Abstract
The formation of the myelin sheath is a crucial step during development since it enables fast and efficient propagation of signals within the limited space of the mammalian central nervous system (CNS). During the process of myelination, oligodendrocytes actively interact with the extracellular matrix (ECM), and these interactions are considered crucial for proper and timely completion of the myelin sheath. However, the exact regulatory circuits involved in the signaling events that occur between the ECM and oligodendrocytes are currently not fully understood. In the present study we, therefore, investigated the role of a known integrator of cell-ECM signaling, namely focal adhesion kinase (FAK), in CNS myelination via the use of conditional (oligodendrocyte-specific) and inducible FAK knock-out mice (Fakflox/flox:PLP/CreERT mice). When inducing FAK knock-out just prior to and during active myelination of the optic nerve, we observed a significant reduction in the number of myelinated fibers at postnatal day 14. In addition, our data revealed a decreased number of primary processes extending from oligodendrocyte cell bodies at this postnatal age and upon induction of FAK knock-out. In contrast, myelination appeared normal at postnatal day 28. Thus, our data suggest that FAK controls the efficiency and timing of CNS myelination during its initial stages by, at least in part, regulating oligodendrocyte process outgrowth and/or remodeling.
Keywords: Oligodendrocyte, myelination, FAK, ECM molecules, process outgrowth
INTRODUCTION
During development of the central nervous system (CNS), differentiation of the myelinating cells, oligodendrocytes, and the process of active myelination itself are regulated by complex interactions of the oligodendrocytes’ cell surfaces with their extracellular environments (Buttery and ffrench-Constant, 1999; Colognato et al., 2007; Fridman et al., 1985; Lubetzki-Korn et al., 1983; Notterpek and Rome, 1994; Oh and Yong, 1996; Siskova et al., 2006; Szuchet et al., 2000). These interactions are to a large extent mediated by the integrin class of extracellular matrix (ECM) receptors (Baron et al., 2005; Benninger et al., 2006; Colognato et al., 2002; Colognato et al., 2004; Frost et al., 1999; Gudz et al., 2002; Lee et al., 2006; Malek-Hedayat and Rome, 1994; Milner and ffrench-Constant, 1994; Olsen and ffrench-Constant, 2005; Relvas et al., 2001). In agreement with a pivotal role of integrin-ECM signaling for the regulation of oligodendrocyte differentiation and CNS myelination, signaling molecules that are effectors in integrin-mediated signaling cascades have also been implicated in these processes (Chun et al., 2003; Fox et al., 2004; Hoshina et al., 2007; Liang et al., 2004; Sloane and Vartanian, 2007).
One of the main regulators of integrin-ECM signaling is focal adhesion kinase (FAK). FAK, also known as protein tyrosine kinase 2 (PTK2), is an ubiquitously expressed non-receptor protein tyrosine kinase that can be activated by a number of extracellular signals (Hanks et al., 1992; Mitra et al., 2005; Mitra and Schlaepfer, 2006; Parsons, 2003; Schaller et al., 1992; Schlaepfer et al., 1999). FAK has been found to be expressed in cells of the oligodendrocyte lineage and is present in myelin (Bacon et al., 2007; Kilpatrick et al., 2000). Interestingly, phosphorylation of FAK at its Tyr397 site, which represents a critical event for its activation and biological effects, has been described to occur primarily in post-migratory differentiating oligodendrocytes and not migratory oligodendrocyte progenitor cells (Liang et al., 2004). Furthermore, additional phosphorylation events at FAK tyrosine residues regulate its overall function (Cohen and Guan, 2005; Hanks and Polte, 1997; Schlaepfer and Hunter, 1996). The phosphorylation of one of these residues, namely the Tyr925 residue, has been found significantly altered during the initial stages of myelination (Fox et al., 2004). Taken together, these data suggest a role of FAK in regulating oligodendrocyte maturation and/or CNS myelination itself. However, this role of FAK has not yet been well characterized.
Ubiquitous FAK knock-out is early embryonically lethal due to general mesodermal defects (Furuta et al., 1995; Ilic et al., 1995a; Ilic et al., 1995b). To investigate the potential role of FAK in the regulation of oligodendrocyte maturation and/or CNS myelination, we, therefore, generated oligodendrocyte-specific and inducible FAK knock-out mice using the Cre-loxP system (Fakflox/flox:PLP/CreERT mice). When inducing FAK knock-out in these mice just prior to and during the initial stages of myelination of the optic nerve, our results reveal that myelination is reduced at postnatal day 14. In addition, our data show that the induction of FAK knock-out results in a reduced number of primary oligodendrocyte processes at this developmental age. This phenotype, however, appears to be transient since the number of myelinated fibers at postnatal day 28 is comparable under both control and knock-out conditions. Taken together, these data demonstrate that FAK is involved in regulating the efficiency and timing of myelination at its initial stages, and they suggest that this regulatory role may involve the control of oligodendrocyte process outgrowth and/or remodeling.
MATERIALS AND METHODS
Animals and induction of FAK knock-out
Mice in which the second kinase domain exon of Fak is flanked by loxP sites (Fakflox/flox mice) were bred to mice that express, under the control of the PLP promoter, Cre recombinase fused to a tamoxifen-inducible mutated ligand binding domain of the human estrogen receptor (PLP/CreERT mice; kindly provided by B. Popko, University of Chicago) (Beggs et al., 2003; Doerflinger et al., 2003). Both strains are on a C56BL/6 genetic background. Litters used for the present study were derived from males that were homozygous for the floxed Fak locus and heterozygous for the PLP/CreERT locus and females that were homozygous for the floxed Fak locus but negative for the PLP/CreERT locus. Thus, knock-out and control mice were derived from the same breeding pairs. To induce FAK knock-out, 300 µl intraperitoneal injections of 3 mg tamoxifen or vehicle, i.e. sunflower oil, were administered daily into lactating mothers from postnatal day (P)2 through P12, whereby P0 refers to the day of birth (Leone et al., 2003). Animals were analyzed at P14 and P28. To confirm successful Cre-mediated recombination under the above described conditions, PLP/CreERT mice were additionally bred to Gt(ROSA)26Sortm1(EYFP)Cos reporter mice, which contain an Enhanced Yellow Fluorescent Protein gene (EYFP) inserted into the Gt(ROSA)26Sor locus and are also on a C56BL/6 genetic background (The Jackson Laboratory, Bar Harbor, ME; see also Srinivas et al., 2001). In these reporter mice, expression of EYFP is blocked by an upstream loxP-flanked STOP sequence and only induced upon successful Cre-mediated recombination at the ROSA26 locus. Gt(ROSA)26Sortm1(EYFP)Cos:PLP/CreERT mice were treated as described above and analyzed at P14. All animal studies were approved by the Institutional Animal Care and Use Committee at Virginia Commonwealth University.
PCR Analysis
For genotype analysis, DNA was extracted from tail clips using the DNeasy blood and tissue kit (Qiagen, Valencia, CA). For analysis of recombination efficiency upon tamoxifen treatment, optic nerves were dissected from P14 animals and DNA was extracted as described above. Polymerase chain reaction (PCR) was performed using the Taq PCR Core Kit (Qiagen, Valencia, CA) and a PTC-200 DNA Engine Cycler (MJ Research, Waltham, MA). The following primer pairs were used at the indicated annealing temperatures: CreERT forward (5’-GATGTAGCCAGCAGCATGTC-3’) and CreERT reverse (5’-ACTATATCCGTAACCTGGAT-3’), 50 °C; FAK-flox-P1 (5’-GACCTTCAACTTCTCATTTCTCC-3’) and FAK-flox-P2 (5’-GAATGCTACAGGAACCAAATAAC-3’), 55 °C. Custom oligonucleotides were obtained from MWG-Biotech (Huntsville, AL). PCR cycling conditions were as follows: 2 min at 94 °C followed by 35 cycles of amplification (45 sec at 94 °C, 1 min 30 sec at the respective annealing temperature and 45 sec at 72 °C) and 4 min of extension at 72 °C. Amplified DNA was analyzed using agarose gel electrophoresis and the VersaDoc 4000 imaging system (Bio-Rad, Hercules, CA).
Immunohistochemistry
Immunohistochemistry was performed on longitudinal sections of optic nerves dissected from tamoxifen-treated P14 Gt(ROSA)26Sortm1(EYFP)Cos:PLP/CreERT mice. Mice were deeply anesthetized, transcardially perfused with 4% paraformaldehyde in 0.1 M Millonigs buffer (150 mM sodium phosphate monobasic/100mM sodium hydroxide) and postfixed 24 hours. Optic nerves were removed, cryoprotected in 30% sucrose/PBS, embedded in Tissue-Tek on dry ice and 10µm sections were cut on a Shandon SME Cryotome (Thermo Scientific, Philadelphia, Pennsylvania). Sections were immunolabeled after a permeabilization step in ice-cold acetone (Dupree et al., 1999) using the following antibodies: mouse monoclonal anti-APC/CC1 (EMD/Calbiochem, Gibbstown, NJ), rabbit polyclonal anti-GFP/YFP (Millipore, Temecula, CA), secondary Alexa Fluor 594-conjugated donkey anti-mouse IgG and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen/Molecular Probes, Carlsbad, CA). Sections were analyzed by confocal microscopy using a Leica TCS SP2 AOBS System (Leica Microsystems Inc., Bannockburn, IL).
Light and Electron Microscopic Analysis
P14 and P28 mice treated with either tamoxifen or sunflower oil were deeply anesthetized and transcardially perfused with 4% paraformaldehyde/2.5% glutaraldehyde in 0.1 M Millonigs buffer (Dupree et al., 1998; Marcus et al., 2006). Mice were postfixed for 1–2 weeks in aldehyde fixative. Optic nerves were dissected out and incubated for 1 hour in 1% osmium tetroxide/0.1M Na-cacodylate buffer (Electron Microscopy Sciences, Ft. Washington, PA). Specimens were dehydrated with ethanol and embedded in Epon resin (Electron Microscopy Sciences, Ft. Washington, PA).
For light microscopic analysis, semithin (1 µm) transverse sections were taken every 1 mm throughout the length of the optic nerve, stained with toluidine blue and imaged using a Nikon ECLIPSE E800M microscope equipped with a Spot RT CCD camera (Nikon Inc. Melville, NY). The number of myelinated fibers was determined in a blinded fashion for each entire transverse section. The average number for the control sections was set to 100% and each value calculated accordingly. Statistical significance was determined using the Student’s t-test.
For electron microscopic analysis, ultrathin transverse sections (90 nm) were taken 1 mm from the lamina cribrosa and at 1 mm intervals along the length of the optic nerve. Sections were collected on formvar-coated slotted grids and stained with uranyl acetate and lead citrate. Images were taken using a JEOL JEM1230 transmission electron microscope equipped with an Ultrascan 4000 Gatan CCD camera.
RESULTS
Administration of tamoxifen to lactating females induces Cre-mediated recombination in the optic nerve and in cells of the oligodendrocyte lineage in early postnatal offsprings containing the PLP/CreERT locus
To determine the in vivo role of FAK in differentiating oligodendrocytes during the developmental time-period of myelination, spatially and temporally controlled transgenic FAK knock-out mice were generated using an inducible Cre-loxP system. More specifically, mice in which the second kinase domain exon of Fak is flanked by loxP sites (Fakflox/flox mice; Fig. 1B) were bred to mice that express, under the control of the proteolipid protein (PLP) promoter, Cre recombinase fused to a tamoxifen-inducible mutated ligand binding domain of the human estrogen receptor (PLP/CreERT mice; Fig. 1B) (Beggs et al., 2003; Doerflinger et al., 2003). Due to the breeding strategy utilized (see materials and methods), all mice analyzed for potential effects of FAK knock-out induction were homozygous for the floxed Fak locus and either heterozygous for the PLP/CreERT locus (Fakflox/flox:PLP/CreERT mice) or lacking the PLP/CreERT locus (Fakflox/flox littermate controls). It has been previously established that only upon tamoxifen administration is CreERT translocated from the cytoplasm to the nucleus, where it is able to catalyze recombination at loxP sites (Leone et al., 2003). In addition, it has been shown that the PLP promoter used for the generation of the PLP/CreERT mice is well suited to direct transgene expression to differentiating oligodendrocytes (Doerflinger et al., 2003; Fuss et al., 2001; Fuss et al., 2000; Wight et al., 1993). Thus, the use of the above described Fakflox/flox:PLP/CreERT mice allows to design strategies in which to induce Cre-mediated recombination and thus FAK knock-out specifically in differentiating oligodendrocytes and during the developmental time-period of active myelination. Due to the region-specific timing of active myelination in the CNS, however, individual knock-out, i.e. tamoxifen injection, strategies need to be employed for specific CNS regions (Caley and Maxwell 1968; Foran and Peterson 1992; Leone et al. 2003; Thomson et al. 2005).
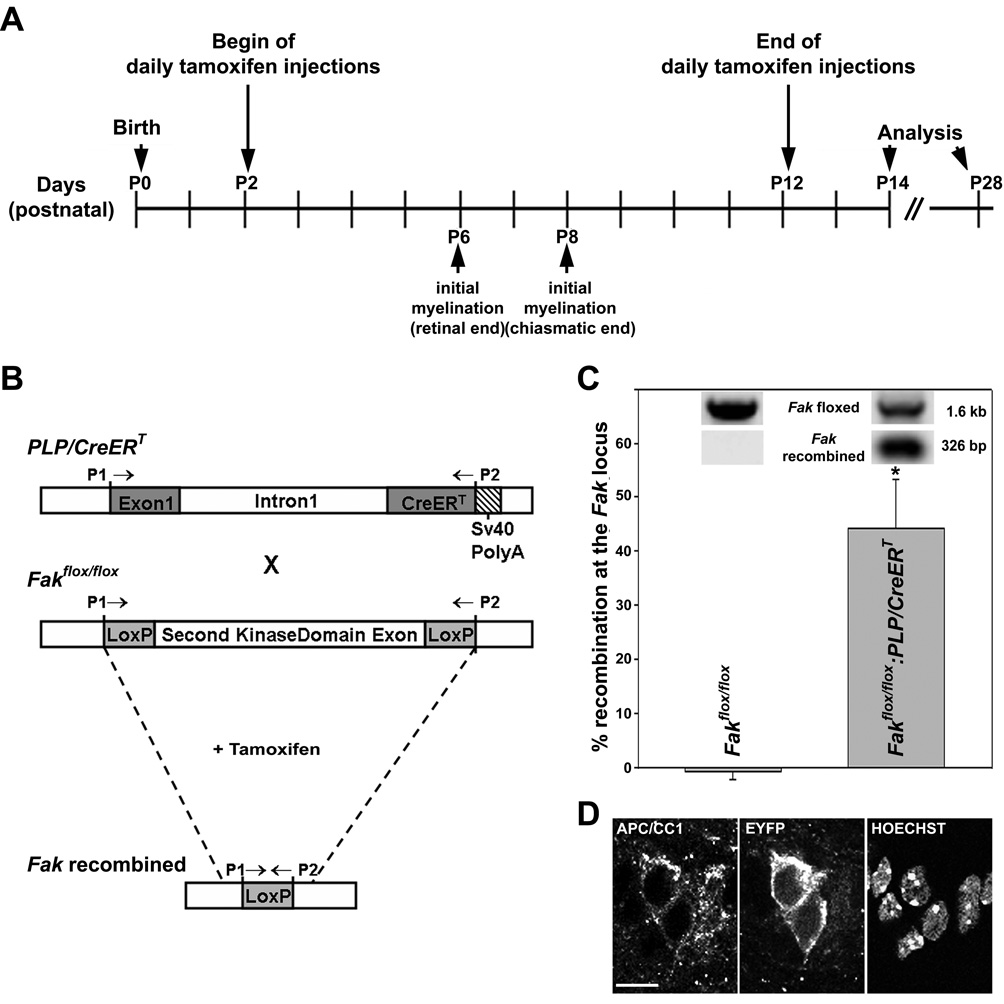
Figure 1.
Induction of Cre-mediated recombination in Fakflox/flox:PLP-CreERT and Gt(ROSA)26Sortm1(EYFP)Cos:PLP-CreERT mice A: Injection paradigm used to induce Cre-mediated recombination at the floxed fak or ROSA26 locus just prior to and during myelination of the optic nerve. Time points of initial myelination are defined as described by Thomson et al. (2005). B: The schematic at the top depicts the transgene construct used to generate the PLP/CreERT mice (Doerflinger et al., 2003). The PLP cassette contains 2.4 kb of the 5’-flanking DNA, exon 1 and intron 1 of the Plp gene. The cDNA sequence coding for CreERT was inserted 3’ of intron 1. For transcription termination a simian virus (SV) 40 poly(A) signal sequence was added. The middle schematic depicts the floxed Fak locus, in which the second kinase domain exon of Fak is flanked by loxP sites (Beggs et al., 2003). Upon cross breeding and tamoxifen application, recombination at the Fak locus is induced as depicted in the bottom schematic. P1 and P2 indicate the location of PCR primers used for genotyping. C: PCR analysis of recombination at the Fak locus in optic nerves of tamoxifen-treated Fakflox/flox:PLP-CreERT and Fakflox/flox mice at P14. Primers P1 and P2 depicted in A were used and the amount of each amplification product was determined using the VersaDoc 4000 imaging system (Bio-Rad, Hercules, CA). The bar graph depicts means ±SEM (n=3 per genotype). The star indicates statistical significance as determined by Student’s t-test. D: Representative confocal images of P14 optic nerve sections taken from tamoxifen-treated Gt(ROSA)26Sortm1(EYFP)Cos:PLP-CreERT mice after double-labeling for APC/CC1 and YFP. Nuclei were stained using Hoechst. Images depict single channel representations of a single optical section (approximately 0.2 µm x–z resolution). Scale Bar: 10 µm.
For the studies presented here, we chose the optic nerve as the anatomical region of interest due to its relatively simple morphology and the well described chronology of oligodendrocyte differentiation and myelination (Butt and Ransom, 1993; Colello et al., 1995; Hildebrand and Waxman, 1984; Hunter and Bedi, 1986; Skoff et al., 1976a; Skoff et al., 1976b; Skoff et al., 1980; Tennekoon et al., 1977; Thomson et al., 2005). In the optic nerve of developing C57Bl/6 mice, i.e. animals of genetic background similar to the one of the Fakflox/flox:PLP/CreERT mice, both oligodendrocyte differentiation and myelination proceed from the retinal to the chiasmatic end of the nerve (Thomson et al., 2005). First axonal contact of oligodendrocyte processes can be seen at the retinal end around postnatal day (P) 6. At this time-point, oligodendrocytes also begin to express the PLP isoform of PLP/DM20 and are thus considered to represent a myelinating stage of the lineage (Tennekoon et al., 1977; Thomson et al., 2005; Trapp et al., 1997). At the chiasmatic end, such initially myelinating cells are first observed at P8. Mature, i.e. compacted myelin can be found extended over the whole length of the nerve around P15 (Foran and Peterson, 1992). Thus, to induce Cre-mediated recombination in the optic nerve just prior to and during the developmental time-period of active myelination, tamoxifen or sunflower oil as vehicle control were administered daily to lactating mothers from two to twelve days postpartum and animals were analyzed during the active stages of myelination, i.e. at P14, and at a mature stage, i.e. at P28 (Fig. 1A). Successful Cre-mediated recombination at the Fak locus was assessed by PCR analysis (Fig. 1C). Recombination could be observed as early as P4 (data not shown). At this time point, oligodendrocytes present in the optic nerve are for the most part at a progenitor stage, at which they express low levels of FAK (Liang et al., 2004). Thus, even in case of a relatively stable FAK protein, FAK protein levels will be reduced upon FAK knock-out induction since it inhibits the normal developmental upregulation of FAK expression. At P14, approximately 40% of the cells within the optic nerve are oligodendrocytes (Barres et al., 1992). Thus, the recombination efficiency of approximately 40% observed in our studies (Fig. 1C) suggests efficient Cre-mediated recombination in cells of the oligodendrocyte lineage, at least at this developmental age. In the other cell types of the optic nerve, which are primarily astrocytes, the PLP promoter is not operative and thus recombination cannot be induced upon tamoxifen administration. To further confirm successful Cre-mediated recombination in cells of the oligodendrocyte lineage under the conditions depicted in Fig. 1A, PLP/CreERT mice were bred to Gt(ROSA)26Sortm1(EYFP)Cos reporter mice, in which expression of EYFP is blocked by an upstream loxP-flanked STOP sequence and only induced upon successful Cre-mediated recombination (Srinivas et al., 2001). As a marker for cells of the oligodendrocyte lineage an antibody to cytoplasmic APC/CC1 was used (Bhat et al., 1996; Fuss et al., 2000). As shown in Fig. 1D, EYFP expression could easily be detected in APC/CC1-positive cells of the optic nerve of a tamoxifen-treated P14 Gt(ROSA)26Sortm1(EYFP)Cos:PLP/CreERT mouse. Taken together, the above data confirm that under the conditions depicted in Fig. 1A Cre-mediated recombination at loxP sites occurs efficiently in the optic nerve and in cells of the oligodendrocyte lineage during early postnatal development (P4 through P14) of offsprings containing the PLP/CreERT locus.
At P14, the number of myelinated axons is decreased in the optic nerve of tamoxifen-treated Fakflox/flox:PLP/CreERT mice
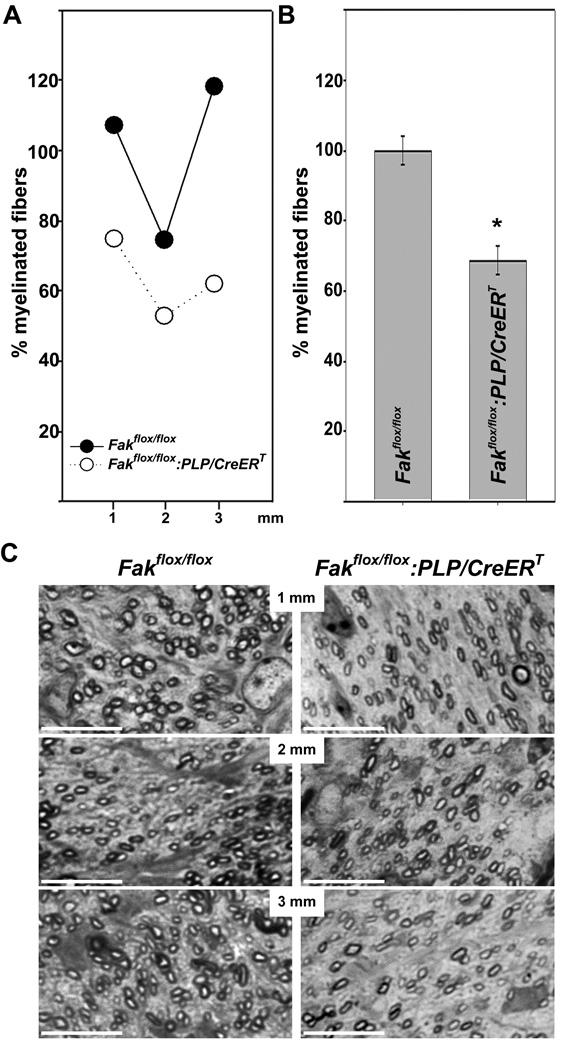
To assess the extent to which FAK may regulate developmental myelination, we determined the number of myelinated fibers in tamoxifen-treated Fakflox/flox:PLP/CreERT and control mice at P14 using light microscopy (Fig. 2). To eliminate the possibility that varying levels of myelination along the length of the optic nerve affects the outcome of our analysis, we examined sections taken at 1 mm intervals along the whole length of the nerve (Skoff et al., 1980). At all levels, the number of myelinated fibers was found to be reduced in the Fakflox/flox:PLP/CreERT compared to Fakflox/flox litter mates (see example in Fig. 2A). When averaging the number of myelinated fibers for all levels, these were found significantly decreased by over 30% in Fakflox/flox:PLP/CreERT compared to Fakflox/flox mice (Figs. 2B, C). No such differences were noted when analyzing vehicle-treated Fakflox/flox:PLP/CreERT and Fakflox/flox mice (data not shown).
Figure 2.
Reduction of the number of myelinated fibers in the optic nerves of tamoxifen treated Fakflox/flox:PLP/CreERT mice at P14 A: Numbers of myelinated fibers within entire transverse sections of P14 optic nerves at approximately 1, 2 and 3 mm from the lamina cribosa. The mean for all control values was set to 100% and the percent values for all data points were calculated accordingly. The results for a representative optic nerve littermate pair are shown. B: Numbers of myelinated fibers averaged over all three intervals of the optic nerve. The percent values were calculated as described in A. The bar graph depicts means ±SEM (n=3 per genotype). The star indicates statistical significance as determined by Student’s t-test. C: Representative pictures of semithin sections from Fakflox/flox (left panel) and Fakflox/flox:PLPCreERT (right panel) optic nerves taken at approximately 1, 2 and 3 mm from the lamina cribosa. Scale Bar: 10 µm.
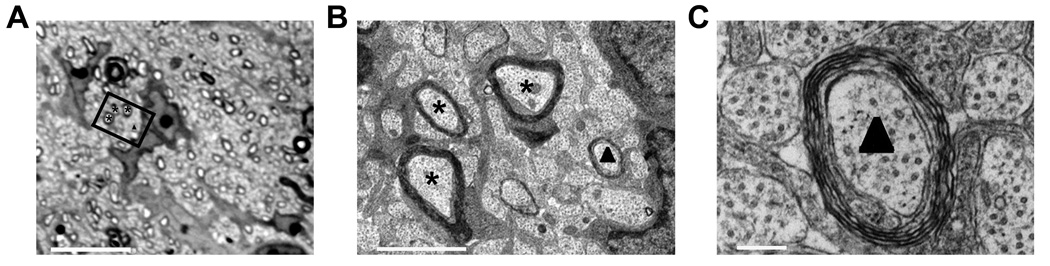
To determine the resolution of our type of analysis, we compared semithin sections (light microcopy) with consecutive ultrathin sections (electron microscopy). As shown in Fig. 3, such analysis revealed that visualizing transverse sections of the optic nerve using light microscopy allows distinction of myelinated axons enwrapped with as few as four layers of myelin. Thus, our data demonstrate that FAK is involved in the regulation of the initial steps of myelination and/or myelin wrapping.
Figure 3.
The resolution, i.e. visual threshold, for the detection of a myelinated fiber in the optic nerve is approximately 4 layers of myelin at the light microscopic level. A: Semithin section of the optic nerve imaged at light microscopic level. Scale Bar: 10 µm. B and C: Ultrathin sections taken immediately adjacent to the semithin section shown in A and imaged at electron microscopic level. Scale Bars: B: 2 µm, C: 200 nm. The rectangle in A marks the area shown in B panel. The stars mark myelinated fibers that can be identified as such at both light and electron microscopic level. The triangle marks a myelinated fiber that can only be identified as such at the electron microscopic level.
At P14, the number of primary oligodendrocyte processes is reduced in the optic nerve of tamoxifen-treated Fakflox/flox:PLP/CreERT mice
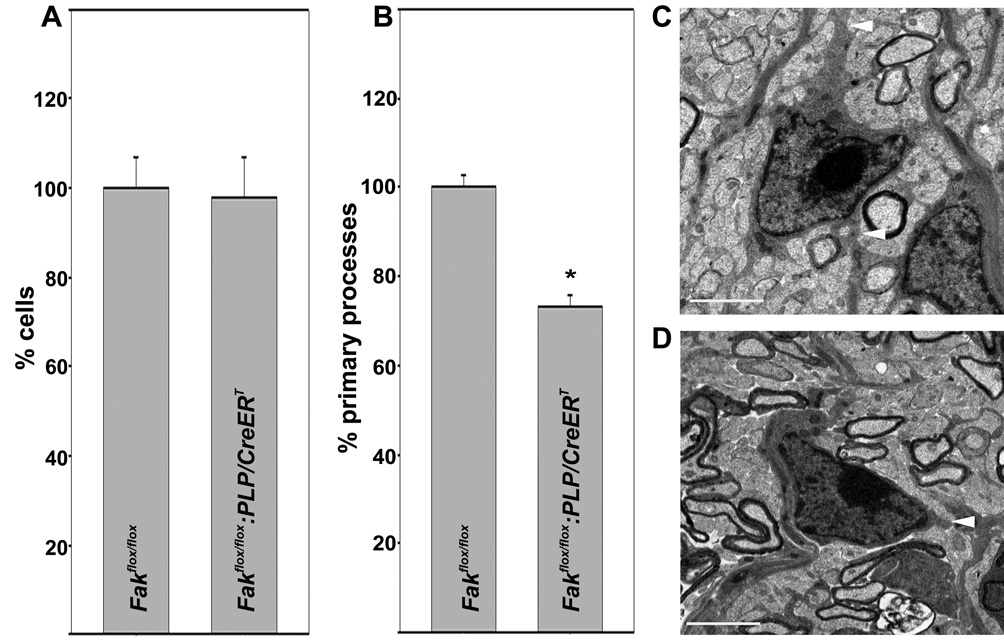
To further investigate the above role of FAK during developmental myelination of the optic nerve, we determined the total number of cells in the semithin 1µm transverse sections taken at the 1 mm interval (measured from the lamina cribrosa). This analysis revealed no differences between P14 Fakflox/flox:PLP/CreERT and Fakflox/flox littermates (Fig. 4A) and thus suggests that the effect of FAK knock-out induction on myelination may not be due to changes in oligodendrocyte numbers. However, when analyzing the number of primary processes, i.e. processes that directly extend from oligodendrocyte cell bodies, a significant decrease of approximately 30 % was observed in P14 Fakflox/flox:PLP/CreERT optic nerves compared to P14 Fakflox/flox littermate optic nerves (Figs. 4B–D). Oligodendrocyte cell bodies can be easily distinguished from cell bodies of the major other cell type found in the optic nerve, namely astrocytes, as astrocytes exhibit abundant glycogen granules and contain intermediate filaments. Thus, we feel confident that our analysis is restricted to a large extent to oligodendrocytes. Taken together, the above data, therefore, suggest that the decrease in the number of myelinated fibers seen upon the induction of FAK knock-out is due, at least in part, to impaired oligodendrocyte process outgrowth and/or remodeling.
Figure 4.
Reduction of the number of primary oligodendrocyte processes in the optic nerves of tamoxifen treated Fakflox/flox:PLP/CreERT. A: Total number of cells counted at light microscopic level in semithin P14 optic nerve sections at the 1 mm interval (see Fig. 2). The mean for all control values was set to 100 % and the percent values for all data points were calculated accordingly. The bar graph depicts means ±SEM (n=3 per genotype). B: Number of primary processes per cell counted at elecron microscopic level and over an entire transverse section for each nerve analyzed. The mean for all control values was set to 100 % and the percent values for all data points were calculated accordingly. The bar graph depicts means ±SEM (n=3 per genotype; 258 cells for each condition). The star indicates statistical significance as determined by Student’s t-test. C: and D: Representative transmission electron micrographs for a Fakflox/flox:PLP/CreERT (C) and a Fakflox/flox (D, control) oligodendrocyte. Scale Bar: 2 µm.
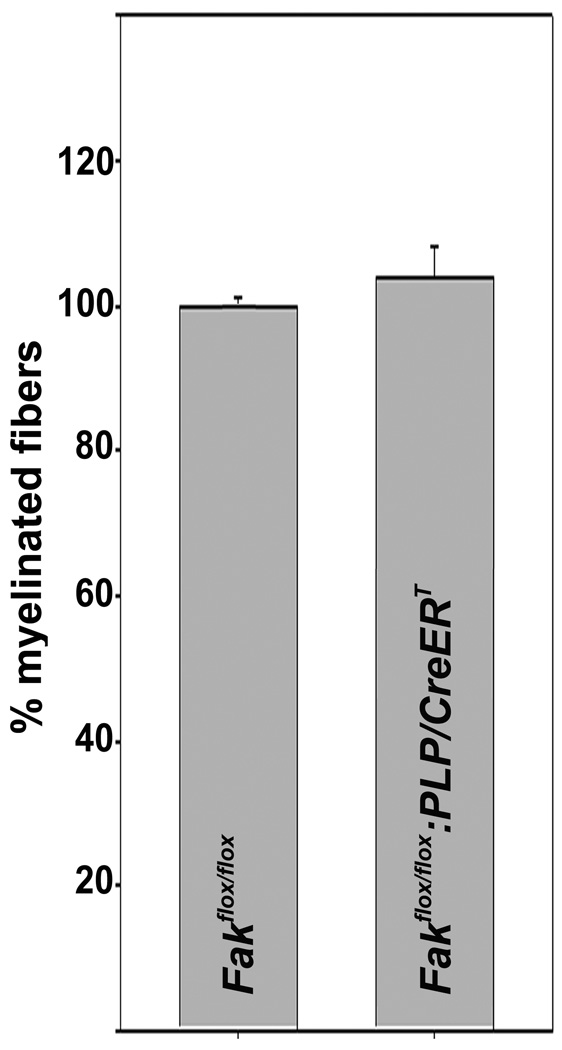
At P28, the number of myelinated axons in the optic nerve is comparable between tamoxifen-treated Fakflox/flox and Fakflox/flox:PLP/CreERT mice
To assess the extent to which the above observed effects of FAK knock-out induction during time-periods of active myelination are persistent into adulthood, we determined the number of myelinated axons at P28. At this age, the number of myelinated axons in the optic nerve was found to be comparable between Fakflox/flox and Fakflox/flox:PLP/CreERT mice (Fig. 5). These data suggest that the role of FAK may be of particular importance for the efficiency and timing of the initial stages of myelination.
Figure 5.
Comparable number of myelinated fibers in the optic nerves of tamoxifen treated Fakflox/flox and Fakflox/flox:PLP/CreERT mice at P28. Numbers of myelinated fibers were determined within entire transverse sections of optic nerves at approximately 1 mm from the lamina cribosa and as described in Fig. 2. The mean for all control values was set to 100% and the percent values for all data points were calculated accordingly. The bar graph depicts means ±SEM (n=3 per genotype).
DISCUSSION
In the present study we demonstrate that induction of FAK knock-out just prior to and during active stages of myelination results in hypomyelination in the optic nerve at a time-point when normally myelinated fibers can be found extended throughout the whole length of the nerve, i.e. at P14. Furthermore, our data suggest that this effect of FAK knock-out induction is due, at least in part, to a reduced outgrowth and/or impaired remodeling of primary oligodendrocyte processes. However, myelination appears to have reached normal levels by P28. Thus, our data suggest that in vivo in the optic nerve FAK promotes efficient and properly timed myelination during the active phases of myelin sheath formation.
In light of the known role of FAK in integrating ECM-integrin signaling events, our data are in support of the previously suggested, but somewhat controversial, regulatory role of integrin signaling during CNS myelination (Benninger et al., 2006; Colognato et al., 2007; Lee et al., 2006; Relvas et al., 2001). Furthermore, they are in good agreement with findings that demonstrate developmental myelination to be controlled by signals up- and downstream of the integrin-FAK axis, such as laminin2/merosin and fyn, respectively (Biffiger et al., 2000; Chun et al., 2003; Sperber et al., 2001). Interestingly, expression of dominant-negative β1 integrin, laminin2/merosin deficiency and fyn knock-out all result in hypomyelination in a region-specific manner with only the optic nerve affected to similar extents in these mutant mice. Thus, the roles of ECM proteins, integrins and FAK may differ in different CNS areas. Those areas that are potentially not controlled by FAK may depend on the FAK-related kinase proline-rich tyrosine kinase 2 (Pyk2). While Pyk2 expression in oligodendrocytes has not been well defined, it represents a good candidate for functionally substituting FAK (Avraham et al., 2000; Klingbeil et al., 2001; Nakamura et al., 2007; Orr and Murphy-Ullrich, 2004). However, future studies will be necessary to dissect the exact roles of FAK and Pyk2 in the regulation of CNS myelination.
FAK has been characterized as a regulator of morphological remodeling, and in particular it has been implicated in the regulation of process outgrowth from both neurons and oligodendrocytes (Beggs et al., 2003; Falk et al., 2005; Hoshina et al., 2007; Robles and Gomez, 2006). Our data demonstrating a reduction in the number of primary processes upon induction of FAK knock-out are consistent with these previous findings. A pivotal role of FAK in the regulation of morphological oligodendrocyte differentiation is further supported by the fact that fyn, a known FAK effector, has been found to be important for the development of the extensive oligodendrocyte process network (Klein et al., 2002; Liang et al., 2004; Osterhout et al., 1999). In the case of fyn, this morphological maturation can be regulated independent of changes in gene expression typically associated with oligodendrocyte differentiation (Buttery and ffrench-Constant, 2001; Osterhout et al., 1999). The extent to which FAK is involved in the control of gene expression in differentiating oligodendrocytes, however, has not yet been characterized. Nevertheless, the above data support the idea that impaired process outgrowth and/or remodeling may at least in part be responsible for the hypomyelination seen in the optic nerve of tamoxifen-treated Fakflox/flox:PLP/CreERT mice.
In our studies, the effect of FAK knock-out induction on myelination was found to be transient with normal levels of myelination detectable at P28. Thus, FAK’s role appears to mainly affect the efficiency and timing of myelination. However, as discussed above, Pyk2 may be able to substitute FAK’s function not only in a region-specific manner but also in case of a loss of FAK. Future studies will be necessary to assess such a potentially important role of Pyk2 in myelination.
Taken together, our data suggest that FAK promotes efficient and properly timed myelination in the optic nerve, where it likely acts as an effector of integrin signaling activated by oligodendrocyte-ECM interactions. Our data further suggest that this signaling event promotes process outgrowth and potentially remodeling during the initial stages of myelination. Impairment of these steps of oligodendrocyte maturation appears at least in part responsible for the limited repair of the myelin sheath seen in lesions of patients suffering from the major demyelinating disease in human, Multiple Sclerosis (Chang et al., 2002; Franklin and ffrench-Constant 2008; Kuhlmann et al. 2008). Thus, further understanding of the role of FAK for CNS myelination does not only further our understanding of normal CNS development but may also reveal novel targets suitable to stimulate remyelination under pathological demyelinating conditions.
ACKNOWLEDGMENTS
The authors thank B. Popko for providing the PLP/CreERT mice, C. Waggener for his aid in analyzing the number of myelinated fibers, J. Williamson for technical assistance in preparing the specimens used for electron microscopy as well as C. Sato-Bigbee and J. Povlishock for helpful discussions and comments.
Grant Information: Electron microscopy was performed at VCU’s Department of Anatomy and Neurobiology Microscopy Facility, which was supported, in part, through NIH-NINDS Center Core grant 5P30NS047463. This work was supported by grants from the National Institute of Health (B.F., L.F.R.) and the National Multiple Sclerosis Society (B.F.).
REFERENCES
- Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal. 2000;12(3):123–133. doi: 10.1016/s0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- Bacon C, Lakics V, Machesky L, Rumsby M. N-WASP regulates extension of filopodia and processes by oligodendrocyte progenitors, oligodendrocytes, and Schwann cells-implications for axon ensheathment at myelination. Glia. 2007;55(8):844–858. doi: 10.1002/glia.20505. [DOI] [PubMed] [Google Scholar]
- Baron W, Colognato H, ffrench-Constant C. Integrin-growth factor interactions as regulators of oligodendroglial development and function. Glia. 2005;49(4):467–479. doi: 10.1002/glia.20132. [DOI] [PubMed] [Google Scholar]
- Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70(1):31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Beggs HE, Schahin-Reed D, Zang K, Goebbels S, Nave KA, Gorski J, Jones KR, Sretavan D, Reichardt LF. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40(3):501–514. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger Y, Colognato H, Thurnherr T, Franklin RJ, Leone DP, Atanasoski S, Nave KA, ffrench-Constant C, Suter U, Relvas JB. Beta1-integrin signaling mediates premyelinating oligodendrocyte survival but is not required for CNS myelination and remyelination. J Neurosci. 2006;26(29):7665–7673. doi: 10.1523/JNEUROSCI.0444-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM. Expression of the APC tumor suppressor protein in oligodendroglia. Glia. 1996;17(2):169–174. doi: 10.1002/(SICI)1098-1136(199606)17:2<169::AID-GLIA8>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Biffiger K, Bartsch S, Montag D, Aguzzi A, Schachner M, Bartsch U. Severe hypomyelination of the murine CNS in the absence of myelin-associated glycoprotein and fyn tyrosine kinase. J Neurosci. 2000;20(19):7430–7437. doi: 10.1523/JNEUROSCI.20-19-07430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. J Comp Neurol. 1993;338(1):141–158. doi: 10.1002/cne.903380110. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Laminin-2/integrin interactions enhance myelin membrane formation by oligodendrocytes. Mol Cell Neurosci. 1999;14(3):199–212. doi: 10.1006/mcne.1999.0781. [DOI] [PubMed] [Google Scholar]
- Buttery PC, ffrench-Constant C. Process extension and myelin sheet formation in maturing oligodendrocytes. Prog Brain Res. 2001;132:115–130. doi: 10.1016/S0079-6123(01)32070-8. [DOI] [PubMed] [Google Scholar]
- Caley DW, Maxwell DS. An electron microscopic study of neurons during postnatal development of the rat cerebral cortex. J Comp Neurol. 1968;133(1):17–44. doi: 10.1002/cne.901330103. [DOI] [PubMed] [Google Scholar]
- Chang A, Tourtellotte WW, Rudick R, Trapp BD. Premyelinating oligodendrocytes in chronic lesions of multiple sclerosis. N Engl J Med. 2002;346(3):165–173. doi: 10.1056/NEJMoa010994. [DOI] [PubMed] [Google Scholar]
- Chun SJ, Rasband MN, Sidman RL, Habib AA, Vartanian T. Integrin-linked kinase is required for laminin-2-induced oligodendrocyte cell spreading and CNS myelination. J Cell Biol. 2003;163(2):397–408. doi: 10.1083/jcb.200304154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen LA, Guan JL. Mechanisms of focal adhesion kinase regulation. Curr Cancer Drug Targets. 2005;5(8):629–643. doi: 10.2174/156800905774932798. [DOI] [PubMed] [Google Scholar]
- Colello RJ, Devey LR, Imperato E, Pott U. The chronology of oligodendrocyte differentiation in the rat optic nerve: evidence for a signaling step initiating myelination in the CNS. J Neurosci. 1995;15(11):7665–7672. doi: 10.1523/JNEUROSCI.15-11-07665.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colognato H, Baron W, Avellana-Adalid V, Relvas JB, Baron-Van Evercooren A, Georges-Labouesse E, ffrench-Constant C. CNS integrins switch growth factor signalling to promote target-dependent survival. Nat Cell Biol. 2002;4(11):833–841. doi: 10.1038/ncb865. [DOI] [PubMed] [Google Scholar]
- Colognato H, Galvin J, Wang Z, Relucio J, Nguyen T, Harrison D, Yurchenco PD, ffrench-Constant C. Identification of dystroglycan as a second laminin receptor in oligodendrocytes, with a role in myelination. Development. 2007;134(9):1723–1736. doi: 10.1242/dev.02819. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167(2):365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35(1):63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Coetzee T, Suzuki K, Popko B. Myelin abnormalities in mice deficient in galactocerebroside and sulfatide. J Neurocytol. 1998;27(9):649–659. doi: 10.1023/a:1006908013972. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147(6):1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk J, Bechara A, Fiore R, Nawabi H, Zhou H, Hoyo-Becerra C, Bozon M, Rougon G, Grumet M, Puschel AW, Sanes JR, Castellani V. Dual functional activity of semaphorin 3B is required for positioning the anterior commissure. Neuron. 2005;48(1):63–75. doi: 10.1016/j.neuron.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Foran DR, Peterson AC. Myelin acquisition in the central nervous system of the mouse revealed by an MBP-Lac Z transgene. J Neurosci. 1992;12(12):4890–4897. doi: 10.1523/JNEUROSCI.12-12-04890.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Alexander JK, Afshari FS, Colello RJ, Fuss B. Phosphodiesterase-I alpha/autotaxin controls cytoskeletal organization and FAK phosphorylation during myelination. Mol Cell Neurosci. 2004;27(2):140–150. doi: 10.1016/j.mcn.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Franklin RJ, ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nat Rev Neurosci. 2008;9(11):839–855. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- Fridman R, Alon Y, Doljanski F, Fuks Z, Vlodavsky I. Cell interaction with the extracellular matrices produced by endothelial cells and fibroblasts. Exp Cell Res. 1985;158(2):461–476. doi: 10.1016/0014-4827(85)90469-0. [DOI] [PubMed] [Google Scholar]
- Frost EE, Buttery PC, Milner R, ffrench-Constant C. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr Biol. 1999;9(21):1251–1254. doi: 10.1016/s0960-9822(99)80506-5. [DOI] [PubMed] [Google Scholar]
- Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11(10):1989–1995. [PubMed] [Google Scholar]
- Fuss B, Afshari FS, Colello RJ, Macklin WB. Normal CNS myelination in transgenic mice overexpressing MHC class I H-2L(d) in oligodendrocytes. Mol Cell Neurosci. 2001;18(2):221–234. doi: 10.1006/mcne.2001.1011. [DOI] [PubMed] [Google Scholar]
- Fuss B, Mallon B, Phan T, Ohlemeyer C, Kirchhoff F, Nishiyama A, Macklin WB. Purification and analysis of in vivo-differentiated oligodendrocytes expressing the green fluorescent protein. Dev Biol. 2000;218(2):259–274. doi: 10.1006/dbio.1999.9574. [DOI] [PubMed] [Google Scholar]
- Gudz TI, Schneider TE, Haas TA, Macklin WB. Myelin proteolipid protein forms a complex with integrins and may participate in integrin receptor signaling in oligodendrocytes. J Neurosci. 2002;22(17):7398–7407. doi: 10.1523/JNEUROSCI.22-17-07398.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks SK, Polte TR. Signaling through focal adhesion kinase. Bioessays. 1997;19(2):137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Waxman SG. Postnatal differentiation of rat optic nerve fibers: electron microscopic observations on the development of nodes of Ranvier and axoglial relations. J Comp Neurol. 1984;224(1):25–37. doi: 10.1002/cne.902240103. [DOI] [PubMed] [Google Scholar]
- Hoshina N, Tezuka T, Yokoyama K, Kozuka-Hata H, Oyama M, Yamamoto T. Focal adhesion kinase regulates laminin-induced oligodendroglial process outgrowth. Genes Cells. 2007;12(11):1245–1254. doi: 10.1111/j.1365-2443.2007.01130.x. [DOI] [PubMed] [Google Scholar]
- Hunter A, Bedi KS. A quantitative morphological study of interstrain variation in the developing rat optic nerve. J Comp Neurol. 1986;245(2):160–166. doi: 10.1002/cne.902450203. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995a;377(6549):539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- Ilic D, Furuta Y, Suda T, Atsumi T, Fujimoto J, Ikawa Y, Yamamoto T, Aizawa S. Focal adhesion kinase is not essential for in vitro and in vivo differentiation of ES cells. Biochem Biophys Res Commun. 1995b;209(1):300–309. doi: 10.1006/bbrc.1995.1503. [DOI] [PubMed] [Google Scholar]
- Kilpatrick TJ, Ortuno D, Bucci T, Lai C, Lemke G. Rat oligodendroglia express c-met and focal adhesion kinase, protein tyrosine kinases implicated in regulating epithelial cell motility. Neurosci Lett. 2000;279(1):5–8. doi: 10.1016/s0304-3940(99)00928-3. [DOI] [PubMed] [Google Scholar]
- Klein C, Kramer EM, Cardine AM, Schraven B, Brandt R, Trotter J. Process outgrowth of oligodendrocytes is promoted by interaction of fyn kinase with the cytoskeletal protein tau. J Neurosci. 2002;22(3):698–707. doi: 10.1523/JNEUROSCI.22-03-00698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingbeil CK, Hauck CR, Hsia DA, Jones KC, Reider SR, Schlaepfer DD. Targeting Pyk2 to beta 1-integrin-containing focal contacts rescues fibronectin-stimulated signaling and haptotactic motility defects of focal adhesion kinase-null cells. J Cell Biol. 2001;152(1):97–110. doi: 10.1083/jcb.152.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann T, Miron V, Cuo Q, Wegner C, Antel J, Bruck W. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–1758. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- Lee KK, de Repentigny Y, Saulnier R, Rippstein P, Macklin WB, Kothary R. Dominant-negative beta1 integrin mice have region-specific myelin defects accompanied by alterations in MAPK activity. Glia. 2006;53(8):836–844. doi: 10.1002/glia.20343. [DOI] [PubMed] [Google Scholar]
- Leone DP, Genoud S, Atanasoski S, Grausenburger R, Berger P, Metzger D, Macklin WB, Chambon P, Suter U. Tamoxifen-inducible glia-specific Cre mice for somatic mutagenesis in oligodendrocytes and Schwann cells. Mol Cell Neurosci. 2003;22(4):430–440. doi: 10.1016/s1044-7431(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24(32):7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubetzki-Korn I, Ovadia H, Vlodavsky I, Fuks Z, Abramsky O. Enhanced growth and morphological differentiation of isolated adult rat oligodendrocytes in vitro: use of a naturally produced extracellular matrix. Brain Res. 1983;267(1):151–155. doi: 10.1016/0006-8993(83)91049-1. [DOI] [PubMed] [Google Scholar]
- Malek-Hedayat S, Rome LH. Expression of a beta 1-related integrin by oligodendroglia in primary culture: evidence for a functional role in myelination. J Cell Biol. 1994;124(6):1039–1046. doi: 10.1083/jcb.124.6.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus J, Honigbaum S, Shroff S, Honke K, Rosenbluth J, Dupree JL. Sulfatide is essential for the maintenance of CNS myelin and axon structure. Glia. 2006;53(4):372–381. doi: 10.1002/glia.20292. [DOI] [PubMed] [Google Scholar]
- Milner R, ffrench-Constant C. A developmental analysis of oligodendroglial integrins in primary cells: changes in alpha v-associated beta subunits during differentiation. Development. 1994;120(12):3497–3506. doi: 10.1242/dev.120.12.3497. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Hanson DA, Schlaepfer DD. Focal adhesion kinase: in command and control of cell motility. Nat Rev Mol Cell Biol. 2005;6(1):56–68. doi: 10.1038/nrm1549. [DOI] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol. 2006;18(5):516–523. doi: 10.1016/j.ceb.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Nakamura I, Duong le T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25(6):337–344. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- Notterpek LM, Rome LH. A protein involved in central nervous system myelination: localization in the extracellular matrix and induction in neuroblastoma cells. Dev Neurosci. 1994;16(5–6):267–278. doi: 10.1159/000112119. [DOI] [PubMed] [Google Scholar]
- Oh LY, Yong VW. Astrocytes promote process outgrowth by adult human oligodendrocytes in vitro through interaction between bFGF and astrocyte extracellular matrix. Glia. 1996;17(3):237–253. doi: 10.1002/(SICI)1098-1136(199607)17:3<237::AID-GLIA6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Olsen IM, ffrench-Constant C. Dynamic regulation of integrin activation by intracellular and extracellular signals controls oligodendrocyte morphology. BMC Biology. 2005;3(25) doi: 10.1186/1741-7007-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr AW, Murphy-Ullrich JE. Regulation of endothelial cell function BY FAK and PYK2. Front Biosci. 2004;9:1254–1266. doi: 10.2741/1239. [DOI] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145(6):1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–1416. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- Relvas JB, Setzu A, Baron W, Buttery PC, LaFlamme SE, Franklin RJ, ffrench-Constant C. Expression of dominant-negative and chimeric subunits reveals an essential role for beta1 integrin during myelination. Curr Biol. 2001;11(13):1039–1043. doi: 10.1016/s0960-9822(01)00292-5. [DOI] [PubMed] [Google Scholar]
- Robles E, Gomez TM. Focal adhesion kinase signaling at sites of integrin-mediated adhesion controls axon pathfinding. Nat Neurosci. 2006 doi: 10.1038/nn1762. [DOI] [PubMed] [Google Scholar]
- Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hauck CR, Sieg DJ. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71(3–4):435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16(10):5623–5633. doi: 10.1128/mcb.16.10.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siskova Z, Baron W, de Vries H, Hoekstra D. Fibronectin impedes "myelin" sheet-directed flow in oligodendrocytes: a role for a beta 1 integrin-mediated PKC signaling pathway in vesicular trafficking. Mol Cell Neurosci. 2006;33(2):150–159. doi: 10.1016/j.mcn.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Price DL, Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. I. Cell proliferation. J Comp Neurol. 1976a;169(3):291–312. doi: 10.1002/cne.901690303. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Price DL, Stocks A. Electron microscopic autoradiographic studies of gliogenesis in rat optic nerve. II. Time of origin. J Comp Neurol. 1976b;169(3):313–334. doi: 10.1002/cne.901690304. [DOI] [PubMed] [Google Scholar]
- Skoff RP, Toland D, Nast E. Pattern of myelination and distribution of neuroglial cells along the developing optic system of the rat and rabbit. J Comp Neurol. 1980;191(2):237–253. doi: 10.1002/cne.901910207. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Vartanian TK. WAVE1 and regulation of actin nucleation in myelination. Neuroscientist. 2007;13(5):486–491. doi: 10.1177/1073858407299423. [DOI] [PubMed] [Google Scholar]
- Sperber BR, Boyle-Walsh EA, Engleka MJ, Gadue P, Peterson AC, Stein PL, Scherer SS, McMorris FA. A unique role for Fyn in CNS myelination. J Neurosci. 2001;21(6):2039–2047. doi: 10.1523/JNEUROSCI.21-06-02039.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szuchet S, Watanabe K, Yamaguchi Y. Differentiation/regeneration of oligodendrocytes entails the assembly of a cell-associated matrix. Int J Dev Neurosci. 2000;18(7):705–720. doi: 10.1016/s0736-5748(00)00034-4. [DOI] [PubMed] [Google Scholar]
- Tennekoon GI, Cohen SR, Price DL, McKhann GM. Myelinogenesis in optic nerve. A morphological, autoradiographic, and biochemical analysis. J Cell Biol. 1977;72(3):604–616. doi: 10.1083/jcb.72.3.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson CE, Vouyiouklis DA, Barrie JA, Wease KN, Montague P. Plp gene regulation in the developing murine optic nerve: correlation with oligodendroglial process alignment along the axons. Dev Neurosci. 2005;27(1):27–36. doi: 10.1159/000084530. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Nishiyama A, Cheng D, Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J Cell Biol. 1997;137(2):459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wight PA, Duchala CS, Readhead C, Macklin WB. A myelin proteolipid protein-LacZ fusion protein is developmentally regulated and targeted to the myelin membrane in transgenic mice. J Cell Biol. 1993;123(2):443–454. doi: 10.1083/jcb.123.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]