytes, and that the expression of Vegf is up-regulated by the binding of HIF-1α to the HRE DNA sequence in the VEGF promoter under hypoxic conditions. VEGF is known to function in angiogenesis, cartilage development and chondrocyte survival [17], and CCN2 is also known to function in chondrocyte proliferation, differentiation and hypertrophy [7]. Therefore, it is thought that Vegf induction via HIF-1α activation by CCN2 promotes chondrocyte differentiation and survival. In fact, in the Ccn2-deficient growth plate, HIF-1α was barely detected in the nucleus of proliferative and pre-hypertrophic layers, regions known to be hypoxic [28]. These findings suggest that HIF-1α activation by hypoxia was inefficient in the Ccn2-deficient growth plate. Therefore, decreased HIF-1α activation by CCN2 deletion cause impaired chondrocyte differentiation and survival, which may eventually lead to the inhibition of Vegf expression in hypertrophic chondrocytes. In this point of view, CCN2 may play as a “signal conductor” in endochondral ossification not only by modulating cell signaling by BMP and TGF-β [42] but also by controlling gene expression of extracellular signaling molecules, such as VEGF. Obviously, further investigation is needed to uncover the entire regulatory network surrounding 2 central regulators of endochondral ossification, CCN2 and VEGF.
Acknowledgments
This work was supported in part by the programs Grants-in-Aid for Young Scientists (B) (to TN) and for Exploratory Research (to MT), the Ministry of Education, Culture, Sports, Science and Technology, Japan, and the Grants-in-Aid for Scientific Research (S) (to MT) and (C) (to SK) from Japan Society for the Promotion of Sciences, a grant from the National Institutes of Health (to KML), and by grants from Ryobi-teien Memorial Foundation (to TN). We thank Drs. Takako Hattori, Eriko Aoyama, Harumi Kawaki, Kazumi Kawata, and Toshihiro Ohgawara for their helpful suggestions; and Yuki Nonami and Yoko Tada for secretarial assistance.
References
- 1.Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–8. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- 2.Maes C, Stockmans I, Moermans K, Van Looveren R, Smets N, Carmeliet P, et al. Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival. J Clin Invest. 2004;113:188–99. doi: 10.1172/JCI19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perbal B, Takigawa M. The CCN family of proteins: an overview. In: Perbal B, Takigawa M, editors. CCN proteins: a new family of cell growth and differentiation regulators. London: Imperial College Press; 2005. pp. 1–18. [Google Scholar]
- 4.Takigawa M. CTGF/Hcs24 as a multifunctional growth factor for fibroblasts, chondrocytes and vascular endothelial cells. Drug News Perspect. 2003;16:11–21. doi: 10.1358/dnp.2003.16.1.829302. [DOI] [PubMed] [Google Scholar]
- 5.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 6.Nakanishi T, Kimura Y, Tamura T, Ichikawa H, Yamaai Y, Sugimoto T, et al. Cloning of a mRNA preferentially expressed in chondrocytes by differential display-PCR from a human chondrocytic cell line that is identical with connective tissue growth factor (CTGF) mRNA. Biochem Biophys Res Commun. 1997;234:206–10. doi: 10.1006/bbrc.1997.6528. [DOI] [PubMed] [Google Scholar]
- 7.Nakanishi T, Nishida T, Shimo T, Kobayashi K, Kubo T, Tamatani T, et al. Effects of CTGF/Hcs24, a product of a hypertrophic chondrocyte-specific gene, on the proliferation and differentiation of chondrocytes in culture. Endocrinology. 2000;141:264–73. doi: 10.1210/endo.141.1.7267. [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, Nakanishi T, Asano M, Shimo T, Takigawa M. Effects of CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, on the proliferation and differentiation of osteoblastic cells in vitro. J Cell Physiol. 2000;184:197–206. doi: 10.1002/1097-4652(200008)184:2<197::AID-JCP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 9.Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, et al. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–45. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- 10.Takigawa M, Nakanishi T, Kubota S, Nishida T. Role of CTGF/Hcs24/ecogenin in skeletal growth control. J Cell Physiol. 2003;194:256–66. doi: 10.1002/jcp.10206. [DOI] [PubMed] [Google Scholar]
- 11.Kubota S, Takigawa M. Role of CCN2/CTGF/Hcs24 in bone growth. Int Rev Cytol. 2007;257:1–41. doi: 10.1016/S0074-7696(07)57001-4. [DOI] [PubMed] [Google Scholar]
- 12.Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, et al. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–19. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 13.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, et al. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–91. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuiper EJ, Roestenberg P, Ehlken C, Lambert V, van Trslong-de Groot HB, Lyons KM, et al. Angiogenesis is not impaired in connective tissue growth factor (CTGF) knock-out mice. J Histochem Cytochem. 2007;55:1139–47. doi: 10.1369/jhc.7A7258.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, et al. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219–21. doi: 10.1096/fj.01-0332fje. [DOI] [PubMed] [Google Scholar]
- 16.Jang HS, Kim HJ, Kim JM, Lee YS, Kim KL, Kim JA, et al. A novel ex vivo angiogenesis assay based on electoroporation-mediated delivery of naked plasmid DNA to skeletal muscle. Mol Ther. 2004;9:464–74. doi: 10.1016/j.ymthe.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Zelzer E, Mamluk R, Ferrara N, Johnson RS, Schipani E, Olsen BR. VEGFA is necessary for chondrocyte survival during bone development. Development. 2004;131:2161–71. doi: 10.1242/dev.01053. [DOI] [PubMed] [Google Scholar]
- 18.Takigawa M, Tajima K, Pan HO, Enomoto M, Kinoshita A, Suzuki F, et al. Establishment of a clonal human chondrosarcoma cell line with cartilage phenotypes. Cancer Res. 1989;49:3996–4002. [PubMed] [Google Scholar]
- 19.Takigawa M, Pan HO, Kinoshita A, Tajima K, Takano Y. Establishment from a human chondrosarcoma of a new immortal cell line with high tumorigenicity in vivo, which is able to form proteoglycan-rich cartilage-like nodules and to respond to insulin in vitro. Int J Cancer. 1991;48:717–25. doi: 10.1002/ijc.2910480515. [DOI] [PubMed] [Google Scholar]
- 20.Zhu JD, Pan HO, Suzuki F, Takigawa M. Proto-oncogene expression in a human chondrosarcoma cell line: HCS-2/8. Jpn J Cancer Res. 1994;85:364–71. doi: 10.1111/j.1349-7006.1994.tb02368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishida T, Nakanishi T, Shimo T, Asano M, Hattori T, Tamatani T, et al. Demonstration of receptors specific for connective tissue growth factor on a human chondrocytic cell line (HCS-2/8) Biochem Biophys Res Commun. 1998;247:905–9. doi: 10.1006/bbrc.1998.8895. [DOI] [PubMed] [Google Scholar]
- 22.Nishida T, Kawaki H, Baxter RM, Deyoung RA, Takigawa M, Lyons KM. CCN2 (connective tissue growth factor) is essential for extracellular matrix production and integrin signaling in chondrocytes. J Cell Commun Signal. 2007;1:45–58. doi: 10.1007/s12079-007-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondo S, Kubota S, Shimo T, Nishida T, Yosimichi G, Eguchi T, et al. Connective tissue growth factor increased by hypoxia may initiate angiogenesis in collaboration with matrix metalloproteinases. Carcinogenesis. 2002;23:769–76. doi: 10.1093/carcin/23.5.769. [DOI] [PubMed] [Google Scholar]
- 24.Nishida T, Kubota S, Nakanishi T, Kuboki T, Yosimichi G, Kondo S, et al. CTGF/Hcs24, a hypertrophic chondrocyte-specific gene product, stimulates proliferation and differentiation, but not hypertrophy of cultured articular chondrocytes. J Cell Physiol. 2002;192:55–63. doi: 10.1002/jcp.10113. [DOI] [PubMed] [Google Scholar]
- 25.Nishida T, Kubota S, Fukunaga T, Kondo S, Yosimichi G, Nakanishi T, et al. CTGF/Hcs24, hypertrophic chondrocyte-specific gene product, interacts with perlecan in regulating the proliferation and differentiation of chondrocytes. J Cell Physiol. 2003;196:265–75. doi: 10.1002/jcp.10277. [DOI] [PubMed] [Google Scholar]
- 26.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondo S, Kubota S, Mukudai Y, Moritani N, Nishida T, Matsushita H, et al. Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-un-tanslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene. 2006;25:1099–110. doi: 10.1038/sj.onc.1209129. [DOI] [PubMed] [Google Scholar]
- 28.Schipani E, Ryan HE, Didrickson S, Kobayashi T, Knight M, Johnson RS. Hypoxia in cartilage: HIF-1α is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–76. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–80. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- 30.Sánchez-Elsner T, Botella LM, Velasco B, Corbi A, Attisano L, Bernabéu C. Synergistic cooperation between hypoxia and transforming growth factor-β pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–35. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]
- 31.Cramert T, Schipani E, Johnson RS, Swoboda B, Pfander D. Expression of VEGF isoforms by epiphyseal chondrocytes during low-oxygen tension is HIF-1α dependent. Osteoarthr Cartil. 2004;12:433–9. doi: 10.1016/j.joca.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Oka M, Kubota S, Kondo S, Eguchi T, Kuroda C, Kawata K, et al. Gene expression and distribution of connective tissue growth factor (CCN2/CTGF) during secondary ossification center formation. J Histochem Cytochem. 2007;55:1245–55. doi: 10.1369/jhc.7A7263.2007. [DOI] [PubMed] [Google Scholar]
- 33.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor165. J Biol Chem. 2002;277:36288–95. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 34.Dean RA, Butler GS, Hamma-Kourbali Y, Delbe J, Brigstock DR, Courty J, et al. Identification of candidate angiogenic inhibitors processed by MMP-2 in cell based proteomic screens: Disruption of VEGF/HARP (pleiotrophin) and VEGF/CTGF angiogenic inhibitory complexes by MMP-2 proteolysis. Mol Cell Biol. 2007;27:8454–65. doi: 10.1128/MCB.00821-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimo T, Nakanishi T, Nishida T, Asano M, Sasaki A, Kanyama M, et al. Involvement of CTGF, a hypertrophic chondrocytes-specific gene product, in tumor angiogenesis. Oncology. 2001;61:315–22. doi: 10.1159/000055339. [DOI] [PubMed] [Google Scholar]
- 36.Kondo S, Tanaka N, Kubota S, Mukudai Y, Yosimichi G, Sugahara T, et al. Novel angiogenic inhibitor DN-9693 that inhibits post-transcriptional induction of connective tissue growth factor (CTGF/CCN2) by vascular endothelial growth factor in human endothelial cells. Mol Cancer Ther. 2006;5:129–37. doi: 10.1158/1535-7163.MCT-05-0097. [DOI] [PubMed] [Google Scholar]
- 37.Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:1223–32. doi: 10.1152/ajprenal.00245.2004. [DOI] [PubMed] [Google Scholar]
- 38.Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol. 2006;146:362–70. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zeng ZJ, Yang LY, Ding X, Wang W. Expression of cystein-rich 61, connective tissue growth factor and Nov genes in hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2004;10:3414–8. doi: 10.3748/wjg.v10.i23.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang CC, Lin MT, Lin BR, Jeng YM, Chen ST, Chu CY, et al. Effect of connective tissue growth factor on hypoxia-inducible factor-1α degradation and tumor angiogenesis. J Natl Cancer Inst. 2006;98:984–95. doi: 10.1093/jnci/djj242. [DOI] [PubMed] [Google Scholar]
- 41.Fang J, Yan L, Shing Y, Moses MA. HIF-1α-mediated up-regulation of vascular endothelial growth factor, independent of basic fibroblast growth factor, is important in the switch to the angiogenic phenotype during early tumorigenesis. Cancer Res. 2001;61:5731–5. [PubMed] [Google Scholar]
- 42.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-β. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]