Abstract
Memory T cells expressing CLA occur in humans and accumulate in normal and inflamed skin. These cells uniformly bind to the vascular adhesion molecule E-selectin, yet only a subset bind to P-selectin. The latter cells are distinguished by the mAb CHO-131, and are enriched in psoriasis lesions. Activated T cells up-regulate CLA expression, but little is currently known about their binding to P-selectin. We observed that CLA+CD4+ T cells derived from stimulated naïve T cells uniformly express the CHO-131 epitope. This occurred as well upon the restimulation of memory CLA+CD4+ T cells. The latter cells also expressed higher levels of PSGL-1 modified by P-selectin glycan ligands; C2GlcNAcT-1 mRNA, a glycosyltransferase critical for such glycan synthesis; and more uniformly bound to P-selectin. Our findings thus indicate that unlike memory CLA+CD4+ T cells, when activated these cells can broadly bind to P-selectin, suggesting a more diverse tissue trafficking capacity.
Keywords: skin, adhesion molecules, inflammation, T cell
INTRODUCTION
Most T cells in normal and diseased skin of humans express the cutaneous lymphocyte-associated antigen (CLA) [1–3]. CLA is a glycan moiety that includes sialyl-LewisX (sLeX) and requires the glycosyltransferase α1,3-fucosyltransferases-VII (FucT-VII) for its biosynthesis [4,5]. CLA is most notably detected by the mAb HECA-452 [6,7], and decorates many of the ligands recognized by the adhesion molecules E- and P-selectin [8–10], which are expressed by vascular endothelial cells at sites of acute inflammation as well as chronically inflamed skin [11]. In contrast to E-selectin, P-selectin binding by leukocytes also requires expression of the O-glycan branching enzyme core 2 β1,6 N-acetylglucosaminyltransferase-I (C2GlcNAcT-I, C2GnT-I, or C2GnT-L), which is involved in the biosynthesis of sLeX-modified, core 2 O-glycans (C2-O-sLeX) [12–14]. C2-O-sLeX decorates P-selectin glycoprotein ligand-1 (PSGL-1, CD162) [12,15], and is directly recognized by the mAb CHO-131 [16]. CHO-131 stains all neutrophils, which uniformly bind to P-selectin, but stains only a subset of resting CLA+ T cells [16]. CHO-131+ CLA+ T cells in the peripheral blood of healthy individuals preferentially bind to P-selectin and are enriched at sites of psoriasis lesions [17].
CLA expression occurs by a subset of memory T cells, but not by naïve T cells, in healthy individuals [7]. CLA+ T cells comprise, in part, Th1, Th2, cytotoxic T cells, and T regulatory cells [18–24], and are important for flexible host immune responses to skin-invading pathogens and cutaneous neoplasms, but have also been implicated in various inflammatory diseases and particular skin-infiltrating T cell malignancies [1,2,22,25,26]. CLA and E-selectin ligand expression are up-regulated by naïve T cells upon their stimulation in vitro or their transition to effector T cells in secondary lymphoid organ [7,27,28]. To the best of our knowledge, however, nothing is currently known about the regulation of P-selectin binding by LA+ T cells, such as by newly formed CLA+ T cells upon naïve cell stimulation or following the reactivation of memory CLA+ T cells. In this study, we examined P-selectin ligand expression by CLA+ CD4+ T cells following the activation of naïve and memory T cells. We report that in contrast to memory CLA+ CD4+ T cells, essentially all newly formed and restimulated CLA+ CD4+ T cells are stained by CHO-131 and bind to P-selectin. Moreover, these cells up-regulate the expression of surface PSGL-1 molecules modified by C2-O-sLeX. Our findings thus provide novel insights into the P-selectin binding activity of recently activated CLA+ T cells, and reveal differential P-selectin binding by resting and activated CLA+ T cells.
MATERIALS AND METHODS
Antibodies
The anti-C2-O-sLeX mAb CHO-131 has been previously described [16,17,29–33]. Biotinylation of CHO-131 was performed using NHS-SS-LC-biotin (Pierce, Rockford, IL), as per the manufacturer’s instructions. FITC, PE, or biotin conjugated HECA-452, anti-CD45RA-FITC, anti-CD45RO-PE, and streptavidin-PerCP-Cy5.5 were purchased from BD Pharmingen (San Diego, CA). Anti-CD69-PE, anti-CD25-PE, anti-CD3ε, and human P-selectin/human IgG Fc chimeric construct (P-selectin/Fc) were purchased from R&D systems (Minneapolis, MN). CD4-FITC, CD4-allophycocyanin (APC) and anti-PSGL-1-biotin (PL1) were purchased from Ancell (Bayport, MN). The anti-human PSGL-1 mAb 215 (unconjugated or conjugated to PE) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-actin (Ab-1) and HRP-conjugated goat anti-mouse IgM were purchased from Oncogene Research Products (San Diego, CA). The appropriately unconjugated and conjugated isotype-matched negative control antibodies were purchased from Caltag Laboratories (Burlingame, CA). FITC or PE-conjugated F(ab')2 goat anti-mouse IgG, PE-conjugated F(ab')2 goat anti-human IgG, APC-conjugated streptavidin, as well as normal mouse and rat serum were purchased from Jackson ImmunoResearch (West Grove, PA).
T cell isolation
Peripheral blood was collected from normal donors in sodium heparin. Fresh tonsil tissue was obtained from the Tissue Procurement Facility at the University of Minnesota’s Masonic Cancer Center. Blood and tissue was obtained in accordance with approved protocols by the Institutional Review Board: Human Subjects Committee at the University of Minnesota. Mononuclear cells were obtained from blood and dissociated tonsil tissue by Ficoll Hypaque density gradient centrifugation using standard procedures. All T cell sorting was perform using Miltenyi microbeads (Auburn, CA), as previously described with some modifications [17]. Enrichment of CD4+ T cells from the isolated mononuclear cells was performed by negative selection using a CD4+ T Cell Isolation Kit II. The enriched CD4+ T cells were then subjected to positive selection using CD45RA microbeads to enrich naïve T cells, or they were stained with anti-CLA (HECA-452-PE, Miltenyi) and subjected to positive selection using anti-PE microbeads to enrich CLA+ CD4+ T cells, or they were stained with CHO-131-biotin and depleted of CHO-131+ cells by anti-biotin microbeads and then stained with HECA-452-PE and subjected to positive selection using anti-PE microbeads to enrich CHO-131− CLA+ CD4+ T cells. All cell sorting procedures were performed according to the manufacture’s instructions and resulted in >93% enrichment of the intended populations with a viability of >90%, as determined by exclusion of the vital dye trypan blue.
T cell activation culture
For T cell activation, 24 well plates were precoated with 5 µg/ml anti-CD3 mAb in PBS at 4°C overnight. Isolated naïve CD4+ T cells, CLA+ CD4+ T cells, or CHO-131− CLA+ CD4+ T cells were plated at 5×105 cells/ml in RPMI 1640 containing 10% FCS, 1% penicillin/streptomycin, 2 mM L-glutamine, and 5 ng/ml rhIL-2 (PeproTech, Rocky Hill, NJ) at 37°C. After two days, the cells were washed with PBS three times, and plated (without adsorbed anti-CD3 mAb) at 5×105 cells/ml in the same media containing 5 ng/ml rhIL-2. Cells were expanded for the indicated time points and used for flow cytometric analyses, RT-PCR, and immunoblotting.
T cell staining for flow cytometric analyses
Multi-color T cell staining was performed as previously described with some modifications [16,17]. To assess HECA-452 staining of sorted naïve CD4+ T cells, the cells were harvested from culture at the indicated time points and sequentially treated with HECA-452-PE and anti-CD4-FITC. To assess simultaneous HECA-452 and CHO-131 staining of sorted naïve CD4+ T cells, sorted CLA+ CD4+ T cells, or sorted CHO-131− CLA+ CD4+ T cells, the respective cells were harvested from culture at the indicated time points and sequentially treated with CHO-131, PE-conjugated F(ab')2 goat anti-mouse IgM, 10% normal mouse and rat serum in PBS, HECA-452-biotin, streptavidin-APC, and then anti-CD4-FITC. To assess CD25, CD69, or PSGL-1 surface expression by sorted CLA+ CD4+ T cells, the cells were harvested from culture at the indicated time points and treated with HECA-452-FITC, anti-CD4-APC, and anti-CD25-PE, anti-CD69-PE, or anti-PSGL-1-PE. Cells dual stained by HECA-452 and anti-CD4 were electronically gated on and examined for their staining by anti-CD25, anti-CD69, or anti-PSGL-1. To assess P-selectin ligand expression by sorted CLA+ CD4+ T cells, the cells were harvested from culture at the indicated time points and treated with 10µg/ml of P-selectin/Fc at 4°C with gentle shaking for 30 min in the presence or absence of EDTA (1mM). The cells were then treated with PE-conjugated F(ab')2 goat anti-human IgG. To assess HECA-452 or CHO-131 staining of tonsil lymphocytes, the isolated mononuclear cells were stained with CHO-131-biotin or HECA-452-biotin, streptavidin-PerCP-Cy5.5, and then anti-CD4-APC, anti-CD45RA-FITC, and anti-CD45RO-PE. Cells triple stained by anti-CD4, anti-CD45RA, and anti-CD45RO were electronically gated on and examined for their staining by CHO-131 or HECA-452. Isotype-matched negative control mAbs were used to evaluate levels of background staining. All antibody staining steps were performed at 4°C with washing (PBS) between each step. Cells were fixed in 1% paraformaldehyde and 5,000 – 50,000 cells were acquired by a FACSCanto flow cytometer (Becton Dickinson, San Jose, CA) and analyzed with CellQuest Pro (Becton Dickinson) or Flowjo (Dako-Cytomation, Ft. Collins Colorado) software. No significant staining of T cells occurred by the antibodies used for microbead positive sorting following two days of activation culture (data not shown).
Semiquantitative RT-PCR analysis of C2GlcNAcT-1
Detection of C2GlcNAcT-I mRNA levels by RT-PCR was performed as described with modifications [34]. Activated T cells were harvested at the indicated time points and total cellular RNA was isolated from 1×106 cells at the indicated time points using a Qiagen RNeasy Mini Kit along with the RNase-Free DNase Set to remove residual amounts of DNA, which were performed according to the manufacturer’s instructions (Qiagen, Valencia, CA). Reverse transcription and PCR were performed sequentially using a Qiagen OneStep RT-PCR Kit and C2GlcNAcT-I gene-specific primers, as per the manufacturer’s instructions. The primers designed for the human gene C2GlcNAcT-I were 5’-GCAGCAACGTCCTCAGCAT-3’and 5’-GATGTCACCTGGAATCAGCA-3’, which generate a 196 bp PCR product and do not amplify genomic DNA [34]. The PCR conditions consisted of 95°C for 5 min and 30 cycles of 94°C 30 sec; 59°C 30 sec; 72°C 30 sec, and a final 72°C for 5 min. Thirty cycles were determined to be below the plateau phase of amplification for all primers (data not shown), giving an accurate reflection of the relative starting levels of mRNA. As an internal control for the RT-PCR analysis, the human eukaryotic translation elongation factor 1 transcripts were amplified from the same RNA samples using the primers 5’-TGTCAAGGATGTTCGTCGTG-3’ and 5’-GCCTGGATGGTTCAGGATAA-3’. PCR products were detected by 1.5% agarose gel electrophoresis.
Immunoprecipitation of PSGL-1 and immunoblotting analysis
Immunoprecipitation and immunoblotting procedures were performed as previously described [35–38]. Briefly, cultured cells were harvested at the indicated time points, washed with PBS, then detergent extracted with cold lysis buffer (HBSS without Ca2+ and Mg2+, 25mM HEPES, 1% Triton-X100, 10mM NaN3 containing 1X fresh Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) for 1 hr under rotation at 4°C. Insoluble materials were removed by centrifugation at 12,000 × g. The protein concentration of the supernatant was determined using a BCA protein assay kit (Pierce), per the manufacture’s instructions. For all samples, a supernatant volume corresponding to 50µg protein (≈ 1×107 cells) was precleared with a Sepharose bead slurry (Sigma-Aldrich, St. Louis, MO), followed by anti-PSGL-1 mAb (215)-conjugated Sepharose bead slurry. After the immunoprecipitation, the beads were extensively washed (HBSS without Ca2+ and Mg2+, 25mM HEPES, 0.1% Triton-X100, and 10mM NaN3), and boiled in SDS sample buffer under reducing conditions to elute PSGL-1. The blots were probed with biotin-conjugated anti-PSGL-1 or biotin conjugated-CHO-131, followed by HRP-conjugated-streptavidin (Pierce, 1:10,000). Antibody reactivity was visualized by addition of SuperSignal chemiluminescent substrate (Pierce) and exposure to film, as per manufacturer’s instructions. Densitometric analysis was performed using NIH ImageJ.
RESULTS
C2-O-sLeX expression is up-regulated following the stimulation of naïve CD4+ T cells and memory CLA+ CD4+ T cells
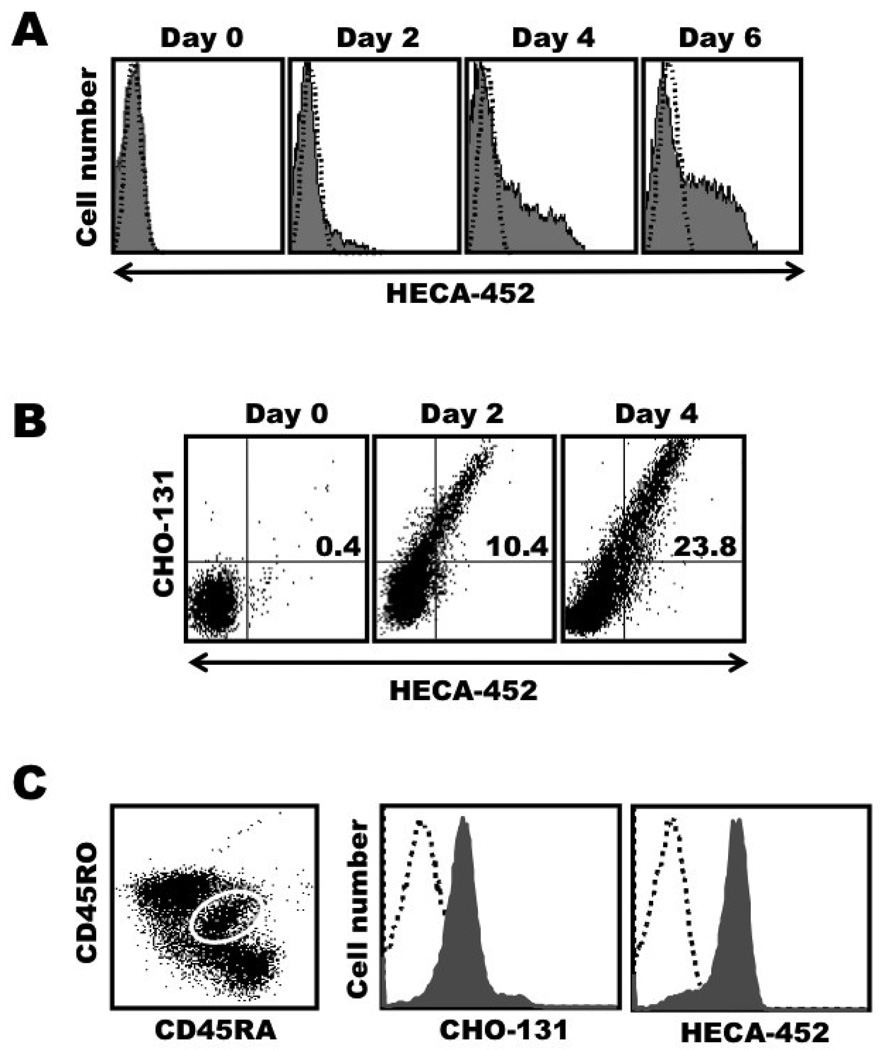
Naïve T cells do not express CLA, yet their stimulation in vitro, for instance by anti-CD3 antibody in the presence of IL-2, results in the up-regulation of FucT-VII expression, E-selectin ligands, and CLA [7,27,28]. We also activated naïve CD4+ T cells isolated from the peripheral blood of healthy individuals with anti-CD3 antibody and IL-2, and observed a similar induction of CLA expression by the newly activated T cells (Fig. 1A). We then stained the activated T cells with CHO-131 and HECA-452 and found that the mAbs were mutually reactive (Fig. 1B). Picker et al. have reported that T cells transitioning from naïve to effector cells in secondary lymphoid organs, identified by a CD45RA/RO double positive phenotype, become CLA+. An examination of this same transitioning population in lymphoid tissues revealed that these cells were essentially uniformly stained by HECA-452 and CHO-131. Indeed, both mAbs shifted the entire stained population to the right with respect to isotype-matched, negative control mAb staining (Fig. 1C). Taken together, the data above reveal that newly formed CLA+ CD4+ T cells broadly express C2-O-sLeX.
Figure 1. Expression kinetics of CLA and the CHO-131 epitope C2-O-sLeX upon the stimulation of naive CD4+ T cells.
Freshly isolated, peripheral blood lymphocytes sorted for naive CD4 T cells (CD45RA+ CD4+ T cells) were stimulated with anti-CD3 mAb and IL-2 for 2 days (activation phase), then cultured in the presence of IL-2 only for 4 more days (expansion phase), for a total cell culture period of 6 days, as described in Materials and Methods. Day 0, freshly isolated cells; Day 2, activation phase; Days 4 and 6, expansion phase. (A) The cultured cells were harvested at the indicated time points and analyzed for the expression of CLA (HECA-452 staining). (B) Expression kinetics of the CHO-131 and HECA-452 epitopes by newly activated CD4 T cells at various time points following their activation. (C) Freshly isolated human lymphocytes from tonsil tissue, electronically gated by morphology (forward and side light scatter profile characteristic of lymphocytes), CD4 expression, and dual expression of CD45RA and CD45RO (left panel, white-lined gate), were analyzed for HECA-452 or CHO-131 staining (right panels). All analyses were performed by flow cytometry. For all plots, the indicated mAb reactivities on the x and y axes represent Log 10 fluorescence. Non-specific antibody labeling was determined using the appropriate isotype negative control antibodies, as indicated and data not shown. Data are representative of at least three independent experiments using T cells isolated from separate donors.
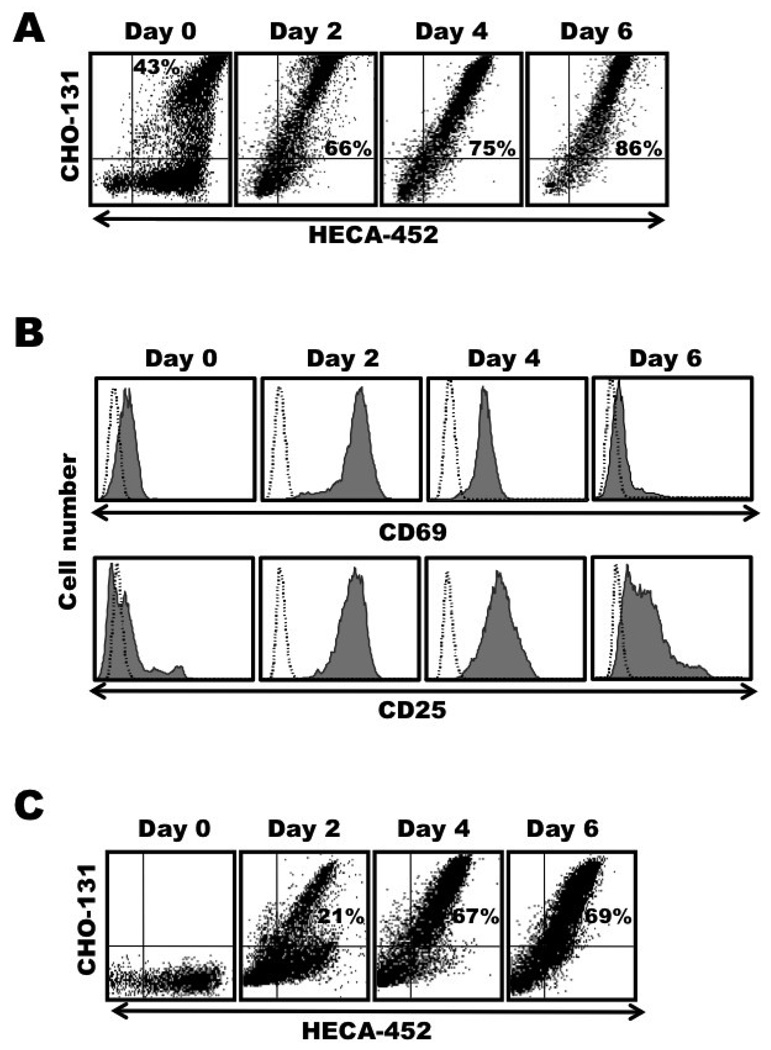
Next we sorted CLA+ CD4+ T cells from the peripheral blood of healthy individuals and stimulated them, as described above. In contrast to resting CLA+ T cells of which only a subset are stained by CHO-131, basically all of the CLA+ CD4+ T cells were reactive with CHO-131 following their stimulation (Fig. 2A). The treated cells also up-regulated expression of the activation markers CD25 and CD69 (Fig. 2B), indicating that the lymphocytes underwent stimulation and not selective cell death. Because it could also be argued that the CHO-131+ CLA+ CD4+ T cells may have undergone preferential expansion during T cell stimulation, we sorted the CHO-131− CLA+ CD4+ T cells and stimulated them as well. We found that following their stimulation; nearly all of the CLA+ cells became CHO-131+ (Fig. 2C).
Figure 2. Memory CLA+ CD4+ T cells are stained in essentially a uniform manner by CHO-131 following their activation.
Freshly isolated, peripheral blood lymphocytes sorted for CLA+ CD4+ T cells or CHO-131− CLA+ CD4+ T cells were stimulated with anti-CD3 mAb plus IL-2, as described in Figure 1. Day 0, freshly isolated cells; Day 2, activation phase; Days 4 and 6, expansion phase. (A) Activated CLA+ CD4+ T cells were harvested at the indicated time points and dual stained with the mAbs CHO-131 and HECA-452. (B) In addition, the activated CLA+ CD4+ T cells were stained for CD69 expression (top panels) or CD25 expression (bottom panels). (C) The expression kinetics of C2-O-sLeX by activated CHO-131− CLA+ CD4+ T cells was analyzed by dual staining with the mAbs CHO-131 and HECA-452. All analyses were performed by flow cytometry. For all plots, the indicated mAb reactivities on the x and y axes represent Log 10 fluorescence. Non-specific antibody labeling was determined using the appropriate isotype negative control antibodies, as indicated and data not shown. Data are representative of at least three independent experiments using T cells isolated from separate donors.
Memory CLA+ CD4+ T cells up-regulate P-selectin ligand expression upon their restimulation
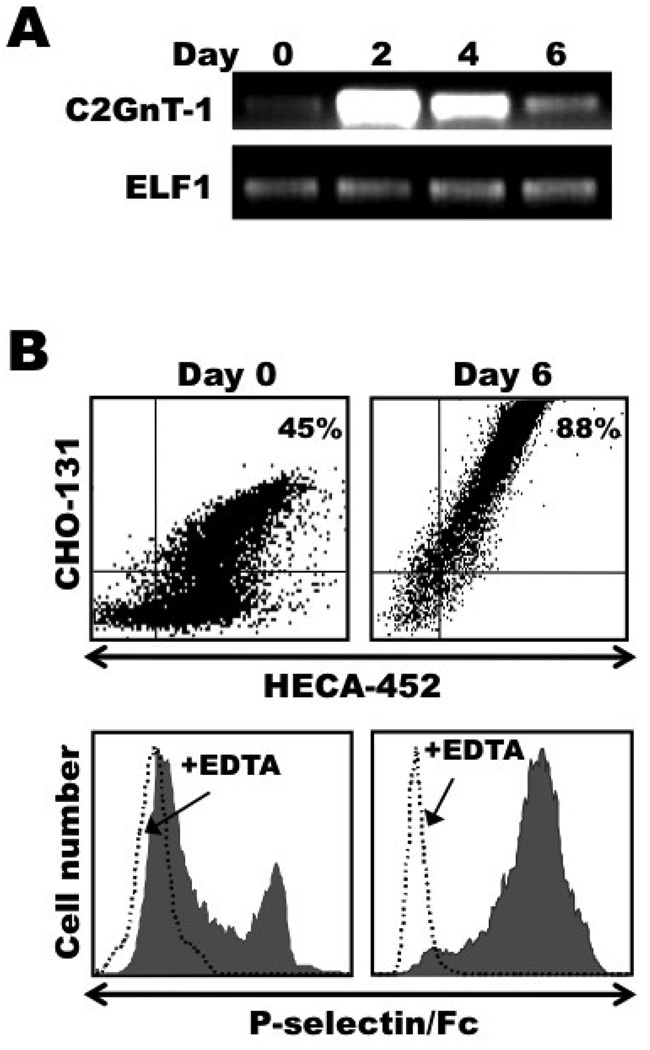
The core 2 branching O-glycan enzyme C2GlcNAcT-I is critical for the biosynthesis of C2-O-sLeX glycans and P-selectin binding [12,16,17,29,30]. Semiquantitative RT-PCR analysis was performed to assess the mRNA levels of C2GlcNAcT-I in resting and activated CLA+ CD4+ T cells. As shown in Figure 3A, the levels of C2GlcNAcT-I mRNA in CLA+ CD4+ T cells were found to be greatly up-regulated following their stimulation with anti-CD3 antibody and IL-2. We also directly examined the P-selectin binding activity of activated CLA+ CD4+ T cells using soluble P-selectin/Fc in flow cytometric assays. Resting memory CLA+ CD4+ T cells were enriched from the peripheral blood of healthy individuals, and, as we have previously described, only a subset of these cells were reactive with P-selectin [Figure 3B and ref.17]. As with CHO-131 staining, following their stimulation with anti-CD3 and IL-2, essentially all of the CLA+ CD4+ T cells were reactive with P-selectin/Fc, which was sensitive to EDTA (Fig. 3B).
Figure 3. Activated CLA+ CD4+ T cells up-regulate their expression of functional P-selectin ligand.
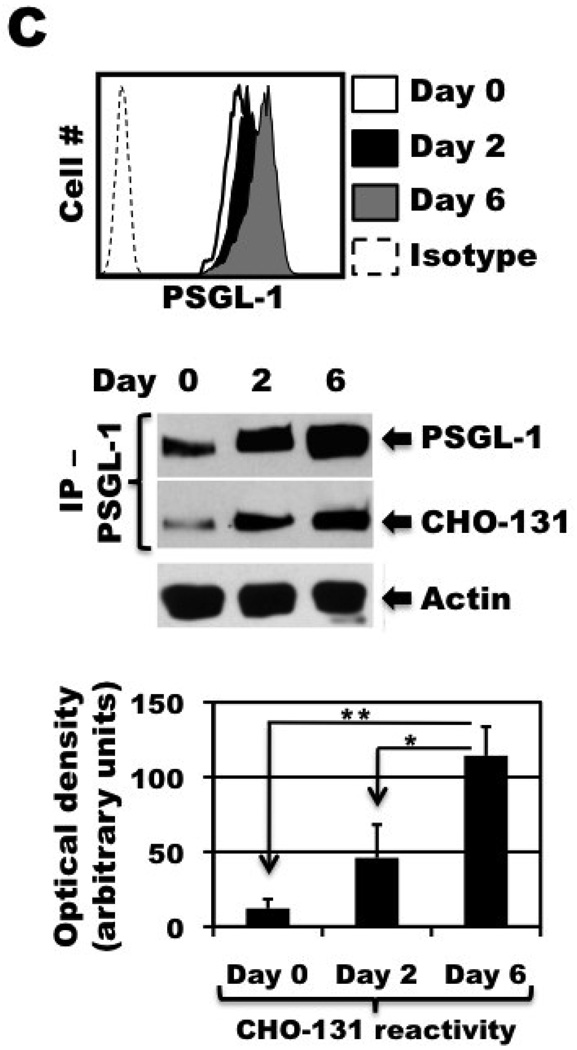
Freshly isolated, peripheral blood lymphocytes sorted for CLA+ CD4+ T cells were stimulated with anti-CD3 mAb and IL-2, as described in Figure 1. Day 0, freshly isolated cells; Day 2, activation phase; Days 4 and 6, expansion phase. (A) Activated CLA+ CD4+ T cells were harvested at the indicated time points and total RNA was isolated. Semiquantitative RT-PCR was then performed, using primers specific for the C2GlcNAcT-I gene (upper panel) or the “housekeeping gene” ELF1 (lower panel), as described in Materials and Methods. (B) Activated CLA+ CD4+ T cells were harvested at the indicated time points and dual stained with the mAbs CHO-131 and HECA-452 (top panels) or stained with P-selectin/Fc in the presence or absence of EDTA, as indicated (bottom panels). Relative protein expression levels were determined by flow cytometry. MAb reactivities on the x and y axes represent Log 10 fluorescence. Non-specific antibody labeling was determined using the appropriate isotype negative control antibodies (data not shown). (C) Activated CLA+ CD4+ T cells were harvested at the indicated time points and analyzed for the expression of PSGL-1 by flow cytometry (left panel). Equivalent cell numbers of the activated CLA+ CD4+ T cells at the indicated time points were detergent extracted and directly subjected to SDS-PAGE for the detection of actin content to assess cell equivalency among the samples, or treated with anti-PSGL-1 mAb for immunoprecipitation (IP - PSGL-1). All samples were subjected to SDS-PAGE and immunoblotting with an anti-PSGL-1 mAb or with CHO-131 (right panel), as described in the Materials and Methods. Non-specific antibody labeling of immunoprecipitated PSGL-1 was determined using the appropriate isotype negative control antibodies, which showed no detectable bands (data not shown). All data are representative of three independent experiments using T cells isolated from separate donors. Densitometric data from three independent experiments are expressed as mean ± SD. *, p = 0.0139 day 6 vs. day 2 stimulated cells; **, p = 0.0065 day 6 vs. day 0 resting cells.
PSGL-1 is the primary protein ligand of P-selectin, but its functional state depends on, in part, C2-O-sLeX modification [12–14]. To determine whether the up-regulation in expression of the CHO-131 epitope by activated CLA+ CD4+ T cells was associated with PSGL-1, the glycoprotein was immunoprecipitated from sorted CLA+ CD4+ T cells prior to and after their stimulation with anti-CD3 antibody and IL-2. Detection of PSGL-1 by immunoblotting with an anti-PSGL-1 mAb revealed increasing levels of its expression at various time points following T cell activation (Fig. 3C), which was also observed by flow cytometry (Fig. 3C). Moreover, CHO-131 demonstrated significantly increased reactivity with immunoprecipitated PSGL-1 for day 6 stimulated T cells compared to day 2 stimulated and resting T cells (Fig. 3C), demonstrating a direct correspondence in P-selectin binding by T cells and CHO-131 reactivity with PSGL-1. For each time point shown in Figure 3C, equal cell numbers of activated CLA+ CD4+ T cells were detergent extracted and equivalent protein concentrations of each cell lysate were subjected to immunoprecipitation, as indicated by the detection of actin (Fig. 3C). Taken together, these findings demonstrate that the expression of C2-O-sLeX-modified PSGL-1 is up-regulated by CLA+ CD4+ T cells upon their activation.
DISCUSSION
Circulating CLA+ T cells in healthy individuals are a component of the memory cell compartment. It is well established that CLA expression by T cells directly correlates with E-selectin binding [6,11,17,18,39]. The CHO-131 epitope comprises the C2-O-sLeX glycan structure [16], and the mAb stains a subset of CLA+ T cells that are enriched in P-selectin binding cells [17]. Upon T cell activation, the expression of CLA can be up-regulated [7,27,28]; however, little is know about the regulation of P-selectin ligand expression by activated CLA+ T cells. This was the focus of the current study.
We examined both newly activated CLA+ CD4+ T cells upon naïve T cell stimulation and re-stimulated, memory CLA+ CD4+ T cells. The induction of CLA expression by naïve T cells following TCR stimulation in vitro is well documented [7,27,28]. We found that the newly generated CLA+ CD4+ T cells up-regulated expression of the CHO-131 epitope in essentially a uniform manner. CD4+ T cells transitioning from naïve cells to effector cells in peripheral lymph nodes and tonsils, identified by their dual expression of CD45RA and CD45RO, also up-regulate CLA expression [7]. Upon examining this same population isolated from human tonsils, it was noted that nearly all of the CD45RA+ CD45RO+ CD4+ T cells were stained by HECA-452 and CHO-131. Moreover, memory CLA+ CD4+ T cells when re-stimulated in vitro essentially all became CHO-131+ as well. It is unlikely that this was due to a preferential outgrowth of the CHO-131+ CLA+ CD4+ T cells or due to a rapid death by the CHO-131− CLA+ CD4+ T cells within the initial cell population, as sorted CHO-131− CLA+ CD4+ T cells also transitioned into CHO-131+ CLA+ CD4+ T cells following their stimulation, with similar kinetics as stimulated CLA+ CD4+ T cells.
Surface expression of the widely described T cell activation markers CD25 and CD69 were up-regulated as well by stimulated CLA+ CD4+ T cells. CD25 and CD69 are known to rapidly down-regulate in expression once TCR stimulation wanes. We observed a similar down-regulation in the expression of these markers soon after the CLA+ CD4+ T cell activation phase, whereas expression of the CHO-131 epitope remained high during the expansion phase. The long-term duration of CHO-131 staining of CLA+ CD4+ T cells as they enter a resting state in vitro could not be determined, however, since the cells ultimately died without continued TCR stimulation. It has been reported that CD43 undergoes increased core 2 O-glycan modification upon the stimulation of murine and human T cells [40,41]. For instance, such modification of CD43 occurred upon the activation of both antigen specific, naïve and memory CD8+ T cells in P14 LCMV TCR-transgenic mice infected with LCMV, which correlated with their acquisition of effector activities [41]. This glycan decoration of CD43 was then lost as the T cells entered a resting memory state, indicating that core 2 O-glycan modification of cell surface proteins is a transient process and characteristic of T cells in the effector, but not memory, cell compartment [41]. Taken together with our findings, we speculate that CHO-131 staining of CLA+ CD4+ T cells may distinguish recently activated (effector) CLA+ CD4+ T cells from CLA+ CD4+ T cells in the memory compartment.
PSGL-1, the primary glycoprotein ligand for P-selectin, is constitutively expressed by T cells; however, it is only functional when properly modified, which includes its decoration by C2-O-sLeX [14,42,43]. In addition to reporting an up-regulation in C2GlcNAcT-I mRNA expression and the biosynthesis of C2-O-sLeX by recently activated CLA+ CD4+ T cells, we also demonstrate that essentially all of these cells bind to P-selectin in contrast to resting CLA+ CD4+ T cells. To determine whether CHO-131 is directly reactive with PSGL-1 expressed by stimulated CLA+ CD4+ T cells, immunoprecipitated PSGL-1 from these cells was immunoblotted with CHO-131. We found that PSGL-1 expression itself was up-regulated following the activation of CLA+ CD4+ T cells. Others have also shown that surface expression of PSGL-1 can be up-regulated upon T cell stimulation in an antigen specific manner using TCR transgenic mice [44,45]. Moreover, we found that CHO-131 directly recognized PSGL-1 and the expression of C2-O-sLeX-modified PSGL-1 significantly increased following the activation of CLA+ CD4+ T cells.
Our studies thus demonstrate that recently activated CLA+ CD4+ T cells express E- and P-selectin ligands in conjunction. The mechanisms regulating expression of the glycosyltransferases involved in the biosynthesis of E- and P-selectin glycan ligands are becoming better understood [11]. Of interest is that the expression of C2GlcNAcT-I and FucT-VII by mouse T cells undergo independent control, which has been proposed to regulate the tissue-selective trafficking of different effector T cell populations [14,46,47]. Moreover, studies involving mice and rats have demonstrated a variable role for E-selectin and P-selectin in T cell trafficking depending on the inflammatory stimulus [48,49]. It is plausible that a differential regulation in expression of E- and P-selectin glycan ligands may also be important for regulating the temporal expression of E- and P-selectin ligands by T cells as they progress from effector cells to memory cells. Interestingly, it has been reported that E- and P-selectin ligand expression can be up-regulated with similar kinetics in stimulated mouse Th1 CD4+ T cells from TCR transgenic mice, yet their P-selectin-binding activity down-regulated much more rapidly than their E-selectin-binding activity [46].
Taken together, we report a difference in the reactivity of CHO-131 with established memory CLA+ T cells in the circulation and newly activated CLA+ T cells or re-stimulated memory CLA+ T cells. CHO-131 stains only a subset of circulating CLA+ CD4+ T cells yet appears to be broadly reactive with activated CLA+ CD4+ T cells. Hence, CHO-131 staining may reveal recently activated CLA+ T cells. Considering that CHO-131+ CLA+ T cells are enriched in P-selectin binding cells, this T cell population may undergo broader and more efficient trafficking than memory CLA+ T cells to sites of inflammation expressing E- and/or P-selectin in the vascular beds. Clark et al. have reported that there is a large population of memory CLA+ T cells in normal skin [3], which is known to constitutively express E-selectin in the vasculature [50]. It will be interesting to determine if these cells are perhaps primarily CHO-131− CLA+ T cells. Upon the initiation of immune reactions in the skin, CHO-131+ CLA+ T cells may then predominate due to the stimulation of resident, memory CLA+ T cells and the recruitment of newly activated CLA+ T cells generated in the draining lymph nodes. CHO-131+ CLA+ T cells may selectively traffic to particular inflammatory stimuli in the skin and/or during certain stages of inflammation progression. Interestingly, our earlier findings demonstrate that essentially all CLA+ T cells localized in psoriasis lesions are reactive with CHO-131 [17].
ACKNOWLEDGEMENTS
The authors thank Dr. Yue Wang for her technical assistance. The study described was supported by Grant Number AR049333 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Robert C, Kupper TS. Inflammatory skin diseases, T cells, and immune surveillance. N. Engl. J. Med. 1999;341:1817–1828. doi: 10.1056/NEJM199912093412407. [DOI] [PubMed] [Google Scholar]
- 2.Santamaria-Babi LF. CLA(+) T cells in cutaneous diseases. Eur. J. Dermatol. 2004;14:13–18. [PubMed] [Google Scholar]
- 3.Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K, Dowgiert RK, Kupper TS. The vast majority of CLA+ T cells are resident in normal skin. J. Immunol. 2006;176:4431–4439. doi: 10.4049/jimmunol.176.7.4431. [DOI] [PubMed] [Google Scholar]
- 4.Berg EL, Robinson MK, Mansson O, Butcher EC, Magnani JL. A carbohydrate domain common to both sialyl Lea and sialyl LeX is recognized by the endothelial cell leukocyte adhesion molecule ELAM-1. J. Biol. Chem. 1991;266:14869–14872. [PubMed] [Google Scholar]
- 5.Knibbs RN, Craig RA, Natsuka S, Chang A, Cameron M, Lowe JB, Stoolman LM. The fucosyltransferase FucT-VII regulates E-selectin ligand synthesis in human T cells. J. Cell Biol. 1996;133:911–920. doi: 10.1083/jcb.133.4.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, Picker LJ, Butcher EC. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J. Exp. Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J. Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 8.Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- 9.Fuhlbrigge RC, King SL, Dimitroff CJ, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on Western blots. J. Immunol. 2002;168:5645–5651. doi: 10.4049/jimmunol.168.11.5645. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto M, Atarashi K, Umemoto E, Furukawa Y, Shigeta A, Miyasaka M, Hirata T. CD43 functions as a ligand for E-Selectin on activated T cells. J. Immunol. 2005;175:8042–8050. doi: 10.4049/jimmunol.175.12.8042. [DOI] [PubMed] [Google Scholar]
- 11.Ley K, Kansas GS. Selectins in T-cell recruitment to non-lymphoid tissues and sites of inflammation. Nat. Rev. Immunol. 2004;4:325–335. doi: 10.1038/nri1351. [DOI] [PubMed] [Google Scholar]
- 12.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Posttranslational modifications of recombinant P-selectin glycoproein ligand-1 required for binding to P- and E-selectin. J. Biol. Chem. 1996;271:3255–3264. [PubMed] [Google Scholar]
- 13.Leppanen A, Mehta P, Ouyang YB, Ju T, Helin J, Moore KL, van Die I, Canfield WM, McEver RP, Cummings RD. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J. Biol. Chem. 1999;274:24838–24848. doi: 10.1074/jbc.274.35.24838. [DOI] [PubMed] [Google Scholar]
- 14.Snapp KR, Heitzig CE, Ellies LG, Marth JD, Kansas GS. Differential requirements for the O-linked branching enzyme core 2 beta1-6-N-glucosaminyltransferase in biosynthesis of ligands for E- selectin and P-selectin. Blood. 2001;97:3806–3811. doi: 10.1182/blood.v97.12.3806. [DOI] [PubMed] [Google Scholar]
- 15.Wilkins PP, McEver RP, Cummings RD. Structures of the O-glycans on P-selectin glycoprotein ligand-1 from HL- 60 cells. J. Biol. Chem. 1996;271:18732–18742. doi: 10.1074/jbc.271.31.18732. [DOI] [PubMed] [Google Scholar]
- 16.Walcheck B, Leppanen A, Cummings RD, Knibbs RN, Stoolman LM, Alexander SR, Mattila PE, McEver RP. The monoclonal antibody CHO-131 binds to a core 2 O-glycan terminated with sialyl-Lewis x, which is a functional glycan ligand for P-selectin. Blood. 2002;99:4063–4069. doi: 10.1182/blood-2001-12-0265. [DOI] [PubMed] [Google Scholar]
- 17.Ni Z, Campbell JJ, Niehans G, Walcheck B. The monoclonal antibody CHO-131 identifies a subset of cutaneous lymphocyte-associated antigen T cells enriched in P-selectin-binding cells. J. Immunol. 2006;177:4742–4748. doi: 10.4049/jimmunol.177.7.4742. [DOI] [PubMed] [Google Scholar]
- 18.Teraki Y, Picker LJ. Independent regulation of cutaneous lymphocyte-associated antigen expression and cytokine synthesis phenotype during human CD4+ memory T cell differentiation. J. Immunol. 1997;159:6018–6029. [PubMed] [Google Scholar]
- 19.Akdis M, Akdis CA, Weigl L, Disch R, Blaser K. Skin-homing, CLA+ memory T cells are activated in atopic dermatitis and regulate IgE by an IL-13-dominated cytokine pattern: IgG4 counter- regulation by CLA- memory T cells. J. Immunol. 1997;159:4611–4619. [PubMed] [Google Scholar]
- 20.Austrup F, Vestweber D, Borges E, Lohning M, Brauer R, Herz U, Renz H, Hallmann R, Scheffold A, Radbruch A, Hamann A. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 21.Wagers AJ, Waters CM, Stoolman LM, Kansas GS. Interleukin 12 and interleukin 4 control T cell adhesion to endothelial selectins through opposite effects on alpha1, 3-fucosyltransferase VII gene expression. J. Exp. Med. 1998;188:2225–2231. doi: 10.1084/jem.188.12.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koelle DM, Liu Z, McClurkan CM, Topp MS, Riddell SR, Pamer EG, Johnson AS, Wald A, Corey L. Expression of cutaneous lymphocyte-associated antigen by CD8(+) T cells specific for a skin-tropic virus. J. Clin. Invest. 2002;110:537–548. doi: 10.1172/JCI15537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iellem A, Colantonio L, D'Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur. J. Immunol. 2003;33:1488–1496. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- 24.Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood. 2003;101:1677–1682. doi: 10.1182/blood-2002-07-2348. [DOI] [PubMed] [Google Scholar]
- 25.Gelb AB, Smoller BR, Warnke RA, Picker LJ. Lymphocytes infiltrating primary cutaneous neoplasms selectively express the cutaneous lymphocyte-associated antigen (CLA) Am. J. Pathol. 1993;142:1556–1564. [PMC free article] [PubMed] [Google Scholar]
- 26.Picker LJ. Control of lymphocyte homing. Curr. Opin. Immunol. 1994;6:394–406. doi: 10.1016/0952-7915(94)90118-x. [DOI] [PubMed] [Google Scholar]
- 27.Armerding D, Kupper TS. Functional cutaneous lymphocyte antigen can be induced in essentially all peripheral blood T lymphocytes. Int. Arch. Allergy Immunol. 1999;119:212–222. doi: 10.1159/000024197. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi R, Mizukawa Y, Yamazaki Y, Hayakawa K, Hayakawa J, Kudo A, Shiohara T. In vitro differentiation from naive to mature E-selectin binding CD4 T cells: acquisition of skin-homing properties occurs independently of cutaneous lymphocyte antigen expression. J. Immunol. 2003;171:5769–5777. doi: 10.4049/jimmunol.171.11.5769. [DOI] [PubMed] [Google Scholar]
- 29.Yago T, Leppanen A, Carlyon JA, Akkoyunlu M, Karmakar S, Fikrig E, Cummings RD, McEver RP. Structurally distinct requirements for binding of P-selectin glycoprotein ligand-1 and sialyl Lewis x to Anaplasma phagocytophilum and P-selectin. J. Biol. Chem. 2003;278:37987–37997. doi: 10.1074/jbc.M305778200. [DOI] [PubMed] [Google Scholar]
- 30.Smith MJ, Smith BR, Lawrence MB, Snapp KR. Functional analysis of the combined role of the O-linked branching enzyme core 2 beta1-6-N-glucosaminyltransferase and dimerization of P-selectin glycoprotein ligand-1 in rolling on P-selectin. J. Biol. Chem. 2004;279:21984–21991. doi: 10.1074/jbc.M402731200. [DOI] [PubMed] [Google Scholar]
- 31.St Hill CA, Bullard KM, Walcheck B. Expression of the high-affinity selectin glycan ligand C2-O-sLeX by colon carcinoma cells. Cancer Lett. 2005;217:105–113. doi: 10.1016/j.canlet.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 32.Descheny L, Gainers ME, Walcheck B, Dimitroff CJ. Ameliorating skin-homing receptors on malignant T cells with a fluorosugar analog of N-acetylglucosamine: P-selectin ligand is a more sensitive target than E-selectin ligand. J. Invest. Dermatol. 2006;126:2065–2073. doi: 10.1038/sj.jid.5700364. [DOI] [PubMed] [Google Scholar]
- 33.St Hill CA, Farooqui M, Mitcheltree G, Gulbahce HE, Jessurun J, Cao Q, Walcheck B. The high affinity selectin glycan ligand C2-O-sLex and mRNA transcripts of the core 2 beta-1,6-N-acetylglusaminyltransferase (C2GnT1) gene are highly expressed in human colorectal adenocarcinomas. BMC Cancer. 2009;9:79. doi: 10.1186/1471-2407-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shimodaira K, Nakayama J, Nakamura N, Hasebe O, Katsuyama T, Fukuda M. Carcinoma-associated expression of core 2 beta-1,6-N- acetylglucosaminyltransferase gene in human colorectal cancer: role of O-glycans in tumor progression. Cancer Res. 1997;57:5201–5206. [PubMed] [Google Scholar]
- 35.Walcheck B, Moore KL, McEver RP, Kishimoto TK. Neutrophil-neutrophil interactions under hydrodynamic shear stress involve L-selectin and PSGL-1. A mechanism that amplifies initial leukocyte accumulation on P-selectin in vitro. J. Clin. Invest. 1996;98:1081–1087. doi: 10.1172/JCI118888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matala E, Alexander SR, Kishimoto TK, Walcheck B. The cytoplasmic domain of L-selectin participates in regulating L- selectin endoproteolysis. J. Immunol. 2001;167:1617–1623. doi: 10.4049/jimmunol.167.3.1617. [DOI] [PubMed] [Google Scholar]
- 37.Mattila PE, Green CE, Schaff U, Simon SI, Walcheck B. Cytoskeletal interactions regulate inducible L-selectin clustering. Am. J. Physiol. Cell. Physiol. 2005;289:C323–C332. doi: 10.1152/ajpcell.00603.2004. [DOI] [PubMed] [Google Scholar]
- 38.Li Y, Brazzell J, Herrera A, Walcheck B. ADAM17 deficiency by mature neutrophils has differential effects on L-selectin shedding. Blood. 2006;108:2275–2279. doi: 10.1182/blood-2006-02-005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni Z, Walcheck B. Varied levels of reactivity by different E-selectin/Fc constructs with cutaneous lymphocyte-associated antigen (CLA)(+) CD4(+) T cells. Immunol. Lett. 2007;108:179–182. doi: 10.1016/j.imlet.2006.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piller F, Piller V, Fox RI, Fukuda M. Human T-lymphocyte activation is associated with changes in O- glycan biosynthesis. J. Biol. Chem. 1988;263:15146–15150. [PubMed] [Google Scholar]
- 41.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 2000;191:1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vachino G, Chang XJ, Veldman GM, Kumar R, Sako D, Fouser LA, Berndt MC, Cumming DA. P-selectin glycoprotein ligand-1 is the major counter-receptor for P-selectin on stimulated T cells and is widely distributed in non-functional form on many lymphocytic cells. J. Biol. Chem. 1995;270(37):21966–21974. doi: 10.1074/jbc.270.37.21966. [DOI] [PubMed] [Google Scholar]
- 43.Hirata T, Merrill-Skoloff G, Aab M, Yang J, Furie BC, Furie B. P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 2000;192:1669–1676. doi: 10.1084/jem.192.11.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deshpande P, King IL, Segal BM. IL-12 driven upregulation of P-selectin ligand on myelin-specific T cells is a critical step in an animal model of autoimmune demyelination. J. Neuroimmunol. 2006;173:35–44. doi: 10.1016/j.jneuroim.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 45.Schumacher A, Liebers U, John M, Gerl V, Meyer M, Witt C, Wolff G. P-selectin glycoprotein ligand-1 (PSGL-1) is up-regulated on leucocytes from patients with chronic obstructive pulmonary disease. Clin. Exp. Immunol. 2005;142:370–376. doi: 10.1111/j.1365-2249.2005.02920.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lim YC, Xie H, Come CE, Alexander SI, Grusby MJ, Lichtman AH, Luscinskas FW. IL-12, STAT4-dependent up-regulation of CD4(+) T cell core 2 beta-1,6-nacetylglucosaminyltransferase, an enzyme essential for biosynthesis of P-selectin ligands. J. Immunol. 2001;167:4476–4484. doi: 10.4049/jimmunol.167.8.4476. [DOI] [PubMed] [Google Scholar]
- 47.White SJ, Underhill GH, Kaplan MH, Kansas GS. Cutting edge: differential requirements for Stat4 in expression of glycosyltransferases responsible for selectin ligand formation in Th1 cells. J. Immunol. 2001;167:628–631. doi: 10.4049/jimmunol.167.2.628. [DOI] [PubMed] [Google Scholar]
- 48.Issekutz AC, Issekutz TB. The role of E-selectin, P-selectin, and very late activation antigen-4 in T lymphocyte migration to dermal inflammation. J. Immunol. 2002;168:1934–1939. doi: 10.4049/jimmunol.168.4.1934. [DOI] [PubMed] [Google Scholar]
- 49.Kulidjian AA, Issekutz AC, Issekutz TB. Differential role of E-selectin and P-selectin in T lymphocyte migration to cutaneous inflammatory reactions induced by cytokines. Int. Immunol. 2002;14:751–760. doi: 10.1093/intimm/dxf045. [DOI] [PubMed] [Google Scholar]
- 50.Groves RW, Allen MH, Barker JN, Haskard DO, MacDonald DM. Endothelial leucocyte adhesion molecule-1 (ELAM-1) expression in cutaneous inflammation. Br. J. Dermatol. 1991;124:117–123. doi: 10.1111/j.1365-2133.1991.tb00419.x. [DOI] [PubMed] [Google Scholar]