Abstract
The present study investigated how trait anxiety alters the balance between attentional control systems to impact performance of a discrete preplanned goal-directed motor task. Participants executed targeted force contractions (engaging the goal-directed attentional system) at the offset of emotional and non-emotional distractors (engaging the stimulus-driven attentional system). High and low anxious participants completed the protocol at two target force levels (10% and 35% of maximum voluntary contraction). Reaction time (RT), performance accuracy, and rate of change of force were calculated. Expectations were confirmed at the 10% but not the 35% target force level: 1) high anxiety was associated with slower RTs, and 2) threat cues lead to faster RTs independently of trait anxiety. These new findings suggest that motor efficiency, but not motor effectiveness is compromised in high relative to low anxious individuals. We conclude that increased stimulus-driven attentional control interferes with movements that require greater attentional resources.
Keywords: attention, force production, trait anxiety, affect, inhibition
Attentional Control Theory: Anxiety, Emotion, and Motor Planning
Impaired attentional processes have been identified as one of the primary cognitive factors underlying the inception and maintenance of anxiety (Eysenck, Derakshan, Santos, & Calvo, 2007). Anxiety related changes in attentional processes manifest in deficits in performance noted across a broad range of tasks including spatial and verbal reasoning (Darke, 1988: Experiments 2 and 3), digit-string short-term memory (Derakshan & Eysenck, 1998) and motor learning (Calvo & Ramos, 1989). The processing efficiency hypothesis (PET: Eysenck & Calvo, 1992) -which was central to the development of the aforementioned studies- has recently been superseded by attentional control theory (ACT: Eysenck et al., 2007). The present study tests the interactions among trait anxiety, emotional distractor cues, and motor planning within the context of two of the six hypotheses proposed in ACT.
Attention Control Theory
ACT (Eysenck et al., 2007) contends that anxiety manifests in impaired attentional control, which leads to performance deficits in tasks involving the central executive of the working memory system. This theoretical position is founded in the assumption that attention is regulated by (1) a goal-directed attentional system, and (2) a stimulus-driven attentional system (Corbetta & Shulman, 2002). The goal-directed attentional system is governed by expectations, knowledge, and current goals and exemplifies top-down attentional control. In contrast, the stimulus-driven attentional system is sensitive to salient stimuli, and exemplifies bottom-up attentional control. Importantly, ACT proposes that anxiety modulates the balance between these two attentional systems with increased anxiety leading to “...an increased influence of the stimulus-driven attentional system and a decreased influence of the goal-directed attentional system” (Eysenck et al., 2007, p.338). This imbalance is reflected in performance deficits on cognitive tasks (Arnell, Killman, & Fijavz, 2007; Blair et al., 2007; Bledowski, Prvulovic, Goebel, Zanella, & Linden, 2004), but the consequences of this imbalance have yet to be investigated when performing discrete goal-directed motor tasks. ACT suggests that inhibition (in addition to shifting and updating) is a distinct function of the central executive. Inhibition refers to one's ability to minimize disruption or interference from task irrelevant stimuli. That is, an increased ability to inhibit interference from task irrelevant stimuli (which engage the stimulus-driven attentional system) allows the goal-directed attentional system to continue to function with minimal disruption.
Motor Planning
When considering goal-directed voluntary movements, a controlled approach to studying how the nervous system regulates motor output is to use tasks that require individuals to produce force against an object. Performing simple functional activities of daily living such as eating, dressing, grooming, and drinking, all require the application of a planned grasping force against an object. Such acts of daily living require the production of a range of sub-maximal forces with most acts necessitating low to moderate levels of force (Marshall & Armstrong, 2004; McPhee, 1987). Motor abnormalities have been associated with affective disorders (Lohr & Caligiuri, 2006; Rossi, Bartalini, Ulivelli, Mantovani, & Di Muro, 2005; Wada, Sunago, & Nagai, 2001; Yardley, Britton, & Lear, 1995), and these motor abnormalities may lead to movements that are repeatedly performed inefficiently and/or inaccurately which, in turn, may compromise quality of life. Moreover, minor variations in force output can lead to dire consequences within military, sport, and medical domains, where anxious states and unexpected distractor stimuli are routinely experienced (Janelle & Hatfield, 2008; Norman, Eva, Brooks, & Hamstra, 2006; Satava, Gallagher, & Pellegrini, 2003).
Much of the current literature that validates PET and ACT is derived from cognitive tasks (e.g., Bonnot & Croizet, 2007; Hardy, Beattie, & Woodman, 2007). Those studies that have tested PET/ACT hypotheses within the motor domain have relied on continuous tasks (Murray & Janelle, 2003; Smith, Bellamy, Collins, & Newell, 2001; Williams, Vickers, & Rodriques, 2002; Wilson, Chattington, Marple-Horvat, & Smith, 2007) or simple reaction time tasks (Elliman, Green, Rogers, & Finch, 1997). Questions therefore remain concerning how emotion and anxiety impact the parameterization of functional motor tasks. By implementing a discrete goal-directed motor task in the current study, force output will be precisely measured and movement errors will be reliably quantified in the context of ACT hypotheses for the first time.
The notion that efficient motor function is susceptible to changes in anxiety and attention has been previously demonstrated (cf., Janelle, 2002). Attentional control and working memory are critical for effective motor planning given that planning voluntary motor action requires the conscious parameterization of movement, which is made possible by the working memory system (Baddeley, 1986; Beilock, Jellison, Rydell, McConnell, & Carr, 2006). Motor planning is generally accepted as a process that occurs prior to movement initiation and uses visual and cognitive information derived from the environment and actor to assist in selecting an appropriate motor response (e.g. Glover, 2004). Before movements are initiated, current information is integrated with memories of past experiences (Rosenbaum, Loukopoulos, Meulenbroek, Vaughan, & Engelbrecht, 1995). Working memory is therefore essential to motor planning. Hence, even well-learned motor behaviors which engage the goal-directed attentional system remain highly susceptible to interference from the stimulus-driven attentional system. Central to the purpose of the current study, we argue that the susceptibility of motor planning to stimulus driven interference will be inflated among anxious individuals (Eysenck et al., 2007).
Anxiety, Attention, and Motor Planning
The current study tested hypotheses three and four proposed in ACT. Hypothesis three predicts that “Anxiety impairs attentional control by increasing the influence of the stimulus-driven attentional system” (Eysenck et al., 2007, p. 342). Evidence for this hypothesis comes from protocols in which performance on a central task is negatively affected by interference from a task commanding attention from the stimulus driven attentional system in high as compared to low anxiety individuals and situations (Fenske & Eastwood, 2003; Hopko, McNeil, Gleason, & Rabalais, 2002; Janelle, Singer, & Williams, 1999). In the current study, the task targeting the goal directed attentional system required the production of a brief force contraction to a predetermined target force level as quickly and accurately as possible. Viewing the distractor images in this case served as the more salient task relative to the motor task. We anticipated that viewing the distractor image would result in a shift in attentional control from the goal-directed attentional system (planning the execution of the goal-directed motor task) to the stimulus-driven attentional system, and this stimulus-driven shift would be less inhibited in high as compared to low anxious individuals. The subsequent question, therefore, is how should increased salience of the stimulus-driven attentional system manifest in performance of the primary goal-directed motor task? ACT predicts that although the effectiveness of a task may be similar between high and low anxious groups, anxiety will lead to a reduction in performance efficiency (Eysenck & Calvo, 1992; Eysenck et al., 2007). Accordingly, presence of distractor cues should result in high anxious individuals performing tasks more slowly, but with similar accuracy to low anxious individuals (Beilock, Kulp, Holt, & Carr, 2004; Fox, Russo, Bowles, & Dutton, 2001; Koster, Croombez, Verschuere, & De Houwer, 2006).
The fourth hypothesis of ACT predicts that “Anxiety impairs efficiency (and often effectiveness) on tasks involving the inhibition function, especially with threat-related distractors” (Eysenck et al., 2007, p. 344). This hypothesis qualifies the third hypothesis based on the characteristics of present distractors, specifically the emotional salience of the distractors. Data from the attentional bias literature demonstrates that highly anxious individuals direct their attention to threat faster than low anxious individuals, and also show deficits in being able to disengage attention from those threatening cues (see Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoom, 2007, for a comprehensive review and meta-analysis). Accordingly, in the present study one may predict that threat images should engage the stimulus-driven attentional system to a greater extent than non-threatening images in anxious individuals, which would be reflected in even slower RTs. However, threat-related attentional effects in high anxious individuals only manifest at very short subliminal or supraliminal time windows immediately following the presentation of threatening cues (Etkin et al., 2004; Fox, 2002). Therefore, a plausible explanation or argument is that, although threatening images engage the stimulus-driven attentional system at the expense of the goal-directed attentional system, this may only be captured at very short time intervals within a single trial. Moreover, when viewing times of emotional images are in the magnitude of seconds as compared to milliseconds, previous evidence in non-anxious individuals suggests that exposure to unpleasant (∼3 s: Hajcak et al., 2007) and threat images prime the motor system for action, and lead to faster RTs (Coombes, Cauraugh, & Janelle, 2007a). Hence, because the primary task in the current study is motoric, and because the presentation window of the distractor image is 2−4 s (to replicate a previous protocol: Coombes et al., 2007a; Coombes, Cauraugh, & Janelle, 2007b), threat cues should prime the motor system in high and low anxious individuals, leading to faster RTs and more efficient performance.
The current project required high and low anxious individuals to execute brief goal-directed force contractions (to engage the goal-directed attentional system) to the offset of emotional and non-emotional distractor cues (to engage the stimulus-driven attentional system). Our primary interest was to determine the extent to which these distractor cues altered the speed, accuracy, and vigor [i.e., rate of change of force production] of a planned motor task. We tested two hypotheses: 1) High anxious, relative to low anxious individuals will display slower RTs, and 2) High and Low anxious individuals will display faster RTs to threat cues as compared to non-threat cues. We expected each of these hypotheses to hold true across two target force levels (10% and 35% of MVC) and we did not expect between group differences in performance accuracy or performance vigor.
Method
Participants
Following power analyses for each hypothesis1 65 (Female = 36, Male = 29, M age = 20.25 years, SD = 1.37) undergraduate volunteers participated in this study for extra course credit. Over 500 potential candidates completed the trait version of the STAI (Spielberger, 1983). Random subsets of participants who reported high (>40) and low (<30) anxiety scores were recruited to participate. One low anxiety group (N = 16) and one high anxiety group (N = 18) completed the protocol at the 10% MVC target force level, and one low anxiety group (N = 16) and one high anxiety group (N = 15) completed the protocol with the target force set at 35% of MVC. Table 1 shows the characteristics of each of the four groups. Data 3 SD from the mean for each group at each target force level were considered outliers and removed prior to statistical analyses. With target force set at 10% of MVC, 4 participants were removed from the RT analysis and 3 participants were removed from the rate of change and RMSE analyses. With target force set at 35% of MVC, 1 participant was removed from the RT and rate of change analyses, and 2 participants were removed from the RMSE analysis. All participants were right handed and reported no central nervous system disorders that would affect movement. All participants provided university approved written informed consent prior to taking part in the experiment.
Table 1.
Group characteristics.
| Target Force | 10% | 35% | ||
|---|---|---|---|---|
| Group | Low | High | Low | High |
| N (of which female) | 16 (7) |
18 (12) |
16 (9) |
15 (8) |
| Ethnicity | ||||
| Hispanic or Latino | 1 | 2 | 1 | 1 |
| Not Hispanic or Latino | 15 | 16 | 15 | 14 |
| M | SD | M | SD | M | SD | M | SD | |
|---|---|---|---|---|---|---|---|---|
| Practice trials | 38.00 | 15.88 | 36.39 | 14.94 | 26.31 | 15.77 | 24.67 | 16.10 |
| Age (yrs) | 19.62 | 1.20 | 19.78 | 1.70 | 21.19 | 1.05 | 20.47 | 0.74 |
| MVC (N) | 48.19 | 13.31 | 37.82 | 13.71 | 56.10 | 20.25 | 46.97 | 8.33 |
| STAI-T score | 25.56 | 1.90 | 46.22 | 4.11 | 25.87 | 2.16 | 46.87 | 6.85 |
Instrumentation and Task
State Trait Anxiety Inventory (Spielberger, 1983)
The trait version of the STAI assesses global levels of anxiety. Although the STAI has been identified as a unideminsional tool for assessing anxiety, one's aggregate STAI score is calculated from scores on multiple questions assessing several different dimensions including apprehension, tension, nervousness, and worry. The inventory is comprised of 20 questions scored on a 4-point Likert scale. Reliability scores range from .65 −.86. Previous studies which have investigated trait anxiety in young adult samples have dichotomized high and low anxious groups using scores ranging between 37.5 to 41 (Bradley, Mogg, Falla, & Hamilton, 1998; Derryberry & Reed, 2002; MacLeod & Mathews, 1988; Mogg & Bradley, 2002). Accordingly, we used trait scores of 40 as a low cut-off for the high trait anxious group, and trait scores of 30 as a high cut-off for the low trait anxious group.
Motor Task
Participants executed isometric ballistic contractions by pinching a force transducer (MLP-75, Transducer Techniques, Temecula, CA, USA) with the thumb and index finger of their right hand while seated in a chair positioned 1 m from a 19” LCD monitor (1024×768 resolution; 70Hz refresh rate). Their elbows were placed at a right angle with their wrists positioned midway between maximum supination and maximum pronation. Analog output from the force transducer was amplified through a 15LT Grass Technologies Physiodata Amplifier System (Astro-Med Inc. West Warwick, RI, USA) at an excitation voltage of 10 V. Custom Labview software (8.1; National Instruments, Austin, TX) controlled trial onset, trial offset, visual stimulus presentation, and also controlled a 16-bit analog-to-digital converter (A/D) (PCI-6220, national Instruments, Austin, TX) which sampled the force at 100Hz. Force data were streamed to disk for offline analysis.
Maximal Voluntary Contraction
Participants’ maximal voluntary contraction (MVC) was assessed using a previously established protocol (Vaillancourt & Newell, 2003). This value was used as the reference for computation of each individual's submaximal goal force level (i.e., 10% or 35% of MVC). MVC values for each high and low anxiety group for each target force level are shown in Table 1. Independent samples t-tests revealed that MVC values between high anxiety and low anxiety groups at each target force level did not differ (10%: t (28) = 1.41, p = .170; 35%: t (28) = 1.51, p = .143).
Emotion Manipulation
Participants viewed 30 digitized photographs selected from the International Affective Picture System2 (IAPS: Lang, Bradley, & Cuthbert, 2005). These pictures represented five emotional categories: 1) erotic couples, 2) mutilation, 3) neutral, 4) adventure, and 5) attack. Images were selected according to affective normative ratings to match arousal between pleasant and unpleasant images while discriminating each from neutral images, and to differentiate valence across all categories. Image presentation order was randomized and counterbalanced between participants.
Procedure
Having provided informed consent, subjects completed the procedure which allowed us to calculate MVC. Task instructions were then verbally communicated to the participant. Participants were informed that they would first see a fixation cross, which would be replaced by an image. They were instructed to look at the image for the entire time it was on the screen. Images were presented for 2−4 s and presentation time was independent of each individual picture. At picture offset the screen went blank and participants were instructed to squeeze the force transducer as quickly and accurately as possible. The goal of the contraction was to match the peak force generated as accurately as possible with the imposed target value (i.e., 10%, 35% of MVC). Completion of the force contraction signaled the end of the trial and the screen remained blank during the intertrial interval (14.5 s). Presentation of the fixation cross marked the beginning of the subsequent trial.
Familiarization with the target force was achieved during a practice session in which participants completed a version of the experimental task with two modifications: 1) all images presented were neutral images taken from the IAPS, and 2) participants received visual feedback following each trial. Following completion of each contraction during the practice period, a horizontal red line representing the target force and a white line representing the force output were visually displayed for 3 s. The practice session was completed once participants were able to execute 4 consecutive contractions within a range of +/− 20% of the target force. The average number of practice trials completed by each group is shown in Table 1. Independent samples t-tests revealed that the number of trials required to obtain the necessary accuracy threshold were not different between groups at each target force level (10%: t (28) = .912, p = .37; 35%: t (28) = .04, p = .967).
Following the practice session, participants completed 30 experimental trials free of experimenter interaction. Performance feedback was not provided during experimental trials. Upon completion of the experimental trials, participants were fully debriefed.
Data Reduction
Dependent measures included RT, root mean square error (RMSE), and peak rate of change of force production (maximum of the first derivative). RT data were calculated by measuring the time between the offset of the image and the onset of force production. Onset of force production was identified as the time point when force production increased 3 fold above baseline. Mean force output during the 100 msec preceding image offset served as the baseline value. The peak rate of change of force was the maximum of the first derivative of force from force onset to when the force level reached its peak force for that trial. Summary statistics for RT and rate of change of force were calculated by averaging these scores within valence category for each participant. RMSE was computed by subtracting peak force for each trial from the target value, squaring that value, and then taking the mean of these values within valence categories. Calculating the square root of each of these values resulted in the RMSE summary statistic. Hence, each valence category was represented by one RMSE value for each participant.
Statistical Analyses
Primary analyses
High and low anxiety group data were compared within each target force level. RT, RMSE, and rate of change of force were each analyzed in separate 2 (Group: high anxiety, low anxiety) × 5 (Valence: adventure, erotica, attack, mutilation, neutral) mixed design ANOVAs with repeated measures on Valence.
Secondary analyses
Although MVC between groups was not statistically significant, the low anxiety group had higher MVCs across both target force levels (see Table 1). Relatively higher MVC levels mandate higher target forces that in turn typically lead to greater error and an increased rate of change of force production, which could have potentially confounded our primary analyses. To guard against this potential confound, secondary analyses were conducted. Specifically, all primary analyses were re-run but with MVC level added as a covariate.
For analyses involving Valence, the Greenhouse-Geisser conservative degrees of freedom adjustment was used if the sphericity assumption was violated. Follow-up analyses were conducted using simple effects tests and Tukey's HSD procedure. The probability value was set at p < 0.05 for all analyses. Results from each target force level are presented consecutively for each dependent variable.
Results
Primary Analyses
Table 2 shows RT, RMSE, and rate of change of force data from the high and low anxiety groups for each target force level across each level of valence.
Table 2.
Force onset, RMSE, and rate of force means (+1SE) for high and low anxiety groups for each target force level across each level of valence.
| Onset | RMSE | Rate of Force | ||||||
|---|---|---|---|---|---|---|---|---|
| Target Force | Group | Valence | M | SE | M | SE | M | SE |
| 10% MVC | low | adventure | 376.82 | 24.74 | 2.72 | 0.35 | 52.45 | 4.30 |
| erotica | 384.04 | 30.95 | 2.66 | 0.31 | 59.03 | 5.13 | ||
| attack | 341.54 | 30.93 | 2.33 | 0.25 | 48.36 | 4.96 | ||
| mutilation | 344.68 | 33.12 | 2.76 | 0.33 | 49.8 | 4.00 | ||
| neutral | 366.66 | 36.27 | 2.45 | 0.23 | 47.37 | 3.84 | ||
| high | adventure | 468.29 | 23.14 | 1.86 | 0.34 | 35.59 | 4.16 | |
| erotica | 486.29 | 28.95 | 1.79 | 0.30 | 35.13 | 4.96 | ||
| attack | 433.1 | 28.94 | 1.43 | 0.24 | 33.03 | 4.80 | ||
| mutilation | 443.31 | 30.98 | 1.53 | 0.32 | 30.9 | 3.87 | ||
| neutral | 484.26 | 33.93 | 1.77 | 0.23 | 33.85 | 3.71 | ||
| 35% MVC | low | adventure | 394.09 | 25.07 | 5.34 | 0.50 | 147.48 | 15.03 |
| erotica | 370.69 | 22.65 | 5.21 | 0.56 | 141.01 | 14.48 | ||
| attack | 356.75 | 27.51 | 5.20 | 0.51 | 144.03 | 15.11 | ||
| mutilation | 400.84 | 27.26 | 6.18 | 0.66 | 141.59 | 15.34 | ||
| neutral | 346.53 | 18.83 | 4.67 | 0.52 | 141.86 | 15.12 | ||
| high | adventure | 403.75 | 26.80 | 4.90 | 0.56 | 121.66 | 16.06 | |
| erotica | 426.23 | 24.22 | 5.19 | 0.62 | 117.9 | 15.48 | ||
| attack | 379.29 | 29.41 | 4.67 | 0.57 | 115.8 | 16.16 | ||
| mutilation | 420.07 | 29.14 | 4.78 | 0.74 | 114.93 | 16.4 | ||
| neutral | 415.93 | 20.13 | 4.95 | 0.58 | 118.76 | 16.16 | ||
Force onset (RT) at 10% MVC
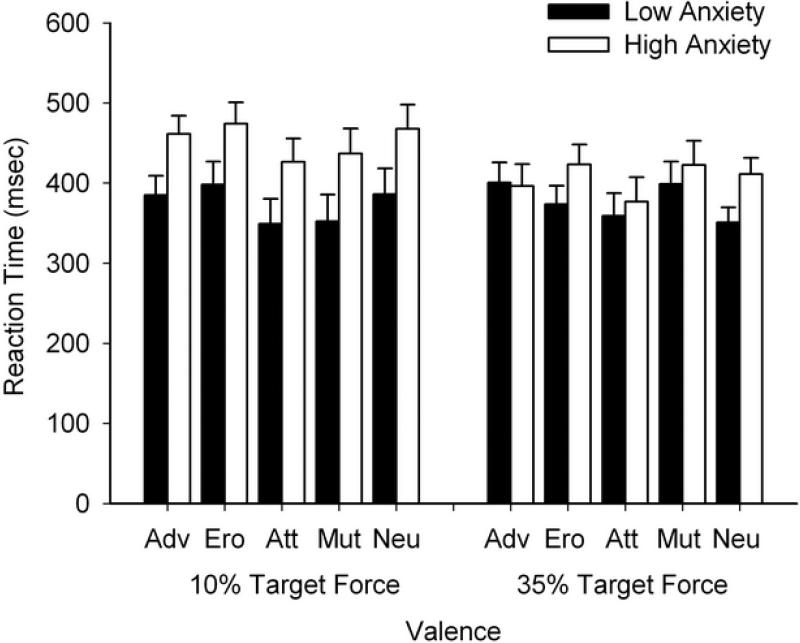
A two-way mixed model ANOVA confirmed the trends shown in Table 2, revealing significant between group differences for RT, F (1, 28) = 6.89, p = .014, η2 = .20. Follow-up tests showed that the high anxiety group was slower than the low anxiety group to initiate movements (HA: M = 362.75, SE = 27.91; LA: M = 463.05, SE = 26.11). A significant main effect of valence was also evidenced, F (3.03, 84.74) = 3.59, p = .017, η2 = .02, with follow-up tests showing a general bias of faster RT's to unpleasant images. Specifically, RT's following the offset of attack images were faster than following adventure, erotica, and neutral images, as well as following mutilation images as compared to erotica images. The Group × Valence interaction was not significant, F (3.03, 84.74) = .24, p = .87, η2 = .01.
Force onset (RT) at 35% MVC
Force onset varied significantly as a function of valence (F (4, 112) = 2.52, p = .045, η2 = .08), with follow-up tests revealing faster responses following exposure to attack as compared to mutilation images. Neither a significant main effect of Group nor a Group × Valence interaction were evidenced (Group: F (1, 28) = 1.34, p = .26, η2 = .05; HA: M = 409.05, SE = 22.25, LA: M = 373.78, SE = 20.82; Group × Valence: F (4, 112) = 1.49, p = .21, η2 = .051).
RMSE at 10% MVC
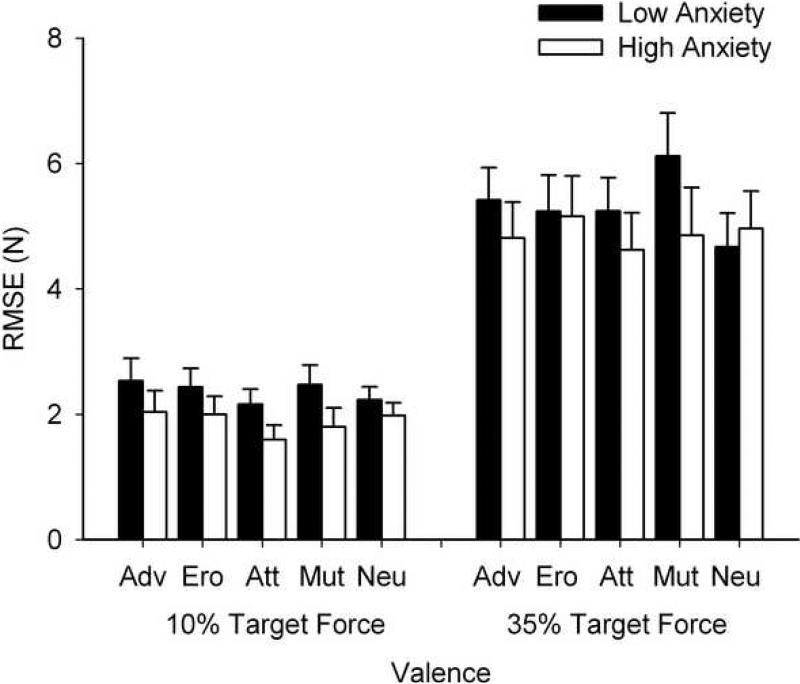
Follow-up analyses on a significant main effect of group (F (1, 29) = 8.26, p = .008 η2 = .22) revealed that the high anxiety group executed the task with greater accuracy as compared to the low anxiety group (HA: M = 1.68, SE = .22; LA: M = 2.58, SE = .23). The main effect of Valence and the Group × Valence interaction were not significant (Valence: F (3.02, 87.52) = 1.08, p = .36, η2 = .04; Group × Valence: F (3.02, 87.52) = .43, p = .73, η2 = .02)
RMSE at 35% MVC
With the target force set to 35% of MVC, analyses revealed that accuracy of performance was not significantly different between groups (F (1, 27) = .35, p = .56. η2 = .01), valence categories (F (4, 108) = 1.22, p = .31, η2 = .04) (HA: M = 4.89, SE = .53; LA: M = 5.32, SE = .48) or between any combination of these two factors (Group × Valence: F (4, 108) = 1.89, p = .12, η2 = .07).
Rate of change of force at 10% MVC
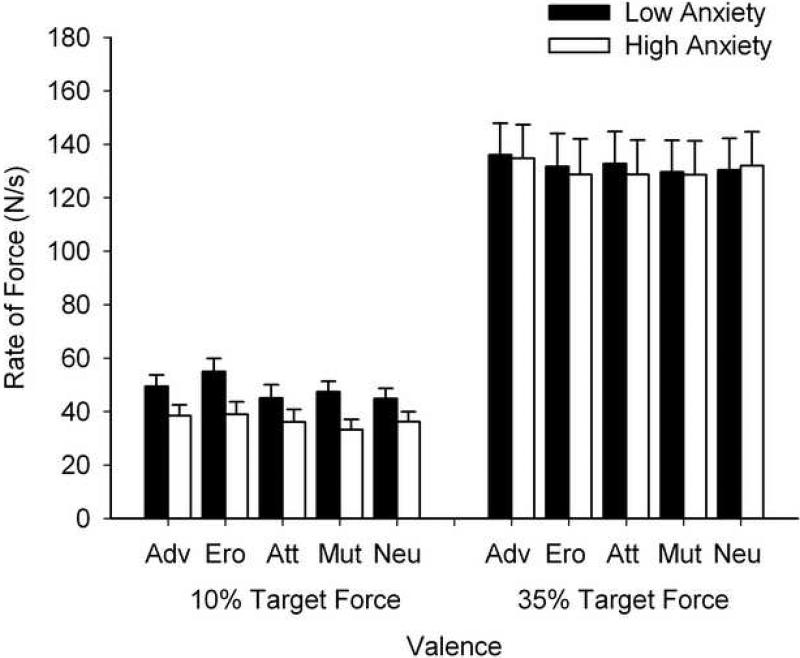
The rate of change of force data for the high and low anxiety groups at each force level for each valence category is shown in Table 2. For contractions made to target force levels at 10% of MVC, rate of change of force differed significantly between groups (F (1, 29) = 9.63, p = .004, η2 = .25), with the high anxiety group displaying an attenuated rate of change of force production relative to the low anxiety group (HA: M = 33.70 SE = 3.97; LA: M = 51.40 SE = 4.10). Rate of change of force production was also significantly altered by valence, (F (2.84, 82.32) = 4.47, p = .007, η2 = .13), with follow-up tests revealing a general increase in rate of change during pleasant images (erotica > attack, mutilation, neutral; adventure > neutral). The Group × Valence interaction was not significant (F (2.84, 82.32) = 2.05, p = .12, η2 = .07).
Rate of change of force at 35% MVC
Main effects of Group and Valence and the interaction between these factors were all non-significant (Group: F (1, 28) = 1.45, p = .24 η2 = .05; HA: M = 117.81, SE = 15.37, LA: M = 143.19, SE = 14.38; Valence: F (4, 112) = .46, p = .77, η2 = .02; Group × Valence: F (4, 112) = .10, p = .92, η2 = .004.
Secondary Analyses
All significant differences are reported for the secondary analyses, as well as non-significant findings that deviated from the primary analyses (covariate: 10%: 42.05N; 35%: 52.01N). Figures 1-3 show the adjusted means for RT (Figure 1), RMSE (Figure 2), and mean rate of change of force (Figure 3). Although data from the 35% target force level are shown in Figures 1-3, note that data from the 35% target force level are not reported below because the critical p-values in the secondary analyses did not differ from those in the primary analysis. As such, Figures 1-3 are presented for each target force level to provide a visual comparison between data for each group, target force level, and valence category.
Figure 1.
Adjusted reaction time data across the valence conditions for each target force level. The black bars represent adjusted mean RT for the low anxiety group. White bars represent adjusted mean RT for the high anxiety group. The high anxiety group were slower than the low anxiety group at 10% of MVC (left), but there was no between group difference at 35% (right). A main effect of valence was evidenced at 10% (attack < adventure, erotica, neutral; mutilation < erotica), and at 35% (attack < mutilation). The error bars are +1SE from the adjusted mean for each group. Valence conditions: Adv = adventure, Ero = erotica, Att = attack, Mut = mutilation, Neu = Neutral.
Figure 3.
Adjusted mean rate of change of force for each target force level across the valence conditions. The black bars represent adjusted mean peak rate of change of force for the low anxiety group and the white bars represent adjusted mean peak rate of change of force for the high anxiety group. Relative to the low anxiety group, the high anxiety group displayed attenuated rate of change at the 10% target force level. No between group effects were found at the 35% target force level. The rate of change of force production at each target force level did not vary as a function of valence, or a combination of trait anxiety and valence. The error bars are +1SE from the adjusted mean for each group. Valence conditions: Adv = adventure, Ero = erotica, Att = attack, Mut = mutilation, Neu = Neutral.
Figure 2.
Adjusted accuracy data (represented as RMSE) across the valence conditions for each target force level. The black bars represent adjusted mean RMSE for the low anxiety group and the white bars represent adjusted mean RMSE for the high anxiety group. Accuracy scores at each target force level did not vary as a function of trait anxiety or valence, or a combination of trait anxiety and valence. The error bars are +1SE from the adjusted mean for each group. Valence conditions: Adv = adventure, Ero = erotica, Att = attack, Mut = mutilation, Neu = Neutral.
Force onset (RT)
RT analyses with the target force at 10% of MVC remained largely unchanged from our primary analyses. A significant main effect of Group (F (1, 27) = 4.57, p = .042, η2 = .15) corroborated our initial findings by showing that the high anxiety group were slower than the low anxiety group (HA: M = 453.32, SE = 24.88; LA: M = 373.97, SE = 26.66). A main effect of Valence was also evidenced (F (4, 108) = 4.35, p = .003, η2 = .14) with the follow-up analyses also unchanged from the primary analyses (attack < adventure, erotica, neutral; mutilation < erotica).
RMSE
With regard to movement accuracy at the 10% target force level, although the trend within the data remained in the same direction as the primary analysis (HA < LA: HA: M = 1.88, SE = .20; LA: M = 2.36, SE = .20), the between group difference was not significant (F (1, 28) = 2.70, p = .11 η2= .09). Hence, when controlling for MVC level, the effect of trait anxiety on performance accuracy was lost, removing the speed-accuracy trade-off that emerged at the 10% MVC target force level in the primary analyses.
Rate of Change of Force
Mean rate of change of force production was altered by Group when the target force was set at 10% of MVC (F (1, 28) = 4.17, p = .05, η2 = .13) with the high anxiety group still displaying attenuated rate of change (M = 36.57, SE = 3.83) as compared to the low anxiety group (M = 48.34, SE = 3.97).
Discussion
The goal of the current study was to determine how the balance between attentional control systems and the inhibition function impact a preplanned goal-directed motor task in individuals with high levels of trait anxiety. Three novel contributions emerged from these findings: 1) High anxiety was associated with attenuated performance efficiency but not performance effectiveness. 2) Threat stimuli lead to faster RTs in high and low anxiety groups. 3) Between group differences were evidenced in the hypothesized direction at relatively low but not at relatively high force levels. Each of these findings is addressed in detail below.
ACT (Eysenck et al., 2007) postulates that high anxiety leads to a decreased ability to inhibit the stimulus-driven attentional system when the goal-directed attentional system is concerned with executing fast and accurate performance. This imbalance is hypothesized to manifest in slower RTs (Arnell et al., 2007; Blair et al., 2007), a prediction that drove our first hypothesis. RT findings with the target force set at 10% of MVC supported this hypothesis. This finding was noted in our primary analyses and was confirmed by the secondary analyses when controlling for between group differences in MVC.
We did not expect movement accuracy to differ between groups. However, the primary analysis revealed that although slower to initiate movements, the high anxiety group was more accurate, and therefore displayed the classic speed-accuracy trade-off (Fitts, 1954, 1966). Such a classic speed-accuracy trade-off may have been driven by between group differences in strategic concerns/regulatory focus (Forster, Higgins, & Bianco, 2003). Previous evidence suggests that highly anxious individuals have a greater tendency to implement a prevention oriented regulatory focus (Forster & Higgins, 2005), and these initial findings support this assertion. The high anxiety group may have employed a prevention regulatory focus rather than a promotion regulatory focus, emphasizing movement accuracy at the sacrifice of movement speed.
Prior to embracing a regulatory focus interpretation, however, secondary analyses were conducted to ensure that MVC, and thus target force level between groups were not driving this speed-accuracy trade-off. Indeed, although MVC between groups was not statistically significant, the low anxiety group had higher MVCs across both target force levels. Relatively higher MVC levels led to higher target forces which in turn typically lead to greater error and an increased rate of change of force production which could have potentially driven between group differences in our primary analyses. This finding is exemplified in Figures 2 and 3, where decreases in absolute response accuracy and increases in absolute response vigor are evidenced when the target force was set at 35% of MVC as compared to 10% of MVC. Once MVC levels were controlled within our secondary analyses, between group accuracy scores were no longer different. In consequence, performance accuracy did not differ between groups which suggested that as theorized, performance efficiency is compromised in high as compared to low trait anxiety, but performance effectiveness is not. Importantly, this finding only held true at the low target force level (to be discussed below). As such, we interpret our data as supporting and extending the scope of ACT (Eysenck et al., 2007), suggesting that following presentation of distractor visual cues, highly anxious individuals are less able to inhibit the influence of the stimulus-driven attentional system on the goal-directed attentional system during a discrete preplanned goal-directed motor task.
Rate of change of force production, reflecting performance vigor, remained unchanged across analyses, suggesting attenuated vigor in the high anxiety group as compared to the low anxiety group at the 10% MVC force level. Although this finding was not expected, the secondary analyses suggested that this effect was partially driven by the lower MVC scores of the high anxiety group. Additionally, this finding may have also been driven by differences in regulatory focus and a general increase in inhibition. Specifically, attenuated vigor is in line with a more prevention biased regulatory focus (Forster et al., 2003) as well as an increase in inhibition (Eysenck et al., 2007). Clearly, further research is needed to clarify the effect of anxiety on the rate of change of force production.
Concerning our second hypothesis, we anticipated that both groups would be faster following the presentation of threat as compared to non-threat images. With the target force set at 10% of MVC our data supported this hypothesis and in doing so corroborated previous evidence that threat images prime the motor system, leading to faster RTs (Coombes et al., 2007a). Importantly, there was no effect of valence on performance accuracy, suggesting that the emotion driven priming of the motor system had no influence on the accuracy of force production. Moreover, in previous studies, increases in the speed of movement during exposure to unpleasant images have been evidenced only when the movement direction and emotional state are congruent (i.e., extension movement + unpleasant image). In the present case, movements were not direction specific (i.e., towards or away from the body), thus suggesting that when no conflict (or congruence) exists between the emotional state and the movement direction, unpleasant emotional states facilitate the speed of contractions. Interestingly, given that previous evidence shows that sustained pinch-grip tasks are equally altered by pleasant and unpleasant states as compared to neutral states (Coombes, Gamble, Cauraugh, & Janelle, 2008), we argue that this specific unpleasant priming may only hold true for short duration discrete movements. Nevertheless, this finding further validates the notion that emotional states alter activity within the motor system (Coombes, Cauraugh, & Janelle, 2006; Coombes, Janelle, & Duley, 2005; de Gelder, Snyder, Greve, Gerard, & Hadjikhani, 2004; Hajcak et al., 2007; Marsh, Ambady, & Kleck, 2005; Pessiglione et al., 2007).
Finally, we found that group differences were only evidenced at low as compared to high target force levels. Three potential explanations may account for this finding. First, effects may not have been similar across target force levels because different subjects composed each group at each target force level. However, this would suggest that at the high target force level, either the low anxiety group performed as one would expect the high anxiety group to perform, or vice versa. While acknowledging this as a possible explanation we argue that it is highly unlikely given the polarization between trait anxiety scores between groups at each target force level. Dissimilar findings across target force level suggest that high anxiety will not always lead to an increase in reaction time and that anxiety driven changes in reaction time are modulated by task demands. Thus, a second possible explanation is task difficulty. Our data suggest that the target force level of 10% MVC was more difficult and required greater planning to achieve greater precision. Consequently, the increased demand on the working memory system was more susceptible to interference from the stimulus driven attentional system. Previous fMRI evidence supports this position. For instance, Ehrsson, Fagergren, and Forssberg (2001) provided evidence that force production at relatively low precision grip forces, relative to larger grip forces, more strongly activated several sensory and motor related fronto-parietal areas involved in higher order sensorimotor integration during the planning and execution of goal-directed actions. Moreover, the average number of practice trials to reach the qualifying pre-experimental performance level suggested that the criterion was easier to reach when the target force level was set at 35% of MVC, and this pattern was stable across groups (see Table 1). Finally, a third explanation is that limited power may have resulted in the null findings at 35% of MVC. Although power analyses were conducted prior to recruitment, they were based on pilot data which probed performance at the 10% target force level. Hence, the null findings at the 35% target force level may well have been the result of a small sample size and limited power.
Greater specification of performance differences as an interaction of trait anxiety and valence of distractor cues may require the manipulation of cue exposure length. Previous evidence suggests that conscious and unconscious processing of threatening information represents distinct operations that are thought to be associated with different neural and behavioral responses (van Honk, Schutter, d' Alfonso, Kessels, & de Haan, 2002; Williams et al., 2006). Indeed, changes in neural and behavioral function in response to unconscious threat appear to be most prominent in individuals with high levels of trait anxiety (Etkin et al., 2004; Fox et al., 2001), which supports the link between trait anxiety and negative affectivity. Therefore, future research should investigate the role that level of awareness (i.e., subliminal vs. supraliminal) of distractor cues may play in modulating the effect of trait anxiety on a ballistic goal-directed motor task. The current findings could also be extended by exploring the effect of emotional distractors on more complex and dynamic motor planning processes, which could be accomplished by alternating the target force level within the same protocol. Further, given the meaningful effects that we have evidenced in a relatively small sub-clinical sample, the replication of these findings in larger clinically anxious samples may accelerate the formulation of practical recommendations related to the clinical assessment of motor performance efficiency in highly trait-anxious individuals.
Additionally, future research efforts could extend our behavioral findings by integrating attentional, neurological, and self-report measures. Although we argue that high anxious individuals’ slower RTs resulted from an inability to inhibit the stimulus driven attentional system, we did not directly assess attention allocation to disparate image content, and we acknowledge this inferential limitation. While we do not believe this limitation compromises the current evidence, we encourage future researchers to verify our behavioral findings with the use of additional attention measures such as eyetracking (e.g., Calvo, Nummenmaa, & Hyona, 2007).
Finally, as previously mentioned, performance differences could have been a product of disparate self regulatory focus between high and low anxiety groups. Although secondary analyses suggested this was not the case, the potential emergence of a speed-accuracy tradeoff which is driven by regulatory focus should not be ruled out in future work given that high anxiety is associated with a prevention regulatory focus (Forster & Higgins, 2005). Thus, future experiments should include self regulatory focus indices if we are to (a) understand how self-regulatory focus impacts the balance between the attentional control systems, and (b) better understand how this (im)balance impacts the motor system.
In conclusion, guided by predictions derived from ACT (Eysenck et al., 2007), the current study investigated how individual differences in trait anxiety alter the planning of force specific motor tasks under varying emotional states. The new findings presented show that motor efficiency, but not motor effectiveness was compromised in high anxious relative to low anxious individuals, but only when the motor task to be performed required greater precision and increased attentional resources. We argue that decreased performance efficiency was driven by attenuated inhibition in the high anxiety group which led to enhanced stimulus driven attentional control at the expense of goal driven attentional control. Finally, our data validate the hypothesis that unpleasant emotional states prime the motor system and do so irrespective of dispositional trait anxiety levels.
Grants
This research was supported by in part by National Institute of Health grants F32-MH-083424, T32-MH-067631, R03-MH-70678, R03-HD-044534, and American Heart Association grant 00061194.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Based on pilot data from 2 subjects who reported high anxiety and 2 subjects who reported low anxiety we conducted a power analysis for each hypothesis. The power analysis (independent t-test, α=.05) for the first hypothesis (Reaction time: HA > LA) used a mean difference of 98 msec (HA = 483, LA = 385; sigma = 100) which was taken from a comparison of RT collapsed across all valence images for each pilot group. To achieve a power goal of 80%, we calculated that we would need to recruit 18 subjects per group. The power analysis (paired t-test, α=.05) for the second hypothesis (threat < non-threat) used a mean difference of 49 msec calculated from RTs to threat images (M = 385, SD = 60.8) and to all non-threat images (M = 434, SD = 80.4) collapsed across all pilot subjects. With a power goal of 80%, we calculated that we would need to recruit a total of 32 subjects for each target force. Taking each power analysis into account, we took the more conservative estimate by setting a recruitment target of 18 subjects per group.
Adventure: 5621, 8180, 8185, 8186, 8490; erotic couples: 4647, 4660, 4800, 4659, 4670; mutilation: 3064, 3030, 3060, 3068, 3071; attack: 3530, 6230, 6250, 6313, 6560; neutral: 7000, 7010, 7030, 7025, 7090, 7059, 7175, 7052, 7050, 7055.
References
- Arnell KM, Killman KV, Fijavz D. Blinded by emotion: Target misses follow attention capture by arousing distractors in RSVP. Emotion. 2007;7(3):465–477. doi: 10.1037/1528-3542.7.3.465. [DOI] [PubMed] [Google Scholar]
- Baddeley AD. Working Memory. Clarendon Press; Oxford, England: 1986. [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoom MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Jellison WA, Rydell RJ, McConnell AR, Carr TH. On the causal mechanisms of stereotype threat: can skills that don't rely heavily on working memory still be threatened? Pers Soc Psychol Bull. 2006;32(8):1059–1071. doi: 10.1177/0146167206288489. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Kulp CA, Holt LE, Carr TH. More on the fragility of performance: Choking under pressure in mathematical problem solving. Journal of Experimental Psychology: General. 2004;133(4):584–600. doi: 10.1037/0096-3445.133.4.584. [DOI] [PubMed] [Google Scholar]
- Blair KS, Smith BW, Mitchell DG, Morton J, Vythilingam M, Pessoa L, et al. Modulation of emotion by cognition and cognition by emotion. Neuroimage. 2007;35(1):430–440. doi: 10.1016/j.neuroimage.2006.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bledowski C, Prvulovic D, Goebel R, Zanella F, Linden DEJ. Attentional systems in target and distractor processing: A combined ERP and fMRI study. NeuroImage. 2004;22:530–540. doi: 10.1016/j.neuroimage.2003.12.034. [DOI] [PubMed] [Google Scholar]
- Bonnot V, Croizet J. Stereotype internalization and women's math performance: The role of interference in working memory. Journal of Experimental Social Psychology. 2007;43:857–866. [Google Scholar]
- Bradley BP, Mogg K, Falla SJ, Hamilton LR. Attentional bias for threatening facial expressions in anxiety: Manipulation of stimulus duration. Cognition and Emotion. 1998;12(6):737–753. [Google Scholar]
- Calvo MG, Nummenmaa L, Hyona J. Emotional and neutral scenes in competition: orienting, efficiency, and identification. Q J Exp Psychol (Colchester) 2007;60(12):1585–1593. doi: 10.1080/17470210701515868. [DOI] [PubMed] [Google Scholar]
- Calvo MG, Ramos PM. Effects of test anxiety on motor learning: The processing efficiency hypothesis. Anxiety Research. 1989;2:45–55. [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Emotion and movement: activation of defensive circuitry alters the magnitude of a sustained muscle contraction. Neurosci Lett. 2006;396(3):192–196. doi: 10.1016/j.neulet.2005.11.048. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Dissociating motivational direction and affective valence: specific emotions alter central motor processes. Psychol Sci. 2007a;18(11):938–942. doi: 10.1111/j.1467-9280.2007.02005.x. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Cauraugh JH, Janelle CM. Emotional state and initiating cue alter central and peripheral motor processes. Emotion. 2007b;7(2):275–284. doi: 10.1037/1528-3542.7.2.275. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Gamble KM, Cauraugh JH, Janelle CM. Emotional states alter force control during a feedback occluded motor task. Emotion. 2008;8(1):104–113. doi: 10.1037/1528-3542.8.1.104. [DOI] [PubMed] [Google Scholar]
- Coombes SA, Janelle CM, Duley AR. Emotion and motor control: movement attributes following affective picture processing. J Mot Behav. 2005;37(6):425–436. doi: 10.3200/JMBR.37.6.425-436. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Darke S. Effects of anxiety on inferential reasoning and task performance. Journal of Personality and Social Psychology. 1988;55:499–505. doi: 10.1037//0022-3514.55.3.499. [DOI] [PubMed] [Google Scholar]
- de Gelder B, Snyder J, Greve D, Gerard G, Hadjikhani N. Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proc Natl Acad Sci U S A. 2004;101(47):16701–16706. doi: 10.1073/pnas.0407042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derakshan N, Eysenck MW. Working memory capacity in high trait-anxious and repressor groups. Cognition and Emotion. 1998;12(5):697–713. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol. 2002;111(2):225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Ehrsson H, Fagergren A, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: An fMRI study. Journal of Neurophysiology. 2001;85:2613–2623. doi: 10.1152/jn.2001.85.6.2613. [DOI] [PubMed] [Google Scholar]
- Elliman NA, Green MW, Rogers PJ, Finch GM. Processing-efficiency theory and the working memory system: Impairments associated with sub-clinical anxiety. Personality and Individual Differences. 1997;23(1):31–35. [Google Scholar]
- Etkin A, Klemenhagen KC, Dudman JT, Rogan MT, Hen R, Kandel ER, et al. Individual differences in trait anxiety predict the response of the basolateral amygdala to unconsciously processed fearful faces. Neuron. 2004;44(1043−1055) doi: 10.1016/j.neuron.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: The processing efficiency theory. Cognition and Emotion. 1992;6:409–434. [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7(2):336–353. doi: 10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Eastwood JD. Modulation of focused attention by faces expressing emotion: Evidence from Flanker tasks. Emotion. 2003;3(4):327–343. doi: 10.1037/1528-3542.3.4.327. [DOI] [PubMed] [Google Scholar]
- Fitts PM. The information capacity of human motor systems in controlling the amplitude of movement. Journal of Experimental Psychology. 1954;47:381–391. [PubMed] [Google Scholar]
- Fitts PM. Cognitive aspects of information processing. 3. Set for speed versus accuracy. J Exp Psychol. 1966;71(6):849–857. doi: 10.1037/h0023232. [DOI] [PubMed] [Google Scholar]
- Forster J, Higgins ET. How global versus local perception fits regulatory focus. Psychol Sci. 2005;16(8):631–636. doi: 10.1111/j.1467-9280.2005.01586.x. [DOI] [PubMed] [Google Scholar]
- Forster J, Higgins ET, Bianco AT. Speed/accuracy decisions in task performance: Built-in trade-off or separate strategic concerns? Organizational Behavior and Human Decision Processes. 2003;90:148–164. [Google Scholar]
- Fox E. Processing emotional facial expressions: The role of anxiety and awareness. Cognitive, Affective, & Behavioral Neuroscience. 2002;2(1):52–63. doi: 10.3758/cabn.2.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety. Journal of Experimental Psychology: General. 2001;130(4):681–700. [PMC free article] [PubMed] [Google Scholar]
- Glover S. Separate visual representations in the planning and control of action. Behav Brain Sci. 2004;27(1):3–24. doi: 10.1017/s0140525x04000020. discussion 24−78. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44(1):91–97. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hardy L, Beattie S, Woodman T. Anxiety-induced performance catastrophes: Investigating effort required as an asymmetry factor. British Journal of Psychology. 2007;98:15–31. doi: 10.1348/000712606x103428. [DOI] [PubMed] [Google Scholar]
- Hopko DR, McNeil DW, Gleason PJ, Rabalais AE. The emotional stroop paradigm: Performance as a function of stimulus properties and self-reported mathematics anxiety. Cognitive Therapy and Research. 2002;26(2):157–166. [Google Scholar]
- Janelle CM. Anxiety, arousal and visual attention: A mechanistic account of performance variability. Journal of Sports Sciences. 2002;20:237–251. doi: 10.1080/026404102317284790. [DOI] [PubMed] [Google Scholar]
- Janelle CM, Hatfield B. Visual attention and brain processes that underlie expert performance: Implications for sport and military psychology. Military Psychology. 2008;20:117–134. [Google Scholar]
- Janelle CM, Singer RN, Williams AM. External distraction and attentional narrowing: Visual search evidence. Journal of Sport and Exercise Psychology. 1999;21:70–91. [Google Scholar]
- Koster E, Croombez G, Verschuere B, De Houwer J. Attention to threat in anxiety-prone individuals: Mechanisms underlying attentional bias. Cognitive Therapy Research. 2006;30:635–643. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida; Gainesville, FL: 2005. Technical Report A-6. [Google Scholar]
- Lohr JB, Caligiuri MP. Abnormalities in motor physiology in bipolar disorder. J Neuropsychiatry Clin Neurosci. 2006;18(3):342–349. doi: 10.1176/jnp.2006.18.3.342. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. Q J Exp Psychol A. 1988;40(4):653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Ambady N, Kleck RE. The effects of fear and anger facial expressions on approach- and avoidance-related behaviors. Emotion. 2005;5(1):119–124. doi: 10.1037/1528-3542.5.1.119. [DOI] [PubMed] [Google Scholar]
- Marshall MM, Armstrong TJ. Observational assessment of forceful exertion and the perceived force demands of daily activities. J Occup Rehabil. 2004;14(4):281–294. doi: 10.1023/b:joor.0000047430.22740.57. [DOI] [PubMed] [Google Scholar]
- McPhee SD. Functional hand evaluations: a review. Am J Occup Ther. 1987;41(3):158–163. doi: 10.5014/ajot.41.3.158. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behav Res Ther. 2002;40(12):1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Murray NP, Janelle CM. Anxiety and performance: A visual search examination of the processing efficiency theory. Journal of Sport and Exercise Psychology. 2003;25:171–187. [Google Scholar]
- Norman G, Eva K, Brooks L, Hamstra S. Expertise in medicine and surgery. In: Ericsson KA, Charness N, Hoffman RR, Feltovich PJ, editors. Cambridge Handbook of Expertise and Expert Performance. Cambridge; New York: 2006. pp. 339–353. [Google Scholar]
- Pessiglione M, Schmidt L, Draganski B, Kalisch R, Lau H, Dolan RJ, et al. How the brain translates money into force: a neuroimaging study of subliminal motivation. Science. 2007;316(5826):904–906. doi: 10.1126/science.1140459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DA, Loukopoulos LD, Meulenbroek RG, Vaughan J, Engelbrecht SE. Planning reaches by evaluating stored postures. Psychol Rev. 1995;102(1):28–67. doi: 10.1037/0033-295x.102.1.28. [DOI] [PubMed] [Google Scholar]
- Rossi S, Bartalini S, Ulivelli A, Mantovani A, Di Muro A. Hypofunctioning of sensory gating mechanisms in patients with Obsessive-Compulsive Disorder. Biological Psychiatry. 2005;57:16–20. doi: 10.1016/j.biopsych.2004.09.023. [DOI] [PubMed] [Google Scholar]
- Satava RM, Gallagher AG, Pellegrini CA. Surgical competence and surgical proficiency: definitions, taxonomy, and metrics. J Am Coll Surg. 2003;196(6):933–937. doi: 10.1016/S1072-7515(03)00237-0. [DOI] [PubMed] [Google Scholar]
- Smith NC, Bellamy M, Collins DJ, Newell D. A test of processing efficiency theory in a team sport context. Journal of Sports Sciences. 2001;19:321–332. doi: 10.1080/02640410152006090. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory (STAI) Consulting Psychologists Press; PaloAlto, CA: 1983. [Google Scholar]
- Vaillancourt DE, Newell KM. Aging and the time and frequency structure of force output variability. J Appl Physiol. 2003;94(3):903–912. doi: 10.1152/japplphysiol.00166.2002. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, d' Alfonso AL, Kessels RPC, de Haan EHF. 1 Hz rTms over the right prefrontal cortex reduces vigilant attention to unmasked but not to masked fearful faces. Biological Psychiatry. 2002;52:312–317. doi: 10.1016/s0006-3223(02)01346-x. [DOI] [PubMed] [Google Scholar]
- Wada MN, Sunago N, Nagai M. Anxiety affects the postural sway of the antero-posterior axis in college students. Neuroscience Letters. 2001;302:157–159. doi: 10.1016/s0304-3940(01)01662-7. [DOI] [PubMed] [Google Scholar]
- Williams AM, Vickers J, Rodriques S. The effects of anxiety on visual search, movement kinematics, and performance in table tennis: A test of Eysenck and Calvo's processing efficiency theory. Journal of Sport & Exercise Psychology. 2002;24:438–456. [Google Scholar]
- Williams LM, Liddell BJ, Kemp AH, Bryant RA, Meares RA, Peduto AS, et al. Amygdala-prefrontal dissociation of subliminal and supraliminal fear. Human Brain Mapping. 2006;27:652–661. doi: 10.1002/hbm.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M, Chattington M, Marple-Horvat DE, Smith NC. A comparison of self-focus versus attentional explanations of choking. Journal of Sport & Exercise Psychology. 2007;29:439–456. doi: 10.1123/jsep.29.4.439. [DOI] [PubMed] [Google Scholar]
- Yardley L, Britton J, Lear S. Relationship between balance system function and agoraphobic avoidance. Behavior Research and Therapy. 1995;33(4):435–439. doi: 10.1016/0005-7967(94)00060-w. [DOI] [PubMed] [Google Scholar]